Salt Formation of the Alliance of Triazole and Oxadiazole Towards Balanced Energy and Safety
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Synthesis
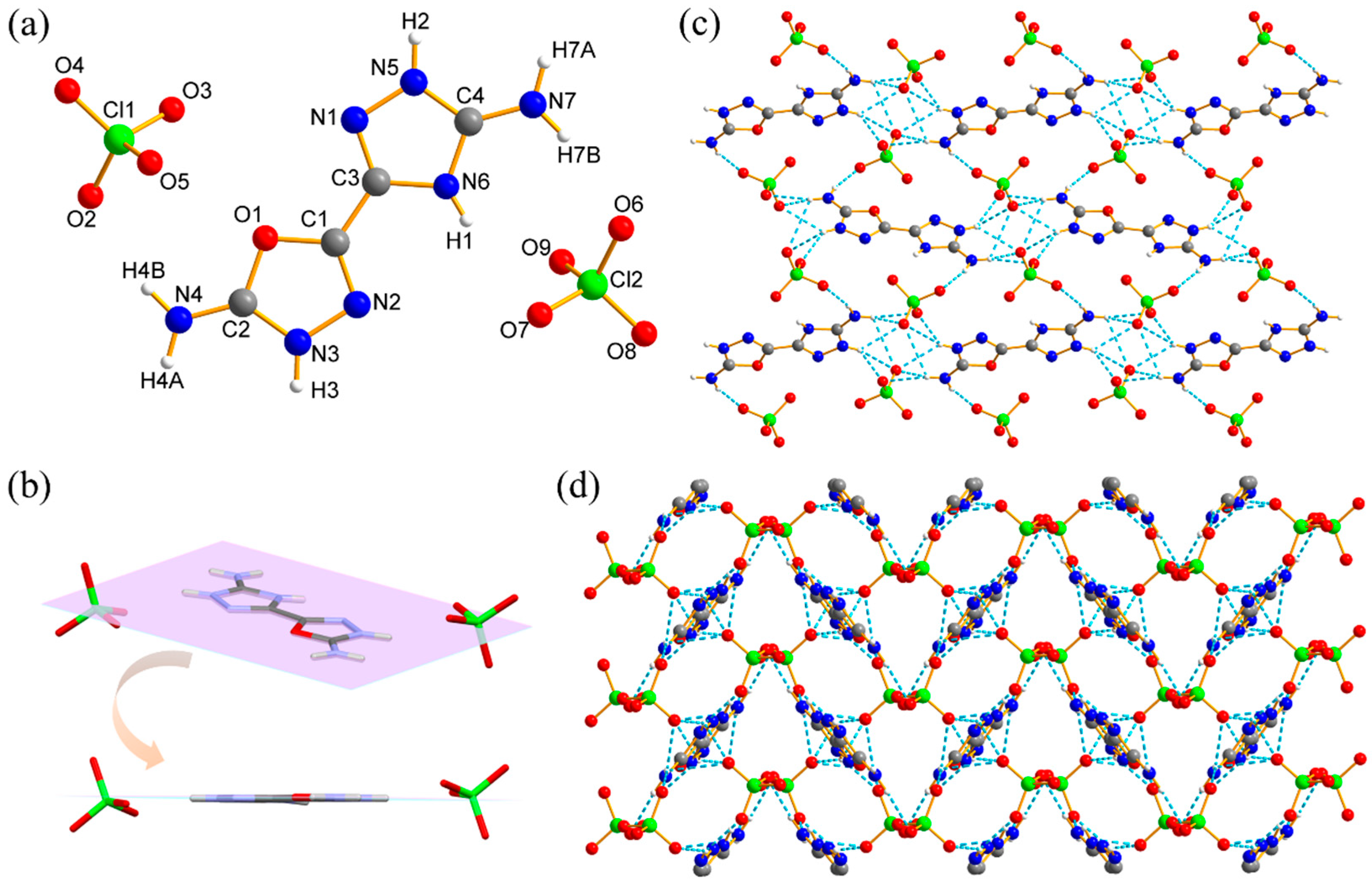
3.2. Crystal Structure Analysis
3.3. Density
3.4. Stabilities
3.5. Detonation Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Staples, R.J.; Shreeve, J.M. A dihydrazone as a remarkably nitrogen-rich thermostable and insensitive energetic material. Org. Lett. 2023, 25, 6082–6086. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.L.; Zhu, Y.H.; Li, J.Z.; Jiang, L.P.; Zhao, X.T.; Fan, X.Z. Preparation, characterization and application of nano-graphene-based energetic materials. Nanomaterials 2021, 11, 2374. [Google Scholar] [CrossRef] [PubMed]
- Sukhanov, G.T.; Filippova, Y.V.; Gatilov, Y.V.; Sukhanova, A.G.; Krupnova, I.A.; Bosov, K.K.; Pivovarova, E.V.; Krasnov, V.I. Energetic materials based on N-substituted 4(5)-nitro-1,2,3-triazoles. Materials 2022, 15, 1119. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Sun, Q.; Wang, Q.; Lin, Q.H.; Lu, M. Embellishing bis-1,2,4-triazole with four nitroamino groups: Advanced high-energy-density materials with remarkable performance and good stability. J. Mater. Chem. A 2020, 8, 11752–11760. [Google Scholar] [CrossRef]
- Schulze, M.C.; Scott, B.L.; Chavez, D.E. A high density pyrazolo-triazine explosive (PTX). J. Mater. Chem. A 2015, 3, 17963–17965. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Lu, Y.W.; Gao, B.; Wang, D.J.; Gou, C.P. Synthesis, characterization, and sensitivity of a CL-20/PNCB spherical composite for security. Materials 2018, 11, 1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.C.; Kumar, D.; Zhang, L.; Shem-Tov, D.; Petrutik, N.; Chinnam, A.K.; Yao, C.; Pang, S.P.; Gozin, M. Energetic butterfly: Heat-resistant diaminodinitro trans-bimane. Molecules 2019, 24, 4324. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.A.; Deng, M.C.; Song, S.W.; Chen, S.T.; Zhang, Q.H.; Shreeve, J.M. Construction of an unusual two-dimensional layered structure for fused-ring energetic materials with high energy and good stability. Engineering 2020, 6, 1006–1012. [Google Scholar] [CrossRef]
- Sultan, M.; Wu, J.Y.; Haq, I.U.; Imran, M.; Yang, L.J.; Wu, J.J.; Lu, J.Y.; Chen, L. Recent progress on synthesis, characterization, and performance of energetic cocrystals: A review. Molecules 2022, 27, 4775. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Jin, B.; Zhang, Q.C.; Shang, Y.; Gou, Z.C.; Tan, B.S.; Peng, R.F. Nitrogen-rich energetic metal-organic framework: Synthesis, structure, properties, and thermal behaviors of Pb (II) complex based on N,N-bis(1H-tetrazole-5-yl)-amine. Materials 2016, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, Y.; Zhu, Z.Q.; Xu, Y.G.; Lu, M. Self-assembly high-energy metal-organic frameworks (HE-MOFs) with sliver-based at ambient temperature: Synthesis, structure and superior explosive performance. Chem. Commun. 