Opportunities and Challenges for Next-Generation Thick Cathodes in Lithium-Ion Batteries
Abstract
1. Introduction
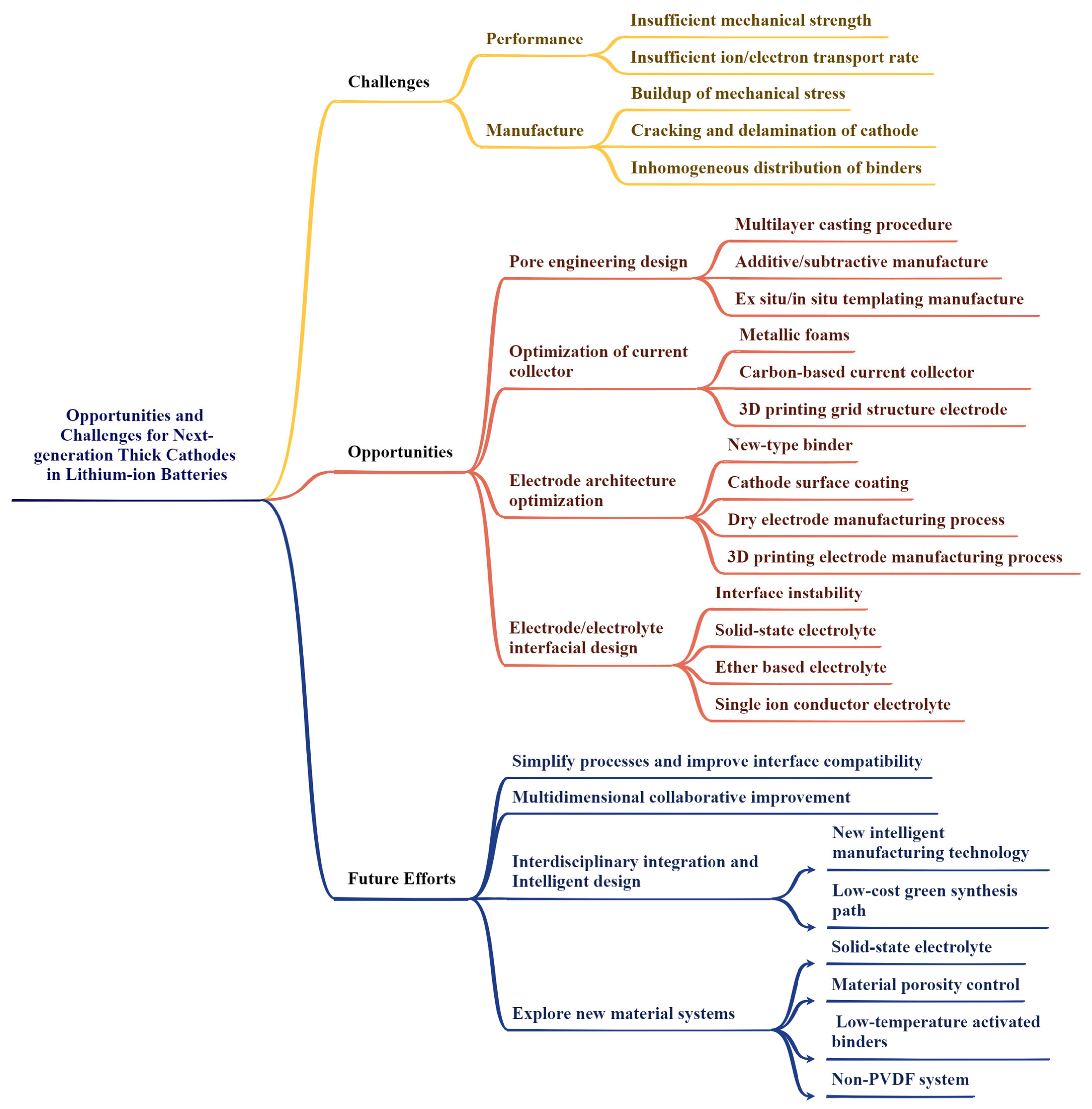
2. Challenges for Thick Cathodes
2.1. Performance-Related Challenges of Thick Cathodes
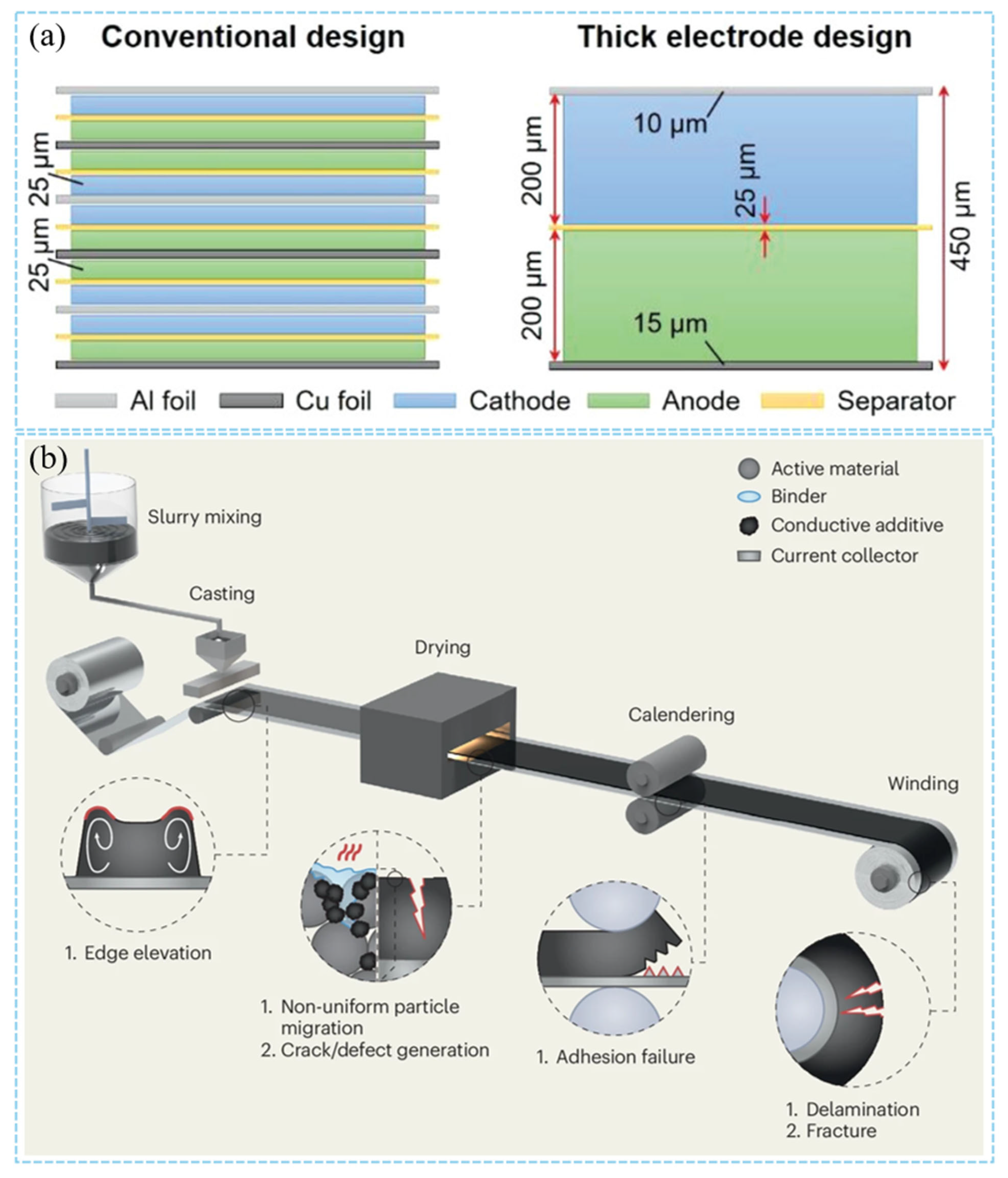
2.2. Manufacture-Related Challenges of Thick Cathodes
3. Strategies to Enhance Thick Cathodes’ Performances
3.1. Pore Engineering
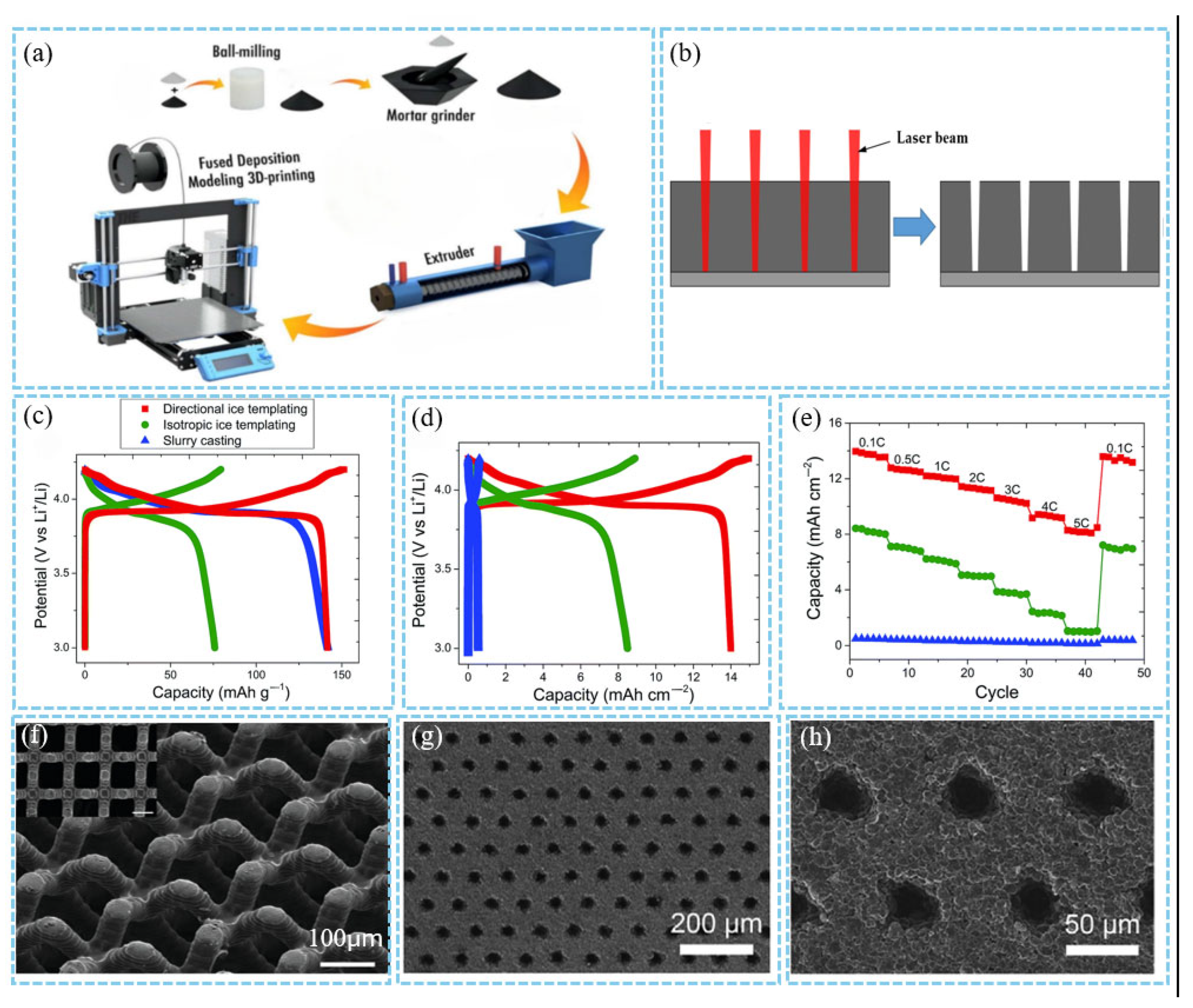
3.1.1. Additive/Subtractive Manufacture
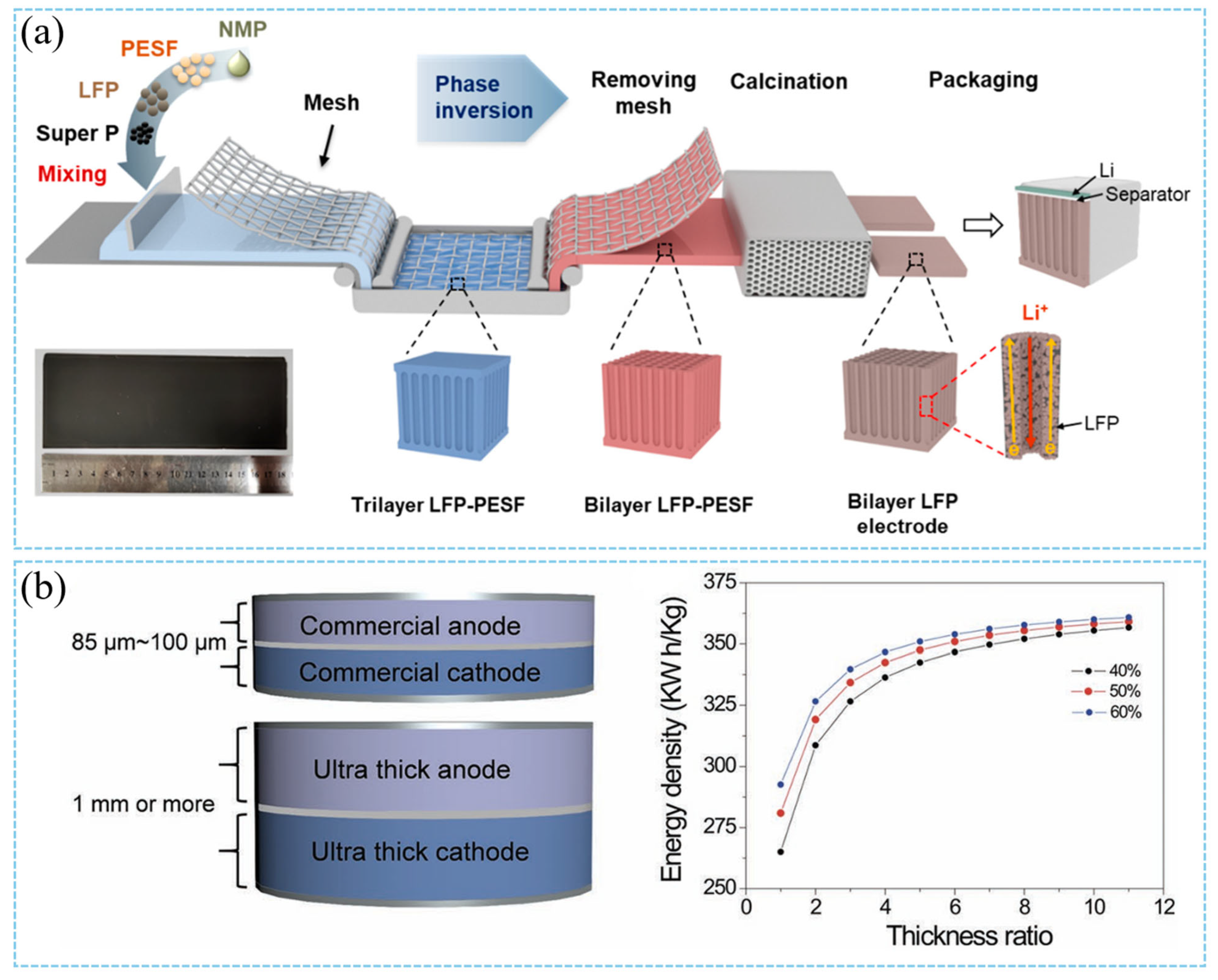
3.1.2. Ex Situ/In Situ Templating Manufacture
3.1.3. Multilayer Casting Procedure
3.2. Innovative Cathode Architecture Design
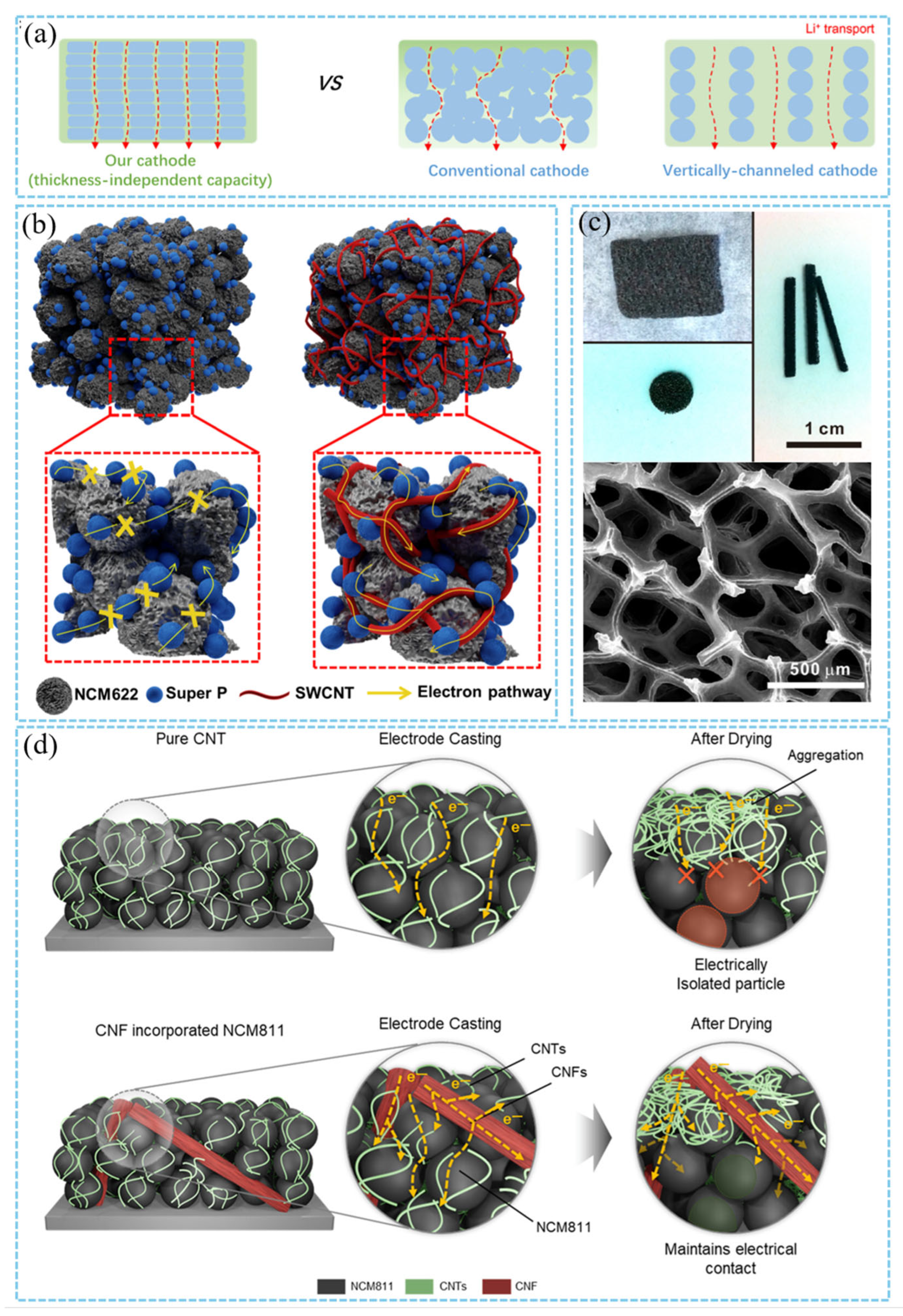
3.2.1. Two-Dimensional Conductive Percolation Network-Based Current Collector
3.2.2. Three-Dimensional Conductive Scaffold-Based Current Collector
3.2.3. Cathode Architecture Optimization
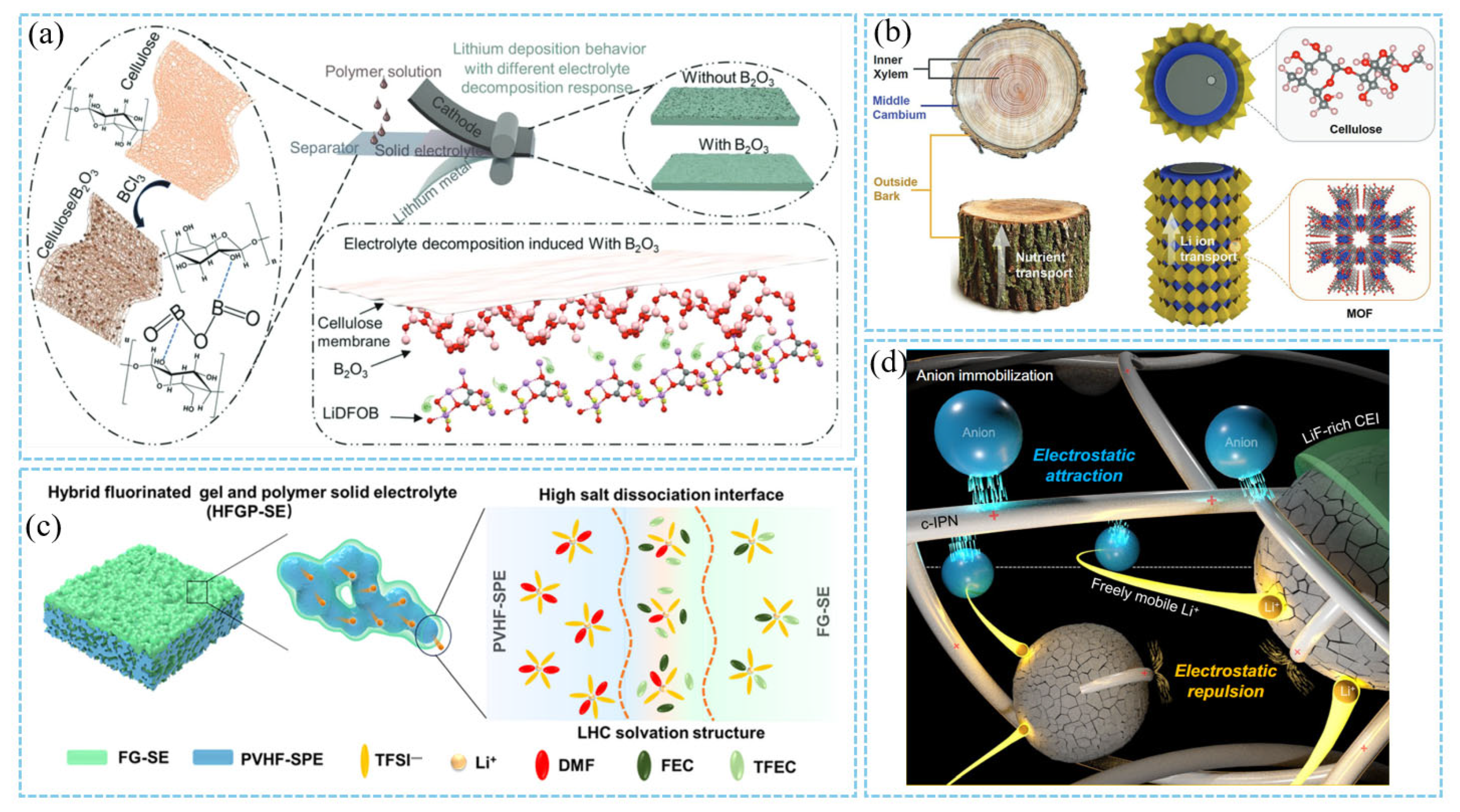
3.3. Electrode/Electrolyte Interfacial Design
3.4. Other Strategies
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, S.; Zhu, L.; He, L.; He, S.; Qin, X.; Zhao, C.; Kang, F.; Li, B. Synthesis Design of Interfacial Nanostructure for Nickel-rich Layered Cathodes. Nano Energy 2022, 97, 107119. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, L.; Wang, S.; Kang, F.; Li, B. Micro- and Nano-structural Design Strategies Towards Polycrystalline Nickel-rich Layered Cathode Materials. J. Mater. Chem. A 2023, 11, 7867–7897. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Zhao, S.; Wang, A.; Luo, J.; Wang, Z.L.; Kang, F.; Lin, Z.; Li, B. Advanced Matrixes for Binder-Free Nanostructured Electrodes in Lithium-Ion Batteries. Adv. Mater. 2020, 32, e1908445. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, L.; Fu, Z.; Lou, J.; Gao, Z.; Wu, J.; Li, C.; Han, C.; Zhou, D.; Wang, Z.; et al. Unraveling Mechanism for Microstructure Engineering toward High-Capacity Nickel-Rich Cathode Materials. Adv. Mater. 2024, 36, e2406175. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, C.; Chen, K.; Wang, Y.; Liu, Q.; Liu, Y.; Ma, B.; Mi, L.; Mao, W. Perspectives on Strategies and Techniques for Building Robust Thick Electrodes for Lithium-ion Batteries. J. Power Sources 2022, 551, 232176. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, N.-Y.; Ju, Z.; Hong, Y.-K.; Kang, K.-D.; Pang, J.-H.; Lee, S.-J.; Chae, S.-S.; Park, M.-S.; Kim, J.-Y.; et al. Upscaling High-areal-capacity Battery Electrodes. Nat. Energy 2025, 10, 295–307. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for Practical High-energy Long-cycling Lithium Metal Batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef]
- Du, M.; Hao, Z.-L.; Liu, Y.; Ma, M.-Y.; Yang, J.-L.; Huang, Z.-X.; Gu, Z.-Y.; Zhang, K.-Y.; Guo, J.-Z.; Wu, X.-L. Architecture Engineering for Thick Electrodes in High-Energy Batteries: Challenges and Strategies. ACS Appl. Mater. Interfaces 2025, 17, 19230–19246. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M.; Cumming, D.J.; Huang, C.; Zankowski, S.P.; Grant, P.S.; Brett, D.J.L.; Shearing, P.R. Design of Scalable, Next-Generation Thick Electrodes: Opportunities and Challenges. ACS Nano 2021, 15, 18624–18632. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Liang, J.; Cui, C.; Zhai, T.; Li, H. Dynamic Investigation of Battery Materials via Advanced Visualization: From Particle, Electrode to Cell Level. Adv. Mater. 2022, 34, 2200777. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Z.; Cheng, S.; Xie, J. Recent Progress and Perspective on Lithium Metal Battery with Nickel-rich Layered Oxide Cathode. eScience 2024, 4, 100265. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.-J.; Jeong, S.; Kim, H.-J. Challenges, Laser Processing and Electrochemical Characteristics on Application of Ultra-thick Electrode for High-energy Lithium-ion Battery. J. Power Sources 2021, 482, 228948. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Marschilok, A.C.; Takeuchi, K.J.; Wang, H.; Takeuchi, E.S.; Yu, G. Gradient Design for High-Energy and High-Power Batteries. Adv. Mater. 2022, 34, e2202780. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Hwang, J.; Song, M.; Song, W.-J.; Lee, K.J. Recent Progress on Solvent-free Electrode Fabrication for Lithium-based Batteries. Chem. Eng. J. 2025, 511, 161888. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Ju, Z.; Wang, L.; Hui, Z.; Mayilvahanan, K.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems. Adv. Mater. 2021, 33, 2101275. [Google Scholar] [CrossRef] [PubMed]
- Joraleechanchai, N.; Sangsanit, T.; Homlamai, K.; Krapong, P.; Sawangphruk, M. Insight into the Effect of Thick Graphite Electrodes Towards High-performance Cylindrical Ni-rich NCA90 Li-ion Batteries. J. Energy Chem. 2023, 87, 322–333. [Google Scholar] [CrossRef]
