Formation of Hybrid Spherical Silica Particles Using a Novel Alkoxy-Functional Polysilsesquioxane Macromonomer as a Precursor in an Acid-Catalyzed Sol-Gel Process
Highlights
- Alkoxy-functional polysilsesquioxane macromonomer was obtained by a click-type reaction in TEOS.
- The reaction mixture was used in a templated sol–gel process yielding spherical microparticles.
- The presence of LPSQ was critical for the spherical morphology of silica.
- The size of the hybrid particles strongly depended on the stirring rate and pH of the reaction medium.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Methodology
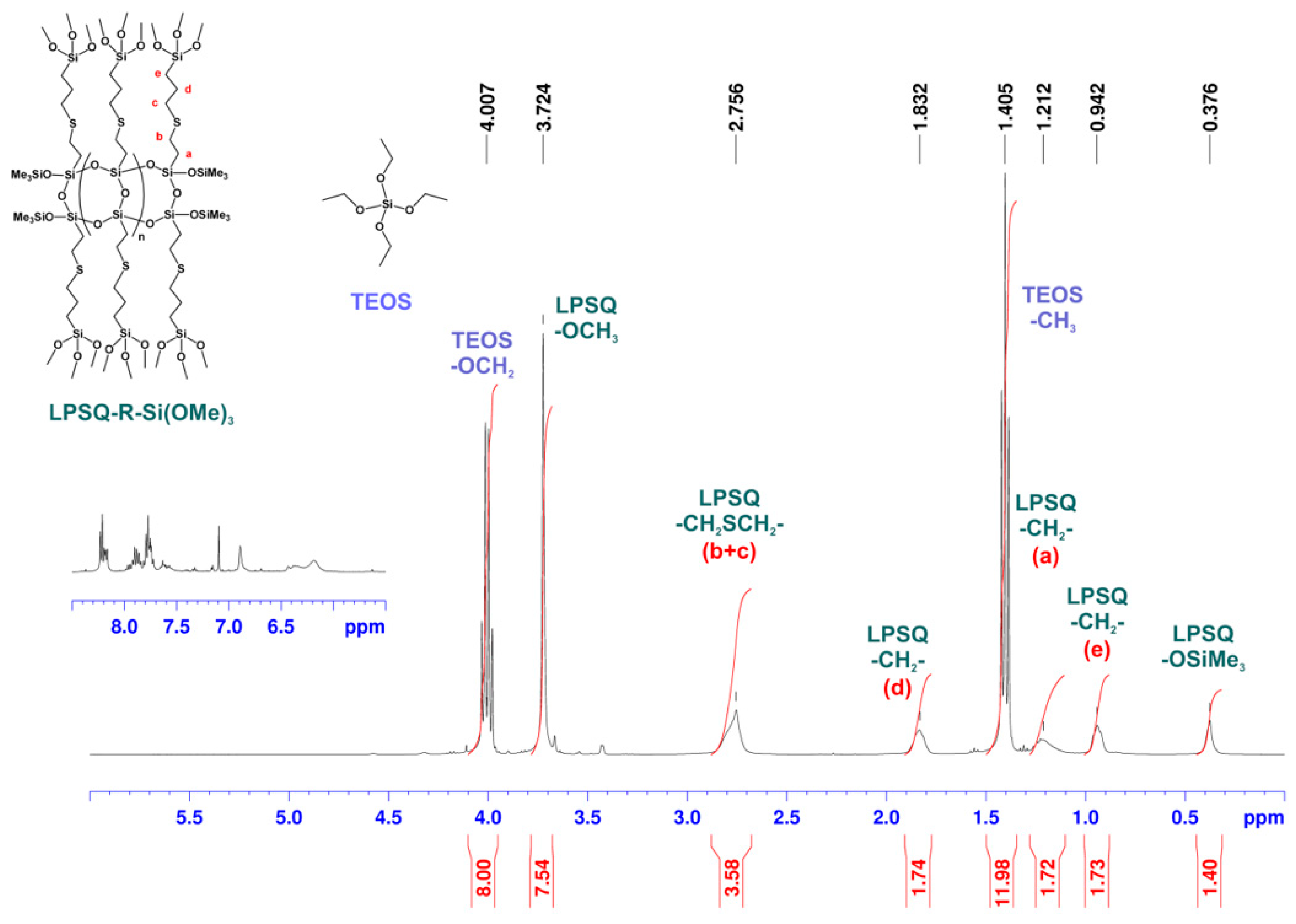
2.2.1. Synthesis of LPSQ-R-Si(OMe)3
2.2.2. Sol–Gel Reaction
- A: Magnetic stirring (70 rpm) over the whole reaction time of 20 h;
- B: Magnetic stirring (70 rpm) only during dropping in the alkoxysilanes;
- C: Magnetic stirring (600 rpm) during dropping in the alkoxysilanes and for additional 30 min afterwards;
- D: Fast stirring (20,000 rpm) with a homogeniser (High Shear Homogeniser Unidrive X 1000 CAT, CAT Scientific, Paso Robles, CA, USA) during addition of alkoxysilanes and for additional for 3 min;
- E: Magnetic stirring at highest rate (1500 rpm) during dropping in the alkoxysilanes and for additional 30 min afterwards;
- F: Slow stirring (4000 rpm) with the homogeniser during addition of alkoxysilanes and for additional 10 min.