2017, 53, 7489–7492. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Huang, R.K.; Chen, S.L.; He, C.T.; Yu, Z.H.; Ye, Z.M.; Zhang, W.X.; Chen, X.M. Metal-free molecular perovskite high-energetic materials. Cryst. Growth Des. 2020, 20, 1891–1897. [Google Scholar] [CrossRef]
- Chen, S.L.; Yang, Z.R.; Wang, B.J.; Shang, Y.; Sun, L.Y.; He, C.T.; Zhou, H.L.; Zhang, W.X.; Chen, X.M. Molecular perovskite high-energetic materials. Sci. China Mater. 2018, 61, 1123–1128. [Google Scholar] [CrossRef]
- Chen, S.L.; Shang, Y.; He, C.T.; Sun, L.Y.; Ye, Z.M.; Zhang, W.X.; Chen, X.M. Optimizing the oxygen balance by changing the A-site cations in molecular perovskite high-energetic materials. CrystEngComm 2018, 20, 7458–7463. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, P.; Zhang, J.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Pushing the limits of oxygen balance in 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2017, 139, 8816–8819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Wang, K.; Li, J.; Zhang, Q.; Shreeve, J.M. Bis(4-nitraminofurazanyl-3-azoxy)azofurazan and derivatives:1,2,5-oxadiazoleStructures and high-performance energetic materials. Angew. Chem. Int. Ed. 2016, 55, 11548–11551. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. gem-Dinitromethyl-functionalized 5-amino-1,3,4-oxadiazolate derivatives: Alternate route, characterization, and property analysis. Org. Lett. 2020, 22, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Kretić, D.S.; Radovanović, J.I.; Veljković, D.Ž. Can the sensitivity of energetic materials be tuned by using hydrogen bonds? Another look at the role of hydrogen bonding in the design of high energetic compounds. Phys. Chem. Chem. Phys. 2021, 23, 7472–7479. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Nitro groups vs. N-oxide linkages: Effects upon some key determinants of detonation performance. Cent. Eur. J. Energetic Mater. 2017, 14, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials– the synthesis and characterization of dihydroxylammonium 5,5’-bistetrazole-1,1’-diolate. J. Mater. Chem. 2012, 22, 20418–20422. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Shen, C.; Lin, Q.; Wang, P.; Lu, M. A series of energetic metal pentazolate hydrates. Nature 2017, 549, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.T.; Wu, Z.Q.; Zhang, Q.; Xu, Y.G.; Lu, G.P. 2-(1,2,4-triazole-5-yl)-1,3,4-oxadiazole as a novel building block for energetic materials. Front. Chem. 2022, 10, 996812. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Jujam, M.; Ghule, V.D.; Dharavath, S. High-performing, insensitive and thermally stable energetic materials from zwitterionic gem-dinitromethyl substituted C-C bonded 1,2,4-triazole and 1,3,4-oxadiazole. Chem. Comm. 2023, 59, 4324–4327. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Y.; Wang, J.H.; Wu, L.L.; Yuan, X.; Yu, Q.; Zhang, J.; Lin, K.F.; Yang, Y.L.; Xia, D.B. Strategy for Balance Energy and Safety: Salt Formation of Nitrogen-Rich Bicyclic Compounds Based on 1,2,4-Triazole. Cryst. Growth Des. 2024, 25, 88–100. [Google Scholar] [CrossRef]
- Nie, X.Y.; Lei, C.J.; Xiong, H.L.; Cheng, G.B.; Yang, H.W. Methylation of a triazole-fused framework to create novel insensitive energetic materials. Energetic Mater. Front. 2020, 1, 165–171. [Google Scholar] [CrossRef]
- Wu, J.T.; Zhang, J.G.; Yin, X.; Cheng, Z.Y.; Xu, C.X. 3,4-Diamino-1,2,4-triazole based energetic salts: Synthesis, characterization, and energetic properties. New. J. Chem. 2015, 39, 5265–5271. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, C.F.; Twamley, B.; Shreeve, J.M. Energetic bicyclic azolium salts. Chem. Eur. J. 2006, 12, 9010–9018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhang, L.A.; Zhu, M.M.; Qin, K.Y.; Lin, Q.H. Insensitive ionic energetic salts and a green metal-free primary explosive based on furazan-triazole. J. Mol. Struct. 2024, 1301, 137369. [Google Scholar] [CrossRef]
- Dai, C.H.; Chen, J.Y.; Tang, J.; Cheng, G.B.; Yang, H.W. Combining 1,2,4-triazole and pyrazole frameworks for new insensitive energetic materials. New J. Chem. 2021, 45, 17960–17965. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Q.J.; Yuan, X.F.; Feng, S.B.; Zhu, S.F.; Chen, Y.H.; Gou, R.J.; Zhang, S.H.; Xu, Y.G.; Lu, M. Tailoring energy performance and stability by self-assembly of explosive and oxidants: Facile synthesis, self-assembled structures and energetic properties. J. Mol. Struct. 2023, 1288, 135767. [Google Scholar] [CrossRef]
- Chen, D.X.; Xiong, H.L.; Yang, H.W.; Tang, J.; Cheng, G.B. Nitropyrazole based tricyclic nitrogen-rich cation salts: A new class of promising insensitive energetic materials. FirePhysChem 2021, 1, 71–75. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, G.J.; Li, J.; Zhang, Z.Q.; Lu, H.C.; Liao, L.Y.; Fan, G.J.; Nie, F.D. Pyrazol-triazole energetic hybrid with high thermal stability and decreased sensitivity: Facile synthesis, characterization and promising performance. Chem. Eur. J. 2020, 379, 122331. [Google Scholar] [CrossRef]