- Alolaywi, H.Y.; Uzun, K.; Cheng, Y.T. “Zero” Porosity High Loading NMC622 Positive Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2024, 171, 010514. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Daniel, C.; Kalnaus, S.; Li, J. Understanding Limiting Factors in Thick Electrode Performance as Applied to High Energy Density Li-ion Batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- Hu, J.T.; Wu, B.B.; Cao, X.; Bi, Y.J.; Chae, S.J.; Niu, C.J.; Xiao, B.W.; Tao, J.H.; Zhang, J.G.; Xiao, J. Evolution of the Rate-limiting Step: From Thin Film to Thick Ni-rich Cathodes. J. Power Sources 2020, 454, 227966. [Google Scholar] [CrossRef]
- Shodiev, A.; Zanotto, F.M.; Yu, J.; Chouchane, M.; Li, J.; Franco, A.A. Designing Electrode Architectures to Facilitate Electrolyte Infiltration for Lithium-ion Batteries. Energy Storage Mater. 2022, 49, 268–277. [Google Scholar] [CrossRef]
- Dai, Y.; Srinivasan, V. On Graded Electrode Porosity as a Design Tool for Improving the Energy Density of Batteries. J. Electrochem. Soc. 2016, 163, A406–A416. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Ju, Z.; Guo, X.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Unraveling the Effects of Hierarchical Bimodal Microscale Porosity on Thick Electrodes. J. Phys. Chem. C 2022, 126, 15135–15143. [Google Scholar] [CrossRef]
- Prasad, M.; Hein, S.; Danner, T.; Prifling, B.; Scurtu, R.; Hoffmann, A.; Hilger, A.; Osenberg, M.; Manke, I.; Wohlfahrt-Mehrens, M.; et al. Influence of Conductive Additives and Binder on the Impedance of Lithium-Ion Battery Electrodes: Effect of an Inhomogeneous Distribution. J. Electrochem. Soc. 2024, 171, 013546. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L. Enabling Aqueous Processing for Crack-free Thick Electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Guo, H.; Qin, Q.; Wang, Z.; Wang, J.; Yan, G. Visualization of Concentration Polarization in Thick Electrodes. Energy Storage Mater. 2022, 51, 476–485. [Google Scholar] [CrossRef]
- Johnson, A.C.; Dunlop, A.J.; Kohlmeyer, R.R.; Kiggins, C.T.; Blake, A.J.; Singh, S.V.; Beale, E.M.; Zahiri, B.; Patra, A.; Yue, X.; et al. Strategies for Approaching One Hundred Percent Dense Lithium-Ion Battery Cathodes. J. Power Sources 2022, 532, 231359. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Liang, L. Thickness Effect on the Mechanical Performance of Cathodes in Lithium-Ion Batteries. J. Energy Storage 2024, 86, 111417. [Google Scholar] [CrossRef]
- Park, S.H.; Naik, K.G.; Vishnugopi, B.S.; Xiao, X.; Drakopoulos, M.; Vo, N.T.; Zhong, Z.; Mukherjee, P.P.; Hatzell, K.B. Chemo-Mechanical Behavior and Stability of High-Loading Cathodes in Solid-State Batteries. ACS Nano 2025, 19, 22262–22269. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Juarez-Yescas, C.; Naik, K.G.; Wang, Y.; Luo, Y.; Puthusseri, D.; Kwon, P.; Vishnugopi, B.S.; Shyam, B.; Yang, H.; et al. Morphological Heterogeneity Impact of Film Solid-State Cathode on Utilization and Fracture Dynamics. ACS Nano 2025, 19, 21878–21890. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Pham, H.Q.; Kang, D.-H.; Park, H.-Y.; Song, S.-W. Improved Rate Capability of Highly Loaded Carbon Fiber-interwoven LiNi0.6Co0.2Mn0.2O2 Cathode Material for High-power Li-ion Batteries. J. Alloys Compd. 2016, 657, 464–471. [Google Scholar] [CrossRef]
- Chen, W.; Wang, K.; Li, Y.; Chen, J.; Wang, H.; Li, L.; Li, H.; Ren, X.; Ouyang, X.; Liu, J.; et al. Minimize the Electrode Concentration Polarization for High-Power Lithium Batteries. Adv. Funct. Mater. 2024, 34, 2410926. [Google Scholar] [CrossRef]
- Wu, J.; Ju, Z.; Zhang, X.; Quilty, C.; Takeuchi, K.J.; Bock, D.C.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Ultrahigh-Capacity and Scalable Architected Battery Electrodes via Tortuosity Modulation. ACS Nano 2021, 15, 19109–19118. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ju, Z.; Zhang, X.; Xu, X.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S.; Yu, G. Low-Tortuosity Thick Electrodes with Active Materials Gradient Design for Enhanced Energy Storage. ACS Nano 2022, 16, 4805–4812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, M.; Chen, W.; Liu, Y.; Zhang, L.; Dongfang, N.; Ruan, Y.; Zhang, J.; Wang, P.; Dong, L.; et al. Sandwich, Vertical-Channeled Thick Electrodes with High Rate and Cycle Performance. Adv. Funct. Mater. 2019, 29, 1809196. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Hu, J.-K.; Huang, J.-Q.; Zhang, Q. Dry Electrode Technology, the Rising Star in Solid-state Battery Industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Li, D.; Li, S.; Xiong, Q.; Huang, Z.; Wang, S.; Hong, H.; Zhu, J.; Lv, H.; et al. Salt Dissociation and Localized High-concentration Solvation at the Interface of a Fluorinated Gel and Polymer Solid Electrolyte. Energy Environ. Sci. 2025, 18, 227–235. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, N.; Gao, R.; Li, Z.; Dou, H.; Li, G.; Qian, L.; Deng, Y.; Liang, J.; Yang, L.; et al. “Tree-Trunk” Design for Flexible Quasi-Solid-State Electrolytes with Hierarchical Ion-Channels Enabling Ultralong-Life Lithium-Metal Batteries. Adv. Mater. 2022, 34, 2203417. [Google Scholar] [CrossRef] [PubMed]
