Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior
Abstract
1. Introduction
- Optimization of surface texture for enhanced triboelectric performance;
- Long-term stability under physiological conditions;
- Scalability of manufacturing processes;
- Integration with existing biomedical device technologies.
2. Review Methodology
2.1. Search Strategy
- Web of Science;
- Scopus;
- IEE Xplore;
- PubMed.
- Studies reporting quantitative performance data;
- Research on biocompatible materials;
- Papers addressing surface texture modification;
- Articles with clear experimental methodologies.
2.2. Analysis Framework
- Material classification;
- Surface modification techniques;
- Performance metrics comparison;
- Biomedical applications assessment.
3. Advanced Biocompatible Materials for Triboelectric Applications
3.1. Synthetic Biocompatible Polymers
3.1.1. Polydimethylsiloxane (PDMS)
3.1.2. Polyfluoroethylene (PTFE)
3.1.3. Ecoflex®
3.1.4. Polyamide Film (Kapton®)
3.1.5. Nylon
3.1.6. Polyethylene Terephthalate (PET)
3.1.7. Comparative Assessment of AM Integration Potential for Synthetic Biocompatible Polymers
3.2. Carbon-Based Biocompatible Materials
3.2.1. Graphene
3.2.2. Carbon Nanotubes (CNTs)
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| hemical Vapor Deposition (CVD) growth of vertically aligned CNT arrays | Aligned CNTs structured for triboelectricity. | Output: 3.2 V, 0.21 µA, 672 nW. | Self-powered weighing systems. | [195] |
| Blending CNTs with cellulose (microcrystalline cellulose–MCC, cellulose powder–CP, cellulose nanofibers–CNF); oven-dried films | Homogeneous nanocarbon dispersion, enhanced with MCC. | Max voltage: 39 V (MCC); current: 3 mA/m2; power: 60 mW/m2 at 40 MΩ. | Biodegradable nanogenerators for low-frequency energy harvesting. | [196] |
| Electrospun polyaniline (PANI)/CNTs/AgNWs composite electrode | Friction layer with hydrophobic surface (contact angle > 120°). | Voc ≈ 150 V; Isc ≈ 60 µA. | Liquid-type sensing systems. | [201] |
3.2.3. Reduced Graphene Oxide (rGO)
3.2.4. Carbon Black (CB)
3.2.5. Comparative Assessment of AM Integration Potential for Carbon-Based Biocompatible Materials
3.3. Advanced Biocompatible Materials and Enhancements
3.3.1. MXenes (Ti3C2Tx)
3.3.2. Ionic Liquids and Hydrogels
3.3.3. Conductive Polymers (PEDOT:PSS)
3.3.4. Comparative Assessment of AM Integration Potential for Advanced Biocompatible Materials and Enhancements
4. Surface Texture and Porosity Engineering for Enhanced Triboelectric Performance
4.1. Surface Roughness and Porosity Control of PDMS
4.2. Surface Roughness and Porosity Control of PTFE
4.3. Surface Roughness and Porosity Control of Ecoflex®
4.4. Surface Roughness and Porosity Control of Kapton®
4.5. Surface Roughness and Porosity Control of Nylon
4.6. Surface Roughness and Porosity Control of PET
4.7. Surface Roughness and Porosity Control of Graphene
4.8. Surface Roughness and Porosity Control of CNTs
4.9. Surface Roughness and Porosity Control of rGOs
4.10. Surface Roughness and Porosity Control of Carbon Black
4.11. Surface Roughness and Porosity Control of MXenes
4.12. Surface Roughness and Porosity Control of Ionic Liquids and Hydrogels
4.13. Surface Roughness and Porosity Control of PEDOT:PSS
4.14. Comparative Assessment of Material Selection for Biomedical TENGs
5. Discussion
- Emerging AM-Compatible Biomaterials: Future research will likely focus on developing novel biocompatible inks and filaments specifically designed for AM, capable of incorporating advanced functionalities. This includes exploring more sophisticated hybrid materials that combine tailored mechanical, electrical, and triboelectric properties with enhanced biocompatibility and bioresorbability. The functionalization of materials during or immediately after the AM process could become standard to achieve desired surface chemistry for specific biological interactions.
- Challenges: The scalability remains a hurdle for complex AM processes, requiring industrial translation from laboratory-scale fabrication. Establishing robust regulatory standards for AM-fabricated, implantable, or long-term wearable TENGs will be critical but challenging, involving stringent requirements for material safety, device performance, and manufacturing reproducibility. Perhaps the most significant challenge is ensuring long-term biocompatibility and reliable performance under dynamic physiological conditions. Devices must withstand mechanical stress, moisture, enzymatic degradation, and the body’s immune response without losing function or causing adverse effects. Materials like CNTs and PET highlight the need to address inherent toxicity or thrombogenicity issues through functionalization and controlled fabrication.
- Future Research Directions: AM offers transformative potential for fabricating the next generation, personalized, self-powered biomedical devices; several research gaps currently limit widespread deployment, necessitating focused future directions. One of the main challenges that remains is the lack of new multifunctional biocompatible inks and filaments that can effectively overcome inherent material limitations, such as toxicity (e.g., with CNTs and PET), agglomeration (e.g., in carbon-based materials), oxidation susceptibility (e.g., in MXenes), and insufficient intrinsic mechanical robustness (e.g., PDMS or PEDOT:PSS) under physiological conditions. In addition, scalability for complex AM processes and the absence of robust regulatory standards for implantable or long-term wearable TENGs remain substantial manufacturing and commercialization hurdles. From a performance validation standpoint, a significant deficiency is the lack of systematic studies on the tribological safety of textured surfaces under simulated physiological conditions, compounded by an indispensable need for long-term in vivo studies to validate overall biocompatibility and device longevity under dynamic physiological conditions (including resistance to mechanical stress, moisture, enzymatic degradation, and the body’s immune response). Addressing these gaps will require more accurate theoretical models predicting triboelectric performance for complex AM-generated morphologies, alongside the development of advanced multi-material, multilayered AM techniques that enable the seamless integration of TENGs with other energy-harvesting modalities or sensing functions for truly multifunctional biomedical devices.
- Strategic Roadmap for Future Development: Future research should prioritize three major areas: enhancing biocompatibility through bioresorbable and AM-compatible materials, improving scalability by refining multi-material printing processes for reproducible, high-resolution fabrication, and enabling multifunctional integration by combining TENGs with sensing, actuation, or therapeutic modules.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arioli, M.; Puiggalí, J.; Franco, L. Nylons with applications in energy generators, 3D printing and biomedicine. Molecules 2024, 29, 2443. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Kim, D.-H.; Seung, W.; Khan, U.; Kim, T.Y.; Kim, T.; Kim, S.-W. 3D-printed biomimetic-villus structure with maximized surface area for triboelectric nanogenerator and dust filter. Nano Energy 2019, 63, 103857. [Google Scholar] [CrossRef]
- Pandey, P.; Seo, M.-K.; Shin, K.H.; Lee, J.; Sohn, J.I. In-situ cured gel polymer/ecoflex hierarchical structure-based stretchable and robust TENG for intelligent touch perception and biometric recognition. Chem. Eng. J. 2024, 499, 156650. [Google Scholar] [CrossRef]
- Xiang, H.; Peng, L.; Yang, Q.; Wang, Z.L.; Cao, X. Triboelectric nanogenerator for high-entropy energy, self-powered sensors, and popular education. Sci. Adv. 2024, 10, eads2291. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.-H.; Li, S.; Qiu, H.; Shi, Y.; Pan, L. Triboelectric nanogenerators based on 2D materials: From materials and devices to applications. Micromachines 2023, 14, 1043. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Liu, Q.; Wang, X.; Cao, J.; Cheng, G.; Zhang, Z.; Ding, J.; Li, K. Origami triboelectric nanogenerator with double-helical structure for environmental energy harvesting. Energy 2020, 212, 118462. [Google Scholar] [CrossRef]
- Sardo, F.R.; Rayegani, A.; Nazar, A.M.; Balaghiinaloo, M.; Saberian, M.; Mohsan, S.A.H.; Alsharif, M.H.; Cho, H.-S. Recent progress of triboelectric nanogenerators for biomedical sensors: From design to application. Biosensors 2022, 12, 697. [Google Scholar] [CrossRef]
- Ikram, M.; Mahmud, M.A.P. Advanced triboelectric nanogenerator-driven drug delivery systems for targeted therapies. Drug Deliv. Transl. Res. 2023, 13, 54–78. [Google Scholar] [CrossRef]
- Kim, D.-W.; Mun, H.; Kang, Y.; Kim, W.-G.; Ahn, D.; Yun, S.-Y.; Han, J.-A.; Lee, D.H.; Lee, T.; Jeong, K.; et al. Synthesis of stretchable triboelectric material with strain-compensating ability using gradient interpenetrating polymer networks. Energy Environ. Sci. 2025, 18, 4080–4096. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Kim, D.H.; Park, J.; Park, K.-I.; Lee, K.J.; Jeong, C.K. Modulation of surface physics and chemistry in triboelectric energy harvesting technologies. Sci. Technol. Adv. Mater. 2019, 20, 758–773. [Google Scholar] [CrossRef]
- Bindhu, A.; Arun, A.P.; Pathak, M. Review on polyvinylidene fluoride-based triboelectric nanogenerators for applications in health monitoring and energy harvesting. ACS Appl. Electron. Mater. 2024, 6, 47–72. [Google Scholar] [CrossRef]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft Material-enabled, flexible hybrid electronics for medicine, healthcare, and human-machine interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef]
- Kim, W.-J.; Nam, K.-W.; Kang, B.-H.; Park, S.-H. Piezoresistive Effect of Conductive and Non-Conductive Fillers in Bi-Layer Hybrid CNT Composites under Extreme Strain. Materials 2023, 16, 6335. [Google Scholar] [CrossRef]
- Wang, F.; Yu, H.; Lv, X.; Ma, X.; Qu, Q.; Wang, H.; Chen, D.; Liu, Y. MXene–MWCNT Conductive Network for Long-Lasting Wearable Strain Sensors with Gesture Recognition Capabilities. Micromachines 2025, 16, 123. [Google Scholar] [CrossRef]
- Yang, S.; Klinkov, V.; Grozova, N.; Shalnova, S.; Larionova, T.; Tolochko, O.; Klimova-Korsmik, O. Nanostructures and Nanomaterials Integrated into Triboelectric Nanogenerators. Micromachines 2025, 16, 403. [Google Scholar] [CrossRef]
- Shanbedi, M.; Ardebili, H.; Karim, A. Polymer-based triboelectric nanogenerators: Materials, characterization, and applications. Prog. Polym. Sci. 2023, 144, 101723. [Google Scholar] [CrossRef]
- Lavazza, J.; Contino, M.; Marano, C. Strain rate, temperature and deformation state effect on Ecoflex 00-50 silicone mechanical behaviour. Mech. Mater. 2023, 178, 104560. [Google Scholar] [CrossRef]
- Wang, G.; Xi, Y.; Xuan, H.; Liu, R.; Chen, X.; Cheng, L. Hybrid nanogenerators based on triboelectrification of a dielectric composite made of lead-free ZnSnO3 nanocubes. Nano Energy 2015, 18, 28–36. [Google Scholar] [CrossRef]
- Chung, J.; Lee, S.; Yong, H.; Moon, H.; Choi, D.; Lee, S. Self-packaging elastic bellows-type triboelectric nanogenerator. Nano Energy 2016, 20, 84–93. [Google Scholar] [CrossRef]
- Singh, H.H.; Khare, N. Flexible ZnO-PVDF/PTFE based piezo-tribo hybrid nanogenerator. Nano Energy 2018, 51, 216–222. [Google Scholar] [CrossRef]
- White, P.; Pankaew, P.; Bavykin, D.; Moshrefi-Torbati, M.; Beeby, S. The investigation of the energy harvesting performance using electrospun PTFE/PVDF based on a triboelectric assembly. Smart Mater. Struct. 2024, 33, 075010. [Google Scholar] [CrossRef]
- Tong, Y.; Feng, Z.; Kim, J.; Robertson, J.L.; Jia, X.; Johnson, B.N. 3D printed stretchable triboelectric nanogenerator fibers and devices. Nano Energy 2020, 75, 104973. [Google Scholar] [CrossRef]
- Li, Z.; Gan, W.C.; Tang, L.; Aw, K.C. Fundamental understanding of multicellular triboelectric nanogenerator with different electrical configurations. Micromachines 2023, 14, 1333. [Google Scholar] [CrossRef]
- Kang, M.; Lee, D.-M.; Hyun, I.; Rubab, N.; Kim, S.-H.; Kim, S.-W. Advances in bioresorbable triboelectric nanogenerators. Chem. Rev. 2023, 123, 11559–11618. [Google Scholar] [CrossRef]
- Qian, C.; Li, L.; Gao, M.; Yang, H.; Cai, Z.; Chen, B.; Xiang, Z.; Zhang, Z.; Song, Y. All-printed 3D hierarchically structured cellulose aerogel based triboelectric nanogenerator for multi-functional sensors. Nano Energy 2019, 63, 103885. [Google Scholar] [CrossRef]
- Dudem, B.; Kim, D.H.; Mule, A.R.; Yu, J.S. Enhanced performance of microarchitectured PTFE-based triboelectric nanogenerator via simple thermal imprinting lithography for self-powered electronics. ACS Appl. Mater. Interfaces 2018, 10, 24181–24192. [Google Scholar] [CrossRef]
- Armitage, J.; Ghanbarzadeh, A.; Wang, C.; Neville, A. An investigation into the influence of tribological parameters on the operation of sliding triboelectric nanogenerators. Tribol. Int. 2021, 155, 106778. [Google Scholar] [CrossRef]
- Xi, Y.; Kaper, H.J.; Choi, C.-H.; Sharma, P.K. Tribological properties of microporous polydimethylsiloxane (PDMS) surfaces under physiological conditions. J. Colloid Interface Sci. 2020, 561, 220–230. [Google Scholar] [CrossRef]
- Park, I.W.; Choi, J.; Kim, K.Y.; Jeong, J.; Gwak, D.; Lee, Y.; Ahn, Y.H.; Choi, Y.J.; Hong, Y.J.; Chung, W.-J.; et al. Vertically aligned cyclo-phenylalanine peptide nanowire-based high-performance triboelectric energy generator. Nano Energy 2019, 57, 737–745. [Google Scholar] [CrossRef]
- Meng, X.; Cai, C.; Luo, B.; Liu, T.; Shao, Y.; Wang, S.; Nie, S. Rational design of cellulosic triboelectric materials for self-powered wearable electronics. Nano-Micro Lett. 2023, 15, 124. [Google Scholar] [CrossRef]
- Yi, Q.; Pei, X.; Das, P.; Qin, H.; Lee, S.W.; Esfandyarpour, R. A self-powered triboelectric MXene-based 3D-printed wearable physiological biosignal sensing system for on-demand, wireless, and real-time health monitoring. Nano Energy 2022, 101, 107511. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zheng, Y.; Zhou, F.; Wang, D. Triboelectrification of interface controlled by photothermal materials based on electron transfer. Nano Energy 2021, 89, 106336. [Google Scholar] [CrossRef]
- Li, J.; Shepelin, N.A.; Sherrell, P.C.; Ellis, A.V. Poly(dimethylsiloxane) for triboelectricity: From mechanisms to practical strategies. Chem. Mater. 2021, 33, 4304–4327. [Google Scholar] [CrossRef]
