Preparation of Foamed Ceramic from Cr Slag and MSWI Fly Ash and Its Cr Leaching Inhibition
Abstract
1. Introduction
1.1. Research Background
1.2. Current Research Status and Limitations
1.3. Main Content and Objectives
2. Materials and Methods
2.1. Preparation Process of CS-MSWI-FA-FC
2.2. Toxicity Leaching Experiment
2.3. Chemical Thermodynamic Calculation
2.4. Testing and Characterization of Foamed Ceramic Properties
3. Results and Discussion
3.1. General Information of Cr Slag, MSWI-FA, and Other Solid Wastes
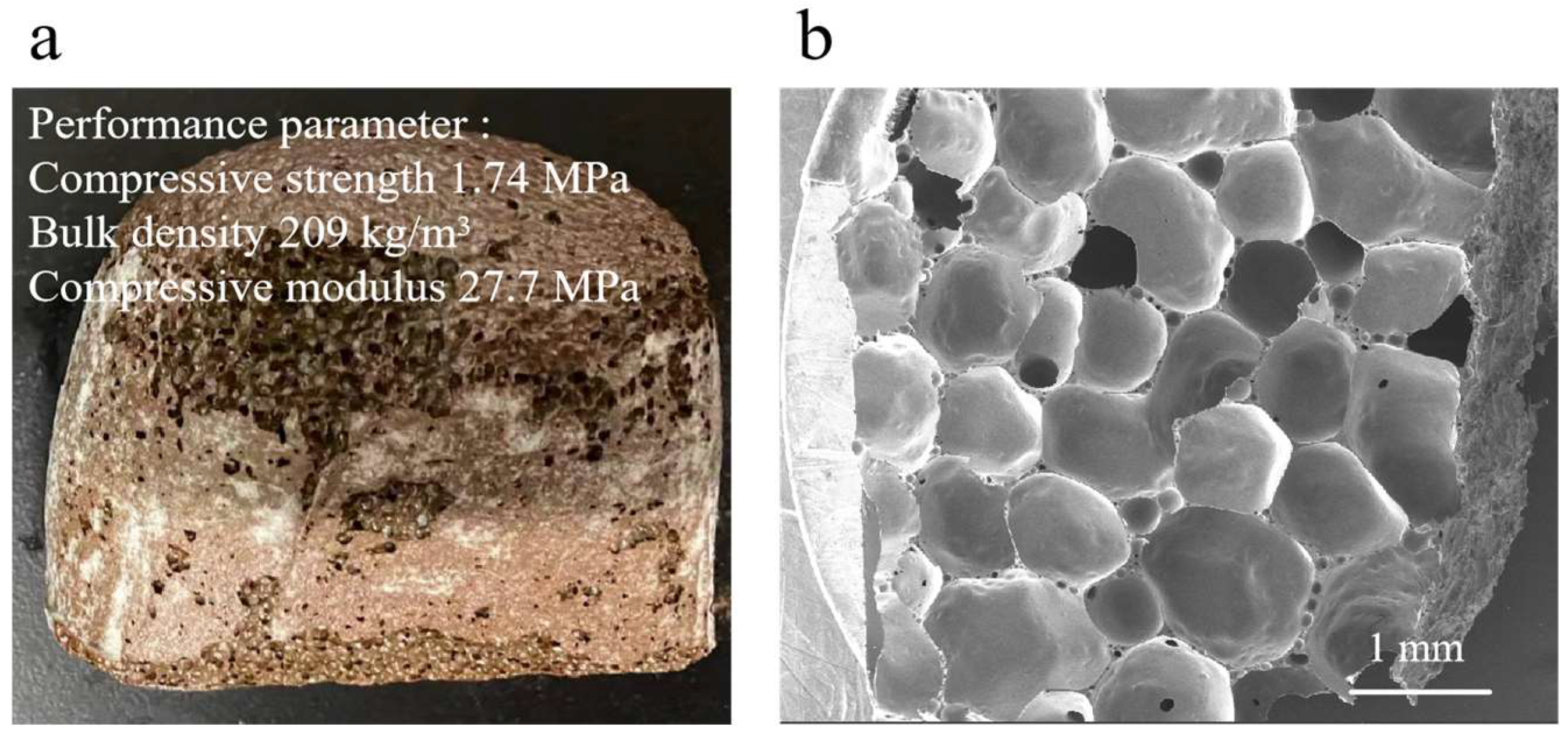
3.2. Physical and Cr Leaching Properties of CS-MSWI-FA-FC Without Inhibitors
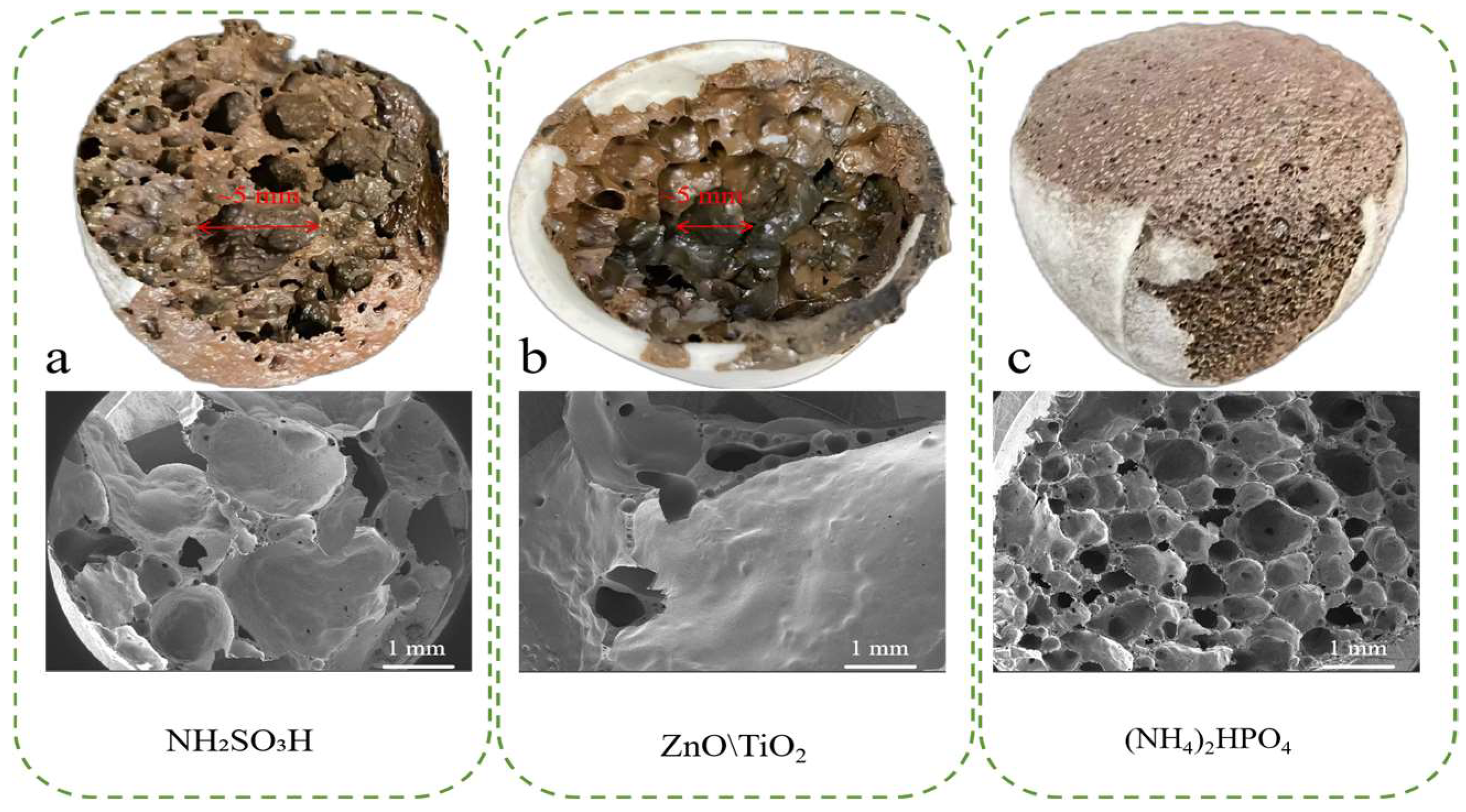
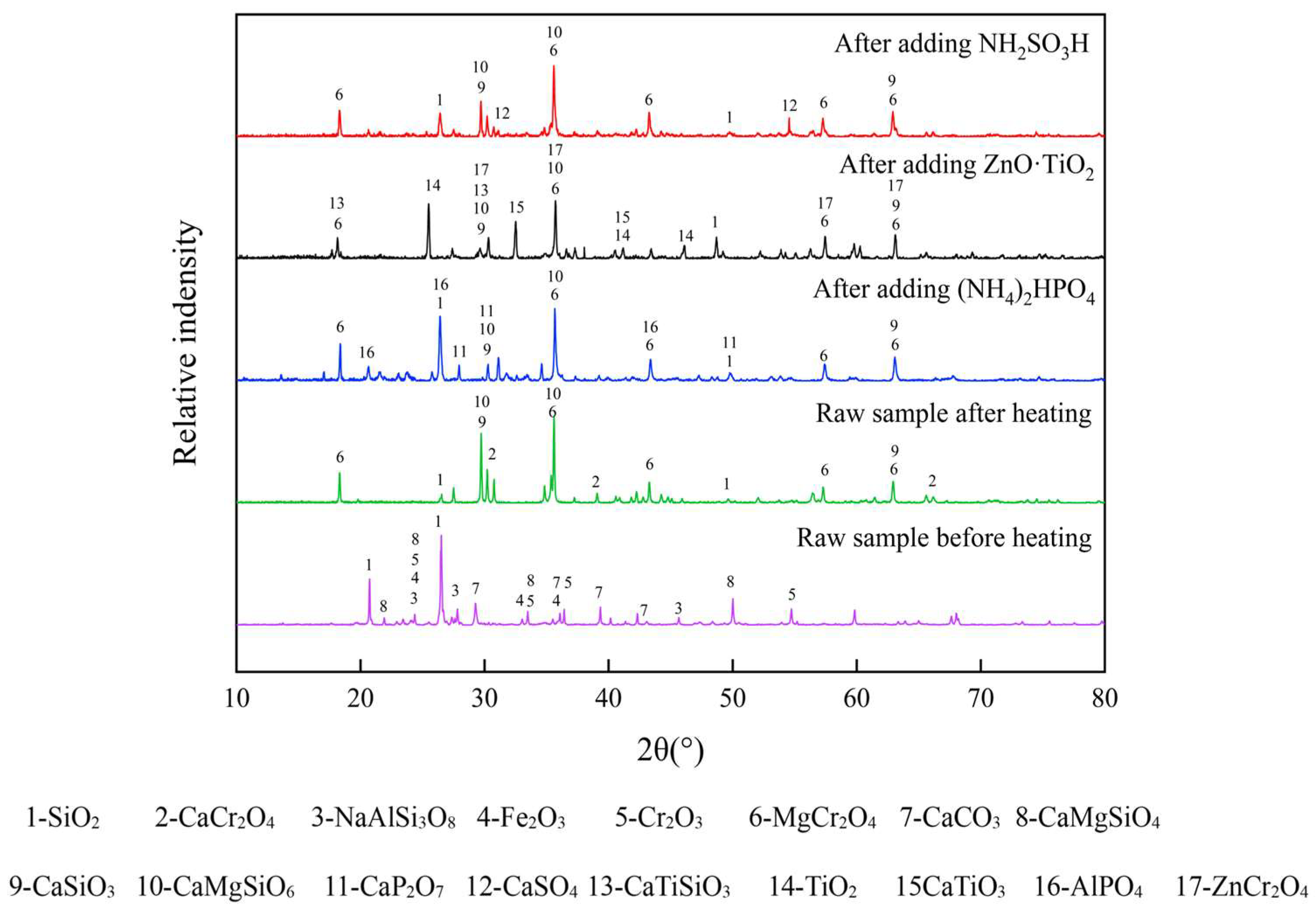
3.3. Effects of Inhibitor Types on the Physical and Cr Leaching Properties
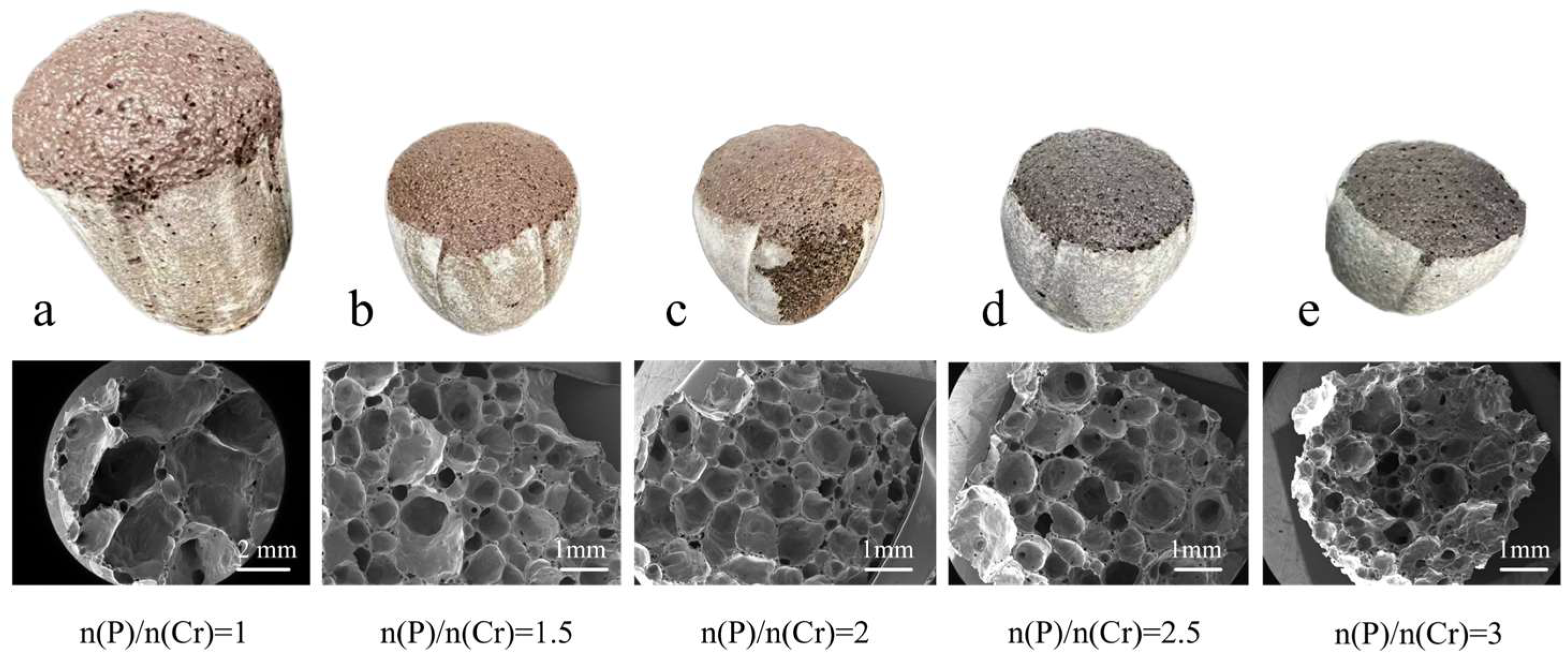
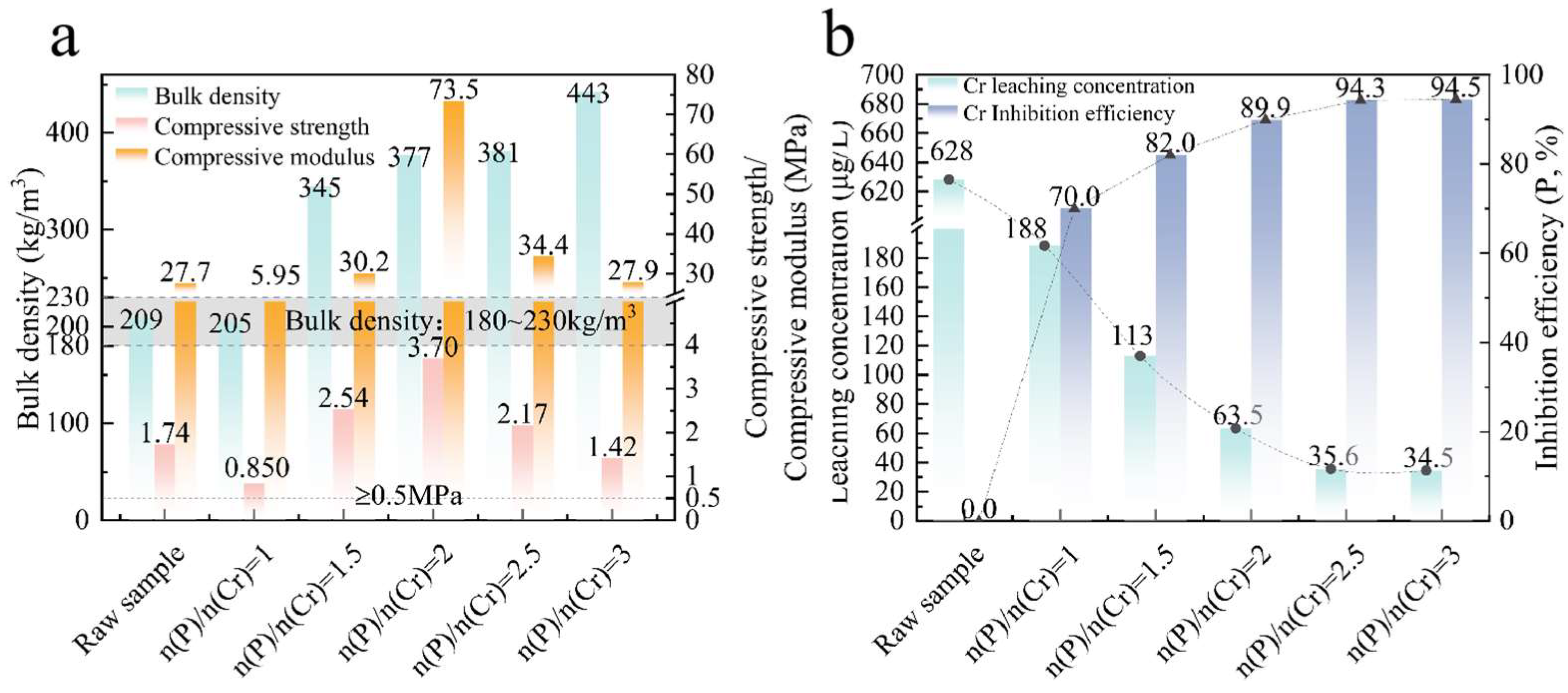
3.4. Effect of (NH4)2HPO4 Amount on the Physical and Cr Leaching Properties
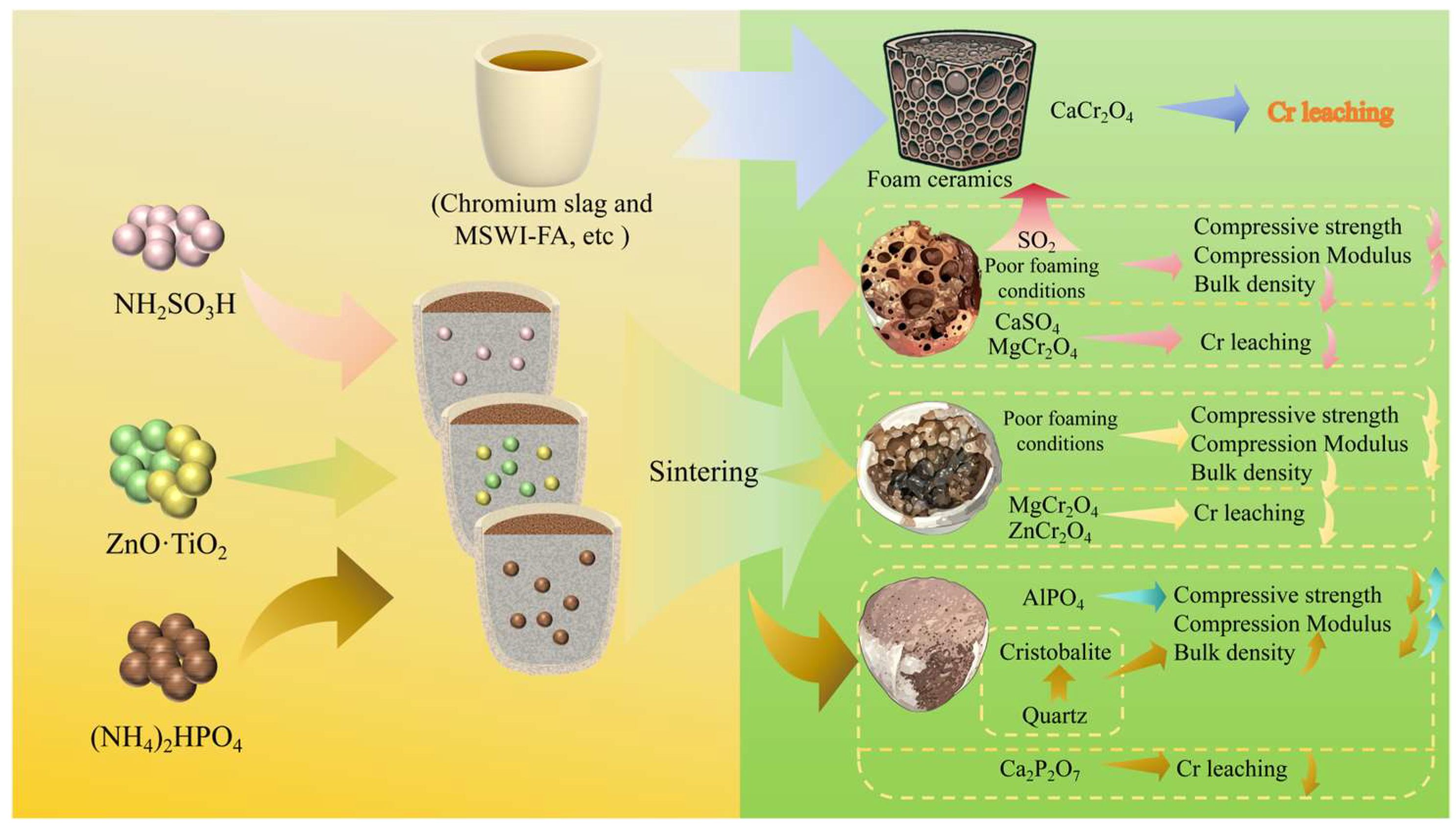
