Abstract
Ground granulated blast-furnace slag (GGBS), a waste product generated during steel production, can be added as a substitute for cement in concrete to mitigate the environmental impact of the cement and steel industries. However, the use of GGBS is limited because it decreases the early strength development of cement or concrete. This study evaluated the performance of incorporating synthesized C-S-H nanoparticles to enhance the compressive strength, early hydration, and microstructure of cement composite. The synthesized C-S-H nanoparticles were produced at standard atmospheric pressure and room temperature. Heat of hydration, X-ray diffraction, and thermogravimetric analyses were conducted to investigate the hydration and mechanical properties of the cement containing the C-S-H nanoparticles. Further, mercury intrusion porosimetry was conducted to examine the pore structures. The experimental finding demonstrated that adding C-S-H nanoparticles accelerated the early hydration progress in the cement composites, thereby increasing their initial compressive strength.
1. Introduction
Concrete is the most commonly used construction material in the world [,], and the cement in concrete is the most important component determining its properties []. The global production of cement exceeds four billion tons annually, rendering it the second-highest contributor to CO2 emissions across all industries [,,]. The growing importance of environmental protection and the various global agreements on CO2 reduction require minimizing CO2 emissions from cement production. However, achieving this reduction has proven to be very challenging [].
Numerous studies have focused on identifying cement substitutes to lower the overall cement content in concrete [,,]. For instance, fly ash (FA), a pozzolanic material, and ground granulated blast-furnace slag (GGBS), a byproduct of steel production, possess latent hydraulic properties and are commonly used as cement substitutes [,]. These materials can partially replace cement, providing environmental benefits and enhancing the mechanical properties and durability of concrete. However, cement and concrete with a high content of FA or GGBS exhibit lower early-age strength compared to ordinary concrete due to a slower initial hydration reaction []. This limitation has led to various studies exploring the use of hydration accelerators or strength enhancers to mitigate this issue.
Some studies confirmed that chloride-based hydration accelerators are effective in improving the initial strength of concrete, however, they reduce the long-term strength as compared to that of ordinary concrete [,]. It was reported that concrete containing sodium-sulfate-based hydration accelerators can help improve the initial strength by rapidly generating ettringite(Aft) hydration products, thereby accelerating the early-age hydration of C3A and C3S [,]. However, sodium-sulfate-based hydration accelerators reduce the long-term strength, similar to chloride-based hydration accelerators. In addition to reducing the long-term strength of concrete, chloride- and sodium-based hydration accelerators shorten the service life of structures by accelerating long-term deterioration, such as the corrosion of steel reinforcement bars [] and alkali–aggregate reactions [].
Recent studies have employed calcium silicate hydrate (C-S-H) seeds as hydration accelerators, as they do not adversely affect the long-term durability of cement-based structures. C-S-H, one of the primary hydration products of Portland cement, plays a critical role in determining the mechanical properties and durability of cementitious materials. It is formed through the hydration of tricalcium silicate (C3S) and dicalcium silicate (C2S), the two major constituents of cement clinker. Although the structure of C-S-H is poorly crystalline and varies depending on hydration conditions, it forms a dense, cohesive gel that fills capillary pores and binds solid particles together. This gel-like phase is responsible for the majority of strength development in hardened cement paste and is widely regarded as the principal strength-giving phase in cement-based systems [,]. Therefore, understanding the morphology, composition, and long-term evolution of C-S-H is essential for the design and performance optimization of durable cementitious materials.
Building on this knowledge, recent approaches have focused on using engineered C-S-H nanocomposites to enhance early-age strength by accelerating the hydration process. These materials incorporate ultra-fine particles smaller than cement grains, which serve as nucleation sites for hydration products, thereby expediting reaction kinetics [,,]. For instance, C-S-H–polycarboxylate nanocomposites with particle sizes below 50 nm were synthesized in an isoprenyloxy poly(ethylene glycol)-based superplasticizer solution by adjusting the pH during synthesis []. Application of these nanocomposites led to a 100% increase in the 12-h compressive strength of cement specimens. Another study fabricated a 329 nm C-S-H–polycarboxylate nanocomposite using a methallyl-ether-based polycarboxylate solution and reported a 55% strength improvement in mortar specimens at 12 h [].
John et al. [] investigated the influence of the Ca/Si ratio in C-S-H nanoparticles on cement hydration. Their findings indicated that lower Ca/Si ratios result in a higher specific surface area for C-S-H, thereby further accelerating cement hydration. Furthermore, it was shown that C-S-H seeds accelerated the hydration reaction of cement and enhanced its compressive strength by at least 20% []. As the use of C-S-H increased, the early strength development of cement composites improved [,]. Moreover, under low-temperature curing conditions, it has been reported that C-S-H nanoparticles can serve as accelerators to enhance compressive strength [].
In this study, C-S-H nanoparticles were synthesized using reagent-class sodium metasilicate (Na2SiO3∙5H2O), cobalt salts (Ca(NO3)2∙4H2O), and a dispersing agent (polycarboxylate based). X-ray photoelectron spectroscopy (XPS) analysis was conducted to analyze the Ca/Si ratios of the synthesized C-S-H nanoparticles. Cement pastes were prepared by adding C-S-H nanoparticles. Hydration heat measurements, thermogravimetry (TG), mercury intrusion porosimetry (MIP), and X-ray diffraction (XRD) analyses were conducted to investigate the cement hydration properties. The hydration behavior of GGBS cement was also examined for its response to the influence of synthesized C-S-H nanoparticles. To this end, a heat of hydration test was conducted along with TG and MIP at each age. Further, the effect of the nanoparticles on the prepared mortar specimens was examined. These findings are expected to promote the wider application of GGBS addition to cement to reduce its environmental impact while ensuring sufficient mechanical properties by modification with C-S-H nanoparticles.
