One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag: Phosphorus Involved in Calcium Alumino-Silicate Hydrate Gel
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Preparation of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag
2.3. Testing Methods
3. Results and Discussion
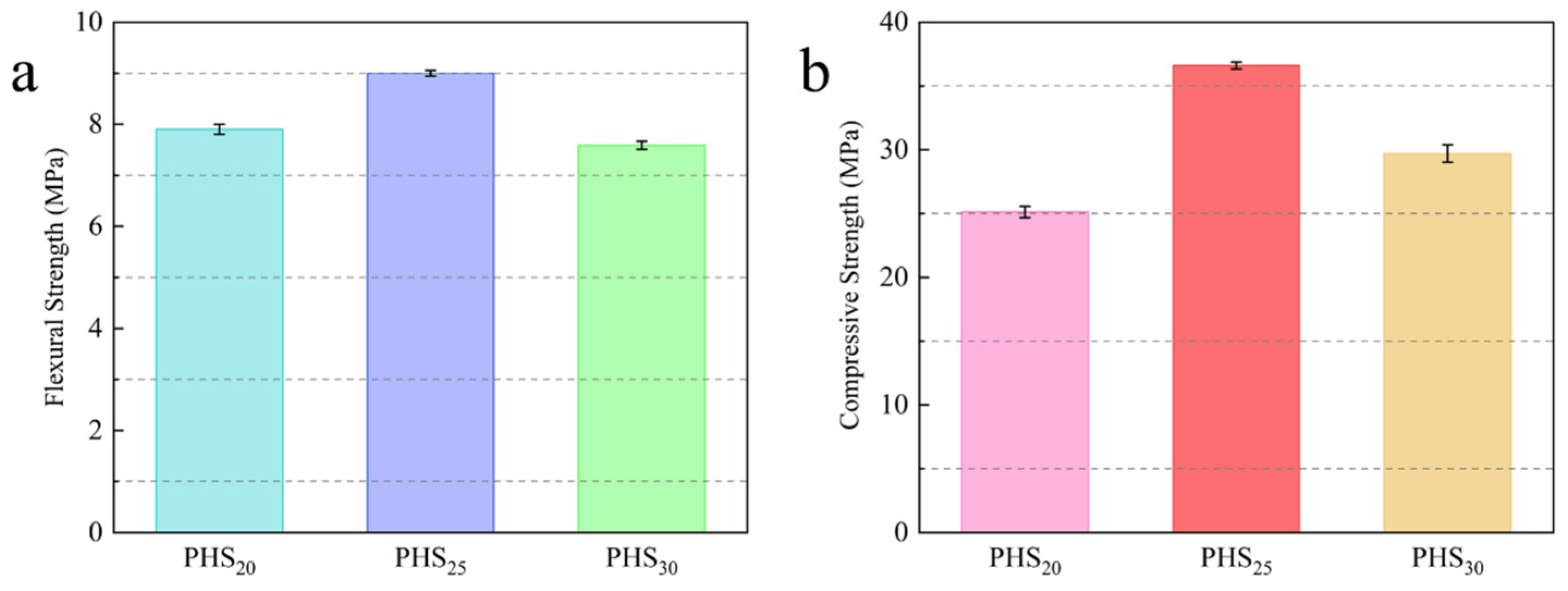
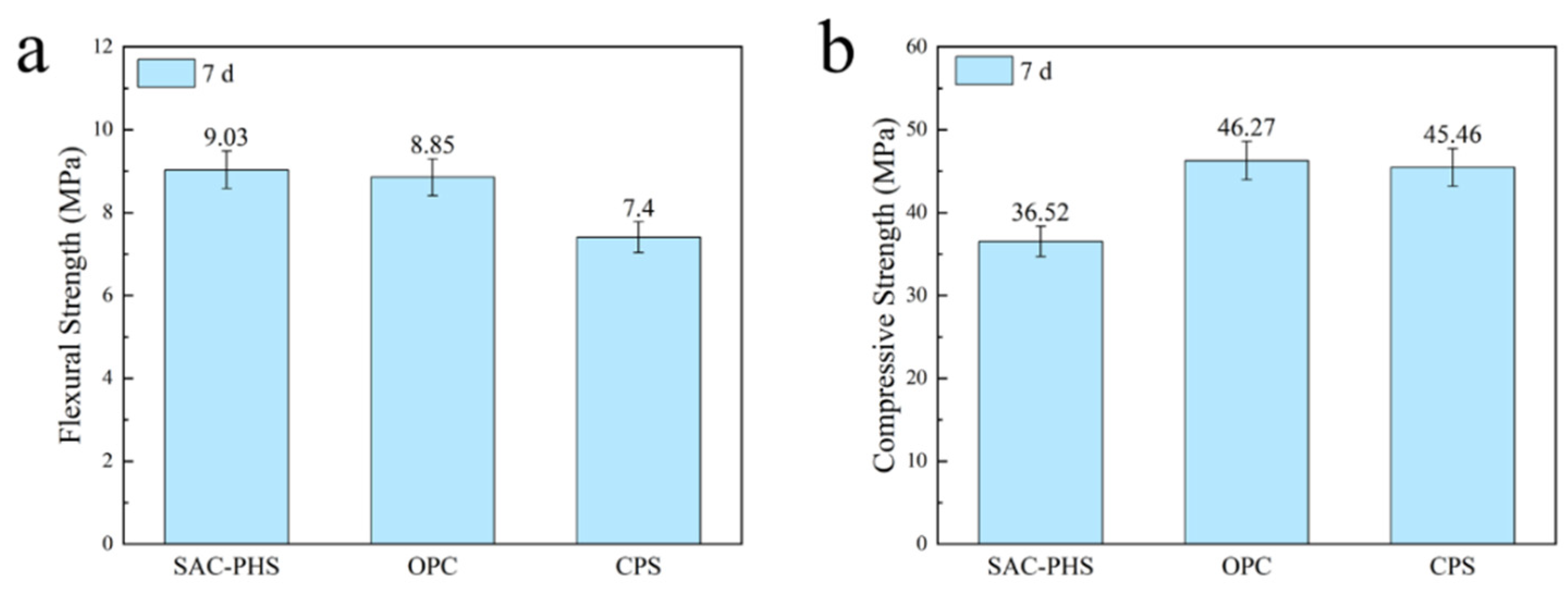
3.1. One-Week Strength of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag
3.2. One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag
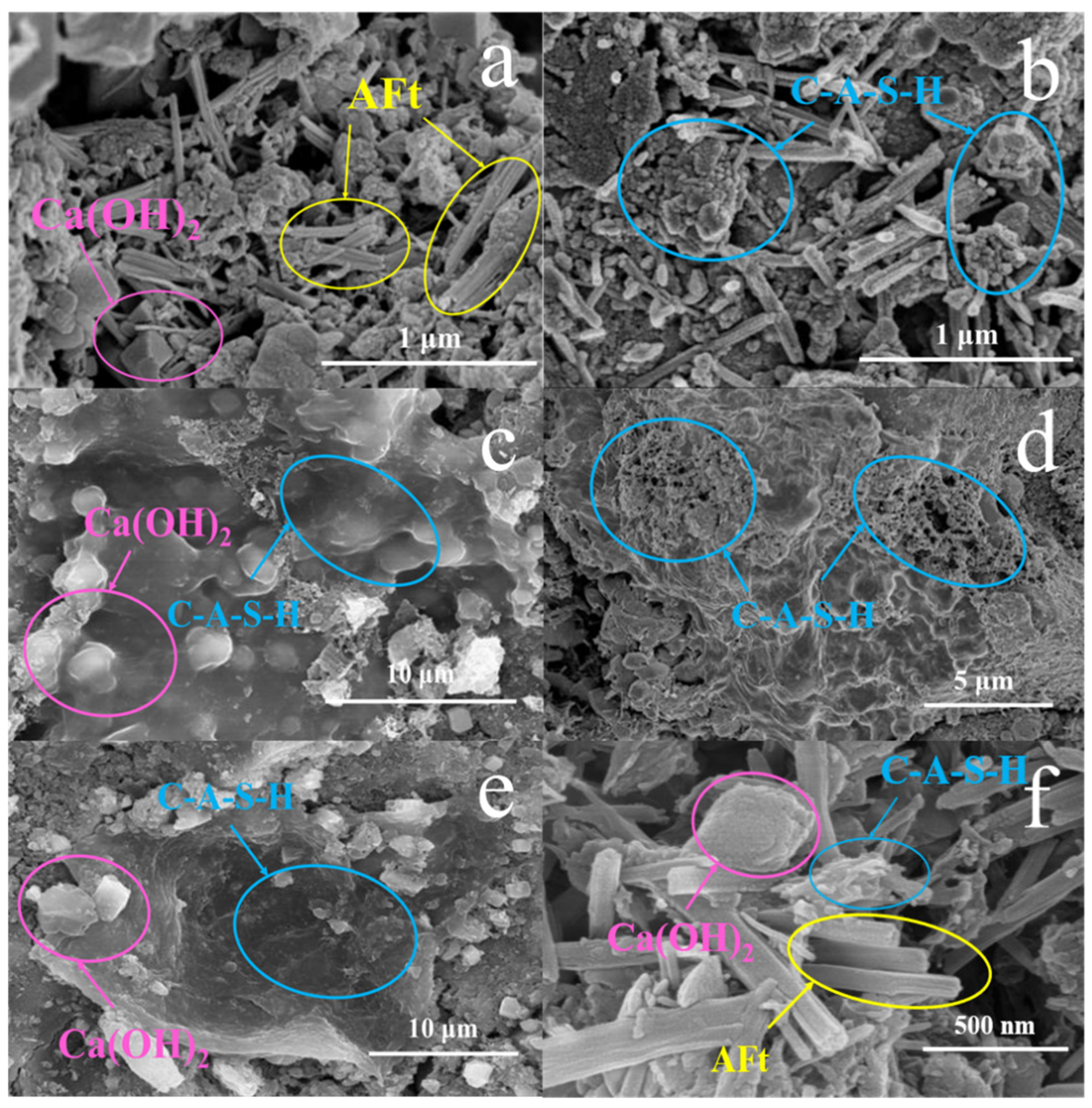
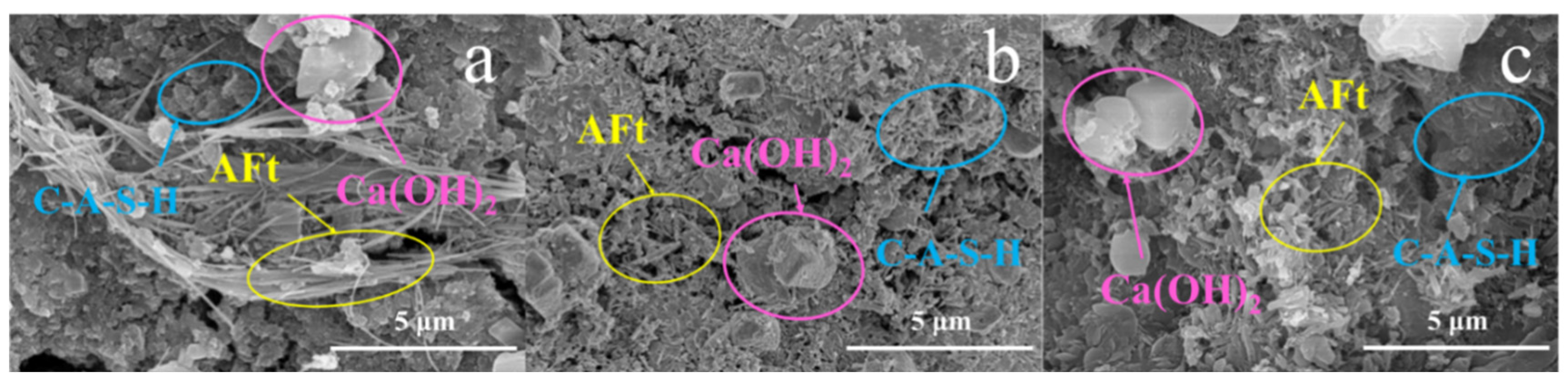
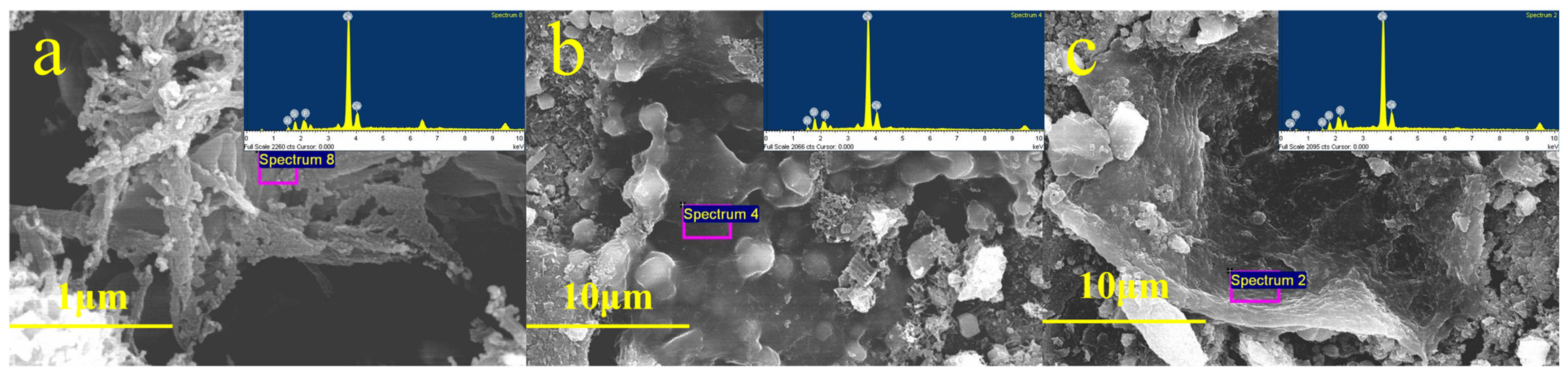
3.2.1. SEM-EDS Analysis
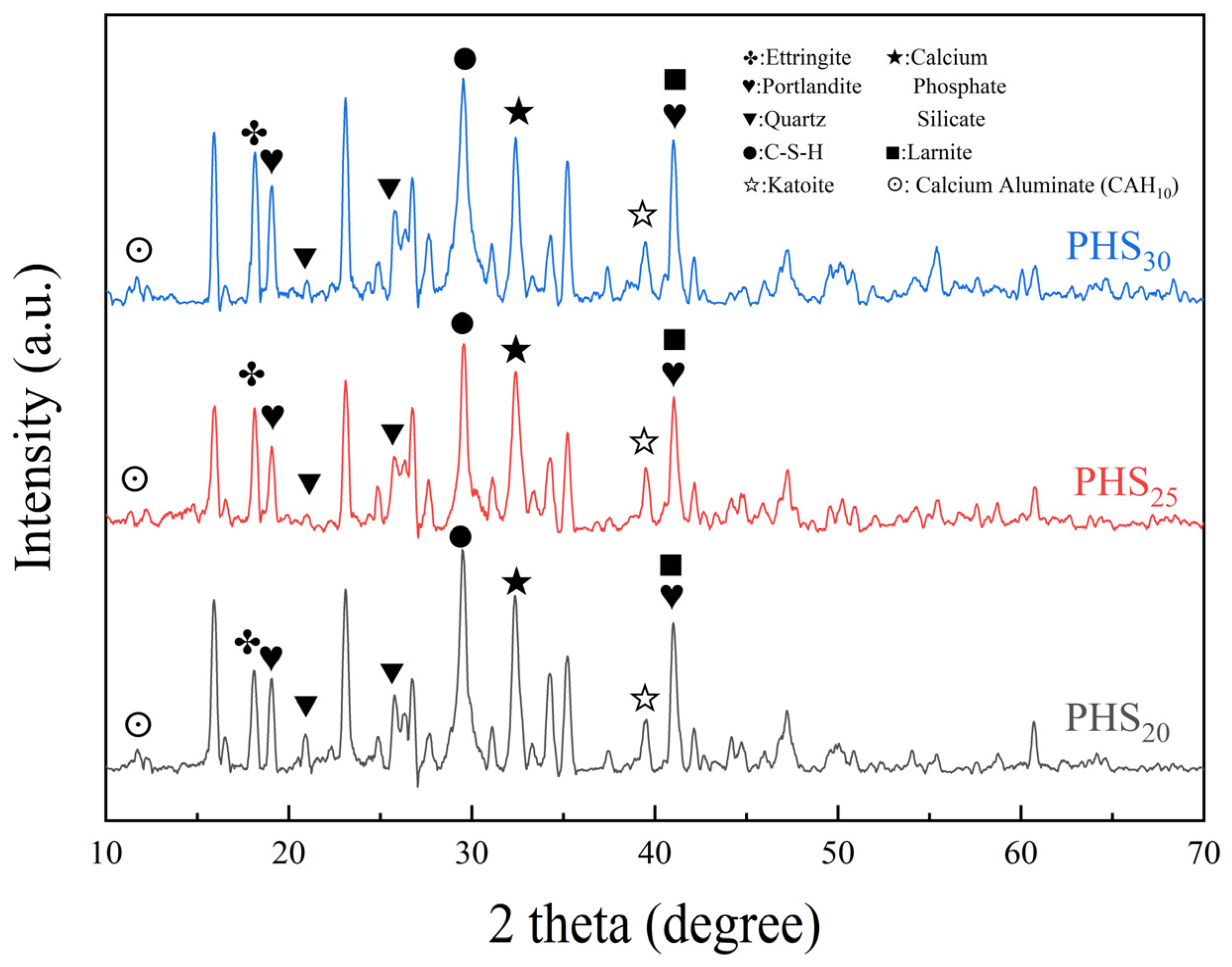
3.2.2. XRD Analysis
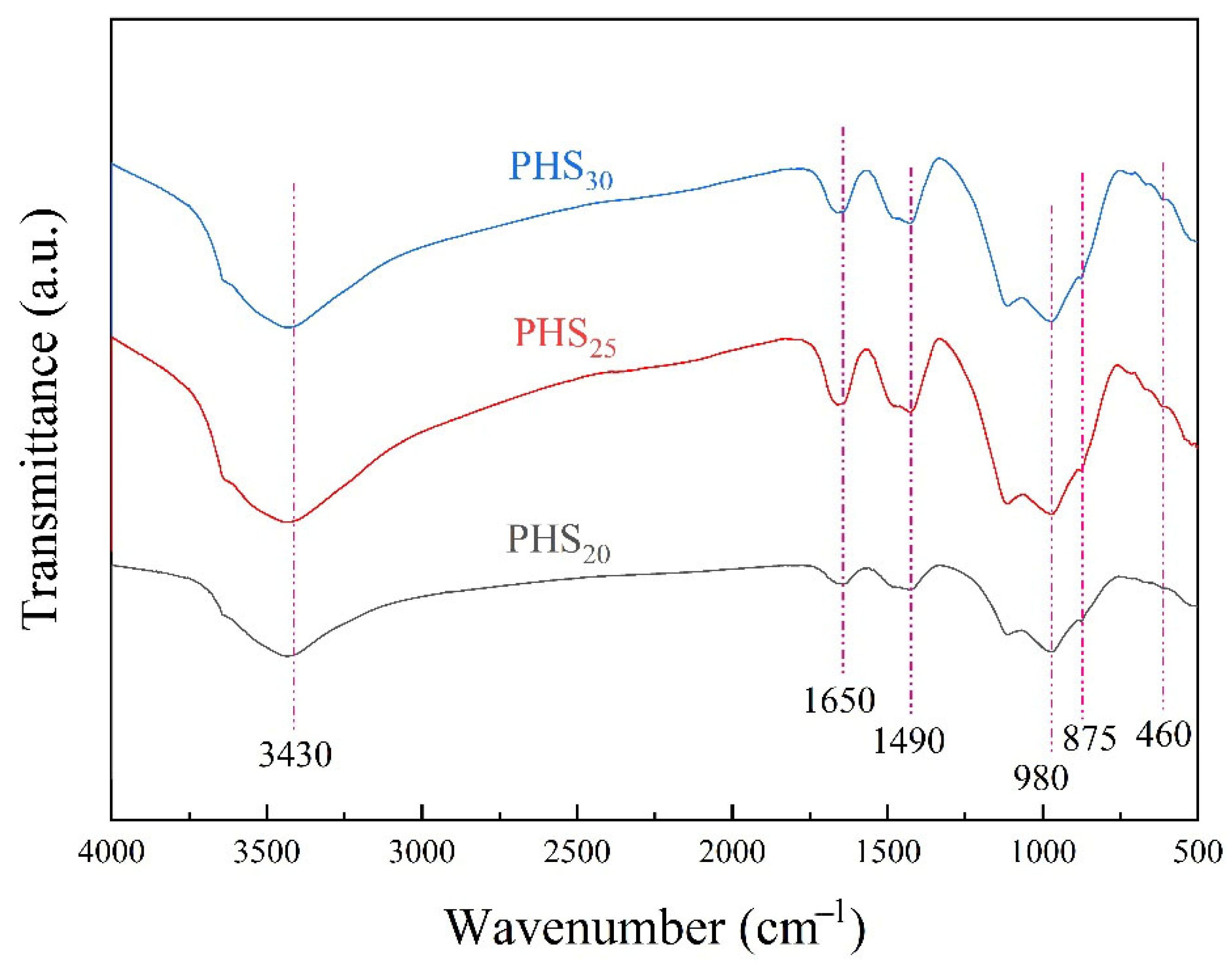
3.2.3. FTIR Analysis
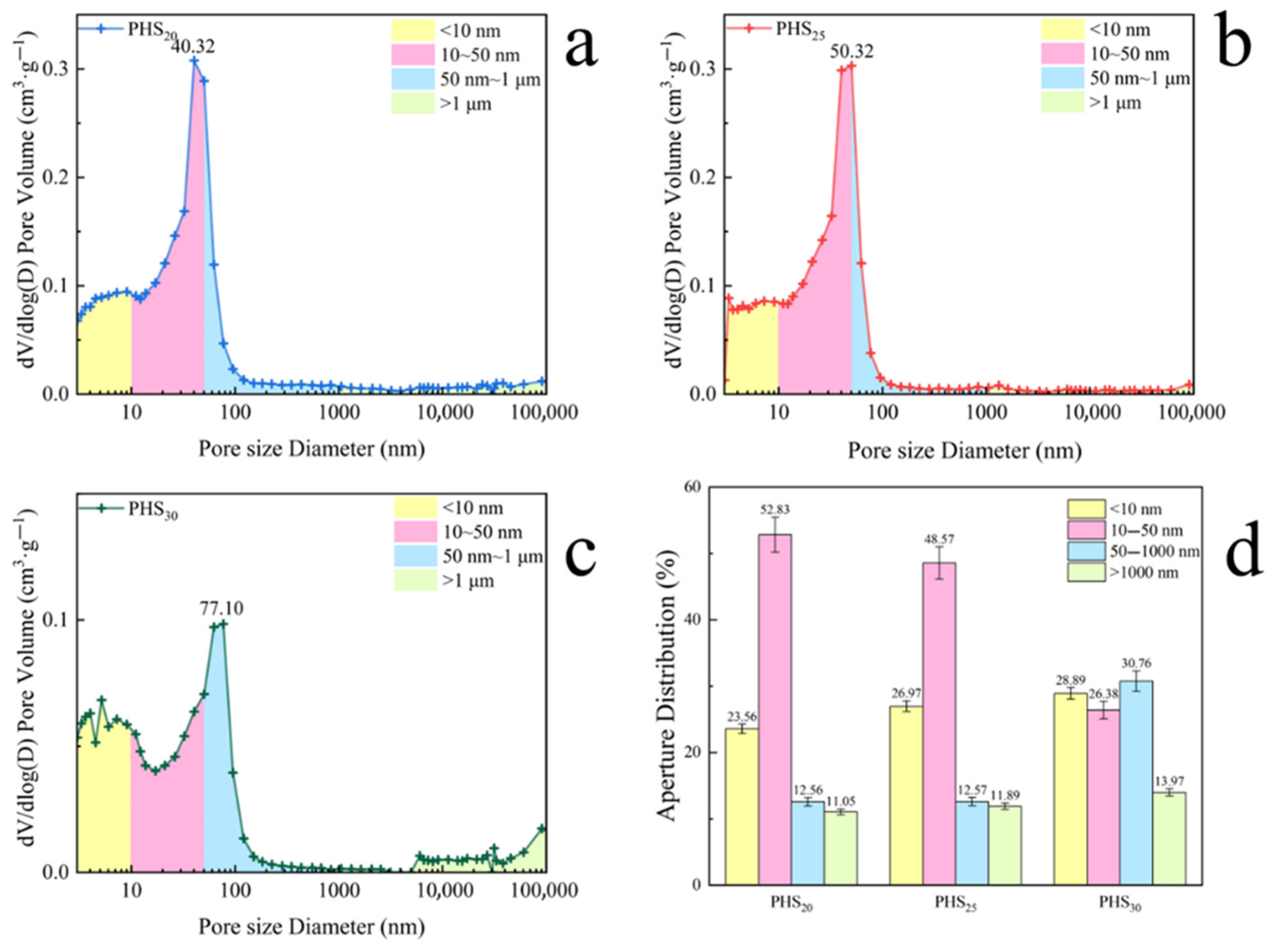
3.2.4. MIP Analysis
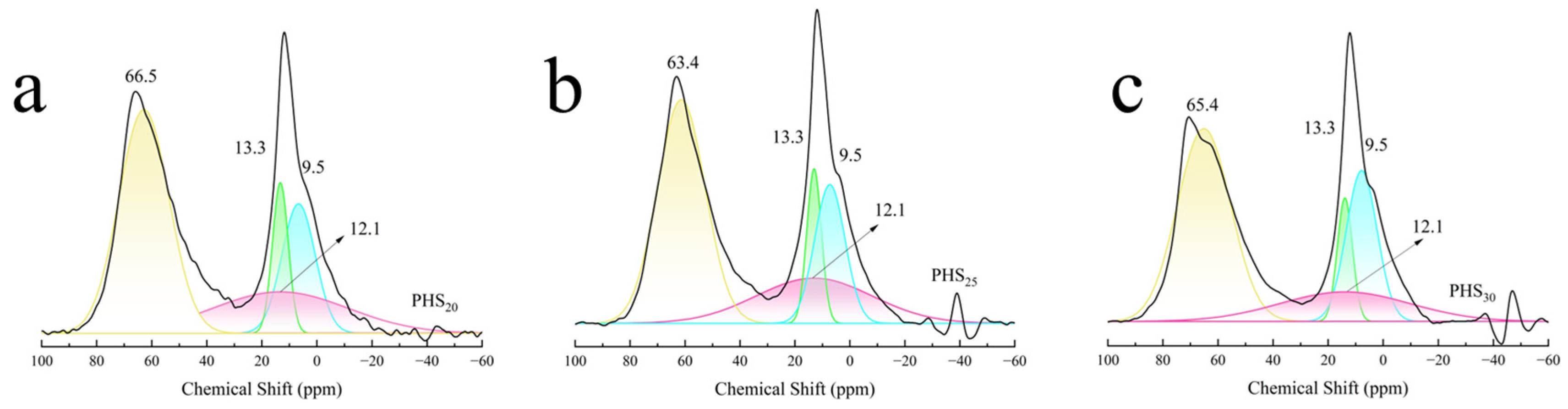
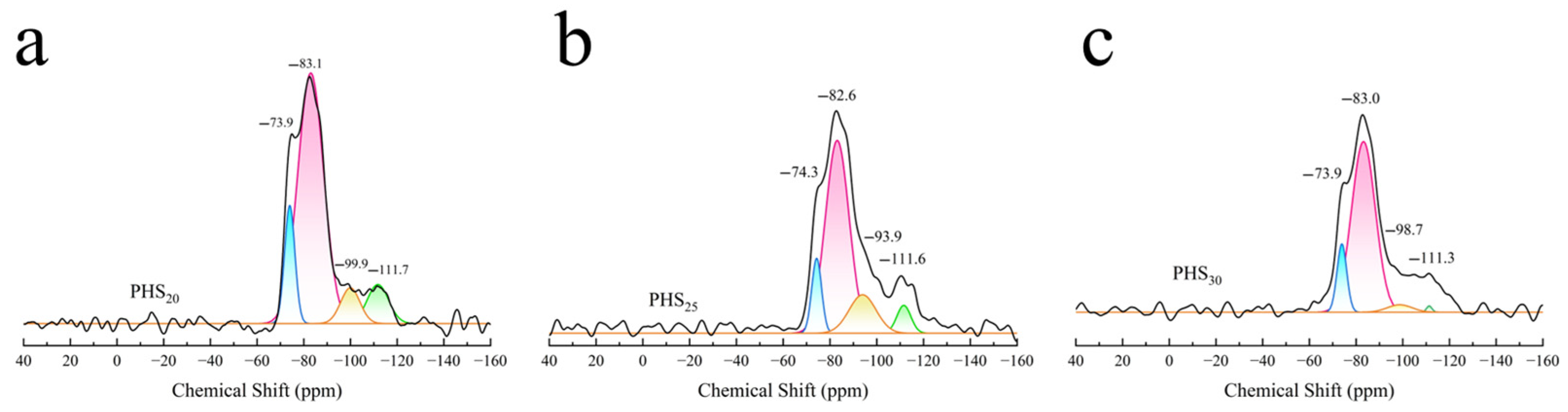
3.2.5. MAS-NMR Analysis
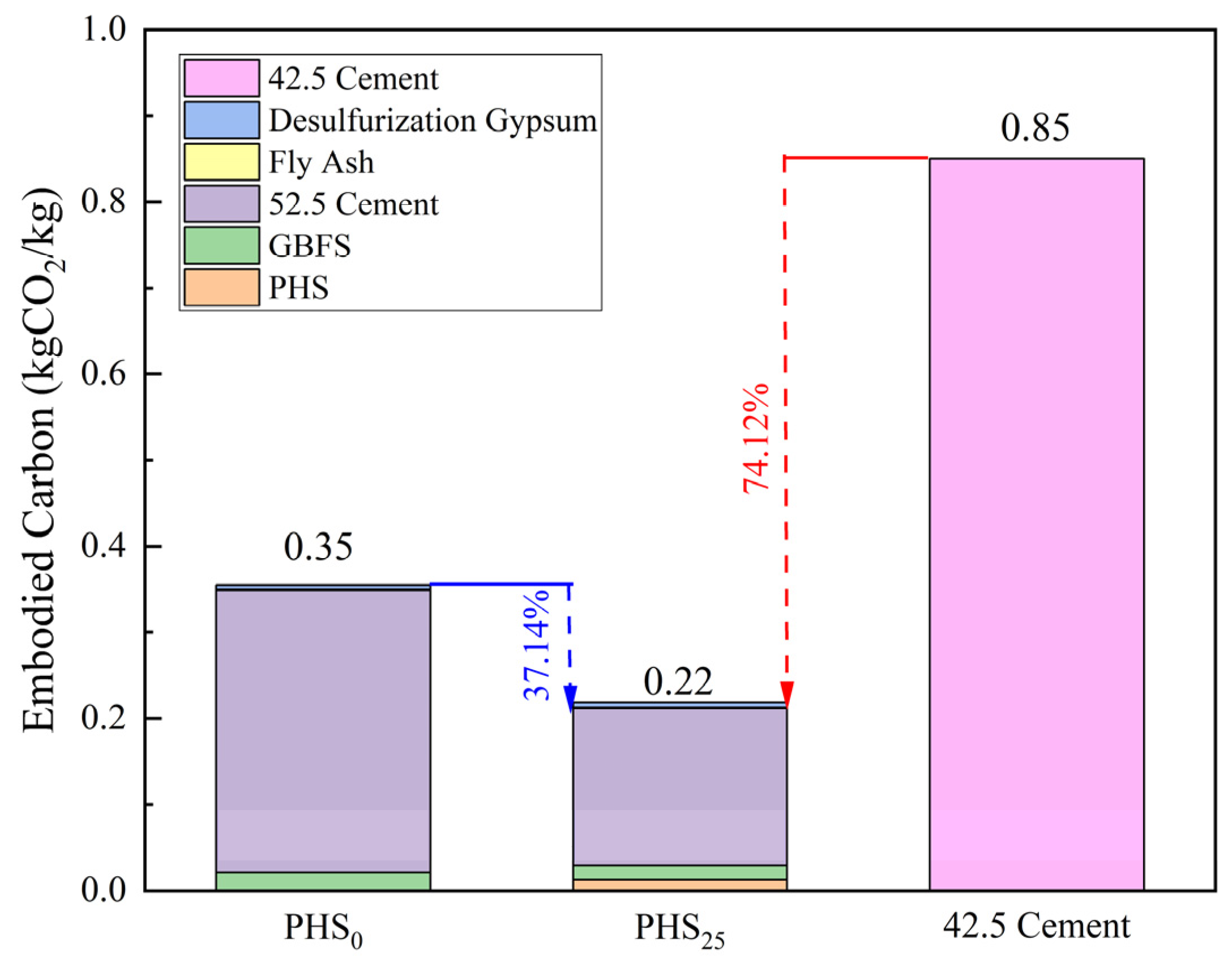
