Reactivity of Shale to Supercritical CO2: Insights from Microstructural Characterization and Mineral Phase Evolution in Caney Shales for CCUS Applications
Abstract
1. Introduction
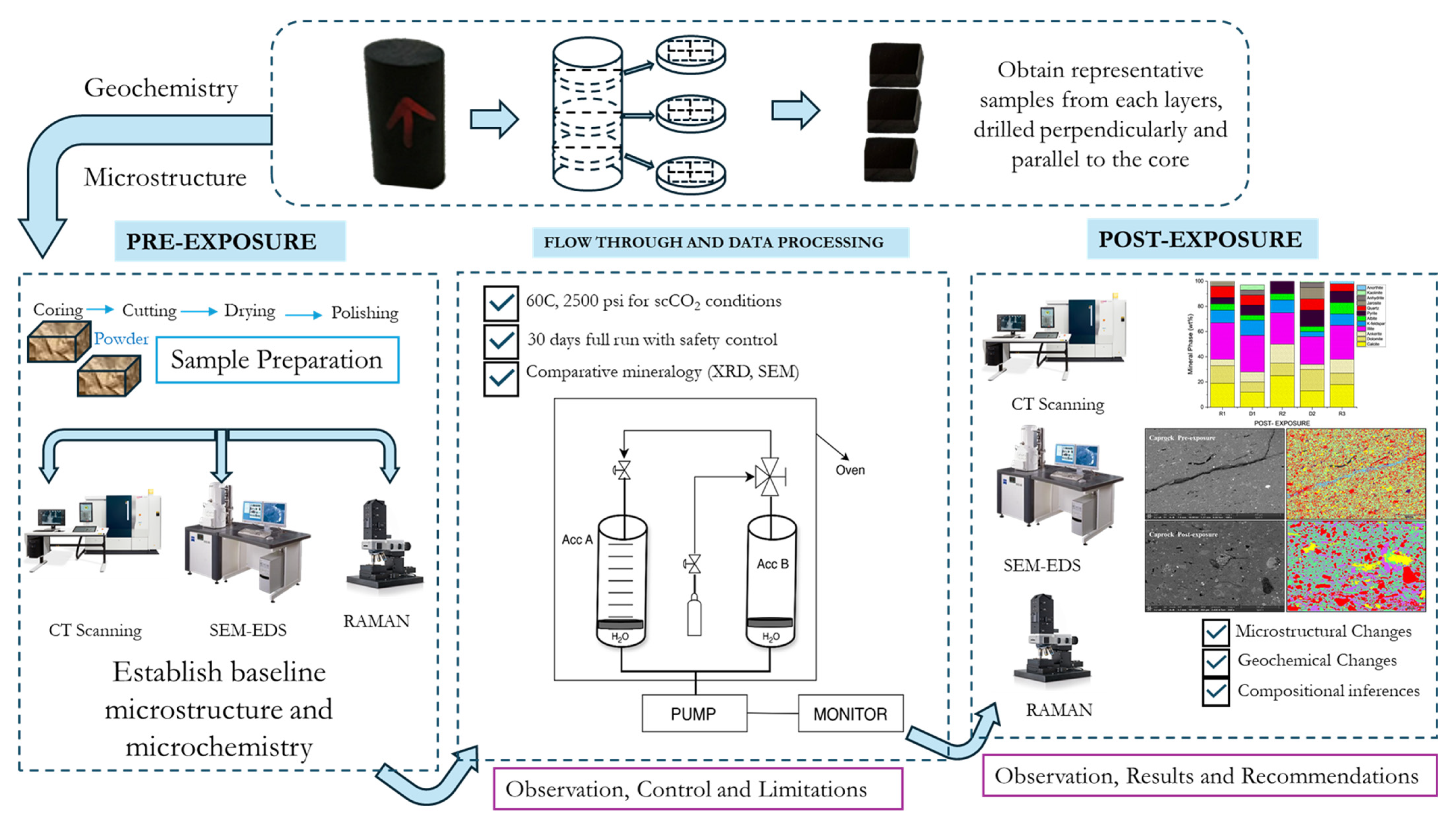
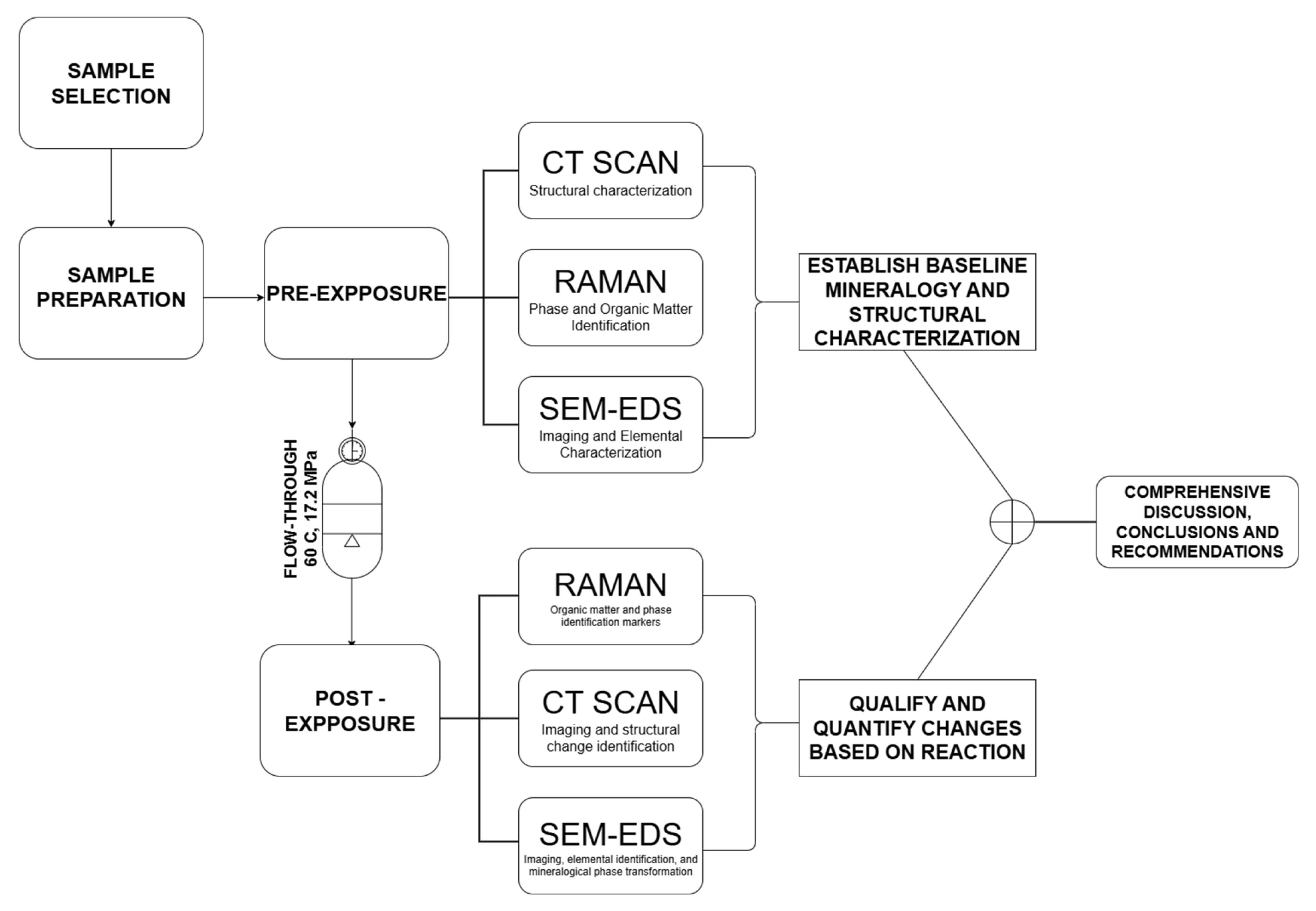
2. Materials and Methods
3. Results
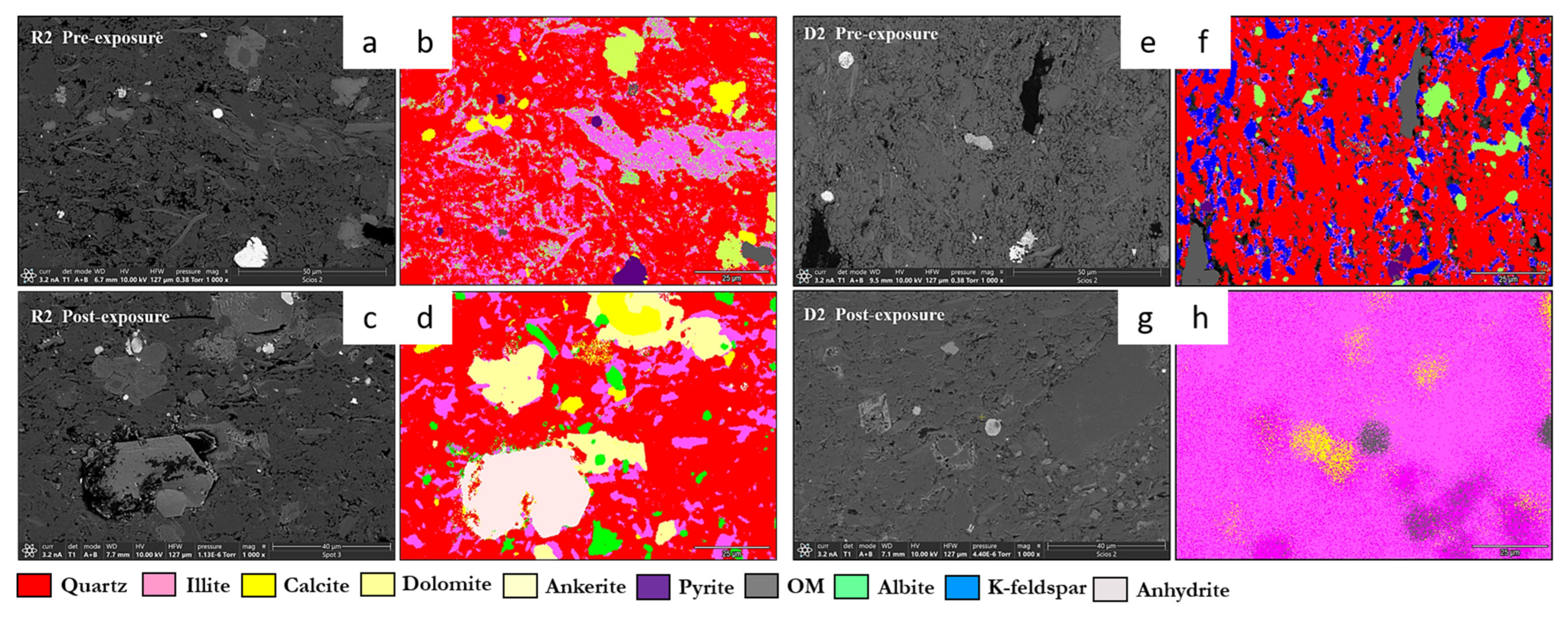
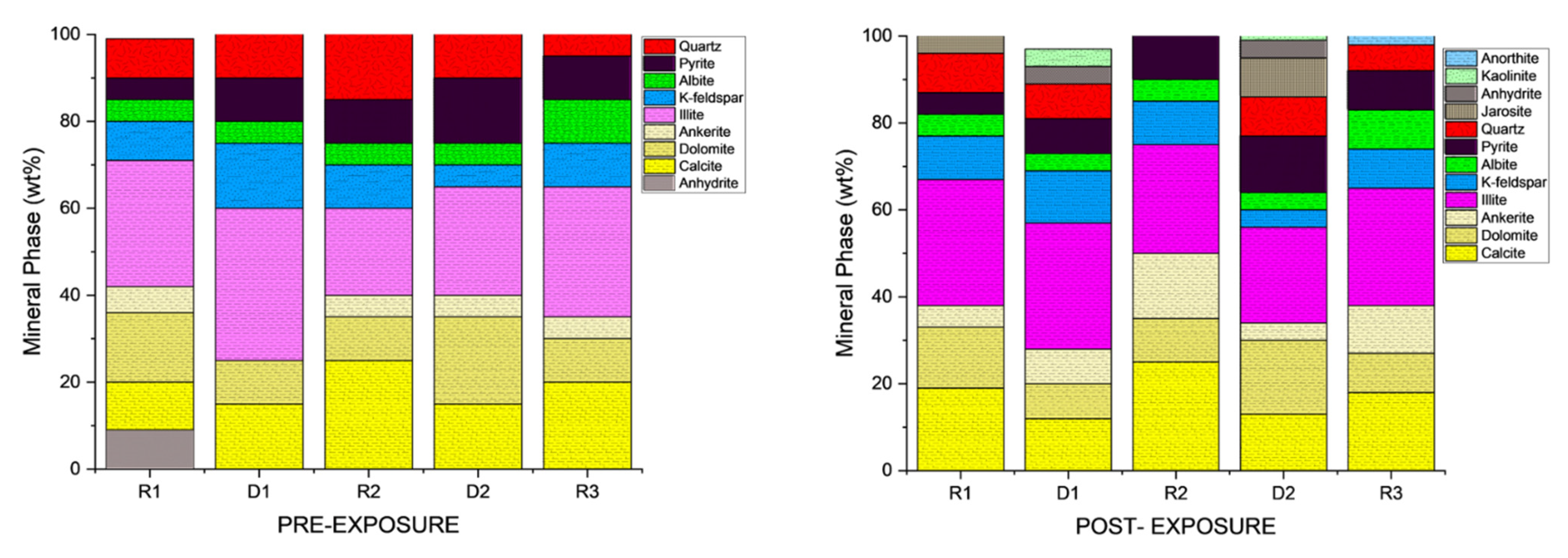