2. Materials and Methods
2.1. Materials
In this research, a cement with no supplementary cementitious materials such as FA or GGBS was used as the binder, referred to as research cement (RC). RC was produced by mixing desulfurized gypsum (5%) with clinker (95%) and then crushing the mixture []. RC used more reliable results when analyzing the cement microstructure. Table 1 presents the chemical compositions of the raw materials and their respective loss-on-ignition (LOI) values. The main mineral components of the RC were identified by XRD (Figure 1a) as C3S, C2S, C3A, C4AF, and gypsum, and Rietveld refinement of the XRD pattern yielded fractions of 61.8, 10.6, 11.3, 8.6, and 7.7 wt%, respectively. The main mineral components of the GGBS were anhydrite and quartz. Most of the GGBS is amorphous, with small amounts of crystalline phases, namely, anhydrite (2.9%) and quartz (2.1%). The densities of RC and GGBS are 3.18 g/cm3 and 2.91 g/cm3, respectively, and their Blaine fineness values are 3870 cm2/g and 4200 cm2/g. The particle size distribution characteristics of RC and GGBS are illustrated in detail in Figure 2, providing a comprehensive depiction of the range and proportion of particle sizes present in each material. C-S-H nanoparticles, used as a hardening accelerator, were synthesized using sodium metasilicate (Na2SiO3∙5H2O), calcium nitrate tetrahydrate (Ca(NO3)2∙4H2O) powder, and a polycarboxylate based dispersant (50 wt% solid content).

Table 1.
Chemical compositions of raw materials (wt%) [].
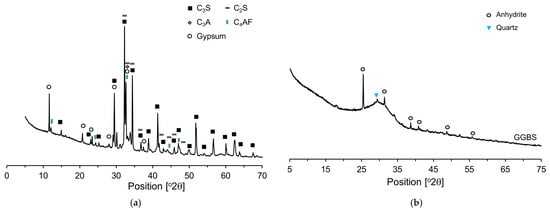
Figure 1.
XRD patterns: (a) RC; (b) GGBS.
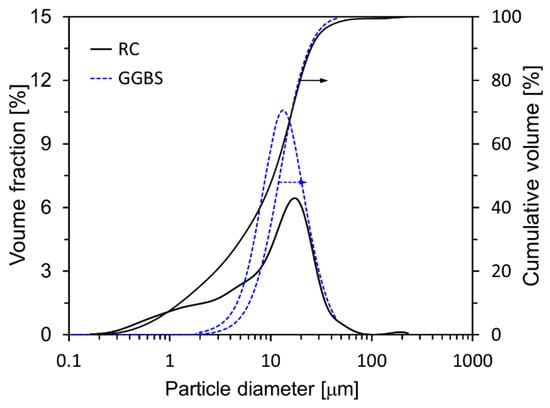
Figure 2.
Particle size distributions of RC and GGBS.
2.2. Synthesis of C-S-H Nanoparticles
C-S-H nanoparticles synthesized in the authors’ previous research were used []. The synthesis of C-S-H nanoparticle was carried out at room temperature using reagent-class Na2SiO3∙5H2O and Ca(NO3)2∙4H2O powders. Figure 3 illustrates an overview of the C-S-H nanoparticle synthesis process. First, 60 g of distilled water was mixed with 22.34 g of the polycarboxylate superplasticizer in a beaker, and the mixture was stirred using an agitator. The content of the polycarboxylate superplasticizer was used to adjust the crystal size of the C-S-H nanoparticles []. The reagent-class Na2SiO3∙5H2O and Ca(NO3)2∙4H2O powders were added to 180 g and 90 g of distilled water, respectively, to achieve a molar concentration ratio of 1:1. Thereafter, automatic flow feeders were fixed to the beaker to supply rates of 4.6 and 2.9 mL/min of Na2SiO3 and Ca(NO3)2 solutions, respectively, under stirring at a speed of at least 1000 rpm. During this synthesis, Na+ and NO3− are generated as impurities, in addition to the C-S-H nanoparticles. These components must be considered because they reduce the durability of concrete via alkali–aggregate reactions that produce NaNO3 [,]. In this study, centrifugal separation was performed at a speed of ~4500 rpm to remove the impurities generated. The supernatant containing the impurities was removed by rinsing the precipitate with distilled water. This process was repeated three times to produce C-S-H nanoparticles as an opaque white gel with a solid content of ~21% (Figure 3). In this study, the particle size of the synthesized C-S-H nanoparticles ranges from 30 nm to 50 nm.
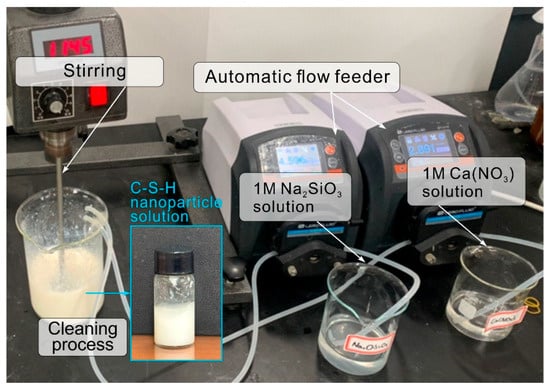
Figure 3.
Setup for synthesizing C-S-H nanoparticles.
2.3. Preparation of Paste and Mortar Samples
Table 2 presents the mixture proportions of specimens. The main variables are the amount of C-S-H added and whether GGBS is used. The PCSH specimens were prepared by adding 0, 1, 2, or 3 wt% of C-S-H nanoparticles, based on the weight of RC, considering the solid content in the nanoparticle solution shown in Figure 3. The GCSH specimens were prepared by adding 0 or 3 wt% C-S-H nanoparticles to cement containing 50 wt% GGBS. The last row in Table 2, labeled “SP (wt%) in the C-S-H nanoparticle solution”, refers to the SP dosage expressed as the weight percentage relative to the total mass of the C-S-H nanoparticle suspension. For all specimens, the water-to-binder (W/B) ratio was fixed at 0.5; the binder-to-sand ratio was fixed at 1:3, and standard sand (ISO 679) was used as sand. Paste specimens were prepared to analyze the effect of the C-S-H nanoparticles on the hydration reaction of cement composites, for which the same recipes summarized in Table 2 were used, but without sand. Paste specimens were prepared following the same mixture proportions outlined in Table 2, with the exception that sand was omitted from the formulation to isolate the effects of the binder and other components on the hydration and strength development processes.