2.2.3. Pyrolysis of the Hybrid Silicas
2.3. Analytic Methods
2.3.1. NMR Spectroscopic Methods
2.3.2. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
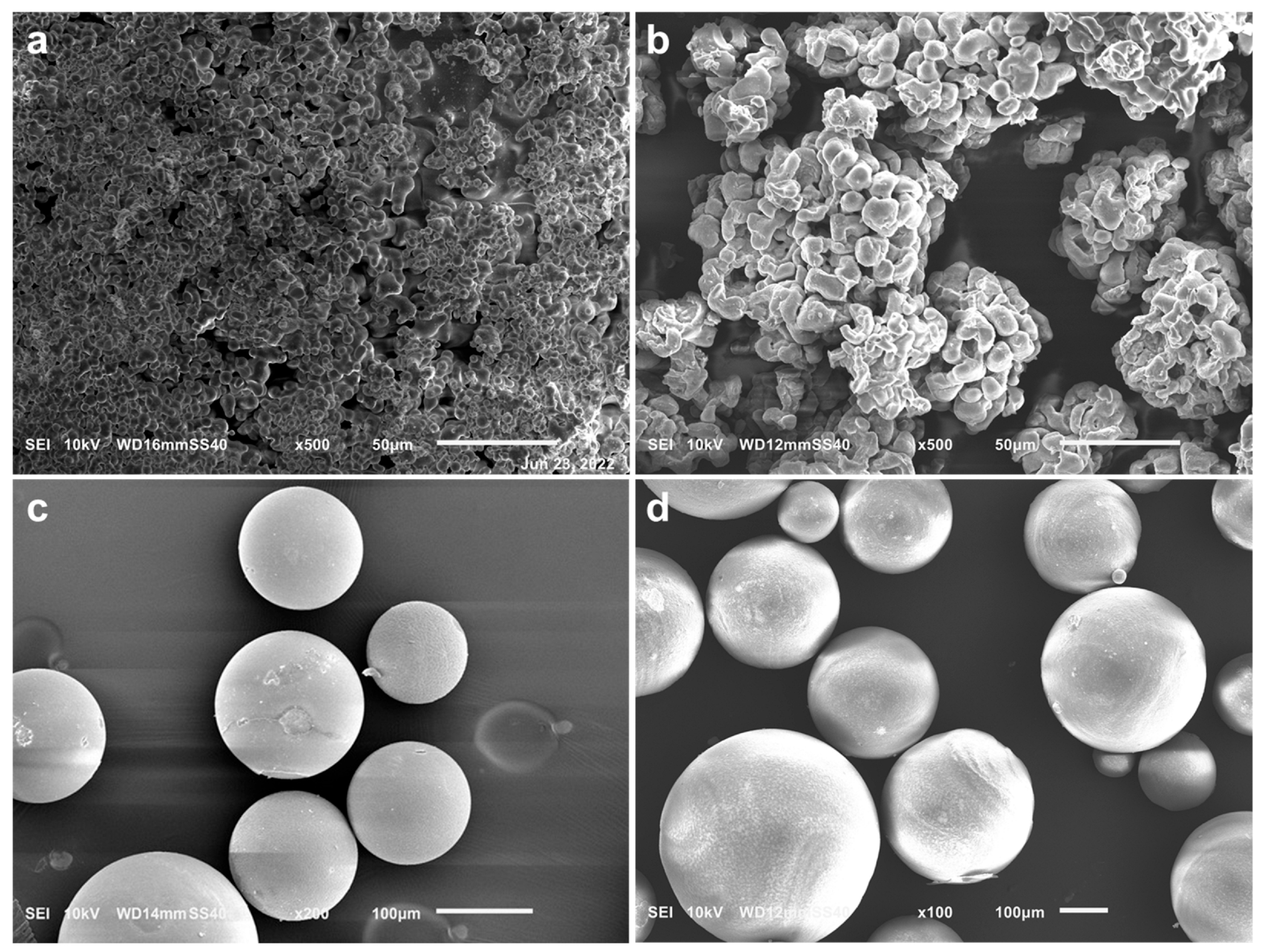
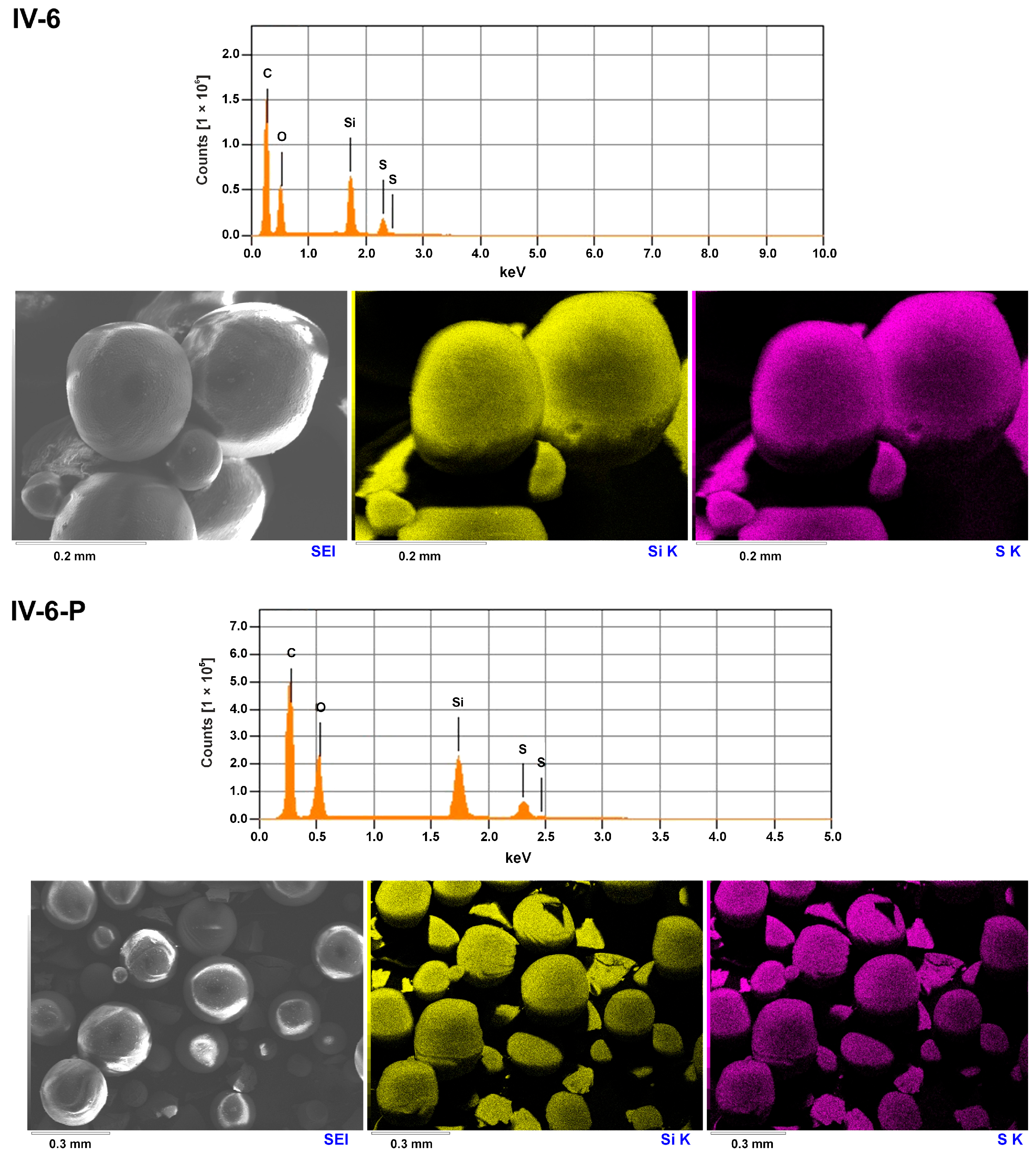
2.3.3. Scanning Electron Microscopy (SEM and SEM-EDS)