- Luo, N.; Feng, Y.; Li, X.; Sun, W.; Wang, D.; Ye, Q.; Sun, X.; Zhou, F.; Liu, W. Manipulating electrical properties of silica-based materials via atomic oxygen irradiation. ACS Appl. Mater. Interfaces 2021, 13, 15344–15352. [Google Scholar] [CrossRef]
- Choi, D.; Yang, S.; Lee, C.; Kim, W.; Kim, J.; Hong, J. Highly surface-embossed polydimethylsiloxane-based triboelectric nanogenerators with hierarchically nanostructured conductive Ni–Cu fabrics. ACS Appl. Mater. Interfaces 2018, 10, 33221–33229. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Li, X.; Zhang, Z. Fabrication of advanced polydimethylsiloxane-based functional materials: Bulk modifications and surface functionalizations. Chem. Eng. J. 2021, 408, 127262. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, X.; Wang, C.; Ye, Z.; Gao, Y.; Xie, J.; Wu, X.; Wen, W. Functionalized PDMS with Versatile and Scalable Surface Roughness Gradients for Cell Culture. ACS Appl. Mater. Interfaces 2015, 7, 17181–17187. [Google Scholar] [CrossRef]
- Vlassov, S.; Oras, S.; Antsov, M.; Sosnin, I.; Polyakov, B.; Shutka, A.; Krauchanka, M.Y.; Dorogin, L.M. Adhesion and mechanical properties of PDMS-based materials probed with AFM: A review. Rev. Adv. Mater. Sci. 2018, 56, 62–78. [Google Scholar] [CrossRef]
- Tropmann, A.; Tanguy, L.; Koltay, P.; Zengerle, R.; Riegger, L. Completely Superhydrophobic PDMS Surfaces for Microfluidics. Langmuir 2012, 28, 8292–8295. [Google Scholar] [CrossRef]
- Jain, A.; Bharadwaj, P.; Heeg, S.; Parzefall, M.; Taniguchi, T.; Watanabe, K.; Novotny, L. Minimizing residues and strain in 2D materials transferred from PDMS. Nanotechnology 2018, 29, 265203. [Google Scholar] [CrossRef]
- Li, Y.; Li, B. Direct ink writing 3D printing of polydimethylsiloxane-based soft and composite materials: A mini review. Oxf. Open Mater. Sci. 2022, 2, itac008. [Google Scholar] [CrossRef]
- Gutierrez, D.B.; Caldona, E.B.; Yang, Z.; Suo, X.; Cheng, X.; Dai, S.; Espiritu, R.D.; Advincula, R.C. 3D-printed PDMS-based membranes for CO2 separation applications. MRS Commun. 2022, 12, 1174–1182. [Google Scholar] [CrossRef]
- Woo, R.; Chen, G.; Zhao, J.; Bae, J. Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane. ACS Appl. Polym. Mater. 2021, 3, 3496–3503. [Google Scholar] [CrossRef]
- Du, K.; Basuki, J.; Glattauer, V.; Mesnard, C.; Nguyen, A.T.; Alexander, D.L.J.; Hughes, T.C. Digital light processing 3D printing of PDMS-based soft and elastic materials with tunable mechanical properties. ACS Appl. Polym. Mater. 2021, 3, 3049–3059. [Google Scholar] [CrossRef]
- Dahlberg, T.; Stangner, T.; Zhang, H.; Wiklund, K.; Lundberg, P.; Edman, L.; Andersson, M. 3D printed water-soluble scaffolds for rapid production of PDMS micro-fluidic flow chambers. Sci. Rep. 2018, 8, 3372. [Google Scholar] [CrossRef]
- Montazerian, H.; Mohamed, M.; Montazeri, M.M.; Kheiri, S.; Milani, A.; Kim, K.; Hoorfar, M. Permeability and mechanical properties of gradient porous PDMS scaffolds fabricated by 3D-printed sacrificial templates designed with minimal surfaces. Acta Biomater. 2019, 96, 149–160. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J. Material extrusion 3D printing of carbon material reinforced PDMS matrix composites and their mechanical properties. Procedia Manuf. 2021, 53, 450–455. [Google Scholar] [CrossRef]
- Sales, F.C.; Ariati, R.M.; Noronha, V.T.; Ribeiro, J.E. Mechanical characterization of PDMS with different mixing ratios. Procedia Struct. Integr. 2022, 37, 383–388. [Google Scholar] [CrossRef]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and applications of PDMS for biomedical engineering: A review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane composites characterization and its applications: A review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Raj M, K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ye, E.; Li, Z.; Wang, D. Contact electrification behaviors of solid–liquid interface: Regulation, mechanisms, and applications. Adv. Energy Sustain. Res. 2023, 4, 2200186. [Google Scholar] [CrossRef]
- Riveiro, A.; Abalde, T.; Pou, P.; Soto, R.; del Val, J.; Comesaña, R.; Badaoui, A.; Boutinguiza, M.; Pou, J. Influence of laser texturing on the wettability of PTFE. Appl. Surf. Sci. 2020, 515, 145984. [Google Scholar] [CrossRef]
- Yang, J.; Su, R.; Ying, H.; Hu, L.; Ruan, X. Preparation and characterization of complex shaped polytetrafluoroethylene (PTFE) parts based on vat photopolymerization 3D printing. Mater. Today Commun. 2023, 36, 106764. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance properties and applications of polytetrafluoroethylene (PTFE)—A review. Adv. Compos. Hybrid Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Johansson, P.; Marklund, P.; Björling, M.; Shi, Y. Effect of roughness on the running-in behavior and tribofilm formation of carbon fiber reinforced PTFE composite in trace moisture environment. Wear 2022, 500–501, 204367. [Google Scholar] [CrossRef]
- Reznickova, A.; Sajdl, P.; Nguyenova, H.Y.; Lacmanova, V.; Kolska, Z.; Kasalkova, N.S.; Kvitek, O.; Svorcik, V. Plasma treatment of PTFE at elevated temperature: The effect of surface properties on its biological performance. Mater. Today Commun. 2022, 31, 103254. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.-J.; Ouyang, X.; Zhang, A.P.; Tam, H.-Y. 3D μ-printing of polytetrafluoroethylene microstructures: A route to superhydrophobic surfaces and devices. Appl. Mater. Today 2020, 19, 100580. [Google Scholar] [CrossRef]
- Tilmatine, O.; Zeghloul, T.; Fatu, A.; Dascalescu, L. Study of the effect of duration of non-thermal plasma treatment on the surface properties of polymers. IOP Conf. Ser. Mater. Sci. Eng. 2020, 724, 012050. [Google Scholar] [CrossRef]
- Jiang, Z.; Erol, O.; Chatterjee, D.; Xu, W.; Hibino, N.; Romer, L.H.; Kang, S.H.; Gracias, D.H. Direct ink writing of poly(tetrafluoroethylene) (PTFE) with tunable mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 28289–28295. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Wu, T.; Liang, H.; Peng, C.; Yang, J.; Jia, P. 3d-Printed Biomimetic Superhydrophobic and Multifunctional Ptfe Structures with Enhanced Wear Resistance. SSRN under review. [CrossRef]
- Ciniero, A.; Fatti, G.; Marsili, M.; Dini, D.; Righi, M.C. Defects drive the tribocharging strength of PTFE: An ab-initio study. Nano Energy 2023, 112, 108502. [Google Scholar] [CrossRef]
- Fatti, G.; Righi, M.C.; Dini, D.; Ciniero, A. Ab initio study of polytetrafluoroethylene defluorination for tribocharging applications. ACS Appl. Polym. Mater. 2020, 2, 5129–5134. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Chi, H.; Qiu, H.; Xue, H.; Bai, H. 3D printing of a polydimethylsiloxane/polytetrafluoroethylene composite elastomer and its application in a triboelectric nanogenerator. ACS Appl. Mater. Interfaces 2020, 12, 57441–57449. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Tao, J.; Hu, M.; Dai, Z. Sensitive self-powered particles detection based on cumulative triboelectric charging. Nano Energy 2021, 89, 106393. [Google Scholar] [CrossRef]
- Chen, F.; Liu, W.; Zhang, D. Femtosecond laser texturing assisted cold plasma hydrophilization of polytetrafluoroethylene surface. Appl. Surf. Sci. 2023, 641, 158488. [Google Scholar] [CrossRef]
- Singh, V.; Singla, A.K.; Bansal, A. Influence of laser texturing along with PTFE topcoat on slurry and cavitation erosion resistance of HVOF sprayed VC coating. Surf. Coat. Technol. 2023, 470, 129858. [Google Scholar] [CrossRef]
- Blanchet, T.A.; Kandanur, S.S.; Schadler, L.S. Coupled effect of filler content and countersurface roughness on PTFE nanocomposite wear resistance. Tribol. Lett. 2010, 40, 11–21. [Google Scholar] [CrossRef]
- Primc, G. Recent advances in surface activation of polytetrafluoroethylene (PTFE) by gaseous plasma treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Armitage, J.L.; Ghanbarzadeh, A.; Bryant, M.G.; Neville, A. Investigating the influence of friction and material wear on triboelectric charge transfer in metal–Polymer contacts. Tribol. Lett. 2022, 70, 46. [Google Scholar] [CrossRef]
- Liao, Z.; Yang, J.; Hossain, M.; Chagnon, G.; Yao, X. The time and temperature dependences of the stress recovery of Ecoflex polymer. Int. J. Non-Linear Mech. 2023, 149, 104338. [Google Scholar] [CrossRef]
- Liao, Z.; Hossain, M.; Yao, X. Ecoflex polymer of different Shore hardnesses: Experimental investigations and constitutive modelling. Mech. Mater. 2020, 144, 103366. [Google Scholar] [CrossRef]
- Lv, K.; Tian, G.; Yan, Y.; Zhou, H.; Fan, Q.; Liang, L.; Liu, N.; Wang, D.; Song, Z.; Xu, F.; et al. Stretchable carbon nanotube/Ecoflex conductive elastomer films toward multifunctional wearable electronics. Chem. Eng. J. 2024, 500, 157534. [Google Scholar] [CrossRef]
- Wang, X.; Tong, W.; Yu, R.; Zhang, J.; Liu, Y.; Gao, C.; Zhang, Y.; Wang, Z.; Liu, S.; An, Q.; et al. Mica’s homo-positive-charging behavior enabled porous elastomer TENG for energy harvesting in high humidity. Nano Energy 2024, 119, 109056. [Google Scholar] [CrossRef]
- Rusu, D.M.; Petrașcu, O.L.; Pascu, A.M.; Mândru, S.D. The Influence of Industrial Environmental Factors on Soft Robot Materials. Materials 2023, 16, 2948. [Google Scholar] [CrossRef]
- Ahmad, D.; Ajaj, R.M. A multiaxial fracture of ecoflex skin with different shore hardness for morphing wing application. Polymers 2023, 15, 1526. [Google Scholar] [CrossRef]
- Song, Z.; Cai, X.; Chen, Z.; Zhu, Z.; Cao, Y.; Li, W. Ultrathin, Stretchable, and Twistable Ferroelectret Nanogenerator for Facial Muscle Detection. Nanoenergy Adv. 2024, 4, 344–354. [Google Scholar] [CrossRef]
- Aw, K.; Budd, J.; Wilshaw-Sparkes, T. Data glove using soft and stretchable piezoresistive sensors. Micromachines 2022, 13, 372. [Google Scholar] [CrossRef]
- Tessier, A.; Zhuo, S.; Ameri, S.K. Ultrasoft Long-Lasting Reusable Hydrogel-Based Sensor Patch for Biosignal Recording. Biosensors 2024, 14, 405. [Google Scholar] [CrossRef]
- Zou, J.; Qiao, Y.; Zhao, J.; Duan, Z.; Yu, J.; Jing, Y.; He, J.; Zhang, L.; Chou, X.; Mu, J. Hybrid pressure sensor based on carbon nano-onions and hierarchical microstructures with synergistic enhancement mechanism for multi-parameter sleep monitoring. Nanomaterials 2023, 13, 2692. [Google Scholar] [CrossRef]
- Eltoukhy, K.A.; Aly, M.F.; Sarquella, M.; Langreo, C.; Serry, M. Fabrication and Optimization of Additively Manufactured Hybrid Nanogenerators for Wearable Devices. Nanomaterials 2025, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- Freire, A.L.; Lima, L.R.; Candido, I.C.M.; Silva, L.G.; Ribeiro, S.J.L.; Carrilho, E.; Oliveira, T.L.; de Oliveira, L.F.C.; Barud, H.S.; de Oliveira, H.P. Metal-Free, Bio-Triboelectric Nanogenerator Based on a Single Electrode of Bacterial Cellulose Modified with Carbon Black. Nanoenergy Adv. 2024, 4, 110–121. [Google Scholar] [CrossRef]
- Zheng, Q.; Jia, C.; Sun, F.; Zhang, M.; Wen, Y.; Xie, Z.; Wang, J.; Liu, B.; Mao, Y.; Zhao, C. Ecoflex Flexible Array of Triboelectric Nanogenerators for Gait Monitoring Alarm Warning Applications. Electronics 2023, 12, 3226. [Google Scholar] [CrossRef]
- Jia, C.; Zhu, Y.; Sun, F.; Wen, Y.; Wang, Q.; Li, Y.; Mao, Y.; Zhao, C. Gas-supported triboelectric nanogenerator based on in situ gap-generation method for biomechanical energy harvesting and wearable motion monitoring. Sustainability 2022, 14, 14422. [Google Scholar] [CrossRef]
- Migliorini, L.; Santaniello, T.; Falqui, A.; Milani, P. Super-stretchable resistive strain sensor based on ecoflex–gold nanocomposites. ACS Appl. Nano Mater. 2023, 6, 8999–9007. [Google Scholar] [CrossRef]
- Huang, H.; Feng, Y.; Yang, X.; Yang, L.; Shen, Y. An insect-inspired terrains-adaptive soft millirobot with multimodal locomotion and transportation capability. Micromachines 2022, 13, 1578. [Google Scholar] [CrossRef]
- Cholleti, E.R.; Stringer, J.; Kelly, P.; Bowen, C.; Aw, K. Mechanical behaviour of large strain capacitive sensor with barium titanate ecoflex composite used to detect human motion. Robotics 2021, 10, 69. [Google Scholar] [CrossRef]
- Fryń, P.; Lalik, S.; Górska, N.; Iwan, A.; Marzec, M. Comparison of the dielectric properties of ecoflex® with L,D-poly(lactic acid) or polycaprolactone in the presence of SWCN or 5CB. Materials 2021, 14, 1719. [Google Scholar] [CrossRef]
- del Bosque, A.; Sánchez-Romate, X.X.F.; Calvo, Á.D.L.L.; Fernández, P.R.; Borromeo, S.; Sánchez, M.; Ureña, A. Highly flexible strain sensors based on CNT-reinforced ecoflex silicone rubber for wireless facemask breathing monitoring via bluetooth. ACS Appl. Polym. Mater. 2023, 5, 8589–8599. [Google Scholar] [CrossRef]
- Truong, T.; Kim, J.-S.; Kim, J. Development of embroidery-type pressure sensor dependent on Interdigitated capacitive method. Polymers 2022, 14, 3446. [Google Scholar] [CrossRef]
- Wicklein, B.; Valurouthu, G.; Yoon, H.; Yoo, H.; Ponnan, S.; Mahato, M.; Kim, J.; Ali, S.S.; Park, J.Y.; Gogotsi, Y.; et al. Influence of MXene Composition on Triboelectricity of MXene-Alginate Nanocomposites. ACS Appl. Mater. Interfaces 2024, 16, 23948–23959. [Google Scholar] [CrossRef]
- Wang, M.; Hou, X.; Qian, S.; Xian, S.; Yu, J.; He, J.; Chou, X. An intelligent glove of synergistically enhanced ZnO/PAN-based piezoelectric sensors for diversified human–machine interaction applications. Electronics 2023, 12, 1782. [Google Scholar] [CrossRef]
- Fortunato, M.; Bellagamba, I.; Tamburrano, A.; Sarto, M.S. Flexible ecoflex®/graphene nanoplatelet foams for highly sensitive low-pressure sensors. Sensors 2020, 20, 4406. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chen, Z.; Wang, S.-J.; Liu, Z.-H.; Liu, Y.-J.; Feng, P.-Y.; Jing, X. A flexible sensor with excellent environmental stability using well-designed encapsulation structure. Polymers 2023, 15, 2308. [Google Scholar] [CrossRef] [PubMed]
- Khomiakova, N.; Hanuš, J.; Kuzminova, A.; Kylián, O. Investigation of wettability, drying and water condensation on polyimide (Kapton) films treated by atmospheric pressure air dielectric barrier discharge. Coatings 2020, 10, 619. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, D.; Liu, G.; Wei, Q. Atomic oxygen adaptability of flexible kapton/Al2O3 composite thin films prepared by ion exchange method. Coatings 2019, 9, 624. [Google Scholar] [CrossRef]
