Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
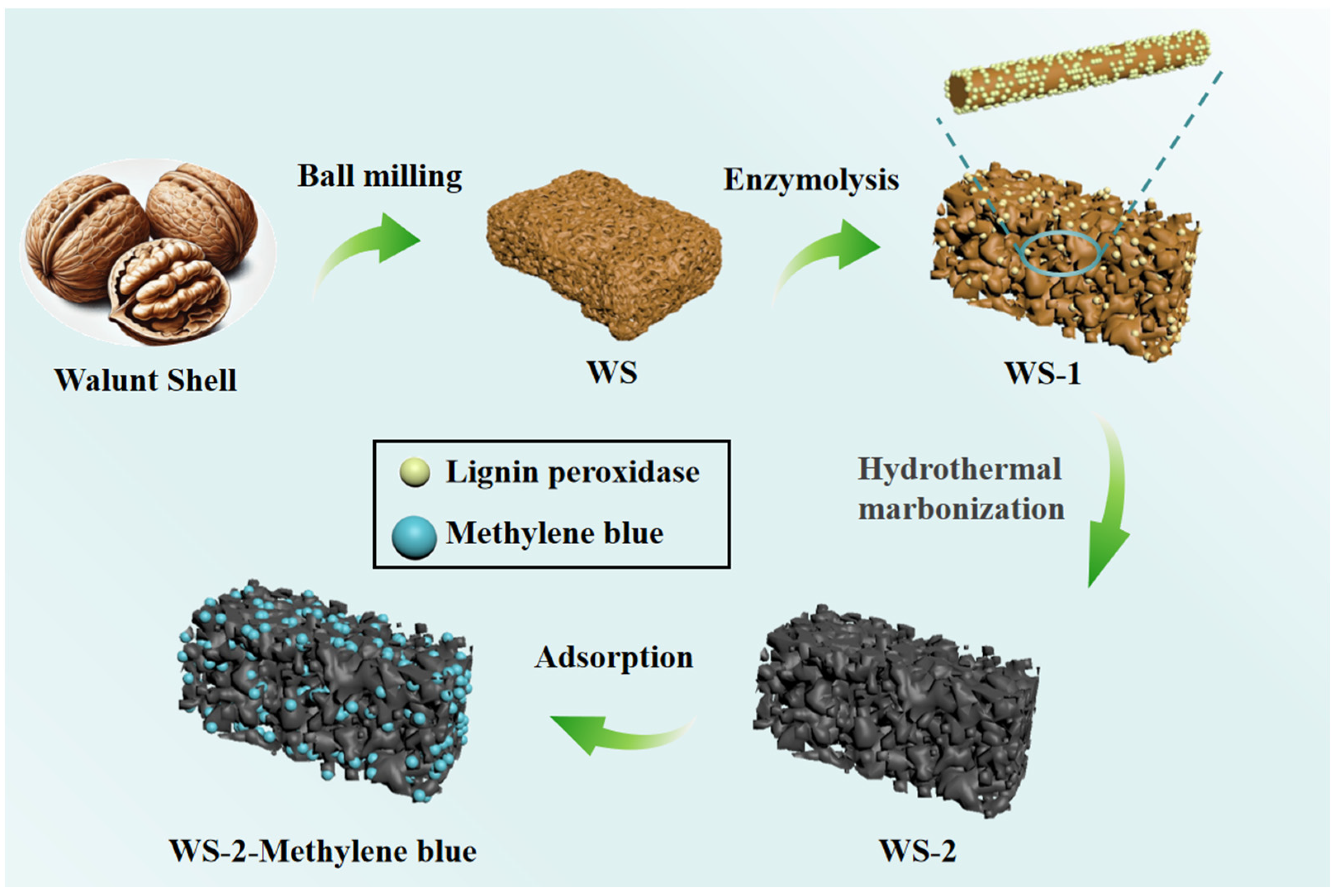
2.2. Preparation of Adsorbents
2.3. Characterization
2.4. Adsorption Experiments
2.5. Adsorption Kinetic and Isotherm Experiments
3. Results and Discussion
3.1. Adsorbent Characterization
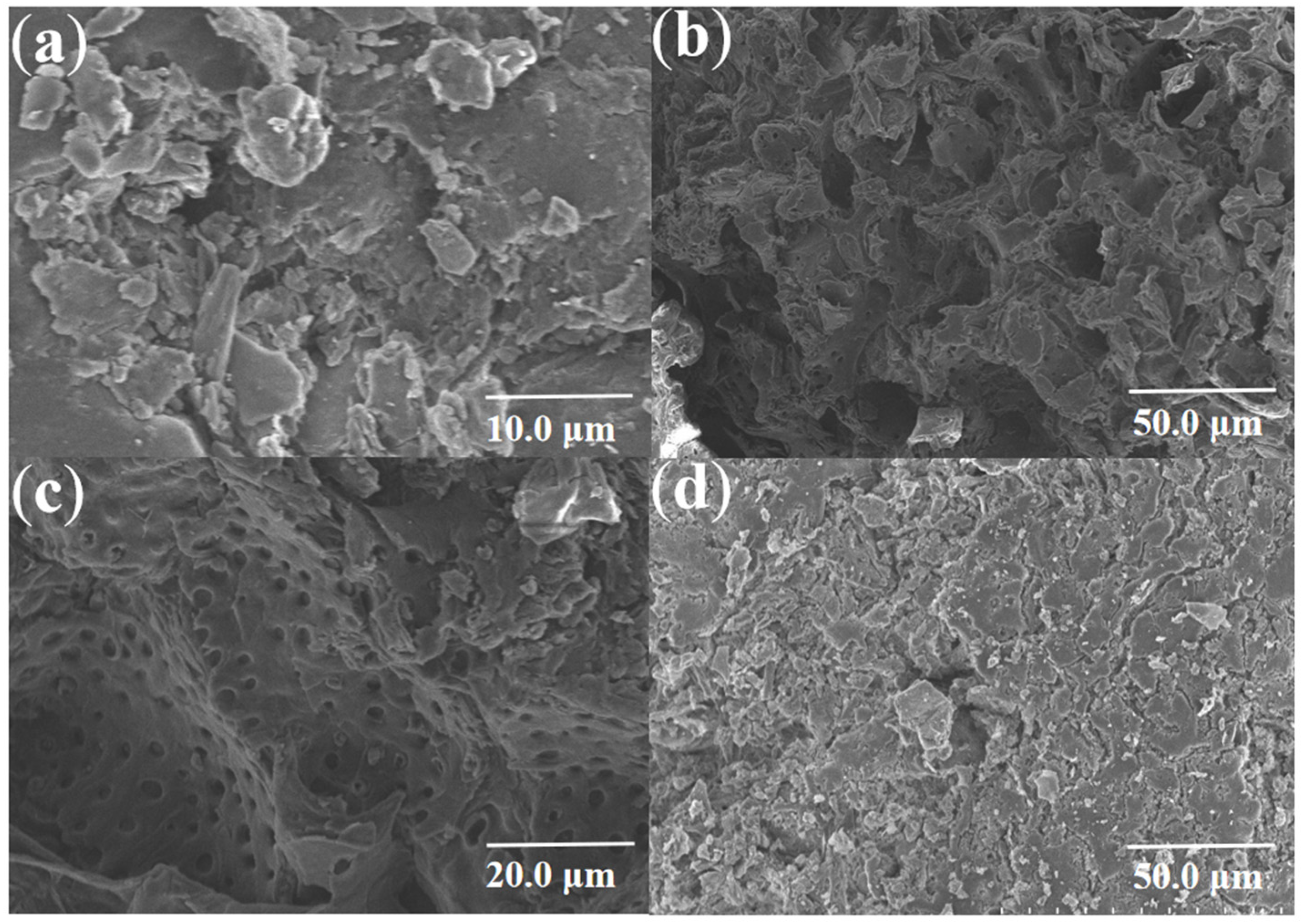
3.1.1. Scanning Electron Microscope Images
3.1.2. Fourier-Transform Infrared Spectroscopy Spectra
3.1.3. Brunauer–Emmett–Teller Surface Area Analysis
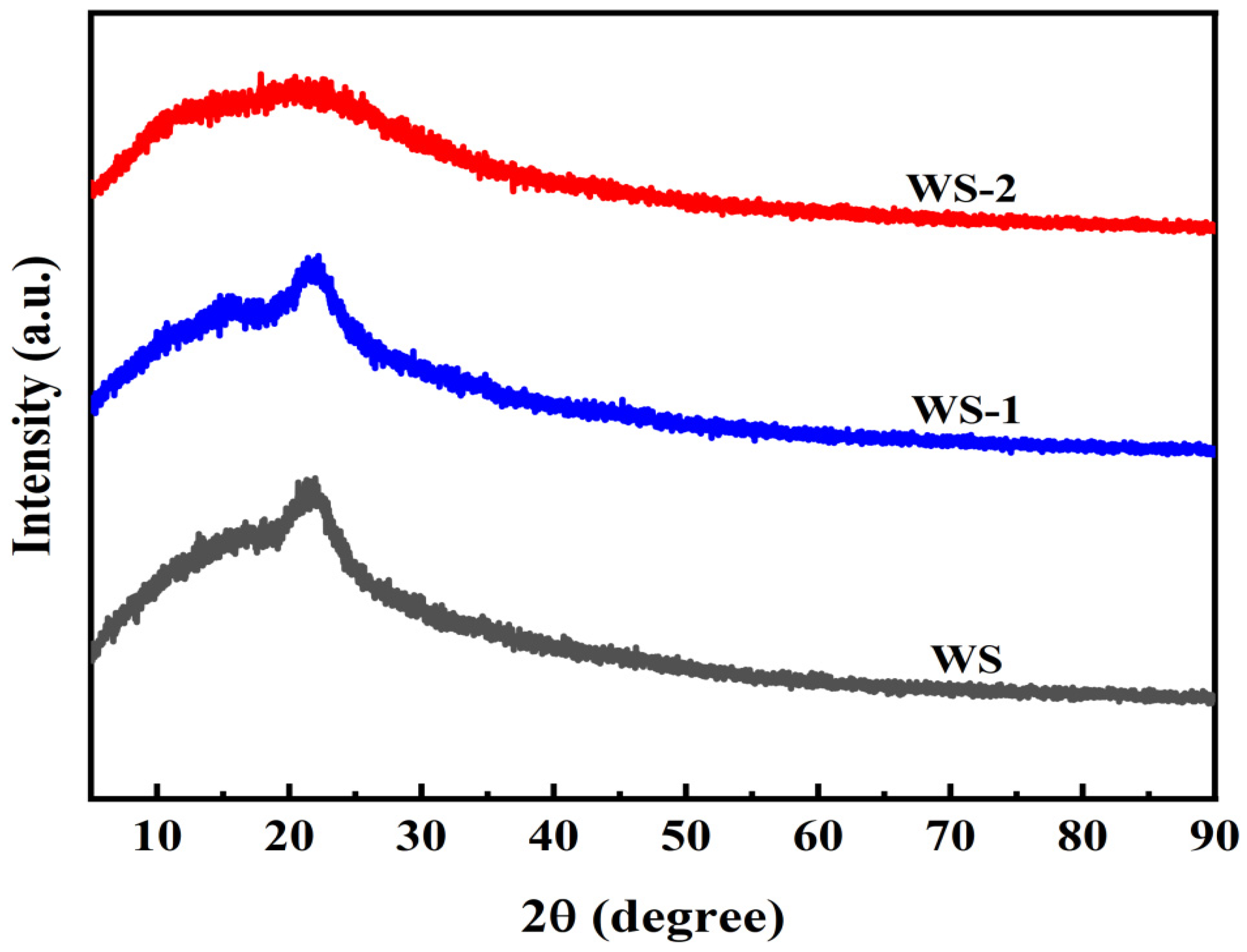
3.1.4. X-Ray Diffraction Patterns
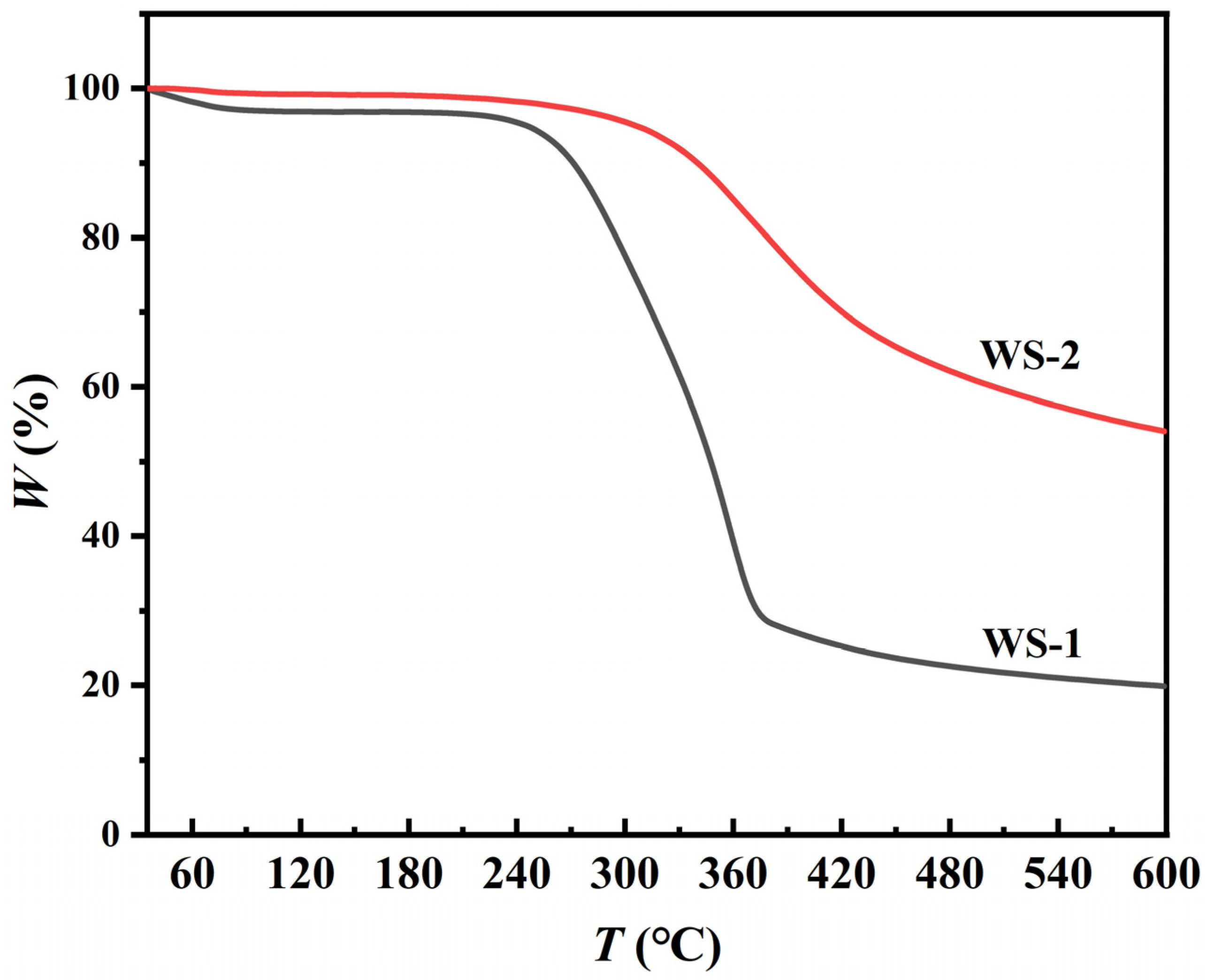
3.1.5. Thermogravimetric Analysis Results
3.2. Adsorption Performances
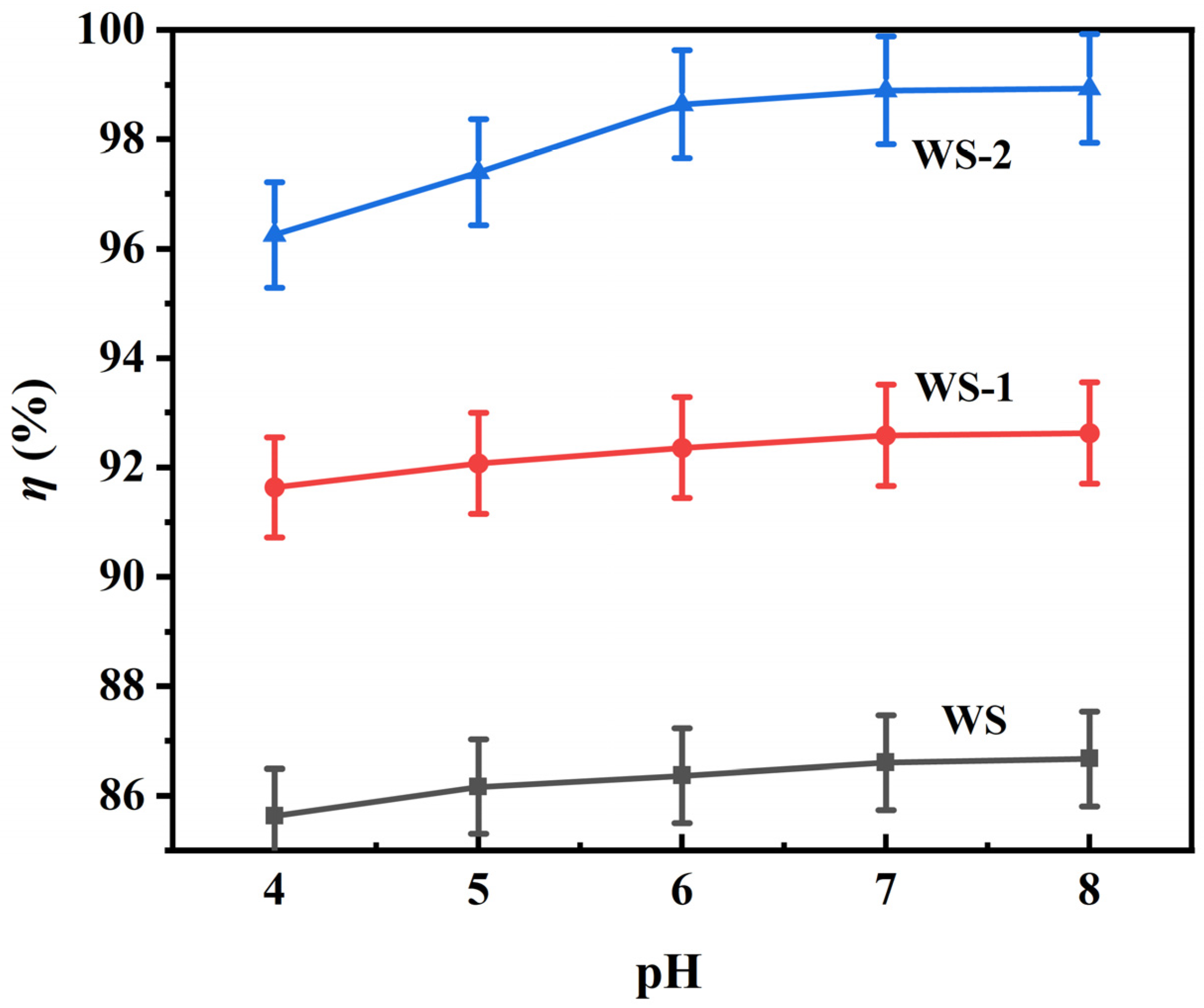
3.2.1. pH-Dependent Adsorption Behavior
3.2.2. Temperature-Dependent Adsorption Profiles
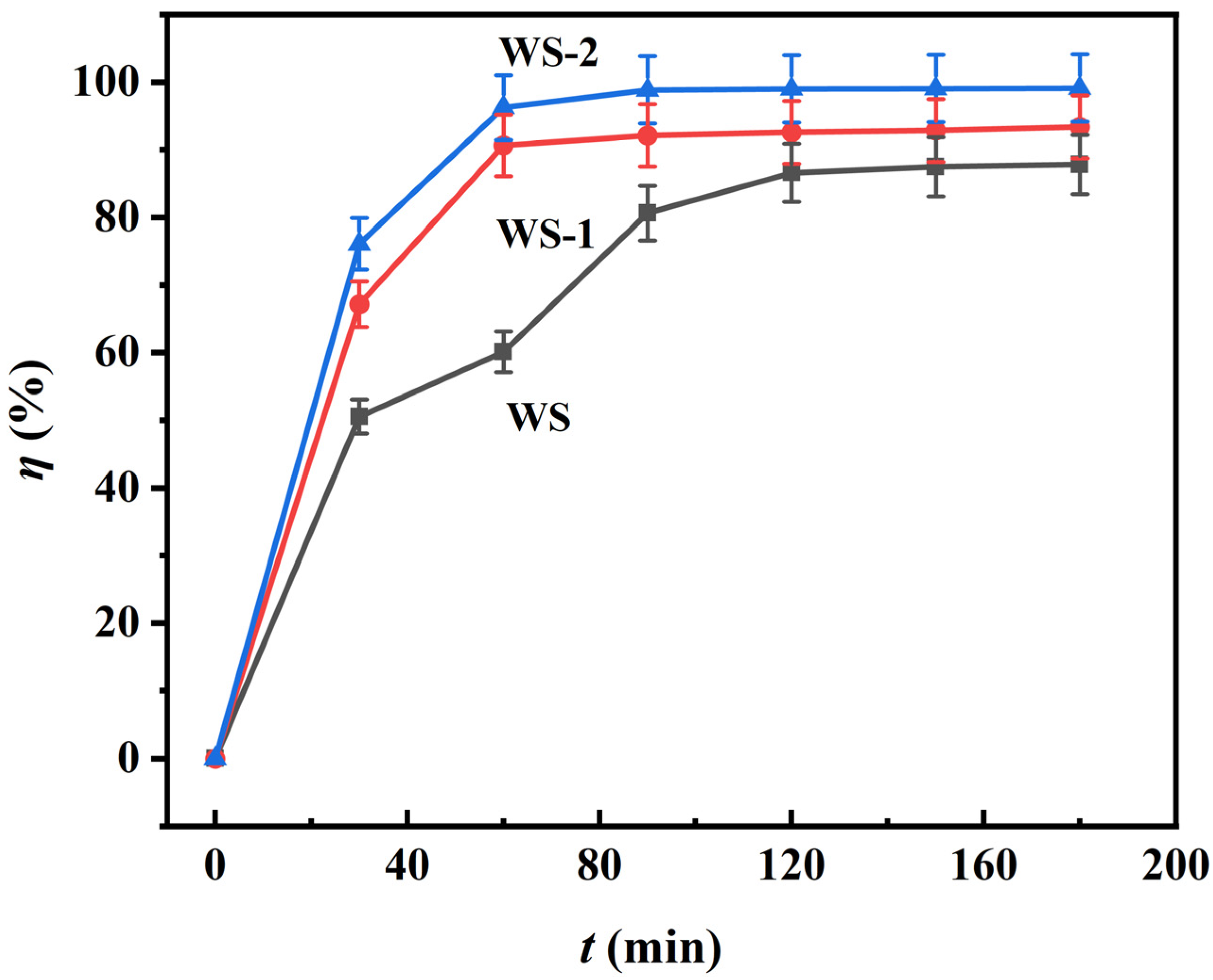
3.2.3. Time-Resolved Adsorption Kinetics
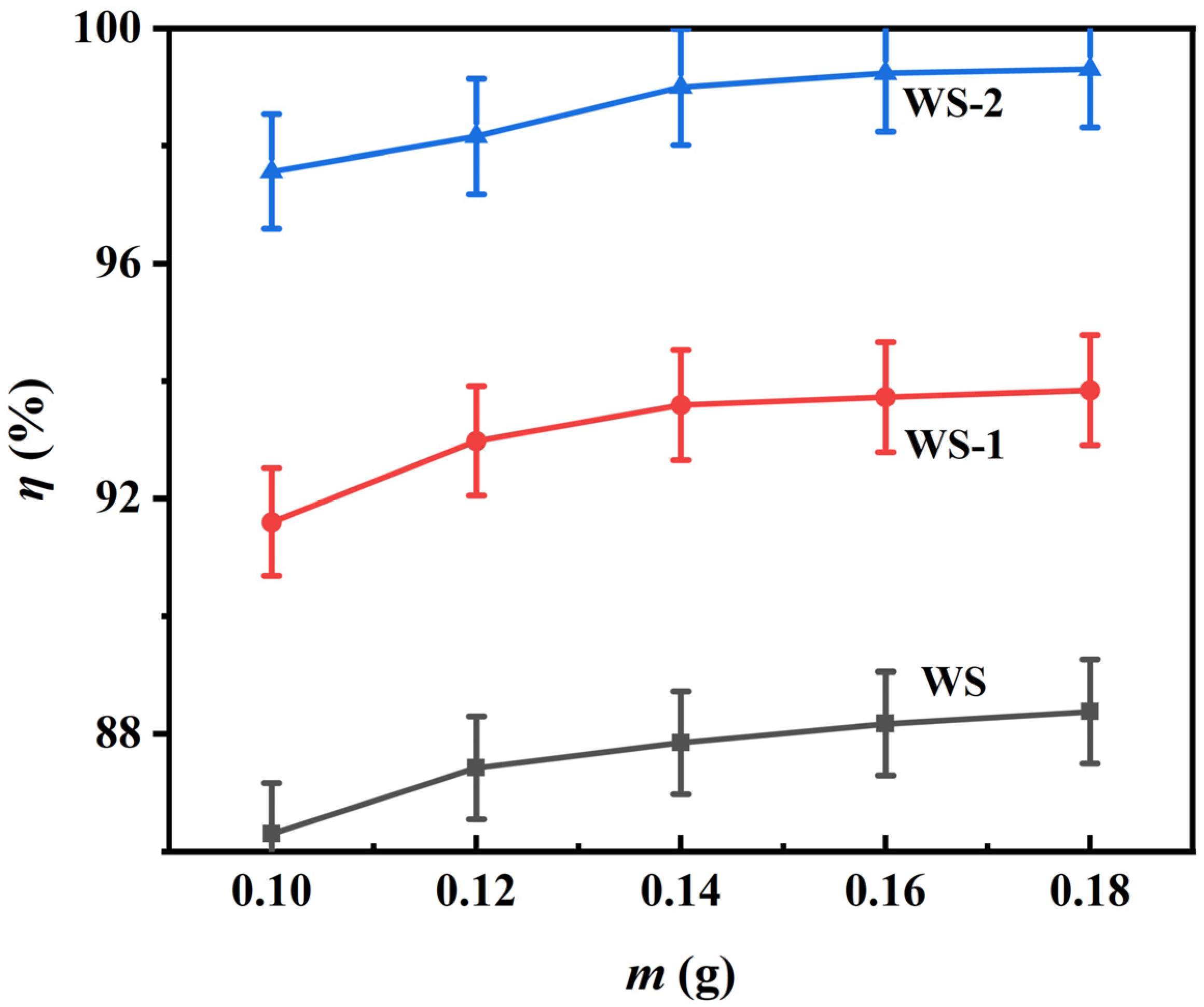
3.2.4. Adsorbent Dosage Optimization
3.3. Adsorption Mechanisms
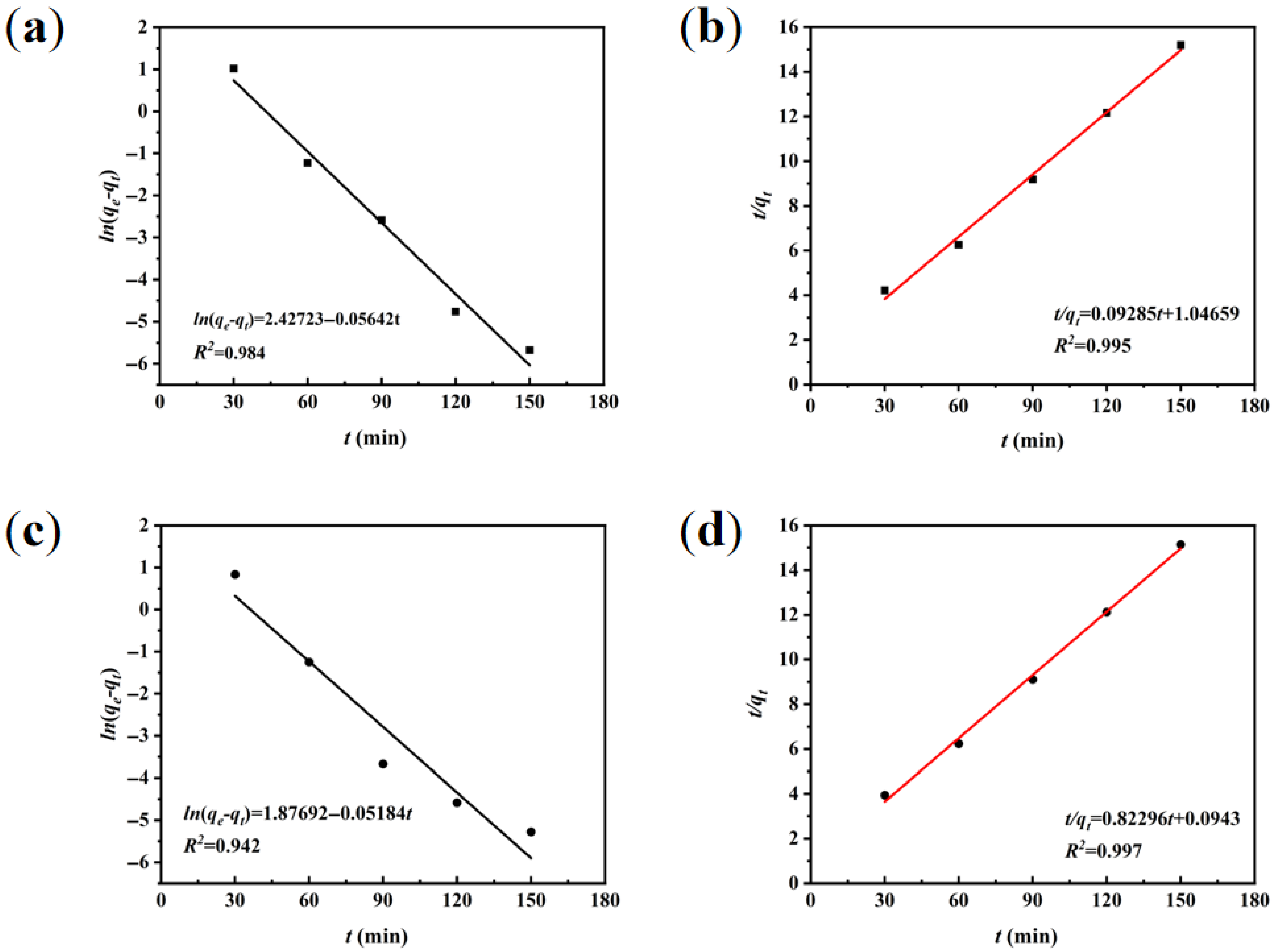
3.3.1. Adsorption Kinetic Modeling
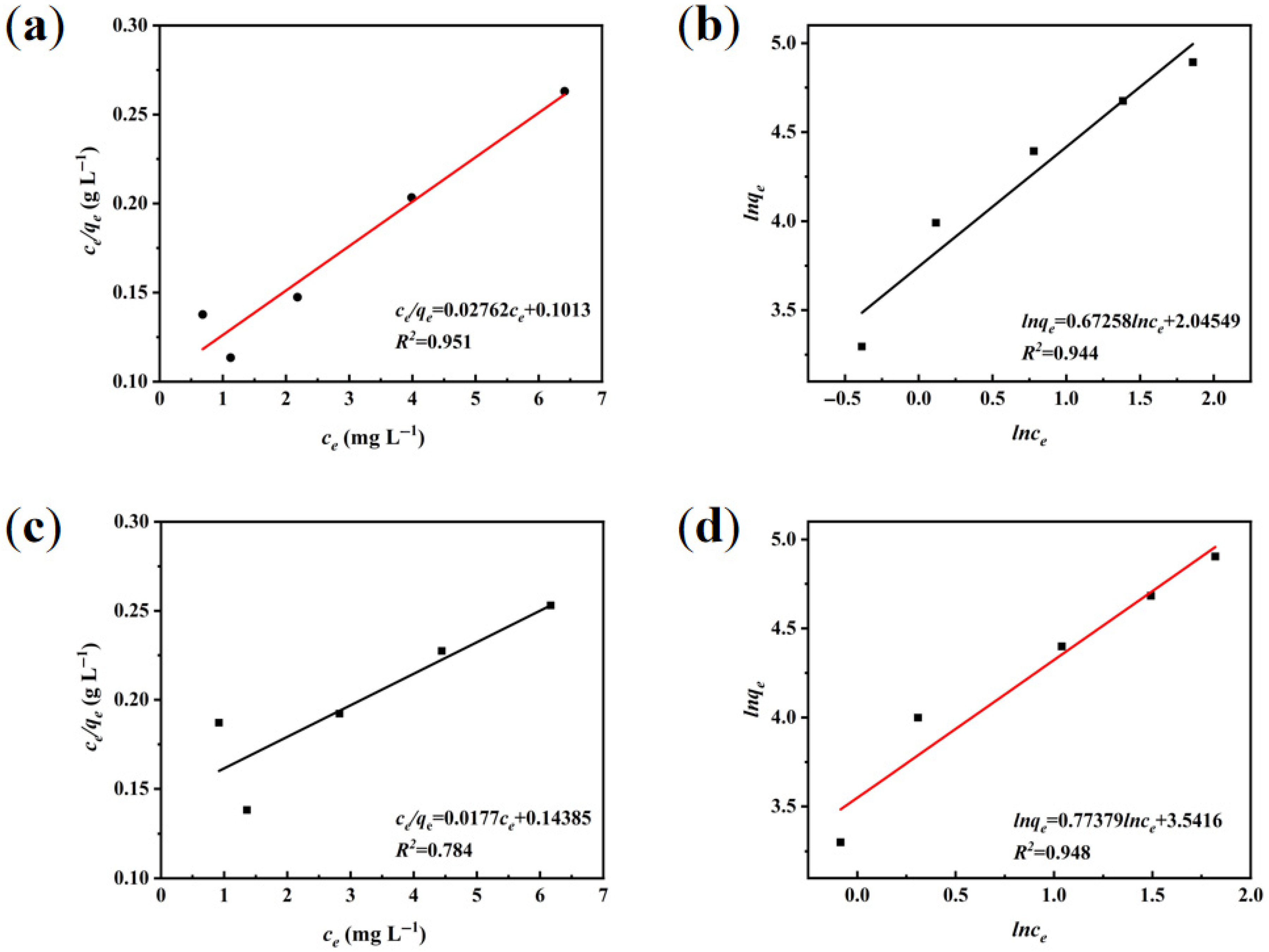
3.3.2. Adsorption Isotherm Modeling
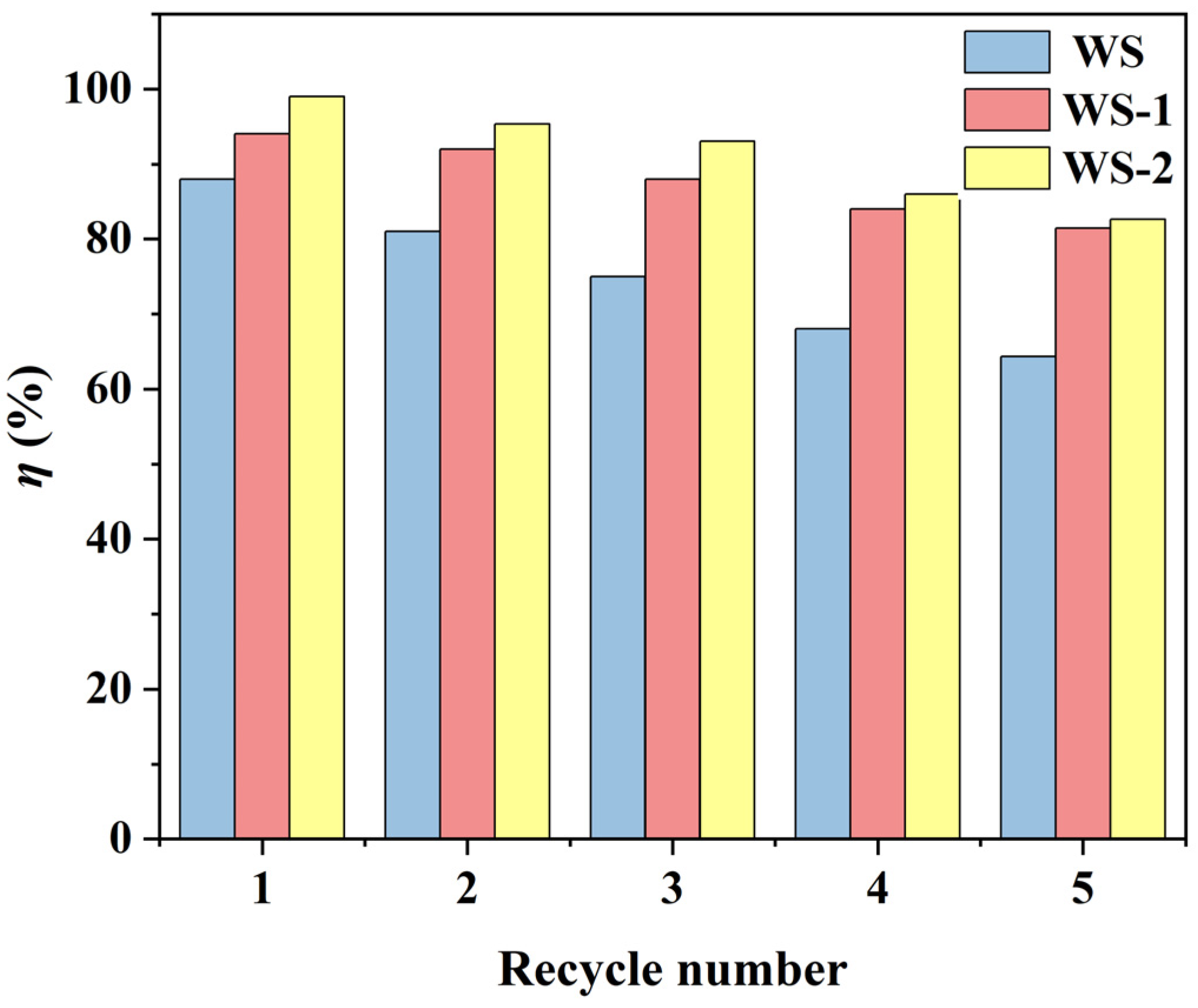
3.4. Analysis of Regenerative Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emmanuel, S.S.; Adesibikan, A.A. Bio-fabricated green silver nano-architecture for degradation of methylene blue water contaminant: A mini-review. Water Environ. Res. 2021, 93, 2873–2882. [Google Scholar] [CrossRef] [PubMed]
