Tailored 3D Lattice SAPO-34/S-PEEK Composite Sorbents by Additive Manufacturing for Sorption Heat Transformation Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Three-Dimensional Printing Technology
- Printing temperature: 180 °C
- Filling percentage: 10%
- Layer height: 0.12 mm
- Slicing software: Ultimaker CURA (version 5.7.2)
- Printing speed: 50 mm/s
- Bed temperature: 60 °C
2.3. Material Characterization
3. Results and Discussion
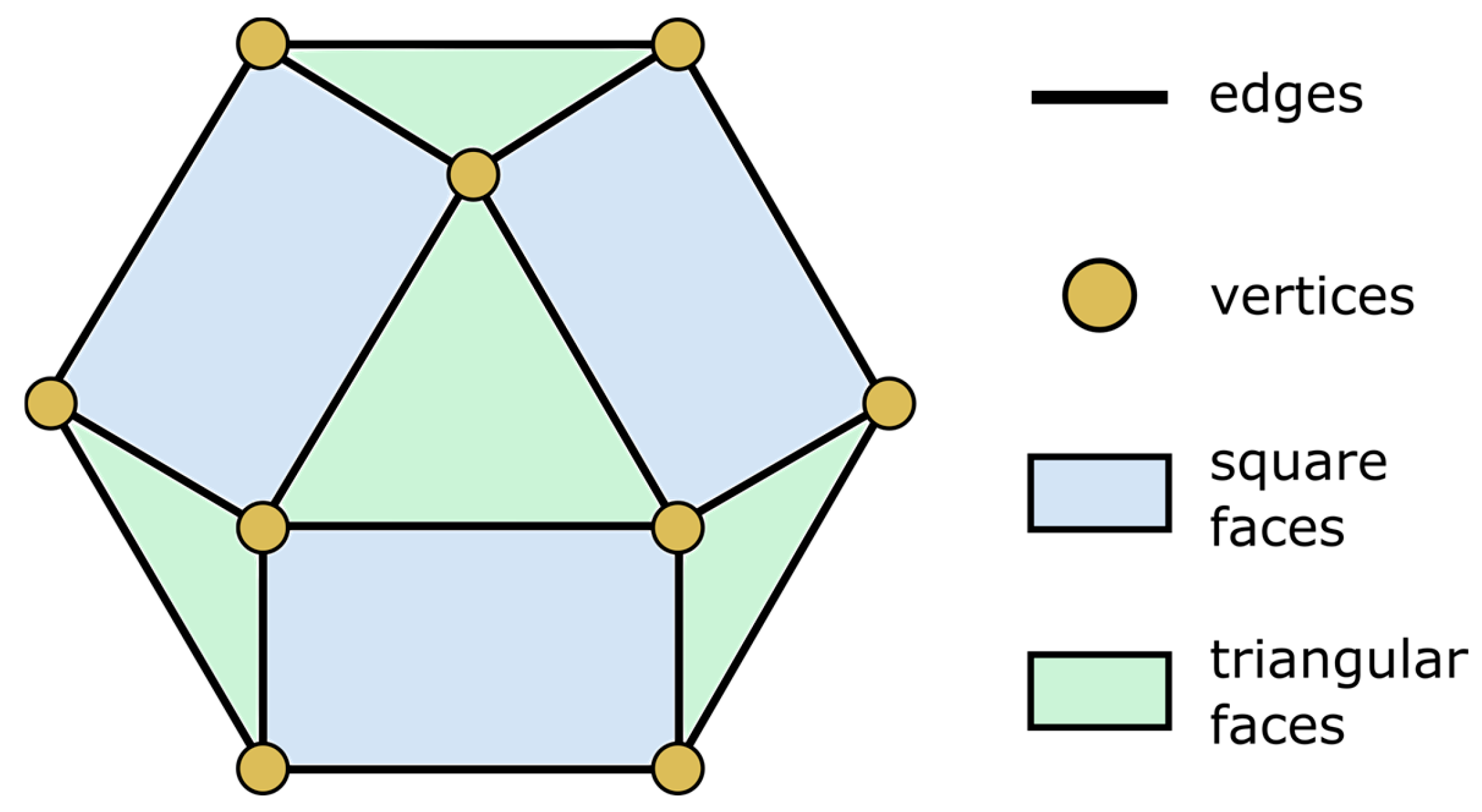
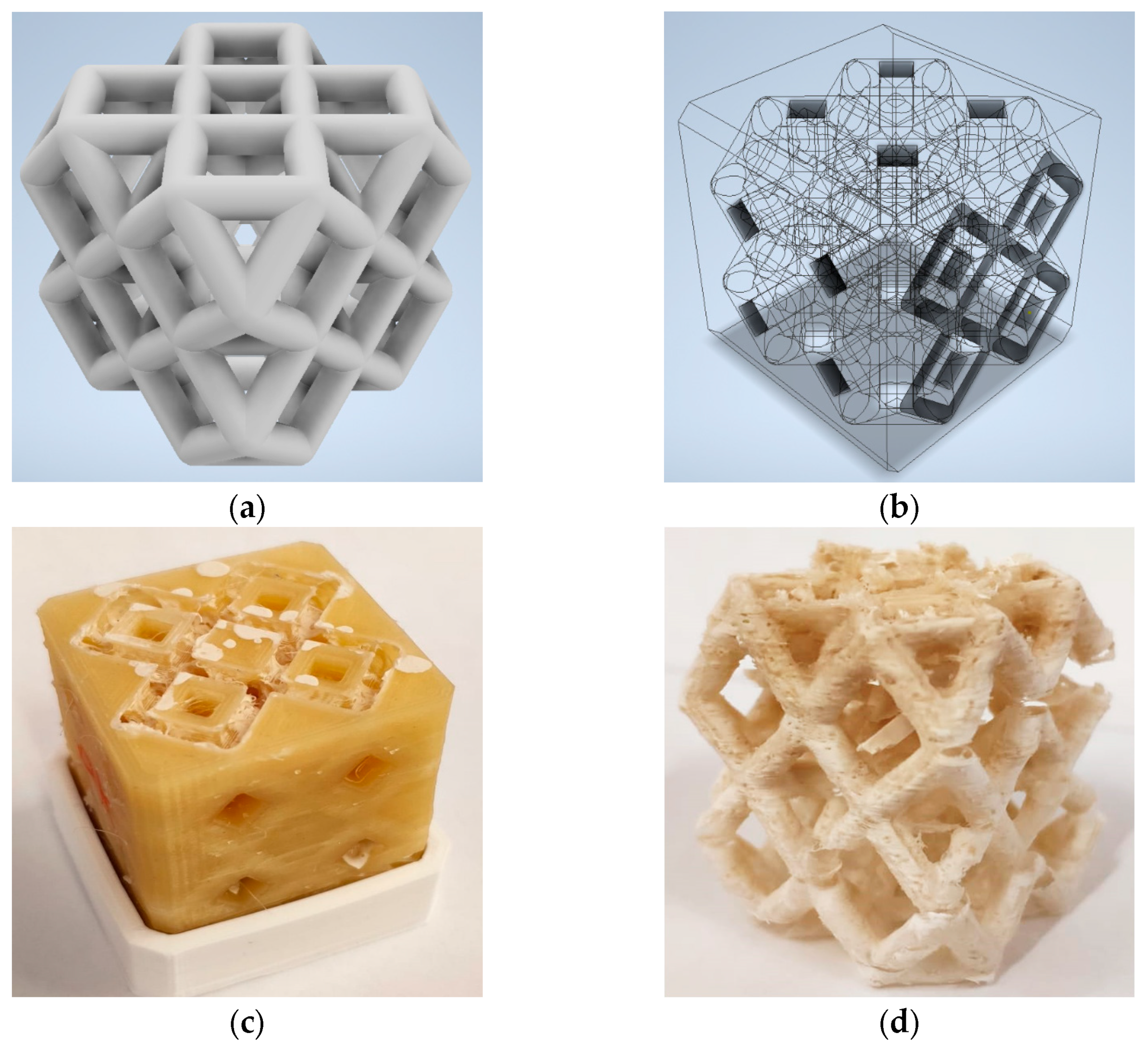
