Evaluation of the Alkali–Silica Reaction Potential of Korean Aggregates: Experimental Insights and Mitigation Strategies for Concrete Durability
Abstract
1. Introduction
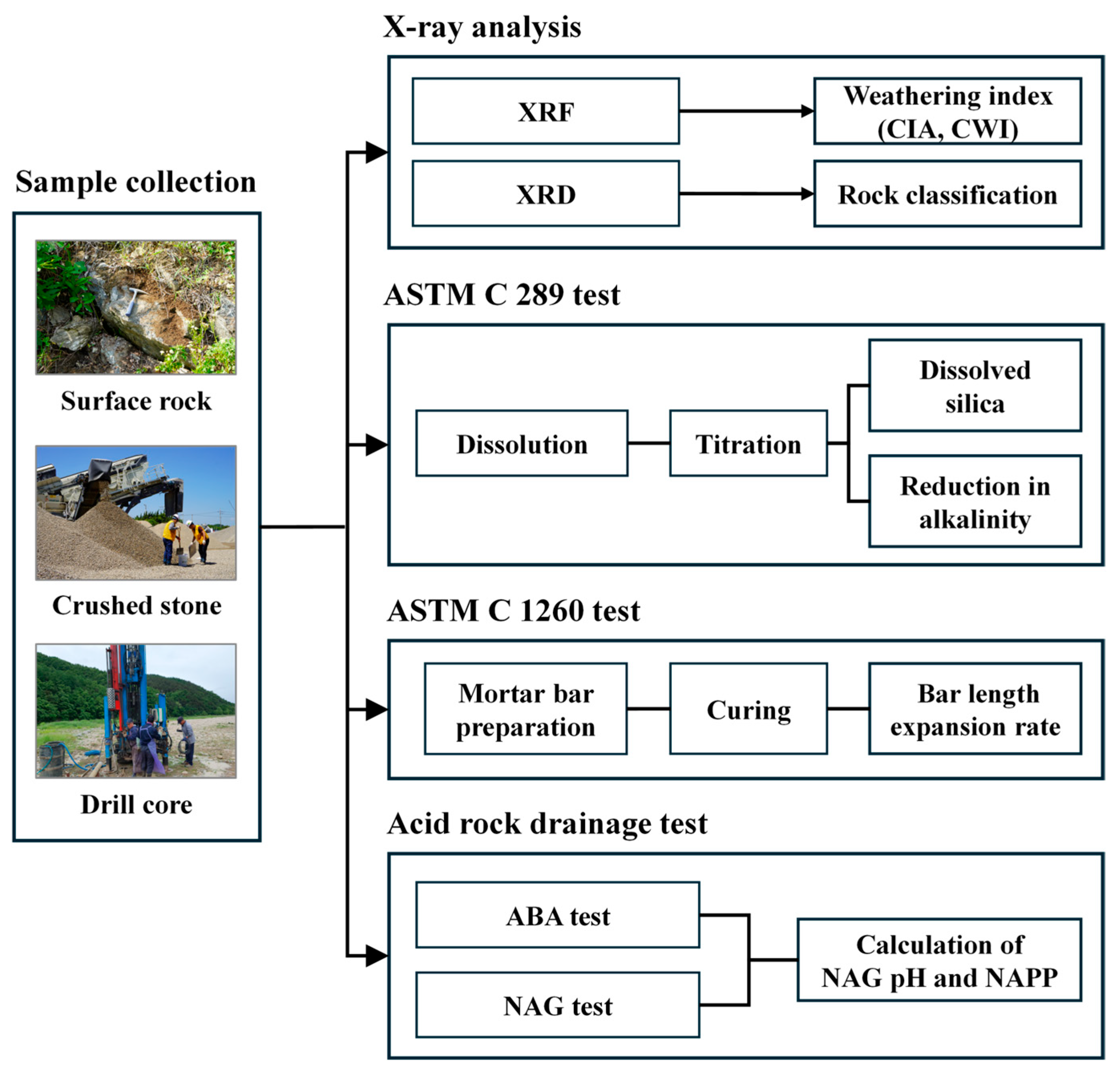
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Testing Methods
2.3. Data Analysis
3. Results
3.1. Aggregate Characterization
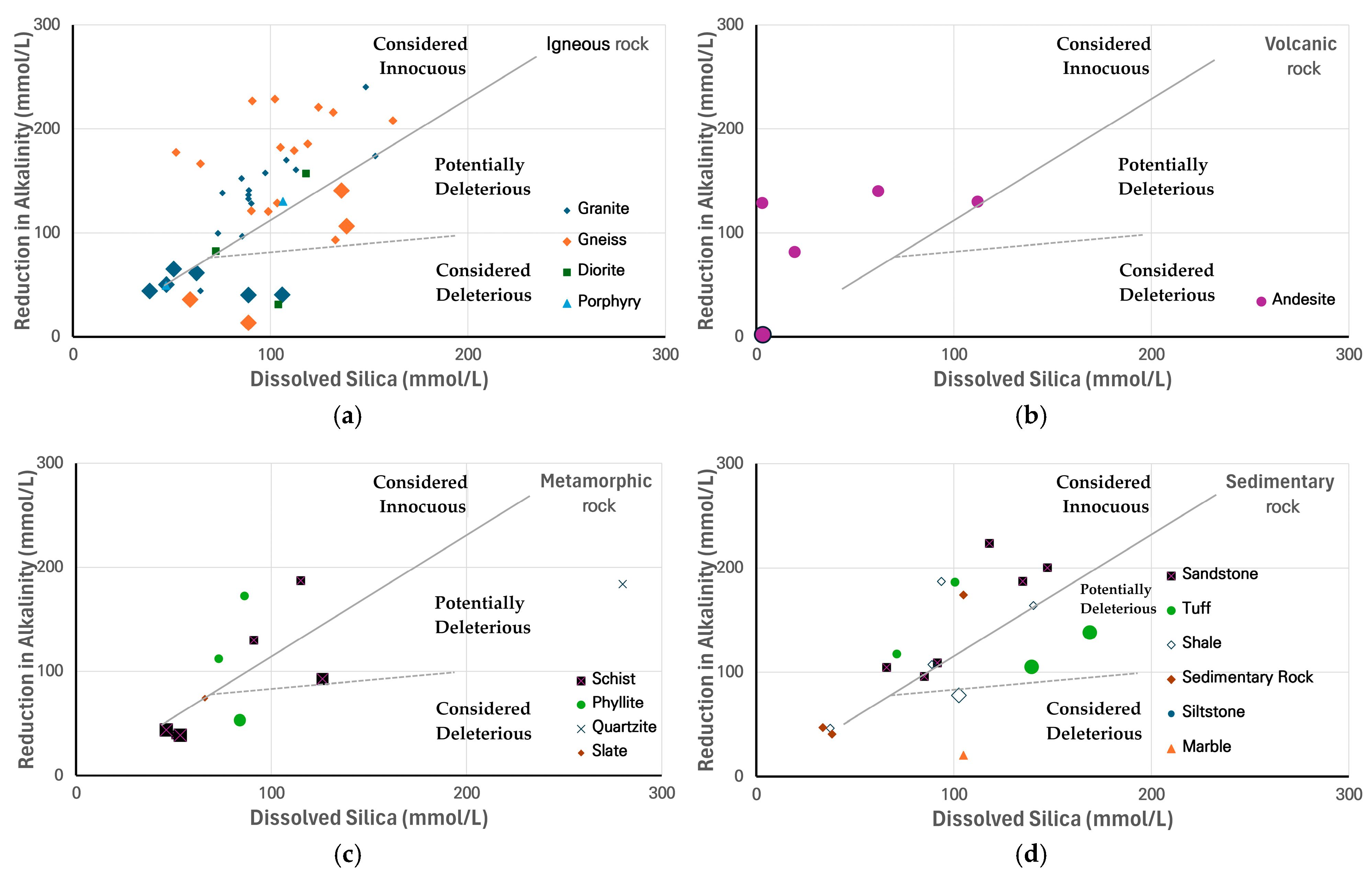
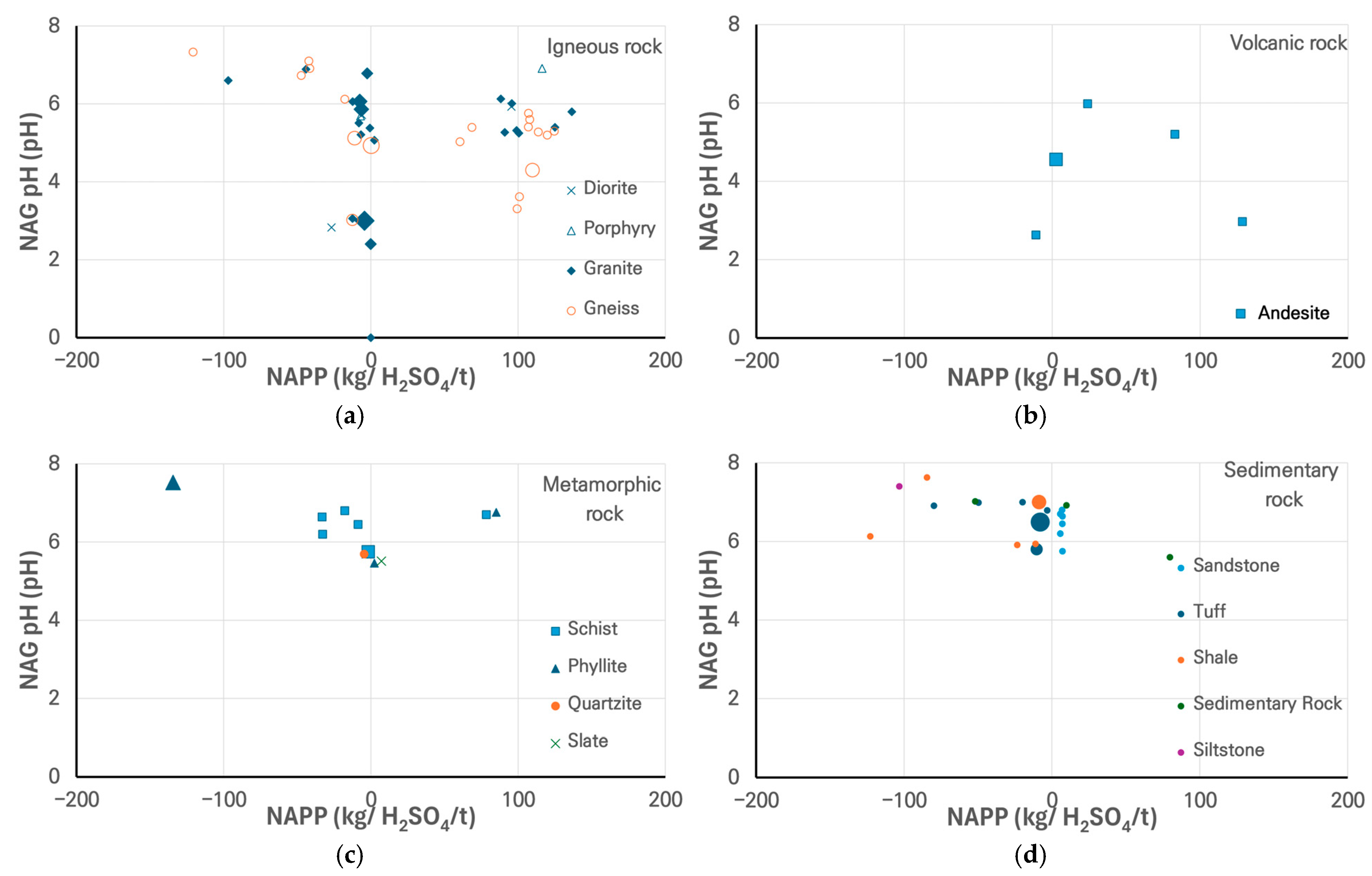
3.2. Assessment of ASR Potential
3.3. Summary of the Reactivity Trends and Anomalies
4. Discussion
4.1. Mechanisms of ASR in Korean Aggregates
4.2. Comparison with Global Studies
4.3. Implications for Concrete Durability
4.4. Mitigation Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baek, C.S.; Seo, J.H.; Kim, Y.J.; Cho, K.H.; Kim, K.K.; Lee, J.Y. A Fundamental Study on the Potential of Alkali-Aggregate Reaction according to KS F 2545 and ASTM C 1260 Test Methods. J. Korean Inst. Resour. Recycl. 2020, 29, 18–27. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-Silica Reaction (ASR) in Concrete: A Review. Case Stud. Constr. Mater. 2021, 15, e00563. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between Mechanical Properties of Concrete and Alkali–Silica Reaction (ASR): A Review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Pedersen, B.M.; Wigun, B.J.; Lindgard, J. Influence of Aggregate Particle Size on Alkali–Silica Reaction: A Literature Review. In Proceedings of the 15th International Conference on Alkali–Aggregate Reaction, São Paulo, Brazil, 3–7 July 2016; Available online: https://icaarconcrete.org/wp-content/uploads/2020/11/15ICAAR-PedersenBM-1.pdf (accessed on 24 April 2025).