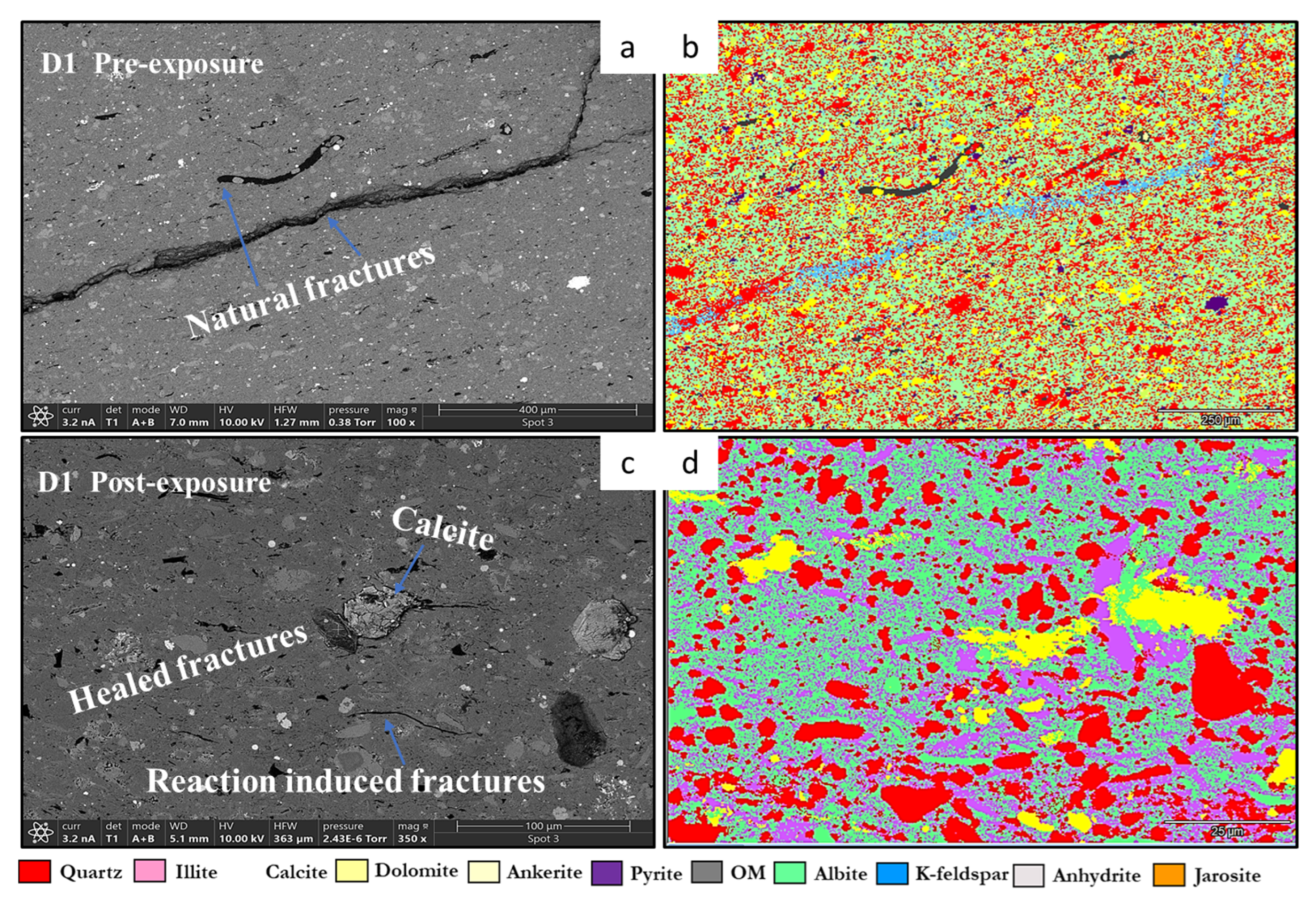
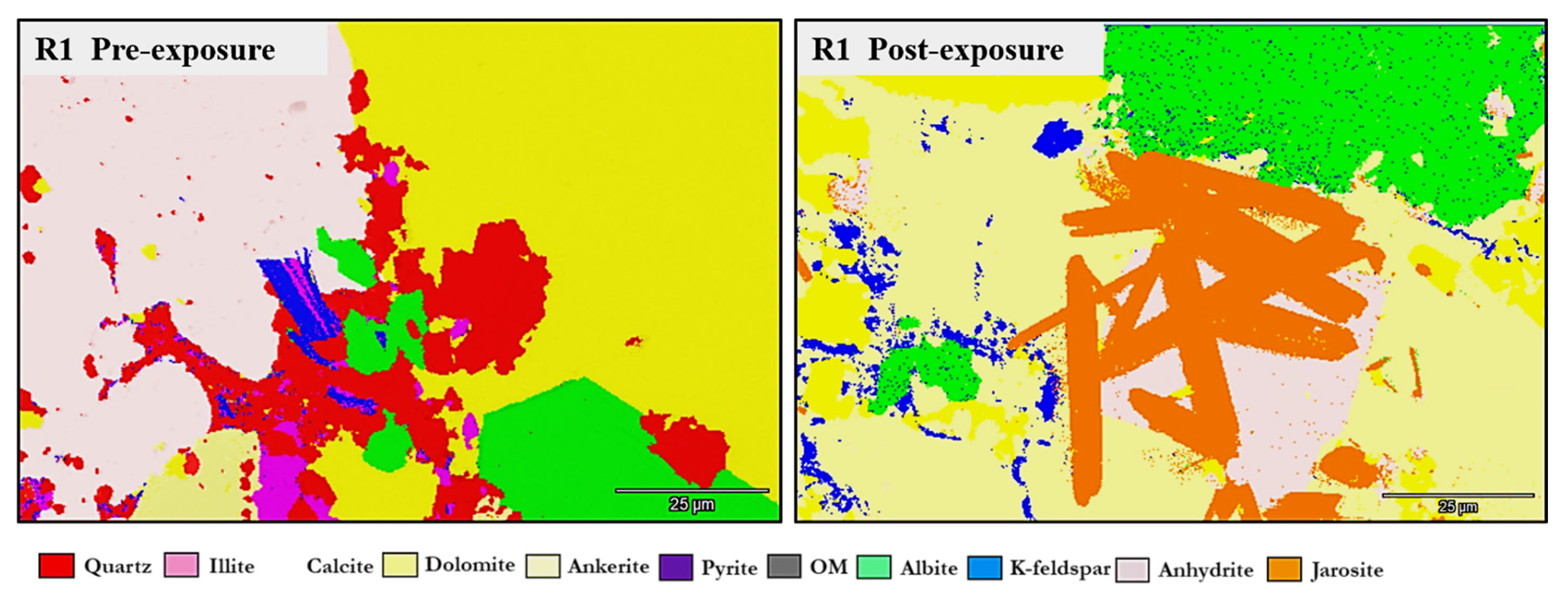
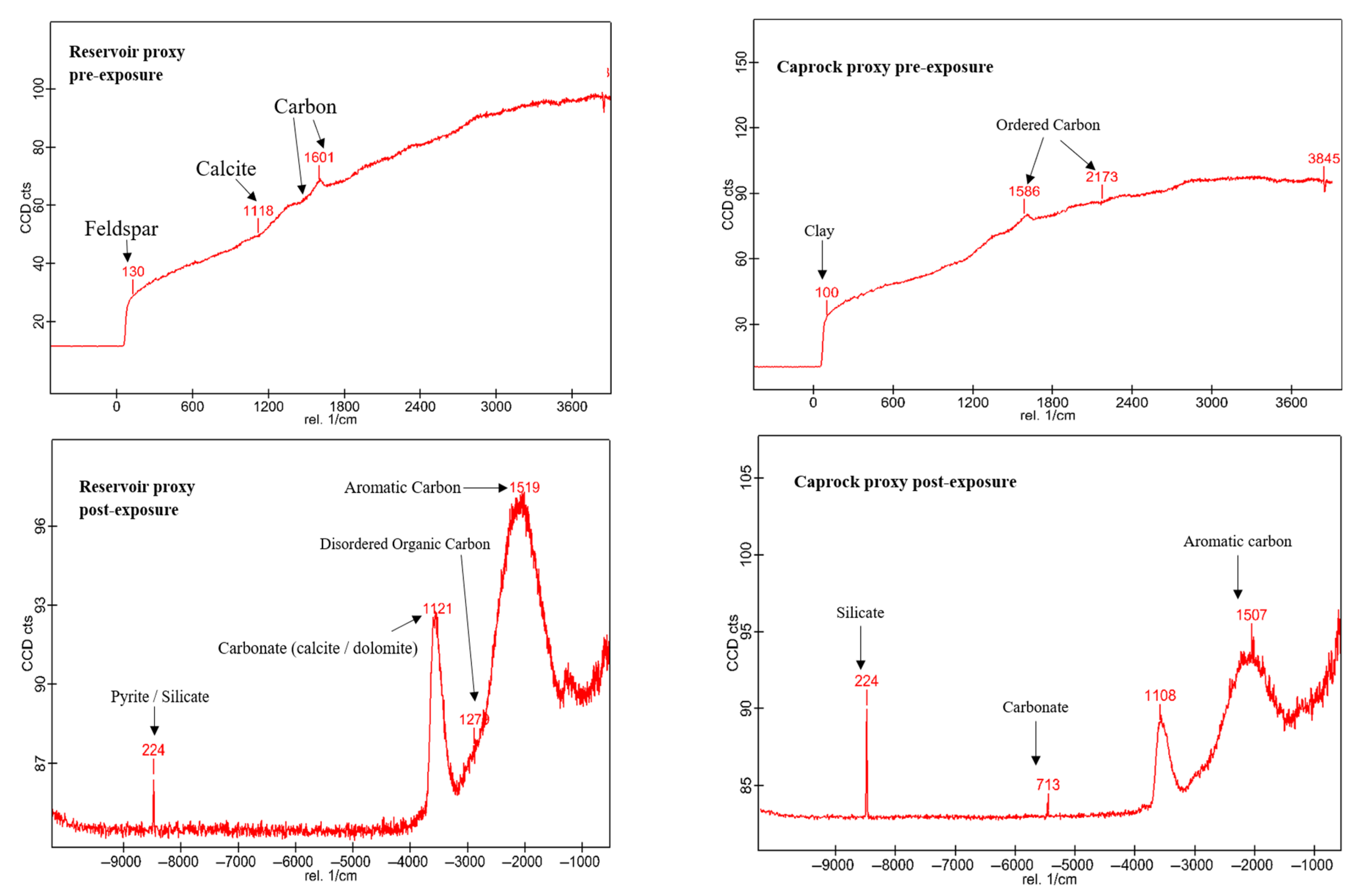
3.1. Mineral Phase Identification
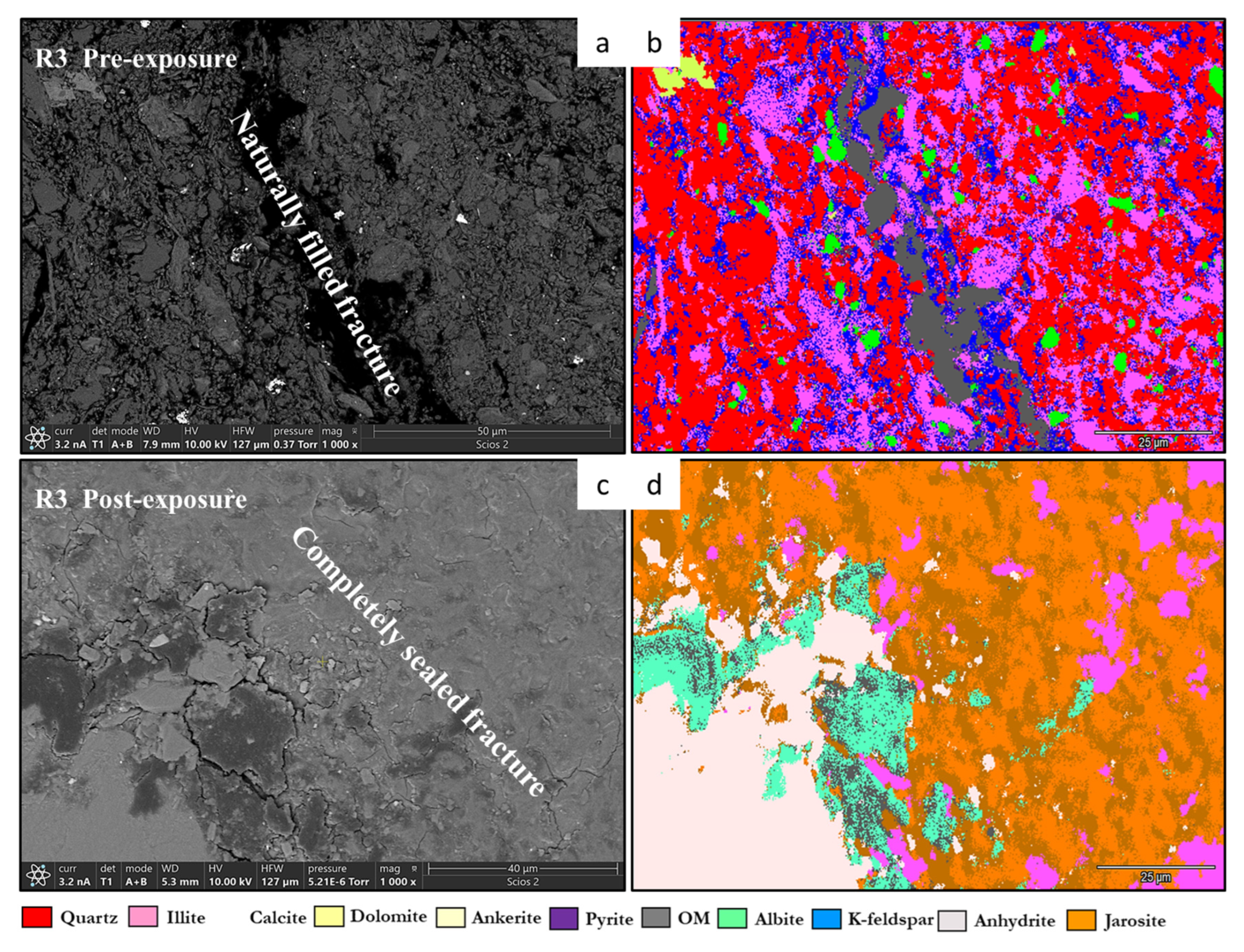
- Quartz remained the principal framework silicate across all facies. In reservoir proxies, pre-exposure abundances were 43.77 wt.% (R1), 42.23 wt.% (R2), and 45.96 wt.% (R3), with post-exposure values of 43.94 wt.%, 42.22 wt.%, and 45.68 wt.%, respectively. In caprock proxies, quartz accounted for 33.79 wt.% (D1) and 33.69 wt.% (D2) prior to exposure, increasing marginally to 34.13 wt.% (D1) and decreasing to 32.75 wt.% (D2) post-exposure. Across all facies, quartz grains retained angular, sharp morphologies with no significantly observable structural or chemical alteration.