3.1. Design and 3D Printing of Adsorbent Lattice Structure
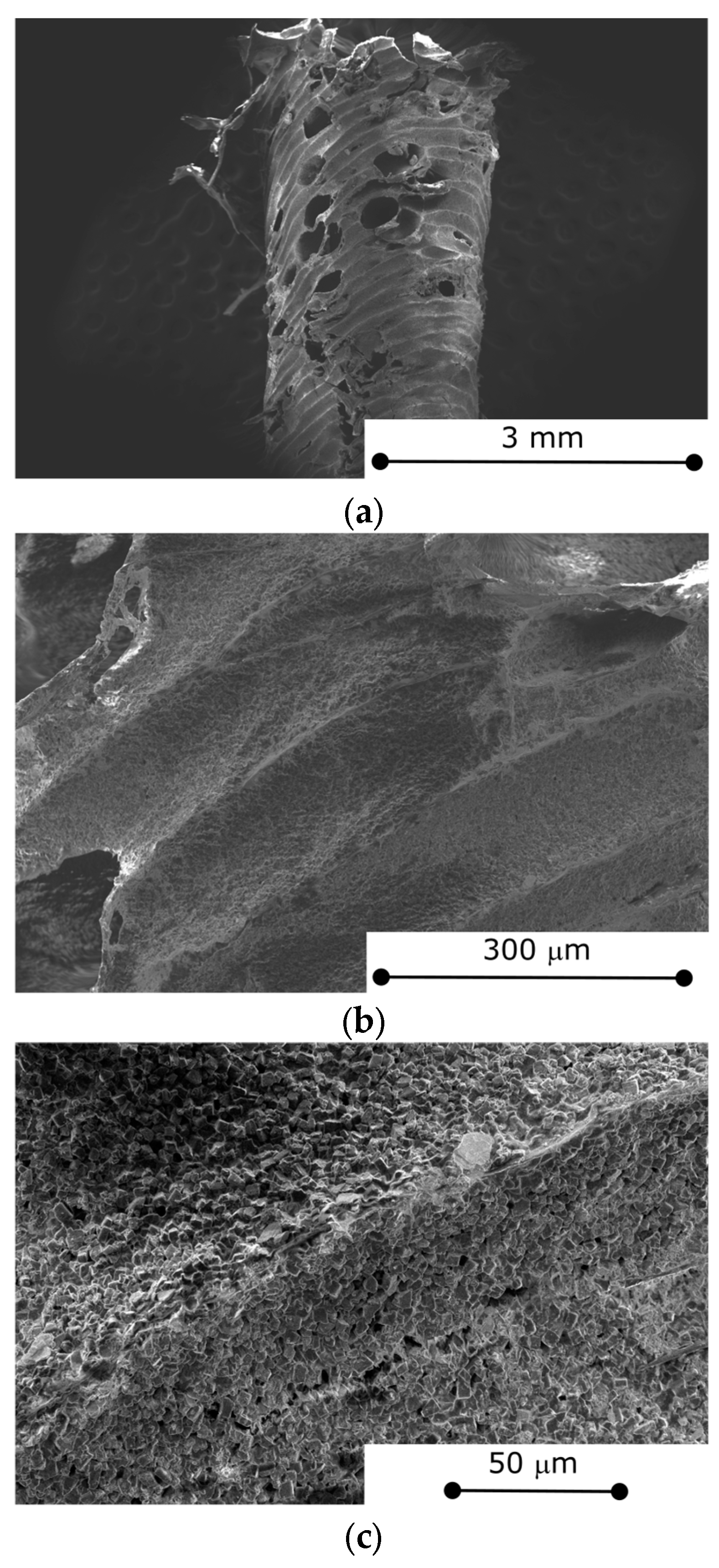
3.2. Morphological Characterization
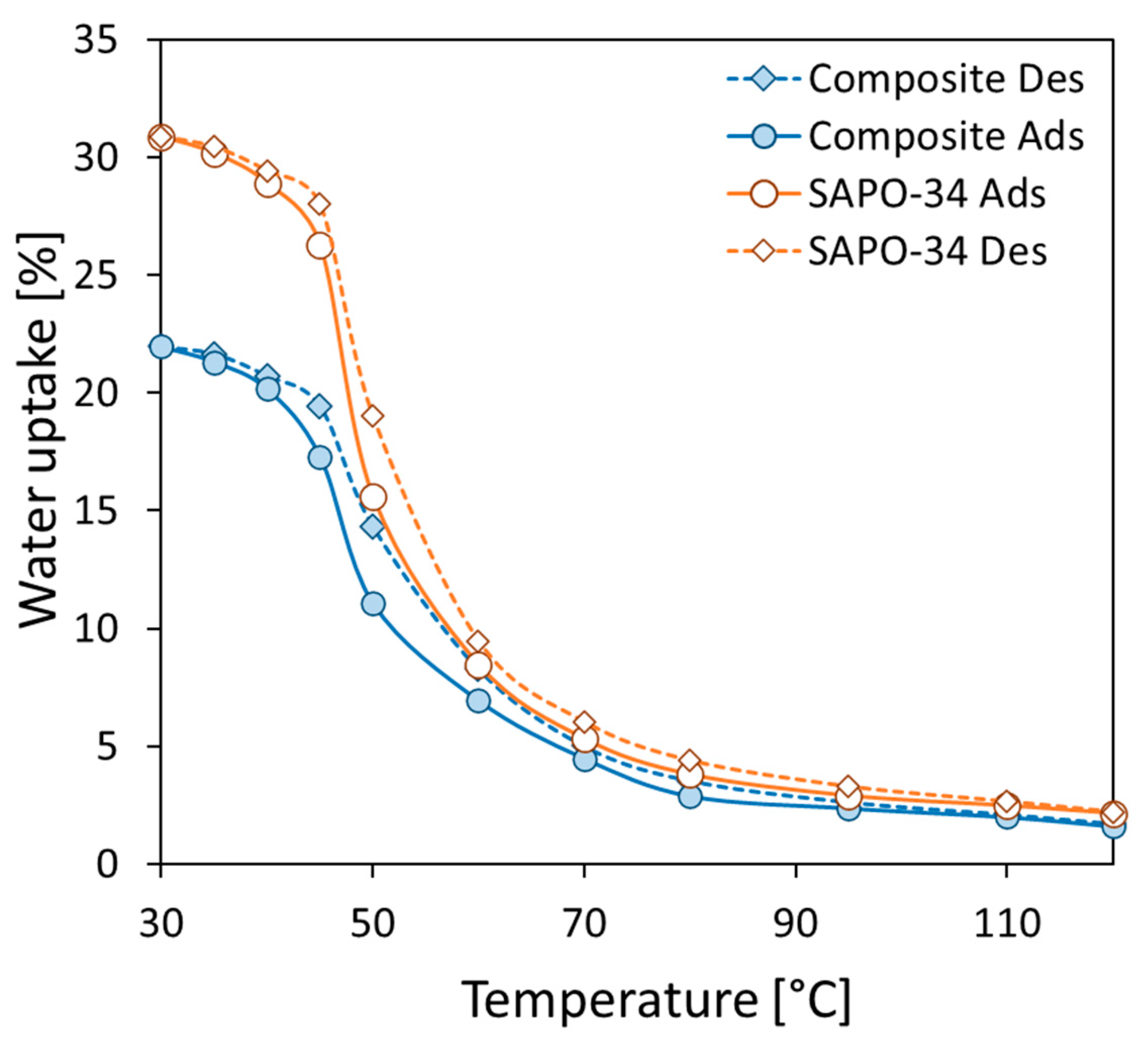
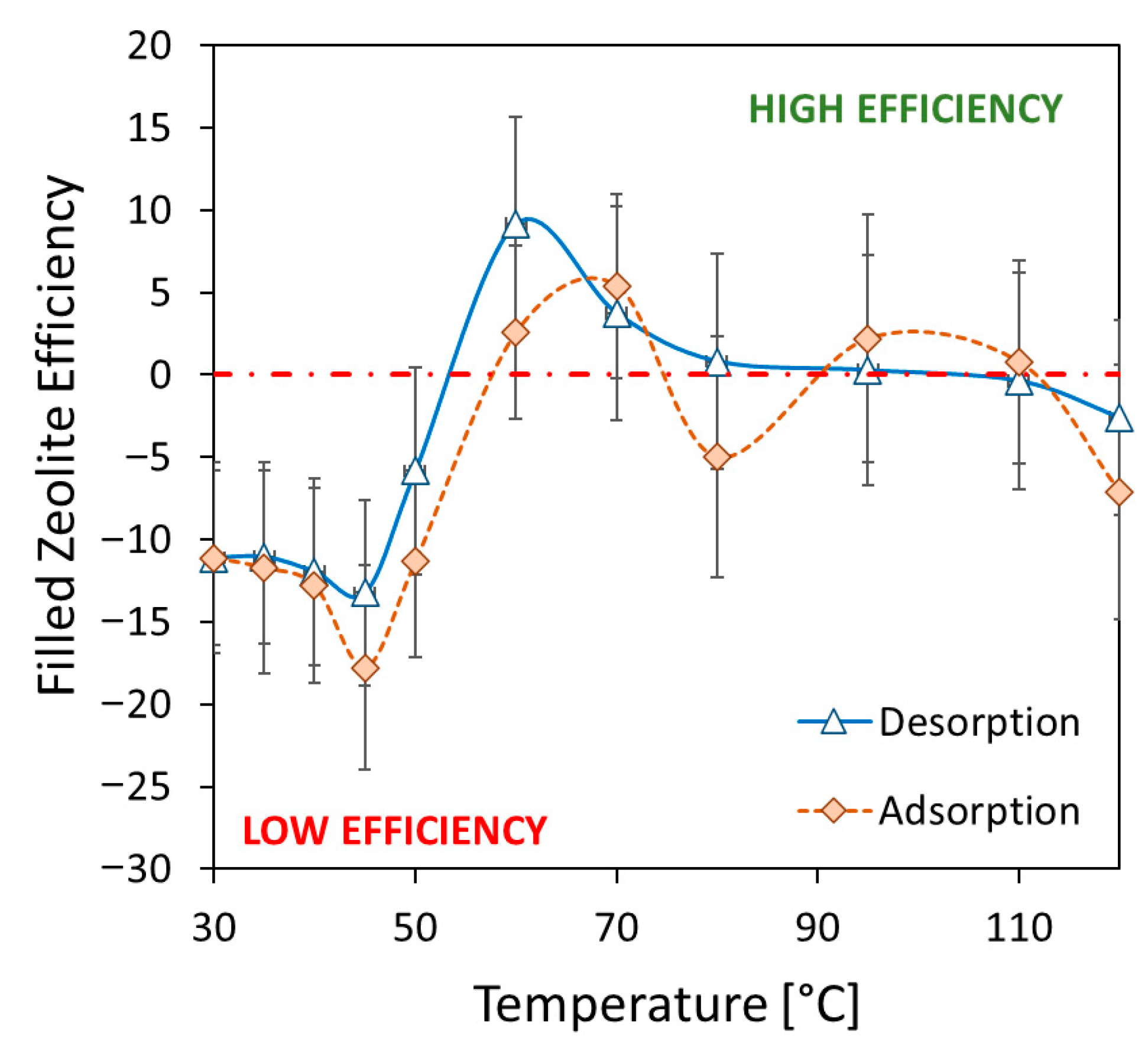
3.3. Adsorption/Desorption Performance
4. Conclusions
- The interconnected three-dimensional lattice structure was created without the need for any metal or plastic reinforcement. The obtained structure was characterized by a void volume ratio equal to 66.14% and a full/composite ratio equal to 2.96.