- Uzun, K.; Alolaywi, H.; Thapa, S.; Frieberg, B.; Wang, M.; Huang, X.; Cheng, Y.-T. Investigating the Effect of Electrode Compositions on Dry-made NMC811 Positive Electrodes. J. Electrochem. Soc. 2024, 171, 080532. [Google Scholar] [CrossRef]
- Stallard, J.C.; Vema, S.; Hall, D.S.; Dennis, A.R.; Penrod, M.E.; Grey, C.P.; Deshpande, V.S.; Fleck, N.A. Effect of Lithiation upon the Shear Strength of NMC811 Single Crystals. J. Electrochem. Soc. 2022, 169, 040511. [Google Scholar] [CrossRef]
- Tang, X.; Jia, Q.; Yang, L.; Bai, M.; Wu, W.; Wang, Z.; Gong, M.; Sa, S.; Tao, S.; Sun, M.; et al. Towards the High-energy-density Battery with Broader Temperature Adaptability: Self-discharge Mitigation of Quaternary Nickel-rich Cathode. Energy Storage Mater. 2020, 33, 239–249. [Google Scholar] [CrossRef]
- Niri, M.F.; Apachitei, G.; Lain, M.; Copley, M.; Marco, J. Machine Learning for Investigating the Relative Importance of Electrodes? N: P Areal Capacity Ratio in the Manufacturing of Lithium-ion Battery Cells. J. Power Sources 2022, 549, 232124. [Google Scholar] [CrossRef]
- Westermeier, M.; Reinhart, G.; Zeilinger, T. Method for Quality Parameter Identification and Classification in Battery Cell Production Quality Planning of Complex Production Chains for Battery Cells. In Proceedings of the 2013 3rd International Electric Drives Production Conference (EDPC), Nuremberg, Germany, 29–30 October 2013. [Google Scholar]
- Tran, H.Y.; Greco, G.; Täubert, C.; Wohlfahrt-Mehrens, M.; Haselrieder, W.; Kwade, A. Influence of Electrode Preparation on the Electrochemical Performance of LiNi0.8Co0.15Al0.05O2 Composite Electrodes for Lithium-ion Batteries. J. Power Sources 2012, 210, 276–285. [Google Scholar] [CrossRef]
- Lee, B.-S.; Wu, Z.; Petrova, V.; Xing, X.; Lim, H.-D.; Liu, H.; Liu, P. Analysis of Rate-Limiting Factors in Thick Electrodes for Electric Vehicle Applications. J. Electrochem. Soc. 2018, 165, A525–A533. [Google Scholar] [CrossRef]
- Roman-Ramirez, L.A.; Apachitei, G.; Faraji-Niri, M.; Lain, M.; Widanage, W.D.; Marco, J. Understanding the Effect of Coating-drying Operating Variables on Electrode Physical and Electrochemical Properties of Lithium-ion Batteries. J. Power Sources 2021, 516, 230689. [Google Scholar] [CrossRef]
- Meyer, C.; Kosfeld, M.; Haselrieder, W.; Kwade, A. Process Modeling of the Electrode Calendering of Lithium-ion Batteries Regarding Variation of Cathode Active Materials and Mass Loadings. J. Energy Storage 2018, 18, 371–379. [Google Scholar] [CrossRef]
- Zhu, P.; Trouillet, V.; Heissler, S.; Pfleging, W. Laser Structuring of High Mass Loaded and Aqueous Acid Processed Li (Ni0.6Mn0.2Co0.2)O2 Cathodes for Lithium-ion Batteries. J. Energy Storage 2023, 66, 107401. [Google Scholar] [CrossRef]
- Narita, K.; Citrin, M.A.; Yang, H.; Xia, X.; Greer, J.R. 3D Architected Carbon Electrodes for Energy Storage. Adv. Energy Mater. 2020, 11, 2002637. [Google Scholar] [CrossRef]
- Bae, C.J.; Erdonmez, C.K.; Halloran, J.W.; Chiang, Y.M. Design of Battery Electrodes with Dual-scale Porosity to Minimize Tortuosity and Maximize Performance. Adv. Mater. 2013, 25, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.S.; Erb, R.M.; Li, L.; Gurijala, A.; Chiang, Y.M. High-performance Battery Electrodes via Magnetic Templating. Nat. Energy 2016, 1, 16099. [Google Scholar] [CrossRef]
- Huang, C.; Grant, P.S. Coral-like Directional Porosity Lithium ion Battery Cathodes by Ice Templating. J. Mater. Chem. A 2018, 6, 14689–14699. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Qin, X.; Wang, S.; He, L.; Qian, K.; Han, T.; Yang, Z.; Kang, F.; Li, B. Heterogeneous Degradation in Thick Nickel-Rich Cathodes During High-Temperature Storage and Mitigation of Thermal Instability by Regulating Cationic Disordering. Small 2021, 17, 2102055. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, L.; Pettes, M.T.; Li, H.; Chen, S.; Shi, L.; Piner, R.; Ruoff, R.S. Ultrathin Graphite Foam: A Three-Dimensional Conductive Network for Battery Electrodes. Nano Lett. 2012, 12, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.-H.; Didwal, P.N.; Kim, H.-J.; Lim, J.-S.; Nguyen, A.-G.; Jin, C.-S.; Chang, D.R.; Park, C.-J. Reinforcing Effect of Single-wall Carbon Nanotubes on the LiNi0.6Co0.2Mn0.2O2 Composite Cathode for High-energy-density All-solid-state Li-ion Batteries. Appl. Surf. Sci. 2021, 568, 150934. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Wang, Z.; Wang, J.; Guo, H.; Yan, G. Free-standing Ultrathick LiMn2O4@Single-wall Carbon Nanotubes Electrode with High Areal Capacity. J. Energy Chem. 2022, 73, 452–459. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, Z.; Zhang, J.; Zhang, C.; Guo, Y.; Cao, Y.; Pan, S.; Liu, Y.; Liu, P.; Ge, Y.; et al. Thick Electrode with Thickness-independent Capacity Enabled by Assembled Two-dimensional Porous Nanosheets. Energy Storage Mater. 2021, 36, 265–271. [Google Scholar] [CrossRef]