- K-Feldspar (KAlSi3O8). K-feldspar was consistently present in all samples. In reservoirs, values ranged from 5.06 to 5.84 wt.% pre-exposure and from 5.89 to 5.99 wt.% post-exposure. In D1 and D2, K-feldspar were 7.06 wt.% and 6.54 wt.% pre-exposure, increasing to 7.13 wt.% in D1 and decreasing mildly to 6.36 wt.% for D2 post-exposure. No dissolution or surface roughening was evident under SEM imaging.
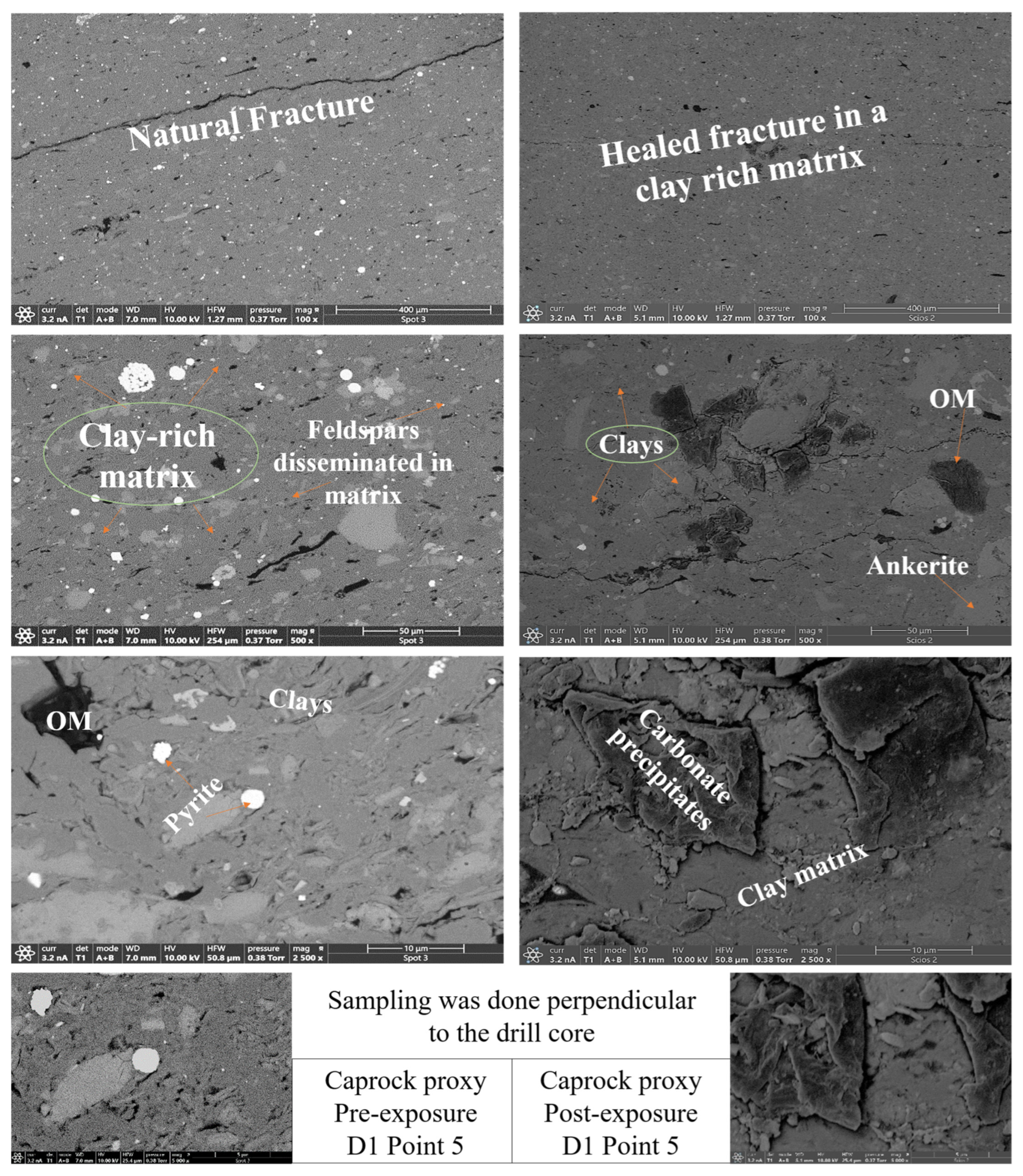
- Illite [(K,H3O)(Al,Mg,Fe)2(Si,Al)4O10(OH)2]. Illite occurred in all facies and was typically distributed along grain boundaries or within clay-rich matrices. Illite content in the reservoir proxies increased from 17.69 to 17.61 wt.% (R3) and 18.40 to 18.67 wt.% (R2). In caprock proxies, illite content increased from 17.26 to 17.47 wt.% (D1) and 16.31 to 16.68 wt.% (D2); pre and postexposure. Platy textures remained intact, although localized thinning and roughening of particle edges were noted in R2 and R3.
- Kaolinite (Al2Si2O5(OH)4). Kaolinite was identified in D1 and D2. Its abundance increased from 10.03 to 10.13 wt.% (D1) and decreased from 9.89 to 9.76 wt.% (D2); pre and post-exposure, respectively. Kaolinite maintained blocky morphology with no signs of chemical erosion or micro-pitting.
- Paragonite (NaAl2(Si3Al)O10(OH)2). Paragonite was not detected in any sample prior to exposure. Post-exposure, it appeared in R2 (0.38 wt.%), R3 (0.88 wt.%), D1 (0.72 wt.%), and D2 (0.90 wt.%). It was typically observed near altered illite flakes and within fine-grained matrix zones, forming as secondary Na-bearing phyllosilicate lamella.
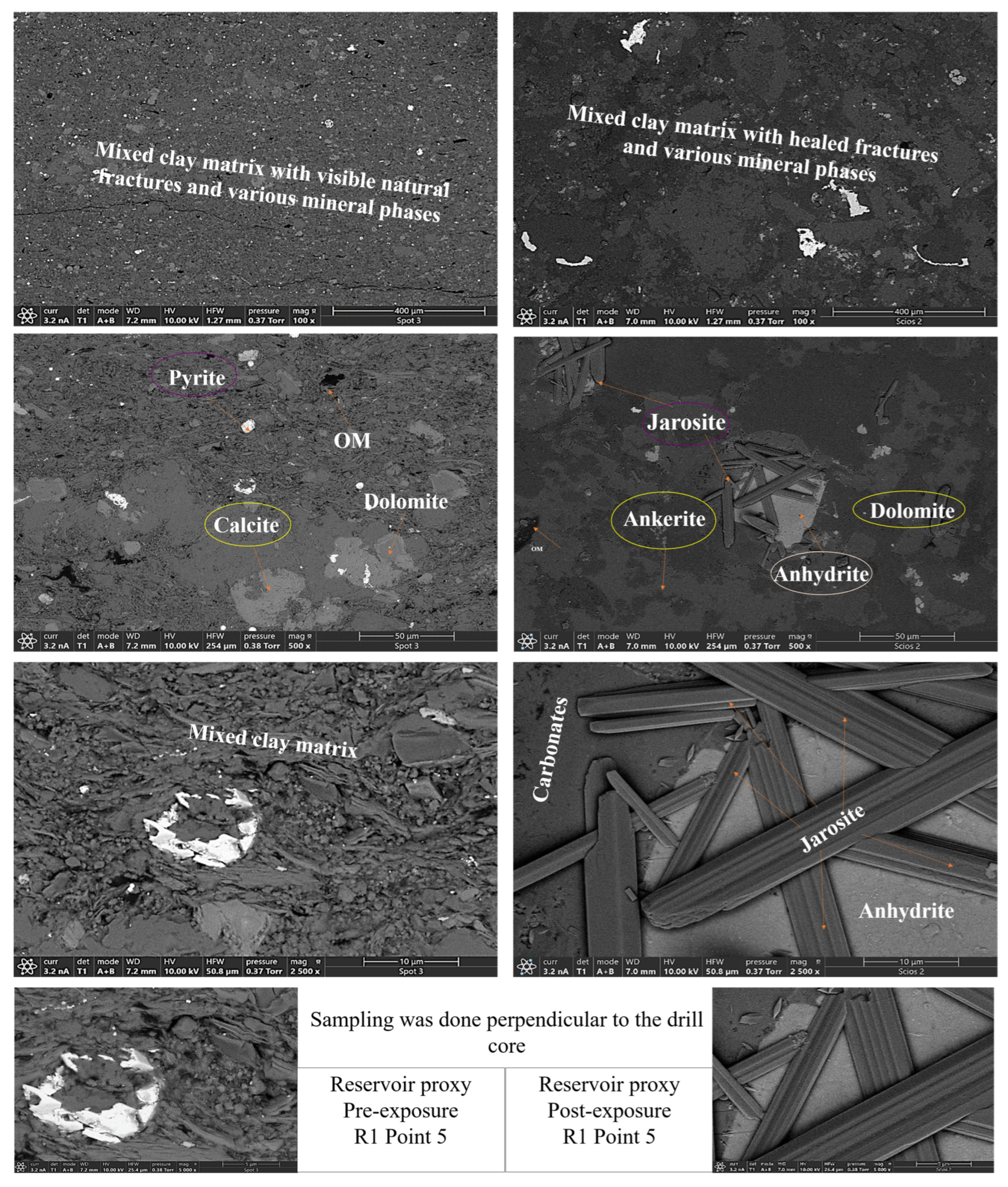
- Calcite (CaCO3). Calcite was present in all samples, particularly in the reservoir facies. Calcite decreased slightly from 9.92 to 9.01 wt.% (R1), 9.97 to 8.76 wt.%, and increased from 8.80 to 9.05 wt.% (R3). In caprocks, it increased from 9.41 to 9.75 wt.% (D1) and decreased from 9.62 to 8.80 wt.% (D2). SEM images revealed surface pitting and edge retreat, especially in R1 and R2.
