Investigation of the Influence of Process Parameters on the Physicochemical and Functional Properties of Oil-Based Composites
Abstract
1. Introduction
2. Methodology
2.1. Oil-Based Binder
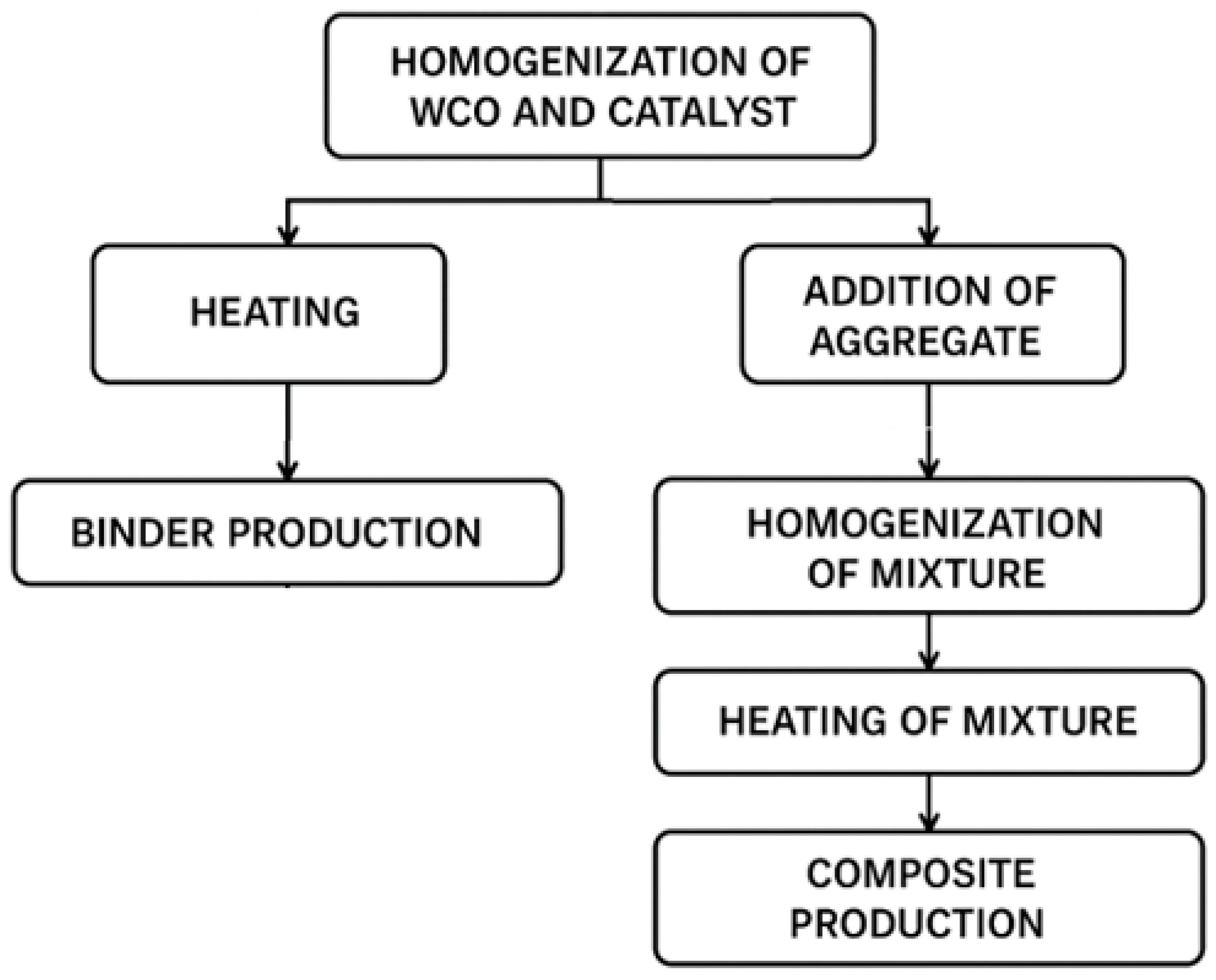
2.2. Preparation of Oil Composites
2.3. Physicochemical Characterisation of the Compositesoil-Based Binder
3. Results and Discussion
3.1. Formation of the Oil Binder
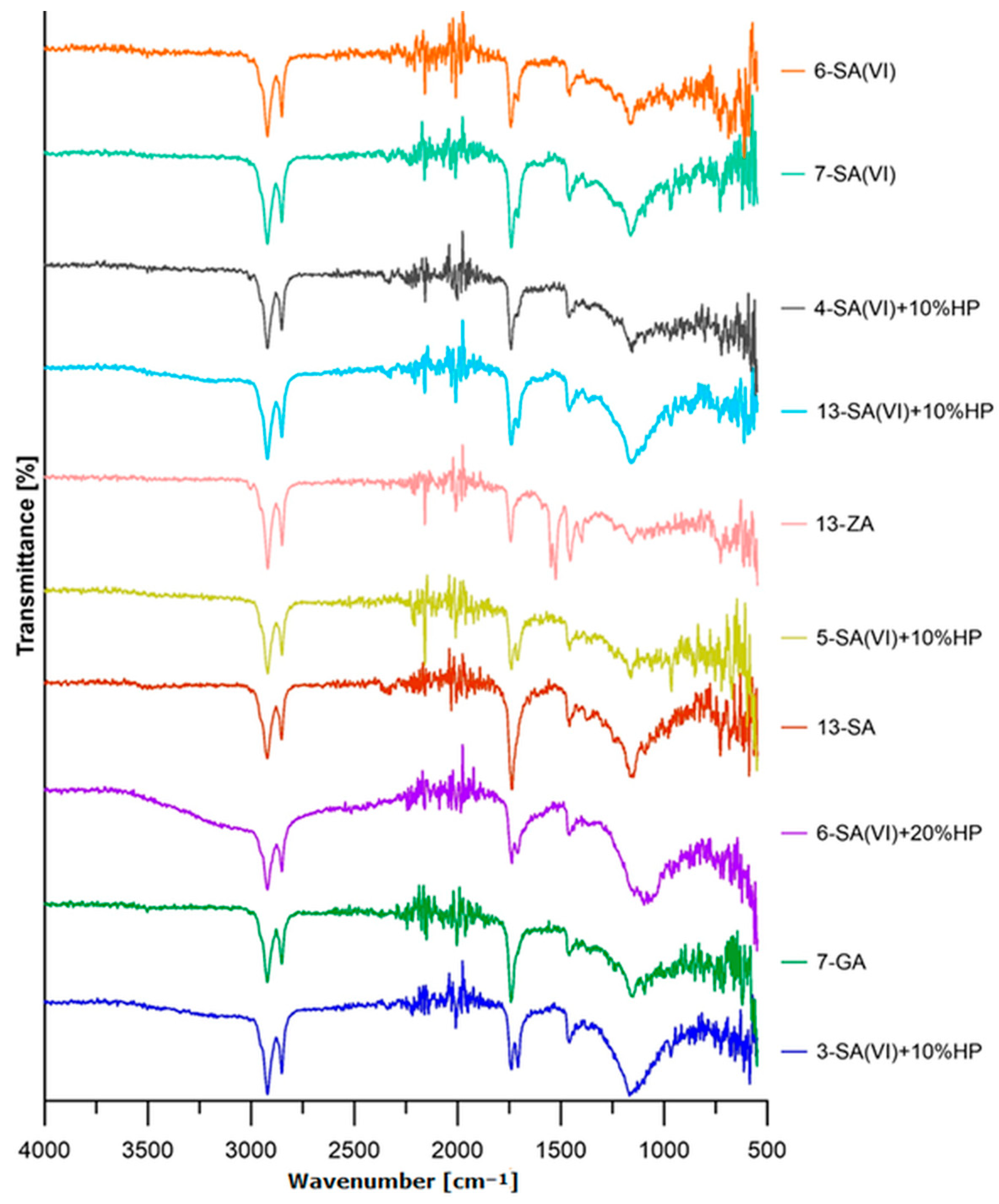
3.2. Composition Analysis of the Oil Binders
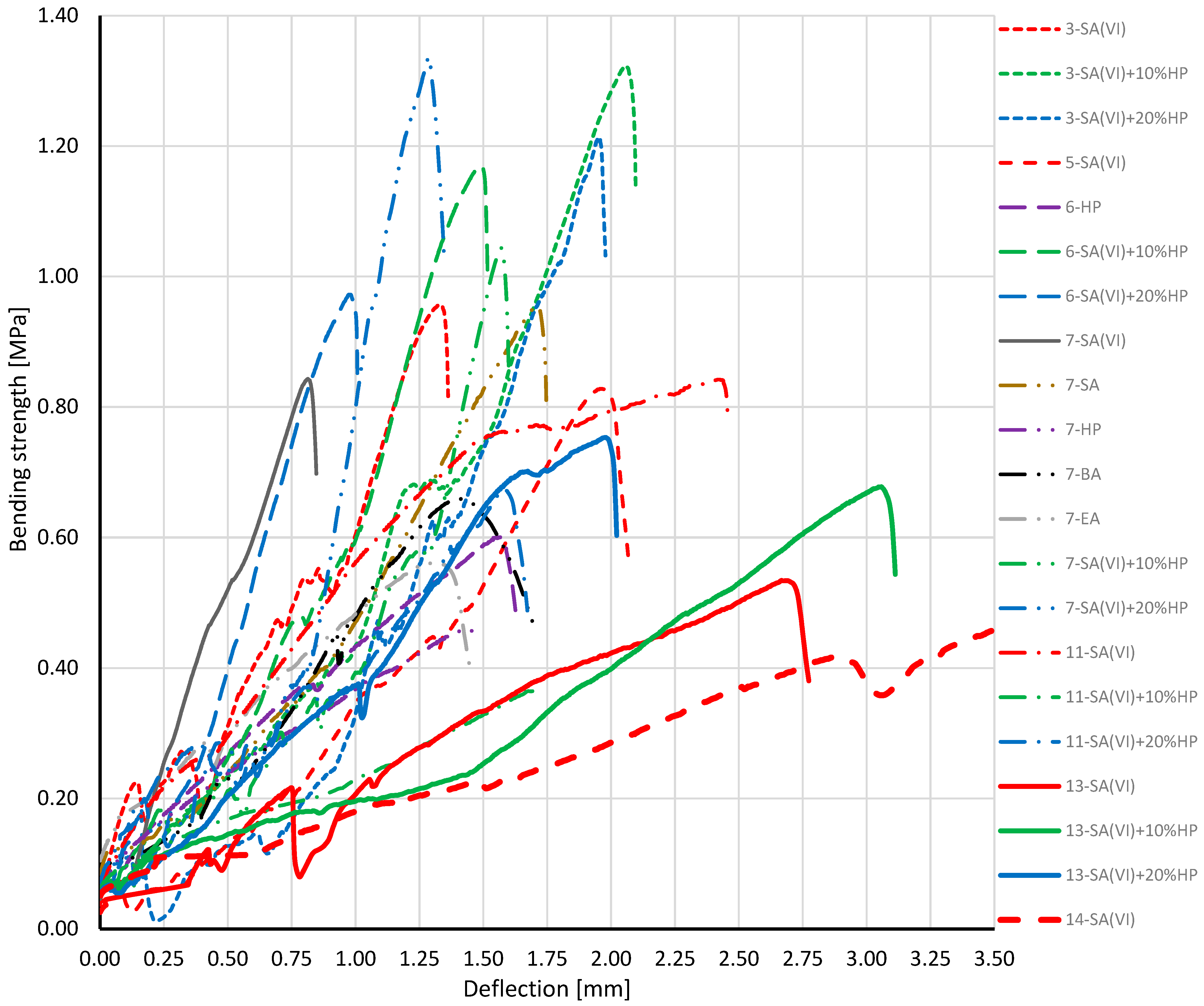
3.3. Mechanical Testing of the Oil Composites
3.4. Water-Absorption Test and Filtrate pH
3.5. Structural Investigation of the Solid Oil-Based Materials
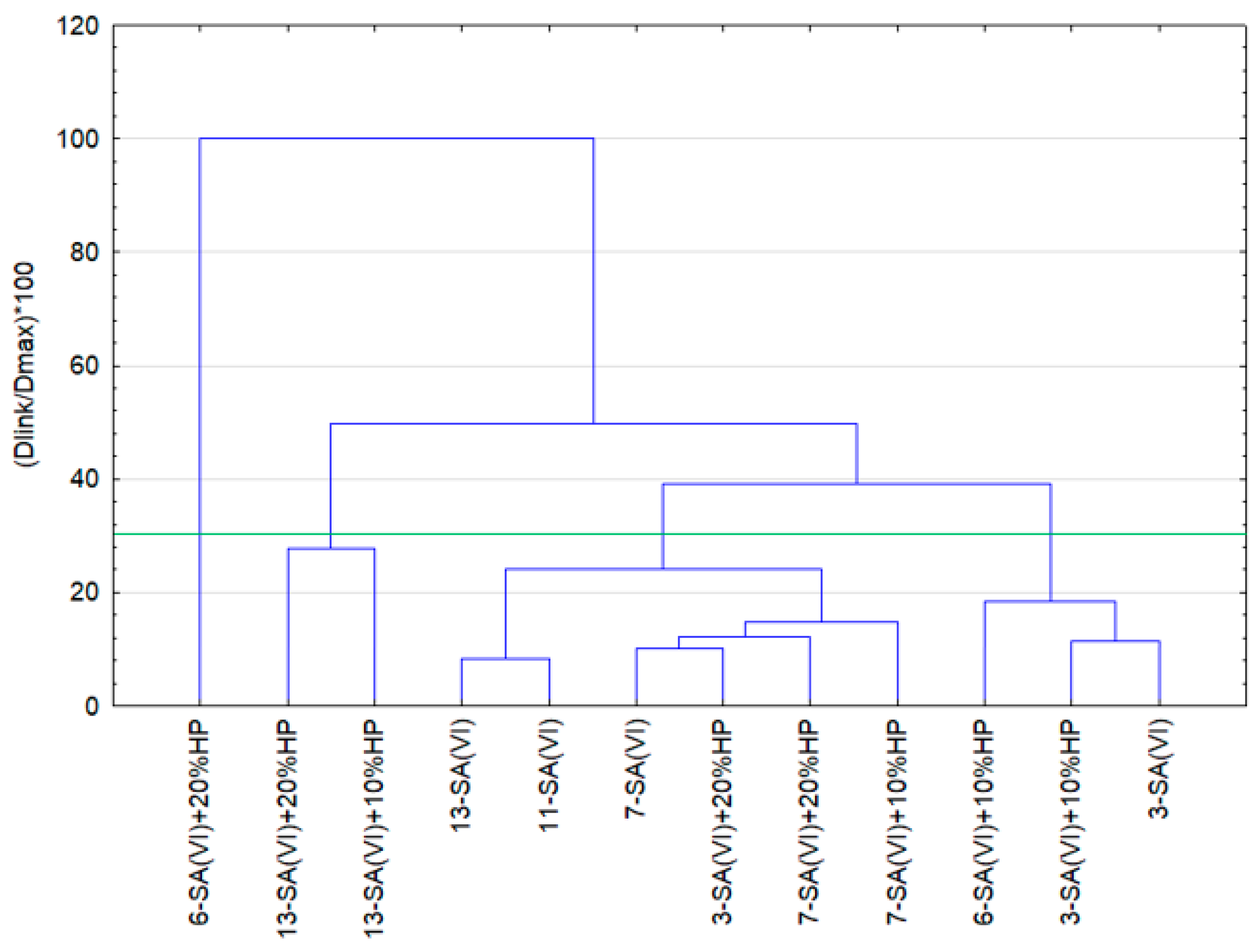
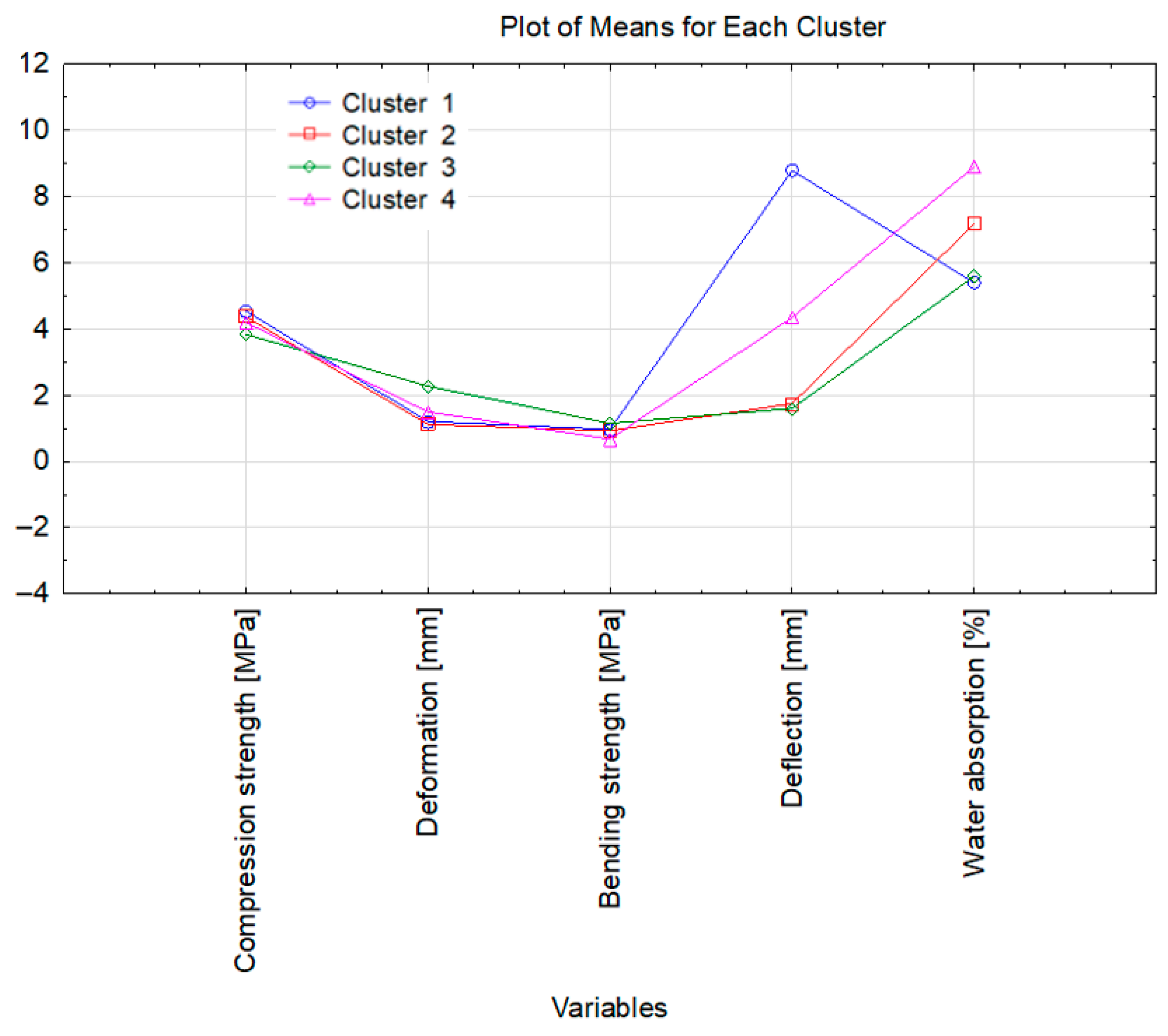
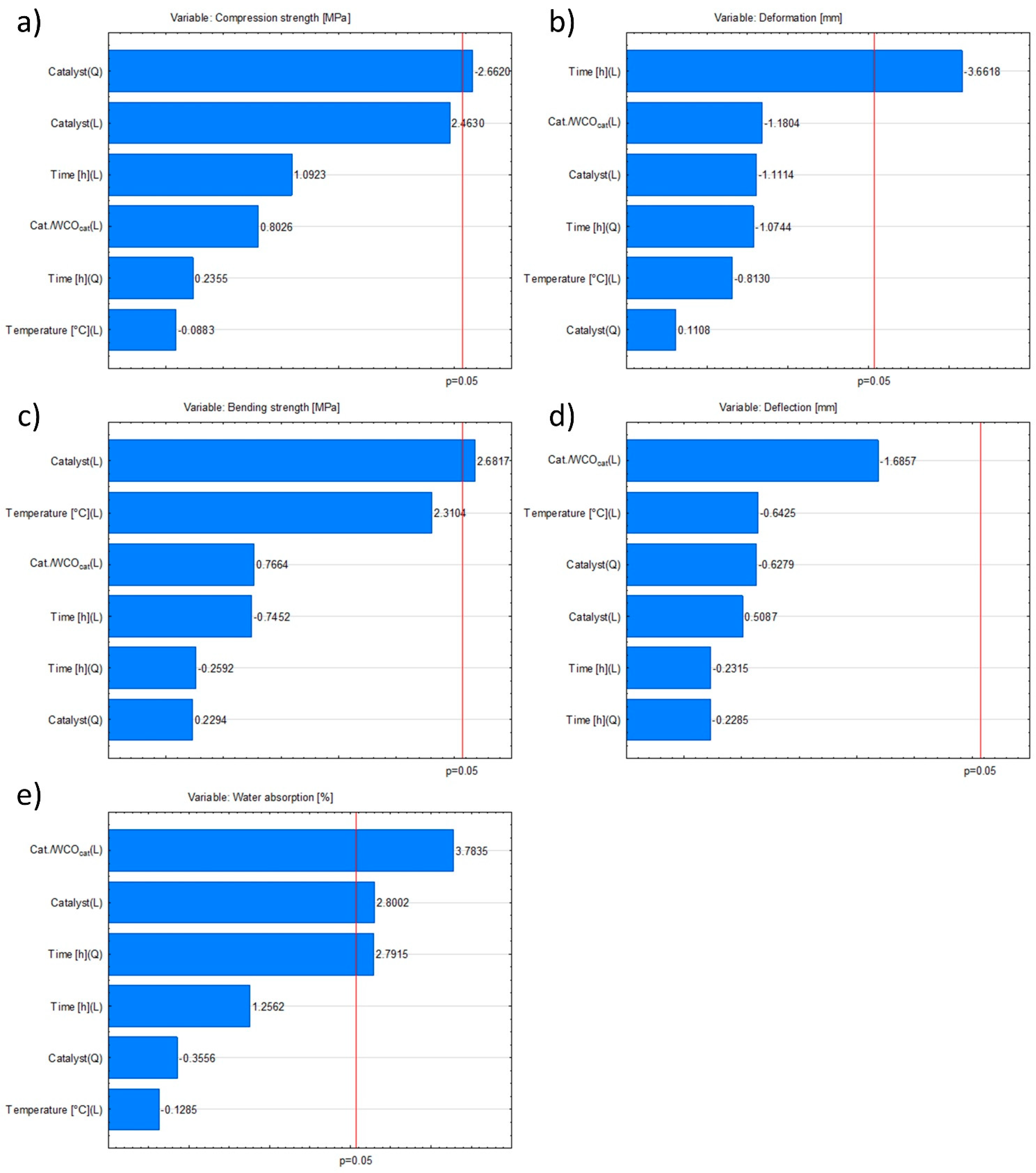
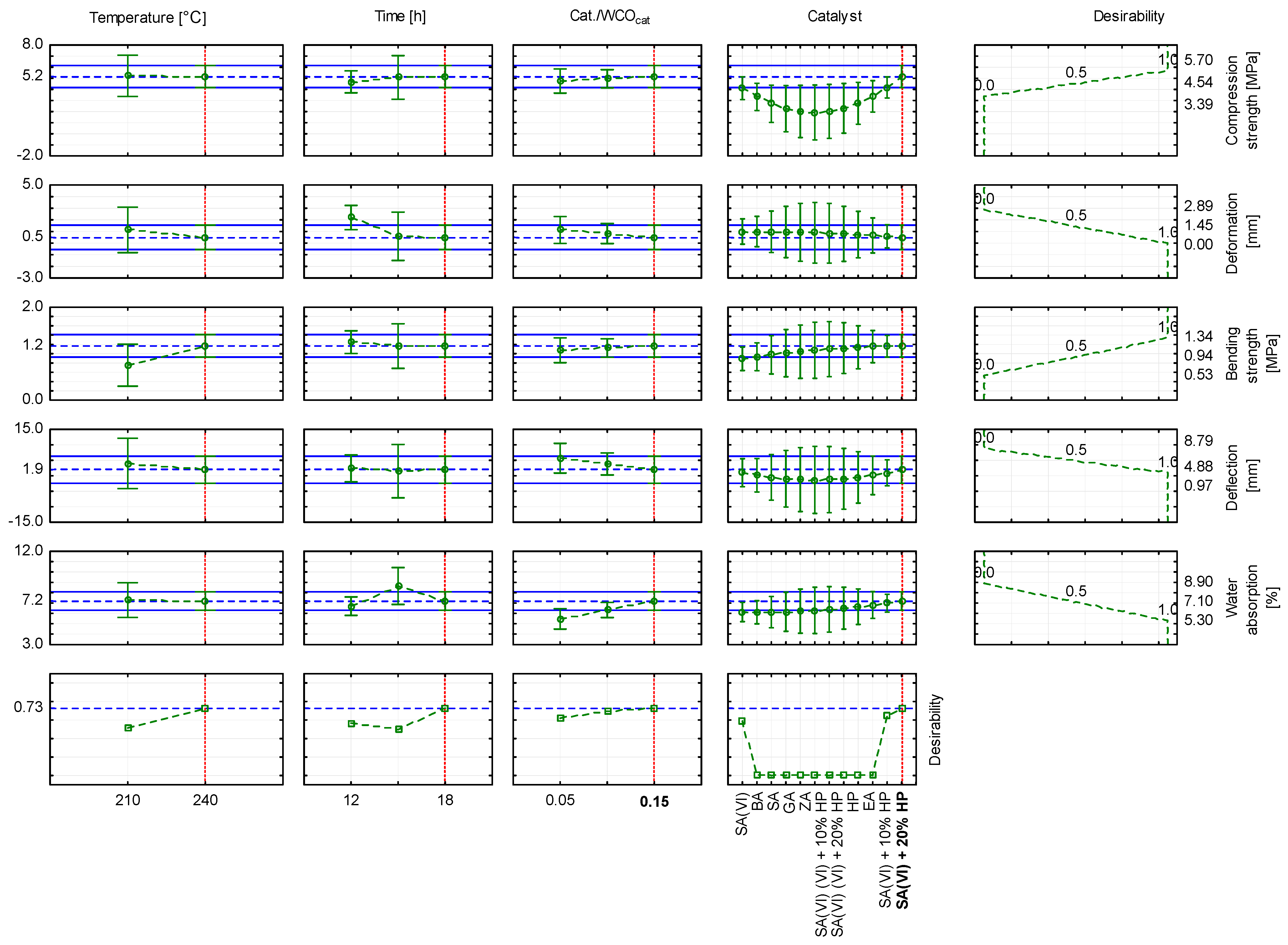
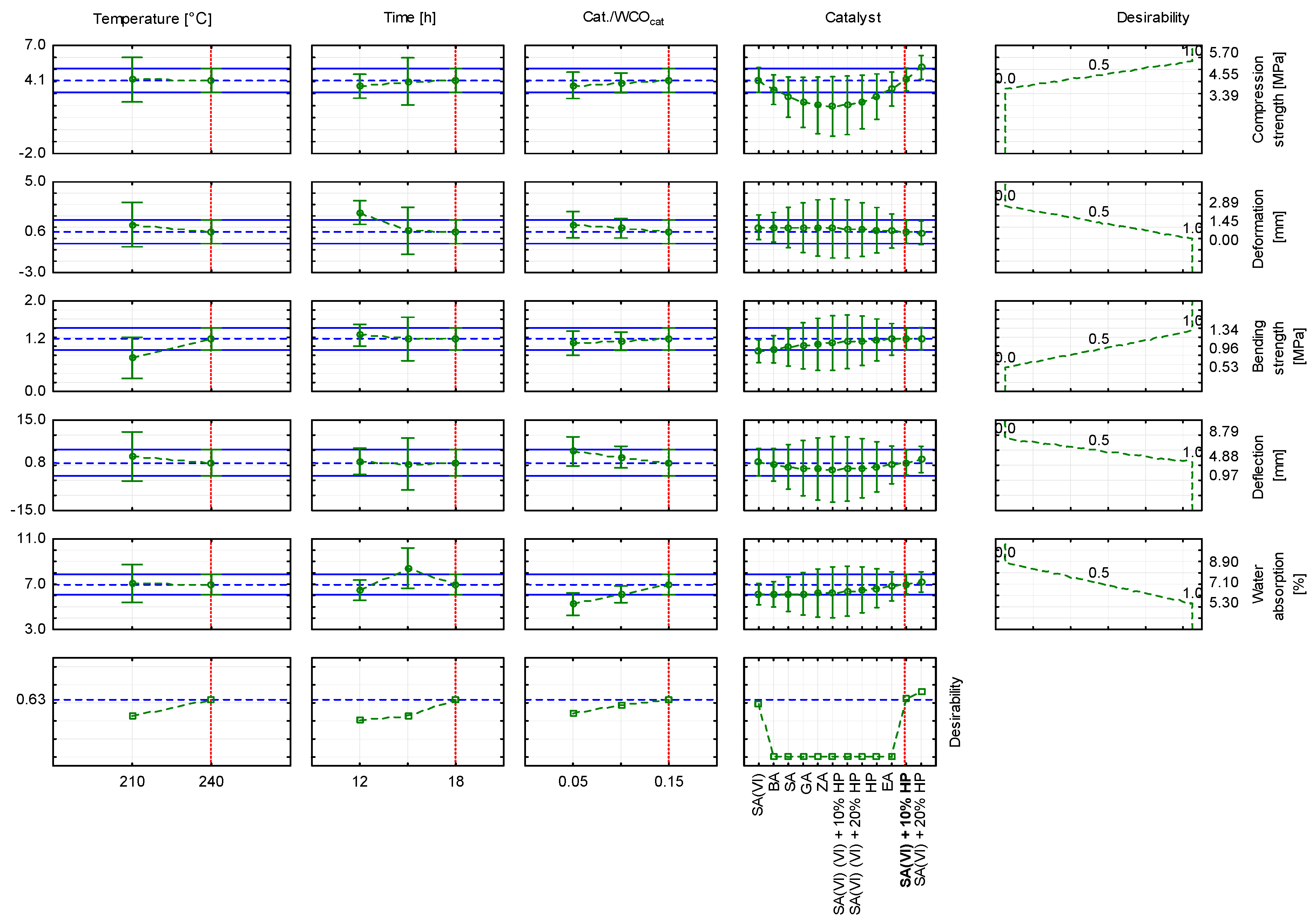
3.6. Statistical Analysis of the Research Results
4. Conclusions