- Cao, W.L.; Wang, T.W.; Dong, W.S.; Lu, Z.J.; Tariq, Q.; Manzoor, S.; Zhang, J.G. Synthesis and characterization of energetic salts based on a new coplanar bicyclic cation-5-amino-3-(5-amino-1,2,4-oxadiazol-3-yl)-1H-1,2,4-triazolium. J. Mol. Struct. 2022, 1248, 131438. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Lu, T.; Chen, Q.X. A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor. Chem. Acc. 2020, 139, 25. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 09; revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sućeska, M. EXPLO5, version 6.01; Brodarski Institute: Zagreb, Croatia, 2013. [Google Scholar]
- Hu, W.; Zhang, G.J.; Yang, P.J.; Yang, H.W.; Cheng, G.B. Intramolecular integration of pyrazole-triazine-triazole heterocyclic skeletons: A novel 5/6/5 fused energetic framework with high energy and low sensitivity. Chem. Eng. J. 2023, 451, 138640. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Tudela, D.; Glasser, L. Lattice potential energy estimation for complex ionic salts from density measurements. Inorg. Chem. 2002, 41, 2364–2367. [Google Scholar] [CrossRef] [PubMed]
- Westwell, M.S.; Searle, M.S.; Wales, D.J. Empirical correlations between thermodynamic properties and intermolecular forces. J. Am. Chem. Soc. 1995, 117, 5013–5015. [Google Scholar] [CrossRef]




| Compounds | Td a (°C) | ρ b (g·cm−3) | Hf c (kJ·mol−1) | D d (m·s−1) | P e (GPa) | IS f (J) | FS g (J) | OB h (%) |
|---|---|---|---|---|---|---|---|---|
| DATOC | 154 | 1.717 | −80.3 | 5984 | 12.4 | 28 | 280 | −63.3 |
| DATOP | 273 | 1.954 | −62.6 | 8624 | 34.4 | 22 | 210 | −6.5 |
| TNT i | 295 | 1.65 | −355.0 | 6881 | 19.5 | 15 | 353 | −74.0 |
| RDX i | 204 | 1.81 | 80.0 | 8795 | 34.9 | 7.4 | 120 | −21.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, M.; Men, J.; Li, B.; Feng, S.; Zhu, S.; Liu, G.; Gou, R.; Zhang, S.; Lu, M.; et al. Salt Formation of the Alliance of Triazole and Oxadiazole Towards Balanced Energy and Safety. Materials 2025, 18, 3435. https://doi.org/10.3390/ma18153435
Liu Y, Wang M, Men J, Li B, Feng S, Zhu S, Liu G, Gou R, Zhang S, Lu M, et al. Salt Formation of the Alliance of Triazole and Oxadiazole Towards Balanced Energy and Safety. Materials. 2025; 18(15):3435. https://doi.org/10.3390/ma18153435
Chicago/Turabian StyleLiu, Yang, Meiqi Wang, Jiawei Men, Bibo Li, Shangbiao Feng, Shuangfei Zhu, Guangrui Liu, Ruijun Gou, Shuhai Zhang, Ming Lu, and et al. 2025. "Salt Formation of the Alliance of Triazole and Oxadiazole Towards Balanced Energy and Safety" Materials 18, no. 15: 3435. https://doi.org/10.3390/ma18153435
APA StyleLiu, Y., Wang, M., Men, J., Li, B., Feng, S., Zhu, S., Liu, G., Gou, R., Zhang, S., Lu, M., & Yang, L. (2025). Salt Formation of the Alliance of Triazole and Oxadiazole Towards Balanced Energy and Safety. Materials, 18(15), 3435. https://doi.org/10.3390/ma18153435