- Ankerite [Ca(Fe2+,Mg)(CO3)2]. Ankerite occurred in both reservoir and caprock proxies. In R1–R3, pre-exposure values ranged from 4.40 wt.% to 5.49 wt.%, decreasing post-exposure to 4.08–4.33 wt.%. In D1, it was not detected pre-exposure and remained absent post-exposure. In D2, it decreased from 4.02 wt.% to 3.55 wt.%. Morphologies were retained but with localized surface dulling near grain boundaries.
- Wollastonite (CaSiO3). Wollastonite was absent prior to exposure and formed in all samples post-exposure. In R1–R3, abundances were 0.67 wt.%, 1.08 wt.%, and 1.02 wt.%, respectively. In D1 and D2, wollastonite was recorded at 0.56 wt.% and 0.93 wt.%. It appeared as fibrous or acicular precipitates localized around sites of prior carbonate dissolution.
- Albite (NaAlSi3O8). Albite was present in every sample, and its presence in R1 had minimal increase from 4.88 to 5.75 wt.% and in R3 it was a negligible change from 5.96 wt.% preexposure to 5.94 wt.% post-exposure. In D1 and D2, albite changed from 4.71 wt.% and 4.82 wt.% to 4.93 wt.% and 4.65 wt.%, respectively, all of which are within experimental error limits due to sample heterogeneity. Grains retained sharp outlines and showed no signs of dissolution.
- Pyrite (FeS2). Pyrite was present across all facies. In reservoirs, pre-exposure values ranged from 4.88 wt.% (R1) to 5.87 wt.% (R2), declining to 4.67–5.41 wt.% post-exposure. In D1 and D2, pyrite decreased from 6.28 wt.% and 6.26 to 5.56 wt.% and 4.84 wt.%, respectively. SEM showed edge diffusion and oxidation halos near OM and clay interfaces in caprock samples.
- Jarosite [KFe3(SO4)2(OH)6]. No jarosite was detected in Caney shale prior to exposure to scCO2, in this study it was identified post-exposure in R1 (0.58 wt.%), D1 (1.24 wt.%), and D2 (1.49 wt.%), with trace detection in R2. No formation was observed in R2. It formed as fine-grained spikelet aggregates, frequently bordering anhydrite, pyrite and organic-rich regions.
- Anhydrite (CaSO4·2H2O). Anhydrite was detected pre-exposure and post-exposure in reservoir and caprock proxies; notably: 0.80 wt.% in D1 and 1.07 wt.% in D2. It appeared as thin, patchy coatings at mineral boundaries. The CaSO4 phase was identified as anhydrite, based on its dehydrated state and the high-temperature SEM-EDS preparation conditions. Given that EDS does not detect hydration state, gypsum or basanite cannot be ruled out entirely, but the thermal and vacuum conditions favor the anhydrite form.
- Organic Matter (CxHyOz). Organic matter was found in all samples, increasing post-exposure in every case. In R1–R3, OM increased from 5.17 wt.%, 6.57 wt.%, and 5.99 wt.% pre-exposure to 10.79 wt.%, 12.77 wt.%, and 11.89 wt.%, respectively. In D1 and D2, OM rose from 6.66 wt.% and 6.99 to 12.15 wt.% and 14.19 wt.%, respectively. Post-exposure OM showed increased surface roughness, irregularity, and porosity development.
3.2. Chemical Elemental Mobilization
4. Discussion
4.1. Mineral Stability and Reactivity
4.1.1. Carbonate Phases
4.1.2. Clays and Feldspars
4.1.3. Sulfide Oxidation and Sulfate Reaction Pathways
4.1.4. Organic Matter: A Chemically Active Interface
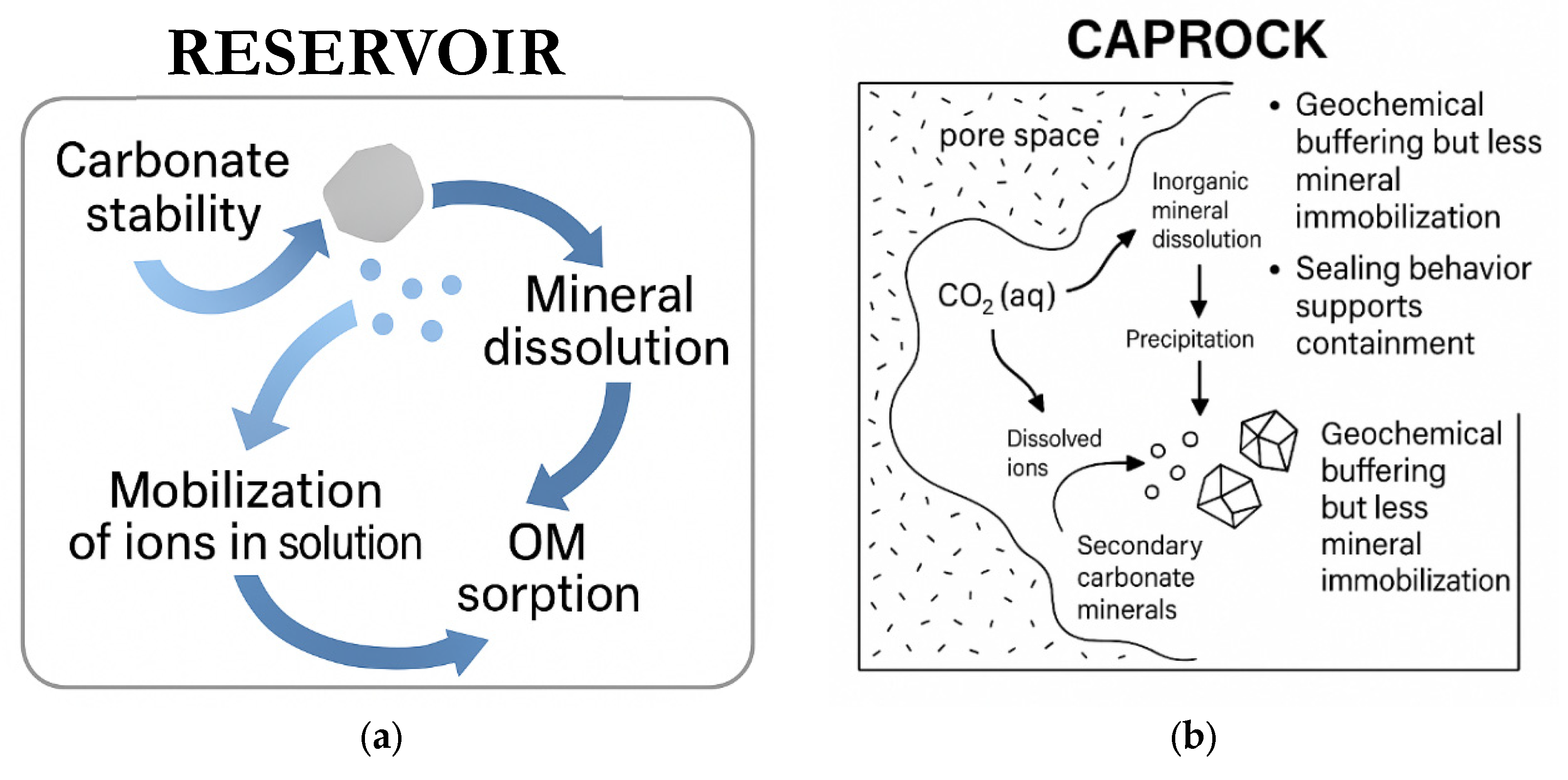
4.2. Relevance for Geochemical Sequestration
4.2.1. Reservoir Proxies
4.2.2. Caprock Proxies
4.2.3. Integrated Storage Performance and Relevance for CCS Design
4.3. Geochemical Insights
5. Conclusions
- Localized porosity development enhances CO2 injectivity, while secondary mineral precipitation at grain contacts and pore throats contributes to self-sealing behavior, supporting containment stability.
- Demonstrated mineral trapping in dry scCO2 (no added brines) systems confirms that water is not a prerequisite for initiating geochemical containment, with in situ precipitation providing a viable mechanism for immobilizing (sequestering) injected CO2.
- Facies-dependent reactivity, mineral phase and ionic species distribution support a naturally evolving balance between fluid migration pathways and geochemical seals. This allows reactive zones (reservoirs) to co-exist with stable, low-permeability zones (caprocks).