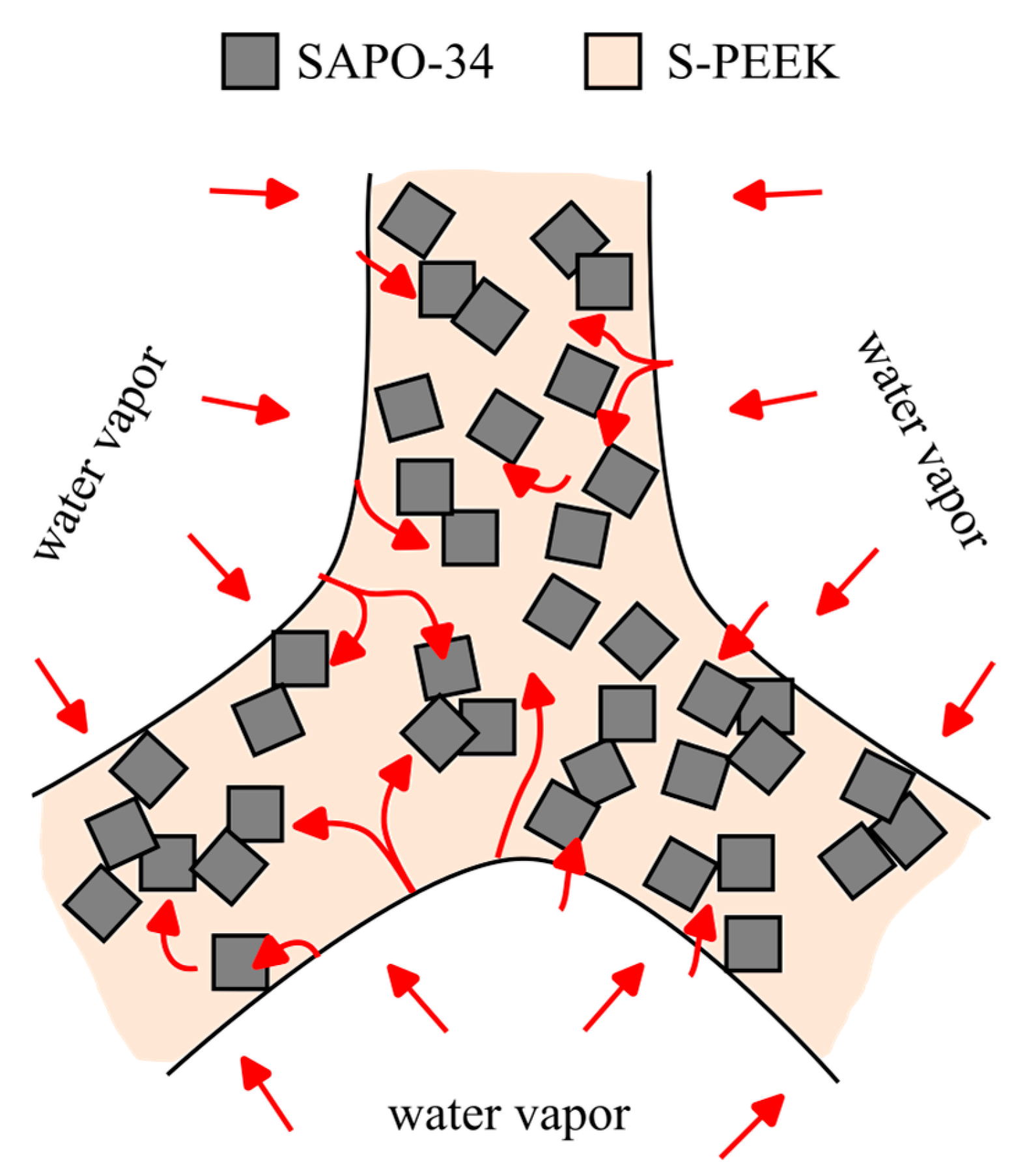
- Morphological investigations evidenced the structural integrity of the composite structure. At the macroscopic level, the cuboctahedron cell’s edge is morphologically a rough tube with holes, representing communication channels from outside to inside the tubes. At the microscopic level, the SAPO-34 filler is well-packed and uniformly arranged in the composite, indicating suitable adhesion with the thermoplastic matrix.
