Assessment of Ammonia Adsorption Capacity on Activated Banana Peel Biochars
Highlights
- Banana peels are considered a promising and sustainable source of biomass for the production of activated biochars.
- Chemical activation is a very effective way to develop surface area and porosity.
- The properties of activated biochars are influenced by process parameters and activation conditions.
- Various applications in environmental remediation and protection are covered.
- The biochars demonstrate high efficiency in NH3 removal.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
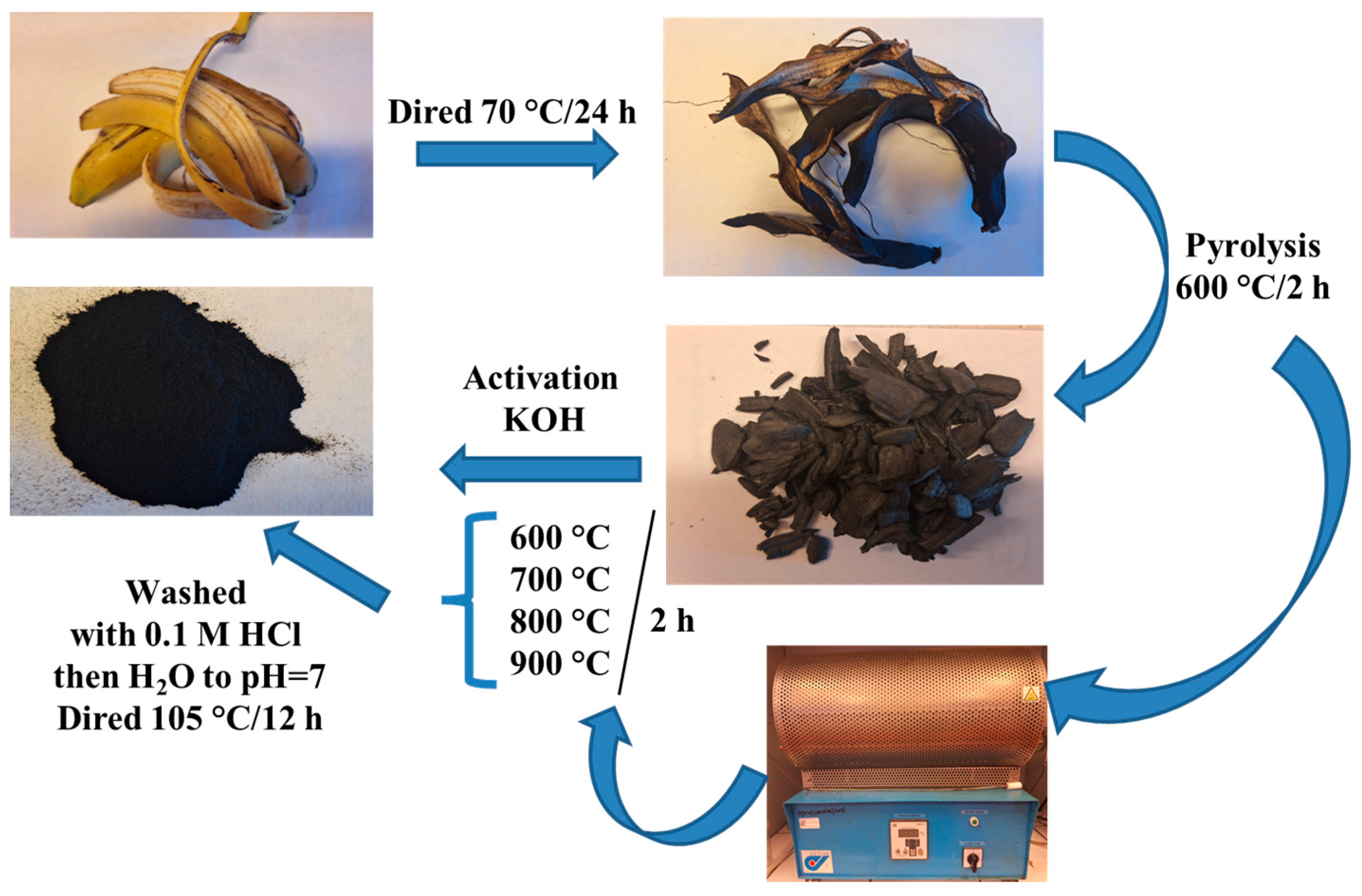
2.2. Biochar Preparation
2.3. Methods
2.3.1. The Nitrogen Low-Temperature Adsorption/Desorption
2.3.2. SEM/EDS Analysis
2.3.3. CHNS Analysis
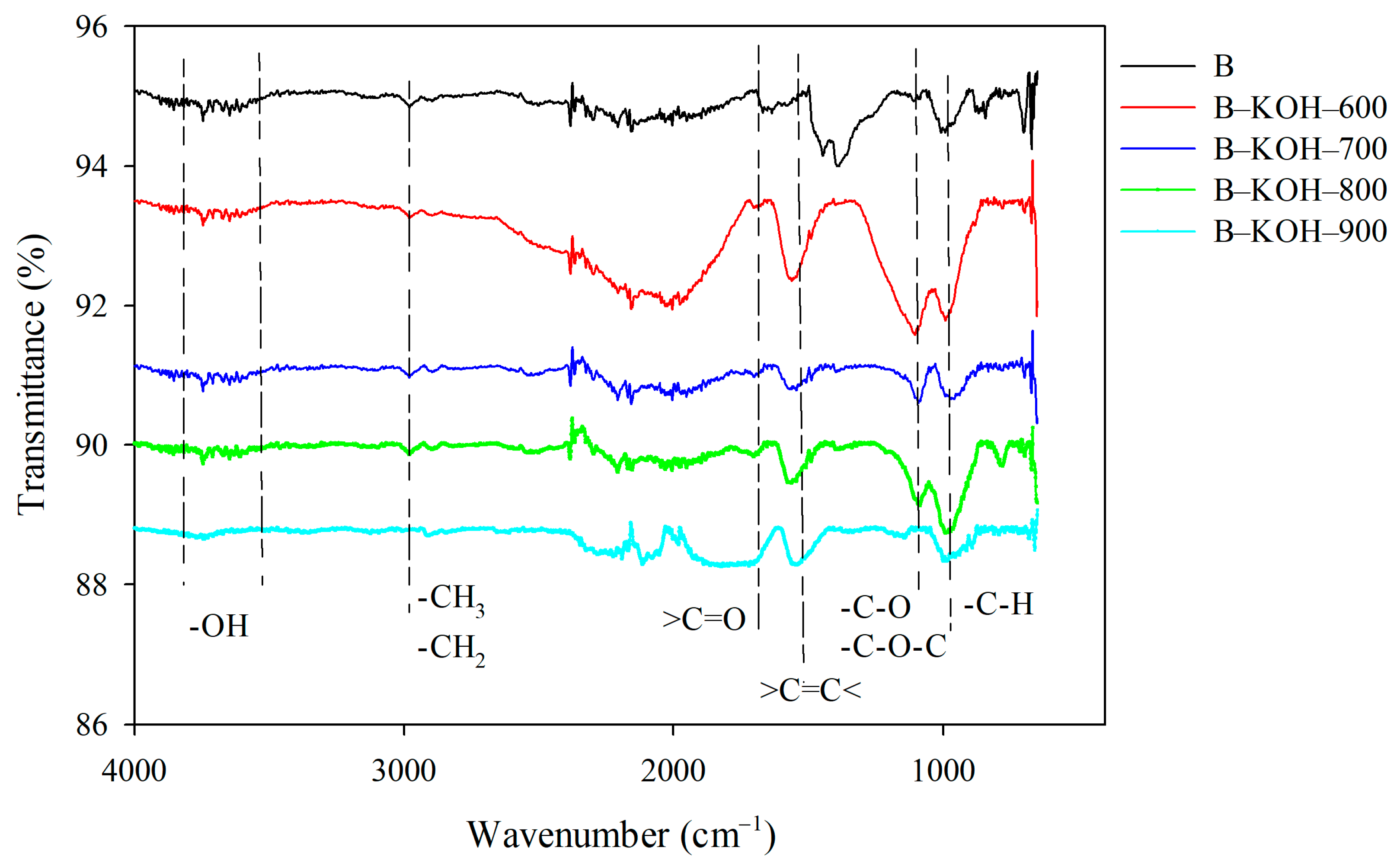
2.3.4. ATR-FTIR
2.3.5. Raman Spectroscopy
2.3.6. Boehm Method
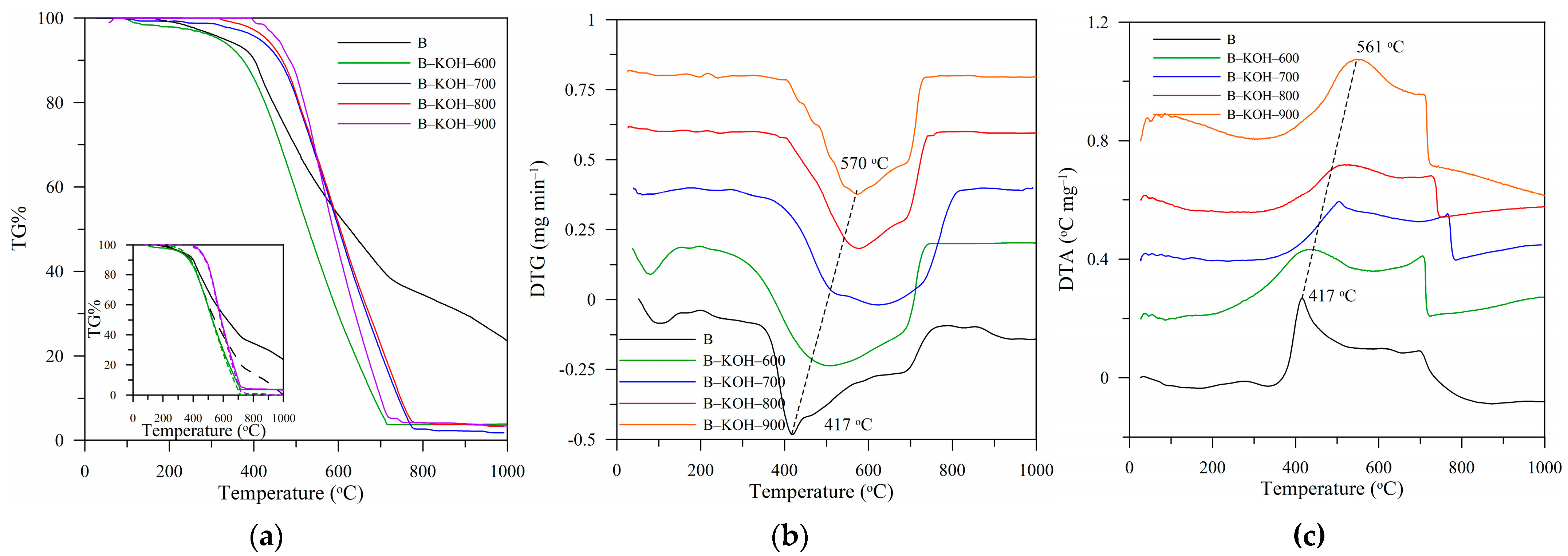
2.3.7. Thermal Analysis
2.3.8. Surface pH
2.3.9. Chemisorption
3. Results
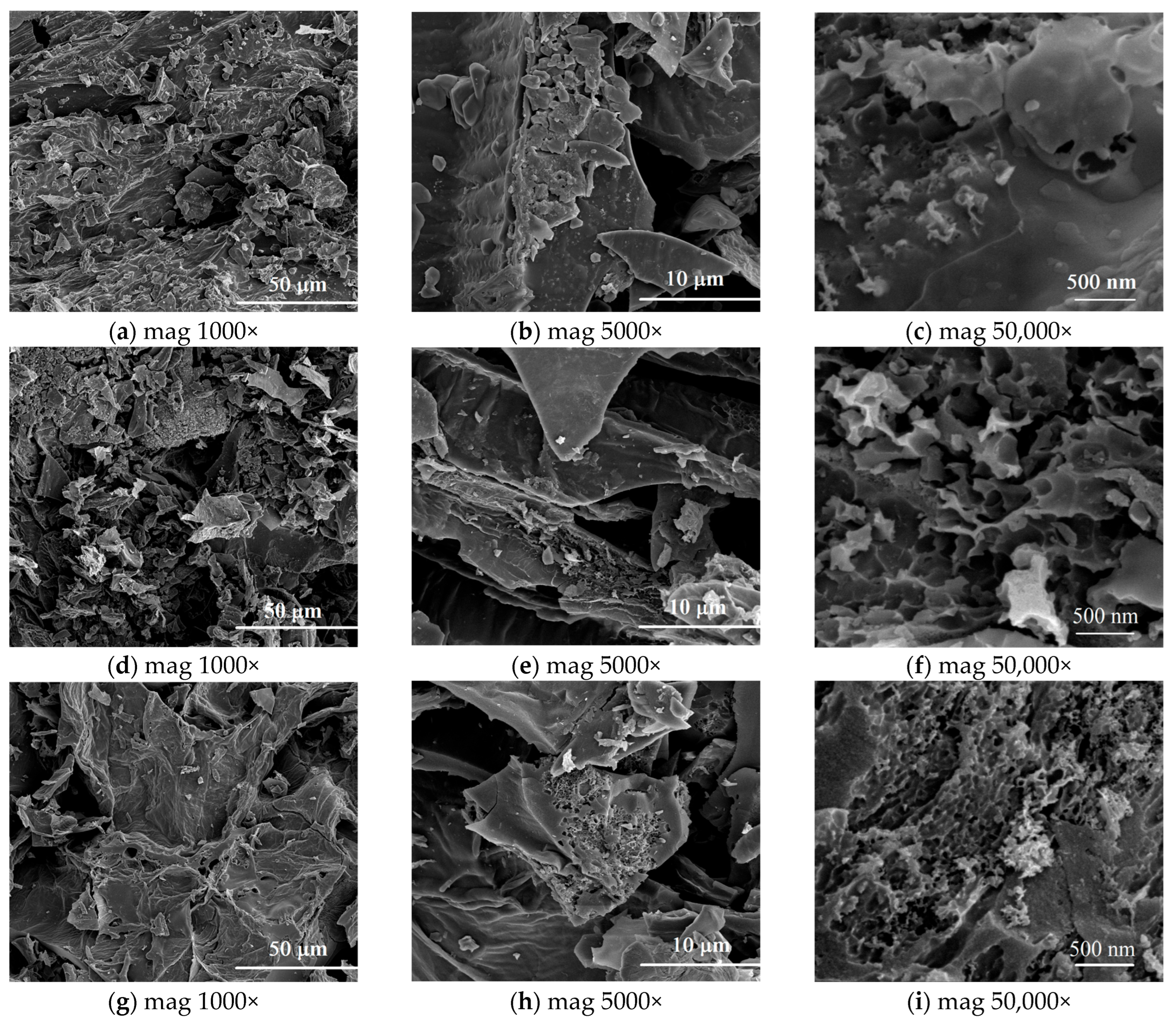
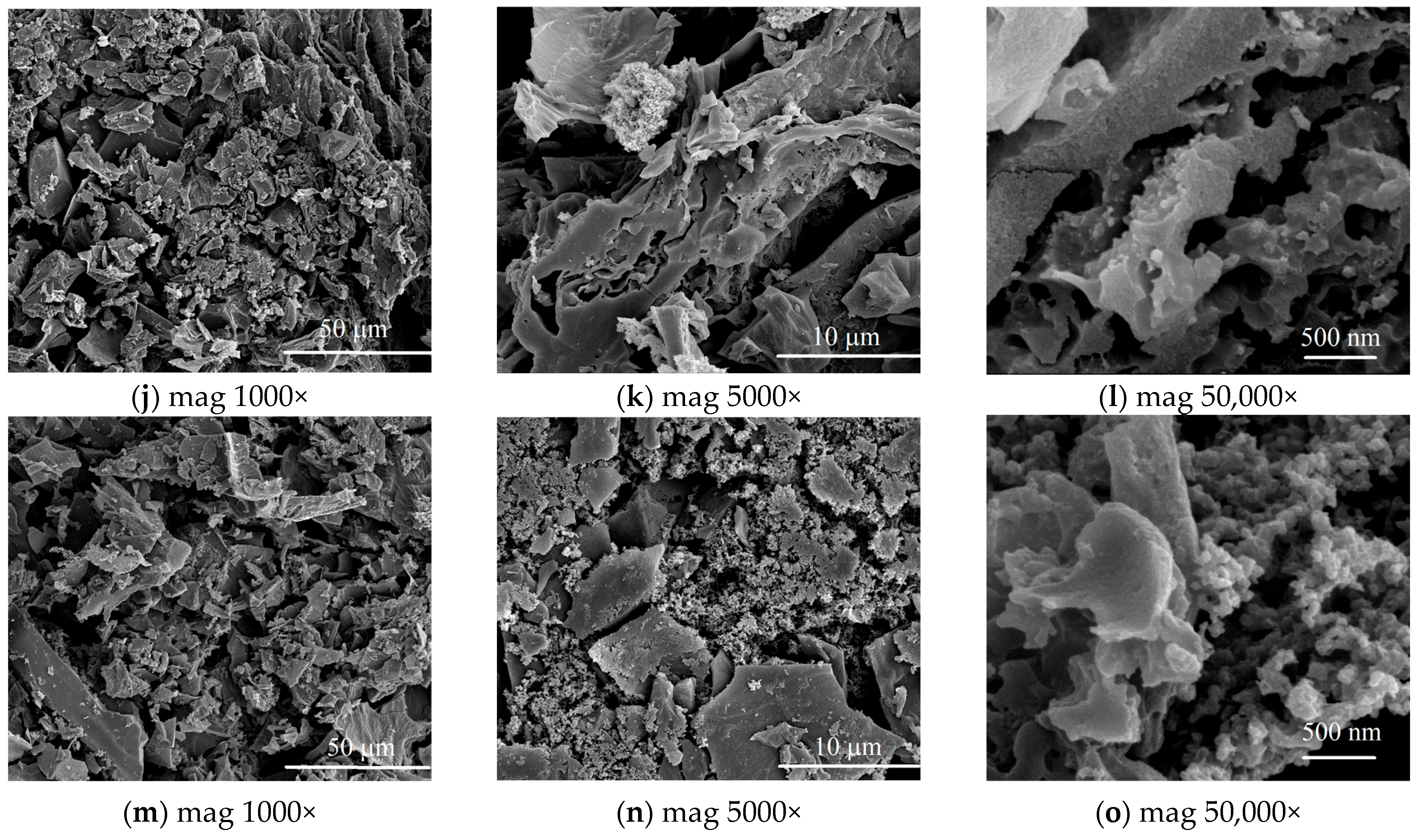
3.1. Characterization of Biochars
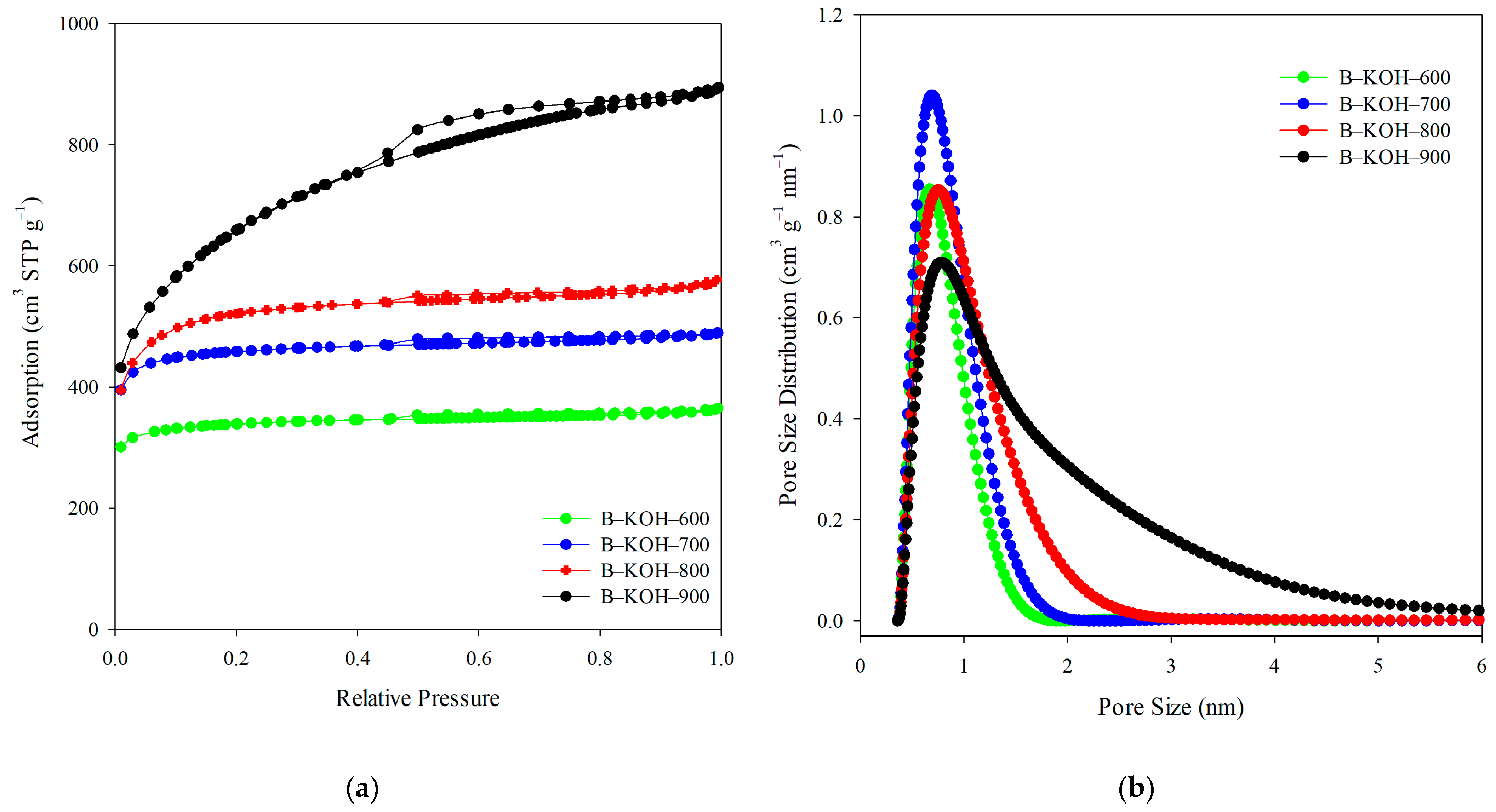
Porous Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Mi, B.; He, J.; Li, Y.; Zhou, Z.; Wu, F. Functionalized Biochars with Highly-Efficient Malachite Green Adsorption Property Produced from Banana Peels via Microwave-Assisted Pyrolysis. Bioresour. Technol. 2023, 376, 128840. [Google Scholar] [CrossRef] [PubMed]
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar Production Techniques Utilizing Biomass Waste-Derived Materials and Environmental Applications—A Review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef] [PubMed]
- Nsamba, H.K.; Hale, S.E.; Cornelissen, G.; Bachmann, R.T. Sustainable Technologies for Small-Scale Biochar Production—A Review. JSBS 2015, 5, 10–31. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent Advances in Engineered Biochar Productions and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent Trends in Biochar Production Methods and Its Application as a Soil Health Conditioner: A Review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Roberts, D.A.; Cole, A.J.; Paul, N.A.; De Nys, R. Algal Biochar Enhances the Re-Vegetation of Stockpiled Mine Soils with Native Grass. J. Environ. Manag. 