- The present study demonstrated the feasibility of producing solid composites based on catalytically modified waste cooking oil (WCO) and sand. The proposed process offers a straightforward method of utilising WCO as a binder precursor, contributing to circular economy strategies and waste valorization.
- Among the tested catalytic systems—including sulfuric(VI) acid, sulfuric(VI) acid/hydrogen peroxide blend, hydrobromic acid, glycolic acid, hydrogen peroxide, salicylic acid, ethyl alcohol, and zinc acetate—the formulations containing sulfuric(VI) acid exhibited the highest efficiency in binder formation and composite cohesion.
- The composite formulated with sulfuric(VI) acid and 20 wt.% hydrogen peroxide and cured at 210 °C exhibited the highest compressive strength (5.70 MPa). The same formulation, when cured at 240 °C, achieved the greatest bending strength (1.34 MPa). These findings confirm the potential to tailor mechanical properties through catalyst selection and process parameters.
- Although the developed materials exhibit lower mechanical strength than conventional construction materials, the values achieved are considered sufficient for selected non-structural applications. Potential uses include temporary construction elements, and fillers for modular systems.
- The proposed composites offer environmental and economic benefits, including the reduction in reliance on virgin resources, mitigation of problematic waste streams, and suitability for low-infrastructure, decentralised production. These factors contribute to the material’s viability in sustainable building applications.
- Certain challenges remain, notably the requirement for elevated curing temperatures and variability in WCO composition, which may affect process reproducibility and energy efficiency.
- Future research should aim to improve binder reactivity at lower temperatures, investigate alternative or synergistic catalytic systems, and establish pre-treatment or standardisation protocols for WCO feedstock. Further studies on long-term durability, water resistance, and