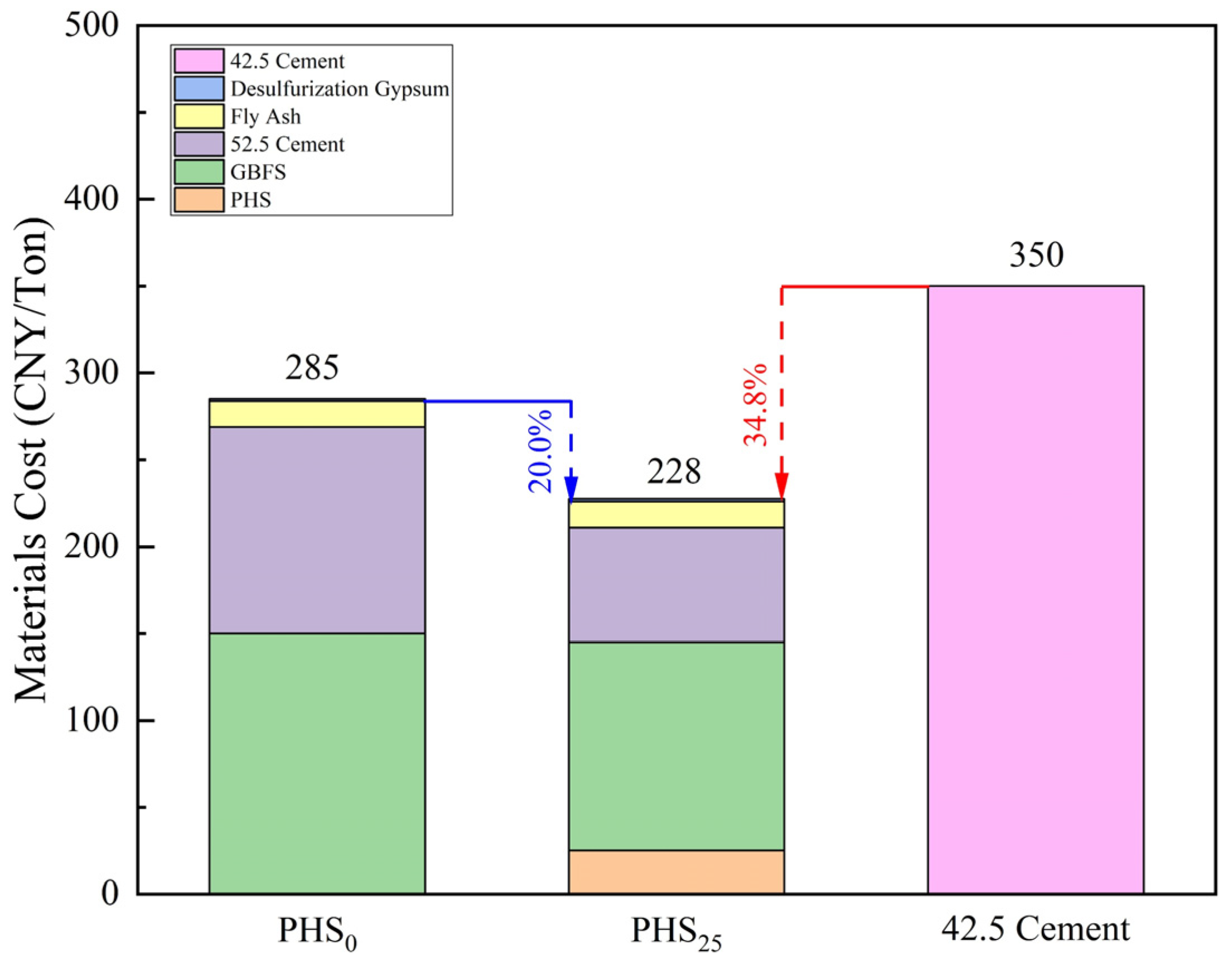
3.3. Carbon Emission and Economic Cost of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag
4. Conclusions
- (1)
- Phosphorous slag could be used as a supplementary cementitious material to prepared silica-aluminum based cementitious materials (SAC-PHS). The PHS25 with good mechanical properties was prepared by mixing 25 wt% phosphorous slag, 40 wt% ground granulated blast furnace slag (GBFS), 20 wt% PII 52.5 cement, 10 wt% fly ash, and 5 wt% desulfurization gypsum. The flexural and compressive strengths of PHS25 at 7 days attained 9.0 MPa and 36.5 MPa, respectively.
- (2)
- The one-week hydration products of SAC-PHS mainly included C-A-S-H gels, Ca(OH)2, and ettringite (AFt). C-A-S-H gels and AFt played an active role in the development of one-week strength. The C-A-S-H gels and AFt produced by SAC-PHS when the content of phosphorous slag reached 25% (PHS25) were the bulk, leading to a smaller pore size and more uniform pore distribution. The P/Si atomic ratio showed that during the formation of C-A-S-H gels, a bond was formed between Si and P, which improved the strength of the SAC-PHS material.
- (3)
- The aluminum in the one-week hydration products of SAC-PHS existed in the form of four and six coordination, proving that the four-coordination Al in the raw material participated in the hydration reaction to form C-A-S-H gels and AFt. The [SiO4] polymerization of C-A-S-H gels was mainly in the form of SiQ2(1Al) in one-week hydration time.