- Rahmaninezhad, S.A.; Mehrdadi, N.; Mahzari, Z. Analysis of the factors controlling the performance of a photoelectrocatalytic cell separated by UF membrane in degrading methylene blue. J. Aust. Ceram. Soc. 2021, 57, 163–172. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Puasa, S.W.; Zulkali, M.M.D. Micellar-enhanced ultrafiltration for removal of reactive dyes from an aqueous solution. Desalination 2006, 191, 153–161. [Google Scholar] [CrossRef]
- Zhao, Z.; Nie, T.; Zhou, W. Enhanced biochar stabilities and adsorption properties for tetracycline by synthesizing silica-composited biochar. Environ. Pollut. 2019, 254, 113015. [Google Scholar] [CrossRef]
- Fan, T.; Yan, Z.; Yang, C.; Qiu, S.; Peng, X.; Zhang, J.; Hu, L.; Chen, L. Preparation of menthol-based hydrophobic deep eutectic solvents for the extraction of triphenylmethane dyes: Quantitative properties and extraction mechanism. Analyst 2021, 146, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Qu, W.Y.; Yuan, T.; Yin, G.J.; Xu, S.A.; Zhang, Q.; Su, H.J. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Chakraborty, S.; Sarkar, D.; Biswas, J.K. Biochar Modification Methods for Augmenting Sorption of Contaminants. Curr. Pollut. Rep. 2022, 8, 519–555. [Google Scholar] [CrossRef]