3.5. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, L.; Yan, X.; Meng, X.; Chen, Y. Processes of chromium (VI) migration and transformation in chromate production site: A case study from the middle of China. Chemosphere 2020, 257, 127282. [Google Scholar] [CrossRef]
- Huang, B.; Gan, M.; Ji, Z.; Fan, X.; Zhang, D.; Chen, X.; Sun, Z.; Huang, X.; Fan, Y. Recent progress on the thermal treatment and resource utilization technologies of municipal waste incineration fly ash: A review. Process Saf. Environ. Prot. 2022, 159, 547–565. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Li, W.; Nie, Y.; Wang, F.; Bian, R.; Wang, H.; Wang, Y.; Gong, Z.; Lu, J.; et al. Solidification/stabilization pre-treatment coupled with landfill disposal of heavy metals in MSWI fly ash in China: A systematic review. J. Hazard. Mater. 2024, 478, 135479. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhi, T.; Liu, L.; Mi, J.; Zhang, M.; Tian, C.; Si, Z.; Liu, X.; Mu, Y. Solidification/stabilization of chromium slag in red mud-based geopolymer. Constr. Build. Mater. 2022, 316, 125813. [Google Scholar] [CrossRef]
- Aharchaou, I.; Py, J.S.; Cambier, S.; Loizeau, J.L.; Cornelis, G.; Rousselle, P.; Battaglia, E.; Vignati, D.A.L. Chromium hazard and risk assessment: New insights from a detailed speciation study in a standard test medium. Environ. Toxicol. Chem. 2018, 37, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Den Braver-Sewradj, S.P.; Van Benthem, J.; Staal, Y.C.M.; Ezendam, J.; Piersma, A.H.; Hessel, E.V.S. Occupational exposure to hexavalent chromium. Part II. Hazard assessment of carcinogenic effects. Regul. Toxicol. Pharmacol. 2021, 126, 105045. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Li, M.; Zhang, Q.; Zhang, W. Production of high-strength eco-conscious ceramics exclusively from municipal solid waste. Ceram. Int. 2024, 50, 47851–47863. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, J.; Li, Z.; Hai, Y.; Xu, J.; Zheng, D. Preparation of black ceramic tiles with chromium slag and copper smelting waste slag. Metals 2023, 13, 537. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, J.; Zheng, D.; Xu, J.; Ma, G.; Khojiev, S.; Kadirov, N. Preparation and chromatic performance of black ceramic tiles from chromium slag, copper slag and silicon manganese slag. J. Ceram. Process. Res. 2025, 26, 139. [Google Scholar]
