Corrosion Response of Steel to Penetration of Chlorides in DC-Treated Hardened Portland Cement Mortar
Abstract
1. Introduction
2. Materials and Methods
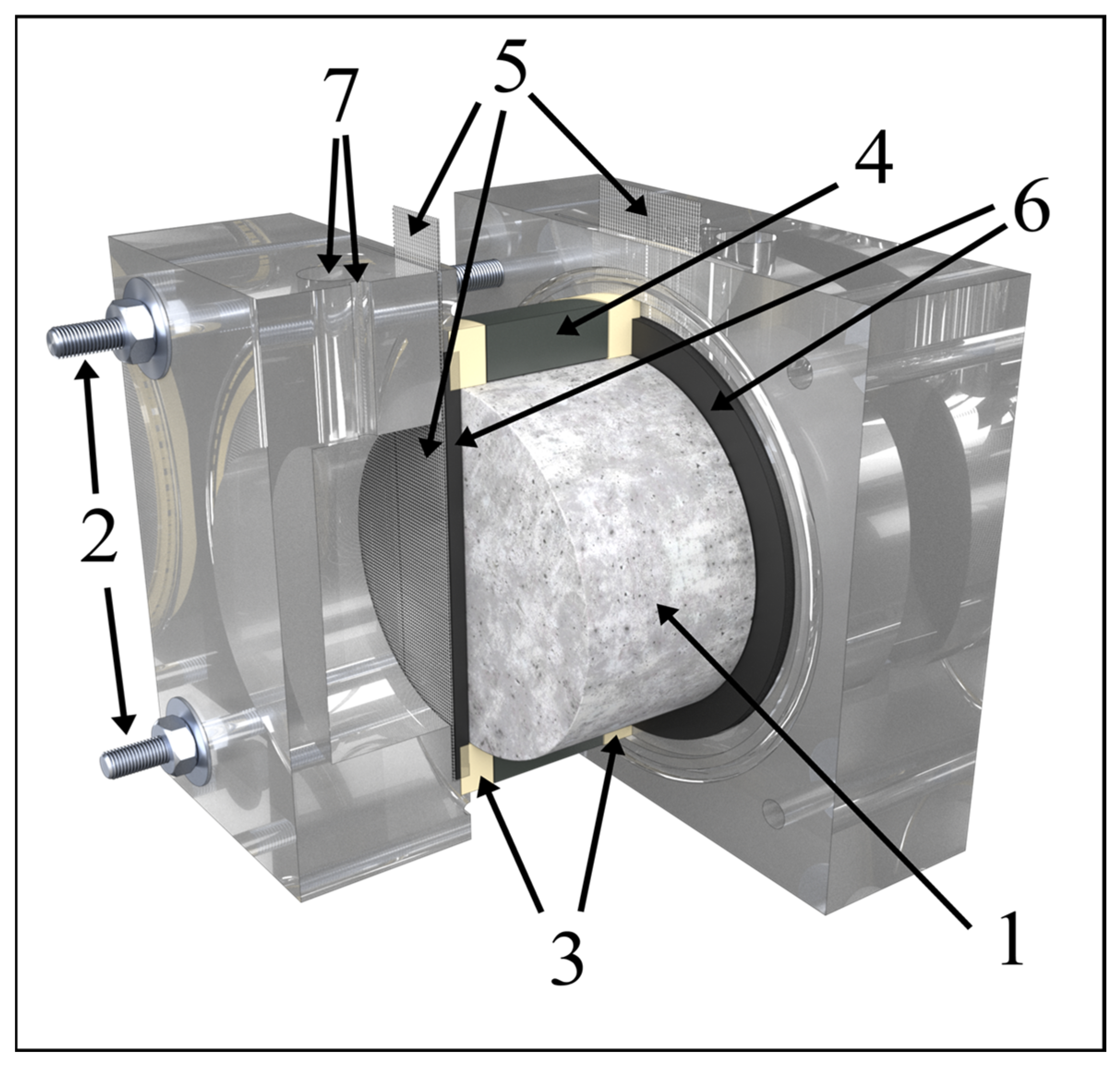
2.1. Materials and Samples
2.2. Electrical Current Treatment