2015, 161, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.N.; Peltz, C.D.; Stanton, M.; Rutherford, D.W.; Rostad, C.E. Biochar Application to Hardrock Mine Tailings: Soil Quality, Microbial Activity, and Toxic Element Sorption. Appl. Geochem. 2014, 43, 35–48. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Toxicity of Metals and Arsenic in a Naturally Contaminated Mine Soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Rey-Salgueiro, L.; Omil, B.; Merino, A.; Martínez-Carballo, E.; Simal-Gándara, J. Organic Pollutants Profiling of Wood Ashes from Biomass Power Plants Linked to the Ash Characteristics. Sci. Total Environ. 2016, 544, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Deng, Y.; Peng, Y.; Yue, K. Effects of Biochar Addition on Toxic Element Concentrations in Plants: A Meta-Analysis. Sci. Total Environ. 2018, 616–617, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Shakil, S.; Din, S.U.; Yasin, K.A.; Ahmad, M.S.; Imran, M.; Hirad, A.H.; Razzokov, J.; Haq, S. Sustainable Transformation of Cotton Stalk Waste as a Biochar-Based Copper-Silicon Composite for Chromium (VI) Detoxification. Desalination Water Treat. 2025, 322, 101166. [Google Scholar] [CrossRef]
- Singh Karam, D.; Nagabovanalli, P.; Sundara Rajoo, K.; Fauziah Ishak, C.; Abdu, A.; Rosli, Z.; Melissa Muharam, F.; Zulperi, D. An Overview on the Preparation of Rice Husk Biochar, Factors Affecting Its Properties, and Its Agriculture Application. J. Saudi Soc. Agric. Sci. 2022, 21, 149–159. [Google Scholar] [CrossRef]
- Chebbi, M.; Ounoki, S.; Youcef, L.; Amrane, A. Synthesis and Characterization of Pine Cones Biochar for the Removal of an Antibiotic (Metronidazole) from Aqueous Solutions. J. Ind. Eng. Chem. 2023, 126, 327–339. [Google Scholar] [CrossRef]