- (4)
- The embodied carbon of PHS25 was 0.22 kgCO2/kg, which is 74.12% lower than the embodied carbon of 42.5 Portland cement and 37.14% lower than that of PHS0. The material cost of PHS25 was 228 CNY/Ton, which is 20.0% lower than PHS0 and 34.8% lower than 42.5 Portland cement, presenting significant environmental and ecological values.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassankhani-Majd, Z.; Anbia, M. Recovery of valuable materials from phosphorus slag using nitric acid leaching followed by precipitation method. Resour. Conserv. Recycl. 2021, 169, 105547. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T. Mineral carbonation of yellow phosphorus slag and characterization of carbonated product. Energy 2019, 188, 116102. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wu, A.; Shi, D.; Zhang, M.; Ruan, Z.; Wang, S. Study on the performance of alkali-activated phosphorus slag cemented paste backfill material: Effect of activator type and amount. Constr. Build. Mater. 2024, 425, 136036. [Google Scholar] [CrossRef]
- Yu, H.; Li, S.; Qian, G.; Gong, X.; Wang, K. Experiment evaluation on the surface treatment of phosphorus slag (PS) micropowder for asphalt modification. Constr. Build. Mater. 2020, 262, 119931. [Google Scholar] [CrossRef]
- Xie, F.; Liu, Z.; Zhang, D.; Wang, J.; Huang, T.; Wang, D. Reaction kinetics and kinetics models of alkali activated phosphorus slag. Constr. Build. Mater. 2020, 237, 117728. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Z. Effect of Na2O and heat-treatment on crystallization of glass–ceramics from phosphorus slag. J. Alloys. Compd. 2013, 557, 190–195. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Zhang, Z. Recycling and comprehensive utilization of yellow phosphorus slag in building materials: A review. Constr. Build. Mater. 2023, 396, 132384. [Google Scholar] [CrossRef]
- Chen, G.; Huang, Y.; Yang, R.; Yu, R.; Xiao, R.; Wang, Z.; Ke, X.; Xie, G.; Cheng, J.; Bao, M. Comparative study on mechanical properties and microstructure development of ultra-high performance concrete incorporating phosphorous slag under different curing regimes. Constr. Build. Mater. 2023, 392, 131963. [Google Scholar] [CrossRef]
- Islam, M.S.; Mohr, B.J.; VandenBerge, D. Performance of natural clinoptilolite zeolite in the cementitious materials: A comparative study with metakaolin, fly ash, and blast furnace slag. J. Build. Eng. 2022, 53, 104535. [Google Scholar] [CrossRef]
- Pan, H.; Geng, Z.; Huang, P.; Zhou, Y.; Tang, J.; She, W.; Liu, J. A bionic solution to make cement matrix tough. Cem. Concr. Compos. 2023, 136, 104881. [Google Scholar] [CrossRef]
- Carvalho, V.R.; Costa, L.C.B.; Elói FPda, F.; Bezerra ACda, S.; Carvalho JMFde Peixoto, R.A.F. Performance of low-energy steel slag powders as supplementary cementitious materials. Constr. Build. Mater. 2023, 392, 131888. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Reuse of copper slag as a supplementary cementitious material: Reactivity and safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- Yang, R.; Yu, R.; Shui, Z.; Gao, X.; Xiao, X.; Zhang, X.; Wang, Y.; He, Y. Low carbon design of an Ultra-High Performance Concrete (UHPC) incorporating phosphorous slag. J. Clean. Prod. 2019, 240, 118157. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, C.; Qu, G.; Liu, S.; Ren, Y.; Chen, B.; Li, J.; Liu, L. A critical review of the typical by-product clean ecology links in the Chinese phosphorus chemical industry in China: Production technologies, fates and future directions. J. Environ. Chem. Eng. 2022, 10, 106685. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, H.; Liu, X.; Zhang, J. Early-age characteristics of red mud-coal gangue cementitious material. J. Hazard. Mater. 2009, 167, 927–932. [Google Scholar] [CrossRef]
- Hamsashree, P.; Pandit, P.; Prashanth, S.; Katpady, D.N. Durability of alkali-activated fly ash-slag concrete—state of art. Innov. Infrastruct. Solut. 2024, 9, 15. [Google Scholar] [CrossRef]
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh mechanical and durability properties of alkali-activated fly ash-slag concrete: A review. Innov. Infrastruct. Solut. 2021, 6, 124. [Google Scholar] [CrossRef]
- ISO 679:2009; Cements—Test Methods—Determination of Strength. International Organization for Standardization: Geneva, Switzerland, 2009.
- Wang, Y.; Zhang, N.; Xiao, H.; Zhao, J.; Zhang, Y.; Liu, X. Structural Characterization of Phosphorous Slag Regarding Occurrence State of Phosphorus in Dicalcium Silicate. Materials 2022, 15, 7450. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength. Standardization Administration of China: Beijing, China, 2021.
- Szabo, G.L.; Jany, B.R.; Muckenhuber, H.; Niggas, A.; Lehner, M.; Janas, A.; Szabo, P.S.; Gan, Z.; George, A.; Turchanin, A.; et al. Charge-State-Enhanced Ion Sputtering of Metallic Gold Nanoislands. Small 2023, 19, 2207263. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Liu, X.; Lan, M.; Lei, B.; Chen, Q.; Xue, X. Mechanical properties of eco-cement mortar containing MgO-modified phosphorous slag. Constr. Build. Mater. 2024, 428, 136223. [Google Scholar] [CrossRef]
- Tran, V.-A.; Nguyen, H.-A. Evaluation on comprehensive properties and bonding performance of practical slag-fly ash blending based alkali-activated material. J. Build. Eng. 2022, 62, 105350. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Mechanical and microstructural evolutions of fly ash/slag-based geopolymer at high temperatures: Effect of curing conditions. Ceram. Int. 2022, 49, 2091–2101. [Google Scholar] [CrossRef]
- Alharbi, N.; Varela, B.; Hailstone, R. Alkali-activated slag characterization by scanning electron microscopy, X-ray microanalysis and nuclear magnetic resonance spectroscopy. Mater. Charact. 2020, 168, 110504. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Yang, K.; Liu, C.; Guan, X.; Liu, S.; Jing, G. Effects of synthetic CSH-tartaric acid nanocomposites on the properties of ordinary Portland cement. Cem. Concr. Compos. 2022, 129, 104466. [Google Scholar] [CrossRef]
- Emmanuel, A.C.; Krishnan, S.; Bishnoi, S. Influence of curing temperature on hydration and microstructural development of ordinary Portland cement. Constr. Build. Mater. 2022, 329, 127070. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, H.; He, X.; Zheng, Z.; Ma, Q.; Ding, J.; Bao, M. The effect of wet-grinding phosphorus slag on the hydration kinetics of Portland cement. Constr. Build. Mater. 2023, 364, 129942. [Google Scholar] [CrossRef]
- He, X.; Ye, Q.; Yang, J.; Dai, F.; Su, Y.; Wang, Y.; Bohumír, S. Physico-chemical Characteristics of Wet-milled Ultrafine-granulated Phosphorus Slag as a Supplementary Cementitious Material. J. Wuhan. Univ. Technol-Mater. Sci. Ed. 2018, 33, 625–633. [Google Scholar] [CrossRef]
- Qi, C.; Xia, C.; Dyskin, A.; Zhao, F. Effect of crack interaction and friction on the dynamic strength of rock-like materials with many cracks. Eng. Fract. Mech. 2021, 257, 108006. [Google Scholar] [CrossRef]