- Qiu, S.X.; Li, Q.L.; Li, X.Y.; Ma, J.X.; Wu, L.Q.; Xie, X.J.; Wu, L.X.; Askari, S.; Dewangan, N.; Ashok, J.; et al. Biomass-Derived Carbon Materials for the Adsorption of Organic Pollutants. Adv. Sustain. Syst. 2024, 8, 2300340. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, Y.; Cheng, M.Q.; Yang, J.Z.; Huang, Q.F.; Huang, Z.C.; Cui, Y.T.; Zhang, L.P.; Wang, Y.S.; Cen, Q.H.; et al. Effective adsorption performance and mechanism of methylene blue from dye wastewater by humic acid sucrose-modified red mud. Process Saf. Environ. Prot. 2024, 191, 1168–1180. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, S.; Wang, Y.; Yang, H. Adsorption Properties and Mechanisms of Methylene Blue by Modified Sphagnum Moss Bio-Based Adsorbents. Materials 2024, 17, 4329. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Li, F.Y.; Yu, Z.P.; Ji, L.C.; Jiang, F.; Chen, C.X.; Yang, J. Enhanced Adsorption of Methylene Blue Using H2O2-Modified Hydrochar. Water Air Soil Pollut. 2022, 233, 422. [Google Scholar] [CrossRef]
- Wei, J.; Liang, G.; Alex, J.; Zhang, T.; Ma, C. Research Progress of Energy Utilization of Agricultural Waste in China: Bibliometric Analysis by Citespace. Sustainability 2020, 12, 812. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; Riazanova, A.V.; Pasalskiy, B.; Budnyak, T.; Lindström, M.E.; Kartel, M. Walnut shells as a potential low-cost lignocellulosic sorbent for dyes and metal ions. Cellulose 2018, 25, 4729–4742. [Google Scholar] [CrossRef]
- Higuchi, T. Microbial degradation of lignin: Role of lignin peroxidase, manganese peroxidase, and laccase. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci. 2004, 80, 204–214. [Google Scholar] [CrossRef]