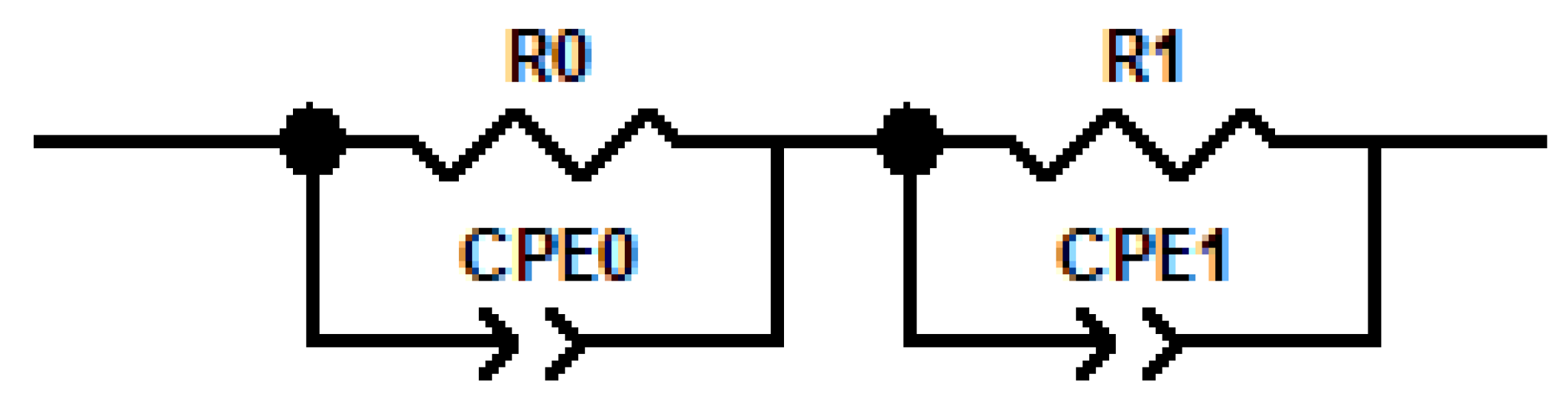
2.3. Electrochemical Impedance Spectroscopy
2.4. Mercury Intrusion Porosimetry
2.5. X-Ray Diffraction
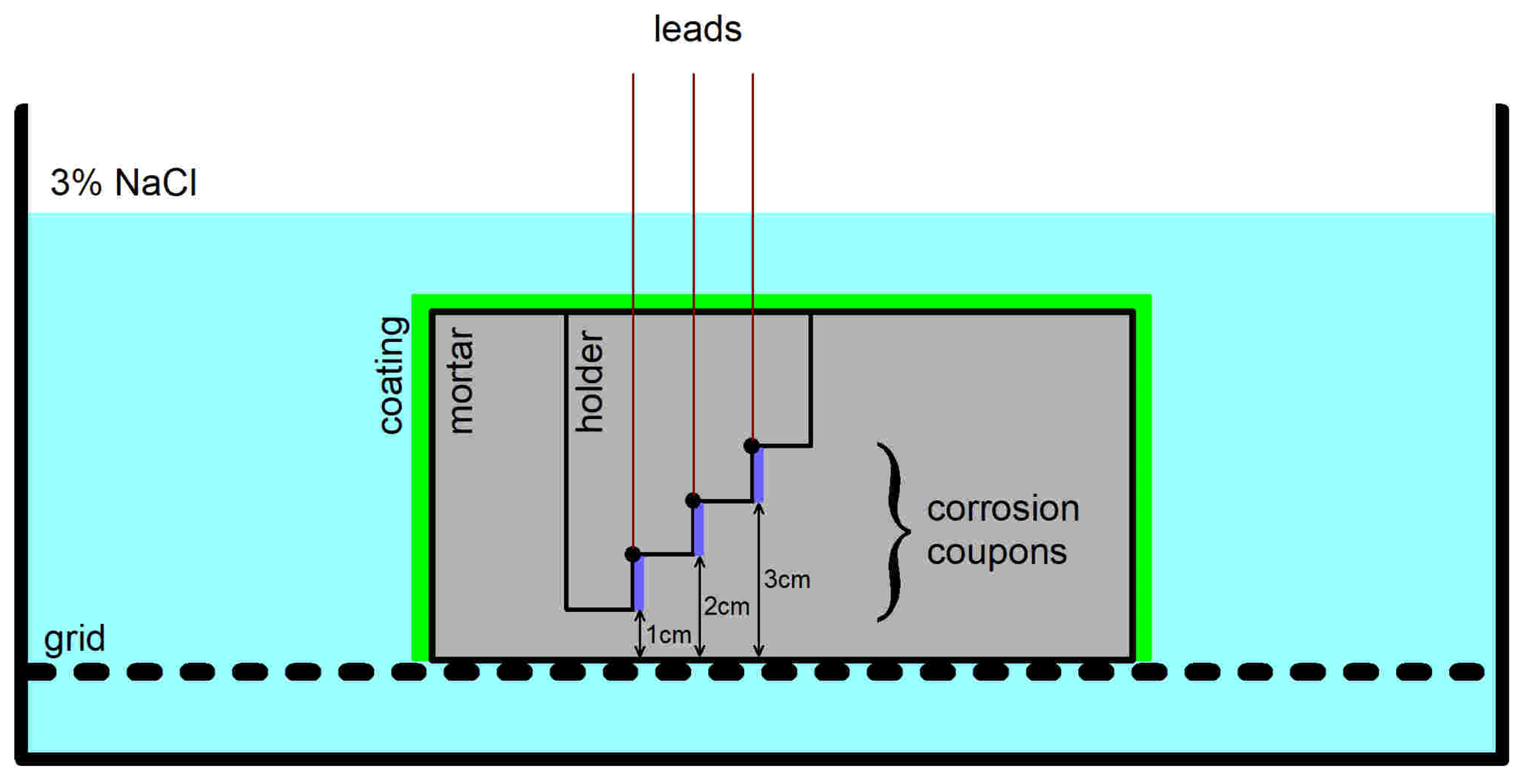
2.6. Corrosion Test
3. Results and Discussion