- Furthermore, water vapor adsorption isobars at 11 mbar at equilibrium in the temperature range 30–120 °C were used to establish strong adsorption/desorption capability. The composite sorbent exhibited a maximum water uptake equal to 21.96% at 30 °C. The observed water uptake is consistent with the filler content. In particular, at low temperatures, the zeolite filler efficiency index is around −10, demonstrating that about 90% of the SAPO-34 is actively participating in the adsorption process.
- It was furthermore theoretically assessed that increasing the filler content (80 wt.% to 95 wt.%) enhanced adsorption heat by 60%, while raising the aspect ratio (2 to 4) decreased it by 50% due to increased internal voids reducing effective adsorption. Optimal performance was estimated with 95 wt.% filler and an aspect ratio of 2, achieving an adsorption heat equal to 208.2 kJ for the chosen geometry.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| DVS | Dynamic vapor Sorption |
| ESEM | Environmental scanning electron microscope |
| PVA | Polyvinyl alcohol |
| RH | Relative humidity |
| S-PEEK | Sulfonated polyether ether ketone |
| T | Temperature |
| W | Water content |
| Wt. | Weight |
| Δw | Water-uptake variation |
References
- Sadeghi, G. Energy storage on demand: Thermal energy storage development, materials, design, and integration challenges. Energy Storage Mater. 2022, 46, 192–222. [Google Scholar] [CrossRef]
- Gbenou, T.R.; Fopah-Lele, A.; Wang, K. Recent Status and Prospects on Thermochemical Heat Storage Processes and Applications. Entropy 2021, 23, 953. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, B.; Wang, R. Water based adsorption thermal battery: Sorption mechanisms and applications. Energy Storage Mater. 2023, 54, 794–821. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Calderón, A.; Svobodova-Sedlackova, A.; Fernández, A.I.; Barreneche, C. The relevance of thermochemical energy storage in the last two decades: The analysis of research evolution. J. Energy Storage 2022, 51, 104377. [Google Scholar] [CrossRef]
- Desai, F.; Prasad, J.S.; Muthukumar, P.; Rahman, M.M. Thermochemical energy storage system for cooling and process heating applications: A review. Energy Convers. Manag. 2021, 229, 113617. [Google Scholar] [CrossRef]
- Tran, T.V.; Gemechu, E.; Oni, A.O.; Carrier, Y.; Tezel, H.; Kumar, A. Developing a framework to evaluate the life cycle energy and greenhouse gas emissions of space heating systems using zeolite 13× as an adsorbent material. J. Energy Storage 2023, 74, 109443. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Hu, P.; Wang, J.; Sun, Y.; Ma, Z. Performance of sorption thermal energy storage in zeolite bed reactors: Analytical solution and experiment. J. Energy Storage 2023, 64, 107154. [Google Scholar] [CrossRef]
- Humbert, G.; Sciacovelli, A. Design of effective heat transfer structures for performance maximization of a closed thermochemical energy storage reactor through topology optimization. Appl. Therm. Eng. 2024, 239, 122146. [Google Scholar] [CrossRef]
- Atakan, A.; Fueldner, G.; Munz, G.; Henninger, S.; Tatlier, M. Adsorption kinetics and isotherms of zeolite coatings directly crystallized on fibrous plates for heat pump applications. Appl. Therm. Eng. 2013, 58, 273–280. [Google Scholar] [CrossRef]
- Freni, A.; Bonaccorsi, L.; Calabrese, L.; Caprì, A.; Frazzica, A.; Sapienza, A. SAPO-34 coated adsorbent heat exchanger for adsorption chillers. Appl. Therm. Eng. 2015, 82, 1–7. [Google Scholar] [CrossRef]