biodegradation under real environmental conditions are essential to validate the composites’ performance in practical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WCO | Waste Cooking Oil |
| SA(VI) | Sulfuric(VI) Acid |
| AA | Acetic Acid |
| SA | Salicylic Acid |
| GA | Glycolic Acid |
| ZA | Zinc Acetate |
| HP | Hydrogen Peroxide |
| BA | Hydrobromic Acid |
| EA | Ethyl Alcohol |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| Cat./WCOcat | Catalyst-to-Waste-Cooking-Oil Mass Ratio |
| MPa | Megapascal |
References
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A Review on CO2 Capture and Sequestration in the Construction Industry: Emerging Approaches and Commercialised Technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Khaiyum, M.Z.; Sarker, S.; Kabir, G. Evaluation of Carbon Emission Factors in the Cement Industry: An Emerging Economy Context. Sustainability 2023, 15, 15407. [Google Scholar] [CrossRef]
- Ferrández, D.; Saiz, P.; Zaragoza-Benzal, A.; Zúñiga-Vicente, J.A. Towards a More Sustainable Environmentally Production System for the Treatment of Recycled Aggregates in the Construction Industry: An Experimental Study. Heliyon 2023, 9, e16641. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.; Dissanayake, H.; Mansi, E.; Stancu, A. Eco Breakthroughs: Sustainable Materials Transforming the Future of Our Planet. Sustainability 2024, 16, 10790. [Google Scholar] [CrossRef]
- Maguire, S.; Robson, I. Sustainable Production and Consumption Within the Circular Economy. In Sustainable Development Through Global Circular Economy Practices; Emerald Publishing Limited: Leeds, UK, 2023; pp. 59–78. [Google Scholar] [CrossRef]
- Foo, W.H.; Chia, W.Y.; Tang, D.Y.Y.; Koay, S.S.N.; Lim, S.S.; Chew, K.W. The Conundrum of Waste Cooking Oil: Transforming Hazard into Energy. J. Hazard. Mater. 2021, 417, 126129. [Google Scholar] [CrossRef] [PubMed]
- Edible Oil Market Outlook 2024–2028. Available online: https://www.reportlinker.com/clp/global/2354 (accessed on 1 April 2025).