- Pan, Z.; Yuan, S.; Ren, X.; He, Z.; Wang, Z.; Han, S.; Qi, Y.; Yu, H.; Liu, J. Preparation and characterization of fluorine-containing polyimide films with enhanced output performance for potential applications as negative friction layers for triboelectric nanogenerators. Technologies 2023, 11, 136. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Q.; Huang, C.; Zhu, Y.; Hu, N. Dependence of incidence angle and flux density in the damage effect of atomic oxygen on kapton film. Polymers 2022, 14, 5444. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, R.; Singh, P.; Kumar, V.; Jaiswal, M.K.; Chakarvarti, S.; Kumar, R. Modifications in physico-chemical properties of 100 MeV oxygen ions irradiated polyimide Kapton-H polymer. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 406, 188–192. [Google Scholar] [CrossRef]
- Khelifa, M.; Yaakoubi, N.; Dridi, C.; Picart, P.; Fakri-Bouchet, L. Realization of Flexible NMR Microcoils. Proceedings 2017, 1, 625. [Google Scholar] [CrossRef]
- Fang, Y.; Hester, J.G.D.; Su, W.; Chow, J.H.; Sitaraman, S.K.; Tentzeris, M.M. A bio-enabled maximally mild layer-by-layer Kapton surface modification approach for the fabrication of all-inkjet-printed flexible electronic devices. Sci. Rep. 2016, 6, 39909. [Google Scholar] [CrossRef]
- Signore, M.A.; Rescio, G.; Francioso, L.; Casino, F.; Leone, A. Aluminum nitride thin film piezoelectric pressure sensor for Respiratory rate detection. Sensors 2024, 24, 2071. [Google Scholar] [CrossRef]
- Plis, E.A.; Engelhart, D.P.; Cooper, R.; Johnston, W.R.; Ferguson, D.; Hoffmann, R. Review of radiation-induced effects in polyimide. Appl. Sci. 2019, 9, 1999. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ye, E.; Li, Z.; Wang, D. Control methods and applications of interface contact electrification of triboelectric nanogenerators: A review. Mater. Res. Lett. 2022, 10, 97–123. [Google Scholar] [CrossRef]
- Henning, L.M.; Abdullayev, A.; Vakifahmetoglu, C.; Simon, U.; Bensalah, H.; Gurlo, A.; Bekheet, M.F. Review on polymeric, inorganic, and composite materials for air filters: From processing to properties. Adv. Energy Sustain. Res. 2021, 2, 2100005. [Google Scholar] [CrossRef]
- Arrington, C.B.; Rau, D.A.; Vandenbrande, J.A.; Hegde, M.; Williams, C.B.; Long, T.E. 3D printing carbonaceous objects from polyimide pyrolysis. ACS Macro Lett. 2021, 10, 412–418. [Google Scholar] [CrossRef]
- Zhang, Z.; Gurtaran, M.; Li, X.; Un, H.-I.; Qin, Y.; Dong, H. Characterization of magnetron sputtered BiTe-based thermoelectric thin films. Nanomaterials 2023, 13, 208. [Google Scholar] [CrossRef]
- Tong, P.; Wei, Q.; Hu, N.; Chen, X. Asynchronous synergistic damage effect of atomic oxygen and space micro debris on kapton film. Coatings 2022, 12, 179. [Google Scholar] [CrossRef]
- Bardakas, A.; Segkos, A.; Tsamis, C. Zinc Oxide-Based Rotational–Linear Triboelectric Nanogenerator. Appl. Sci. 2024, 14, 2396. [Google Scholar] [CrossRef]
- Rodrigues-Marinho, T.; Castro, N.; Correia, V.; Costa, P.; Lanceros-Méndez, S. Triboelectric energy harvesting response of different polymer-based materials. Materials 2020, 13, 4980. [Google Scholar] [CrossRef]
- Ray, A.; Roth, J.; Saruhan, B. Laser-induced interdigital structured graphene electrodes based flexible micro-supercapacitor for efficient peak energy storage. Molecules 2022, 27, 329. [Google Scholar] [CrossRef]
- Massaglia, G.; Spisni, G.; Serra, T.; Quaglio, M. Laser-Induced Graphene Electrodes for Flexible pH Sensors. Nanomaterials 2024, 14, 2008. [Google Scholar] [CrossRef]
- Kolomijec, A.; Jankowski-Mihułowicz, P.; Węglarski, M.; Bailiuk, N. Study on the impact of laser settings on parameters of induced graphene layers Constituting the ANTENNA of UHF RFIDLIG transponders. Sensors 2025, 25, 1906. [Google Scholar] [CrossRef]
- Jia, X.; Herrera-Alonso, M.; McCarthy, T.J. Nylon surface modification. Part Targeting the amide groups for selective introduction of reactive functionalities. Polymer 2006, 47, 4916–4924. [Google Scholar] [CrossRef]
- Dasgupta, S.; Hammond, W.B.; Goddard, W.A. Crystal structures and properties of nylon polymers from theory. J. Am. Chem. Soc. 1996, 118, 12291–12301. [Google Scholar] [CrossRef]
- Siddikali, P.; Sreekanth, P.S.R. Evaluation of Mechanical, Thermal, and Tribological Properties of 3D-Printed Nylon (PA6) Hybrid Composites Reinforced with MWCNTs and Carbon Fibers. J. Compos. Sci. 2025, 9, 155. [Google Scholar] [CrossRef]
- Randhawa, K.S.; Patel, A.D. Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers. e-Polymers 2020, 20, 733–745. [Google Scholar] [CrossRef]
- Rodríguez, M.; Vázquez-Vélez, E.; Martinez, H.; Torres, A. Superficial surface treatment using atmospheric plasma on recycled nylon 6. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2021, 8, 191–196. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Chattopadhyay, J.; Billups, W.E.; Winey, K.I. Tuning the mechanical properties of SWNT/Nylon 6,10 composites with flexible spacers at the interface. Nano Lett. 2007, 7, 1178–1185. [Google Scholar] [CrossRef]
- Calignano, F.; Lorusso, M.; Roppolo, I.; Minetola, P. Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing. Machines 2020, 8, 52. [Google Scholar] [CrossRef]
- de Jager, B.; Moxham, T.; Besnard, C.; Salvati, E.; Chen, J.; Dolbnya, I.P.; Korsunsky, A.M. Synchrotron X-ray scattering analysis of Nylon-12 crystallisation variation depending on 3D printing conditions. Polymers 2020, 12, 1169. [Google Scholar] [CrossRef]
- Le Bars, P.; Bandiaky, O.N.; Le Guéhennec, L.; Clouet, R.; Kouadio, A.A. Different polymers for the base of removable dentures? part I: A narrative review of Mechanical and physical properties. Polymers 2023, 15, 3495. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, Y.; Wen, J.; Yin, L.; Zhang, Q. Study on the Tribological Properties of MC Nylon Composites Filled with Hydraulic Oil. IOP Conf. Ser. Mater. Sci. Eng. 2018, 317, 012072. [Google Scholar] [CrossRef]
- Kovrigina, E.; Poletaeva, Y.; Zheng, Y.; Chubarov, A.; Dmitrienko, E. Nylon-6-coated doxorubicin-loaded magnetic nanoparticles and nanocapsules for cancer treatment. Magnetochemistry 2023, 9, 106. [Google Scholar] [CrossRef]
- Sun, B.; Xu, D.; Wang, Z.; Zhan, Y.; Zhang, K. Interfacial structure design for triboelectric nanogenerators. Battery Energy 2022, 1, 20220001. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, J.; Gao, H.; Loo, L.S. Substrate effects on the surface properties of nylon. Appl. Surf. Sci. 2013, 282, 115–120. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhao, J.P.; Kang, E.T.; Neoh, K.G.; Li, J. Functionalization of nylon membranes via surface-initiated atom-transfer Radical polymerization. Langmuir 2007, 23, 8585–8592. [Google Scholar] [CrossRef]
- Antonova, O.Y.; Kochetkova, O.Y.; Kanev, I.L. Light-to-heat converting ECM-mimetic nanofiber scaffolds for neuronal differentiation and neurite outgrowth guidance. Nanomaterials 2022, 12, 2166. [Google Scholar] [CrossRef]
- Čapková, P.; Čajka, A.; Kolská, Z.; Kormunda, M.; Pavlík, J.; Munzarová, M.; Dopita, M.; Rafaja, D. Phase composition and surface properties of nylon-6 nanofibers prepared by nanospider technology at various electrode distances. J. Polym. Res. 2015, 22, 101. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Chubarov, A.; Pyshnyi, D.; Dmitrienko, E. Doxorubicin-loaded silica nanocomposites for cancer treatment. Coatings 2023, 13, 324. [Google Scholar] [CrossRef]
- Mlinarić, N.M.; Wawrzaszek, B.; Kowalska, K.; Selmani, A.; Učakar, A.; Vidmar, J.; Kušter, M.; Van de Velde, N.; Trebše, P.; Škapin, A.S.; et al. Poly(allylamine hydrochloride) and ZnO Nanohybrid coating for the development of hydrophobic, antibacterial, and biocompatible textiles. Nanomaterials 2024, 14, 570. [Google Scholar] [CrossRef]
- Balan, G.S.; Raj, S.A.; Adithya, R.N. Effect of post-heat treatment on the mechanical and surface properties of nylon 12 produced via material extrusion and selective laser sintering processes. Polym. Bull. 2024, 81, 10149–10174. [Google Scholar] [CrossRef]
- Zhang, J.; Ferrie, S.; Zhang, S.; Vogel, Y.B.; Peiris, C.R.; Darwish, N.; Ciampi, S. Single-electrode electrochemistry: Chemically engineering surface adhesion and hardness to maximize redox work extracted from tribocharged silicon. ACS Appl. Nano Mater. 2019, 2, 7230–7236. [Google Scholar] [CrossRef]
- Zhang, J.; Coote, M.L.; Ciampi, S. Electrostatics and electrochemistry: Mechanism and scope of charge-transfer reactions on the surface of tribocharged insulators. J. Am. Chem. Soc. 2021, 143, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Gandha, K.; Li, L.; Nlebedim, I.; Post, B.K.; Kunc, V.; Sales, B.C.; Bell, J.; Paranthaman, M.P. Additive manufacturing of anisotropic hybrid NdFeB-SmFeN nylon composite bonded magnets. J. Magn. Magn. Mater. 2018, 467, 8–13. [Google Scholar] [CrossRef]
- Mushtaq, R.T.; Wang, Y.; Khan, A.M.; Rehman, M.; Li, X.; Sharma, S. A post-processing laser polishing method to improve process performance of 3D printed new Industrial Nylon-6 polymer. J. Manuf. Process. 2023, 101, 546–560. [Google Scholar] [CrossRef]
- Hartomacioğlu, S. Optimization of Production Parameters for Impact Strength of 3D-Printed Carbon/Glass Fiber-Reinforced Nylon Composite in Critical ZX Printing Orientation. Polymers 2024, 16, 3006. [Google Scholar] [CrossRef]
- Ramanathan, A.; Thippanna, V.; Kumar, A.S.; Sundaravadivelan, B.; Zhu, Y.; Ravichandran, D.; Yang, S.; Song, K. Highly loaded carbon fiber filaments for 3D-printed composites. J. Polym. Sci. 2023, 62, 2670–2682. [Google Scholar] [CrossRef]
- Zárybnická, L.; Machotová, J.; Pagáč, M.; Rychlý, J.; Vykydalová, A. The effect of filling density on flammability and mechanical properties of 3D-printed carbon fiber-reinforced nylon. Polym. Test. 2023, 120, 107944. [Google Scholar] [CrossRef]
- Wang, S.; Ge, S.; Zhang, D. Comparison of tribological behavior of nylon composites filled with zinc oxide particles and whiskers. Wear 2009, 266, 248–254. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, Y.; Zhang, H.; Zhang, W.; Tu, S. Preparation and biocompatibility of grafted functional β-cyclodextrin copolymers from the surface of PET films. Mater. Sci. Eng. C 2014, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Kim, J.H.; Nam, S.Y.; Kwon, D.J. Triboelectric properties of recycled poly (ethylene terephthalate) nano-fibrous web from waste clothes. In Colloids and Surfaces A: Physicochemical and Engineering Aspects; Elsevier: Amsterdam, The Netherlands, 2025; Volume 717, p. 136803. [Google Scholar] [CrossRef]
- Szafran, K.; Jurak, M.; Mroczka, R.; Wiącek, A.E. Surface properties of the polyethylene terephthalate (PET) substrate modified with the phospholipid-polypeptide-antioxidant films: Design of functional biocoatings. Pharmaceutics 2022, 14, 2815. [Google Scholar] [CrossRef] [PubMed]
- Keykha, M.; Sheikholeslami, T.F. The comparison of triboelectric power generated by electron-donating polymers KAPTON and PDMS in contact with PET polymer. Energy Harvest. Syst. 2022, 9, 53–61. [Google Scholar] [CrossRef]
- Thakur, V.N.; Han, J.I. Combined triboelectric and piezoelectric effect in ZnO/PVDF hybrid-based fiber-structured nanogenerator with Pdms: Carbon black electrodes. Polymers 2022, 14, 4414. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Lü, X.; Wang, Y.; Cui, N.; Li, B.; Ding, M.; Liu, J.; Guo, Z.; Gu, L. Mitigating public hygiene anxiety in waste material applications: Development of an antibacterial and high performance triboelectric nanogenerator from recycled PET. Nano Energy 2025, 134, 110533. [Google Scholar] [CrossRef]
- Roy, S.; Maji, P.K.; Goh, K.-L. Sustainable design of flexible 3D aerogel from waste PET bottle for wastewater treatment to energy harvesting device. Chem. Eng. J. 2021, 413, 127409. [Google Scholar] [CrossRef]
- Lai, W.L.; Sharma, S.; Roy, S.; Maji, P.K.; Sharma, B.; Ramakrishna, S.; Goh, K.L. Roadmap to sustainable plastic waste management: A focused study on recycling PET for triboelectric nanogenerator production in Singapore and India. Environ. Sci. Pollut. Res. 2022, 29, 51234–51268. [Google Scholar] [CrossRef]
- Slobodian, P.; Riha, P.; Hausnerova, B. Unsorted Postconsumer Plastic Waste in Energy Conversion Using Piezoelectric, Triboelectric, and Pyroelectric Generation Mechanisms. ACS Sustain. Chem. Eng. 2025, 13, 2683–2693. [Google Scholar] [CrossRef]
- Lim, N.; Hong, D.; Kim, C.; Jeong, J.; Lee, H.W.; Kwon, K.-H. Inductively coupled plasma surface modification of polyethylene terephthalate and application in a triboelectric generator. Thin Solid Films 2017, 637, 27–31. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kwon, Y.H.; Kim, Y.-H.; Jung, J.-Y.; Lee, M.H.; Nah, J. Triboelectric charging sequence induced by surface functionalization as a method to fabricate high performance triboelectric generators. ACS Nano 2015, 9, 4621–4627. [Google Scholar] [CrossRef]
- Shin, S.-H.; Bae, Y.E.; Moon, H.K.; Kim, J.; Choi, S.-H.; Kim, Y.; Yoon, H.J.; Lee, M.H.; Nah, J. Formation of triboelectric series via atomic-level surface functionalization for triboelectric energy harvesting. ACS Nano 2017, 11, 6131–6138. [Google Scholar] [CrossRef]
- Yu, K.-K.; Zhao, T.-Q.; Luo, Q.-L.; Ping, Y. Recycled PET Fibers with Dopamine Surface Modification for Enhanced Interlayer Adhesion in 3D Printed Concrete. Materials 2024, 17, 5126. [Google Scholar] [CrossRef] [PubMed]
- Celik, Y.; Shamsuyeva, M.; Endres, H.J. Thermal and mechanical properties of the recycled and virgin PET—Part I. Polymers 2022, 14, 1326. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, M.; Ptak, A. The Experimental Comparison of Abrasion Resistance of Extruded and 3D Printed Plastics. Materials 2025, 18, 1592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Scaraggi, M.; Zheng, Y.; Li, X.; Wu, Y.; Wang, D.; Dini, D.; Zhou, F. Quantifying wetting dynamics with triboelectrification. Adv. Sci. 2022, 9, e2200822. [Google Scholar] [CrossRef]
- Reisinger, B.; Fahrner, M.; Frischauf, I.; Yakunin, S.; Svorcik, V.; Fiedorowicz, H.; Bartnik, A.; Romanin, C.; Heitz, J. EUV micropatterning for biocompatibility control of PET. Appl. Phys. A 2010, 100, 511–516. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Q.; Xu, X.; Song, M.; Lu, Y.; Yang, L.; Wang, W.; Wang, Y.; Li, M.; Wang, D. Construction Strategy for Flexible and Breathable SiO2/Al/NFs/PET Composite Fabrics with Dual Shielding against Microwave and Infrared–Thermal Radiations for Wearable Protective Clothing. Polymers 2023, 16, 6. [Google Scholar] [CrossRef]