- Chen, B.; Pang, L.; Zhou, Z.; Chang, Q.; Fu, P. Study on the hydration properties of a ternary cementitious material system containing activated gold tailings and granulated blast furnace slag. J. Build. Eng. 2023, 63, 105574. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, M.; Yan, S.; Gao, Z.; Hu, Y.; Peng, L.; Zhang, Y.; Wang, Z. Study on the influence mechanism of calcium carbonate particles on mechanical properties of microcrack cement. Constr. Build. Mater. 2024, 411, 134563. [Google Scholar] [CrossRef]
- Wang, L.; Guo, F.; Lin, Y.; Yang, H.; Tang, S.W. Comparison between the effects of phosphorous slag and fly ash on the C-S-H structure, long-term hydration heat and volume deformation of cement-based materials. Constr. Build. Mater. 2020, 250, 118807. [Google Scholar] [CrossRef]
- Yang, J.; Hou, D.; Ding, Q.; Zhang, G.; Zhang, Y.; Hu, H. Insight on the nanoscale chemical degradation mechanism of MgCl2 attack in cement paste. Constr. Build. Mater. 2020, 238, 117777. [Google Scholar] [CrossRef]
- Zheng, Q.; Jiang, J.; Chen, C.; Yu, J.; Li, X.; Tang, L.; Li, S. Nanoengineering Microstructure of Hybrid C–S–H/Silicene Gel. ACS Appl. Mater. Interfaces 2020, 12, 17806–17814. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, L.; Huang, A.; Li, B.; Sun, Y.; Jiang, Z.; Li, W.; Zhu, H. Healing of concrete cracks by in-situ synthesis of ettringite induced by electric field. Constr. Build. Mater. 2022, 352, 128685. [Google Scholar] [CrossRef]
- Gollop, R.S.; Taylor, H.F.W. Microstructural and microanalytical studies of sulfate attack. I. Ordinary portland cement paste. Cem. Concr. Res. 1992, 22, 1027–1038. [Google Scholar] [CrossRef]
- Yang, J.; Ding, Q.; Zhang, G.; Hou, D.; Zhao, M.; Cao, J. Effect of sulfate attack on the composition and micro-mechanical properties of C-A-S-H gel in cement-slag paste: A combined study of nanoindentation and SEM-EDS. Constr. Build. Mater. 2022, 345, 128275. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Wu, H.; Cao, C. Chloride permeability of concrete mixed with activity recycled powder obtained from C&D waste. Constr. Build. Mater. 2019, 199, 652–663. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Xu, Z.; Li, S.; Luo, X.; Chen, G. Long-term hydration and microstructure evolution of blended cement containing ground granulated blast furnace slag and waste clay brick. Cem. Concr. Compos. 2021, 118, 103982. [Google Scholar] [CrossRef]
- Prochon, P.; Piotrowski, T.; Kępniak, M. The effects of phosphate compounds on the microstructure and mechanical properties of fly ash geopolymer mortars. Materials 2024, 17, 5451. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Z.; Liu, X. Comprehensive understanding of aluminosilicate phosphate geopolymers: A critical review. Materials 2022, 15, 5961. [Google Scholar] [CrossRef]
- Furlani, E.; Aneggi, E.; Rondinella, A.; Zanocco, M.; Fedrizzi, L.; Maschio, S. The effect of the P/Si ratio on the preparation and properties of phosphoric acid-metakaolin geopolymers. J. Ceram. Sci. Technol. 2021, 12, 19–28. [Google Scholar] [CrossRef]
- Yan, C.; Ma, H.; Luo, Z.; Zhou, X.; Wang, L. Influence of phosphorus sources on the compressive strength and microstructure of ferronickel slag-based magnesium phosphate cement. Materials 2022, 15, 1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Z.; Zhu, H.; Wang, X.; Gao, J. Effects of silane on reaction process and microstructure of metakaolin-based geopolymer composites. J. Build. Eng. 2020, 32, 101695. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Rao, F.; Yin, W. Mechanical property and structural evolution of alkali-activated slag-phosphate mine tailings mortars. Chemosphere 2020, 251, 126367. [Google Scholar] [CrossRef] [PubMed]
- Talero, R. Comparative XRD analysis ettringite originating from pozzolan and from portland cement. Cem. Concr. Res. 1996, 26, 1277–1283. [Google Scholar] [CrossRef]
- Fang, D.; Huang, L.; Fang, Z.; Zhang, Q.; Shen, Q.; Li, Y.; Xu, X.; Ji, F. Evaluation of porous calcium silicate hydrate derived from carbide slag for removing phosphate from wastewater. Chem. Eng. J. 2018, 354, 1–11. [Google Scholar] [CrossRef]
- Padilla-Encinas, P.; Palomo, A.; Blanco-Varela, M.T.; Fernández-Jiménez, A. Calcium sulfoaluminate clinker hydration at different alkali concentrations. Cem. Concr. Res. 2020, 138, 106251. [Google Scholar] [CrossRef]
- Wang, F.; Kong, X.; Jiang, L.; Wang, D. The acceleration mechanism of nano-C-S-H particles on OPC hydration. Constr. Build. Mater. 2020, 249, 118734. [Google Scholar] [CrossRef]
- De Matos, P.R.; Andrade Neto, J.S.; Campos, C.E.M. Is the R index accurate to assess the preferred orientation of portlandite in cement pastes? Constr. Build. Mater. 2021, 292, 123471. [Google Scholar] [CrossRef]
- Marinoni, N.; Broekmans, M.A.T.M. Microstructure of selected aggregate quartz by XRD, and a critical review of the crystallinity index. Cem. Concr. Res. 2013, 54, 215–225. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Swamiappan, S. Biomineralization, mechanical, antibacterial and biological investigation of larnite and rankinite bioceramics. Mater. Sci. Eng. C. 2021, 118, 111466. [Google Scholar] [CrossRef]
- Zheng, H.; Duan, Y.; Li, M.; Hou, D.; Wang, P.; Chen, J.; Li, S. Reaction molecular dynamics study of calcium alumino-silicate hydrate gel in the hydration deposition process at the calcium silicate hydrate interface: The influence of Al/Si. J. Build. Eng. 2024, 86, 108823. [Google Scholar] [CrossRef]
- Hou, D.; Wu, C.; Yang, Q.; Wang, P.; Ding, Q. Temperature Effect of Hydration and Microstructure of Tricalcium Silicate–Slag Powder Hydrated Composites: An Experimental and Molecular Dynamics Investigation. ACS Sustain. Chem. Eng. 2021, 9, 13773–13787. [Google Scholar] [CrossRef]
- Zhu, D.; Wen, A.; Tang, A. Mechanical properties, durability and environmental assessment of low-carbon cementitious composite with natural fibrous wollastonite. Environ. Res. 2023, 234, 116552. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Li, S.; Lin, C.; Gao, Y.; Liu, C. Feasibility of preparing red mud-based cementitious materials: Synergistic utilization of industrial solid waste, waste heat, and tail gas. J. Clean. Prod. 2021, 285, 124896. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W.; Jiang, Y.; Wan, Y.; Du, R.; Li, H. Solidification of heavy metals in lead smelting slag and development of cementitious materials. J. Clean. Prod. 2022, 359, 132134. [Google Scholar] [CrossRef]
- Tan, Q.; Yang, Q.; Ye, C.; Wang, D.; Xie, N. Utilization of red mud in high-performance grouting material for semi-flexible pavement. J. Clean. Prod. 2024, 454, 142240. [Google Scholar] [CrossRef]
- Li, Z.; Corr, D.J.; Han, B.; Shah, S.P. Investigating the effect of carbon nanotube on early age hydration of cementitious composites with isothermal calorimetry and Fourier transform infrared spectroscopy. Cem. Concr. Compos. 2020, 107, 103513. [Google Scholar] [CrossRef]