Table 2.
Mixture proportions of cement composites containing C-S-H nanoparticles.
2.4. Characterization Methods
XPS analysis was conducted to analyze the calcium-to-silicon (Ca/Si) ratio in the C-S-H nanoparticles. Samples of dried C-S-H nanoparticles were pressed into pellets, and the sample surface was subjected to qualitative and quantitative analyses. A NEXSA XPS system (ThermoFisher Scientific, Waltham, MA, USA) was used, with the X-ray spot diameter set to 400 μm.
The hydration heat measurements were carried out via a Calmtrix I-Cal 2000 (Calmetrix, Inc., Boston, MA, USA). Pastes were prepared following the mixtures shown in Table 2. Subsequently, approximately 70 g of each specimen were carefully transferred into a dedicated test container designed to prevent external contamination. The container was then placed into the calorimeter, where, under controlled conditions of 20 ± 1 °C, the heat of hydration was continuously measured for 72 h.
Mortar specimens, prepared in accordance with the specifications outlined in ISO 679 [], were subjected to compressive strength tests. Steel molds were employed to fabricate mortar specimens measuring 40 mm × 40 mm × 160 mm. The specimens were demolded after curing in a constant temperature (20 ± 1 °C) and relative humidity (≥90%) chamber for 24 h. During the first 24 h after casting, the specimens were cured exclusively in a controlled temperature and humidity chamber, without any exposure to water. After demolding at 1 day, they were transferred to a water tank maintained at 20 ± 1 °C for subsequent curing until the designated testing age. According to ISO 679, one specimen was split into two pieces, and the compressive strength was measured for six specimens per group. The compressive strength results were reported as average values. However, the compressive strength at ages of 8 h, 16 h, and 1 d was measured without water curing; the specimens were demolded in the chamber at the respective testing ages and tested immediately.
XRD analysis (Empyrean, Malvern Panalytical, Chipping Norton, Australia) was conducted to identify the crystalline hydration products at each age. Diffraction patterns were obtained over a 2θ range of 5–75°, with a step size of 0.01° and a counting time of 5 s, respectively. Rietveld refinement of the X-ray diffraction (XRD) data was carried out using X’Pert HighScore Plus 5.3a (PANalytical, Almelo, The Netherlands). For the quantitative analysis of mineral composition, 10% corundum (α-Al2O3) was used as an internal standard. The synthesized C-S-H nanoparticles were subjected to freezing and drying preprocessing. The cement pastes were carefully submerged in isopropanol at specific ages. Then, the samples were kept under vacuum for 24 h to halt the hydration reaction, and then dried in a furnace at 40 °C for an additional 24 h. Finally, the samples were crushed into powder before testing.
TG analysis was performed using an SDT Q600 (TA Instruments, New Castle, DE, USA) to investigate the paste’s hydration behavior at different ages. The weight change was measured while the temperature was increased to 1000 °C at a rate of 10 °C/min in a nitrogen atmosphere (100 mL/min). The samples were prepared using the same method as that used for preprocessing the paste specimens for XRD.
MIP was performed using an Autopore V system (Micromeritics, Norcross, GA, USA) to measure the pore size distribution inside the paste specimens. The specimens were fabricated by drying the paste at 40 °C for 24 h after stopping hydration by crushing it into a size of ~5 mm.
3. Results and Discussion
3.1. Effect of C-S-H Nanoparticles on Hydration Properties
Figure 4 shows the XPS results for the synthesized C-S-H nanoparticles. According to a previous study, the properties of nano C-S-H crystals vary depending on the Ca/Si ratio []. Figure 4a shows that the Ca 2p spectrum has two peaks at binding energies of 350.1 and 346.5 eV with intensities of 16,247 and 28,363, respectively. The Si 2p spectrum has one peak at a binding energy of 101.4 eV, with an intensity of 8141. From these results, it was determined that the Ca/Si ratio of the synthesized C-S-H nanoparticles was 0.78. A comparison of the Ca and Si peaks among the various types of C-S-H revealed that they are similar to those of 11 Å Tobermorite (Ca4.5(Si6O16)(OH)·5H2O) [].
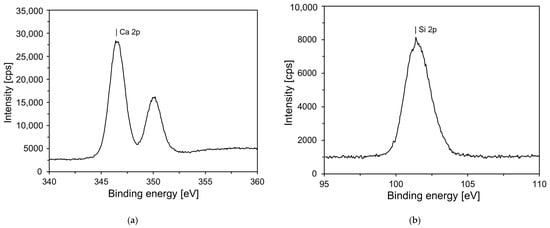
Figure 4.
XPS analysis of C-S-H nanoparticles, showing the elemental peaks: (a) calcium (Ca); (b) silicon (Si).
Figure 5 illustrates the specific heat flow and cumulative heat release results. Figure 5a,b show the experimental results for PCSH, and Figure 5c,d show the results for GCSH. As depicted in Figure 5a, the second peak shifted to an earlier time with increasing C-S-H nanoparticle content. This effect can be explained by the shortened induction period in the cement paste resulting from the incorporation of C-S-H nanoparticles. Furthermore, as the C-S-H content increased, the second peak grew in magnitude, indicating that C-S-H nanoparticles enhance the cement hydration reaction []. As shown in Figure 5b, the initial cumulative heat release of the PCSH samples increases with higher C-S-H nanoparticle content. This tendency is noticeable between 6 and 24 h and is related to the C-S-H nanoparticles accelerating the hydration reaction of cement by serving as nucleation sites []. However, the increment of cumulative heat release of the specimens containing the C-S-H nanoparticles decreased gradually after 24 h and was lower than that of PCSH0 at 72 h. This is attributed to the rapid and increased formation of outer C-S-H hydration products on the surface of cement particles induced by the C-S-H nanoparticles. This hydration layer hinders the contact between unreacted cement and water, thereby slowing down the hydration process.