- Existing shale development from hydraulic fracturing offers an operational advantage, enabling CO2 storage to leverage established well infrastructure, reservoir access strategies, and field-scale monitoring systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ndlovu, P.; Bulannga, R.; Mguni, L.L. Progress in Carbon Dioxide Capture, Storage and Monitoring in Geological Landform. Front. Energy Res. 2024, 12, 1450991. [Google Scholar] [CrossRef]
- Olabode, A.; Radonjic, M. Characterization of Shale Cap-Rock Nano-Pores in Geologic CO2 Containment. Environ. Eng. Geosci. 2014, 20, 361–370. [Google Scholar] [CrossRef]
- Busch, A.; Amann, A.; Bertier, P.; Waschbusch, M.; Krooss, B.M. The Significance of Caprock Sealing Integrity for CO2 Storage. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010; p. SPE-139588-MS. [Google Scholar]
- Du, H.; Carpenter, K.; Hui, D.; Radonjic, M. Microstructure and Micromechanics of Shale Rocks: Case Study of Marcellus Shale. Facta Univ. Ser. Mech. Eng. 2017, 15, 331–340. [Google Scholar] [CrossRef][Green Version]
- Hazra, B.; Vishal, V.; Sethi, C.; Chandra, D. Impact of Supercritical CO2 on Shale Reservoirs and Its Implication for CO2 Sequestration. Energy Fuels 2022, 36, 9882–9903. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, G.; Achang, M.; Cains, J.; Wethington, C.; Katende, A.; Grammer, G.M.; Puckette, J.; Pashin, J.; Castagna, M.; et al. Multiscale Characterization of the Caney Shale—An Emerging Play in Oklahoma. Midcont. Geosci. 2021, 2, 33–53. [Google Scholar] [CrossRef]
- Xie, W.; Chen, S.; Wang, M.; Yu, Z.; Wang, H. Progress and Prospects of Supercritical CO2 Application in the Exploitation of Shale Gas Reservoirs. Energy Fuels 2021, 35, 18370–18384. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, F.; Kang, Y.; Hu, Y.; Liu, Y. SC-CO2 and Brine Exposure Altering the Mineralogy, Microstructure, and Micro- and Macromechanical Properties of Shale. Energy Fuels 2024, 38, 11064–11077. [Google Scholar] [CrossRef]
- Deehan, M.; Renta, I.; Yaary, S.; Fillingham, J.; Sarkar, S.; Matlock, G.; Newcomb, L.; Bayler, E.; Ghirardelli, J.; Grasso, M. 2023 NOAA Science Report; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2024. [Google Scholar]
- Boudreau, K.; Robinson, M.; Farooqi, Z. IPCC Sixth Assessment Report: Climate Change 2021: The Physical Science Basis Summary For Policymakers. Can. J. Emerg. Manag. 2022, 2. [Google Scholar] [CrossRef]
- Dechamps, P. The IEA World Energy Outlook 2022—A brief analysis and implications. Eur. Energy Clim. J. 2023, 11, 100–103. [Google Scholar] [CrossRef]
- Dong, M.; Gong, H.; Sang, Q.; Zhao, X.; Zhu, C. Review of CO2-Kerogen Interaction and Its Effects on Enhanced Oil Recovery and Carbon Sequestration in Shale Oil Reservoirs. Resour. Chem. Mater. 2022, 1, 93–113. [Google Scholar] [CrossRef]
- Pearce, J.; Raza, S.; Baublys, K.; Hayes, P.; Firouzi, M.; Rudolph, V. Unconventional CO2 Storage: CO2 Mineral Trapping Predicted in Characterized Shales, Sandstones, and Coal Seam Interburden. SPE J. 2022, 27, 3218–3239. [Google Scholar] [CrossRef]
- Sanguinito, S.; Goodman, A.; Tkach, M.; Kutchko, B.; Culp, J.; Natesakhawat, S.; Fazio, J.; Fukai, I.; Crandall, D. Quantifying Dry Supercritical CO2-Induced Changes of the Utica Shale. Fuel 2018, 226, 54–64. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Xing, H.; Zou, Y. CO2–Brine–Rock Interactions Altering the Mineralogical, Physical, and Mechanical Properties of Carbonate-Rich Shale Oil Reservoirs. Energy 2022, 256, 124608. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 20. [Google Scholar] [CrossRef]
- Jiang, X. A Review of Physical Modelling and Numerical Simulation of Long-Term Geological Storage of CO2. Appl. Energy 2011, 88, 3557–3566. [Google Scholar] [CrossRef]
- Bielinski, A. Numerical Simulation of CO2 Sequestration in Geological Formations; Institut für Wasserbau, Universität Stuttgart: Stuttgart, Germany, 2007; ISBN 978-3-933761-58-3. [Google Scholar]
- Ehlig-Economides, C.A. Geologic Carbon Dioxide Sequestration Methods, Opportunities, and Impacts. Curr. Opin. Chem. Eng. 2023, 42, 100957. [Google Scholar] [CrossRef]
- Anderson, S.T.; Jahediesfanjani, H. Estimating the Pressure-Limited Dynamic Capacity and Costs of Basin-Scale CO2 Storage in a Saline Formation. Int. J. Greenh. Gas Control. 2019, 88, 156–167. [Google Scholar] [CrossRef]
- Shukla, R.; Ranjith, P.; Haque, A.; Choi, X. A Review of Studies on CO2 Sequestration and Caprock Integrity. Fuel 2010, 89, 2651–2664. [Google Scholar] [CrossRef]
- Olabode, A.; Radonjic, M. Shale Caprock/Acidic Brine Interaction in Underground CO2 Storage. J. Energy Resour. Technol. 2014, 136, 042901. [Google Scholar] [CrossRef]
- Zhou, X.; Sang, S.; Niu, Q.; Zhang, K.; Liu, F.; Wang, W.; Chang, J. Changes of Multiscale Surface Morphology and Pore Structure of Mudstone Associated with Supercritical CO2-Water Exposure at Different Times. Energy Fuels 2021, 35, 4212–4223. [Google Scholar] [CrossRef]
- Gao, H.; Xie, Y.; Cheng, Z.; Wang, C.; Li, T.; Zhu, X.; Luo, K.; Cao, J.; Li, N. A Minireview of the Influence of CO2 Injection on the Pore Structure of Reservoir Rocks: Advances and Outlook. Energy Fuels 2023, 37, 118–135. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, M.; Sun, X.; Liu, B.; Ostadhassan, M.; Huang, W.; Chen, X.; Pan, Z. Pore Network Characterization of Shale Reservoirs Through State-of-the-Art X-Ray Computed Tomography: A Review. Gas Sci. Eng. 2023, 113, 204967. [Google Scholar] [CrossRef]
- Carpenter, K.C.; Dje, L.B.; Achang, M.; Radonjic, M. Comparative Laboratory Study of the Geochemical Reactivity of the Marcellus Shale: Rock–Fluid Interaction of Drilled Core Samples vs. Outcrop Specimens. Water 2023, 15, 1940. [Google Scholar] [CrossRef]
- Loring, J.S.; Qafoku, O.; Thompson, C.J.; McNeill, A.S.; Vasiliu, M.; Dixon, D.A.; Miller, Q.R.S.; McGrail, B.P.; Rosso, K.M.; Ilton, E.S.; et al. Synergistic Coupling of CO2 and H2O during Expansion of Clays in Supercritical CO2–CH4 Fluid Mixtures. Environ. Sci. Technol. 2021, 55, 11192–11203. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Zou, R.; Zou, R.; Huang, L.; Liu, Y.; Meng, Z.; Wang, Z.; Lei, H. A Systematic Review of CO2 Injection for Enhanced Oil Recovery and Carbon Storage in Shale Reservoirs. Int. J. Hydrogen Energy 2023, 48, 37134–37165. [Google Scholar] [CrossRef]
- Chang, X.; Lin, S.; Yang, C.; Wang, K.; Liu, S.; Guo, Y. A Critical Review of ScCO2-Enhanced Gas Recovery and Geologic Storage in Shale Reservoirs. Gas Sci. Eng. 2024, 125, 205317. [Google Scholar] [CrossRef]
- Awejori, G.A.; Dong, W.; Doughty, C.; Spycher, N.; Radonjic, M. Mineral and Fluid Transformation of Hydraulically Fractured Shale: Case Study of Caney Shale in Southern Oklahoma. Geomech. Geophys. Geo-energ. Geo-resour. 2024, 10, 128. [Google Scholar] [CrossRef]
- Ma, J.-F.; Li, L.; Wang, H.-F.; Tan, M.-Y.; Cui, S.-L.; Zhang, Y.-Y.; Qu, Z.-P.; Jia, L.-Y.; Zhang, S.-H. Geophysical Monitoring Technology for CO2 Sequestration. Appl. Geophys. 2016, 13, 288–306. [Google Scholar] [CrossRef]
- Knauss, K.G.; Johnson, J.W.; Steefel, C.I. Evaluation of the Impact of CO2, Co-Contaminant Gas, Aqueous Fluid and Reservoir Rock Interactions on the Geologic Sequestration of CO2. Chem. Geol. 2005, 217, 339–350. [Google Scholar] [CrossRef]
- Tapriyal, D.; Haeri, F.; Crandall, D.; Horn, W.; Lun, L.; Lee, A.; Goodman, A. Caprock Remains Water Wet Under Geologic CO2 Storage Conditions. Geophys. Res. Lett. 2024, 51, e2024GL109123. [Google Scholar] [CrossRef]
- Choo, T.K.; Etschmann, B.; Selomulya, C.; Zhang, L. Behavior of Fe2+/3+ Cation and Its Interference with the Precipitation of Mg2+ Cation upon Mineral Carbonation of Yallourn Fly Ash Leachate under Ambient Conditions. Energy Fuels 2016, 30, 3269–3280. [Google Scholar] [CrossRef]
- Stubbs, A.R.; MacDonald, J.; Neill, I. Mechanisms of Secondary Carbonate Precipitation on Felsic, Intermediate and Mafic Igneous Rocks: A Case Study for NW Scotland. Scott. J. Geol. 2024, 60, sjg2024-003. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, S.; Xian, X.; Zheng, Y.; Yang, K.; Liu, J. Comprehensive Review of Property Alterations Induced by CO2–Shale Interaction: Implications for CO2 Sequestration in Shale. Energy Fuels 2022, 36, 8066–8080. [Google Scholar] [CrossRef]