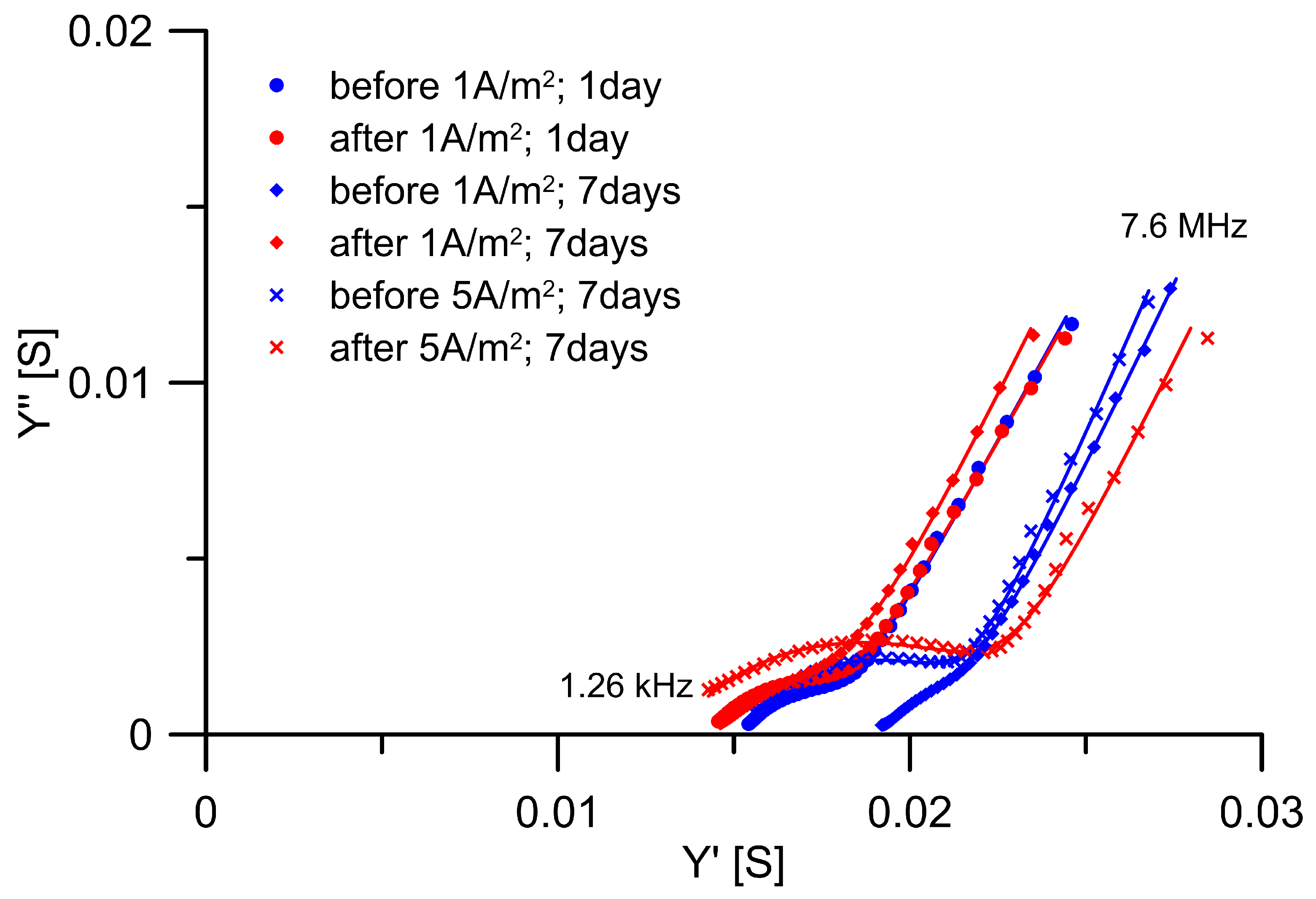
3.1. Conductivity Properties of Mortars
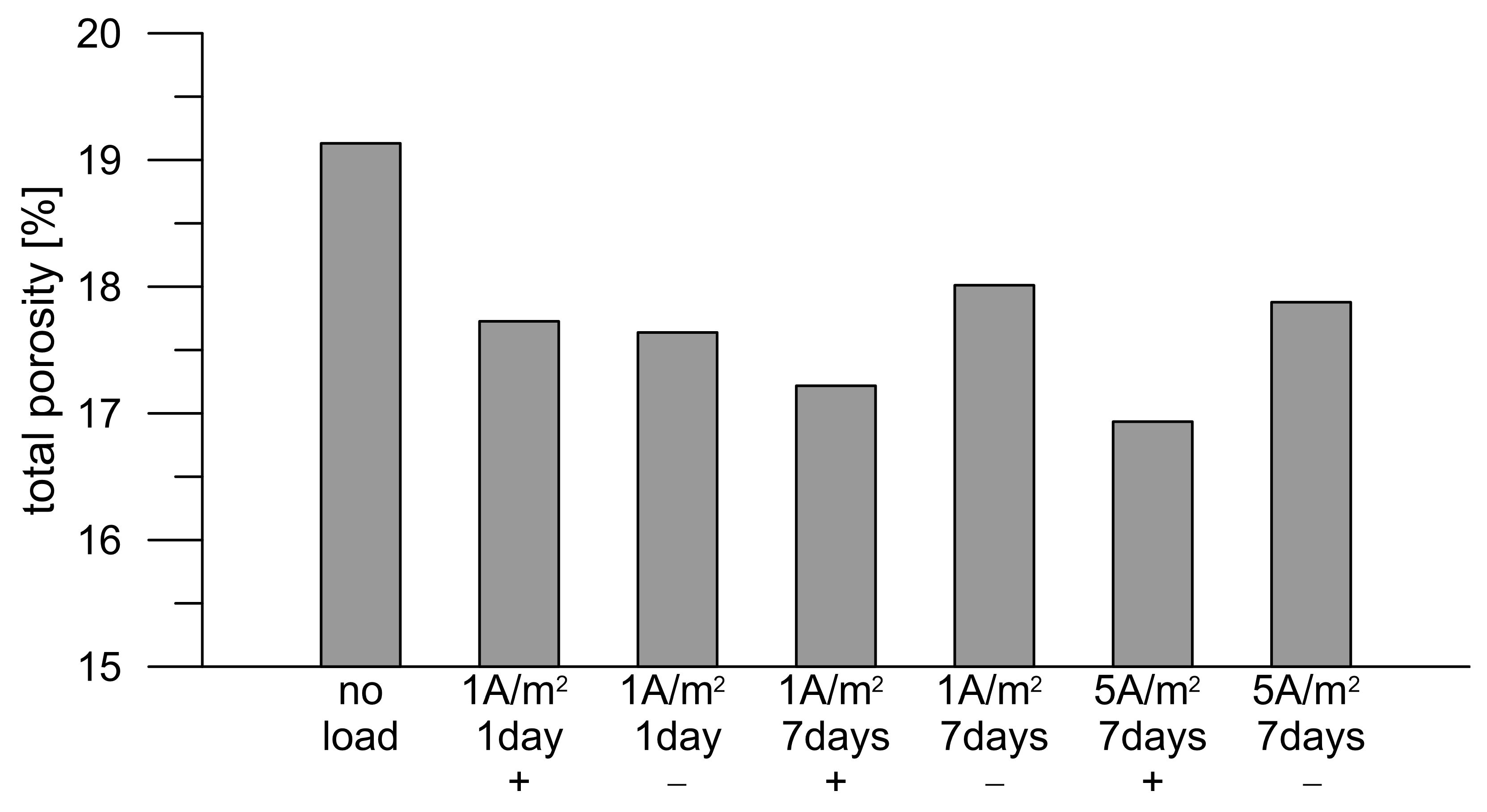
3.2. Porosity of Mortars
3.3. X-Ray Diffraction Analysis of the Mortar Surface
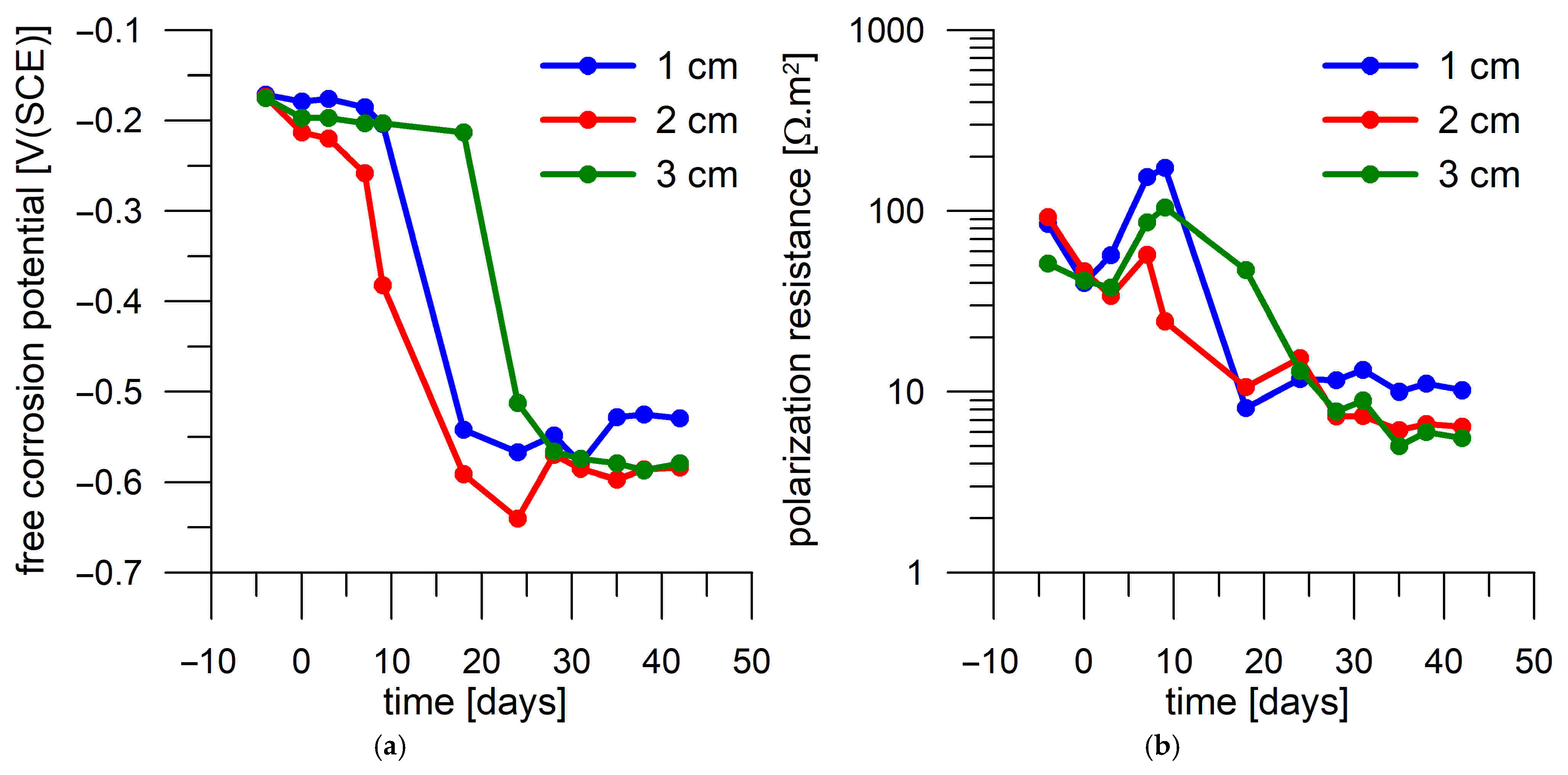