- Olmez, O.B.; Gultekin, C.; Balcik, B.; Ekici, A.; Özener, O.Ö. A Variable Neighborhood Search Based Matheuristic for a Waste Cooking Oil Collection Network Design Problem. Eur. J. Oper. Res. 2022, 302, 187–202. [Google Scholar] [CrossRef]
- Lopresto, C.G.; De Paola, M.G.; Calabrò, V. Importance of the Properties, Collection, and Storage of Waste Cooking Oils to Produce High-Quality Biodiesel—An Overview. Biomass Bioenergy 2024, 189, 107363. [Google Scholar] [CrossRef]
- Bruun, N.; Tesfaye, F.; Hemming, J.; Dirbeba, M.J.; Hupa, L. Effect of Storage Time on the Physicochemical Properties of Waste Fish Oils and Used Cooking Vegetable Oils. Energies 2020, 14, 101. [Google Scholar] [CrossRef]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible Oil Parameters during Deterioration Processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Guo, X.; Qiang, J.; Cao, Y.; Zhang, S.; Yu, X. Influence of Triacylglycerol Structure on the Formation of Lipid Oxidation Products in Different Vegetable Oils during Frying Process. Food Chem. 2025, 464, 141783. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef]
- Onn, M.; Jalil, M.J.; Mohd Yusoff, N.I.S.; Edward, E.B.; Wahit, M.U. A Comprehensive Review on Chemical Route to Convert Waste Cooking Oils to Renewable Polymeric Materials. Ind. Crops Prod. 2024, 211, 118194. [Google Scholar] [CrossRef]
- Bardella, N.; Facchin, M.; Fabris, E.; Baldan, M.; Beghetto, V. Waste Cooking Oil as Eco-Friendly Rejuvenator for Reclaimed Asphalt Pavement. Materials 2024, 17, 1477. [Google Scholar] [CrossRef]
- Dou, Z.; Dierenfeld, E.S.; Wang, X.; Chen, X.; Shurson, G.C. A Critical Analysis of Challenges and Opportunities for Upcycling Food Waste to Animal Feed to Reduce Climate and Resource Burdens. Resour. Conserv. Recycl. 2024, 203, 107418. [Google Scholar] [CrossRef]
- Beghetto, V. Waste Cooking Oils into High-Value Products: Where Is the Industry Going? Polymers 2025, 17, 887. [Google Scholar] [CrossRef] [PubMed]
- Mihail, I.; Zoran, P.S. Polymerization of Soybean Oil with Superacids. In Soybean Applications and Technology; INTECH: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Staroń, A. Composite Materials Based on Waste Cooking Oil for Construction Applications. Buildings 2023, 13, 994. [Google Scholar] [CrossRef]
- Staroń, A.; Chwastowski, J.; Kijania-Kontak, M.; Wiśniewski, M.; Staroń, P. Bio-Enriched Composite Materials Derived from Waste Cooking Oil for Selective Reduction of Odour Intensity. Sci. Rep. 2024, 14, 16311. [Google Scholar] [CrossRef]
- Li, H.; Beghetto, V. Strategies for the Transformation of Waste Cooking Oils into High-Value Products: A Critical Review. Polymers 2025, 17, 368. [Google Scholar] [CrossRef]
- Staroń, A.; Pucelik, B.; Barzowska, A.; Pulit-Prociak, J. Synthesis and Characteristics of Biocidal Oil Composites Enhanced with Thymol and Salicylic Acid. Clean Technol. Environ. Policy 2024, 26, 3481–3505. [Google Scholar] [CrossRef]
- Kumar, A.; Bhayana, S.; Singh, P.K.; Tripathi, A.D.; Paul, V.; Balodi, V.; Agarwal, A. Valorization of Used Cooking Oil: Challenges, Current Developments, Life Cycle Assessment and Future Prospects. Discov. Sustain. 2025, 6, 1–31. [Google Scholar] [CrossRef]
- S, A.; Suresh, T.; Sahoo, S.K. Development of Moisture/Oil-Resistant Biocoatings from Waste Cooking Oil for Packaging Applications: Scientific Upcycling with Circular Economy Potential. ACS Sustain. Resour. Manag. 2024, 1, 2612–2620. [Google Scholar] [CrossRef]
- Mangala, D.; Saputra, E.; Sedayu, B.B.; Pujiastuti, D.Y.; Syamani, F.A.; Novianto, T.D.; Pamungkas, A.; Irianto, H.E. Utilization of Waste Cooking Oil as a Substitute for Plasticizers in the Production of Carrageenan/Cornstarch Bioplastic. Green Mater. 2025, 13, 28–36. [Google Scholar] [CrossRef]
- Staroń, A.; Ciuruś, J.; Kijania-Kontak, M. Effect of Waste Cooking Oil-Based Composite Materials on Radish Growth and Biochemical Responses. Materials 2023, 16, 7350. [Google Scholar] [CrossRef] [PubMed]
- Staroń, A.; Kijania-Kontak, M.; Kozak, A.; Banach, M. Obtaining of Oil Blocks as a Way to Manage Hazardous Asbestos. Waste Manag. 2020, 105, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Staroń, A.; Papla, A.; Midura, A.; Kijania-Kontak, M.; Świergosz, T.; Banach, M. Physicochemical Properties, Strength and Phytotoxicity of Building Blocks with Waste Cooking Oil as Binder. J. Clean. Prod. 2022, 335, 130316. [Google Scholar] [CrossRef]
- Adebayo, J.O.; Napiah, M.; Ibrahim, K.; Raduan Kabit, M. Evaluation of Waste Cooking Oil as Sustainable Binder for Building Blocks. In Proceedings of the International Conference on Civil and Environmental Engineering (ICCEE 2018), Kuala Lumpur, Malaysia, 2–5 October 2018; EDP Sciences: Les Ulis, France, 2018; Volume 65. [Google Scholar]
- Attia, M.I.; Zoorob, S.; Hassan, K.; El-Husseini, H.; Reid, J.M.; Al Kuwari, M.S. Development of Building Blocks Using Vegetable Oil and Recycled Aggregate. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 120. [Google Scholar]
- Staroń, A.; Pucelik, B.; Barzowska, A.; Kijania-Kontak, M.; Staroń, P. Antimicrobial Properties of WCO-Based Composites Enriched with Hops and Curly Sorrel for Green Building Solutions. PLoS ONE 2024, 19, e0307452. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhang, S.; Gao, Q.; Lu, Q.; Peng, R.; Xu, P.; Shang, H.; Yuan, Y.; Zou, H. Micro-FTIR Combined with Curve Fitting Method to Study Cellulose Crystallinity of Developing Cotton Fibers. Anal. Bioanal. Chem. 2021, 413, 1313–1320. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Temperature Dependences of Ir Spectra of Humic Substances of Brown Coal. Agronomy 2021, 11, 1822. [Google Scholar] [CrossRef]