- Aziz, S.; Anbreen, S.; Iftikhar, I.; Fatima, T.; Iftikhar, A.; Ali, L. Green Technology: Synthesis of Iron-Modified Biochar Derived from Pine Cones to Remove Azithromycin and Ciprofloxacin from Water. Front. Environ. Sci. 2024, 12, 1353267. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.P.; Moni, C.; Dieguez-Alonso, A.; De La Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of Properties of Biochar Produced from Different Types of Lignocellulosic Biomass by Slow Pyrolysis at 600 °C. Appl. Energy Combust. Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Latchman, K.; Fox, D.; Ketwaru, P.; Singh, D.; Seecharan, D. Characterization of Biochar Produced from Sawdust for Environmental Management. World Environ. 2024, 14, 20–30. [Google Scholar]
- Rashad, E.; Saleh, H.N.; Eltaweil, A.S.; Saleh, M.E.; Sillanpaa, M.; Mostafa, A.R. Pinewood Sawdust Biochar as an Effective Biosorbent for PAHs Removal from Wastewater. Biomass Conv. Bioref. 2023, 13, 13443–13459. [Google Scholar] [CrossRef]
- Petala, A.; Bampos, G.; Frontistis, Z. Using Sawdust Derived Biochar as a Novel 3D Particle Electrode for Micropollutants Degradation. Water 2022, 14, 357. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin Materials for Adsorption: Current Trend, Perspectives and Opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, T.; Boichuk, A.; Vashchynskyi, V.; Changarath, M.E.; Krečmarová, M.; Abargues, R.; Martínez-Pastor, J.P.; Sánchez-Royo, J.F. Highly Porous Activated Carbon Derived from Plant Raw Material as High-Performance Anode for Aqueous Sodium-Ion Supercapacitors. J. Power Sources 2024, 622, 235356. [Google Scholar] [CrossRef]
- Volfkovich, Y.M. High Power Supercapacitors. Review. J. Electroanal. Chem. 2024, 963, 118290. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Adsorption-Pyrolysis Technology for Recovering Heavy Metals in Solution Using Contaminated Biomass Phytoremediation. Resour. Conserv. Recycl. 2018, 129, 20–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chen, K.; Shen, S.; Hu, H.; Chang, M.; Chen, D.; Wu, Y.; Yuan, H.; Wang, Y. Engineering Banana-Peel-Derived Biochar for the Rapid Adsorption of Tetracycline Based on Double Chemical Activation. Resour. Conserv. Recycl. 