- Santos, M.B.; Brito, J.D.; Cilva, A.S. A Review on Alkali–Silica Reaction Evolution in Recycled Aggregate Concrete. Materials 2020, 13, 2625. [Google Scholar] [CrossRef]
- Islam, M.S.; Akhtar, S.A. Critical Assessment of ASR Testing Methods. Can. Chem. Trans. 2013, 1, 253–266. [Google Scholar] [CrossRef]
- Fournier, B.; Bérubé, M.A.; Folliard, K.J.; Thomas, M. Report on the Diagnosis, Prognosis, and Mitigation of Alkali–Silica Reaction (ASR) in Transportation Structures; U.S. Department of Transportation, Federal Highway Administration: Washington, DC, USA, 2010; pp. 11–20. Available online: https://www.fhwa.dot.gov/pavement/concrete/pubs/hif09004/hif09004.pdf (accessed on 24 April 2025).
- Ministry of Land, Infrastructure and Transport. Aggregate Resource Information Management System (AGRIS)–Report on Aggregate Resource Survey. Available online: https://www.agris.go.kr/agris2/info/new_report.do (accessed on 24 April 2025).
- Yun, K.K.; Hong, S.S.; Han, S.H.; Kim, S.K. Evaluation of Alkali–Silica Reactivity for Aggregates in Korea according to Test Methods. J. Korea Concr. Inst. 2008, 20, 689–696. [Google Scholar] [CrossRef]
- You, B.H.; Baek, C.S.; Joo, K.Y. Physical Properties of Volcanic Rocks in Jeju–Ulleung Area as Aggregates. Econ. Environ. Geol. 2024, 57, 205–217. [Google Scholar] [CrossRef]
- KS F 2545; Method of Test for Potential Alkali-Silica Reactivity of Aggregates—Chemical Method. Korean Standards Association: Seoul, Republic of Korea, 2022.
- ASTM C 289-07; Standard Test Method for Potential Alkali-Silica Reactivity of Aggregates (Chemical Method). ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM C1260-22; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2022.