- Stoyanova, N.; Paneva, D.; Mincheva, R.; Toncheva, A.; Manolova, N.; Dubois, P.; Rashkov, I. Poly(L-lactide) and poly(butylene succinate) immiscible blends: From electrospinning to biologically active materials. Mater. Sci. Eng. C 2014, 41, 119–126. [Google Scholar] [CrossRef]
- Kang, J.-W.; Ji, I.S.; Lee, S.K.; Lee, J.M.; Hong, J. Flexible Polytetrafluoroethylene (PTFE) Tube-Based Curved Serpentine Micromixer Guided by 3D-Printed Frames. BioChip J. 2025, 19, 287–300. [Google Scholar] [CrossRef]
- Jucius, D.; Guobienė, A.; Grigaliūnas, V. Surface texturing of polytetrafluoroethylene by hot embossing. Appl. Surf. Sci. 2010, 256, 2164–2169. [Google Scholar] [CrossRef]
- Ovid’Ko, I.A. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial mechanisms and effectiveness of graphene and graphene-functionalized biomaterials. A scope review. Front. Bioeng. Biotechnol. 2020, 8, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, A.-I.; Aghasoleimani, K.; Semertsidou, A.; Vyas, J.; Roșca, A.-L.; Ficai, D.; Ficai, A. Graphene-Related Nanomaterials for Biomedical Applications. Nanomaterials 2023, 13, 1092. [Google Scholar] [CrossRef]
- Laraba, S.R.; Luo, W.; Rezzoug, A.; Zahra, Q.U.A.; Zhang, S.; Wu, B.; Chen, W.; Xiao, L.; Yang, Y.; Wei, J.; et al. Graphene-based composites for biomedical applications. Green Chem. Lett. Rev. 2022, 15, 724–748. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Comprehensive application of graphene: Emphasis on biomedical concerns. Nano-Micro Lett. 2019, 11, 6. [Google Scholar] [CrossRef]
- Han, S.; Sun, J.; He, S.; Tang, M.; Chai, R. The application of graphene-based biomaterials in biomedicine. Am. J. Transl. Res. 2019, 11, 3246–3260. [Google Scholar]
- Chen, C.; Xi, Y.; Weng, Y. Progress in the development of graphene-based biomaterials for tissue engineering and regeneration. Materials 2022, 15, 2164. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Caminero, M.Á.; Chacón, J.M.; García-Plaza, E.; Núñez, P.J.; Reverte, J.M.; Becar, J.P. Additive manufacturing of Pla-based composites using fused filament fabrication: Effect of graphene nanoplatelet reinforcement on mechanical properties, dimensional accuracy and texture. Polymers 2019, 11, 799. [Google Scholar] [CrossRef]
- Jiao, Y.; Lin, Z.; Guo, X.; Zhou, L.; Yang, Y.; Hu, X.; Hu, Z.; Zhao, X.; Xiao, J.; Li, T.; et al. Compositional engineering of hybrid organic–inorganic lead-halide perovskite and PVDF-graphene for high-performance triboelectric nanogenerators. ACS Appl. Mater. Interfaces 2024, 16, 3532–3541. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Guo, Y.; Chen, Y.; Zhang, H.; Xiao, J.; Hou, M.; Liu, H.; Ma, L.; Chen, X.; Wong, C. Advances in graphene-based electrode for triboelectric nanogenerator. Nano-Micro Lett. 2025, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M. Theoretical study on a graphene triboelectric nanogenerator with metal contacts. Eur. Phys. J. Plus 2018, 133, 334. [Google Scholar] [CrossRef]
- Zhang, R.; Hummelgård, M.; Örtegren, J.; Andersson, H.; Olsen, M.; Chen, D.; Li, J.; Eivazi, A.; Dahlström, C.; Norgren, M.; et al. Triboelectric nanogenerators with ultrahigh current density enhanced by hydrogen bonding between nylon and graphene oxide. Nano Energy 2023, 115, 108737. [Google Scholar] [CrossRef]
- Parvez, A.N.; Rahaman, H.; Kim, H.C.; Ahn, K.K. Optimization of triboelectric energy harvesting from falling water droplet onto wrinkled polydimethylsiloxane-reduced graphene oxide nanocomposite surface. Compos. Part B Eng. 2019, 174, 106923. [Google Scholar] [CrossRef]
- Deepak, D.; Soin, N.; Roy, S.S. Optimizing the efficiency of triboelectric nanogenerators by surface nanoarchitectonics of graphene-based electrodes: A review. Mater. Today Commun. 2023, 34, 105412. [Google Scholar] [CrossRef]
- Stanford, M.G.; Li, J.T.; Chyan, Y.; Wang, Z.; Wang, W.; Tour, J.M. Laser-induced graphene triboelectric nanogenerators. ACS Nano 2019, 13, 7166–7174. [Google Scholar] [CrossRef]
- Shin, J.; Ji, S.; Cho, H.; Park, J. Highly flexible triboelectric nanogenerator using porous carbon nanotube composites. Polymers 2023, 15, 1135. [Google Scholar] [CrossRef]
- Harnchana, V.; Van Ngoc, H.; He, W.; Rasheed, A.; Park, H.; Amornkitbamrung, V.; Kang, D.J. Enhanced power output of a triboelectric nanogenerator using poly(dimethylsiloxane) modified with graphene oxide and sodium dodecyl sulfate. ACS Appl. Mater. Interfaces 2018, 10, 25263–25272. [Google Scholar] [CrossRef]
- Gaihre, B.; Potes, M.A.; Serdiuk, V.; Tilton, M.; Liu, X.; Lu, L. Two-dimensional nanomaterials-added dynamism in 3D printing and bioprinting of biomedical platforms: Unique opportunities and challenges. Biomaterials 2022, 284, 121507. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Fan, Z.; Wang, W.; Wang, B.; Guo, Z. Ink-based 3D printing technologies for graphene-based materials: A review. Adv. Compos. Hybrid Mater. 2019, 2, 1–33. [Google Scholar] [CrossRef]
- García-Tuñón, E.; Feilden, E.; Zheng, H.; D’eLia, E.; Leong, A.; Saiz, E. Graphene oxide: An all-in-one processing additive for 3D printing. ACS Appl. Mater. Interfaces 2017, 9, 32977–32989. [Google Scholar] [CrossRef]
- Poosala, A.; Hrimchum, K.; Aussawasathien, D.; Pentrakoon, D.; Voicu, S.I. The Effect of Oxygen-Plasma Treated Graphene Nanoplatelets upon the Properties of Multiwalled Carbon Nanotube and Polycarbonate Hybrid Nanocomposites Used for Electrostatic Dissipative Applications. J. Nanomater. 2015, 2015, 470297. [Google Scholar] [CrossRef]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Villate-Beitia, I.; Zárate, J.; Puras, G.; Pedraz, J.L. Carbon-based nanostructures as emerging materials for gene delivery applications. Pharmaceutics 2024, 16, 288. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.; Baltatu, M.S.; Vizureanu, P. The effectiveness mechanisms of carbon nanotubes (CNTs) as reinforcements for magnesium-based composites for biomedical applications: A review. Nanomaterials 2024, 14, 756. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Hirlekar, R.; Yamagar, M.; Garse, H.; Vij, M.; Kadam, V. Carbon nanotubes and its applications: A review. Asian J. Pharm. Clin. Res. 2009, 2, 17–27. [Google Scholar]
- Ghoshal, S. Polymer/carbon nanotubes (CNT) nanocomposites processing using additive manufacturing (three-dimensional printing) technique: An overview. Fibers 2017, 5, 40. [Google Scholar] [CrossRef]
- Ujah, C.O.; Von Kallon, D.V.; Aigbodion, V.S. tribological properties of CNTs-reinforced nano composite materials. Lubricants 2023, 11, 95. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Văduva, M.; Nila, A.; Udrescu, A.; Cramariuc, O.; Baibarac, M. Nanocomposites Based on Iron Oxide and Carbonaceous Nanoparticles: From Synthesis to Their Biomedical Applications. Materials 2024, 17, 6127. [Google Scholar] [CrossRef] [PubMed]
- Il’ina, M.V.; Soboleva, O.I.; Khubezov, S.A.; Smirnov, V.A.; Il’in, O.I. Study of nitrogen-doped carbon nanotubes for creation of piezoelectric nanogenerator. J. Low Power Electron. Appl. 2023, 13, 11. [Google Scholar] [CrossRef]
- González, J.; Ghaffarinejad, A.; Ivanov, M.; Ferreira, P.; Vilarinho, P.M.; Borrás, A.; Amorín, H.; Wicklein, B. Advanced cellulose–nanocarbon composite films for high-performance triboelectric and piezoelectric nanogenerators. Nanomaterials 2023, 13, 1206. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Verma, P.; Kumar, S. Electrical conductivity of CNT/polymer composites: 3D printing, measurements and modeling. Compos. Part B Eng. 2020, 183, 107600. [Google Scholar] [CrossRef]
- Podsiadły, B.; Matuszewski, P.; Skalski, A.; Słoma, M. Carbon nanotube-based Composite FILAMENTS for 3D printing of structural and conductive elements. Appl. Sci. 2021, 11, 1272. [Google Scholar] [CrossRef]
- Murakami, T.; Yada, N.; Yoshida, S. Carbon Nanotube-Based Printed All-Organic Microelectrode Arrays for Neural Stimulation and Recording. Micromachines 2024, 15, 650. [Google Scholar] [CrossRef]
- Golovakhin, V.; Kim, E.Y.; Novgorodtseva, O.N.; Maksimovskiy, E.A.; Ukhina, A.V.; Ishchenko, A.V.; Bannov, A.G. Treatment of multi-walled carbon nanotubes with dichromic acid: Oxidation and appearance of intercalation. Membranes 2023, 13, 729. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Xu, T.; Cui, H.; Li, X. Triboelectric nanogenerator for droplet energy harvesting based on hydrophobic composites. Materials 2023, 16, 5439. [Google Scholar] [CrossRef]
- Patil, R.; Alimperti, S. Graphene in 3D bioprinting. J. Funct. Biomater. 2024, 15, 82. [Google Scholar] [CrossRef]
- Adami, R.; Lamberti, P.; Casa, M.; D’avanzo, N.; Ponticorvo, E.; Cirillo, C.; Sarno, M.; Bychanok, D.; Kuzhir, P.; Yu, C.; et al. Synthesis and electrical percolation of highly amorphous polyvinyl alcohol/reduced graphene oxide nanocomposite. Materials 2023, 16, 4060. [Google Scholar] [CrossRef]
- Ge, K.; Shao, H.; Raymundo-Piñero, E.; Taberna, P.-L.; Simon, P. Cation desolvation-induced capacitance enhancement in reduced graphene oxide (rGO). Nat. Commun. 2024, 15, 1935. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Reduced graphene oxide (rGO)-loaded metal-oxide nanofiber gas sensors: An overview. Sensors 2021, 21, 1352. [Google Scholar] [CrossRef] [PubMed]
- Perumal, D.; Albert, E.L.; Saad, N.; Hin, T.Y.Y.; Zawawi, R.M.; Teh, H.F.; Abdullah, C.A.C. Fabrication and characterization of clinacanthus nutans mediated reduced graphene oxide using a green approach. Crystals 2022, 12, 1539. [Google Scholar] [CrossRef]
- Singh, R.; Ullah, S.; Rao, N.; Singh, M.; Patra, I.; Darko, D.A.; Issac, C.P.J.; Esmaeilzadeh-Salestani, K.; Kanaoujiya, R.; Vijayan, V.; et al. Synthesis of Three-Dimensional Reduced-Graphene Oxide from Graphene Oxide. J. Nanomater. 2022, 2022, 8731429. [Google Scholar] [CrossRef]
- Viprya, P.; Kumar, D.; Kowshik, S. Study of different properties of graphene oxide (GO) and reduced graphene oxide (rGO). Eng. Proc. 2023, 59, 84. [Google Scholar] [CrossRef]
- Jagódka, P.; Matus, K.; Łamacz, A. On the HKUST-1/GO and HKUST-1/rGO composites: The impact of synthesis method on Physicochemical properties. Molecules 2022, 27, 7082. [Google Scholar] [CrossRef]
- Rawat, S.S.; Harsha, A.P.; Chouhan, A.; Khatri, O.P. Effect of graphene-based nanoadditives on the tribological and rheological performance of paraffin grease. J. Mater. Eng. Perform. 2020, 29, 2235–2247. [Google Scholar] [CrossRef]
- Das, P.; Deoghare, A.B.; Maity, S.R. A novel approach to synthesize reduced graphene oxide (RGO) at low thermal conditions. Arab. J. Sci. Eng. 2021, 46, 5467–5475. [Google Scholar] [CrossRef]
- Smirnov, A.; Pristinskiy, Y.; Pinargote, N.W.S.; Meleshkin, Y.; Podrabinnik, P.; Volosova, M.; Grigoriev, S. Mechanical Performance and Tribological Behavior of WC-ZrO2 Composites with Different Content of Graphene Oxide Fabricated by Spark Plasma Sintering. Sci 2024, 6, 82. [Google Scholar] [CrossRef]
- Scarpa, D.; Iuliano, M.; Cirillo, C.; Iovane, P.; Borriello, C.; Portofino, S.; Ponticorvo, E.; Galvagno, S.; Sarno, M. Self-assembled monolayers of reduced graphene oxide for robust 3D-printed supercapacitors. Sci. Rep. 2024, 14, 14998. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Prabhakaran, A.; Gupta, S.; Subramanian, V.R. Boosting photocatalytic activity using reduced graphene oxide (RGO)/semiconductor nanocomposites: Issues and future scope. ACS Omega 2021, 6, 8734–8743. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Kong, L.; He, J. Tribological Behavior of Reduced Graphene Oxide–Al2O3 Nanofluid: Interaction among Testing Force, Rotational Speed and Nanoparticle Concentration. Materials 2022, 15, 5177. [Google Scholar] [CrossRef] [PubMed]
- Öztekin, D.; Arbağ, H.; Yaşyerli, S. Preparation of RGO with Enhanced Electrical Conductivity: Effects of Sequential Reductions of L-Ascorbic Acid and Thermal. Arab. J. Sci. Eng. 2025, 50, 9905–9918. [Google Scholar] [CrossRef]
- Andrade, S.K.S.; Lakshmi, S.S.; Bakos, I.; Klébert, S.; Kun, R.; Mohai, M.; Nagy, B.; László, K. The influence of reduced graphene oxide on the texture and chemistry of N,S-doped porous carbon. Implications for electrocatalytic and energy storage applications. Nanomaterials 2023, 13, 2364. [Google Scholar] [CrossRef]
- Sha, M.S.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Alahmad, J.K.; Cabibihan, J.-J.; Karnan, M.; Sadasivuni, K.K. Photocatalytic degradation of organic dyes using reduced graphene oxide (rGO). Sci. Rep. 2024, 14, 3608. [Google Scholar] [CrossRef]
- Wilczek, P.; Major, R.; Lipinska, L.; Lackner, J.; Mzyk, A. Thrombogenicity and biocompatibility studies of reduced graphene oxide modified acellular pulmonary valve tissue. Mater. Sci. Eng. C 2015, 53, 310–321. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wu, Z.; Kang, Y.; Xie, S.; Cai, Z.; Shan, X.; Li, Q. Pulsed electromagnetic field-assisted reduced graphene oxide composite 3D printed nerve scaffold promotes sciatic nerve regeneration in rats. Biofabrication 2024, 16, 035013. [Google Scholar] [CrossRef]
- Kang, M.S.; Jeong, S.J.; Lee, S.H.; Kim, B.; Hong, S.W.; Lee, J.H.; Han, D.-W. Reduced graphene oxide coating enhances osteogenic differentiation of human mesenchymal stem cells on Ti surfaces. Biomater. Res. 2021, 25, 1–9. [Google Scholar] [CrossRef]
- Sarno, M.; Scarpa, D.; Senatore, A.; Mustafa, W.A.A. rGO/GO Nanosheets in tribology: From the state of the art to the Future prospective. Lubricants 2020, 8, 31. [Google Scholar] [CrossRef]
- Sieradzka, M.; Fabia, J.; Biniaś, D.; Graczyk, T.; Fryczkowski, R. High-impact polystyrene reinforced with reduced graphene oxide as a filament for fused filament fabrication 3D printing. Materials 2021, 14, 7008. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Zhang, L.; Shah, A.H.; Chen, Z. Material extrusion 3D printing of synergistically enhanced conductive poly(lactic) acid polymer composites with reduced graphene oxide and glass fibers for high-performance electronic applications. Compos. Struct. 2025, 362, 119104. [Google Scholar] [CrossRef]
- Silva, M.V.C.O.; Carvalho, M.S.; Silva, L.R.G.; Rocha, R.G.; Cambraia, L.V.; Janegitz, B.C.; Nossol, E.; Muñoz, R.A.A.; Richter, E.M.; Stefano, J.S. Tailoring 3D-printed sensor properties with reduced-graphene oxide: Improved conductive filaments. Microchim. Acta 2024, 191, 633. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, C.; Osendi, M.I.; Moyano, J.J.; Mosa, J.; Aparicio, M. Electrochemical response of 3D-printed free-standing reduced graphene oxide electrode for sodium ion batteries using a three-electrode glass cell. Materials 2023, 16, 5386. [Google Scholar] [CrossRef]