- Tatlier, M. Theoretical investigation of performances of zeolite Y and SAPO-34 coatings for adsorption heat pump applications. Heat Mass Transf./Waerme Stoffuebertragung 2021, 57, 975–984. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Bonaccorsi, L.; Frazzica, A.; Caprì, A.; Freni, A.; Proverbio, E. Development and characterization of silane-zeolite adsorbent coatings for adsorption heat pump applications. Appl. Therm. Eng. 2017, 116, 364–371. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Silicone composite foams for adsorption heat pump applications. Sustain. Mater. Technol. 2017, 12, 27–34. [Google Scholar] [CrossRef]
- Palamara, D.; Palomba, V.; Calabrese, L.; Frazzica, A. Evaluation of ad/desorption dynamics of S-PEEK/Zeolite composite coatings by T-LTJ method. Appl. Therm. Eng. 2022, 208, 118262. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 zeolite filled macrocellular foams for adsorption heat pump applications: A preliminary study. Appl. Therm. Eng. 2017, 124, 1312–1318. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Innovative composite sorbent for thermal energy storage based on a SrBr2·6H2O filled silicone composite foam. J. Energy Storage 2019, 26, 100954. [Google Scholar] [CrossRef]
- Jia, L.; Xu, X.; Zhang, H.; Xu, J. Sulfonation of polyetheretherketone and its effects on permeation behavior to nitrogen and water vapor. J. Appl. Polym. Sci. 1996, 60, 1231–1237. [Google Scholar] [CrossRef]
- Palamara, D.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Effect of degree of sulfonation on the performance of adsorbent SAPO-34/S-PEEK composite coatings for adsorption heat pumps. Prog. Org. Coat. 2021, 154, 106193. [Google Scholar] [CrossRef]
- Calabrese, L.; Palamara, D.; Bruzzaniti, P.; Proverbio, E. Assessment of high performance SAPO-34/S-PEEK composite coatings for adsorption heat pumps. J. Appl. Polym. Sci. 2020, 138, 50076. [Google Scholar] [CrossRef]
- Scherle, M.; Nowak, T.A.; Welzel, S.; Etzold, B.J.M.; Nieken, U. Experimental study of 3D-structured adsorbent composites with improved heat and mass transfer for adsorption heat pumps. Chem. Eng. J. 2022, 431, 133365. [Google Scholar] [CrossRef]
- Gado, M.G.; Al-Ketan, O.; Aziz, M.; Al-Rub, R.A.; Ookawara, S. Triply Periodic Minimal Surface Structures: Design, Fabrication, 3D Printing Techniques, State-of-the-Art Studies, and Prospective Thermal Applications for Efficient Energy Utilization. Energy Technol. 2024, 12, 2301287. [Google Scholar] [CrossRef]
- Gado, M.G.; Ookawara, S. 3D-printed triply periodic minimal surface (TPMS) structures: Towards potential application of adsorption-based atmospheric water harvesting. Energy Convers. Manag. 2023, 297, 117729. [Google Scholar] [CrossRef]
- Thompson, J.F.; Bellerjeau, C.; Marinick, G.; Osio-Norgaard, J.; Evans, A.; Carry, P.; Street, R.A.; Petit, C.; Whiting, G.L. Intrinsic Thermal Desorption in a 3D Printed Multifunctional Composite CO2 Sorbent with Embedded Heating Capability. ACS Appl. Mater. Interfaces 2019, 11, 43337–43343. [Google Scholar] [CrossRef]
- Wang, Y.; Rim, G.; Song, M.; Holmes, H.E.; Jones, C.W.; Lively, R.P. Cold Temperature Direct Air CO2 Capture with Amine-Loaded Metal–Organic Framework Monoliths. ACS Appl. Mater. Interfaces 2024, 16, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.S.; Seyedkanani, A.; Najafi, G.; Sasmito, A.P.; Akbarzadeh, A. Multiscale architected porous materials for renewable energy conversion and storage. Energy Storage Mater. 2023, 59, 102768. [Google Scholar] [CrossRef]
- Zhakeyev, A.; Wang, P.; Zhang, L.; Shu, W.; Wang, H.; Xuan, J. Additive Manufacturing: Unlocking the Evolution of Energy Materials. Adv. Sci. 2017, 4, 1700187. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Bruzzaniti, P.; Palamara, D.; Freni, A.; Proverbio, E. New SAPO-34-SPEEK composite coatings for adsorption heat pumps: Adsorption performance and thermodynamic analysis. Energy 2020, 203, 117814. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Design and Optimization of Lattice Structures: A Review. Appl. Sci. 2020, 10, 6374. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.; Qian, M.; Faruque, O.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Des. 2019, 183, 108137. [Google Scholar] [CrossRef]