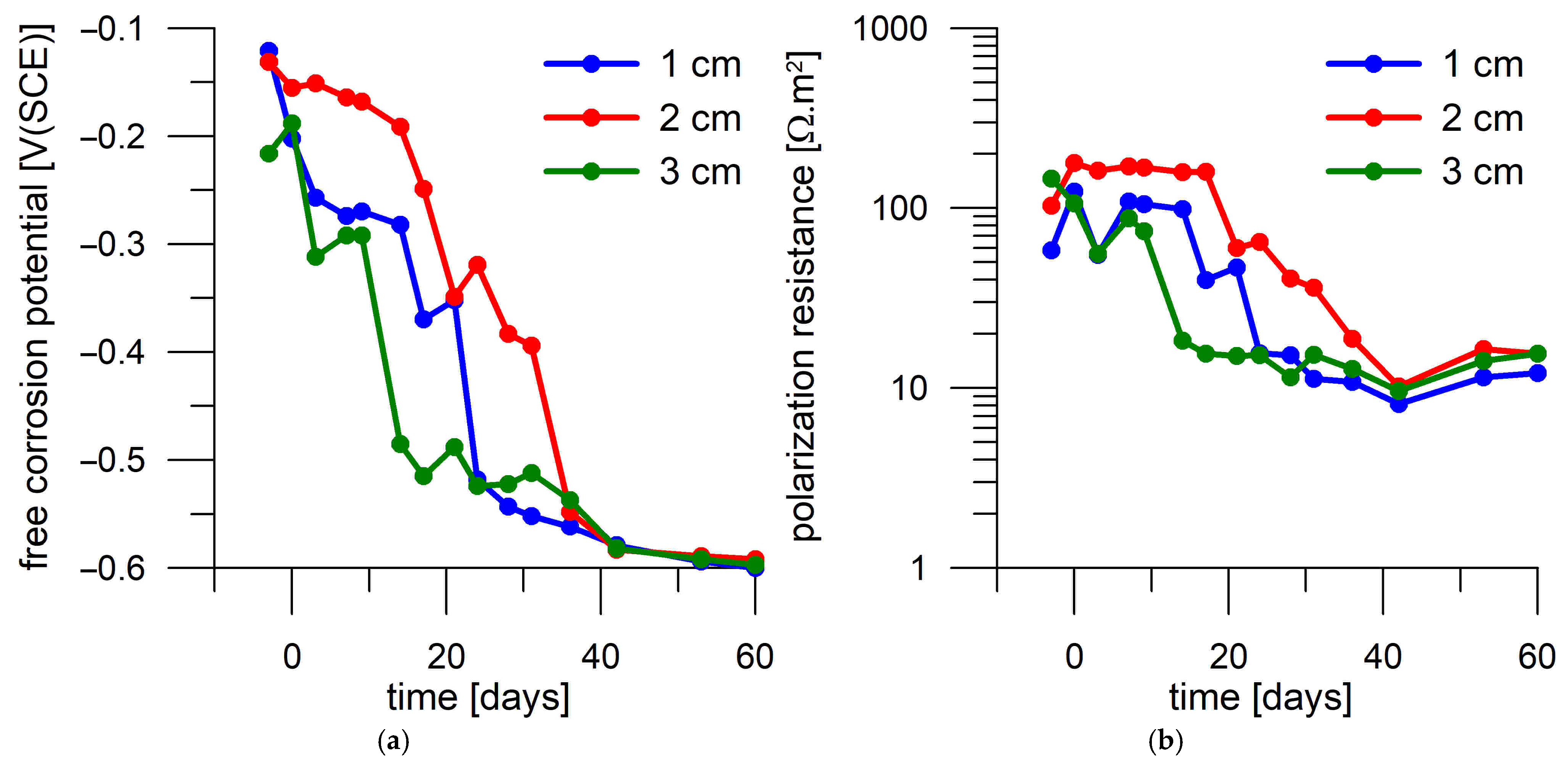
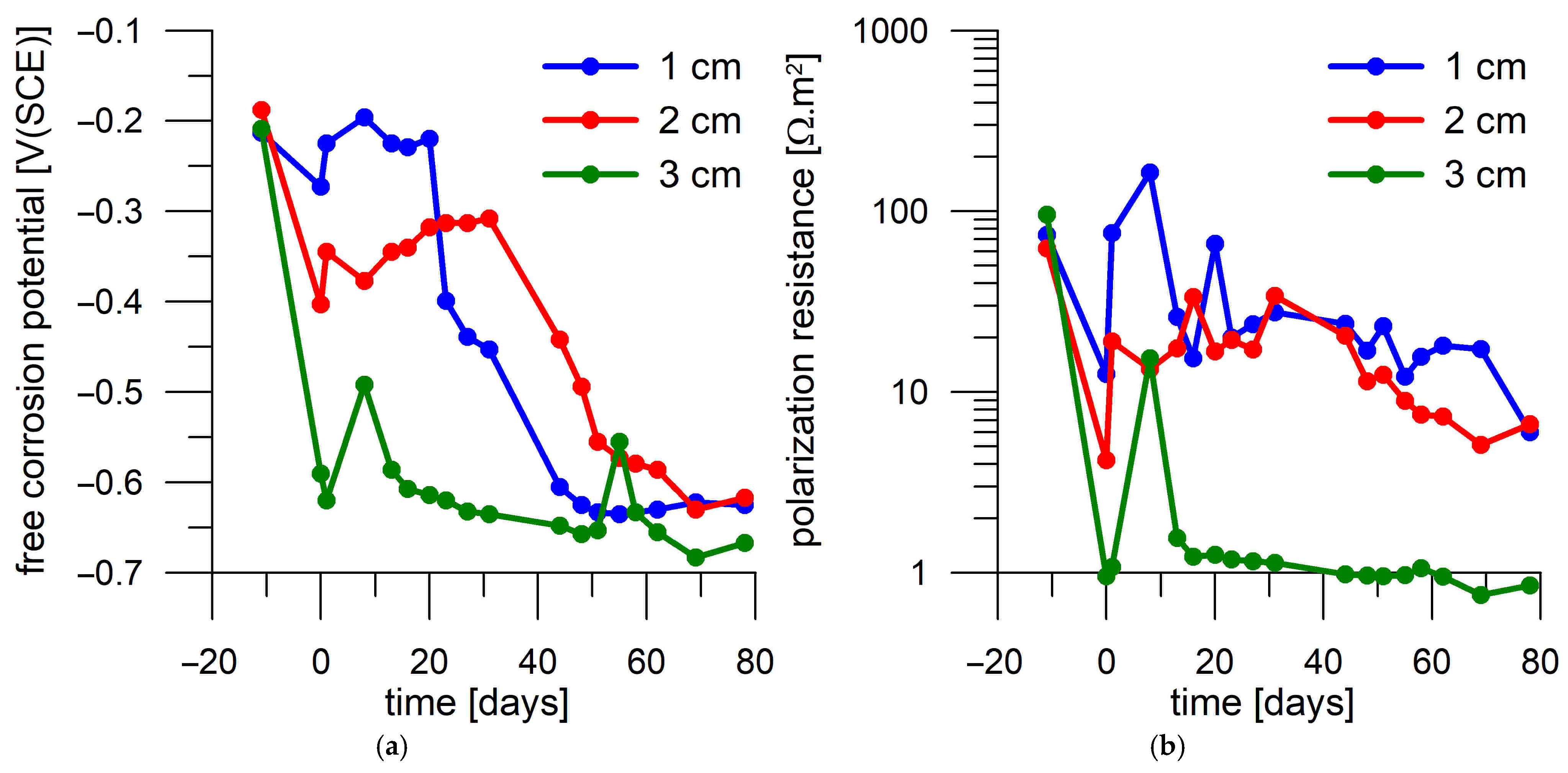
3.4. Corrosion Test in Mortar
4. Conclusions
- The conductivity properties of Portland cement mortar were influenced by DC treatment. The largest decrease (26%) in the conductivity of the mortar’s continuous pathways was observed for the less intensive DC treatment, 1 A/m2 applied for 7 days. Under more intensive DC treatments, a lower decrease in conductivity was identified.