Figure 5.
Heat of hydration results for PCSH and GCSH samples: (a) specific heat flow of PCSH samples; (b) cumulative heat release of PCSH samples; (c) specific heat flow of GCSH samples; (d) cumulative heat release of GCSH samples.
The specific heat flow results for GCSH0 and GCSH3 are shown in Figure 5c, where the accelerated hydration reaction in GGBS cement due to the presence of C-S-H nanoparticles is observed as the earlier appearance of the second and third peaks with slightly higher intensities. At 72 h, the GCSH samples exhibited a lower cumulative heat release than the PCSH samples as shown in Figure 5d. This difference can be explained by the influence of GGBS []. C-S-H nanoparticles significantly enhanced the cumulative heat release in cement mixtures with a high GGBS content. This increase is likely due to the nanoparticles providing additional nucleation sites, which accelerate the hydration reaction of both the cement and the GGBS particles, thereby improving the overall reactivity of the system.
3.2. Effect of C-S-H Nanoparticles on the Compressive Strength
The compressive strength tests were conducted at ages of 8 h, 16 h, 1 d, 3 d, 7 d, and 28 d. As illustrated in Figure 6, the compressive strength of mortar specimens increases with the incorporation of higher concentrations of C-S-H nanoparticles. This trend is particularly evident at early ages, though the rate of strength gain diminishes over time. All specimens containing C-S-H nanoparticles exhibited higher compressive strengths than did PCSH0 from 8 h to 7 d of age, with the largest difference observed at 16 h. At this age, the compressive strengths of PCSH1, PCSH2, and PCSH3 were 5.3, 6.6, and 7.2 MPa higher than that of PSCH0, respectively. This phenomenon can be explained by the accelerated hydration process observed in specimens with C-S-H nanoparticles, along with a reduced compressive strength gap after 16 h. By 7 d, the compressive strengths of PCSH1, PCSH2, and PCSH3 demonstrated notable improvements, exceeding the strength of PCSH0 by 0.1 MPa, 2.4 MPa, and 3.4 MPa, respectively. However, by 28 d, the compressive strengths of all specimens incorporating C-S-H nanoparticles were observed to be lower than that of PCSH0. For example, PCSH3 had the lowest compressive strength of all samples at 28 d, which was 1.6 MPa lower than that of PCSH0.
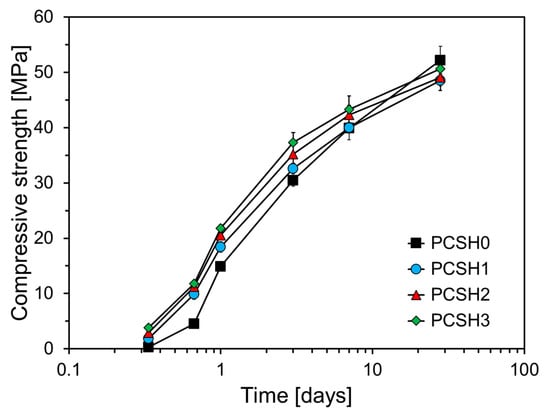
Figure 6.
Compressive strength development over time of PCSH samples with different C-S-H nanoparticle contents.
During early-age hydration, the reaction in cement samples is mainly confined to the surfaces of the cement particles [,]. However, C-S-H nanoparticles—smaller than the cement particles—supply extra nucleation sites that speed up the cement’s initial hydration reaction []. Specifically, the C-S-H nanoparticles fill the voids between cement particles and act as additional nucleation sites, facilitating the formation of hydration products through their interaction with the surrounding matrix. This increases the density of the structure compared to that of the samples without C-S-H nanoparticles, resulting in a higher initial strength up to 7 d. The hydration product layer resulting from the rapid hydration reaction reduces the diffusion rate of Ca2+ inside the cement particles, thereby impeding the mid-to-long-term hydration reaction []. This explains the significant early-age strength development observed in specimens containing C-S-H nanoparticles, however, their compressive strength beyond 28 d was relatively low.
Figure 7 shows the compressive strength of the GGBS cement specimens containing C-S-H nanoparticles. The major limitation of cement composites containing GGBS is that their early-age strength is low compared to that of ordinary cement composites []. This limits the mass utilization of GGBS in the construction industry because it increases the construction time. Many studies have focused on increasing the early-age strength of cement composites containing GGBS [,,,]. As an example, Xu et al. conducted a study on the influence of nanosilica in cement composites containing high volume GGBS []. Figure 7 shows that the compressive strength of GCSH0, in which GGBS replaced 50% of the cement weight, is lower than that of PCSH0. The compressive strengths of GCSH0 at 1 d, 3 d, 7 d of age were 10.2, 15.3, and 16.0 MPa lower than that of PCSH0, respectively, however, the difference in compressive strength decreased to 2.9 MPa at 28 d. Furthermore, GCSH3 containing 3 wt% C-S-H nanoparticles exhibited an improved compressive strength, which was noticeable at 7 d of age. At 8 h, the compressive strength of GCSH3 surpassed that of PCSH0 by 0.57 MPa, however, it was 1.19, 5.4, and 6 MPa lower at 16 h, 1 d, and 3 d, respectively. This tendency was reversed after 3 d; at 7 and 28 d, the compressive strengths of GCSH3 were 4.1 and 1.6 MPa higher than that of PCSH0. GCSH3 consistently demonstrated higher compressive strength than GCSH0 at all ages, with the maximum difference of 20.1 MPa observed at 7 d. However, from 8 h to 3 d, the compressive strength of GCSH3 was 2.96–12.8 MPa lower than that of PCSH3. This is because the C-S-H nanoparticles act as additional nucleation sites, which accelerate the early hydration of the cement. As a result, an alkaline environment is rapidly established, which in turn activates the latent hydraulic properties of GGBS, leading to earlier and greater strength development [].