- Yang, G.-F.; Joo, S.-K. Calendering Effect on the Electrochemical Performances of the Thick Li-ion Battery Electrodes Using a Three Dimensional Ni Alloy Foam Current Collector. Electrochim. Acta 2015, 170, 263–268. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Cui, C.; Jin, Y.; Zhang, G.; Zhou, H.; Qian, W. High Power Density & Energy Density Li-ion Battery with Aluminum Foam Enhanced Electrode: Fabrication and Simulation. J. Power Sources 2022, 524, 230977. [Google Scholar] [CrossRef]
- Jo, S.; Lee, K.; Jung, Y.; Woo, D.; Kim, T.; Kim, P.J. Direct-spun Carbon Nanotube Sheet: A Flexible, Ultralight, Stackable Three-dimensional Current Collector for High-performance Lithium-ion Batteries. Carbon 2024, 219, 118786. [Google Scholar] [CrossRef]
- Alolaywi, H.; Uzun, K.; Cheng, Y.-T. Low Porosity NMC622 and NMC811 Electrodes Made by Severe Calendering. J. Energy Storage 2025, 105, 114559. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.-K.; Lee, S.-Y.; Park, J.H. Ultrahigh Loading dry-process for Solvent-free Lithium-ion Battery Electrode Fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, Y.; Yuan, W.; Zhang, G.; Zheng, H.; Chen, Z. Advances in Multi-scale Design and Fabrication Processes for Thick Electrodes in Lithium-ion Batteries. Energy Rev. 2024, 3, 100066. [Google Scholar] [CrossRef]
- Tjaden, B.; Cooper, S.J.; Brett, D.J.L.; Kramer, D.; Shearing, P.R. On the Origin and Application of the Bruggeman Correlation for Analysing Transport Phenomena in Electrochemical Systems. Curr. Opin. Chem. Eng. 2016, 12, 44–51. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Tan, C.; Heenan, T.M.M.; Lagnoni, M.; O’Regan, K.; Daemi, S.; Bertei, A.; Jones, H.G.; Hinds, G.; et al. Multi-length Scale Microstructural Design of Lithium-ion Battery Electrodes for Improved Discharge Rate Performance. Energy Environ. Sci. 2021, 14, 5929–5946. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Hung, F.-Y.; Lui, T.-S.; Tan, H.-P. Microstructure and High Temperature Charge-Discharge Characteristics of 3D Additive Manufacturing Produced Mg-Ni Anode. Mater. Trans. 2018, 59, 685–689. [Google Scholar] [CrossRef]
- Zhu, P.; Sterzl, Y.; Pfleging, W. Impact of Laser Ablation Strategies on Electrochemical Performances of 3D Batteries Containing Aqueous Acid Processed LiNi0.6Mn0.2Co0.2O2 Cathodes with High Mass Loading. Batteries 2024, 10, 354. [Google Scholar] [CrossRef]
- Chen, K.-H.; Namkoong, M.J.; Goel, V.; Yang, C.; Kazemiabnavi, S.; Mortuza, S.M.; Kazyak, E.; Mazumder, J.; Thornton, K.; Sakamoto, J.; et al. Efficient Fast-charging of Lithium-ion Batteries Enabled by Laser-patterned Three-dimensional Graphite aAnode Architectures. J. Power Sources 2020, 471, 228475. [Google Scholar] [CrossRef]
- Fu, K.; Li, X.; Sun, K.; Zhang, Z.; Yang, H.; Gong, L.; Qin, G.; Hu, D.; Li, T.; Tan, P. Rational Design of Thick Electrodes in Lithium-Ion Batteries by Re-Understanding the Relationship Between Thermodynamics and Kinetics. Adv. Funct. Mater. 2024, 34, 2409623. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ju, Z.; Yu, X.; Wang, Y.; Du, Y.; Bai, Z.; Dou, S.; Yu, G. Thickness-independent Scalable High-performance Li-S Batteries with High Areal Sulfur Loading via Electron-enriched Carbon Framework. Nat. Commun. 2021, 12, 4519. [Google Scholar] [CrossRef] [PubMed]
- Delattre, B.; Amin, R.; Sander, J.; De Coninck, J.; Tomsia, A.P.; Chiang, Y.-M. Impact of Pore Tortuosity on Electrode Kinetics in Lithium Battery Electrodes: Study in Directionally Freeze-Cast LiNi0.8Co0.15Al0.05O2(NCA). J. Electrochem. Soc. 2018, 165, A388–A395. [Google Scholar] [CrossRef]
- Lu, L.L.; Lu, Y.Y.; Xiao, Z.J.; Zhang, T.W.; Zhou, F.; Ma, T.; Ni, Y.; Yao, H.B.; Yu, S.H.; Cui, Y. Wood-Inspired High-Performance Ultrathick Bulk Battery Electrodes. Adv. Mater. 2018, 30, e1706745. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Erb, R.M.; Wang, J.; Wang, J.; Chiang, Y.M. Fabrication of Low-Tortuosity Ultrahigh-Area-Capacity Battery Electrodes through Magnetic Alignment of Emulsion-Based Slurries. Adv. Energy Mater. 2018, 9, 1802472. [Google Scholar] [CrossRef]
- Huang, C.; Leung, C.L.A.; Leung, P.; Grant, P.S. A Solid-State Battery Cathode with a Polymer Composite Electrolyte and Low Tortuosity Microstructure by Directional Freezing and Polymerization. Adv. Energy Mater. 2021, 11, 2002387. [Google Scholar] [CrossRef]