2.3.4. Wide-Angle X-Ray Diffraction
3. Results
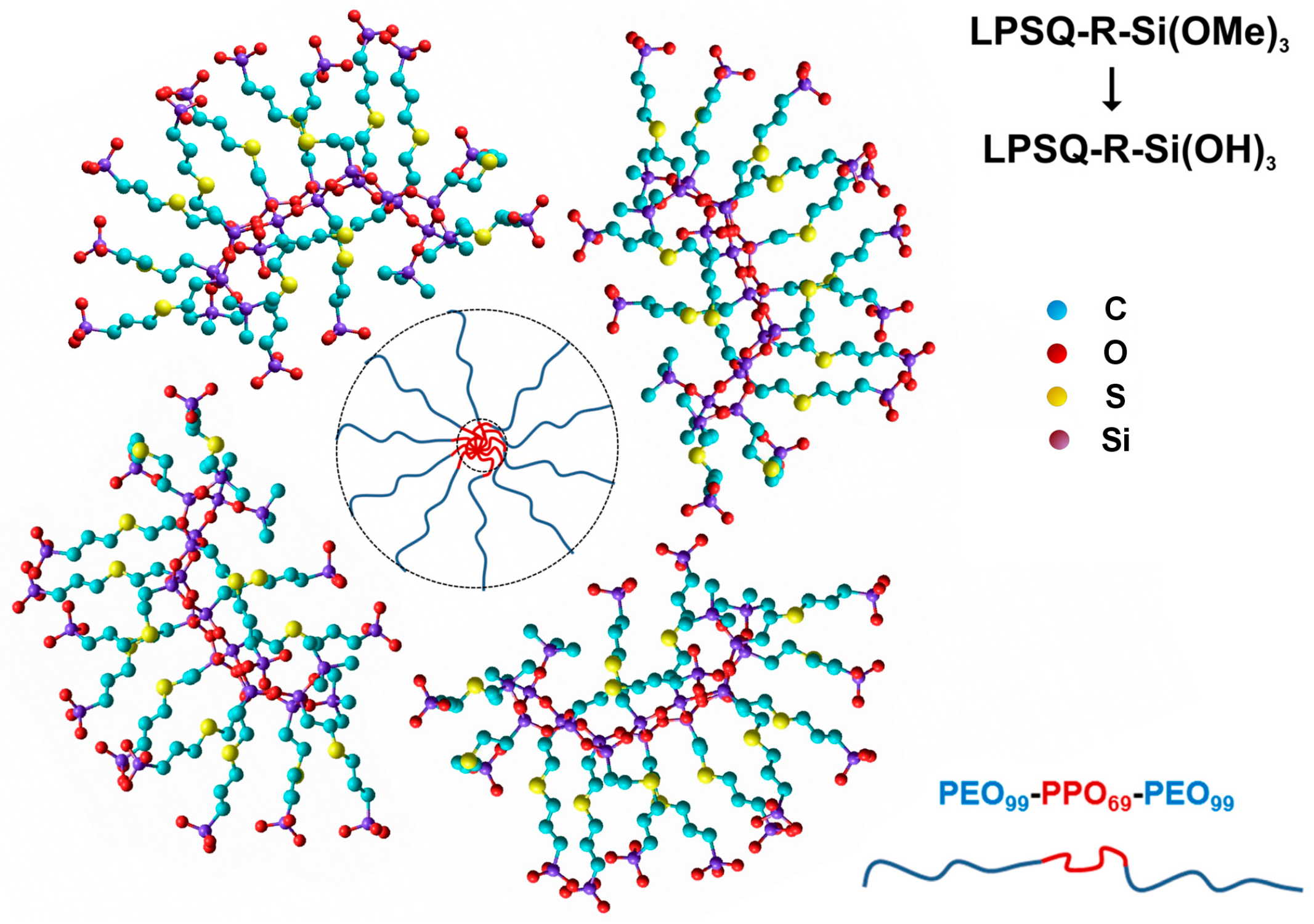
3.1. Synthesis of LPSQ-R-Si(OMe)3
3.2. Preparation of Hybrid