2023, 190, 106821. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kamińska, A.; Miądlicki, P.; Wróblewska, A.; Kiełbasa, K.; Wróbel, R.J.; Serafin, J.; Michalkiewicz, B. Activated Carbon Modification towards Efficient Catalyst for High Value-Added Products Synthesis from Alpha-Pinene. Materials 2021, 14, 7811. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.A.F.; Uemura, Y.; Yusup, S.; Elhassan, F.; Uddin, A.; Hiwada, A.; Demiya, M. Activated Carbon from Rubber Wood Sawdust by Carbon Dioxide Activation. Procedia Eng. 2016, 148, 530–537. [Google Scholar] [CrossRef]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. A Comparative Study of CO2 and CH4 Adsorption Using Activated Carbon Prepared from Pine Cone by Phosphoric Acid Activation. Korean J. Chem. Eng. 2016, 33, 2943–2952. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Oporto, G.; Dawson-Andoh, B.; McDonald, L.; Sabolsky, E. Influence of One-Step and Two-Step KOH Activation on Activated Carbon Characteristics. Bioresour. Technol. Rep. 2019, 7, 100266. [Google Scholar] [CrossRef]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of Methylene Blue by Ultrasonic Surface Modified Chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, G.Y.; Nam, H.; Nam, K.-W. Ammonia Gas Adsorption Study Using Copper Impregnated on Mesoporous Activated Carbon from Seaweed Waste for Indoor Air Purification. Build. Environ. 2024, 261, 111737. [Google Scholar] [CrossRef]
- Niu, Y.; Zheng, C.; Xie, Y.; Kang, K.; Song, H.; Bai, S.; Han, H.; Li, S. Efficient Adsorption of Ammonia by Surface-Modified Activated Carbon Fiber Mesh. Nanomaterials 2023, 13, 2857. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Tang, X.; Zhu, J.; Wang, Z.; Pan, Y.; Wu, H.; Wu, L.; Wu, Q.; He, Y.; Tian, S.; et al. Improved Inversion of Monthly Ammonia Emissions in China Based on the Chinese Ammonia Monitoring Network and Ensemble Kalman Filter. Environ. Sci. Technol. 2019, 53, 12529–12538. [Google Scholar] [CrossRef] [PubMed]