- Neidhart, L.; Froehlich, K.; Eshraghi, N.; Cupid, D.; Winter, F.; Jahn, M. Aqueous Manufacturing of Defect-Free Thick Multi-Layer NMC811 Electrodes. Nanomaterials 2022, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hui, Z.; King, S.T.; Wu, J.; Ju, Z.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; Wang, L.; et al. Gradient Architecture Design in Scalable Porous Battery Electrodes. Nano Lett. 2022, 22, 2521–2528. [Google Scholar] [CrossRef]
- Kim, S.; Park, K. Electrode Design to Mitigate the Kinetic Issue of Cathodes in High Energy Lithium-ion Batteries. J. Power Sources 2022, 547, 231916. [Google Scholar] [CrossRef]
- Cai, D.; Gao, M.; Luo, S.; Wu, X.; Yang, Y.; Xie, Y.; Zhu, L.; Deng, X.; Ji, Y.; Yuan, Z. Scalable Thick Ni-rich Layered Oxide Cathode Design for High Energy/Power Balanced Lithium-ion Battery. J. Power Sources 2024, 602, 234276. [Google Scholar] [CrossRef]
- Neidhart, L.; Fröhlich, K.; Winter, F.; Jahn, M. Implementing Binder Gradients in Thick Water-Based NMC811 Cathodes via Multi-Layer Coating. Batteries 2023, 9, 171. [Google Scholar] [CrossRef]
- Neidhart, L.; Froehlich, K.; Boz, B.; Winter, F.; Jahn, M. Layer by Layer: Improved Tortuosity in High Loading Aqueous NMC811 Electrodes. J. Electrochem. Soc. 2024, 171, 050532. [Google Scholar] [CrossRef]
- Song, Z.; Zhu, P.; Pfleging, W.; Sun, J. Electrochemical Performance of Thick-Film Li(Ni0.6Mn0.2Co0.2)O2 Cathode with Hierarchic Structures and Laser Ablation. Nanomaterials 2021, 11, 2962. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Li, J.; Du, Z.; Daniel, C.; Dunlop, A.R.; Polzin, B.J.; Jansen, A.N.; Krumdick, G.K.; Wood, D.L. Impact of Secondary Particle Size and Two-layer Architectures on the High-rate Performance of Thick Electrodes in Lithium-ion Battery Pouch Cells. J. Power Sources 2021, 515, 230429. [Google Scholar] [CrossRef]
- Song, K.; Li, W.; Chen, Z.; Wu, X.; Zhou, Q.; Snyder, K.; Zhang, L. An Effective Approach to Improve Electrochemical Performance of Thick Electrodes. Ionics 2021, 27, 1261–1270. [Google Scholar] [CrossRef]
- Ali, J.; Embleton, T.J.; Choi, J.H.; Won, S.-J.; Saqib, K.S.; Ko, K.; Choi, S.; Jo, M.; Hwang, J.; Park, S.; et al. Overcoming Through-Plane Resistance in Lithium-Ion Battery Cathode Electrodes via the Application of Trace High-Aspect-Ratio Carbon Nanofiber Carbon Additives with Carbon Nanotube-Coated LiNi0.8Co0.1Mn0.1O2. ACS Appl. Energy Mater. 2024, 7, 10134–10148. [Google Scholar] [CrossRef]
- Hallot, M.; Demortiere, A.; Roussel, P.; Lethien, C. Sputtered LiMn1.5Ni0.5O4 thin Films for Li-ion Micro-batteries with High Energy and Rate Capabilities. Energy Storage Mater. 2018, 15, 396–406. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Li, Y.; Kuang, Y.; Song, J.; Luo, W.; Wang, Y.; Yao, Y.; Pastel, G.; Xie, J.; et al. Highly Conductive, Lightweight, Low-Tortuosity Carbon Frameworks as Ultrathick 3D Current Collectors. Adv. Energy Mater. 2017, 7, 1700595. [Google Scholar] [CrossRef]
- Zankowski, S.P.; Chaykina, D.; Vereecken, P.M. Interconnected Ni Nanowires Integrated with LixMnO2 as Fast Charging and High Volumetric Capacity Cathodes for Li-ion Batteries. J. Mater. Chem. A 2020, 8, 14178–14189. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, H.; Busbee, J.; Shen, Z.; Kiggins, C.; Hua, Y.; Eaves, J.; Davis, J.; Shi, T.; Shao, Y.-T.; et al. Electroplating Lithium Transition Metal Oxides. Sci. Adv. 2025, 3, e1602427. [Google Scholar] [CrossRef] [PubMed]
- Oehm, J.; Kamlah, M.; Knoblauch, V. Ultra-Thick Cathodes for High-Energy Lithium-Ion Batteries Based on Aluminium Foams—Microstructural Evolution during Densification and Its Impact on the Electrochemical Properties. Batteries 2023, 9, 303. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Z.; Zhang, H.; Liu, Z.; Zheng, J.; Zhou, J.; Jia, Z.; Zhang, Q.; Lai, C.; Wu, Y.; et al. Stress-Tolerant Printed Architectures Toward Stable Cycling of Ultrahigh-Loading Ni-Rich Layered Oxide Cathodes for WearableEnergy Storage Devices. Energy Fuels 2022, 36, 5009–5017. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-M.; Cho, S.-K.; Kim, N.-Y.; Lee, S.-Y. Redox-homogeneous, Gel Electrolyte-embedded High-mass-loading Cathodes for High-energy Lithium Metal Batteries. Nat. Commun. 2022, 13, 2541. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, R.; Chen, Z.; Wang, X.; Tu, S.; Liu, S.; Zeng, Y.; Dong, T.; Cheng, K.; Ou, Y.; et al. Enhancing Fast-Charging Capability of Thick Electrode in Lithium-Ion Batteries Through Electronic/Ionic Hybrid Conductive Additive Engineering. Adv. Energy Mater. 2025, 15, 2500242. [Google Scholar] [CrossRef]