- Jena, K.K.; Mayyas, A.T.; Mohanty, B.; Jena, B.K.; Jos, J.R.; AlFantazi, A.; Chakraborty, B.; Almarzooqi, A.A. Recycling of electrode materials from spent lithium-ion batteries to develop graphene Nanosheets and Graphene–molybdenum disulfide nanohybrid: Environmental benefits, analysis of supercapacitor performance, and influence of density functional theory calculations. Energy Fuels 2022, 36, 2159–2170. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cordero-Lanzac, T.; Gonzalez, A.; Zangi, R.; Tomovska, R. Reduced graphene oxide/polymer monolithic materials for selective CO2 capture. Polymers 2020, 12, 936. [Google Scholar] [CrossRef]
- Wang, R.; Fang, B.; Liang, H.; Mo, R. Advanced partially holey reduced graphene oxide inks for 3D printing self-healing thick electrodes with ultra-high areal desalination capacity. Desalination 2025, 602, 118605. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Chitvuttichot, S.; Tuantranont, A.; Treeratanaphitak, T.; Gostick, J. Development of 3D-Printed Electrodes Using Polyacrylonitrile/Graphene Composites for Application in Polysulfide Bromide Flow Battery. In Chemical Engineering and Processing-Process Intensification; Elsevier: Amsterdam, The Netherlands, 2025; p. 110233. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Wang, S.; Fang, D.; Huang, J.; Zhao, G.; Liu, Y. Facile in situ preparation of Cu/RGO nanohybrid for enhancing the tribological performance of phenolic resins nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5, 1280–1293. [Google Scholar] [CrossRef]
- Kelesidis, G.A.; Benz, S.; Pratsinis, S.E. Process design for carbon black size and morphology. Carbon 2023, 213, 118255. [Google Scholar] [CrossRef]
- Long, C.M.; Nascarella, M.A.; Valberg, P.A. Carbon black vs. black carbon and other airborne materials containing elemental carbon: Physical and chemical distinctions. Environ. Pollut. 2013, 181, 271–286. [Google Scholar] [CrossRef]
- Drozdov, V.A.; Gulyaeva, T.I.; Trenikhin, M.V. Effect of irradiation with a continuous beam of accelerated electrons on the texture and nanostructure of carbon black: A study by adsorption and high-resolution transmission Electron microscopy. Russ. J. Appl. Chem. 2018, 91, 1635–1641. [Google Scholar] [CrossRef]
- So, S.-Y.; Park, S.-H.; Park, S.-H.; Gwak, G.-M.; Lyu, S.-K. Additive-manufactured flexible triboelectric sensor based on porous PDMS sponge for highly detecting joint movements. Int. J. Precis. Eng. Manuf. Technol. 2023, 10, 97–107. [Google Scholar] [CrossRef]
- Koterwa, A.; Kaczmarzyk, I.; Mania, S.; Cieslik, M.; Tylingo, R.; Ossowski, T.; Bogdanowicz, R.; Niedziałkowski, P.; Ryl, J. The role of electrolysis and enzymatic hydrolysis treatment in the enhancement of the electrochemical properties of 3D-printed carbon black/poly(lactic acid) structures. Appl. Surf. Sci. 2022, 574, 151587. [Google Scholar] [CrossRef]
- Kim, N.P. 3D-printed conductive carbon-infused thermoplastic polyurethane. Polymers 2020, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Carbon fiber biocompatibility for implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Taşlı, A.E.; Knapczyk-Korczak, J.; Stachewicz, U. Steering triboelectric and mechanical properties of polymer fibers with carbon black. Compos. Sci. Technol. 2023, 243, 110247. [Google Scholar] [CrossRef]
- Kosmidou, T.V.; Vatalis, A.S.; Delides, C.G.; Logakis, E.; Pissis, P.; Papanicolaou, G.C. Structural, mechanical and electrical characterization of epoxy-amine/carbon black nanonocomposites. Express Polym. Lett. 2008, 2, 364–372. [Google Scholar] [CrossRef]
- Brunella, V.; Rossatto, B.G.; Mastropasqua, C.; Cesano, F.; Scarano, D. Thermal/electrical properties and texture of carbon black PC polymer composites near the electrical percolation threshold. J. Compos. Sci. 2021, 5, 212. [Google Scholar] [CrossRef]
- Karásek, L.; Sumita, M. Characterization of dispersion state of filler and polymer-filler interactions in rubber-carbon black composites. J. Mater. Sci. 1996, 31, 281–289. [Google Scholar] [CrossRef]
- Fan, L.; Wang, G.; Wang, W.; Lu, H.; Yang, F.; Rui, X. Size effect of carbon black on the structure and mechanical properties of magnetorheological elastomers. J. Mater. Sci. 2018, 54, 1326–1340. [Google Scholar] [CrossRef]
- Tavakoli, Y.; Barmouz, M.; Azarhoushang, B. Electrically actuated 4D printed hybrid copper fiber-carbon black rein-forced composites. Polym. Test. 2025, 147, 108799. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Grammatikos, S.; Tzounis, L. Multi-functional medical grade Polyamide12/Carbon black nanocomposites in material extrusion 3D printing. Compos. Struct. 2023, 311, 116788. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, B.; Fu, F.; You, F.; Dong, X.; Dai, M. Resistivity and its anisotropy characterization of 3D-printed acrylonitrile butadiene styrene copolymer (ABS)/carbon black (CB) composites. Appl. Sci. 2017, 7, 20. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Tzounis, L.; Velidakis, E.; Mountakis, N.; Grammatikos, S.A. Polyamide 12/Multiwalled carbon nanotube and carbon black Nanocomposites manufactured by 3D printing fused filament fabrication: A comparison of the electrical, thermoelectric, and mechanical properties. Compos. Struct. 2021, 7, 38. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Děkanovský, L.; Chacko, L.; Wu, B.; Luxa, J.; Regner, J.; Paštika, J.; Koňáková, D.; Sofer, Z. Unraveling the Versatility of Carbon Black–Polylactic Acid (CB/PLA) 3D-Printed Electrodes via Sustainable Electrochemical Activation. Small Methods 2025, e2402214. [Google Scholar] [CrossRef]
- Keel, E.; Ejaz, A.; Mckinlay, M.; Garcia, M.P.; Caffio, M.; Gibson, D.; Núñez, C.G. Three-dimensional graphene foam based triboelectric nanogenerators for energy systems and autonomous sensors. Nano Energy 2023, 112, 108475. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Sintusiri, J.; Hongsrichan, P.; Boonsri, P.; Tongjune, P.; Sriwong, C.; Ruttanapun, C.; Thongbai, P.; Harnchana, V. Development of functional construction materials from cement–reduced graphene oxide composite capable of generating electricity with improved mechanical strength. J. Mater. Sci. 2024, 59, 16568–16582. [Google Scholar] [CrossRef]
- Dey, A.; Varagnolo, S.; Power, N.P.; Vangapally, N.; Elias, Y.; Damptey, L.; Jaato, B.N.; Gopalan, S.; Golrokhi, Z.; Sonar, P.; et al. Doped MXenes—A new paradigm in 2D systems: Synthesis, properties and applications. Prog. Mater. Sci. 2023, 139, 101166. [Google Scholar] [CrossRef]
- Pabba, D.P.; Satthiyaraju, M.; Ramasdoss, A.; Sakthivel, P.; Chidhambaram, N.; Dhanabalan, S.; Abarzúa, C.V.; Morel, M.J.; Udayabhaskar, R.; Mangalaraja, R.V.; et al. MXene-based nanocomposites for piezoelectric and triboelectric energy harvesting applications. Micromachines 2023, 14, 1273. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Mao, Y.; Li, Z. Progress and biomedical applications of MXenes. Nano Sel. 2021, 2, 1480–1508. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Wetting of MXenes and Beyond. Nano-Micro Lett. 2023, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Bilibana, M.P. Electrochemical properties of MXenes and applications. Adv. Sens. Energy Mater. 2023, 2, 100080. [Google Scholar] [CrossRef]
- Rahman, U.U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as emerging materials: Synthesis, properties, and applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Lai, W.; Guo, M. MXene Surface Architectonics: Bridging Molecular Design to Multifunctional Applications. Molecules 2025, 30, 1929. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, X.; Wang, N. Beyond energy harvesting: A review on the critical role of MXene in triboelectric nanogenerator. Energy Mater. 2024, 4, 400035. [Google Scholar] [CrossRef]
- Aghayar, Z.; Malaki, M.; Zhang, Y. MXene-based ink design for printed applications. Nanomaterials 2022, 12, 4346. [Google Scholar] [CrossRef]
- Matias, M.L.; Pereira, C.; Almeida, H.V.; Jana, S.; Panigrahi, S.; Menda, U.D.; Nunes, D.; Fortunato, E.; Martins, R.; Nandy, S. 3D printed MXene architectures for a plethora of smart applications. Mater. Today Adv. 2024, 23, 100512. [Google Scholar] [CrossRef]
- Jung, S.; Zafar, U.; Achary, L.S.K.; Koo, C.M. Ligand chemistry for surface functionalization in MXenes: A review. EcoMat 2023, 5, 12395. [Google Scholar] [CrossRef]
- Li, Y.; Kankala, R.K.; Chen, A.-Z.; Wang, S.-B. 3D Printing of Ultrathin MXene toward Tough and Thermally Resistant Nanocomposites. Nanomaterials 2022, 12, 2862. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Huang, F.; Tao, X.; Ouyang, Y.; Zhou, Y.; Mo, X. High-output lotus-leaf-bionic triboelectric nanogenerators based on 2D MXene for health monitoring of human feet. Nanomaterials 2022, 12, 3217. [Google Scholar] [CrossRef]
- Dehaghi, F.M.; Aberoumand, M.; Sundararaj, U. A Review on Multifunctional Polymer–MXene Hybrid Materials for Electronic Applications. Molecules 2025, 30, 1955. [Google Scholar] [CrossRef] [PubMed]
- Firouzjaei, M.D.; Karimiziarani, M.; Moradkhani, H.; Elliott, M.; Anasori, B. MXenes: The two-dimensional influencers. Mater. Today Adv. 2022, 13, 100202. [Google Scholar] [CrossRef]
- Ferreira, M.P.S.; Ferreira, I.; Pais, V.; Leite, L.; Bessa, J.; Cunha, F.; Fangueiro, R. Towards Perfluoroalkyl and Polyfluoroalkyl Substance (PFAS)-Free Energy Harvesting: Recent Advances in Triboelectric Nanogenerators for Sports Applications. Micromachines 2025, 16, 313. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, S.; Zou, J.; Liu, S.; Hsu, J.-P.; Li, N.; Zhu, G.; Zhuang, Z.; Kang, Q.; Zhang, Y. Improving stability of MXenes. Nano Res. 2022, 15, 6551–6567. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Szuplewska, A.; Chudy, M.; Woźniak, J.; Petrus, M.; Wojciechowski, T.; Vasilchenko, A.S.; Jastrzębska, A.M. Engineering of 2D Ti3C2 MXene surface charge and its Influence on biological properties. Materials 2020, 13, 2347. [Google Scholar] [CrossRef]
- Sheikh, K.; Hossain, K.R.; Hossain, A.; Sagar, S.I.; Raju, R.H.; Haque, F. 3D printed ionic liquids based hydrogels and applications. J. Ion. Liq. 2024, 4, 100093. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, Y.; Chen, Z.; Wang, S.; Ren, Y.; Wang, Q.; Shao, J.; Wang, W.; Dong, X. Thermoresponsive lignin-reinforced poly(ionic liquid) hydrogel wireless strain sensor. Research 2021, 2021, 9845482. [Google Scholar] [CrossRef]
- Calandra, P.; Szerb, E.I.; Lombardo, D.; Algieri, V.; De Nino, A.; Maiuolo, L. A presentation of ionic liquids as lubricants: Some critical comments. Appl. Sci. 2021, 11, 5677. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, Z.; Wu, H.; Lu, Y.; Wang, N. Porous polymer materials in triboelectric nanogenerators: A review. Polymers 2023, 15, 4383. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Cuthbert, T.J.; Shokurov, A.V.; Chu, P.K.; Feng, S.; Menon, C. Hydrogels as soft ionic conductors in flexible and wearable triboelectric nanogenerators. Adv. Sci. 2022, 9, 2106008. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, C.; Niu, M.; Zhu, P.; Pan, Z.; Mao, Y. Ionic hydrogels-based triboelectric nanogenerators for self-powered human–machine interfaces. J. Physics Mater. 2023, 7, 012001. [Google Scholar] [CrossRef]
- Xie, S.; Yan, H.; Qi, R. A Review of polymer-based environment-induced nanogenerators: Power generation performance and polymer material manipulations. Polymers 2024, 16, 555. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, P.; Tan, M.; Niu, M.; Liang, S.; Mao, Y. Recent Advances in Hydrogel-Based Self-Powered Artificial Skins for Human–Machine Interfaces. Adv. Intell. Syst. 2023, 5, 2300162. [Google Scholar] [CrossRef]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Sani, E.S.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering biodegradable and biocompatible bio-ionic liquid conjugated hydrogels with tunable conductivity and mechanical properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef]
- Krishnadoss, V.; Kanjilal, B.; Masoumi, A.; Banerjee, A.; Dehzangi, I.; Pezhouman, A.; Ardehali, R.; Martins-Green, M.; Leijten, J.; Noshadi, I. Programmable bio-ionic liquid functionalized hydrogels for in situ 3D bioprinting of electronics at the tissue interface. Mater. Today Adv. 2023, 17, 100352. [Google Scholar] [CrossRef]
- Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Ribeiro, M.C.C.; Smirnov, M.A. Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers 2022, 14, 5148. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D. Nanotechnology Applied to Cellulosic Materials. Materials 2023, 16, 3104. [Google Scholar] [CrossRef]
- Lu, P.; Liao, X.; Guo, X.; Cai, C.; Liu, Y.; Chi, M.; Du, G.; Wei, Z.; Meng, X.; Nie, S. Gel-based triboelectric nanogenerators for flexible sensing: Principles, properties, and applications. Nano-Micro Lett. 2024, 16, 206. [Google Scholar] [CrossRef]
- Yin, J.; Jia, P.; Ren, Z.; Zhang, Q.; Lu, W.; Yao, Q.; Deng, M.; Zhou, X.; Gao, Y.; Liu, N. Recent Advances in Self-Powered Sensors Based on Ionic Hydrogels. Research 2025, 8, 0571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, N.; Zhang, Z.; Cui, X.; Zhang, H. Hydrogel-based energy harvesters and Self-powered sensors for wearable applications. Nanoenergy Adv. 2023, 3, 315–342. [Google Scholar] [CrossRef]
- Baig, M.M.; Khan, S.A.; Ahmad, H.; Liang, J.; Zhu, G.; Pang, H.; Zhang, Y. 3D printing of hydrogels for flexible micro-supercapacitors. FlexMat 2024, 1, 79–99. [Google Scholar] [CrossRef]
- Salehi, S.M.; Santagada, R.; Depietra, S.; Fontananova, E.; Curcio, E.; Di Profio, G. Ionic liquid hydrogel composite membranes (IL-HCMs). Chemengineering 2019, 3, 47. [Google Scholar] [CrossRef]
- Chen, C.-K.; Chen, P.-W.; Wang, H.-J.; Yeh, M.-Y. Alkyl chain length effects of imidazolium ionic liquids on electrical and mechanical performances of polyacrylamide/alginate-based hydrogels. Gels 2021, 7, 164. [Google Scholar] [CrossRef]
- Hu, J.; Andablo-Reyes, E.; Soltanahmadi, S.; Sarkar, A. Synergistic microgel-reinforced hydrogels as high-performance lubricants. ACS Macro Lett. 2020, 9, 1726–1731. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, L.; Wu, J.; Shi, Y.; Feng, X.; Lu, X.; Zhu, J.; Mu, L. Versatile ionic gel driven by dual Hydrogen bond networks: Toward advanced lubrication and self-healing. ACS Appl. Polym. Mater. 2021, 3, 5932–5941. [Google Scholar] [CrossRef]