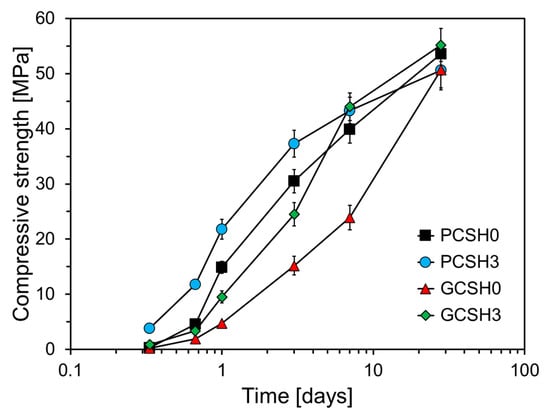
Figure 7.
Compressive strength development over time of PCSH and GCSH samples with different C-S-H nanoparticle contents.
3.3. Hydration Reaction Analysis by XRD
Figure 8 shows the XRD patterns of the PCSH0 and PCSH3 specimens obtained at different ages. Alite, belite, aluminoferrite, portlandite, ettringite, and C-S-H were detected in both specimens. The PCSH3 specimen had higher consumptions of alite, belite, and aluminoferrite than did PCSH0 at early-age hydration. The same tendency was observed in a previous study [] when the results of the two samples were compared. This implies that C-S-H nanoparticles increase hydrate formation by accelerating the early-age hydration reaction [].
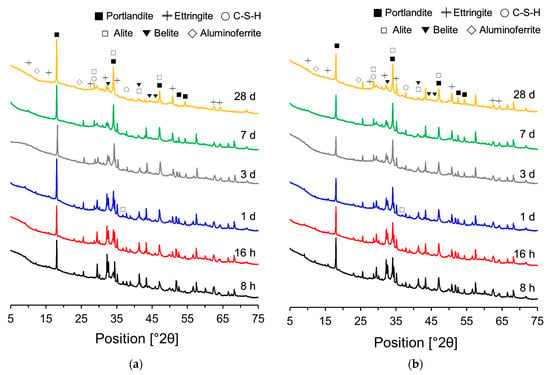
Figure 8.
XRD patterns measured at different ages: (a) PCSH0; (b) PCSH3.
Figure 9 shows the Rietveld refinement analysis results based on the XRD patterns in Figure 8. Figure 9a,b show that the generation of portlandite and C-S-H hydration products increased with age. The composition ratio (y-axis) was calculated by weight fraction. The quantity of C-S-H generated at the early stages of hydration was found to be approximately 9% higher in PCSH3 compared to PCSH0.
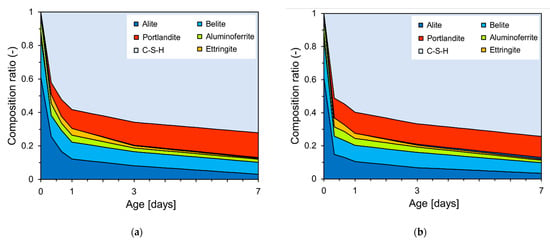
Figure 9.
Fractions of the various crystalline components determined from Rietveld analysis of the XRD patterns: (a) PCSH0; (b) PCSH3.
The generation of C-S-H and portlandite increased with increasing consumption of alite as shown in Figure 10. The PCSH0 and PCSH3 specimens showed the most noticeable differences in alite reduction at early ages, as depicted in Figure 10. The reacted alite contents of PCSH3 were 10.9, 3.8, 1.7, and 0.4% higher than those of PCSH0 at 8 h, 16 h, 1 d, and 3 d, respectively. This is related to the finding that the PCSH3 specimen had a higher early-age compressive strength than that of PCSH0 [].
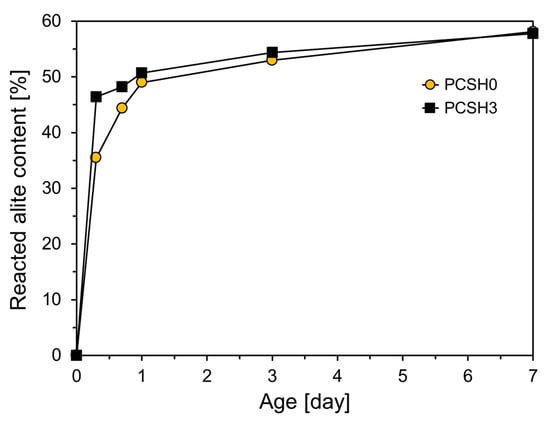
Figure 10.
Reacted alite contents of PCSH0 and PCSH3.
3.4. Thermal Gravimetric Analysis (TGA) Results
Figure 11 shows the TG results for PCSH series paste specimens tested at different ages. For all samples, the amount of hydration products tended to increase with increasing age. This is based on the weight changes in the 70–200 °C temperature range, which corresponds to C-S-H and ettringite [,]. In addition, this tendency is observed in the 400–500 °C temperature range, which corresponds to Ca(OH)2 [].
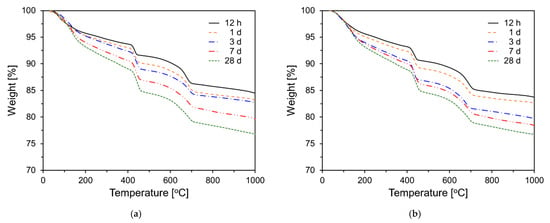
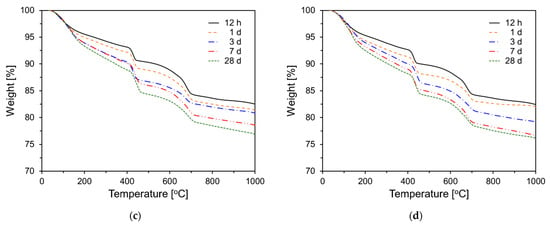
Figure 11.