- Kim, W.; Hwang, C.; Kim, Y.M.; Yu, J.-S.; Kim, Y.-J.; Kim, K.J.; Kim, H.-s. Modulation of Lithium Iron Phosphate Electrode Architecture by Magnetic Ordering for Lithium-ion Batteries. J. Mater. Chem. A 2024, 12, 14786–14791. [Google Scholar] [CrossRef]
- Karanth, P.; Weijers, M.; Ombrini, P.; Ripepi, D.; Ooms, F.; Mulder, F.M. A phase Inversion Strategy for Low-tortuosity and Ultrahigh-mass-loading Nickel-rich Layered Oxide Electrodes. Cell Rep. Phys. Sci. 2024, 5, 101972. [Google Scholar] [CrossRef]
- Wei, T.-S.; Ahn, B.Y.; Grotto, J.; Lewis, J.A. 3D Printing of Customized Li-Ion Batteries with Thick Electrodes. Adv. Mater. 2018, 30, 1703027. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Kim, J.; Han, S.; Lee, J.; Lee, H.; Kwon, J.; Lee, J.; Seo, J.; Kim, P.J.; Song, T.; et al. Low-Resistance LiFePO4 Thick Film Electrode Processed with Dry Electrode Technology for High-Energy-Density Lithium-Ion Batteries. Small Sci. 2024, 4, 2300302. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, G.-S.; Hwang, B.U.; Bang, J.; Kim, J.; Jeong, K.-M. Development of a Feasible and Scalable Manufacturing Method for PTFE-based Solvent-free Lithium-ion Battery Electrodes. Chem. Eng. J. 2024, 491, 151957. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Kim, M.; Lee, H.; Choi, J.; Park, H.B.; Kim, H.; Jang, J.; Kim, Y.H.; Song, T.; et al. 10 mAh cm−2 Cathode by Roll-to-Roll Process for Low Cost and High Energy Density Li-Ion Batteries. Adv. Energy Mater. 2024, 14, 2303455. [Google Scholar] [CrossRef]
- Embleton, T.J.; Choi, J.H.; Won, S.-J.; Ali, J.; Saqib, K.S.; Ko, K.; Jo, M.; Hwang, J.; Park, J.; Lee, J.H.; et al. High-Energy Density Ultra-Thick Drying-Free Ni-Rich Cathode Electrodes for Application in Lithium-Ion Batteries. Energy Storage Mater. 2024, 71, 103542. [Google Scholar] [CrossRef]
- Kim, H.; Lim, J.H.; Lee, T.; An, J.; Kim, H.; Song, H.; Lee, H.; Choi, J.W.; Kang, J.H. Ozone-Treated Carbon Nanotube as a Conductive Agent for Dry-Processed Lithium-Ion Battery Cathode. ACS Energy Lett. 2023, 8, 3460–3466. [Google Scholar] [CrossRef]
- Linh, C.N.T.; Thuc, V.D.; Mai, D.D.; Nguyen, M.C.; Pham, D.T.; Yu, W.J.; Kim, D. Dispersion-assisted Carbon Nanotubes as a Conductive Agent for Dry-processed Cathode for Lithium-ion Battery. Chem. Eng. J. 2025, 509, 161183. [Google Scholar] [CrossRef]
- Jeong, D.; Kwon, D.-S.; Kim, H.J.; Shim, J. Striking a Balance: Exploring Optimal Functionalities and Composition of Highly Adhesive and Dispersing Binders for High-Nickel Cathodes in Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2302845. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Moon, J.; Ryou, M.-H.; Kim, S.-H.; Kim, J.-H.; Kim, J.-M.; Bang, J.; Lee, S.-Y. Amphiphilic Bottlebrush Polymeric Binders for High-Mass-Loading Cathodes in Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2102109. [Google Scholar] [CrossRef]
- Jang, J.; Ahn, J.; Ahn, J.; Jeong, U.; Yoon, J.; Park, J.K.; Shin, W.; Kang, M.J.; Cho, M.-k.; Kang, D.J.; et al. A Fluorine-Free Binder with Organic-Inorganic Crosslinked Networks Enabling Structural Stability of Ni-Rich Layered Cathodes in Lithium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2410866. [Google Scholar] [CrossRef]
- Zahiri, B.; Patra, A.; Kiggins, C.; Bin Yong, A.X.; Ertekin, E.; Cook, J.B.; Braun, P.V. Revealing the role of the cathode–electrolyte interface on solid-state batteries. Nat. Mater. 2021, 20, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.; Lee, Y.M.; Shin, D.O.; Kim, J.Y.; Lee, Y.-G.; Kim, K.M. High-rate Cycling Performance and Surface Analysis of LiNi1−xCox/2Mnx/2O2 (x = 2/3, 0.4, 0.2) Cathode Materials. Mater. Chem. Phys. 2019, 222, 1–10. [Google Scholar] [CrossRef]
- Miao, R.; Yang, J.; Xu, Z.; Wang, J.; Nuli, Y.; Sun, L. A New Ether-based Electrolyte for Dendrite-free Lithium-Metal Based Rechargeable Batteries. Sci. Rep. 2016, 6, 21771. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Ma, S.B.; Lee, D.J.; Im, D.; Doo, S.-G.; Yamamoto, O. A Highly Reversible Lithium Metal Anode. Sci. Rep. 2014, 4, 3815. [Google Scholar] [CrossRef] [PubMed]
- Dato, M.; Hafiz, H.; Liu, Z.; Hung, C.; Lopez, J.; Guo, J.; Amine, K.; He, M.; Su, C.-C. Nonsolvating Fluoroaromatic Cosolvent Enabled Long-Term Cycling of High-Voltage Lithium-Ion Batteries with Organosulfur Electrolytes. ACS Appl. Mater. Interfaces 2024, 16, 42069–42079. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Qu, K.; Wang, Y.; Shen, K.; Jiang, C.; Yu, B.; Luo, P.; Li, Z.; Chen, M.; et al. Concentrated Ternary Ether Electrolyte Allows for Stable Cycling of a Lithium Metal Battery with Commercial Mass Loading High-nickel NMC and Thin Anodes. Carbon Energy 2023, 5, e275. [Google Scholar] [CrossRef]