- Wu, Y.; Cuthbert, T.J.; Luo, Y.; Chu, P.K.; Menon, C. Cross-Link-Dependent Ionogel-Based Triboelectric Nanogenerators with Slippery and Antireflective Properties. Small 2023, 19, e2301381. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Liu, Q.; Chen, Z. Hydrogel-Based Functional Materials for Thermoelectric Applications: Progress and Perspectives. Adv. Funct. Mater. 2024, 34, 202410127. [Google Scholar] [CrossRef]
- Li, G.; Guo, C.F. PEDOT:PSS-based intrinsically soft and stretchable bioelectronics. Soft Sci. 2022, 2, 7. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; Janssen, R.A.J.; Bastiaansen, J.A.M.; Kiggen, N.M.M.; Langeveld, B.M.W.; van Breemen, A.J.J.M.; de Kok, M.M. Microscopic understanding of the anisotropic conductivity of PEDOT:PSS thin films. Adv. Mater. 2007, 19, 1196–1200. [Google Scholar] [CrossRef]
- Kaur, G.; Collis, G.E.; Adhikari, R.; Gunatillake, P. Electrical Conductivity, Thermo-Mechanical Properties, and Cytotoxicity of Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) (PEDOT:PSS)/Sulfonated Polyurethane Blends. Materials 2024, 17, 4602. [Google Scholar] [CrossRef] [PubMed]
- Hrehorova, E.; Rebros, M.; Pekarovicova, A.; Fleming, P.D.; Bliznyuk, V.N. Characterization of conductive polymer inks based on PEDOT: PSS. TAGA J. 2008, 4, 219–231. [Google Scholar]
- Alamer, F.A.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y.; et al. Review on PEDOT:PSS-based conductive fabric. ACS Omega 2022, 7, 35371–35386. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-K.; Kim, J.; Park, J.-S.; Moon, J.B.; Oh, J.; Lee, W.-K.; Kang, M.-G. Synthesis and characterization of a conductive polymer blend based on PEDOT:PSS and its electromagnetic applications. Polymers 2022, 14, 393. [Google Scholar] [CrossRef]
- Li, K.; Jia, X.; Cao, J.; Xu, J.; Wang, H.; Liu, X. High-Resolution Electrohydrodynamic Printing with Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Conductive Polymers. Coatings 2024, 14, 1610. [Google Scholar] [CrossRef]
- Guzzo, S.; Carli, S.; Pavan, B.; Lunghi, A.; Murgia, M.; Bianchi, M. Evaluation of the in vitro biocompatibility of PEDOT:Nafion Coatings. Nanomaterials 2021, 11, 2022. [Google Scholar] [CrossRef]
- Sultana, N.; Chang, H.C.; Jefferson, S.; Daniels, D.E. Application of conductive poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) polymers in potential biomedical engineering. J. Pharm. Investig. 2020, 50, 437–444. [Google Scholar] [CrossRef]
- Brendgen, R.; Grethe, T.; Schwarz-Pfeiffer, A. Straightforward Production Methods for Diverse Porous PEDOT:PSS Structures and Their Characterization. Sensors 2024, 24, 4919. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Malengier, B.; Fante, K.A.; Nigusse, A.B.; Van Langenhove, L. Development of a flex and stretchy conductive cotton fabric via flat screen printing of PEDOT:PSS/PDMS conductive polymer composite. Sensors 2020, 20, 1742. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Q.; Zhang, Y.; Sun, H.; Chen, S.; Liang, L.; An, S.; Yang, X.; Zang, L. Recent Advances in the Tunable Optoelectromagnetic Properties of PEDOTs. Molecules 2025, 30, 179. [Google Scholar] [CrossRef]
- Slejko, E.A.; Carraro, G.; Huang, X.; Smerieri, M. Advances in the fabrication, properties, and applications of electrospun PEDOT-based conductive nanofibers. Polymers 2024, 16, 2514. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yoo, S.; Kim, J.-H. Water-based highly stretchable PEDOT:PSS/nonionic WPU transparent electrode. Polymers 2022, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Kim, S.Y.; Hyun, J.H.; Park, B.; Choy, S.; Koirala, G.R.; Kim, T.-I. Fibrillary gelation and dedoping of PEDOT:PSS fibers for interdigitated organic electrochemical transistors and circuits. npj Flex. Electron. 2022, 6, 31. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Bu, T.; Liu, Y.; Qi, Y.; Xie, Y.; Xu, S.; Deng, W.; Yang, W.; Zhang, C. MXene based mechanically and electrically enhanced film for triboelectric nanogenerator. Nano Res. 2021, 14, 4833–4840. [Google Scholar] [CrossRef]
- Ryan, K.R.; Down, M.P.; Hurst, N.J.; Keefe, E.M.; Banks, C.E. Additive manufacturing (3D printing) of electrically conductive polymers and polymer nanocomposites and their applications. eScience 2022, 2, 365–381. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Dominguez-Alfaro, A.; Lopez-Larrea, N.; Alegret, N.; Mecerreyes, D. Additive manufacturing of conducting polymers: Recent advances, challenges, and opportunities. ACS Appl. Polym. Mater. 2021, 3, 2865–2883. [Google Scholar] [CrossRef]
- Tseghai, G.B.; Mengistie, D.A.; Malengier, B.; Fante, K.A.; Van Langenhove, L. PEDOT:PSS-based conductive textiles and their applications. Sensors 2020, 20, 1881. [Google Scholar] [CrossRef]
- Lv, N.; Liu, S.; Liu, G.; Liu, X. Direct Ink Writing of Highly Conductive and Strongly Adhesive PEDOT:PSS-EP Coatings for Antistatic Applications. Colloids Interfaces 2024, 8, 48. [Google Scholar] [CrossRef]
- Hofmann, A.I.; Östergren, I.; Kim, Y.; Fauth, S.; Craighero, M.; Yoon, M.-H.; Lund, A.; Müller, C. All-polymer conducting fibers and 3D prints via melt processing and templated polymerization. ACS Appl. Mater. Interfaces 2020, 12, 8713–8721. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Environmentally friendly synthesis of poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate)/SnO2 nanocomposites. Polymers 2021, 13, 2445. [Google Scholar] [CrossRef]
- Bianchi, M.; Guzzo, S.; Lunghi, A.; Greco, P.; Pisciotta, A.; Murgia, M.; Carnevale, G.; Fadiga, L.; Biscarini, F. Synergy of nanotopography and electrical conductivity of PEDOT/PSS for enhanced neuronal development. ACS Appl. Mater. Interfaces 2023, 15, 59224–59235. [Google Scholar] [CrossRef]
- Gascón, N.C.; Wouters, B.; Terryn, H.; Hubin, A. Effect of Impregnation of PEDOT:PSS in Etched Aluminium Electrodes on the Performance of Solid State Electrolytic Capacitors. Inorganics 2024, 12, 185. [Google Scholar] [CrossRef]
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials 2009, 30, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; D’AMone, S.; Manca, M.; Viola, I.; Cingolani, R.; Gigli, G. Superhydrophobicity due to the hierarchical scale roughness of PDMS surfaces. Langmuir 2008, 24, 2712–2718. [Google Scholar] [CrossRef]
- Ji, M.G.; Bazroun, M.; Cho, I.H.; Slafer, W.D.; Biswas, R.; Kim, J. Mechano-Triboelectric Analysis of Surface Charge Generation on Replica-Molded Elastomeric Nanodomes. Micromachines 2021, 12, 1460. [Google Scholar] [CrossRef]
- Villegas, M.; Cetinic, Z.; Shakeri, A.; Didar, T.F. Fabricating smooth PDMS microfluidic channels from low-resolution 3D printed molds using an omniphobic lubricant-infused coating. Anal. Chim. Acta 2018, 1000, 248–255. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, L.; Zhou, C.; Wang, X. The lubricant retaining effect of micro-dimples on the sliding surface of PDMS. Tribol. Int. 2012, 52, 87–93. [Google Scholar] [CrossRef]
- Kang, M.; Park, Y.M.; Kim, B.H.; Seo, Y.H. Micro- and nanoscale surface texturing effects on surface friction. Appl. Surf. Sci. 2015, 345, 344–348. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Wang, X. Modify the friction between steel ball and PDMS disk under water lubrication by surface texturing. Meccanica 2011, 46, 499–507. [Google Scholar] [CrossRef]
- Ji, M.G.; Li, Q.; Biswas, R.; Kim, J. Stability and temporal decay of nanopatterned tribocharge on nanotextured elastomer surfaces. Nano Energy 2021, 79, 105441. [Google Scholar] [CrossRef]
- Zhang, J.; Rogers, F.J.M.; Darwish, N.; Gonçales, V.R.; Vogel, Y.B.; Wang, F.; Gooding, J.J.; Peiris, M.C.R.; Jia, G.; Veder, J.-P.; et al. Electrochemistry on tribocharged polymers is governed by the stability of surface charges rather than charging magnitude. J. Am. Chem. Soc. 2019, 141, 5863–5870. [Google Scholar] [CrossRef] [PubMed]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Goh, Q.-L.; Chee, P.-S.; Lim, E.-H.; Ng, D.W.-K. An AI-assisted and self-powered smart robotic gripper based on eco-egain nanocomposite for pick-and-place operation. Nanomaterials 2022, 12, 1317. [Google Scholar] [CrossRef]
- Tilmatine, O. Étude Des Phénomènes Tribologiques Et Électrostatiques Couplés à La Surface Des Polymères. Ph.D. Dissertation, Université de Poitiers, Poitiers, France, 2022. [Google Scholar]
- Armitage, J.L. Tribology and the Triboelectric Effect: A Study Into the Influence of Tribological Factors on Frictional Electrification. Ph.D. Dissertation, University of Leeds, Leeds, UK, 2022. [Google Scholar]
- Šutka, A.; Lapčinskis, L.; He, D.; Kim, H.; Berry, J.D.; Bai, J.; Knite, M.; Ellis, A.V.; Jeong, C.K.; Sherrell, P.C. Engineering polymer interfaces: A review toward controlling triboelectric surface charge. Adv. Mater. Interfaces 2023, 10, 202300323. [Google Scholar] [CrossRef]
- Ko, Y.; Vu, C.C.; Kim, J. Carbonized cotton fabric-based flexible Capacitive PRESSURE sensor using a porous dielectric layer with tilted air gaps. Sensors 2021, 21, 3895. [Google Scholar] [CrossRef]
- Zhang, J.; Su, E.; Li, C.; Xu, S.; Tang, W.; Cao, L.N.; Li, D.; Wang, Z.L. Enhancing Artifact Protection in Smart Transportation Monitoring Systems via a Porous Structural Triboelectric Nanogenerator. Electronics 2023, 12, 3031. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Zhang, G.; Wang, L.; Chen, L.; Yang, J.; Wang, Z. Flexible sensors array based on frosted microstructured ecoflex film and TPU nanofibers for epidermal pulse wave monitoring. Sensors 2023, 23, 3717. [Google Scholar] [CrossRef]
- Yin, B.; Liu, F.; Chen, Q.; Liu, M.; Wang, F. Flexible strain sensors Based on bionic parallel vein-like structures for human Motion monitoring. Sensors 2024, 24, 468. [Google Scholar] [CrossRef]
- Truong, T.; To, T.T.L.; Yoon, C.; Kim, J.H.; Park, Y.; Youn, H.; Rho, J. Highly Stretchable, Robust, and Floatable Broadband Light Absorber of Carbon Nanotubes–Ecoflex Composite on 3D Mogul-Pattern for Photothermal Conversion. Energy Technol. 2025, 2402169, early review. [Google Scholar] [CrossRef]
- Seeböck, R.; Esrom, H.; Charbonnier, M.; Romand, M.; Kogelschatz, U. Surface modification of polyimide using dielectric barrier discharge treatment. Surf. Coatings Technol. 2001, 142–144, 455–459. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Ananth, A.; Gupta, N.; Anandan, C. Superhydrophobic nanostructured Kapton® surfaces fabricated through Ar+O2 plasma treatment: Effects of different environments on wetting behaviour. Appl. Surf. Sci. 2013, 268, 464–471. [Google Scholar] [CrossRef]
- Shu, M.; Li, Z.; Man, Y.; Liu, K.; Liu, H.; Gao, Y. Surface modification of poly (4,4′-oxydiphenylene pyromellitimide) (Kapton) by alkali solution and its applications to atomic oxygen protective coating. Corros. Sci. 2016, 112, 418–425. [Google Scholar] [CrossRef]
- Orazi, L.; Pelaccia, R.; Siciliani, V.; Oubellaouch, K.; Mazzonetto, M.; Reggiani, B. Ultrafast laser texturing to improve wettability of polyimide (Kapton) films. J. Manuf. Process. 2023, 107, 368–375. [Google Scholar] [CrossRef]
- Potu, S.; M, N.; Rajaboina, R.K.; Gollapelli, B.; Vallamkondu, J.; Mishra, S.; Divi, H.; Babu, A.; K, U.K.; Kodali, P. High-performance and low-cost overhead projector sheet-based triboelectric nanogenerator for self-powered cholesteric liquid crystal, electroluminescence, and portable electronic devices. ACS Appl. Energy Mater. 2022, 5, 13702–13713. [Google Scholar] [CrossRef]
- Gouzman, I.; Girshevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Thin film oxide barrier layers: Protection of kapton from space environment by liquid phase deposition of titanium oxide. ACS Appl. Mater. Interfaces 2010, 2, 1835–1843. [Google Scholar] [CrossRef]
- Alouani, M.A.; Casanova-Chafer, J.; de Bernardi-Martín, S.; García-Gómez, A.; Salehnia, F.; Santos-Ceballos, J.C.; Santos-Betancourt, A.; Vilanova, X.; Llobet, E. The Effect of Doping rGO with Nanosized MnO2 on Its Gas Sensing Properties. Chemosensors 2024, 12, 256. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, G.; Liu, G.; Jiang, H.; Zhang, T. Effects and mechanism on Kapton film under ozone exposure in a ground near space simulator. Appl. Surf. Sci. 2018, 440, 1083–1090. [Google Scholar] [CrossRef]
- Labiano, I.I.; Alomainy, A. Flexible inkjet-printed graphene antenna on Kapton. Flex. Print. Electron. 2021, 6, 025010. [Google Scholar] [CrossRef]
- Vázquez-González, P.J.; Paniagua-Chávez, M.L.; Zebadua-Chavarria, L.A.; Mota-Grajales, R.; Meza-Avendaño, C.A.; Campos-González, E.; Escobosa-Echavarría, A.; Hu, Y.; Pérez-Ramos, A.E.; Matuz, M.; et al. Comprehensive Structural, Chemical, and Optical Characterization of Cu2ZnSnS4 Films on Kapton Using the Automated Successive Ionic Layer Adsorption and Reaction Method. Nanomaterials 2025, 15, 85. [Google Scholar] [CrossRef]
- Guo, J.; Asli, A.E.N.; Williams, K.R.; Lai, P.L.; Wang, X.; Montazami, R.; Hashemi, N.N. Viability of neural cells on 3D printed graphene bioelectronics. Biosensors 2019, 9, 112. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Hajnys, J.; Kozior, T.; Gogolewski, D.; Zmarzły, P. Quality of surface texture and mechanical properties of PLA and PA-based material reinforced with carbon fibers manufactured by FDM and CFF 3D printing technologies. Polymers 2021, 13, 1671. [Google Scholar] [CrossRef]
- Masood, M.T.; Papadopoulou, E.L.; Heredia-Guerrero, J.A.; Bayer, I.S.; Athanassiou, A.; Ceseracciu, L. Graphene and polytetrafluoroethylene synergistically improve the tribological properties and adhesion of nylon 66 coatings. Carbon 2017, 123, 26–33. [Google Scholar] [CrossRef]
- Clavería, I.; Gimeno, S.; Miguel, I.; Mendoza, G.; Lostalé, A.; Fernández, Á.; Castell, P.; Elduque, D. Tribological performance of nylon composites with nanoadditives for self-lubrication purposes. Polymers 2020, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- RG, A.P.; Bajaj, G.; John, A.E.; Chandran, S.; Kumar, V.V.; Ramakrishna, S. A review on the recent applications of synthetic biopolymers in 3D printing for biomedical applications. J. Mater. Sci. Mater. Med. 2023, 34, 62. [Google Scholar] [CrossRef]