Thermogravimetric analysis results: (a) PCSH0; (b) PCSH1; (c) PCSH2; (d) PCSH3.
As shown in Figure 12a, the mass losses in the 70–200 °C temperature range (M70-200) of specimens containing the C-S-H nanoparticles are higher than those of PCSH0 up to 7 d. This trend is the most noticeable at 3 d, and it increases with an increase in the content of the C-S-H nanoparticles. However, at 28 d, the M70-200 values of PCSH1 and PCSH2 were similar to that of PCSH0. Figure 12b depicts the Ca(OH)2 content for each sample at various ages. The largest difference in the Ca(OH)2 content was observed at 12 h. Samples containing C-S-H nanoparticles exhibited higher Ca(OH)2 contents, with similar trends observed up to 7 d. However, at 28 d, this trend was completely reversed, with the PCSH0 showing the highest Ca(OH)2 content and the PCSH3 the lowest. These findings are consistent with the compressive strength results. The compressive strength of PCSH3 exceeded that of PCSH0 for the first three days. However, by seven days, the strength difference had diminished, and at 28 d, PCSH3 exhibited lower compressive strength than PCSH0.
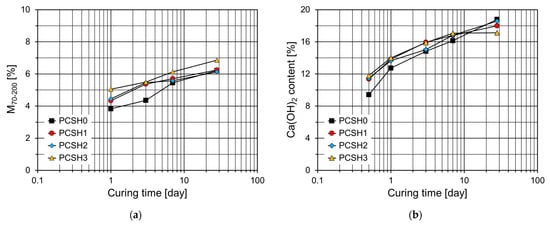
Figure 12.
Content of PCSH specimens: (a) M70-200; (b) Ca(OH)2.
3.5. Pore Structure of Specimens Containing C-S-H Nanoparticles
Figure 13 shows the pore structure of PCSH paste specimens determined by MIP. Figure 13a shows that the cumulative pore volumes of PCSH0, PCSH1, PCSH2, and PCSH3 at 3 d were 0.25, 0.23, 0.21, and 0.20 mL/g, respectively. The unit mL/g represents the volume of mercury intruded per gram of sample, indicating the total pore volume per unit mass of the specimen. The cumulative pore volume exhibits a decreasing trend as the content of C-S-H nanoparticles increases. This is because the microstructure was densified as the C-S-H nanoparticles that act as nucleation sites accelerated the generation of hydration products. A tendency similar to that of 3 d was observed at 7 d, however, the magnitude of the difference decreased. PCSH2 and PCSH3 show very similar values (Figure 13c). All samples exhibit similar values (0.16 to 0.18 mg/L) for the cumulative pore volume at 28 d, similar to the compressive strength tendency shown in Figure 6. The dV/dlogD graphs in Figure 13 show that the pore diameter decreases over time for all samples. Figure 13b shows that the main peak near 1 μm occurs at a smaller pore diameter with an increase in the content of the C-S-H nanoparticles at 3 d; this is attributed to a reduction in the pore size due to the accelerated production of hydration products in the presence of the C-S-H nanoparticles. However, at curing ages of 7 days and 28 days, this trend was not clearly evident. These findings are consistent with the compressive strength results.
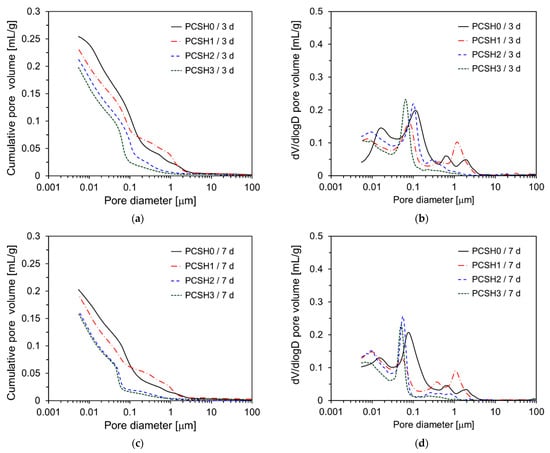

Figure 13.
Cumulative pore volume and dV/dlogD curves of specimens: (a) Cumulative pore volume at 3 days; (b) dV/dlogD curves at 3 days; (c) Cumulative pore volume at 7 days; (d) dV/dlogD curves at 7 days; (e) Cumulative pore volume at 28 days; (f) dV/dlogD curves at 28 days.
4. Conclusions
This study investigated the influence of synthesized C–S–H nanoparticles on the early hydration behavior and microstructural development of cement composites. The main findings are summarized as follows:
- The chemical structure of the synthesized C–S–H nanoparticles, as determined by XPS analysis, was found to be similar to that of 11 Å tobermorite (Ca4.5(Si6O16)(OH)·5H2O).
- From the heat of hydration results, it was confirmed that the C–S–H nanoparticles effectively accelerate early hydration reactions. Isothermal calorimetry revealed that incorporating C–S–H nanoparticles shortened the induction period and increased the intensity of the second exothermic peak.
- C-S-H nanoparticles enhanced the early-age compressive strength of mortar specimens. However, the 28-day strength slightly decreased due to the formation of a dense hydration product layer in the early stage, which impeded Ca2+ diffusion into the un-hydrated cement core, thereby slowing mid-to-late hydration. These trends were consistent with the TG and MIP analyses.
- C-S-H nanoparticles significantly improved compressive strength by acting as nucleation sites and creating an alkaline environment that promotes the reaction between cement and GGBS.