- Cheng, H.; Cao, J.; Li, F.; Geng, X.; Li, D.; Wei, Y.; Lin, X.; Xu, H.; Huang, Y. Inorganic-Rich Interphase Induced by Boric Oxide Solid Acid toward Long Cyclic Solid-State Lithium-Metal Batteries. Adv. Funct. Mater. 2024, 34, 2307677. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.M.; Kim, J.W.; Kweon, S.H.; Moon, H.-S.; Yim, T.; Kwak, S.K.; Lee, S.-Y. Regulating Electrostatic Phenomena by Cationic Polymer Binder for Scalable High-areal-capacity Li Battery Electrodes. Nat. Commun. 2023, 14, 5721. [Google Scholar] [CrossRef] [PubMed]
- Hallot, M.; Caja-Munoz, B.; Leviel, C.; Lebedev, O.I.; Retoux, R.; Avila, J.; Roussel, P.; Asensio, M.C.; Lethien, C. Atomic Layer Deposition of a Nanometer-Thick Li3PO4 Protective Layer on LiNi0.5Mn1.5O4 Films: Dream or Reality for Long-Term Cycling? ACS Appl. Mater. Interfaces 2021, 13, 15761–15773. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, L.; Zhai, L.; Oh, K.S.; Seo, J.M.; Li, C.; Han, D.; Baek, J.B.; Lee, S.Y. Olefin-Linked Covalent Organic Frameworks with Electronegative Channels as Cationic Highways for Sustainable Lithium Metal Battery Anodes. Angew. Chem. Int. Ed. 2023, 62, e202307459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meng, X.; Hou, W.; Hu, W.; Mo, J.; Yang, T.; Zhang, W.; Fan, Q.; Liu, L.; Jiang, B.; et al. Solid Polymer Electrolytes: Ion Conduction Mechanisms and Enhancement Strategies. Nano Res. Energy 2023, 2, e9120050. [Google Scholar] [CrossRef]
- Dong, X.; Mayer, A.; Liu, X.; Passerini, S.; Bresser, D. Single-Ion Conducting Multi-block Copolymer Electrolyte for Lithium-Metal Batteries with High Mass Loading NCM811 Cathodes. ACS Energy Lett. 2023, 8, 1114–1121. [Google Scholar] [CrossRef]
- Liang, H.-P.; Zarrabeitia, M.; Chen, Z.; Jovanovic, S.; Merz, S.; Granwehr, J.; Passerini, S.; Bresser, D. Polysiloxane-Based Single-Ion Conducting Polymer Blend Electrolyte Comprising Small-Molecule Organic Carbonates for High-Energy and High-Power Lithium-Metal Batteries. Adv. Energy Mater. 2022, 12, 2200013. [Google Scholar] [CrossRef]
- He, Y.; Shan, X.; Li, Y.; Li, Z.; Li, L.; Zhao, S.; Gao, S.; Qu, J.; Yang, H.; Cao, P.-F. In-situ Formation of Quasi-solid Polymer Electrolyte for Wide-temperature Applicable Li-metal Batteries. Energy Storage Mater. 2024, 68, 103281. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Y.; Su, Q.; Lu, Y.; Liu, K.; Li, Z.; Liu, H.; Zhang, L. Dual Modified NCMA Cathode with Enhanced Interface Stability Enabled High-performance Sulfide-based All-solid-state Lithium Battery. Chem. Eng. J. 2023, 471, 144405. [Google Scholar] [CrossRef]
- Chen, K.; Tang, Y.; Zhang, S.; Hao, X.; Zhao, X.; Cheng, L.-Q.; Xiao, Y.; Wen, Z. Promoted Stability and Reaction Kinetics in Ni-Rich Cathodes via Mechanical Fusing Multifunctional LiZr2(PO4)3 Nanocrystals for High Mass Loading All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 45459–45472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, T.; Gao, S.; Ding, H.; Li, Z.; Li, L.; Yang, D.; Yang, H.; Cao, P.-F. Construction of an Ultrathin Multi-functional Polymer Electrolyte for Safe and Stable All-solid-state Batteries. Mater. Horiz. 2025, 12, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Wang, Z.; Liao, J.; Zhang, H.; Song, D.; Zhu, L.; Zhang, L. High-Voltage Stability of Small-Size Single Crystal Ni-Rich Layered Cathode for Sulfide-Based All-Solid-State Lithium Battery at 4.5 V. Adv. Energy Mater. 2023, 13, 2300850. [Google Scholar] [CrossRef]
- Kim, U.H.; Yu, T.Y.; Lee, J.W.; Lee, H.U.; Belharouak, I.; Yoon, C.S.; Sun, Y.K. Microstructure- and Interface-Modified Ni-Rich Cathode for High-Energy-Density All-Solid-State Lithium Batteries. ACS Energy Lett. 2023, 8, 809–817. [Google Scholar] [CrossRef]
- Plateau, T.P.; Pham, H.; Zhu, Y.; Leu, M.; Park, J. Enabling Ultrathick Electrodes via a Microcasting Process for High Energy and Power Density Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201353. [Google Scholar] [CrossRef]
- Chen, Y.; Alder, J.; Song, T.; Chen, L.; Sheridan, R.; Davenport, A.; Kendrick, E. Influence of Magnetic Field upon Electrode Kinetics and Ionic Transport. J. Power Sources 2024, 602, 234323. [Google Scholar] [CrossRef]





| Active Materials | Improvement Strategy | Cycling Stability | Electrode Capacity [mAh g−1/C] | Voltage Window [V vs. Li/Li+] | Loading [mg/cm2] | Scalability Level [1~5] (a) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Pore engineering | LiFePO4 | Templated phase inversion | - | 156/0.1 C | 2.5–4 V | 100 | 4 | [35] |
| Li(Ni0.6Mn0.2Co0.2)O2 | Laser structure | 72%/80 (0.5 C) | 130/0.5 C | 3–4.3 V | 35 | 3.5 | [50] | |
| LCO | Ice-template | 90%/200 (1 C) | 124/1 C | 3–4.2 V | 30~35 | 4 | [54] | |
| LiNi0.8Mn0.1Co0.1O2 | Multilayer coating process | - | 171/0.2 C | 2.8–4.5 V | 20~25 | 4 | [77] | |
| Ni-rich NCM | (Single crystal/polycrystalline) double layer cathode | 55.5%/50 (0.5 C) | - | 2.8–4.3 V | 21 | 4.5 | [79] | |
| LiNi0.9Co0.05Mn0.05O2 | Gradient pore structure | 88.24%/100 (1 C/2 C) | 62.09/4 C | 2.5–4.2 V | 25 | 4.5 | [80] | |