- DC treatment alters the porosity of mortar surface layers, especially affecting the distribution of pore volume in relation to pore size. Approximately 2/3 of small pores (0.001–0.01 µm) were replaced by medium-sized pores (0.01–0.06 µm) as a result of 1 A/m2 DC treatment. The porosity alteration was not that pronounced for the higher-intensity DC treatment, a finding that is in agreement with the above-mentioned changes in conductivity properties.
- Alteration of the mortar surface was also confirmed by a phase analysis revealing an increased carbonate phase content, likely attributable to the carbonation of Portlandite precipitated in catholyte.
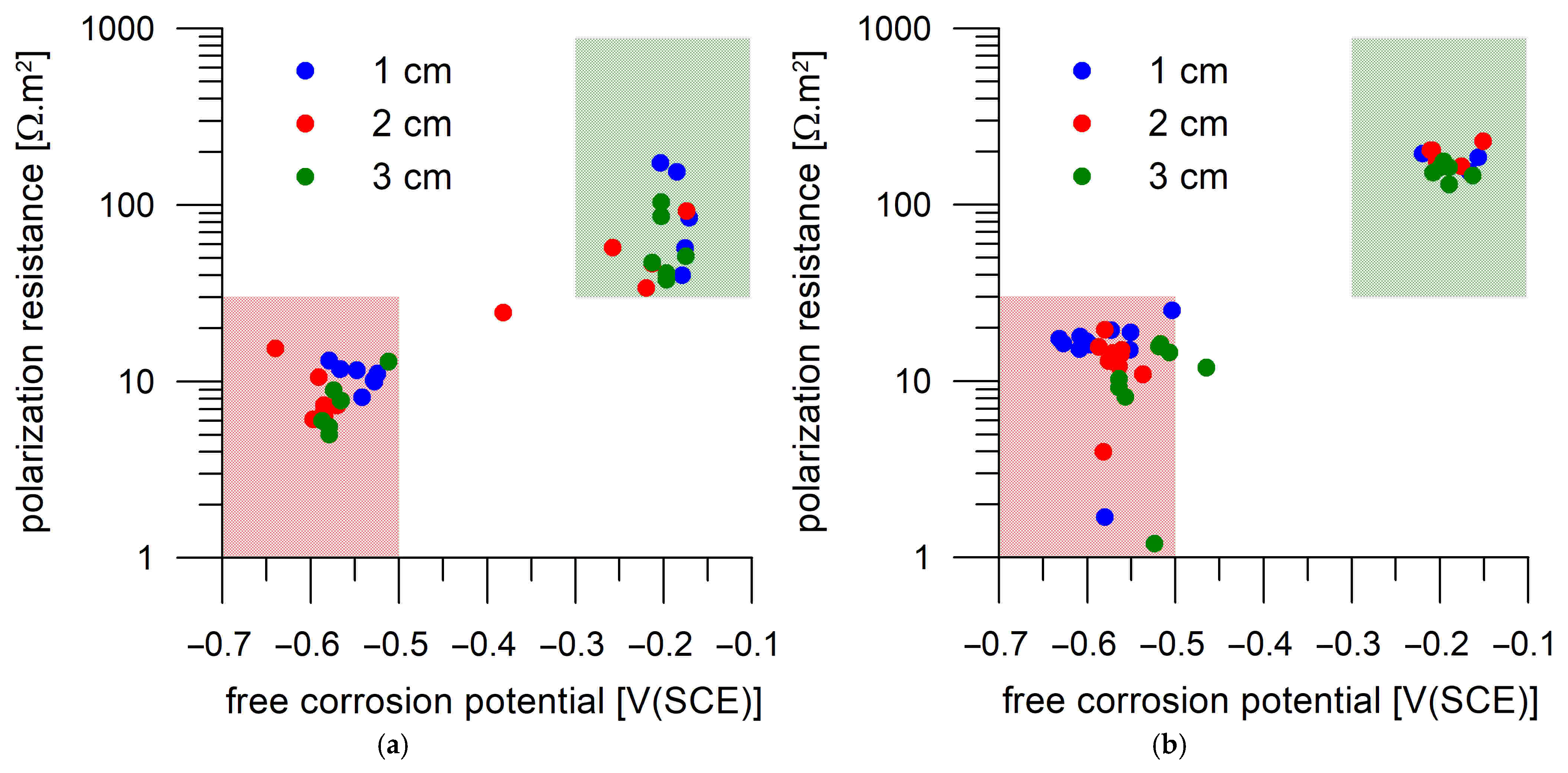
- Corrosion monitoring of the steel coupons embedded in Portland cement mortar showed a clear relation between the free corrosion potential (Eoc) and the resistance to polarization (Rp). In most cases, the passive state was characterized by Rp > 30 Ω.m2 and Eoc > −300 mV(SCE), while the active state was characterized by Rp < 30 Ω.m2 and Eoc < −500 mV(SCE).