- The use of C-S-H nanoparticles as early-strength promoters can support the broader adoption of GGBS in construction materials, thereby contributing to reduced environmental impact from both the cement and steel industries.
Author Contributions
Conceptualization, Y.-C.C.; data curation, S.W.; formal analysis, Y.H.; funding acquisition, Y.-C.C.; investigation, Y.H. and S.W.; methodology, Y.H. and S.W.; project administration, Y.-C.C.; supervision, Y.-C.C.; validation, Y.-C.C.; visualization, Y.H. and S.W.; writing—original draft, Y.H.; writing—review and editing, S.W. and Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT) (RS-2025-00520142). This work was also supported by the Industrial Technology Innovation Program (no. RS-2024-00438194, Development of technology of raw material substitute of using recycling resource to reduce sintering temperature by 100 degrees Celsius) funded by the Ministry of Trade, Industry & Energy (MOTIE, KOREA).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed toward the authors.
Conflicts of Interest
Author Suji Woo was employed by the company GS E&C. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Shetty, M.S. Concrete Technology: Theory and Practice, 7th ed.; S. Chand & Company Pty. Ltd.: New Delhi, India, 1982. [Google Scholar]
- Domone, P.; Illston, J. Construction Materials: Their Nature and Behaviour, 4h ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Zhang, C.; Yu, B.; Chen, J.; Wei, Y. Green transition pathways for cement industry in China. Resour. Conserv. Recycl. 2021, 166, 105355. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon Dioxide Emissions from the Global Cement Industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the Eco-Efficiency of Cement Use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Ige, O.E.; Von Kallon, D.V.; Desai, D. Carbon Emissions Mitigation Methods for Cement Industry Using a Systems Dynamics Model. Clean Technol. Environ. Policy 2024, 26, 579–597. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Z.M.; Yao, Y.H.; Tang, R.F.; Li, S.T.; Liu, X.; Sun, D.W. The Effect and Mechanism of C–S–H-PCE Nanocomposites on the Early Strength of Mortar under Different Water-to-Cement Ratio. J. Build. Eng. 2021, 44, 103360. [Google Scholar] [CrossRef]
- Gartner, E. Industrially Interesting Approaches to “Low-CO2” Cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Mohammadi, A.M.; Ramezanianpour, A.A. Investigating the Environmental and Economic Impacts of Using Supplementary Cementitious Materials (SCMs) Using the Life Cycle Approach. J. Build. Eng. 2023, 79, 107934. [Google Scholar] [CrossRef]
- Nedunuri, S.S.S.A.; Sertse, S.G.; Muhammad, S. Microstructural Study of Portland Cement Partially Replaced with Fly Ash, Ground Granulated Blast Furnace Slag and Silica Fume as Determined by Pozzolanic Activity. Constr. Build. Mater. 2020, 238, 117561. [Google Scholar] [CrossRef]
- Mehta, P.K. Pozzolanic and Cementitious By-Products as Mineral Admixtures for Concrete—A Critical Review. ACI Symp. Publ. 1983, 79, 1–46. [Google Scholar]
- Ravina, D.; Mehta, P.K. Compressive Strength of Low Cement/High Fly Ash Concrete. Cem. Concr. Res. 1988, 18, 571–583. [Google Scholar] [CrossRef]
- Jain, S.; Pradhan, B. Effect of Cement Type on Hydration, Microstructure and Thermo-Gravimetric Behaviour of Chloride Admixed Self-Compacting Concrete. Constr. Build. Mater. 2019, 212, 304–316. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Dong, B.; Yang, X. Chlorine Immobilization and Performances of Cement Paste/Mortar with C-S-Hs-PCE and Calcium Chloride. Constr. Build. Mater. 2020, 262, 120694. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, H.; Zhou, Q.; Zhang, Y.; Zhao, P. Influence of Sulfates on Formation of Ettringite during Early C3A Hydration. Materials 2022, 15, 6934. [Google Scholar] [CrossRef] [PubMed]
- Mota, B.; Matschei, T.; Scrivener, K.L. Impact of NaOH and Na2SO4 on the Kinetics and Microstructural Development of White Cement Hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Song, Z.; Jiang, L.; Chu, H. Impact of Calcium Leaching on Chloride Diffusion Behavior of Cement Pastes Exposed to Ammonium Chloride Aqueous Solution. Constr. Build. Mater. 2017, 153, 211–215. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Impacts of Hexitols on the Hydration of a Tricalcium Aluminate-Calcium Sulfate Mixture. Cem. Concr. Res. 2016, 89, 177–186. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of Nucleation Seeding on the Hydration Mechanisms of Tricalcium Silicate and Cement. J. Phys. Chem. C. 2009, 113, 4327–4334. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Wang, J.; He, H. Effects of Highly Crystalized Nano CSH Particles on Performances of Portland Cement Paste and Its Mechanism. Crystals 2020, 10, 816. [Google Scholar] [CrossRef]
- Verma, P.; Chowdhury, R.; Chakrabarti, A. Early Strength Development of Cement Composites Using Nano-Calcium Silicate Hydrate (CSH) Based Hardening Accelerator. Mater. Today Proc. 2023, 93, 91–98. [Google Scholar] [CrossRef]
- Kanchanason, V.; Plank, J. Role of pH on the Structure, Composition and Morphology of C-S-H–PCE Nanocomposites and Their Effect on Early Strength Development of Portland Cement. Cem. Concr. Res. 2017, 102, 90–98. [Google Scholar] [CrossRef]