- Kang, C.; Zhu, L.; Wang, Y.; Wang, Y.; Xiao, K.; Tian, T. Adsorption of Basic Dyes Using Walnut Shell-based Biochar Produced by Hydrothermal Carbonization. Chem. Res. Chin. Univ. 2018, 34, 622–627. [Google Scholar] [CrossRef]
- Negahdar, L.; Delidovich, I.; Palkovits, R. Aqueous-phase hydrolysis of cellulose and hemicelluloses over molecular acidic catalysts: Insights into the kinetics and reaction mechanism. Appl. Catal. B Environ. 2016, 184, 285–298. [Google Scholar] [CrossRef]
- Li, X.; Cen, K.; Wang, L.; Jia, D.; Zhu, X.; Chen, D. Co-pyrolysis of cellulose and lignin: Effects of pyrolysis temperature, residence time, and lignin percentage on the properties of biochar using response surface methodology. Ind. Crops Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Saini, R.; Pandey, M.; Mishra, R.K.; Kumar, P. Adsorption potential of hydrochar derived from hydrothermal carbonization of waste biomass towards the removal of methylene blue dye from wastewater. Biomass Convers. Biorefinery 2024, 15, 9229–9249. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, M.; Zhang, S.; Min, Z.H.; Yimsiri, P.; Li, C.Z. Effects of biomass char structure on its gasification reactivity. Bioresour. Technol. 2010, 101, 7935–7943. [Google Scholar] [CrossRef]
- Yang, D.; Wu, X.; Qiu, X.; Chang, Y.; Lou, H. Polymerization reactivity of sulfomethylated alkali lignin modified with horseradish peroxidase. Bioresour. Technol. 2014, 155, 418–421. [Google Scholar] [CrossRef]