- Olabode, A.; Radonjic, M. Geochemical Markers in Shale-CO2 Experiment at Core Scale. Energy Procedia 2017, 114, 3840–3854. [Google Scholar] [CrossRef]
- Goodman, A.; Fukai, I.; Dilmore, R.; Frailey, S.; Bromhal, G.; Soeder, D.; Gorecki, C.; Peck, W.; Rodosta, T.; Guthrie, G. Methodology for Assessing CO2 Storage Potential of Organic-Rich Shale Formations. Energy Procedia 2014, 63, 5178–5184. [Google Scholar] [CrossRef]
- Dje, L.B.; Awejori, G.A.; Radonjic, M. Comparison of Geochemical Reactivity of Marcellus and Caney Shale Based on Effluent Analysis. In Proceedings of the 58th U.S. Rock Mechanics/Geomechanics Symposium, Golden, CO, USA, 23–26 June 2024; p. D041S053R004. [Google Scholar]
- Fatah, A.; Bennour, Z.; Ben Mahmud, H.; Gholami, R.; Hossain, M.M. A Review on the Influence of CO2/Shale Interaction on Shale Properties: Implications of CCS in Shales. Energies 2020, 13, 3200. [Google Scholar] [CrossRef]
- Peter, A.; Yang, D.; Eshiet, K.I.-I.I.; Sheng, Y. A Review of the Studies on CO2–Brine–Rock Interaction in Geological Storage Process. Geosciences 2022, 12, 168. [Google Scholar] [CrossRef]
- Song, Y.; Yang, L.; Wang, H.; Sun, X.; Bai, S.; Wang, N.; Liang, J.; Zhou, L. The Coupling Reaction of Fe2+ Bio-Oxidation and Resulting Fe3+ Hydrolysis Drastically Improve the Formation of Iron Hydroxysulfate Minerals in AMD. Environ. Technol. 2021, 42, 2325–2334. [Google Scholar] [CrossRef]
- Ghosh, S.; Adsul, T.; Varma, A.K. Organic Matter and Mineralogical Acumens in CO2 Sequestration. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Adetunji, C.O., Asiri, A.M., Eds.; Elsevier: Leiden, The Netherlands, 2023; pp. 561–594. ISBN 978-0-323-99429-3. [Google Scholar]
- Zhou, Q.; Liu, J.; Ma, B.; Li, C.; Xiao, Y.; Chen, G.; Lyu, C. Pyrite Characteristics in Lacustrine Shale and Implications for Organic Matter Enrichment and Shale Oil: A Case Study from the Triassic Yanchang Formation in the Ordos Basin, NW China. ACS Omega 2024, 9, 16519–16535. [Google Scholar] [CrossRef]
- Adua Awejori, G.; Doughty, C.; Xiong, F.; Paronish, T.; Spycher, N.; Radonjic, M. Integrated Experimental and Modeling Study of Geochemical Reactions of Simple Fracturing Fluids with Caney Shale. Energy Fuels 2022, 36, 10064–10081. [Google Scholar] [CrossRef]
- Katende, A.; Rutqvist, J.; Benge, M.; Seyedolali, A.; Bunger, A.; Puckette, J.O.; Rhin, A.; Radonjic, M. Convergence of Micro-Geochemistry and Micro-Geomechanics towards Understanding Proppant Shale Rock Interaction: A Caney Shale Case Study in Southern Oklahoma, USA. J. Nat. Gas Sci. Eng. 2021, 96, 104296. [Google Scholar] [CrossRef]
- Geisler, T.; Dohmen, L.; Lenting, C.; Fritzsche, M.B.K. Real-Time in Situ Observations of Reaction and Transport Phenomena during Silicate Glass Corrosion by Fluid-Cell Raman Spectroscopy. Nat. Mater. 2019, 18, 342–348. [Google Scholar] [CrossRef]
- Massion, C.; Vissa, V.S.K.; Lu, Y.; Crandall, D.; Bunger, A.; Radonjic, M. Geomimicry-Inspired Micro-Nano Concrete as Subsurface Hydraulic Barrier Materials: Learning from Shale Rocks as Best Geological Seals. In Proceedings of the REWAS 2022: Energy Technologies and CO2 Management (Volume II); Tesfaye, F., Zhang, L., Guillen, D.P., Sun, Z., Baba, A.A., Neelameggham, N.R., Zhang, M., Verhulst, D.E., Alam, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–138. [Google Scholar]
- Vialle, S.; Ajo-Franklin, J.; Carey, J.W. Geological Carbon Storage: Subsurface Seals and Caprock Integrity; John Wiley & Sons: New York, NY, USA, 2018; ISBN 978-1-119-11867-1. [Google Scholar]
- Darkwah-Owusu, V.; Yusof, M.A.M.; Sokama-Neuyam, Y.A.; Turkson, J.N.; Fjelde, I. A Comprehensive Review of Remediation Strategies for Mitigating Salt Precipitation and Enhancing CO2 Injectivity during CO2 Injection into Saline Aquifers. Sci. Total Environ. 2024, 950, 175232. [Google Scholar] [CrossRef]
| Author(s) | Focus | Research Gaps |
|---|---|---|
| [17,18] | Numerical Simulations of CO2 in Geological Settings | Limited empirical data on physicochemical interactions at the mineralogical level in shales. Need for experimental validation of simulated predictions and theoretical models. |
| [19,20] | Geologic Carbon Sequestration Review | High costs and energy requirements for CO2 capture; need for cost reduction and efficiency enhancement. |
| [3,5,17,21,22] | Caprock Integrity and Fracture Dynamics | Need for long-term studies need to understand the evolution of fissures under continuous CO2 flow. The importance of considering hydrological factors in geological stability assessments. |
| [2,23,24,25] | Pore Structure Alterations | Microscale and nanoscale analysis, shale-specific studies, and controlled experiments are vital to assess structural changes and ensure long-term CO2 storage integrity. |
| [5,7,14,26,27] | Subcritical and Supercritical CO2 Effects on Shale | Robust simulations and further studies are essential to understand shale sensitivity to CO2 under varying conditions and optimize EOR strategies. |
| [28,29,30,31] | CO2 Storage Capacity and Monitoring | Targeted modeling, localized studies, and field validation are essential to predict CO2–shale interactions, refine capacity estimates, and assess long-term storage risks. |
| [5,15,26,27,32,33] | Impact of CO2—Rock Interactions | Comprehensive experimental and modeling studies are needed to understand shale reactivity, nanoconfinement, water-chemistry interactions, and long-term CO2 impacts across diverse geological settings. |
| Phase | Main Elements (wt.%) |
|---|---|
| Quartz | Si (34.5), O (49.9), Fe (6.0), C (3.9), Al (2.9) |
| Organic Matter (OM) | C (43.0), O (34.5), Si (16.6), Ca (2.6), Al (2.0) |
| Calcite | Ca (21.3), O (52.0), Si (16.3), C (6.3), Al (2.0) |
| Pyrite | Fe (35.8), S (24.1), O (20.8), Si (12.0), C (3.2) |
| Illite | Si (26.6), O (50.9), Al (10.0), K (3.5), Fe (3.0) |
| Dolomite | Ca (31.5), Mg (20.4), O (45.1), C (2.1) |
| Kaolinite | Si (29.5), O (52.7), Al (9.0), C (3.7), Fe (1.5) |
| Paragonite | Na (4.5), Al (12.6), Si (31.0), O (48.0), Fe (1.2) |
| Wollastonite | Ca (22.0), Si (28.5), O (44.0), C (3.5), minor Al |
| Ankerite | Ca (22.5), Fe (12.4), Mg (10.2), C (5.8), O (49.1) |
| Albite | Na (6.8), Al (19.1), Si (35.2), O (37.5), trace Ca |
| Jarosite | K (4.5), Fe (23.4), S (13.2), O (50.0), OH present |
| Anhydrite | Ca (26.2), S (18.0), O (55.8) |
| Ionic Species | Primary Mineral Phase Sources | Facies Observed | Post-Exposure Observation (Quantified in wt.%) | Possible Geochemical Path |
|---|---|---|---|---|
| K+ | K-feldspar, Illite | R1, R2, D1, D2, R3 | K-feldspar reduction (13.6 to 7–10%); slight Illite shift | Leaching from feldspars and clay edges |
| Na+ | Albite | R1, R3, D2 | Minor Albite decline (5.0% to 2.6–3.4%) | Limited Na+ exchange |
| Ca2+ | Calcite, Dolomite, Ankerite | R1, R2, R3, D1, D2 | Redistribution among carbonate phases; net Ca2+ preserved | Partial dissolution and re-precipitation |
| Mg2+ | Dolomite, Illite, Ankerite | R2, R3, D2, D1 | Mg-bearing carbonates reduced; Dolomite often retained | Phase transition and reallocation |
| Fe2+/Fe3+ | Pyrite, Illite, Ankerite | D1, D2, R2, R3 | Pyrite decreased (up to 50%); Fe detected near former grains | Oxidation and surface destabilization |
| SO42− | Anhydrite, Pyrite | R1, D1, D2, R2, R3 | Anhydrite loss, S redistributed | Sulfate release from dissolution/oxidation |
| Al3+ | K-feldspar, Albite, Illite | All facies | No significant compositional change | Structurally retained in aluminosilicates |
| Si4+ | Quartz, K-feldspar, Illite, Albite | All facies | Quartz (~24–25%) stable throughout | Framework remains chemically inert |
| C (elemental) CO32− | Calcite, Dolomite, Ankerite | All facies | Carbon and carbonates retained via phase shifts, not net loss | Re-precipitation or phase conversion |
| S (Elemental) | Pyrite, Anhydrite | D2, R2, R3 | Sulfur detected post-Anhydrite; diffused spatially | Sulfate migration from sulfates/sulfides |