- Smart, R.; Skinner, W.; Levay, G.; Gerson, A.; Thomas, J.; Schumann, R.; Weisener, C.; Weber, P.; Miller, S. ARD Test Handbook: Prediction and Kinetic Control of Acid Mine Drainage; AMIRA International: Melbourne, Australia, 2002; Project P387A. [Google Scholar]
- Yun, K.K.; Hong, S.S.; Han, S.H. Expansion Behavior of Aggregate of Korea due to Alkali–Silica Reaction Using ASTM C 1260 Method. J. Korea Concr. Inst. 2008, 20, 431–437. [Google Scholar] [CrossRef]
- Rajabipour, F. Alkali–Silica Reaction: Current Understanding of the Reaction Mechanisms and the Knowledge Gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B. Role of Aluminum and Lithium in Mitigating Alkali–Silica Reaction: A Review. Front. Mater. 2021, 8, 796396. [Google Scholar] [CrossRef]
- Hong, S.H. A Case Study for Deterioration due to Alkali-Silica Reaction in the Cement Concrete Pavement. J. Korea Concr. Inst. 2006, 18, 355–360. [Google Scholar] [CrossRef]
- Bakare, A.T.; Alexander, M.G. Use of Metakaolin as Supplementary Cementitious Material in Concrete. RILEM Tech. Lett. 2019, 4, 89–102. [Google Scholar] [CrossRef]
- Gillott, J.E.; Wang, H. Improved Control of Alkali–Silica Reaction by Combined Use of Admixtures. Cem. Concr. Res. 1993, 23, 973–980. [Google Scholar] [CrossRef]
- Gregerová, M.; Štulířová, J.; Frýbort, A.; Grošek, J. Impact of light mica on the intensity of the alkali-silica reactions in cement concrete pavements containing cataclased granite aggregates. Case Stud. Constr. Mater. 2024, 21, e03521. [Google Scholar] [CrossRef]
- Nixon, P.J.; Sims, I. RILEM Recommended Test Method: AAR-2—Detection of Potential Alkali-Reactivity—Accelerated Mortar-Bar Test Method for Aggregates. In RILEM State-of-the-Art Reports; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–77. [Google Scholar]
- KS F 2507; Test Method for Soundness of Aggregates by Use of Sodium Sulfate. Korean Standards Association: Seoul, Republic of Korea, 2024.
- Olajide, O.; Nokken, R.; Sanchez, L.F.M. Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature. Materials 2023, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Figueira, R.B.; Sousa, R.; Coelho, L.; Azenha, M.; Almeida, J.M.D.; Jorge, P.A.S.; Silva, A.S. Alkali-silica reaction in concrete: Mechanisms, mitigation and test methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Francklin, I.; Ribeiro, R.P.; Corrêa, F.A. Quartzite Mining Waste: Diagnosis of ASR Alkali–Silica Reaction in Mortars and Portland Cement Concrete. Materials 2021, 14, 7642. [Google Scholar] [CrossRef] [PubMed]
- Šachlová, S.; Kuchařová, A.; Pertold, Z.; Přikryl, R.; Fridrichová, M. Quantitative Assessment of Alkali–Silica Reaction Potential of Quartz-Rich Aggregates: Comparison of chemical test and accelerated mortar bar test improved by SEM-PIA. Bull. Eng. Geol. Environ. 2017, 76, 133–144. [Google Scholar] [CrossRef]