- Viatte, C.; Abeed, R.; Yamanouchi, S.; Porter, W.C.; Safieddine, S.; Van Damme, M.; Clarisse, L.; Herrera, B.; Grutter, M.; Coheur, P.-F.; et al. NH3 Spatiotemporal Variability over Paris, Mexico City, and Toronto, and Its Link to PM2.5 during Pollution Events. Atmos. Chem. Phys. 2022, 22, 12907–12922. [Google Scholar] [CrossRef]
- Vainio, E.; Yrjas, P.; Hupa, L.; Hupa, M. Cold-End Corrosion Caused by Hygroscopic Ammonium Chloride in Thermal Conversion of Biomass and Waste. Fuel 2023, 346, 128061. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kamińska, A.; Serafin, J.; Michalkiewicz, B. Chemical Activation of Banana Peel Waste-Derived Biochar Using KOH and Urea for CO2 Capture. Materials 2024, 17, 872. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.R.; Mahmoud, R.K.; Shaban, M.; Fahim, I.S.; Abd El-Salam, H.M.; Mahmoud, H.M. Towards a Circular Economy: Valorization of Banana Peels by Developing Bio-Composites Thermal Insulators. Sci. Rep. 2023, 13, 12756. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption Models for Carbon Slit-Shaped Pores with Surface Energetical Heterogeneity and Geometrical Corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon Slit Pore Model Incorporating Surface Energetical Heterogeneity and Geometrical Corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Adsorption Properties of Biochars Obtained by KOH Activation. Adsorption 2024, 30, 167–183. [Google Scholar] [CrossRef]
- Charmas, B.; Wawrzaszek, B.; Jedynak, K.; Jawtoszuk, A. Low-Cost Chestnut-Based Biocarbons Physically Activated via CO2 or Steam: Evaluation of the Structural and Adsorption Properties. Materials 2025, 18, 1497. [Google Scholar] [CrossRef] [PubMed]
- Calvelo Pereira, R.; Kaal, J.; Camps Arbestain, M.; Pardo Lorenzo, R.; Aitkenhead, W.; Hedley, M.; Macías, F.; Hindmarsh, J.; Maciá-Agulló, J.A. Contribution to Characterisation of Biochar to Estimate the Labile Fraction of Carbon. Org. Geochem. 2011, 42, 1331–1342. [Google Scholar] [CrossRef]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Skrzypczak, M.; Pietrzak, R. Effect of Activation Method on the Physicochemical Properties and NO2 Removal Abilities of Sorbents Obtained from Plum Stones (Prunus Domestica). Chem. Eng. J. 2010, 162, 723–729. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zuo, J.; Li, W.; Xia, Z.; Zhao, T.; Tan, C.; Wang, Y.; Li, J. Preparation of Modified Biochar and Its Adsorption of Cr(VI) in Aqueous Solution. Coatings 2023, 13, 1884. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, Kinetics and Mechanism of Removal of Methylene Blue from Aqueous Solution by Adsorption onto Pine Cone Biomass of Pinus Radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of Biochars Derived from Wood Pellets and Pelletized Wheat Straw as Replacements for Peat in Potting Substrates. Ind. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Marciniak, M.; Goscianska, J.; Pietrzak, R. Physicochemical Characterization of Ordered Mesoporous Carbons Functionalized by Wet Oxidation. J. Mater. Sci. 2018, 53, 5997–6007. [Google Scholar] [CrossRef]
- Ma, R.; Ma, Y.; Gao, Y.; Cao, J. Preparation of Micro-Mesoporous Carbon from Seawater-Impregnated Sawdust by Low Temperature One-Step CO2 Activation for Adsorption of Oxytetracycline. SN Appl. Sci. 2020, 2, 171. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the Surface Chemistry of Activated Carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Motejadded Emrooz, H.B.; Maleki, M.; Rashidi, A.; Shokouhimehr, M. Adsorption Mechanism of a Cationic Dye on a Biomass-Derived Micro- and Mesoporous Carbon: Structural, Kinetic, and Equilibrium Insight. Biomass Conv. Bioref. 2021, 11, 943–954. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2. Materials 2021, 14, 3859. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Chai, X.; Song, X.; He, H.; Fan, H.; Liang, D. Effects of Adsorbate Molecular Space Conformation on the Adsorption Capacity of Porous Carbon Materials: A Case Study of Propylene Glycol Methyl Ether. Sci. Total Environ. 2020, 712, 135495. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Li, H.-S.; Chen, C.