- Pelagade, S.; Singh, N.; Qureshi, A.; Rane, R.; Mukherjee, S.; Deshpande, U.; Ganesan, V.; Shripathi, T. Investigation of surface properties of Ar-plasma treated polyethylene terephthalate (PET) films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 289, 34–38. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Guo, Y.; Zhang, Z. Surface modification of a biomedical polyethylene terephthalate (PET) by air plasma. Appl. Surf. Sci. 2009, 255, 4446–4451. [Google Scholar] [CrossRef]
- Xia, L.; Cao, D.; Zhang, H.; Zhang, M.; Shan, L.; Zhang, H.; Wang, T. Surface modification of recycled polyester fiber and performance evaluation of its asphalt mastic and mixture. Sustainability 2023, 16, 278. [Google Scholar] [CrossRef]
- Marciel, A.; Bastos, A.; Pereira, L.; Jakka, S.K.; Borges, J.; Vaz, F.; Peres, M.; Lorenz, K.; Bafti, A.; Pavić, L.; et al. Niobium oxide thin films grown on flexible ITO-coated PET substrates. Coatings 2024, 14, 1127. [Google Scholar] [CrossRef]
- Pelisser, F.; Montedo, O.R.K.; Gleize, P.J.P.; Roman, H.R. Mechanical properties of recycled PET fibers in concrete. Mater. Res. 2012, 15, 679–686. [Google Scholar] [CrossRef]
- Tzaneva, B.; Aleksandrova, M.; Mateev, V.; Stefanov, B.; Iliev, I. Electrochemical Properties of PEDOT:PSS/Graphene Conductive Layers in Artificial Sweat. Sensors 2023, 24, 39. [Google Scholar] [CrossRef]
- Singh, S.P.; Ramanan, S.; Kaufman, Y.; Arnusch, C.J. Laser-induced graphene biofilm inhibition: Texture does matter. ACS Appl. Nano Mater. 2018, 1, 1713–1720. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Zhang, J.; Wu, W.; Song, G. Enhanced stretchable graphene-based triboelectric nanogenerator via control of surface nanostructure. Nano Energy 2019, 58, 304–311. [Google Scholar] [CrossRef]
- Xue, P.; Huang, Z.; Chen, C. Effect of substrate roughness on the friction and wear behaviors of laser-induced graphene film. Lubricants 2022, 10, 239. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Liu, M.; Valentin, T.M.; Wang, Z.; Steinberg, R.S.; Sodhi, J.; Wong, I.Y.; Hurt, R.H. Hierarchical metal oxide topographies replicated from highly textured graphene oxide by intercalation templating. ACS Nano 2016, 10, 10869–10879. [Google Scholar] [CrossRef]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface properties of graphene oxide reinforced polyvinyl chloride nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Reddy, S.; Xu, X.; Guo, T.; Zhu, R.; He, L.; Ramakrishana, S. Allotropic carbon (graphene oxide and reduced graphene oxide) based biomaterials for neural regeneration. Curr. Opin. Biomed. Eng. 2018, 6, 120–129. [Google Scholar] [CrossRef]
- Adekoya, G.J.; Ezika, A.C.; Adekoya, O.C.; Sadiku, E.R.; Hamam, Y.; Ray, S.S. Recent advancements in biomedical application of polylactic acid/graphene nanocomposites: An overview. BMEMat 2023, 1, 12042. [Google Scholar] [CrossRef]
- Reinert, L.; Schütz, S.; Suárez, S.; Mücklich, F. Influence of surface roughness on the lubrication effect of carbon nanoparticle-coated steel surfaces. Tribol. Lett. 2018, 66, 45. [Google Scholar] [CrossRef]
- Gbordzoe, S.; Yarmolenko, S.; Hsieh, Y.-Y.; Adusei, P.K.; Alvarez, N.T.; Fialkova, S.; Shanov, V. Three-dimensional texture analysis of aligned carbon nanotube structures. Carbon 2017, 121, 591–601. [Google Scholar] [CrossRef]
- Tu, J.; Yang, Y.; Wang, L.; Ma, X.; Zhang, X. Tribological properties of carbon-nanotube-reinforced copper composites. Tribol. Lett. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Han, G.; Xia, J.; Wang, L.; Tu, J.; Xu, Z. Tribological behavior of carbon-nanotube-filled PTFE composites. Tribol. Lett. 2003, 15, 275–278. [Google Scholar] [CrossRef]
- Maimaitituersun, N.; Wang, J.; Wang, D.; Ning, Z. Mechanical and Electrical Properties of Cementitious Composites Reinforced with Multi-Scale Carbon Fibers. Materials 2025, 18, 1830. [Google Scholar] [CrossRef]
- Abreu, S.; Vale, N.; Soares, O.S.G.P. Combination of CNTs with Classical Drugs for Treatment in Human Colorectal Adenocarcinoma (HT-29) Cell Line. Nanomaterials 2023, 13, 1933. [Google Scholar] [CrossRef]
- Huang, X.; Sun, C.; Liu, S.; Zhao, B.; Ge, M.; Zhang, H. Regulating the Structures of Carbon Cloth and Carbon Nanotubes to Boost the Positive Electrode Reaction of Vanadium Redox Flow Batteries. Catalysts 2025, 15, 345. [Google Scholar] [CrossRef]
- Wang, Y. 3D Porous Sponge/Carbon Nanotube/Polyaniline/Chitosan Capacitive Bioanode Material for Improving the Power Generation and Energy Storage Performance of Microbial Fuel Cells. Coatings 2024, 14, 152. [Google Scholar] [CrossRef]
- Cao, Q.; Hensley, D.K.; Lavrik, N.V.; Venton, B.J. Carbon nanospikes have better electrochemical properties than carbon nanotubes due to greater surface roughness and defect sites. Carbon 2019, 155, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Mohanavel, V.; Kannan, S.; Raman, M.; Kulandaivel, A.; Seikh, A.H.; Iqbal, A. Impact of CNT addition on surface roughness and dimensional characteristics of polymer nano-composite fabricated by FDM method. Int. J. Adv. Manuf. Technol. 2023, 1–13. [Google Scholar] [CrossRef]
- Yun, D.; Gao, Q.; Jia, D.; Zhao, B. Study on Milling Mechanism and Surface Roughness of CNTS/2009Al Composites. Int. J. Precis. Eng. Manuf. 2025, 26, 1061–1073. [Google Scholar] [CrossRef]
- Sangeetha, M.; Vasanthaprabhu, A.; Sivaprakasam, K.; Nithya, S.; Dhinakaran, V.; Gunasekar, P. Surface roughness analysis for newly prepared CNT-coated metal matrix: RSM approach. Appl. Nanosci. 2023, 13, 693–705. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials 2020, 10, 264. [Google Scholar] [CrossRef]
- Bhunia, R.; Gupta, S.; Fatma, B.; Prateek; Gupta, R.K.; Garg, A. Milli-watt power harvesting from dual triboelectric and piezoelectric effects of multifunctional green and robust reduced graphene oxide/P(VDF-TrFE) composite flexible films. ACS Appl. Mater. Interfaces 2019, 11, 38177–38189. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, F.; Zhao, Z.; Bai, P.; Ma, Y.; Alhadhrami, A.; Mersal, G.A.M.; Lin, Z.; Ibrahim, M.M.; El-Bahy, Z.M. Direct ink printing reduced graphene oxide/KCu7S4 electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 2022, 5, 1516–1526. [Google Scholar] [CrossRef]
- Manickam, S.; Muthoosamy, K.; Bai, R.G.; Abubakar, I.B.; Sudheer, S.M.; Hongngee, L.; Hwei-San, L.; Nayming, H.; Ch, C. Exceedingly biocompatible and thin-layered reduced graphene oxide nanosheets using an eco-friendly mushroom extract strategy. Int. J. Nanomed. 2015, 10, 1505–1519. [Google Scholar] [CrossRef]
- Mindivan, F. The synthesis and charecterization of graphene oxide (GO) and reduced graphene oxide (rGO). Machines. Technologies. Materials 2016, 10, 32–35. [Google Scholar]
- Othman, F.E.C.; Yusof, N.; González-Benito, J.; Fan, X.; Ismail, A.F. Electrospun composites made of reduced graphene oxide and polyacrylonitrile-based activated carbon nanofibers (rGO/ACNF) for enhanced CO2 adsorption. Polymers 2020, 12, 2117. [Google Scholar] [CrossRef]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Mounasamy, V.; Madanagurusamy, S. Porous reduced graphene oxide (rGO)/WO3 nanocomposites for the enhanced detection of NH3 at room temperature. Nanoscale Adv. 2019, 1, 1799–1811. [Google Scholar] [CrossRef]
- Patel, J.; Kiani, A. Effects of reduced graphene oxide (rGO) at different concentrations on tribological properties of liquid base lubricants. Lubricants 2019, 7, 11. [Google Scholar] [CrossRef]
- MacDonald, A.F.; Harley-Troxell, M.E.; Newby, S.D.; Dhar, M.S. 3D-printing graphene scaffolds for bone tissue engineering. Pharmaceutics 2022, 14, 1834. [Google Scholar] [CrossRef]
- Seyedsalehi, A.; Daneshmandi, L.; Barajaa, M.; Riordan, J.; Laurencin, C.T. Fabrication and characterization of mechanically competent 3D printed polycaprolactone-reduced graphene oxide scaffolds. Sci. Rep. 2020, 10, 22210. [Google Scholar] [CrossRef]
- Hu, J.; Yu, J.; Li, Y.; Liao, X.; Yan, X.; Li, L. Nano carbon black-based high performance wearable pressure sensors. Nanomaterials 2020, 10, 664. [Google Scholar] [CrossRef]
- Jankovská, Z.; Večeř, M.; Koutník, I.; Matějová, L. A case study of waste scrap tyre-derived carbon black tested for nitrogen, carbon dioxide, and cyclohexane adsorption. Molecules 2020, 25, 4445. [Google Scholar] [CrossRef]
- Liu, B.; Du, T.; Xu, X.; Liu, J.; Zhu, P.; Guo, L.; Li, Y.; Wang, T.; Zou, Y.; Wang, H.; et al. An Underwater Triboelectric Biomechanical Energy Harvester to Power the Electronic Tag of Marine Life. J. Mar. Sci. Eng. 2023, 11, 1766. [Google Scholar] [CrossRef]
- Samoei, V.K.; Jayatissa, A.H.; Sano, K. Flexible Pressure Sensor Based on Carbon Black/PVDF Nanocomposite. Chem. Sci. Int. J. 2024, 33, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Ru, H. Effect of Lubricating Phase on Microstructure and Properties of Cu–Fe Friction Materials. Materials 2019, 12, 313. [Google Scholar] [CrossRef]
- Guo, S.; Radecka, I.; Eissa, A.M.; Ivanov, E.; Stoeva, Z.; Tchuenbou-Magaia, F. Recent advances in carbon-based sensors for food and medical packaging under transit: A focus on humidity, temperature, mechanical, and multifunctional sensing technologies—A systematic review. Materials 2025, 18, 1862. [Google Scholar] [CrossRef]
- Göçerler, H.; Danecker, C.; Gachot, C.; Sui, X.; Tomala, A.; Rosenkranz, A.; Nastran, M.; Bayer, B.C.; Loni, E.; Schwarz, S.; et al. Unlocking the synergistic impact of laser texturing and Ti3C2Tx MXene coatings—Substrate-specific tribological insights. Carbon 2025, 238, 120270. [Google Scholar] [CrossRef]
- Anasori, B.; Naguib, M.; Editors, G. Two-dimensional MXenes. MRS Bull. 2023, 48, 238–244. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Righi, M.C.; Sumant, A.V.; Anasori, B.; Mochalin, V.N. Perspectives of 2D MXene tribology. Adv. Mater. 2023, 35, e2207757. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Recent advances in two-dimensional MXene for supercapacitor applications: Progress, challenges, and perspectives. Nanomaterials 2023, 13, 919. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A review on the synthesis of MXenes and their lubrication performance and mechanisms. Tribol. Int. 2023, 179, 108170. [Google Scholar] [CrossRef]
- Yue, F.; Liu, M.; Bai, M.; Hu, M.; Li, F.; Guo, Y.; Vrublevsky, I.; Sun, X. Novel electrochemical aptasensor based on ordered mesoporous Carbon/2D Ti3C2 MXene as nanocarrier for simultaneous detection of aminoglycoside antibiotics in milk. Biosensors 2022, 12, 626. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Wu, M.; Yang, W.; Zhao, J.; Wang, Z.; Guo, S.; Wang, H.; Wang, W.; Wang, J. A flexible triboelectric nanogenerator based on multilayer MXene/cellulose nanofibril composite film for patterned electroluminescence display. Materials 2022, 15, 6770. [Google Scholar] [CrossRef]
- Gibertini, E.; Lissandrello, F.; Bertoli, L.; Viviani, P.; Magagnin, L. All-inkjet-printed Ti3C2 MXene capacitor for textile energy storage. Coatings 2023, 13, 230. [Google Scholar] [CrossRef]
- Hossain, K.R.; Jiang, P.; Yao, X.; Yang, X.; Hu, D.; Wang, X. Ionic liquids for 3D printing: Fabrication, properties, applications. J. Ion. Liq. 2023, 3, 100066. [Google Scholar] [CrossRef]
- Kotsiras, A.; Whitley, J.; Orozco, E.; Guymon, C.A. Influence of Ionic Liquid and Light Intensity on a Polymer Nanostructure Formed in Lyotropic Liquid Crystalline Templates. ACS Appl. Polym. Mater. 2024, 6, 2442–2452. [Google Scholar] [CrossRef] [PubMed]
- Arafune, H.; Muto, F.; Kamijo, T.; Honma, S.; Morinaga, T.; Sato, T. Tribological properties of double-network gels substituted by ionic liquids. Lubricants 2018, 6, 89. [Google Scholar] [CrossRef]
- Li, J.; Cao, J.; Lu, B.; Gu, G. 3D-printed PEDOT:PSS for soft robotics. Nat. Rev. Mater. 2023, 8, 604–622. [Google Scholar] [CrossRef]
- Li, L.; Jiang, C.; Li, L. Hierarchical structures on platinum–iridium substrates enhancing conducting polymer adhesion. Bio-Design Manuf. 2024, 7, 889–898. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.-J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D printable conductive hydrogel with crystallized PEDOT:PSS for neural tissue engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, J.; Han, R.; Chen, C.; Jiang, H.; Li, X.; Peng, Y.; Wang, D.; Wang, K. A Cost-Effective Strategy to Modify the Electrical Properties of PEDOT:PSS via Femtosecond Laser Irradiation. Crystals 2024, 14, 775. [Google Scholar] [CrossRef]
- Vergara, M.E.S.; Correa, O.J.; Ballinas-Indilí, R.; Cosme, I.; Bada, J.R.Á.; Álvarez-Toledano, C. Innovative Application of Salophen Derivatives in Organic Electronics as a Composite Film with a Poly(3,4-Ethylenedioxythiophene)-poly(styrenesulfonate) Matrix. Polymers 2024, 16, 2622. [Google Scholar] [CrossRef]
- Chandran, A.C.S.; Schneider, J.; Nair, R.; Bill, B.; Gadegaard, N.; Hogg, R.; Kumar, S.; Manjakkal, L. Enhancing Supercapacitor Electrochemical Performance with 3D Printed Cellular PEEK/MWCNT Electrodes Coated with PEDOT: PSS. ACS Omega 2024, 9, 33998–34007. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Hu, G.; Lu, S.; Li, B.; Zhang, Y.; Ru, J. Single-walled carbon nanotube-reinforced PEDOT: PSS hybrid Electrodes for high-performance ionic electroactive polymer actuator. Materials 2024, 17, 2469. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Roy, S.S.; Giri, P. Phosphorus-doped Ti3C2Tx MXene/PEDOT:PSS aerogel-based electromagnetic interference shields with unique combination of high green index and shielding effectiveness. Mater. Today Adv. 2025, 26, 00576. [Google Scholar] [CrossRef]
- Gao, C.; Cui, X.; Wang, C.; Wang, M.; Wu, S.; Quan, Y.; Wang, P.; Zhao, D.; Li, S. 3D-printed hierarchical porous and multidimensional conductive network based on conducting polymer/graphene oxide. J. Materiomics 2024, 10, 234–244. [Google Scholar] [CrossRef]
- Yousefi, M.A.; Rahmatabadi, D.; Baniassadi, M.; Bodaghi, M.; Baghani, M. 4D printing of multifunctional and biodegradable PLA-PBAT-Fe3O4 nanocomposites with supreme mechanical and shape memory properties. Macromol. Rapid Commun. 2025, 46, e2400661. [Google Scholar] [CrossRef]
- Meng, X.; Mo, L.; Han, S.; Zhao, J.; Pan, Y.; Wang, F.; Fang, Y.; Li, L. Pressure-Temperature Dual-Parameter Flexible Sensors Based on Conformal Printing of Conducting Polymer PEDOT:PSS on Microstructured Substrate. Adv. Mater. Interfaces 2023, 10, 202201927. [Google Scholar] [CrossRef]
- Vara, H.; Hernández-Labrado, G.R.; Alves-Sampaio, A.; Collazos-Castro, J.E. Stability of conducting polymer-coated carbon microfibers for long-term electrical stimulation of Injured neural tissue. Polymers 2024, 16, 2093. [Google Scholar] [CrossRef]