- Mohamed, O.A. A Review of Durability and Strength Characteristics of Alkali–Activated Slag Concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef]
- Piccinali, A.; Diotti, A.; Plizzari, G.; Sorlini, S. Impact of Recycled Aggregate on the Mechanical and Environmental Properties of Concrete: A Review. Materials 2022, 15, 1818. [Google Scholar] [CrossRef] [PubMed]
- Behravan, A.; Arce, G.; Ozyildirim, H.C.; Spradley, E.; Davenport, C. Repair and Treatment of Alkali–Silica Reaction (ASR)-Affected Transportation Infrastructures: Review and Interview. Infrastructures 2025, 10, 101. [Google Scholar] [CrossRef]
- Nikmehr, B.; Al-Ameri, R.A. State-of-the-Art Review on the Incorporation of Recycled Concrete Aggregates in Geopolymer Concrete. Recycling 2022, 7, 51. [Google Scholar] [CrossRef]
- Wongpaun, A.; Tangchirapat, W.; Suwan, T.; Fan, M. Factors affecting compressive strength and expansion due to alkali-silica reaction of fly ash-based alkaline activated mortar. Case Stud. Constr. Mater. 2023, 19, e02595. [Google Scholar] [CrossRef]


| Rock Classification | Samples | ||
|---|---|---|---|
| Igneous | Granite | Granite | 17 |
| Biotite granite | 2 | ||
| Porphyritic granite | 1 | ||
| Gneissic granite | 2 | ||
| Gneiss | Gneiss | 4 | |
| Banded gneiss | 5 | ||
| Porphyroblastic gneiss | 2 | ||
| Granite gneiss | 2 | ||
| Granitic gneiss | 4 | ||
| Migmatitic gneiss | 2 | ||
| Diorite | Diorite | 2 | |
| Granodiorite | 1 | ||
| Porphyry | Granite porphyry | 1 | |
| Quartz porphyry | 1 | ||
| Volcanic | Andesite | Andesite | 1 |
| Microcrystalline andesite | 1 | ||
| Tuffaceous andesite | 1 | ||
| Andesite rocks | 2 | ||
| Metamorphic | Schist | Schist | 5 |
| Siliceous schist | 1 | ||
| Phyllite | Phyllite | 1 | |
| Sand-rich phyllite | 1 | ||
| Calcite-rich phyllite | 1 | ||
| Quartzite | Quartzite | 1 | |
| Slate | Slate | 1 | |
| Sedimentary | Sandstone | Sandstone | 5 |
| Sandstone rocks | 1 | ||
| Tuff | Tuff | 6 | |
| Shale | Shale | 4 | |
| Shale rocks | 1 | ||
| Sedimentary Rock | Granitic sedimentary rocks | 1 | |
| Sand-rich sedimentary rocks | 1 | ||
| Metamorphic sedimentary rocks | 1 | ||
| Siltstone | Siltstone | 1 | |
| Marble | Marble | 1 | |
| Total | 84 | ||
| Sample Name | Rock Type | Composition Minerals |
|---|---|---|
| 24JP-MG05 | Phyllite | Biotite, Ca-plagioclase, Quartz |
| 24GS-MG02 | Gneiss | Muscovite, Quartz, Pennantite, Albite, Biotite |
| 24ES-MG05 | Shale | Muscovite, Quartz, Albite, Calcite, Pennantite, Hematite |
| 23NS-MG06 | Schist | Quartz, Muscovite, Biotite, Pennantite, Calcite |
| 23BA-MG03 | Granite | Albite, Quartz, Microcline, Muscovite, Pennantite, Biotite, Magnetite, Calcite |
| 22PC-MG24 | Marble | Calcite, Dolomite, Serpentine |
| 22PC-MG20 | Granite | Plagioclase, Quartz, K-feldspar, Muscovite, Biotite |