- Seharing, A.; Azman, A.H.; Abdullah, S. A review on integration of lightweight gradient lattice structures in additive manufacturing parts. Adv. Mech. Eng. 2014, 12, 1687814020916951. [Google Scholar] [CrossRef]
- Palamara, D.; Calabrese, L. Sulfonated-Recycled-PEEK as Matrix of Water Vapor Adsorbent SAPO-34 Based Composite Coatings for Adsorption Heat Pumps: Mechanical and Thermochemical Characterization. Materials 2022, 15, 8439. [Google Scholar] [CrossRef] [PubMed]
- Frazzica, A.; Sapienza, A.; Freni, A. Novel experimental methodology for the characterization of thermodynamic performance of advanced working pairs for adsorptive heat transformers. Appl. Therm. Eng. 2014, 72, 229–236. [Google Scholar] [CrossRef]
- Calabrese, L.; Bruzzaniti, L.B.P.; Freni, A.F.A. Adsorption performance and thermodynamic analysis of SAPO–34 silicone composite foams for adsorption heat pump applications. Mater. Renew. Sustain. Energy 2018, 7, 24. [Google Scholar] [CrossRef]
- Jansen, J.C.; Drioli, E. Poly(ether ether ketone) derivative membranes—A review of their preparation, properties and potential. Polym. Sci. Ser. A 2009, 51, 1355. [Google Scholar] [CrossRef]
- Calabrese, L.; De Antonellis, S.; Vasta, S.; Brancato, V.; Freni, A. Modified silicone-SAPO34 composite materials for adsorption thermal energy storage systems. Appl. Sci. 2020, 10, 8715. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Proverbio, E.; Freni, A. SAPO-34 based zeolite coatings for adsorption heat pumps. Energy 2019, 187, 115981. [Google Scholar] [CrossRef]
- Santori, G.; Frazzica, A.; Freni, A.; Galieni, M.; Bonaccorsi, L.; Polonara, F.; Restuccia, G. Optimization and testing on an adsorption dishwasher. Energy 2013, 50, 170–176. [Google Scholar] [CrossRef]

| Geometrical Feature | Value |
|---|---|
| Composite structure volume | 6.59 cm3 |
| 3D full volume | 19.46 cm3 |
| Empty volume | 12.87 cm3 |
| Aspect ratio * | 2.96 |
| Empty volume | 66.14% |
| Pipe diameter/length | 3 mm/8.3 mm |
| Weight | 1.936 g |
| Filler Content wt.% | Density kg/m3 | Δw wt.% | Aspect Ratio | Volume cm3 | W g | Qads J |
|---|---|---|---|---|---|---|
| 80 | 257.6 | 18.4 | 2 | 9.73 | 2.51 | 1300 |
| 3 | 6.49 | 1.67 | 866 | |||
| 4 | 4.87 | 1.25 | 650 | |||
| 85 | 288.2 | 20.4 | 2 | 9.73 | 2.80 | 1613 |
| 3 | 6.49 | 1.87 | 1076 | |||
| 4 | 4.87 | 1.40 | 807 | |||
| 90 | 293.5 | 22.4 | 2 | 9.73 | 2.86 | 1805 |
| 3 | 6.49 | 1.90 | 1204 | |||
| 4 | 4.87 | 1.43 | 903 | |||
| 95 | 310.6 | 24.5 | 2 | 9.73 | 3.02 | 2082 |
| 3 | 6.49 | 2.01 | 1388 | |||
| 4 | 4.87 | 1.51 | 104.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marabello, G.; Mastronardo, E.; Palamara, D.; Frazzica, A.; Calabrese, L. Tailored 3D Lattice SAPO-34/S-PEEK Composite Sorbents by Additive Manufacturing for Sorption Heat Transformation Applications. Materials 2025, 18, 3428. https://doi.org/10.3390/ma18153428
Marabello G, Mastronardo E, Palamara D, Frazzica A, Calabrese L. Tailored 3D Lattice SAPO-34/S-PEEK Composite Sorbents by Additive Manufacturing for Sorption Heat Transformation Applications. Materials. 2025; 18(15):3428. https://doi.org/10.3390/ma18153428
Chicago/Turabian StyleMarabello, Gabriele, Emanuela Mastronardo, Davide Palamara, Andrea Frazzica, and Luigi Calabrese. 2025. "Tailored 3D Lattice SAPO-34/S-PEEK Composite Sorbents by Additive Manufacturing for Sorption Heat Transformation Applications" Materials 18, no. 15: 3428. https://doi.org/10.3390/ma18153428
APA StyleMarabello, G., Mastronardo, E., Palamara, D., Frazzica, A., & Calabrese, L. (2025). Tailored 3D Lattice SAPO-34/S-PEEK Composite Sorbents by Additive Manufacturing for Sorption Heat Transformation Applications. Materials, 18(15), 3428. https://doi.org/10.3390/ma18153428