- Lunghi, A.; Velluto, F.; Di Lisa, L.; Genitoni, M.; Biscarini, F.; Focarete, M.L.; Gualandi, C.; Bianchi, M. Fabrication and characterization of bioresorbable, electroactive and highly regular nanomodulated cell interfaces. Nanotechnology 2024, 36, 015301. [Google Scholar] [CrossRef]
- Aleksandrova, M.; Mateev, V.; Iliev, I. Behavior of Polymer Electrode PEDOT:PSS/Graphene on Flexible Substrate for Wearable Biosensor at Different Loading Modes. Nanomaterials 2024, 14, 1357. [Google Scholar] [CrossRef]
- Liu, J.; Garcia, J.; Leahy, L.M.; Song, R.; Mullarkey, D.; Fei, B.; Dervan, A.; Shvets, I.V.; Stamenov, P.; Wang, W.; et al. 3D printing of multifunctional conductive polymer composite hydrogels. Adv. Funct. Mater. 2023, 33, 202214196. [Google Scholar] [CrossRef]
- Jain, K.; Wang, Z.; Garma, L.D.; Engel, E.; Ciftci, G.C.; Fager, C.; Larsson, P.A.; Wågberg, L. 3D printable composites of modified cellulose fibers and conductive polymers and their use in wearable electronics. Appl. Mater. Today 2023, 30, 101703. [Google Scholar] [CrossRef]
- Amruth, C.; Singh, A.K.; Sharma, A.; Corzo, D.; Baran, D. Micro-3D Printed Conductive Polymer Composite via Two-Photon Polymerization for Sensing Applications. Adv. Mater. Technol. 2024, 9, 202400290. [Google Scholar] [CrossRef]
- Shafagh, S.H.; Deen, I.; Panneerselvam, D.M.; Packirisamy, M. PEDOT:PSS-MWCNT Nanocomposite Wire for Routing in Energy Harvesting Devices. Micromachines 2025, 16, 382. [Google Scholar] [CrossRef] [PubMed]
- Boratto, M.H.; Graeff, C.F.O.; Han, S. Highly Stable Flexible Organic Electrochemical Transistors with Natural Rubber Latex Additives. Polymers 2024, 16, 2287. [Google Scholar] [CrossRef]
- Zheng, Q.; Shi, B.; Li, Z.; Wang, Z.L. Recent Progress on piezoelectric and triboelectric energy harvesters in biomedical systems. Adv. Sci. 2017, 4, 1700029. [Google Scholar] [CrossRef]
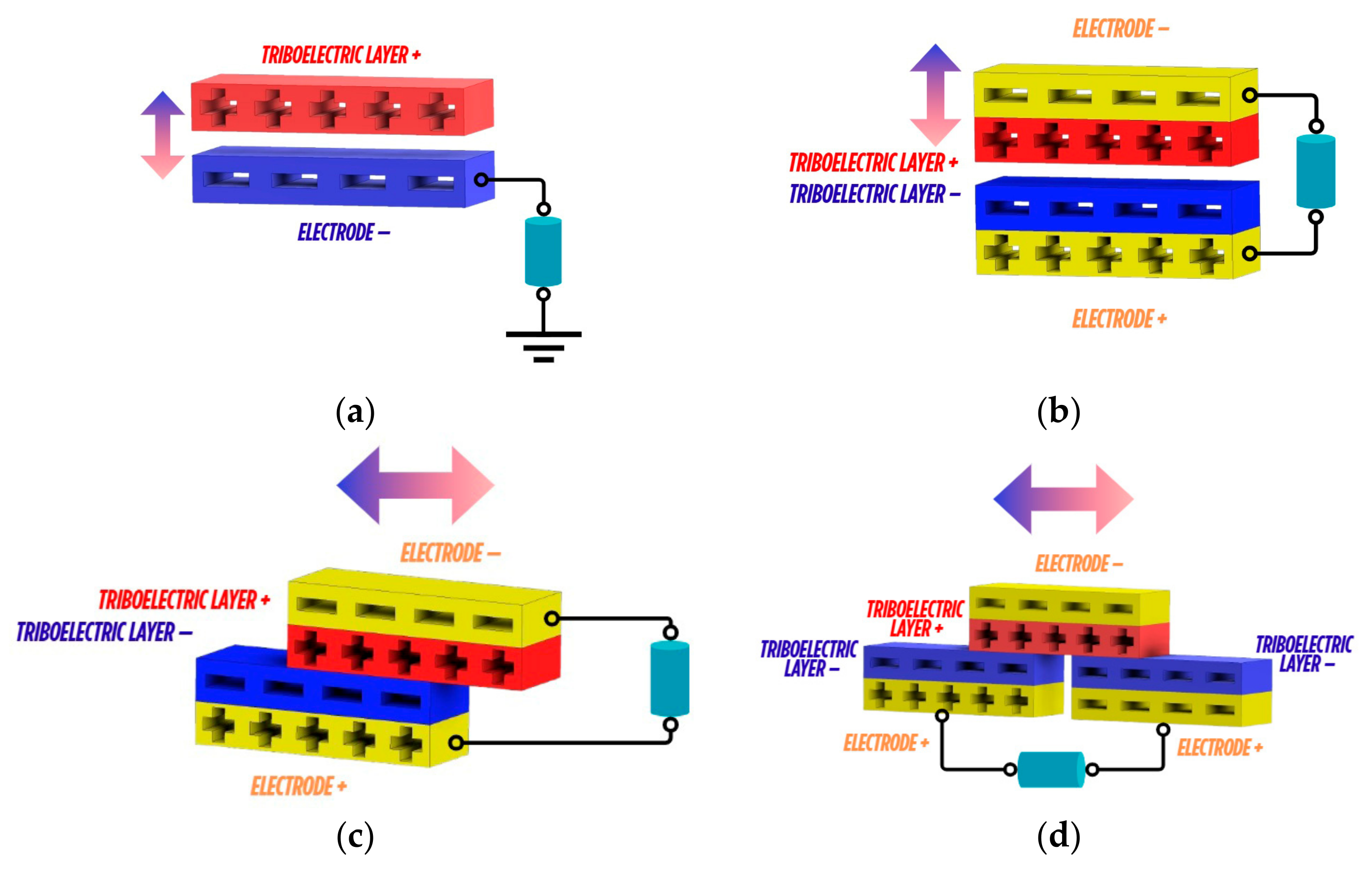


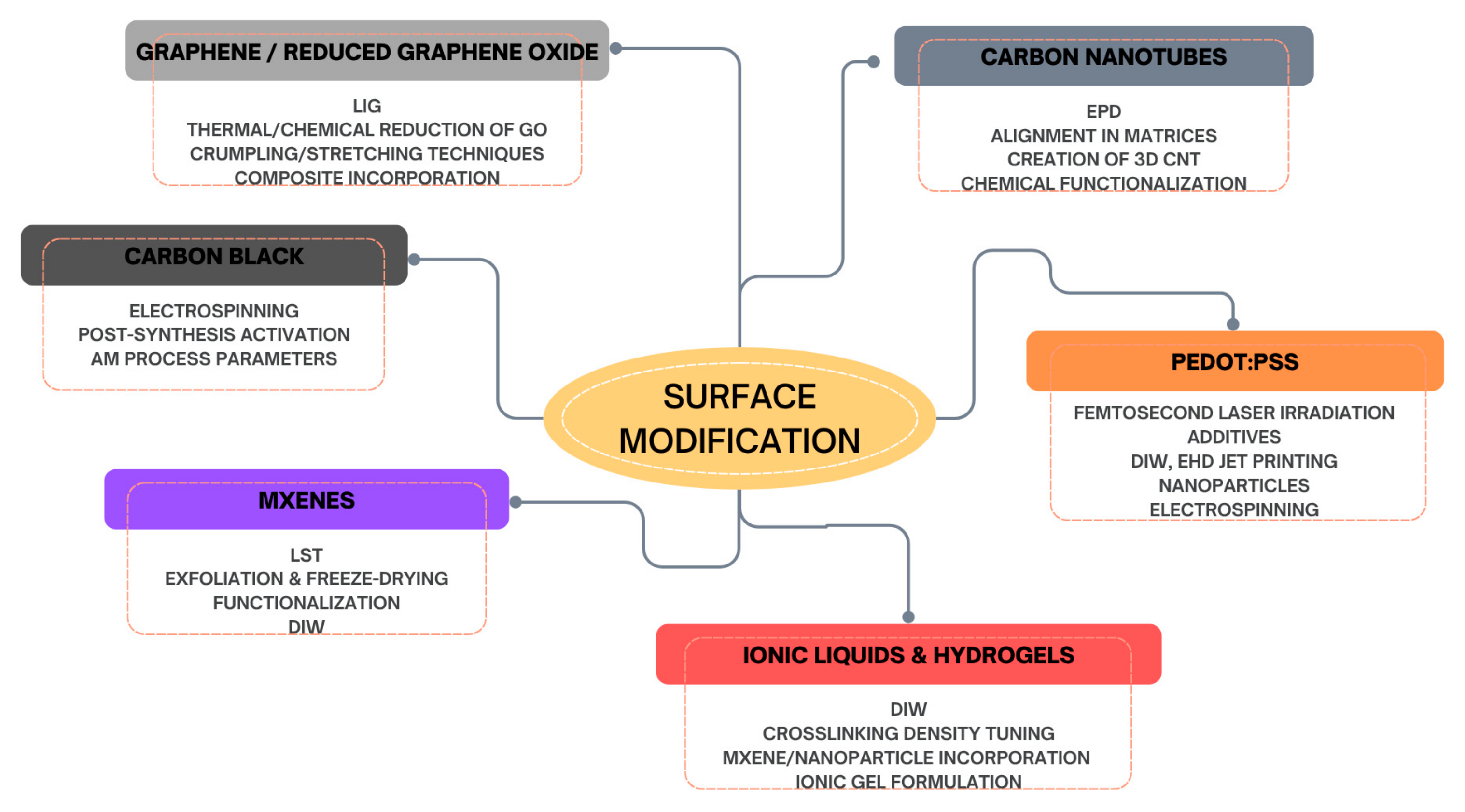
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Direct Ink Writing, DIW | ZnSnO3 nanocubes (6 wt%) | Max output: 400 V, 28 µA, 7 µA/cm2; 3 mW @ 20 MΩ; 6.2× higher than pure PDMS; stable over 500 cycles. | Flexible sensors; biological devices; Light-Emitting Diodes, LEDs. | [18] |
| Carbon fiber (4 wt%) | Tensile modulus increased by 52.4% compared to pure PDMS. | Generally, for composites and 3D models. | [47] | |
| Porous structures (using DBP removal and fumed silica) | In total, +378% stiffness, +267% strength, +14% ductility vs. nonporous PDMS; 75% infill = best performance. | Soft robotics; biomedical devices; designing adaptive soft robots/actuators. | [43] | |
| DLP | Thiourea groups (1.1–7.2%) | Max elongation: 1000%; excellent cyclic compression durability. | Medical devices; wearable devices; soft robotics. | [44] |
| Sacrificial FDM Mold | PDMS porous scaffolds with minimal surface design | Reversible under 40% strain; cell viability > 90% after 4 days (3D culture). | Scaffolding for soft tissue engineering; biocompatible porous organ-shaped scaffolds. | [43] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| DIW | PTFE micropowder for improved rheology and enhanced electron affinity | Elongation at break: 483%; low strain loss after 1000 cycles; excellent elasticity and shape stability. | Biomedical devices; soft robotics; stretchable wearable devices. | [64] |
| Vat Photopolymerization, VPP/DLP 3D Printing | Hydrophobic microstructures (striped and cylindrical) on PTFE; 3D-printed micro-ball and micro-mushroom arrays | Tensile strength: 22.04 MPa; yield strength: 12.57 MPa; WCA: 130.6°–132.8° (vs. 99.8° for unmodified PTFE). | Microfluidic containers; biocompatible implants; energy-harvesting TENGs. | [54,58] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Molding/casting with 3D-printed mold | Prismatic mold for Ecoflex®/Carbon Nano-Onions, CNO composite | N/A, used as a negative electrode material for TENG. | General TENG applications. | [80] |
| Polylactic Acid, PLA mold for pristine Ecoflex® | Output voltage: up to 17 V with Cu; similar for Al; lower electrification vs. PTFE/PDMS due to lower electron affinity. | General TENG applications and for comparative studies. | [81] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Commercial Kapton® film; 3D-printed PLA (20% infill) substrate via Sigma R19 BCN3D | Unmodified Kapton® (negative triboelectric layer) paired with Mica (positive). | Mica: Kapton® at 5 MΩ load: 50.1 V, 10.2 µA, 0.5 mW. | Energy harvesting for low-power electronics: LED lighting, capacitor charging. | [110] |
| Commercial Kapton® film (not AM) | Kapton® paired with rGO-modified polyimide or Ti3C2Tx MXene-hydrogel systems. | PI: rGO/PI: 190 V, 630 µW/cm2; MXene hydrogel: enhanced output (N/A). | Wearable electronics; handwriting recognition. | [5] |
| CO2 laser-structured Kapton® foil | Laser-induced graphene (LIG) interdigital porous electrodes on Kapton. | Not TENG specific but produced flexible MSCs: 1.75 mF/cm2 (areal capacitance), 0.256 µWh/cm2 (energy density), 0.11 mW/cm2 (power density). | Energy storage in flexible micro-supercapacitors; potential in textiles, biomedical, and food systems. | [111,112,113] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Commercial PA66 film; 3D-printed PLA (20% infill) substrates using Sigma R19 BCN3D | Unmodified PA66 paired with PP or SEBS | At 5 MΩ load: PA66:PP—172.5 V, 34.4 µA, 5.9 mW; PA66:SEBS—68.1 V, 13.2 µA, 0.9 mW. | Low-power energy harvesting (LED lighting and capacitor charging). | [110] |
| Electrospun Nylon 66 nanofibers | Nanostructured PTFE (nanopillars) as a tribopositive pair | Voltage ↑ 6×; current ↑ 700%; charge ↑ 500% vs. unstructured pairs; stable with only 2.78% drop after 24,000 cycles. | Biomechanical harvesting (keyboard typing); wearable and sensing systems. | [125] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Recycled PET processed via blending, melt mixing, and compression molding | Surface modified with polyhexamethylene guanidine (PHMG) to enhance antibacterial activity and tribopolarity. | Maximum charge density: 22.1 nC/cm2; among the highest reported for waste-derived TENGs. | Self-powered wearable and hygiene-monitoring sensors in direct/indirect human contact. | [146] |
| Electrospinning of recycled PET into nanofibrous aerogel structures | Formation of high-porosity, amine-rich nanofiber web. | Output: 67.7 V, 9.4 µA; power density: 5.47 W/m2; mechanical durability >10,000 cycles with 99% signal retention. | Lightweight wearable energy harvesters; sustainable small-scale devices. | [147,148] |
| Compression molding and melt mixing of unsorted plastic waste (PET, polystyrene–PS, high-density polyethylene–HDPE, polypropylene–PP) | Surface morphology altered by multi-material blending (e.g., PET bottle film and PS/HDPE/PP). | PET:B + PET (60% PET): charge density: 390 nC/m2; voltage: ~58 V (stable over 1000 cycles). | Green energy generation from mixed post-consumer plastic waste. | [149] |
| Material | Triboelectric Polarity | Biocompatibility | AM Compatibility | Ref. |
|---|---|---|---|---|
| PDMS | Strongly negative | Excellent; long-term cytocompatibility | DIW, DLP, molding, templates | [34] |
| PTFE | Extremely negative | Moderate (inert but not adhesive to tissue) | DIW, DLP, electrospinning | [21] |
| Ecoflex® | Slightly positive | Excellent; highly stretchable, skin-friendly | 3D-printed molds (indirect) | [160,161] |
| Kapton® | Moderately negative | Good; stable and used in bioelectronics | Substrate-based only | [97,125] |
| Nylon | Positive | High; widely used in biomedical components | FFF and SLS | [110] |
| PET | Slightly negative | Moderate; enhanced through surface treatment | Electrospinning and molding | [149] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| AM (3D BioPlotter) | Graphene scaffolds (20–60 vol%) forming a continuous conductive network | Conductivity: ↑ from 200 to 600 S/m (20–60% graphene); strain range: ↓ from 210% to 81%; resistance ↑ with tensile strain; irreversible after large bending. | Nerve conduits; support for hMSC proliferation and neuronal induction. | [165,170] |
| AM (FFF) | PLA–graphene composites using HDPlas® functionalized graphene nanoplatelets (0.3–5 µm) for improved bonding | Tensile and flexural strength: ~1.5–1.7× vs. PLA; shear strength: ~1.2× vs. PLA; slightly lower impact resistance than unfilled PLA. | Load-bearing biomedical scaffolds (e.g., bone tissue engineering). | [171] |
| Electrospinning | PVDF–graphene nanofibers (0–0.25 wt%) with optimized perovskite-based tribopositive layer | Power density: 11.23 W/m2; VOC: 245 V; ISC: 24 μA; QSC: 80.2 nC; durable: stable after 12,000 cycles and 2 months’ storage. | Energy harvesting; self-powered LEDs, capacitors, digital tubes, photodetectors. | [172] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| PI (Kapton)/PI:rGO/PI multilayer | rGO as an electron trap in PI; facilitates electron transfer and reduces the energy gap (confirmed by red shift in absorption). | Voltage: 190 V; power density: 6.3 W/m2; stable output across frequencies due to consistent charge retention. | Energy harvesting; self-powered sensing (no specific biomedical application noted). | [5] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| Multilayered fiber TENG on PET via coating (PDMS:CB, ZnO, PVDF) | CB dispersed in PDMS coated on PET fiber; followed by ZnO and PVDF layering | Under 10 g load: ~5.1 V, ~92.5 nA; up to 10× voltage enhancement vs. prior devices; output linked to synergistic piezo- and triboelectric effects between CB-PDMS, ZnO, and PVDF | Wearable pressure sensors for smartwatches and fitness devices | [145] |
| Material extrusion-based AM | CB integrated in porous PDMS matrix for charge generation | N/A; device fabricated as high-efficiency triboelectric sensor | Finger-thimble sensor for potential use in healthcare monitoring | [235] |
| Material | Triboelectric Polarity | Biocompatibility | AM Compatibility | Ref. |
|---|---|---|---|---|
| Graphene | Positive electron affinity | Conditional; depends on surface functionalization | DIW, electrospinning, bioplotter | [226,249,250] |
| CNTs | Negative | Limited; concerns exist regarding cytotoxicity | Mixed into PDMS/Ecoflex® composites | [233,239] |
| rGO | Slightly negative | Improved vs. pristine graphene; good with hydrogel matrix | Inkjet printing, casting, hydrogel formation | [5,167,251] |
| CB | Neutral to slightly negative | Acceptable in low concentrations; used in elastomer blends | Dispersed into PDMS, Ecoflex®, thermoplastics | [149,235,239] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| 3D Aerogel | MXene/cellulose aerogel lightweight, flexible matrix | Voltage: 115.3 V; current: 0.78 µA; power density: 402.94 mW/m2. | Electromagnetic shielding, energy harvesting, and self-powered sensing. | [264] |
| DIW (Liquid Electrode) | Stretchable MXene electrode with high electronegativity | Voltage: 300 V; current: 5.5 µA; power density: 504 mW/m2. | Stretchable high-output TENG applications. | [264] |
| Fabrication Strategy | Surface Engineering | Functional Performance | Biomedical Applications | Reference |
|---|---|---|---|---|
| 3D Bioprinting | Bio-ionic ink (BIL) photo-crosslinked in hydrogel backbone; BIL content modulates properties | Enhanced conductivity, tunable mechanics, cell adhesion, anti-fouling. | Electroconductive tissue scaffolds, bioelectronics, in situ printing on tissue. | [279] |
| DIW | UV-curable ionogel ink forming self-supporting structures | High transparency, mechanical strength, improved electrochemical traits. | Strain sensors, tissue engineering, drug screening. | [270] |
| Mixed 3D Printing | PAM–LiCl ionic hydrogel integrated with 3D-printed frictional layers | N/A; optimized for low-frequency energy harvesting. | Powering low-frequency wearable/implantable electronics. | [277] |
| Electrospinning | PEI-modified cellulose nanofibers (CNFs), AgNP-coated, ionic liquid-dissolved cellulose matrix | Voc: 286 V, Isc: 4 µA; load density: 13.3–85.8 µC/m2 depending on film type. | Wearable electronics, energy harvesting, self-powered sensors. | [280,281] |
| Material | Triboelectric Polarity | Biocompatibility | AM Compatibility | Ref. |
|---|---|---|---|---|
| MXenes (e.g., Ti3C2Tx) | Negative | Good when embedded in hydrogel/elastomer matrix | Inkjet printing and hydrogel embedding | [264] |
| ILs | Tunable (depends on pairing) | Moderate to good; cytotoxicity depends on ion pair | Dip coating, casting, electrostatic spraying | [273,274,275,276,277] |
| Hydrogels (e.g., PVA and polyacrylic acid–PAA) | Variable (matrix-dependent) | Excellent; highly biocompatible and skin-compliant | Hydrogel casting and inkjet printing | [270,277,279] |
| PEDOT:PSS | Slightly positive to neutral | Good; conductive polymer used in bioelectronics | Spin coating, screen printing, inkjet printing | [293,294,301] |
| Material | Biocompatibility | Conductivity | Surface Modifiability | AM Compatibility | Limitations |
|---|---|---|---|---|---|
| PDMS | High | Low | High | High | Softness |
| PTFE | High | Low | Moderate | Moderate | Difficult Processing |
| Ecoflex® | High | Low | Moderate | Moderate | Insulating |
| Kapton® | Moderate | Low | Moderate | Moderate | Low Output |
| Nylon | High | Low | Moderate | High | Hydrophilicity |
| PET | Moderate | Low | High | High | Hydrophobicity |
| Graphene | Variable | High | High | Moderate | Toxicity |
| CNTs | Variable | High | High | Moderate | Agglomeration |
| rGO | Variable | Moderate | High | Moderate | Dispersion |
| MXene | Tunable | High | High | High | Oxidation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brăileanu, P.I.; Pascu, N.E. Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior. Materials 2025, 18, 3366. https://doi.org/10.3390/ma18143366
Brăileanu PI, Pascu NE. Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior. Materials. 2025; 18(14):3366. https://doi.org/10.3390/ma18143366
Chicago/Turabian StyleBrăileanu, Patricia Isabela, and Nicoleta Elisabeta Pascu. 2025. "Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior" Materials 18, no. 14: 3366. https://doi.org/10.3390/ma18143366
APA StyleBrăileanu, P. I., & Pascu, N. E. (2025). Influence of Surface Texture in Additively Manufactured Biocompatible Materials and Triboelectric Behavior. Materials, 18(14), 3366. https://doi.org/10.3390/ma18143366