| 22PC-MG19 | Diorite | Plagioclase, Quartz, Chlorite, Biotite, Hornblende |
| 22MJ-MG05 | Granite | Plagioclase, Quartz, K-feldspar, Muscovite, Chlorite |
| 22DDC-MG03 | Gneiss | Plagioclase, Quartz, Muscovite, Biotite |
| 21YP-MG06 | Gneiss | Plagioclase, Quartz, Pennantite, Biotite, Garnet |
| 21KJ-MG20 | Tuff | Plagioclase, Quartz, K-feldspar, Chlorite, Epidote |
| 21KJ-MG14 | Tuff | Plagioclase, Quartz, K-feldspar, Muscovite, Hematite, Chlorite |
| 21KJ-MG01 | Schist | Quartz, Plagioclase, Muscovite, K-feldspar |
| 21ICN-MG08 | Schist | Biotite, Plagioclase, K-feldspar, Quartz |
| 21CJ-MG06 | Gneiss | K-feldspar, Pennantite, Magnetite, Hematite, Biotite |
| 21AS-MG04 | Gneiss | Quartz, Muscovite |
| 24GJ-MG08 | Andesite | Quartz, Albite, Pennantite, Muscovite, Orthoclase, Calcite |
| 23YD-MG04 | Granite | Quartz, Orthoclase, Hornblende, Biotite, Pennantite, Magnetite |
| 23YD-MG01 | Granite | Albite, Quartz, Hornblende, Microcline, Pennantite |
| 22IC-MG04 | Granite | Plagioclase, Quartz, K-feldspar, Muscovite, Pennantite, Biotite |
| Sample Name | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO | P2O5 | Lg. Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24JP-MG05 | 59.9 | 15.35 | 6.38 | 6.58 | 3.95 | 3.27 | 0.86 | 0.73 | 0.09 | 0.15 | 1.74 |
| 24GS-MG02 | 59.03 | 18.82 | 7.35 | 0.3 | 2.91 | 4.61 | 1.06 | 0.81 | 0.07 | 0.05 | 3.84 |
| 24ES-MG05 | 61.82 | 14.21 | 5.56 | 3.34 | 2.88 | 3.44 | 1.52 | 0.8 | 0.06 | 0.16 | 5.91 |
| 23NS-MG06 | 69.41 | 11.95 | 3.46 | 5.23 | 3.36 | 3.12 | 0.46 | 0.65 | 0.07 | 0.13 | 1.61 |
| 23BA-MG03 | 72.04 | 13.85 | 1.76 | 1.64 | 0.36 | 3.95 | 3.92 | 0.29 | 0.02 | 0.08 | 1.43 |
| 22PC-MG24 | 5.49 | 1.24 | 0.5 | 45.88 | 7.07 | 0.29 | 0.02 | 0.07 | 0.02 | 0.01 | 38.53 |
| 22PC-MG20 | 76.18 | 13.08 | 0.91 | 0.71 | 0.05 | 4.78 | 3.65 | 0.06 | 0.04 | 0.02 | 0.32 |
| 22PC-MG19 | 50.09 | 17.98 | 10.36 | 8.07 | 4.45 | 2.14 | 2.83 | 1.64 | 0.15 | 0.32 | 1.06 |
| 22MJ-MG05 | 78.55 | 11.45 | 1.49 | 0.32 | 0.27 | 4.91 | 2.14 | 0.05 | 0.03 | 0.04 | 0.65 |
| 22DDC-MG03 | 64.49 | 16.79 | 5.54 | 3.0 | 1.96 | 2.90 | 2.92 | 0.49 | 0.13 | 0.08 | 0.77 |
| 21YP-MG06 | 68.98 | 13.97 | 5.46 | 3.07 | 1.87 | 1.26 | 3.29 | 0.53 | 0.08 | 0.1 | 0.96 |
| 21KJ-MG20 | 71.81 | 13.45 | 2.86 | 1.66 | 0.61 | 3.85 | 4.04 | 0.29 | 0.1 | 0.09 | 0.86 |
| 21KJ-MG14 | 72.77 | 13.04 | 2.77 | 0.79 | 0.28 | 4.81 | 4.09 | 0.2 | 0.09 | 0.04 | 0.64 |
| 21KJ-MG01 | 78.31 | 10.53 | 2.47 | 1.3 | 1.13 | 3.76 | 0.68 | 0.34 | 0.04 | 0.06 | 1.64 |
| 21ICN-MG08 | 59.81 | 15.4 | 7.97 | 3.86 | 2.23 | 3.71 | 3.41 | 1.53 | 0.11 | 0.66 | 0.89 |
| 21CJ-MG06 | 64.97 | 15.15 | 6.17 | 1.9 | 1.01 | 5.41 | 3.3 | 0.78 | 0.1 | 0.2 | 0.57 |
| 21AS-MG04 | 95.5 | 2.64 | 0.33 | 0.03 | 0.06 | 0.72 | 0.01 | 0.05 | 0.01 | 0.01 | 0.61 |
| 24GJ-MG08 | 68.84 | 13.56 | 5.1 | 2.02 | 2.14 | 2.44 | 2.82 | 0.91 | 0.03 | 0.05 | 1.37 |