- Paksung, N.; Pfersich, J.; Arauzo, P.J.; Jung, D.; Kruse, A. Structural Effects of Cellulose on Hydrolysis and Carbonization Behavior during Hydrothermal Treatment. ACS Omega 2020, 5, 12210–12223. [Google Scholar] [CrossRef]
- Bestani, B.; Benderdouche, N.; Benstaali, B.; Belhakem, M.; Addou, A. Methylene blue and iodine adsorption onto an activated desert plant. Bioresour. Technol. 2008, 99, 8441–8444. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Zhou, H.; Li, Z.; Fu, Y.; Qin, M. Utilization of lignin separated from pre-hydrolysis liquor via horseradish peroxidase modification as an adsorbent for methylene blue removal from aqueous solution. Ind. Crops Prod. 2021, 167, 113535. [Google Scholar] [CrossRef]
- Li, B.Y.; Li, K.Q. Effect of nitric acid pre-oxidation concentration on pore structure and nitrogen/oxygen active decoration sites of ethylenediamine -modified biochar for mercury(II) adsorption and the possible mechanism. Chemosphere 2019, 220, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Sonoda, K.; Maruoka, K.; Yamazaki, Y.; Naragino, H.; Honda, K.; Murafuji, T.; Sumimoto, M. Porous TiO2 adsorbed with squaraine dye as visible-light-responsive photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 421, 113543. [Google Scholar] [CrossRef]
- Liu, X.-J.; Li, M.-F.; Ma, J.-F.; Bian, J.; Peng, F. Chitosan crosslinked composite based on corncob lignin biochar to adsorb methylene blue: Kinetics, isotherm, and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128621. [Google Scholar] [CrossRef]