- Shahirah Ahmad Zamanhuri, N.; Farhan Hanafi, M.; Sapawe, N. Characterization and Physicochemical Properties of Biodiesel Produced from Waste Cooking Oil (WCO) Using Magnetic Alumina-Ferric Oxide Nanoparticles Catalyst. Mater. Today Proc. 2020, 31, A122–A125. [Google Scholar] [CrossRef]
- Fernández-Silva, S.D.; Delgado, M.A.; Ruiz-Méndez, M.V.; Giráldez, I.; García-Morales, M. Potential Valorization of Waste Cooking Oils into Sustainable Bio-Lubricants. Ind. Crops Prod. 2022, 185, 115109. [Google Scholar] [CrossRef]
- Asemani, M.; Rabbani, A.R. Detailed FTIR Spectroscopy Characterization of Crude Oil Extracted Asphaltenes: Curve Resolve of Overlapping Bands. J. Pet. Sci. Eng. 2020, 185, 106618. [Google Scholar] [CrossRef]
- Zahran, M.K.; Kamel, M.A.; Farag, R.K.; Mohamed, N.H.; El-Sherbiny, S. Assessment of Green Biodiesel Production Based on Egyptian Waste Cooking Oil Using Different Porous Membranes. Egypt. J. Chem. 2021, 64, 4401–4415. [Google Scholar] [CrossRef]
- Staroń, A.; Kijania-Kontak, M.; Dziadas, M.; Banach, M. Assessment of the Environmental Impact of Solid Oil Materials Based on Pyrolysis Oil. Materials 2023, 16, 5847. [Google Scholar] [CrossRef] [PubMed]
- Van Nam, H.; Viet, D.Q.; Tam, T.T.; Tho, V.D.S. Chemical Composition of Pyrolysis Oil through Thermal Decomposition of Sugarcane Biomass. Vietnam J. Chem. 2020, 58, 770–778. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and Proportions of Waste Tyre Pyrolysis Products Depending on the Reactor Type—A Review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, X.; Qiao, A.; Mu, Y. Compressive Properties and Degradable Behavior of Biodegradable Porous Zinc Fabricated with the Protein Foaming Method. J. Funct. Biomater. 2022, 13, 151. [Google Scholar] [CrossRef]
- Marks, J.H.; Wang, J.; Evseev, M.M.; Kuznetsov, O.V.; Antonov, I.O.; Kaiser, R.I. Complex Reactive Acids from Methanol and Carbon Dioxide Ice: Glycolic Acid (HOCH2COOH) and Carbonic Acid Monomethyl Ester (CH3OCOOH). Astrophys. J. 2023, 942, 43. [Google Scholar] [CrossRef]
- Wu, H.; Duan, M.; Ning, Z.; Gan, H.; Jiang, N. Reactive Toughening of Poly(Glycolic Acid)/Poly(ε-Caprolactone) Blends Using Environmentally Friendly and Cost-Effective Bio-Based Chain Extenders. J. Appl. Polym. Sci. 2025, e57016. [Google Scholar] [CrossRef]
- Li, C. Relationship between Water Absorption and Porosity in Concrete with Limestone Powder Addition. Struct. Concr. 2022, 23, 3284–3293. [Google Scholar] [CrossRef]
- Farhana, Z.F.; Kamarudin, H.; Rahmat, A.; Al Bakri, A.M.M. The Relationship between Water Absorption and Porosity for Geopolymer Paste. Mater. Sci. Forum 2015, 803, 166–172. [Google Scholar] [CrossRef]
- Ngaosuwan, K.; Lotero, E.; Suwannakarn, K.; Goodwin, J.G.; Praserthdam, P. Hydrolysis of Triglycerides Using Solid Acid Catalysts. Ind. Eng. Chem. Res. 2009, 48, 4757–4767. [Google Scholar] [CrossRef]
- Ma, G.; Dai, L.; Liu, D.; Du, W. A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production. Catalysts 2018, 8, 527. [Google Scholar] [CrossRef]
- Adam, A.A.; Deviana, L.; Siregar, A.P.N. Mustofa Water Absorption of Ambient-Cured Geopolymer Concrete. IOP Conf. Ser. Earth Environ. Sci. 2023, 1157, 012025. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Atif, R.; Shyha, I.; Inam, F. The Degradation of Mechanical Properties in Polymer Nano-Composites Exposed to Liquid Media—A Review. RSC Adv. 2015, 6, 1076–1089. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Esterification of Free Fatty Acids Using Sulfuric Acid as Catalyst in the Presence of Triglycerides. Biomass Bioenergy 2008, 32, 892–895. [Google Scholar] [CrossRef]
- Shu, D.; Zhang, J.; Ruan, R.; Lei, H.; Wang, Y.; Moriko, Q.; Zou, R.; Huo, E.; Duan, D.; Gan, L.; et al. Insights into Preparation Methods and Functions of Carbon-Based Solid Acids. Molecules 2024, 29, 247. [Google Scholar] [CrossRef]
- Salimon, J.; Abdullah, B.M.; Yusop, R.M.; Salih, N. Synthesis, Reactivity and Application Studies for Different Biolubricants. Chem. Cent. J. 2014, 8, 16. [Google Scholar] [CrossRef]
- Reyes, L.; Nikitine, C.; Vilcocq, L.; Fongarland, P.; Autocatalyzed, P.F. Catalyzed Esterification Kinetics of Glycolic Acid with Ethanol. React. Chem. Eng. 2022, 2022, 460–474. [Google Scholar] [CrossRef]
- Rahim, N.H.; Jalil, M.J.; Mubarak, N.M.; Azmi, I.S.; Anbuchezhiyan, G. Catalytic Epoxidation of Unsaturated Fatty Acids in Palm Stearin via in Situ Peracetic Acids Mechanism. Sci. Rep. 2025, 15, 4789. [Google Scholar] [CrossRef]
- Abdullah, B.M.; Salimon, J. Epoxidation of Vegetable Oils and Fatty Acids: Catalysts, Methods and Advantages. J. Appl. Sci. 2010, 10, 1545–1553. [Google Scholar] [CrossRef]
- Reda, S.Y. Evaluation of Antioxidants Stability by Thermal Analysis and Its Protective Effect in Heated Edible Vegetable Oil. Food Sci. Technol. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Rojas-Avellaneda, D.; Ramirez-Caballero, G.E. Effect of Temperature and Catalyst Concentration on Polyglycerol during Synthesis. Int. J. Polym. Sci. 2015, 2015, 910249. [Google Scholar] [CrossRef]
- PN-EN 12390-3:2019-07; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens 2019. iTeh, Inc.: Newark, DE, USA, 2019.
- PN-EN 12390-5:2019-08; Testing Hardened Concrete—Part 5: Flexural Strength of Test Specimens 2019. iTeh, Inc.: Newark, DE, USA, 2019.