-H. Effect of Surface Acidic Oxides of Activated Carbon on Adsorption of Ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption Behaviors of Ammonia and Hydrogen Sulfide on Activated Carbon Prepared from Petroleum Coke by KOH Chemical Activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Takaya, C.A.; Parmar, K.R.; Fletcher, L.A.; Ross, A.B. Biomass-Derived Carbonaceous Adsorbents for Trapping Ammonia. Agriculture 2019, 9, 16. [Google Scholar] [CrossRef]
- Liu, C.Y.; Aika, K. Effect of Surface Oxidation of Active Carbon on Ammonia Adsorption. Bull. Chem. Soc. Jpn. 2003, 76, 1463–1468. [Google Scholar] [CrossRef]
- Ro, K.; Lima, I.; Reddy, G.; Jackson, M.; Gao, B. Removing Gaseous NH3 Using Biochar as an Adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Z.; Huang, J.; Hu, W.; Xie, D.; Qiao, Y. Efficient Adsorption of Ammonia on Activated Carbon from Hydrochar of Pomelo Peel at Room Temperature: Role of Chemical Components in Feedstock. J. Clean. Prod. 2023, 406, 137076. [Google Scholar] [CrossRef]

| Biochar | SBET (m2 g−1) | Vt (cm3 g−1) | Vultra (cm3 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | wmi (nm) | Micro porosity (%) |
|---|---|---|---|---|---|---|---|
| B | 0.040 | 0.0014 | - | - | - | - | - |
| B-KOH-600 | 1134 | 0.56 | 0.18 | 0.50 | 0.06 | 0.67 | 89 |
| B-KOH-700 | 1540 | 0.76 | 0.22 | 0.68 | 0.08 | 0.70 | 90 |
| B-KOH-800 | 1770 | 0.89 | 0.16 | 0.74 | 0.15 | 0.76 | 83 |
| B-KOH-900 | 2332 | 1.38 | 0.13 | 0.77 | 0.61 | 0.78 | 56 |
| Biochar | NH3 Adsorption Capacity (mmol g−1) Pressure ~750 mmHg | TPD NH3 (mmol g−1) Pressure ~750 mmHg | ||
|---|---|---|---|---|
| Temperature | 0 °C | 20 °C | 0 °C | 20 °C |
| B | 0.85 | 0.74 | 0.25 | 0.23 |
| B–KOH–600 | 5.94 | 3.83 | 0.71 | 0.5 |
| B–KOH–700 | 4.83 | 3.74 | 0.65 | 0.55 |
| B–KOH–800 | 3.66 | 3.42 | 0.59 | 0.51 |
| B–KOH–900 | 2.43 | 2.38 | 0.45 | 0.43 |
| Biochar | %A | %VC | %FC | Cthermo |
|---|---|---|---|---|
| B | 18.6 | 33.0 | 48.4 | 0.594 |
| B–KOH–600 | 5.7 | 26.0 | 68.3 | 0.724 |
| B–KOH–700 | 1.7 | 12.1 | 86.2 | 0.877 |
| B–KOH–800 | 1.5 | 8.8 | 89.7 | 0.914 |
| B–KOH–900 | 1.5 | 8.4 | 90.0 | 0.915 |
| Biochar | C (%) | H (%) | N (%) | S (%) |
|---|---|---|---|---|
| B | 62.43 | 1.561 | 1.26 | 0.076 |