- The transition of the corrosion coupons embedded in the Portland cement mortar from the passive to the active corrosion state occurred as early as after 13 days of immersion in 3 wt.% NaCl solution or later (up to 36 days) regardless of the coupons’ distance from the mortar surface (1–3 cm) and the DC treatment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DC | Direct current |
| MIP | Mercury intrusion porosimetry |
| EIS | Electrochemical impedance spectroscopy |
| XRD | X-ray diffraction |
| Eoc | Free corrosion potential or open-circuit potential |
| Rp | Polarization resistance |
| ECE | Electrochemical chloride extraction |
| C-S-H | Calcium hydroxyzincate |
| SCE | Saturated calomel electrode |
| R | Resistance |
| CPE | Constant phase element |
| RCCP | Resistance of the continuously conductive path |
| GCCP | Conductivity of the continuously conductive path |
| Rcp | Resistance of discontinuous pores |
| Gcp | Conductivity of discontinuous pores |
| CDP | Interfacial capacitance in the discontinuous path |
| Cmat | Capacitance of the non-conductive matrix |
References
- Pedeferri, P. Cathodic protection and cathodic prevention. Constr. Build. Mater. 1996, 10, 391. [Google Scholar] [CrossRef]
- Glass, G.K.; Hassanein, A.M.; Buenfeld, N.R. Cathodic protection afforded by an intermittent current applied to reinforced concrete. Corros. Sci. 2001, 43, 1111–1131. [Google Scholar] [CrossRef]
- Karthick, S.P.; Madhavamayandi, A.; Muralidharan, S.; Saraswathy, V. Electrochemical process to improve the durability of concrete structures. J. Build. Eng. 2016, 7, 273–280. [Google Scholar] [CrossRef]
- Orellan, J.C.; Escadeillas, J.C.; Arliguie, G. Electrochemical chloride extraction: Efficiency and side effects. Cem. Concr. Res. 2004, 34, 227–234. [Google Scholar] [CrossRef]
- Banfill, P. Re-alkalization of carbonated concrete—Effect on concrete properties. Constr. Build. Mater. 1997, 11, 255. [Google Scholar] [CrossRef]
- Miranda, M.; González, J.; Cobo, A.; Otero, E. Several questions about electrochemical rehabilitation methods for reinforced concrete structures. Corros. Sci. 2006, 48, 2172. [Google Scholar] [CrossRef]
- Topçu, I.B.; Uygunoglu, T.; Hocaoglu, I. Electrical conductivity of setting cement paste with different mineral admixtures. Constr. Build. Mater. 2012, 28, 414–420. [Google Scholar] [CrossRef]
- Sawada, S.; Page, C.L.; Page, M.M. Electrochemical injection of organic corrosion inhibitors into concrete. Corros. Sci. 2005, 47, 2063–2078. [Google Scholar] [CrossRef]
- Němeček, J.; Li, L.; Xi, Y. Electrokinetic nanoparticle injection for remediating leaks in oil well cement. Constr. Build. Mater. 2017, 156, 63–72. [Google Scholar] [CrossRef]
- Cecini, D.; Austin, S.A.; Cavalaro, S.; Palmeri, A. Accelerated electric curing of steel-fibre reinforced concrete. Constr. Build. Mater. 2018, 189, 192–204. [Google Scholar] [CrossRef]
- Wang, J.; An, J.; Li, Y.; Xiang, Y.; Xiao, Q.; Tang, Z.; Long, G. Influencing factors of the temperature rise of direct electric curing concrete and its effect on concrete properties. Constr. Build. Mater. 2024, 438, 137110. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, Y.; He, J.; Wang, F.; Zeng, X.; Ma, K.; Long, G. Influence of direct electric curing on the hydration and microstructure of cement paste excluding ohmic heating. Constr. Build. Mater. 2023, 369, 130546. [Google Scholar] [CrossRef]
- Siegwart, M.; Lyness, J.F.; McFarland, B.J. Change of pore size in concrete due to electrochemical chloride extraction and possible implications for the migration of ions. Cem. Concr. Res. 2003, 33, 1211–1221. [Google Scholar] [CrossRef]
- Zhu, X.; Peng, W.; Xiao, C.; Ma, L.; Hou, J. Effect of electric field intensity on chloride binding capacity of concrete. J. Mater. Res. Technol. 2023, 23, 4466–4478. [Google Scholar] [CrossRef]
- Susanto, A. Stray current-induced development of cement-based microstructure in water-submerged, Ca(OH)2-submerged and sealed conditions. J. Adv. Concr. Technol. 2017, 15, 244–268. [Google Scholar] [CrossRef]
- Kamisho, T.; Ishii, R.; Tsuda, M. Prediction of Hydrogen Embrittlement of Reinforcing Steel Bars in Concrete Poles. NTT Tech. Rev. 2020, 18, 43–47. [Google Scholar] [CrossRef]
- Thi, H.; Yokota, H.; Hashimoto, K. Effects of electrochemical chloride extraction on hydrated products of various cement paste systems. J. Adv. Concr. Technol. 2015, 13, 564–582. [Google Scholar] [CrossRef]
- Fajardo, G.; Esca-deillas, G.; Arliguie, G. Electrochemical chloride extraction (ECE) from steel-reinforced concrete specimens contaminated by “artificial” seawater. Corros. Sci. 2006, 48, 110–125. [Google Scholar] [CrossRef]
- Sawada, S.; Kubo, J.; Page, C.L.; Page, M.M. Electrochemical injection of organic corrosion inhibitors into carbonated cementitious materials: Part 1. Effects on pore solution chemistry. Corros. Sci. 2007, 49, 1205–1227. [Google Scholar] [CrossRef]
- Xu, C.; Jin, W.L.; Wang, H.L.; Wu, H.; Huang, N.; Li, Z.; Mao, J. Organic corrosion inhibitor of triethylenetetramine into chloride contaminated concrete by electroinjection method. Constr. Build. Mater. 2016, 115, 602–617. [Google Scholar] [CrossRef]
- Fan, W.; Mao, J.; Jin, W.; Xia, J.; Zhang, J.; Li, Q. Repair effect of bidirectional electromigration rehabilitation on concrete structures at different durability deterioration stages. Constr. Build. Mater. 2020, 251, 118872. [Google Scholar] [CrossRef]
- Susanto, A.; Peng, G.; Koleva, D.A.; van Breugel, K. Electrical Current Flow and Cement Hydration: Implications on Cement-Based Microstructure. Int. J. Struct. Civ. Eng. Res. 2017, 6, 75–82. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, Y.; He, J.; Wang, F.; Zeng, X.; Ma, K.; Long, G. A Comparative Study on the Mechanical Properties and Microstructure of Cement-Based Materials by Direct Electric Curing and Steam Curing. Materials 2021, 14, 7407. [Google Scholar] [CrossRef] [PubMed]
- EN 14629; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Chloride Content in Hardened Concrete. Comité Européen de Normalisation: Brussel, Belgium, 2007.
- EN 10130; Cold Rolled Low Carbon Steel Flat Products for Cold Forming—Technical Delivery Conditions. Comité Européen de Normalisation: Brussel, Belgium, 2006.