| Designation | Substance | Physical State |
|---|---|---|
| SA(VI) | sulfuric(VI) acid | liquid solid (crystalline powder) solid |
| AA | acetic acid | liquid |
| SA | salicylic acid | solid (crystalline powder) |
| GA | glycolic acid | solid |
| ZA | zinc acetate | solid |
| HP | hydrogen peroxide | liquid |
| BA | hydrobromic acid | liquid |
| EA | ethyl alcohol | liquid |
| SA(VI) + 10% HP | sulfuric(VI) acid + 10% hydrogen peroxide | liquid |
| SA(VI) + 20% HP | sulfuric(VI) acid + 20% hydrogen peroxide | liquid |
| 10% HP + SA(VI) | 10% hydrogen peroxide + sulfuric(VI) acid | liquid |
| 20% HP + SA(VI) | 20% hydrogen peroxide + sulfuric(VI) acid | liquid |
| 10% HP + AA | 10% hydrogen peroxide + acetic acid | liquid |
| 20% HP + AA | 20% hydrogen peroxide + acetic acid | liquid |
| Process Number | Temperature [°C] | Time [h] | Cat./WCOcat |
|---|---|---|---|
| 1 | 180 | 12 | 0.15 |
| 2 | 240 | 12 | 0.05 |
| 3 | 240 | 12 | 0.15 |
| 4 | 180 | 18 | 0.05 |
| 5 | 180 | 18 | 0.15 |
| 6 | 240 | 18 | 0.05 |
| 7 | 240 | 18 | 0.15 |
| 8 | 210 | 12 | 0.10 |
| 9 | 210 | 18 | 0.10 |
| 10 | 180 | 15 | 0.10 |
| 11 | 240 | 15 | 0.10 |
| 12 | 210 | 15 | 0.05 |
| 13 | 210 | 15 | 0.15 |
| 14 | 210 | 15 | 0.10 |
| Catalyst | Compressive Strength [MPa] for Samples from a Given Process | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 6 | 7 | 9 | 11 | 13 | 14 | |
| SA (VI) | 3.30 | 4.24 | 4.08 | 3.77 | 4.19 | 4.13 | 3.83 | 3.42 | 2.76 |
| SA (VI) + 10%HP | 3.44 | 3.39 | 1.07 | 3.89 | 3.98 | 1.90 | 4.20 | ||
| SA (VI) + 20%HP | 3.11 | 4.30 | 1.41 | 4.55 | 5.20 | 2.35 | 5.70 | 2.43 | |
| BA | 2.16 | ||||||||
| GA | 3.52 | 2.50 | |||||||
| HP | 2.38 | 2.11 | |||||||
| SA | 1.77 | ||||||||
| EA | 1.67 | ||||||||
| ZA | 0.74 | ||||||||
| Catalyst | Bending Strength [MPa] for Samples from a Given Process | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 5 | 6 | 7 | 11 | 13 | 14 | |
| SA (VI) | 0.96 | 0.83 | 0.84 | 0.84 | 0.53 | 0.48 | |
| SA (VI) + 10%HP | 1.32 | 1.17 | 1.04 | 0.37 | 0.68 | ||
| SA (VI) + 20%HP | 1.18 | 0.97 | 1.34 | 0.67 | 0.75 | ||
| BA | 0.66 | ||||||
| HP | 0.60 | 0.46 | |||||
| SA | 0.95 | ||||||
| EA | 0.57 | ||||||
| Sample | Process Parameters | Water Absorption [%] | pH |
|---|---|---|---|
| 3-SA(VI) | 240 °C, 12 h, Cat./WCOcat: 0.15 | 5.4 | 3.71 |
| 6-SA(VI) | 240 °C, 18 h, Cat./WCOcat: 0.05 | 5.8 | 3.99 |
| 2-SA(VI) | 240 °C, 12 h, Cat./WCOcat: 0.05 | 5.9 | 4.17 |
| 11-SA(VI) | 240 °C, 15 h, Cat./WCOcat: 0.10 | 6.7 | 4.03 |
| 13-SA(VI) | 210 °C, 15 h, Cat./WCOcat: 0.15 | 7.4 | 3.74 |
| 7-SA(VI) | 240 °C, 18 h, Cat./WCOcat: 0.15 | 6.6 | 3.78 |
| 9-SA(VI) | 210 °C, 18 h, Cat./WCOcat: 0.10 | 8.4 | 3.83 |
| 6-SA(VI) + 10%HP | 240 °C, 18 h, Cat./WCOcat: 0.05 | 5.3 | 4.10 |
| 2-SA(VI) + 10%HP | 240 °C, 12 h, Cat./WCOcat: 0.05 | 5.8 | 4.09 |
| 3-SA(VI) + 10%HP | 240 °C, 12 h, Cat./WCOcat: 0.15 | 6.1 | 3.61 |
| 7-SA(VI) + 10%HP | 240 °C, 18 h, Cat./WCOcat: 0.15 | 6.9 | 3.89 |
| 13-SA(VI) + 10%HP | 210 °C, 15 h, Cat./WCOcat: 0.15 | 8.9 | 3.76 |
| 6-SA(VI) + 20%HP | 240 °C, 18 h, Cat./WCOcat: 0.05 | 5.4 | 4.14 |
| 3-SA(VI) + 20%HP | 240 °C, 12 h, Cat./WCOcat: 0.15 | 7.3 | 4.02 |
| 7-SA(VI) + 20%HP | 240 °C, 18 h, Cat./WCOcat: 0.15 | 6.8 | 3.84 |
| 13-SA(VI) + 20%HP | 210 °C, 15 h, Cat./WCOcat: 0.15 | 8.6 | 3.69 |
| 6-GA | 240 °C, 18 h, Cat./WCOcat: 0.05 | 3.0 | 4.81 |
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| Case | 6-SA(VI) + 20%HP | 7-SA(VI) + 10%HP | 3-SA(VI) | 13-SA(VI) + 10%HP |
| 7-SA(VI) + 20%HP | 3-SA(VI) + 10%HP | |||
| 3-SA(VI) + 20%HP | 6-SA(VI) + 10%HP | |||
| 7-SA(VI) | ||||
| 11-SA(VI) | ||||
| 13-SA(VI) | ||||
| 13-SA(VI) + 20%HP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadzka, A.; Kijania-Kontak, M. Investigation of the Influence of Process Parameters on the Physicochemical and Functional Properties of Oil-Based Composites. Materials 2025, 18, 3447. https://doi.org/10.3390/ma18153447
Zawadzka A, Kijania-Kontak M. Investigation of the Influence of Process Parameters on the Physicochemical and Functional Properties of Oil-Based Composites. Materials. 2025; 18(15):3447. https://doi.org/10.3390/ma18153447
Chicago/Turabian StyleZawadzka, Anita, and Magda Kijania-Kontak. 2025. "Investigation of the Influence of Process Parameters on the Physicochemical and Functional Properties of Oil-Based Composites" Materials 18, no. 15: 3447. https://doi.org/10.3390/ma18153447
APA StyleZawadzka, A., & Kijania-Kontak, M. (2025). Investigation of the Influence of Process Parameters on the Physicochemical and Functional Properties of Oil-Based Composites. Materials, 18(15), 3447. https://doi.org/10.3390/ma18153447



.png)