| P/PO43− | Apatite, trace organics | D2, R3 (trace levels) | Stable in isolated inclusions | Largely inert under dry CO2 |
| Mineral Phase | Chemical Formula | Rationale in CCUS | Occurrence in Shales | Relevance to CCUS |
|---|---|---|---|---|
| Calcite | CaCO3 | Forms during CO2 sequestration via reaction with calcium-bearing minerals. | Common carbonate mineral in shales. | Relevant due to carbonate precipitation under CO2-rich conditions. |
| Dolomite | CaMg(CO3)2 | Forms from interactions of CO2 with calcium and magnesium-rich minerals. | Present in some shale formations; associated with carbonate deposits. | Plays a role in carbonate mineralization under CO2 sequestration. |
| Magnesite | MgCO3 | Forms when CO2 reacts with magnesium-bearing minerals. | Rare in shales, mainly found in magnesium-rich environments. | Forms stable carbonate phases during CO2 sequestration. |
| Siderite | FeCO3 | Iron carbonate that forms in CO2-rich environments. | Occasionally found in Fe-rich shales, but more common in sedimentary rocks. | Can store CO2 in carbonate form but limited occurrence in shales. |
| Quartz | SiO2 | Stable silicate mineral in shales, largely unreactive to CO2. | Common silicate mineral in shales, a major constituent of sandstones. | Mechanically stable but chemically inert under CO2 exposure. |
| Illite | (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2 | Clay mineral influencing shale porosity and permeability under CO2 exposure. | Frequent in shales as a clay mineral affecting permeability. | Affects shale permeability and reactivity with CO2. |
| Montmorillonite | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O | Swelling clay mineral that absorbs CO2, altering shale properties. | Found in clay-rich shales, particularly those with high swelling potential. | Modifies pore structure and water retention upon CO2 exposure. |
| Kaolinite | Al2Si2O5(OH)4 | Clay mineral with minor interactions with CO2. | Occurs in some shales but not a dominant mineral. | Minor role in CO2 reactivity, mainly affects shale composition. |
| Ankerite | Ca(Fe2+,Mg,Mn)(CO3)2 | Iron and magnesium carbonate forming under CO2 sequestration conditions. | Found in iron-rich sedimentary formations, including some shales. | Potentially relevant for mineral trapping of CO2. |
| Chlorite | (Mg,Fe2+,Fe3+,Al)6(Si,Al)4O10(OH)8 | Clay mineral influencing CO2-induced alterations in shales. | Occurs in some shales, affecting fluid interactions. | Affects CO2-rock interactions by modifying clay stability. |
| Pyrite | FeS2 | Common sulfide in shales, oxidizing under CO2 influence. | Common in organic-rich shales, particularly those with high sulfur content. | Oxidation influences acid generation, affecting mineral trapping. |
| Feldspar | KAlSi3O8—NaAlSi3O8—CaAl2Si2O8 | Silicate mineral that weathers in CO2 environments. | Common framework silicate mineral in various shales. | Minor role in CO2 sequestration; undergoes limited chemical change. |
| Hematite | Fe2O3 | Iron oxide that forms from pyrite oxidation during CO2 sequestration. | Minor iron oxide phase in shales formed from oxidation processes. | May form secondary precipitates upon CO2 exposure. |
| Anhydrite | CaSO4 | Sulfate mineral present in caprocks affecting CO2 storage integrity. | Common in evaporite-bearing shales and caprocks. | Contributes to caprock integrity in sequestration sites. |
| Anhydrite | CaSO4·2H2O | Hydrated sulfate mineral influenced by CO2-rich fluids. | Hydrated form of anhydrite, often found in caprocks overlying shales. | Influences CO2 migration in formations containing gypsum. |
| Halite | NaCl | Salt mineral forming low-permeability barriers in caprocks. | Evaporite mineral occasionally present in shale formations. | Enhances caprock sealing potential, reducing CO2 leakage. |
| Serpentine | (Mg,Fe)3Si2O5(OH)4 | Silicate mineral reacting with CO2 to form magnesite. | Occurs in some altered shales with high magnesium content. | Can interact with CO2 under specific geochemical conditions. |
| Olivine | (Mg,Fe)2SiO4 | Silicate mineral reacting with CO2 to facilitate mineral sequestration. | Found in ultramafic environments but rare in shales. | Minor direct role in CO2 sequestration in shales. |
| Plagioclase | (Na,Ca)(Si,Al)4O8 | Silicate feldspar undergoing carbonation reactions with CO2. | Common in feldspar-rich shales and sandstones. | Participates in feldspar weathering reactions under CO2 influence. |
| Smectite | (Ca,Na)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O | Clay group mineral swells upon CO2 exposure, modifying rock properties. | Occurs in clay-rich shale formations, affecting fluid movement. | Clay swelling may alter CO2 migration pathways. |
| Brucite | Mg(OH)2 | Magnesium hydroxide that reacts with CO2 forming magnesite. | Rare in shales but found in magnesium-rich alteration zones. | Relevant in carbonation processes for CO2 trapping. |
| Forsterite | Mg2SiO4 | High-Mg silicate reacting with CO2 for mineral sequestration. | More common in ultramafic formations, rare in shales. | Limited relevance in shales; reacts with CO2 in ultramafic rocks. |
| Talc | Mg3Si4O10(OH)2 | Magnesium silicate that alters during CO2 interactions. | Occurs in talc-carbonate altered zones; uncommon in shales. | Plays minor role in mineral transformations in CO2 storage. |
| Mariposite | Cr-muscovite | Chromium-bearing mica associated with carbonated ultramafic rocks. | Occasionally found in altered metamorphic environments, rare in shales. | Not directly involved in CO2 trapping but alters rock properties. |
| Fuchsite | Cr-muscovite | Green, chromium-bearing mica found in carbonated environments. | Rarely found in shales; more common in metamorphic terrains. | Limited role in CO2 interactions due to mineral stability. |
| Zeolites | Mx/n [(AlO2)x(SiO2)y] · zH2O | Adsorbs CO2, enhancing storage capacity in shales. | Uncommon in natural shale formations but widely used in CO2 capture studies. | Relevant in artificial CO2 capture applications but rare in shales. |
| Muscovite | KAl2(AlSi3O10)(OH)2 | Stable mineral in shales, does not significantly react with CO2 under sequestration conditions. | Common in shales as a mica mineral, contributing to overall mineral composition. | Minimal role in CO2 sequestration due to chemical stability. |
| Jarosite | KFe3(SO4)2(OH)6 | Forms in acidic environments and is not relevant for CO2 sequestration in typical shale formations. | Not common in shales; forms in oxidizing, acidic conditions, often as a sulfide weathering product. | Not relevant for CCUS in shales due to formation constraints. |
| Dawsonite | NaAlCO3(OH)2 | Potential mineral for CO2 trapping in sandstone formations through carbonate precipitation. | Rare; more common in sandstone reservoirs where CO2 mineral trapping occurs. | Relevant in sandstone-hosted sequestration but not typically found in shale settings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dje, L.B.; Radonjic, M. Reactivity of Shale to Supercritical CO2: Insights from Microstructural Characterization and Mineral Phase Evolution in Caney Shales for CCUS Applications. Materials 2025, 18, 3382. https://doi.org/10.3390/ma18143382
Dje LB, Radonjic M. Reactivity of Shale to Supercritical CO2: Insights from Microstructural Characterization and Mineral Phase Evolution in Caney Shales for CCUS Applications. Materials. 2025; 18(14):3382. https://doi.org/10.3390/ma18143382
Chicago/Turabian StyleDje, Loic Bethel, and Mileva Radonjic. 2025. "Reactivity of Shale to Supercritical CO2: Insights from Microstructural Characterization and Mineral Phase Evolution in Caney Shales for CCUS Applications" Materials 18, no. 14: 3382. https://doi.org/10.3390/ma18143382
APA StyleDje, L. B., & Radonjic, M. (2025). Reactivity of Shale to Supercritical CO2: Insights from Microstructural Characterization and Mineral Phase Evolution in Caney Shales for CCUS Applications. Materials, 18(14), 3382. https://doi.org/10.3390/ma18143382