| 23YD-MG04 | 65.52 | 14.99 | 4.38 | 4.81 | 2.04 | 2.11 | 3.55 | 0.45 | 0.09 | 0.12 | 1.21 |
| 23YD-MG01 | 60.83 | 15.91 | 6.65 | 4.32 | 2.59 | 1.86 | 4.24 | 0.71 | 0.13 | 0.19 | 1.78 |
| 22IC-MG04 | 65.52 | 14.99 | 4.38 | 4.81 | 2.04 | 2.11 | 3.55 | 0.45 | 0.09 | 0.12 | 1.21 |
| Sample Name | Alkali Aggregate Reaction | Soundness | Acid Rock Drainage | Weathering Index | |||
|---|---|---|---|---|---|---|---|
| Chemical | Mortar Bar Expansion (%) | (%) | NAGpH (pH) | NAPP (kg H2SO4/t) | CIA | CWI | |
| 24JP-MG05 | potentially deleterious | 0.13 | 2.3 | 7.52 | −134.3 | 48.1 | 54.1 |
| 24GS-MG02 | 0.12 | 1.6 | 4.3 | 109.8 | 72.4 | 89.7 | |
| 24ES-MG05 | 0.12 | 3.5 | 7 | −8.7 | 54.4 | 63.4 | |
| 23NS-MG06 | 0.11 | 0.1 | 5.75 | −1.75 | 47.3 | 54.6 | |
| 23BA-MG03 | 0.1 | 0.1 | 6.78 | −2.48 | 50.6 | 60 | |
| 22PC-MG24 | 0.06 | 0.5 | 8.14 | −888.4 | 1.3 | 1.3 | |
| 22PC-MG20 | 0.1 | 7.3 | 2.4 | −0.04 | 66.8 | 90.8 | |
| 22PC-MG19 | 0.07 | 2.3 | 2.83 | −26.78 | 43.3 | 45.9 | |
| 22MJ-MG05 | 0.17 | 0.3 | 3 | −4.36 | 55.1 | 74.1 | |
| 22DDC-MG03 | 0.1 | 0.8 | 3.02 | −12.48 | 59.1 | 66.4 | |
| 21YP-MG06 | 0.08 | 5.2 | 6.12 | −17.69 | 53.5 | 56.5 | |
| 21KJ-MG20 | 0.1 | 2.9 | 5.8 | −10.24 | 49.7 | 58.7 | |
| 21KJ-MG14 | 0.16 | 3.6 | 6.49 | −7.79 | 49.6 | 61.8 | |
| 21KJ-MG01 | 0.12 | 0.5 | 6.8 | −17.75 | 58.7 | 75.9 | |
| 21ICN-MG08 | 0.1 | 11.9 | 6.2 | −32.75 | 50.6 | 58.2 | |
| 21CJ-MG06 | 0.12 | 3 | 5.12 | −11.01 | 51.5 | 64.3 | |
| 21AS-MG04 | 0.14 | 11 | 4.93 | 0.18 | 76.2 | 98.2 | |
| 24GJ-MG08 | considered potentially deleterious | 0.11 | 3.1 | 4.56 | 2.6 | 54.6 | 58.1 |
| 23YD-MG04 | 0.13 | 0.1 | 5.86 | −6.43 | 47.5 | 51.2 | |
| 23YD-MG01 | 0.11 | 0.1 | 6.11 | −5.99 | 49.3 | 52.5 | |
| 22IC-MG04 | 0.13 | 0.6 | 6.06 | −12.43 | 51.3 | 61.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, C.S.; You, B.W. Evaluation of the Alkali–Silica Reaction Potential of Korean Aggregates: Experimental Insights and Mitigation Strategies for Concrete Durability. Materials 2025, 18, 3373. https://doi.org/10.3390/ma18143373
Baek CS, You BW. Evaluation of the Alkali–Silica Reaction Potential of Korean Aggregates: Experimental Insights and Mitigation Strategies for Concrete Durability. Materials. 2025; 18(14):3373. https://doi.org/10.3390/ma18143373
Chicago/Turabian StyleBaek, Chul Seoung, and Byoung Woon You. 2025. "Evaluation of the Alkali–Silica Reaction Potential of Korean Aggregates: Experimental Insights and Mitigation Strategies for Concrete Durability" Materials 18, no. 14: 3373. https://doi.org/10.3390/ma18143373
APA StyleBaek, C. S., & You, B. W. (2025). Evaluation of the Alkali–Silica Reaction Potential of Korean Aggregates: Experimental Insights and Mitigation Strategies for Concrete Durability. Materials, 18(14), 3373. https://doi.org/10.3390/ma18143373