- Ge, X.; Zhou, M.; Wang, H.; Liu, Z.; Wu, H.; Chen, X. Preparation and characterization of ceramic foams from chromium slag and coal bottom ash. Ceram. Int. 2018, 44, 11888–11891. [Google Scholar] [CrossRef]
- Xu, X.; Gao, W.; Bai, X. A Novel Foamed Ceramic and Its Preparation Method. CN Patent CN118812240A, 22 October 2024. [Google Scholar]
- Liu, C.; Liu, L.; Tan, K.; Zhang, L.; Tang, K.; Shi, X. Fabrication and characterization of porous cordierite ceramics prepared from ferrochromium slag. Ceram. Int. 2016, 42, 734–742. [Google Scholar] [CrossRef]
- Luo, J.; Sheng, B.; Shi, Q. A review on the migration and transformation of heavy metals influence by alkali/alkaline earth metals during combustion. J. Fuel Chem. Technol. 2020, 48, 1318–1326. [Google Scholar] [CrossRef]
- Zhao, G.; Tian, C.; Wu, P.; Zhang, X.; Wang, Z.; Chen, X.; Xiong, Z.; Zhao, Y.; Zhang, J. Effect of oxide interactions on chromium speciation transformation during simulated municipal solid waste incineration. J. Environ. Sci. 2024, 142, 11–20. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, H.; Chen, H.; Peng, M.; Zhang, W.; Hu, L.; Mao, L. The introduction of sulfates to suppress Cr(III) oxidation during incineration of tannery sludge and reduce leachability toxicity of incineration residue. J. Clean. Prod. 2023, 382, 135272. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, Y.; Huang, X.; Qian, J.; Wu, Y.; Li, Z.; Lu, S. Synergistic immobilization of chromium in tannery sludge by ZnO and TiO2 and the oxidation mechanism of Cr(III) under alkaline in high temperature. J. Hazard. Mater. 2022, 424, 127290. [Google Scholar] [CrossRef]
- Mao, L.; Su, P.; Huang, B.; Zhang, W. Detoxification of solid waste containing Cr(VI) with phosphate by thermal treatment. Chem. Eng. J. 2017, 314, 114–122. [Google Scholar] [CrossRef]
- HJ/T 300-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Acetic Acid Buffer Solution Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2007.
- Degterov, S.; Pelton, A.D. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CrO-Cr2O3-SiO2-CaO system. Metall. Mater. Trans. B-Proc. Metall. Mater. Proc. Sci. 1997, 28, 235–242. [Google Scholar] [CrossRef]
- Klemme, S.; van Miltenburg, J.C.; Javorsky, P.; Wastin, F. Thermodynamic properties of uvarovite garnet (Ca3Cr2Si3O12). Am. Mineral. 2005, 90, 663–666. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Anovitz, L.M.; Castelli, R.A.; Porter, W.D. Thermodynamics of Cr2O3, FeCr2O4, ZnCr2O4, and CoCr2O4. J. Chem. Thermodyn. 2007, 39, 1474–1492. [Google Scholar] [CrossRef]
- Pan, Y.; Shao, H.J. Preparation of β-TCP with high thermal stability by solid reaction route. J. Mater. Sci. 2003, 38, 1049–1056. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Zhang, W.; Heikkilä, M.J.; Koivula, R.T.; Friedrich, B. Solid-state conversion of scandium phosphate into scandium oxide with sodium compounds. Ind. Eng. Chem. Res. 2019, 58, 14609–14620. [Google Scholar] [CrossRef]
- Pérez, F.J.; Hierro, M.P.; Pedraza, F.; Gómez, C.; Carpintero, M.C.; Trilleros, J.A. Kinetic studies of Cr and Al deposition using CVD-FBR on different metallic substrates. Surf. Coat. Tech. 1999, 122, 281–289. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Liang, X.; Li, J.; Liu, C.; Ji, F.; Sun, Z. Transformation and environmental chemical characteristics of hazardous trace elements in an 800 t/d waste incineration thermal power plant. Sci. Total Environ. 2024, 918, 170693. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shi, M.; Yang, Y.; Liu, H.; Xu, M.; Shen, J.; Yao, H. Further insight into the formation and oxidation of CaCr2O4 during solid fuel combustion. Environ. Sci. Tech. 2018, 52, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Yin, Z.; Shi, W.; Tao, M.; Liu, W.; Gao, Z.; Ma, C. Foam-gelcasting preparation and properties of high-strength mullite porous ceramics. Ceram. Int. 2023, 49, 6873–6879. [Google Scholar] [CrossRef]
- JG/T 511-2017; Foamed Ceramic Thermal Insulation Board in Building. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2017.
- Li, C.; Zhang, P.; Zeng, L.; Yu, L.; Li, D. Study on preparation of glass-ceramics from municipal solid waste incineration (MSWI) fly ash and chromium slag. J. Build. Eng. 2023, 68, 106080. [Google Scholar] [CrossRef]
- GB 16889-2008; Standard for Pollution Control on the Landfill Site of Municipal Solid Waste. Ministry of Ecology and Environment: Beijing, China, 2008.