- Sun, J.F.; Shi, H.; Qian, B.B.; Xu, Z.Q.; Li, W.F.; Shen, X.D. Effects of Synthetic C-S-H/PCE Nanocomposites on Early Cement Hydration. Constr. Build. Mater. 2017, 140, 282–292. [Google Scholar] [CrossRef]
- John, E.; Epping, J.D.; Stephan, D. The Influence of the Chemical and Physical Properties of C-S-H Seeds on Their Potential to Accelerate Cement Hydration. Constr. Build. Mater. 2019, 228, 116723. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhang, W.X.; Xu, K.; Monteiro, P.J.M. Fibrillar Calcium Silicate Hydrate Seeds from Hydrated Tricalcium Silicate Lower Cement Demand. Cem. Concr. Res. 2020, 137, 106195. [Google Scholar] [CrossRef]
- Das, S.; Ray, S.; Sarkar, S. Early Strength Development in Concrete Using Preformed CSH Nano Crystals. Constr. Build. Mater. 2020, 233, 117214. [Google Scholar] [CrossRef]
- Pedrosa, H.C.; Reales, O.M.; Reis, V.D.; Paiva, M.D.; Fairbairn, E.M.R. Hydration of Portland Cement Accelerated by C-S-H Seeds at Different Temperatures. Cem. Concr. Res. 2020, 129, 105978. [Google Scholar] [CrossRef]
- Woo, S.; Choi, Y.C. Synthesis of Calcium Silicate Hydrate Nanoparticles and Their Effect on Cement Hydration and Compressive Strength. Constr. Build. Mater. 2023, 407, 133559. [Google Scholar] [CrossRef]
- Schönlein, M.; Plank, J. A TEM Study on the Very Early Crystallization of C-S-H in the Presence of Polycarboxylate Superplasticizers: Transformation from Initial C-S-H Globules to Nanofoils. Cem. Concr. Res. 2018, 106, 33–39. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali–Silica Reaction: Current Understanding of the Reaction Mechanisms and the Knowledge Gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Pan, J.; Wang, W.; Wang, J.; Bai, Y.; Wang, J. Influence of Coarse Aggregate Size on Deterioration of Concrete Affected by Alkali-Aggregate Reaction. Constr. Build. Mater. 2022, 329, 127228. [Google Scholar] [CrossRef]
- ISO 679; Cement—Test Methods—Determination of Strength. ISO: Geneva, Switzerland, 2009.
- Black, L.; Garbev, K.; Stemmermann, P.; Hallam, K.R.; Allen, G.C. Characterisation of Crystalline C-S-H Phases by X-ray Photoelectron Spectroscopy. Cem. Concr. Res. 2003, 33, 899–911. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Ma, Z.; Wang, F.; Hu, C. Novel C-F-S-H/PCE Nanocomposites with High-Efficiency Seeding Effect on the Hydration of Portland Cement. Cem. Concr. Compos. 2023, 140, 105077. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, J.; Zhao, Y.; Li, S.; Guo, Z.; Luo, X.; Chen, G. Promoting Utilization Rate of Ground Granulated Blast Furnace Slag (GGBS): Incorporation of Nanosilica to Improve the Properties of Blended Cement Containing High Volume GGBS. J. Clean. Prod. 2022, 332, 130096. [Google Scholar] [CrossRef]
- Jennings, H.M. A Model for the Microstructure of Calcium Silicate Hydrate in Cement Paste. Cem. Concr. Res. 2000, 30, 101–116. [Google Scholar] [CrossRef]
- Hu, C.; Ruan, Y.; Yao, S.; Wang, F.; He, Y.; Gao, Y. Insight into the Evolution of the Elastic Properties of Calcium-Silicate-Hydrate (C–S–H) Gel. Cem. Concr. Compos 2019, 104, 103342. [Google Scholar] [CrossRef]
- Oner, A.; Akyuz, S. An Experimental Study on Optimum Usage of GGBS for the Compressive Strength of Concrete. Cem. Concr. Compos. 2007, 29, 505–514. [Google Scholar] [CrossRef]
- Barnett, S.J.; Soutsos, M.N.; Millard, S.G.; Bungey, J.H. Strength Development of Mortars Containing Ground Granulated Blast-Furnace Slag: Effect of Curing Temperature and Determination of Apparent Activation Energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Soutsos, M.; Kanavaris, F. Applicability of the Modified Nurse-Saul (MNS) Maturity Function for Estimating the Effect of Temperature on the Compressive Strength of GGBS Concretes. Constr. Build. Mater. 2023, 381, 131250. [Google Scholar] [CrossRef]
- Li, B.; Hou, P.; Chen, H.; Zhao, P.; Du, P.; Wang, S.; Cheng, X. GGBS Hydration Acceleration Evidence in Supersulfated Cement by NanoSiO2. Cem. Concr. Compos. 2022, 132, 104609. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Yang, X. Effect and Characterization of the Nucleation C-S-H Seed on the Reactivity of Granulated Blast Furnace Slag Powder. Constr. Build. Mater. 2020, 238, 117726. [Google Scholar] [CrossRef]
- Zhou, Z.; Sofi, M.; Liu, J.; Li, S.; Zhong, A.; Mendis, P. Nano-CSH Modified High Volume Fly Ash Concrete: Early-Age Properties and Environmental Impact Analysis. J. Clean. Prod. 2021, 286, 124924. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Pan, G.; Meng, H.; Li, D. Novel In-Situ Controllably Grown CSH: Synthesis, Characterization and the Effect on Cement Hydration. Cem. Concr. Compos. 2023, 139, 105044. [Google Scholar] [CrossRef]
- Collier, N.C. Transition and Decomposition Temperatures of Cement Phases—A Collection of Thermal Analysis Data. Ceram. Silik. 2016, 60, 338–343. [Google Scholar] [CrossRef]
- Wang, R.; Yu, J.; Gu, S.; Han, X.; He, P.; Liu, Q.; Xue, L. Effect of Ion Chelator on Hydration Process of Portland Cement. Constr. Build. Mater. 2020, 259, 119727. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I.G. Nonevaporable Water from Neat OPC and Replacement Materials in Composite Cements Hydrated at Different Temperatures. Cem. Concr. Res. 2003, 33, 1883–1888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).