- Si, J.; Yuan, T.Q.; Cui, B.K. Exploring strategies for adsorption of azo dye Congo Red using free and immobilized biomasses of Trametes pubescens. Ann. Microbiol. 2015, 65, 411–421. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.Y.; Feng, C.; Qin, B.J.; Zhang, W.H.; Zhao, N.; Li, Y.Y.; Ni, Z.B.; Xu, Z.H.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qin, C.X.; Wang, T.; Chen, F.Y.; Li, X.L.; Hou, H.B.; Zhou, M. Study on the adsorption of dyestuffs with different properties by sludge-rice husk biochar: Adsorption capacity, isotherm, kinetic, thermodynamics and mechanism. J. Mol. Liq. 2019, 285, 62–74. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Teng, S.X.; Song, R.H.; Wang, S.G. Adsorption of methylene blue on anaerobic granular sludge: Effect of functional groups. Desalination 2010, 263, 11–17. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chen, J.; Lin, J.H. Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J. Environ. Chem. Eng. 2020, 8, 103929. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A. Dyes Adsorption from Aqueous Solutions by Chitosan. Sep. Sci. Technol. 2015, 50, 1101–1107. [Google Scholar] [CrossRef]
- Sun, D.H.; Zhang, X.D.; Wu, Y.D.; Liu, X. Adsorption of anionic dyes from aqueous solution on fly ash. J. Hazard. Mater. 2010, 181, 335–342. [Google Scholar] [CrossRef]