| LiNi0.8Mn0.1Co0.1O2 | Adhesive gradient | 93%/100 (0.2 C) | 156/1 C | 3–4.2 V | 20~25 | 4 | [81] | |
| LiNi0.8Co0.15Al0.05O2 | Gradient porosity | 99.5%/100 (0.2 C) | 180.7/0.2 C | 4.25 V | 13~16 | 4 | [85] | |
| LiNi0.83Mn0.12Co0.05O2 | Particle size gradient | 73.3%/150.05 mA h/g (1 C) | 176.1/1 C | 2.7–4.3 V | 29.6 | 4.5 | [34] | |
| NMC532 | Particle size gradient | 80%/1000 (0.5 C) | - | 2.5–4.2 V | 25 | 4 | [84] | |
| 2D conductive percolation network | LiNi1/3Co1/3Mn1/3O2 | 2D porous nanosheets | 92.8%/100 (0.1 C) | 147.2/0.1 | 2.8–4.3 V | 320 | 4 | [59] |
| LiNi0.6Co0.2Mn0.2O2 | Single-walled carbon nanotubes | 80%/300 (0.5 C) | 4.7 mA h cm−2/0.5 C | 2.5–4.3 V | 36.4 | 4 | [57] | |
| LiNi0.8Co0.1Mn0.1O2 | Carbon nanofiber | 93.7%/100 (1 C) | 208.02/0.1 C | 2.75–4.3 V | 20 | 4 | [86] | |
| LiMn2O4 | Single-walled carbon nanotubes | 95%/50 (0.1 C) | 106/0.1 C | 3–4.3 V | 60 | 3 | [58] | |
| 3D conductive scaffold | LiFePO4 | Nickel alloy foam current collector | 90%/100 (0.3 C) | 102/0.3 C | 2.5–4 V | 32 | 4 | [60] |
| NCM811 | CNTS prepared by spinning technology | 89.6%/100 (2 C) | 211/0.1 C | 3–4.3 V | 6.3 | 4 | [62] | |
| LiFe0.7Mn0.3PO4 | Vertical channel sandwich structure | 60%/1000 (1 C) | 146.8/0.5 C | 2–4.5 V | 21.2 | 4.5 | [37] | |
| LiNi0.8Co0.1Mn0.1O2 | 3D printing grid structure | 77.68%/100 (200 mA/g) | 204.3/25 mA/g | 2.8–4.3 V | 36.6 | 4 | [92] | |
| LiNi0.6Co0.2Mn0.2O2 | 3D carbon fiber network | 84%/50 (1 C) | 165/1 C | 3–4.6 V | 11 | 4 | [33] | |
| LFP | Bionic multi-channel carbon framework | 76%/140 (2 mA cm−2) | 5 mA h cm−2/2 mA cm−2 | 2.15–4.2 V | 60 | 4 | [88] | |
| Cathode architecture optimization | LiNi0.8Co0.1Mn0.1O2 | Bicontinuous electron/ion conduction network | 90%/80 (0.05 C) | 191/0.05 C | 3–4.2 V | 36 | 4 | [93] |
| NMC811 | Severe calendering process | 87.7%/100 (0.33 C) | 181/0.33 C | 3–4.2 V | 19.13 | 4 | [63] | |
| NMC811 | Non-solvent induced phase transformation technology | 98.65%/100 (0.1 C) | 160.3/1 C | 3.6–4.3 V | 60 | 4.5 | [96] | |
| LFP | 3D printing technology | - | 133/0.2 mA cm−2 | - | 108 | 4 | [97] | |
| LiNi0.8Co0.15Al0.05O2 | Roll-to-roll drying process | 82.1%/100 (0.5 C) | 190.1/0.5 C | - | 50 | 4 | [100] | |
| NCM811 | PTFE Adhesive/Dry Process | - | 160/0.5 C | - | 52 | 4.5 | [99] | |
| LiNi0.8Co0.1Mn0.1O2 | Carbon nanotube dispersion/dry process | 66.1%/50 (0.33 C) | 211.47/0.2 C | 2.8~4.4 V | 50 | 4 | [103] | |
| LiNi0.8Mn0.1Co0.1O2 | Adhesive optimization | 75%/300 (0.5 C) | - | 3.0–4.5 V | 21.7 | 4.5 | [104] | |
| LiNi0.8Mn0.1Co0.1O2 | Design of new adhesive | 80.6%/240 (0.5 C) | 190/0.1 C | 3.0–4.2 V | 27 | 4 | [105] | |
| Electrode/electrolyte interfacial design | LiNi0.8Mn0.1Co0.1O2 | Nonsolvating fluoroaromatic cosolvent | 71.9%/500 (0.33 C) | 218.9/0.33 C | 3.0–4.5 V | 13.75 | 4 | [111] |
| NMC622 | Concentrated ternary salt ether-based electrolyte | 80%/430 (0.2 C/0.5 C) | - | 2.7–4.4 V | 13.8 | 4 | [112] | |
| NCM811 | “Tree-Trunk” design | 80%/300 (1 C) | 207/0.1 C | - | 14.8 | 4 | [40] | |
| LiNi0.8Co0.1Mn0.1O2 | Cationic polymer binder | 82%/100 (0.68/1.35 mA cm−2) | 210/0.1 C | 3.0–4.4 V | 65 | 4 | [114] | |
| NCM811 | Single-ion conductor polymer electrolyte | 84.5%/300 (2 C) | 160/0.3 C | 3.0–4.2 V | 10.6 | 4 | [118] | |
| LiFePO4 | Novel quasi-solid polymer electrolyte | 75%/1500 (2 C) | 116/2 C | - | 6~7 | 4.5 | [120] | |
| LiNi0.88Co0.04Mn0.05Al0.03O2 | Core–shell structure engineering and surface coating synergy | 96.4%/300 (0.5 C) | 128.8/2 C (55 °C) | 2.1–3.68 V | 35.6 | 4 | [121] | |
| LiNi0.8Mn0.1Co0.1O2 | Mechanical melting modification | 97%/1300 (1 C) | 194.9/0.05 C | 2.8–4.4 V | 27 | 4 | [122] | |
| NCM811 | Ultrathin multifunctional polymer electrolyte | 84.2%/500 (0.5 C) | 178.6/0.5 C | 2.8~4.3 V | 7~9 | 4.2 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Luo, Y.; Wang, K.; Zhang, L.; Yan, P.; Sui, M. Opportunities and Challenges for Next-Generation Thick Cathodes in Lithium-Ion Batteries. Materials 2025, 18, 3464. https://doi.org/10.3390/ma18153464
Li S, Luo Y, Wang K, Zhang L, Yan P, Sui M. Opportunities and Challenges for Next-Generation Thick Cathodes in Lithium-Ion Batteries. Materials. 2025; 18(15):3464. https://doi.org/10.3390/ma18153464
Chicago/Turabian StyleLi, Shengkai, Yuxuan Luo, Kangchen Wang, Lihan Zhang, Pengfei Yan, and Manling Sui. 2025. "Opportunities and Challenges for Next-Generation Thick Cathodes in Lithium-Ion Batteries" Materials 18, no. 15: 3464. https://doi.org/10.3390/ma18153464
APA StyleLi, S., Luo, Y., Wang, K., Zhang, L., Yan, P., & Sui, M. (2025). Opportunities and Challenges for Next-Generation Thick Cathodes in Lithium-Ion Batteries. Materials, 18(15), 3464. https://doi.org/10.3390/ma18153464