- Sánchez, I.; Nóvoa, X.R.; De Vera, G.; Climent, M.A. Microstructural modications in Portland cement concrete due to forced ionic migration tests. Study by impedance spectroscopy. Cem. Concr. Res. 2008, 38, 1015–1025. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Devi, R.R.; Emmanuel, B.; Palaniswamy, N. Determination of diffusion coefficient of chloride in concrete: An electrochemical impedance spectroscopic approach. Mater. Struct. 2008, 41, 1315–1326. [Google Scholar] [CrossRef]
- Kouřil, M.; Novák, P.; Bojko, M. Limitations of the linear polarization method to determine stainless steel corrosion rate in concrete environment. Cem. Concr. Compos. 2006, 28, 220–225. [Google Scholar] [CrossRef]
- Halamickova, P.; Detwiler, R.J. Water permeability and chloride ion diffusion in Portland cement mortars: Relationship to sand content and critical pore diameter. Cem. Concr. Res. 1995, 25, 790–802. [Google Scholar] [CrossRef]
- Song, G. Equivalent circuit model for ac electrochemical impedance spectroscopy of concrete. Cem. Concr. Res. 2000, 30, 1723–1730. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical Note: Concerning the Conversion of the Constant Phase Element Parameter Y into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Kouřil, M.; Hybasek, V.; Reiser, M.; Msallamova, S.; Sedlarova, I.; Nemecek, J. Microstructural and Transport Properties Changes in Blended Cement Concrete Resulting from DC Treatments. In Proceedings of the AMPP Conference 2023, Denver, CO, USA, 19–23 March 2023. [Google Scholar]
- Novák, P.; Malá, R.; Joska, L. Influence of pre-rusting on steel corrosion in concrete. Cem. Concr. Res. 2001, 31, 589–593. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. RILEM TC 154-EMC: ‘Electrochemical Techniques for Measuring Metallic Corrosion’. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Ňachaj, D.; Němeček, J.; Kouřil, M.; Keppert, M. Effect of electric current on porosity of concrete. Acta Polytech. CTU Proc. 2023, 40, 69–75. [Google Scholar] [CrossRef]





| Lower frequency limit | 1.26 Hz |
| Upper frequency limit | 7.6 MHz |
| Steps per decade | 10 |
| Measure periods | 50 |
| Frequency scan direction | Up–down |
| Frequency scan strategy | Single sine |
| Amplitude | 20 mV |
| Current Load | 1 A/m2; 1 Day | 1 A/m2; 7 Days | 5 A/m2; 7 Days | |||
|---|---|---|---|---|---|---|
| Rcp [Ω] | 349 | 261 | 338 | 227 | 154 | 103 |
| Rccp [Ω] | 65 | 70 | 53 | 70 | 66 | 75 |
| Cdp [F] | 1.3 × 10−8 | 1.6 × 10−8 | 4.6 × 10−9 | 6.3 × 10−9 | 3.6 × 10−8 | 9.1 × 10−8 |
| Cmat [F] | 2.4 × 10−10 | 2.3 × 10−10 | 2.5 × 10−10 | 2.3 × 10−10 | 2.4 × 10−10 | 2.2 × 10−10 |
| Gcp [S] | 2.9 × 10−3 | 3.8 × 10−3 | 3.0 × 10−3 | 4.4 × 10−3 | 6.5 × 10−3 | 9.7 × 10−3 |
| Gccp [S] | 1.5 × 10−2 | 1.4 × 10−2 | 1.9 × 10−2 | 1.4 × 10−2 | 1.5 × 10−2 | 1.3 × 10−2 |
| Current Load | Quartz | Aluminate–Silicate Phases | Carbonate Phases | Rest | Supplementary Material |
|---|---|---|---|---|---|
| No load | 85 | 5 | 5 | 5 | Protocol S1 |
| 1 A/m2; 1 day; + | 80 | 8 | 6 | 6 | Protocol S2 |
| 1 A/m2; 1 day; − | 75 | 10 | 14 | 1 | Protocol S3 |
| 1 A/m2; 7 days; + | 80 | 12 | 5 | 3 | Protocol S4 |
| 1 A/m2; 7 days; − | 50 | 9 | 40 | 1 | Protocol S5 |
| 5 A/m2; 7 days; + | 70 | 17 | 10 | 3 | Protocol S6 |
| 5 A/m2; 7 days; − | 55 | 10 | 35 | - | Protocol S7 |
| Time to Activation [Days] | Depth 1 cm | Depth 2 cm | Depth 3 cm |
|---|---|---|---|
| No load | 20–24 | 17–36 | 14–29 |
| 1 A/m2; 1 day | 18–21 | 13–18 | 16–24 |
| 5 A/m2; 7 days | 13–21 | 17–31 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouřil, M.; Saksa, J.; Hybášek, V.; Sedlářová, I.; Němeček, J.; Kohoutková, M.; Němeček, J. Corrosion Response of Steel to Penetration of Chlorides in DC-Treated Hardened Portland Cement Mortar. Materials 2025, 18, 3365. https://doi.org/10.3390/ma18143365
Kouřil M, Saksa J, Hybášek V, Sedlářová I, Němeček J, Kohoutková M, Němeček J. Corrosion Response of Steel to Penetration of Chlorides in DC-Treated Hardened Portland Cement Mortar. Materials. 2025; 18(14):3365. https://doi.org/10.3390/ma18143365
Chicago/Turabian StyleKouřil, Milan, Jan Saksa, Vojtěch Hybášek, Ivona Sedlářová, Jiří Němeček, Martina Kohoutková, and Jiří Němeček. 2025. "Corrosion Response of Steel to Penetration of Chlorides in DC-Treated Hardened Portland Cement Mortar" Materials 18, no. 14: 3365. https://doi.org/10.3390/ma18143365
APA StyleKouřil, M., Saksa, J., Hybášek, V., Sedlářová, I., Němeček, J., Kohoutková, M., & Němeček, J. (2025). Corrosion Response of Steel to Penetration of Chlorides in DC-Treated Hardened Portland Cement Mortar. Materials, 18(14), 3365. https://doi.org/10.3390/ma18143365