- Tang, R.; Dai, C.; Li, C.; Liu, W.; Gao, S.; Wang, C. Removal of Methylene Blue from Aqueous Solution Using Agricultural Residue Walnut Shell: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. 2017, 2017, 8404965. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Zhang, Y.; Zhao, M.; Zhou, N.; Fan, S. Effect of citric acid modification on the properties of hydrochar and pyrochar and their adsorption performance toward methylene blue: Crucial roles of minerals and oxygen functional groups. Environ. Monit. Assess. 2024, 196, 664. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, G.D.R.; Duarte, C.R.; Barrozo, M.A.S. Hydrothermal carbonization of acerola (Malphigia emarginata DC) wastes and its application as an adsorbent. Waste Manag. 2019, 95, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Rouissi, T.; Das, R.K.; Brar, S.K.; Ramirez, A.A.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag. 2016, 49, 537–544. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Y.; Yang, H.; Zhan, L.; Sun, G.; Luo, L. On the adsorption characteristics and mechanism of methylene blue by ball mill modified biochar. Sci. Rep. 2023, 13, 211174. [Google Scholar] [CrossRef] [PubMed]
- Primaz, C.T.; Ribes-Greus, A.; Jacques, R.A. Valorization of cotton residues for production of bio-oil and engineered biochar. Energy 2021, 235, 121363. [Google Scholar] [CrossRef]
- Mu, Y.; Ma, H. NaOH-modified mesoporous biochar derived from tea residue for methylene Blue and Orange II removal. Chem. Eng. Res. Des. 2021, 167, 129–140. [Google Scholar] [CrossRef]