| B–KOH–600 | 82.78 | 1.361 | 0.69 | 0.127 |
| B–KOH–700 | 89.14 | 0.528 | 0.19 | 0.139 |
| B–KOH–800 | 85.39 | 0.346 | 0.22 | 0.166 |
| B–KOH–900 | 90.60 | 0.288 | 0.21 | 0.147 |
| Biochar | Total Basic Groups (mmol g−1) | Total Acidic Groups (mmol g−1) | Total Groups (mmol g−1) | pHbiochar | ID/IG |
|---|---|---|---|---|---|
| B | 2.818 | 0.290 | 3.108 | 9.65 | 1.18 |
| B-KOH-600 | 0.390 | 2.300 | 2.690 | 6.68 | 1.24 |
| B-KOH-700 | 0.324 | 1.978 | 2.302 | 7.85 | 1.23 |
| B-KOH-800 | 0.816 | 1.416 | 2.232 | 7.65 | 1.38 |
| B-KOH-900 | 0.590 | 1.248 | 1.838 | 7.24 | 1.32 |
| Carbon Materials | Adsorption Capacity (mmol g−1) | Ref. |
|---|---|---|
| B | 0.85 | This study |
| B–KOH–600 | 5.94 | This study |
| B–KOH–700 | 4.83 | This study |
| B–KOH–800 | 3.66 | This study |
| B–KOH–900 | 2.43 | This study |
| Cu/AC 1 | 7.55 | [33] |
| C–C1-4 2 | 2.95 | [46] |
| C–C1-2 3 | 2.61 | [46] |
| C–H1-4 4 | 2.40 | [46] |
| C–H1-2 5 | 2.24 | [46] |
| C–O1-4 6 | 2.78 | [46] |
| C–O1-2 7 | 2.38 | [46] |
| C–A1-4 8 | 3.05 | [46] |
| C–A1-2 9 | 2.59 | [46] |
| OAK–450–KOH 10 | 0.35 | [64] |
| OAK–250–KOH 11 | 1.47 | [64] |
| Na–OH–AC 12 | 1.69 | [65] |
| AC 13 | 1.19 | [65] |
| HNO3–AC 14 | 3.07 | [65] |
| AA–WS250–AR 15 | 3.11 | [66] |
| BC–1–CO2–6h 16 | 5.18 | [59] |
| BC–2–CO2–6h 17 | 3.95 | [59] |
| PP–AC 18 | 4.60 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedynak, K.; Charmas, B. Assessment of Ammonia Adsorption Capacity on Activated Banana Peel Biochars. Materials 2025, 18, 3395. https://doi.org/10.3390/ma18143395
Jedynak K, Charmas B. Assessment of Ammonia Adsorption Capacity on Activated Banana Peel Biochars. Materials. 2025; 18(14):3395. https://doi.org/10.3390/ma18143395
Chicago/Turabian StyleJedynak, Katarzyna, and Barbara Charmas. 2025. "Assessment of Ammonia Adsorption Capacity on Activated Banana Peel Biochars" Materials 18, no. 14: 3395. https://doi.org/10.3390/ma18143395
APA StyleJedynak, K., & Charmas, B. (2025). Assessment of Ammonia Adsorption Capacity on Activated Banana Peel Biochars. Materials, 18(14), 3395. https://doi.org/10.3390/ma18143395