- Du, H.; Xu, D.; Li, X.; Li, J.; Ni, W.; Li, Y.; Fu, P. Application of molten iron desulfurization slag to replace steel slag as an alkaline component in solid waste-based cementitious materials. J. Clean. Prod. 2022, 377, 134353. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Wang, Y.; Lu, P.; Wu, N.; Li, C.; Zhang, Y. Superior reinforcement efficiency of basalt fibers in geopolymers: Hydration interaction and embodied carbon advantage over carbon fibers. Sustain. Mater. Technol. 2024, 43, e01203. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, K.; Wang, C.; Wang, X.; Deng, G.; Wei, P. Influence of the porosity and pore size on the compressive and splitting strengths of cellular concrete with millimeter-size pores. Constr. Build. Mater. 2020, 235, 117508. [Google Scholar] [CrossRef]
- Wei, G.; Dong, B.; Hong, S.; Fang, G.; Xing, F.; Wang, Y. Deep insight into reactive chemical structures of Class F fly ash. Cem. Concr. Compos. 2023, 142, 105195. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Cassino, C.; Gastaldi, D.; Buzzi, L.; Canonico, F.; Marchese, L. Fingerprinting the hydration products of hydraulic binders using snapshots from time-resolved in situ multinuclear MAS NMR spectroscopy. J. Phys. Chem. C 2021, 125, 9261–9272. [Google Scholar] [CrossRef]
- Singh, P.S.; Trigg, M.; Burgar, I.; Bastow, T. Geopolymer formation processes at room temperature studied by 29Si and 27Al MAS-NMR. Mater. Sci. Eng. A 2005, 396, 392–402. [Google Scholar] [CrossRef]
- Berezicka, A.; Szumera, M.; Sułowska, J.; Jeleń, P.; Olejniczak, Z.; Stępień, J.; Zając, M.; Pollastri, S.; Olivi, L. Unraveling the nature of sulfur-bearing silicate-phosphate glasses: Insights from multi-spectroscopic (Raman, MIR, 29Si, 31P MAS-NMR, XAS, XANES) investigation. Ceram. Int. 2022, 48, 4238–4254. [Google Scholar] [CrossRef]
- Guo, X.; Song, M. Micro-nanostructures of tobermorite hydrothermal-synthesized from fly ash and municipal solid waste incineration fly ash. Constr. Build. Mater. 2018, 191, 431–439. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Tawfik, T.A.; Sikora, P.; Abd Elrahman, M. Preparation and characterization of a novel alkali-activated magnesite cement. Constr. Build. Mater. 2022, 345, 128384. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, S.; Liu, C. Phosphorus slags: Structural insights, dissolution behavior, and potential as sustainable supplementary cementitious materials. Chem. Eng. J. 2025, 507, 160463. [Google Scholar] [CrossRef]
- Schneider, J.; Cincotto, M.A.; Panepucci, H. 29Si and 27Al high-resolution NMR characterization of calcium silicate hydrate phases in activated blast-furnace slag pastes. Cem. Concr. Res. 2001, 31, 993–1001. [Google Scholar] [CrossRef]
- Hjorth, J.; Skibsted, J.; Jakobsen, H.J. 29Si MAS NMR studies of portland cement components and effects of microsilica on the hydration reaction. Cem. Concr. Res. 1988, 18, 789–798. [Google Scholar] [CrossRef]
- Škvára, F.; Kopecký, L.; Němeček, J.; Bittnar, Z. Microstructure of geopolymer materials based on fly ash. Ceram.-Silik. 2006, 50, 208–215. [Google Scholar]
- D’Espinose de Lacaillerie, J.-B.; Fretigny, C.; Massiot, D. MAS NMR spectra of quadrupolar nuclei in disordered solids: The Czjzek model. J. Magn. Reson. 2008, 192, 244–251. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Sun, Y.; Zhang, N. Correlation between 29Si polymerization and cementitious activity of coal gangue. J. Zhejiang Univ. 2009, 10, 1334–12240. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. 29Si and 27Al NMR study of alkali-activated slag. Cem. Concr. Res. 2003, 33, 769–774. [Google Scholar] [CrossRef]
- Hammond, G.; Jones, C. Inventory of Carbon & Energy (ICE); University of Bath: Bath, UK, 2008. [Google Scholar]
- Li, Y.; Wang, J.; Han, Y.; Zhao, Q.; Fang, X.; Cao, Z. Robust and opportunistic scheduling of district integrated natural gas and power system with high wind power penetration considering demand flexibility and compressed air energy storage. J. Clean. Prod. 2020, 256, 120456. [Google Scholar] [CrossRef]
- Farkas, A.; Degiuli, N.; Martić, I.; Vujanović, M. Greenhouse gas emissions reduction potential by using antifouling coatings in a maritime transport industry. J. Clean. Prod. 2021, 295, 126428. [Google Scholar] [CrossRef]




| Raw Material | Origin of Raw Material |
|---|---|
| Phosphorous slag | Guizhou Phosphorous Slag, Guiyang, China |
| Ground granulated blast furnace slag | Tangshan Iron and Steel Group, Tangshan, China |
| PII 52.5 Portland cement | Anhui Conch Cement, Wuhu, China |
| Fly ash | Shandong Weiqiao Group, Binzhou, China |
| Desulfurization gypsum | Shandong Weiqiao Group, Binzhou, China |
| Standard sand | Xiamen ISO Standard Sand Co., Ltd., Xiamen, China |
| No. | PHS /wt% | GBFS/wt% | PII 52.5 Portland Cement/wt% | Fly Ash/wt% | Desulfurization Gypsum/wt% |
|---|---|---|---|---|---|
| PHS0 | 0 | 50 | 36 | 10 | 4 |
| PHS20 | 20 | 45 | 20 | 10 | 5 |
| PHS25 | 25 | 40 | 20 | 10 | 5 |
| PHS30 | 30 | 35 | 20 | 10 | 5 |
| PHS35 | 35 | 30 | 20 | 10 | 5 |
| PHS40 | 40 | 25 | 20 | 10 | 5 |
| Sample | AlIV | AlVI | |
|---|---|---|---|
| Chemical Shift (ppm) | 66.5 | 9.5–13.3 | |
| PHS20 | Relative area | 100 | 69.7 |
| Relative content (%) | 58.9 | 41.1 | |
| Chemical Shift (ppm) | 63.4 | 9.5–13.3 | |
| PHS25 | Relative area | 100 | 89.8 |
| Relative content (%) | 52.7 | 47.3 | |
| Chemical Shift (ppm) | 65.4 | 9.5–13.3 | |
| PHS30 | Relative area | 100 | 74.7 |
| Relative content (%) | 57.2 | 42.7 |
| Sample | Chemical Shift (ppm) | Type | Relative Area | RBO |
|---|---|---|---|---|
| PHS20 | −73.9 | SiQ0 | 18.8 | 50.0% |
| −83.1 | SiQ2(1Al) | 100 | ||
| −99.9 | SiQ3 | 10.6 | ||
| −111.7 | SiQ4 | 12.9 | ||
| PHS25 | −74.3 | SiQ0 | 16.1 | 51.0% |
| −82.6 | SiQ2(1Al) | 100 | ||
| −93.9 | SiQ3 | 22.8 | ||
| −111.6 | SiQ4 | 8.6 | ||
| PHS30 | −73.9 | SiQ0 | 16.4 | 45.0% |
| −83.0 | SiQ2(1Al) | 100 | ||
| −98.7 | SiQ3 | 4.5 | ||
| −111.3 | SiQ4 | 0.8 |
| Material | Embodied Carbon | Market Price |
|---|---|---|
| (kgCO2/kg) | (CNY/Ton) | |
| PHS | 0.05 | 100 |
| GBFS | 0.0416 | 300 |
| PII 52.5 Cement | 0.912 | 330 |
| Fly ash | 0.01 | 150 |
| Desulfurization gypsum | 0.12 | 30 |
| 42.5 Portland Cement | 0.85 | 350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, Y.; Zhang, J.; Wang, Y.; Zhang, N.; Liu, X.; Sun, Y. One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag: Phosphorus Involved in Calcium Alumino-Silicate Hydrate Gel. Materials 2025, 18, 3360. https://doi.org/10.3390/ma18143360
Li Z, Wang Y, Zhang J, Wang Y, Zhang N, Liu X, Sun Y. One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag: Phosphorus Involved in Calcium Alumino-Silicate Hydrate Gel. Materials. 2025; 18(14):3360. https://doi.org/10.3390/ma18143360
Chicago/Turabian StyleLi, Zipei, Yu Wang, Jiale Zhang, Yipu Wang, Na Zhang, Xiaoming Liu, and Yinming Sun. 2025. "One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag: Phosphorus Involved in Calcium Alumino-Silicate Hydrate Gel" Materials 18, no. 14: 3360. https://doi.org/10.3390/ma18143360
APA StyleLi, Z., Wang, Y., Zhang, J., Wang, Y., Zhang, N., Liu, X., & Sun, Y. (2025). One-Week Hydration Characteristics of Silica-Alumina Based Cementitious Materials Composed of Phosphorous Slag: Phosphorus Involved in Calcium Alumino-Silicate Hydrate Gel. Materials, 18(14), 3360. https://doi.org/10.3390/ma18143360