| Adsorbents | BET Surface Area |
|---|---|
| WS | 192 m2 g−1 |
| WS-1 | 462 m2 g−1 |
| WS-2 | 630 m2 g−1 |
| Type of Biochar | Pyrolysis Temperature (°C) | Modifying Agent | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|---|
| Pine wood | 525 | Physical pulverization | 25 | [43] |
| Pig manure | 400 | Physical pulverization | 7.9 | [43] |
| Cardboard | 500 | Physical pulverization | 8.9 | [43] |
| Rice straw | 500 | Ball milling | 50.27 | [44] |
| Cotton residue | 550 | NaOH | 23.82 | [45] |
| Tea residue | 700 | NaOH | 105.44 | [46] |
| WS | 25 | None | 31.4 | Present study |
| WS-1 | 40 | Lignin peroxidase | 33.57 | Present study |
| WS-2 | 180 | Citric acid | 35.7 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Zhou, X.; Yang, R.; Cai, D.; Ren, W. Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal. Materials 2025, 18, 3434. https://doi.org/10.3390/ma18153434
Lv X, Zhou X, Yang R, Cai D, Ren W. Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal. Materials. 2025; 18(15):3434. https://doi.org/10.3390/ma18153434
Chicago/Turabian StyleLv, Xifeng, Xuejian Zhou, Ruiqi Yang, Di Cai, and Wenqiang Ren. 2025. "Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal" Materials 18, no. 15: 3434. https://doi.org/10.3390/ma18153434
APA StyleLv, X., Zhou, X., Yang, R., Cai, D., & Ren, W. (2025). Enzymatic Modification of Walnut Shell for High-Efficiency Adsorptive Methylene Blue Removal. Materials, 18(15), 3434. https://doi.org/10.3390/ma18153434