- Yang, Y.; Zhu, C.; Wang, H.; Hao, Y.; Yan, L.; Zhang, Z.; Yan, H.; Chen, X.; Ma, H. Synergistic immobilization of Cr from real tannery sludge by formation of spinel phases with TiO2 and ZnO. J. Environ. Chem. Eng. 2022, 10, 108679. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y.; Suvorov, D. Gas-releasing reactions in foam-glass formation using carbon and MnxOy as the foaming agents. Ceram. Int. 2017, 43, 4638–4646. [Google Scholar] [CrossRef]
- Zhou, M.; Ge, X.; Wang, H.; Chen, L.; Chen, X. Effect of the CaO content and decomposition of calcium-containing minerals on properties and microstructure of ceramic foams from fly ash. Ceram. Int. 2017, 43, 9451–9457. [Google Scholar] [CrossRef]
- Jia, C.Y.; Ding, R.; Liu, J.C.; Liu, A.F.; Teng, D.X. Effects of Al(OH)3 on the structure and properties of foam ceramics cemented by phosphate. Adv. Mater. Res. 2015, 1120–1121, 21–26. [Google Scholar] [CrossRef]
- Khamkongkaeo, A.; Bootchanont, A.; Klysubun, W.; Amonpattaratkit, P.; Boonchuduang, T.; Tuchinda, N.; Phetrattanarangsi, T.; Nuntawong, N.; Kuimalee, S.; Lohwongwatana, B. Effect of phosphate compound on physical and mechanical properties of SiO2 ceramic. Ceram. Int. 2019, 45, 1356–1362. [Google Scholar] [CrossRef]
- Kavouras, P.; Pantazopoulou, E.; Varitis, S.; Vourlias, G.; Chrissafis, K.; Dimitrakopulos, G.P.; Mitrakas, M.; Zouboulis, A.I.; Karakostas, T.; Xenidis, A. Incineration of tannery sludge under oxic and anoxic conditions: Study of chromium speciation. J. Hazard. Mater. 2015, 283, 672–679. [Google Scholar] [CrossRef]



| Phase | Components | Ref. |
|---|---|---|
| Gas phase | Cr, CrO, CrO2, CrO3, CrCl4, CrCl3, CrCl2 | [24,25] |
| Solid phase | Ca3Cr2(SiO4)3, CaCrO4, CaCr2O4, Cr2O3, MgCr2O4, K2CrO4, Na2CrO4, CrO2, FeCr2O4, Cr2(SO4)3 | [15,25,26] |
| Na2O | MgO | Al2O3 | SiO2 | Cl | K2O | CaO | TiO2 | Cr2O3 | Fe2O3 | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr slag | 1.32 | 1.90 | 15.4 | 62.6 | 0.0500 | 2.60 | 0.670 | 0.560 | 0.0700 | 14.1 | 0.750 |
| MSWI-FA | 0.920 | 2.18 | 1.63 | 9.55 | 0.0700 | 0.560 | 72.8 | 0.310 | 0.290 | 1.39 | 10.4 |
| Kaoline | 7.43 | 1.44 | 15.9 | 66.2 | 0.00 | 4.28 | 3.88 | 0.0600 | 0.0200 | 0.620 | 0.210 |
| Feldspar | 6.77 | 0.160 | 13.8 | 70.4 | 0.0100 | 7.88 | 0.330 | 0.0600 | 0.0200 | 0.260 | 0.350 |
| Fluorspar tailings | 0.160 | 0.310 | 3.63 | 91.8 | 0.0400 | 1.10 | 1.01 | 0.00 | 0.0200 | 0.860 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, C.; Tang, Y.; Zhao, S. Preparation of Foamed Ceramic from Cr Slag and MSWI Fly Ash and Its Cr Leaching Inhibition. Materials 2025, 18, 3372. https://doi.org/10.3390/ma18143372
Li H, Liu C, Tang Y, Zhao S. Preparation of Foamed Ceramic from Cr Slag and MSWI Fly Ash and Its Cr Leaching Inhibition. Materials. 2025; 18(14):3372. https://doi.org/10.3390/ma18143372
Chicago/Turabian StyleLi, Hesong, Cheng Liu, Yikun Tang, and Shilin Zhao. 2025. "Preparation of Foamed Ceramic from Cr Slag and MSWI Fly Ash and Its Cr Leaching Inhibition" Materials 18, no. 14: 3372. https://doi.org/10.3390/ma18143372
APA StyleLi, H., Liu, C., Tang, Y., & Zhao, S. (2025). Preparation of Foamed Ceramic from Cr Slag and MSWI Fly Ash and Its Cr Leaching Inhibition. Materials, 18(14), 3372. https://doi.org/10.3390/ma18143372