Silica by Sol–Gel Method
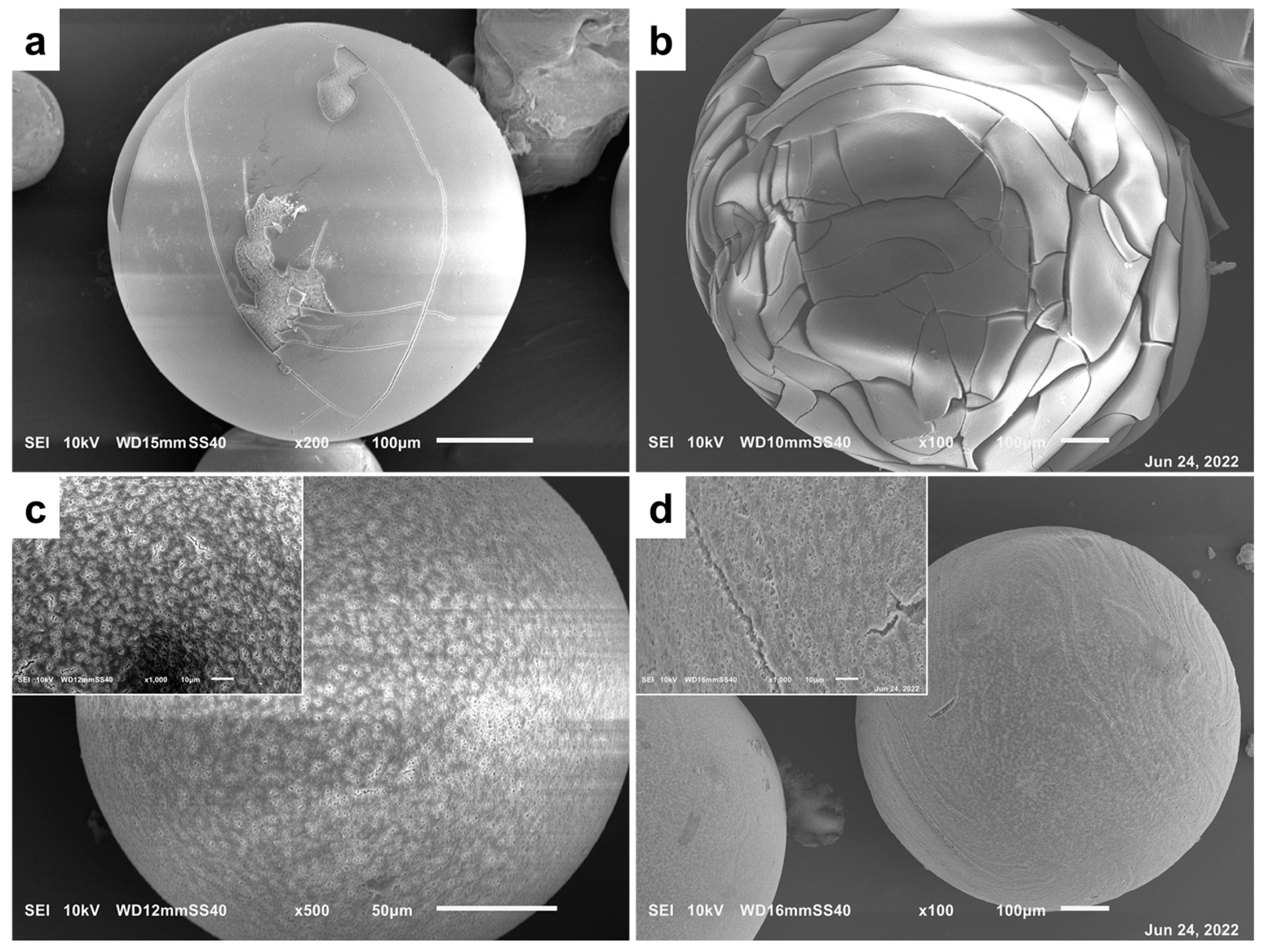
3.2.1. The Effect of Sol–Gel Conditions on the Morphology of Silica Particles
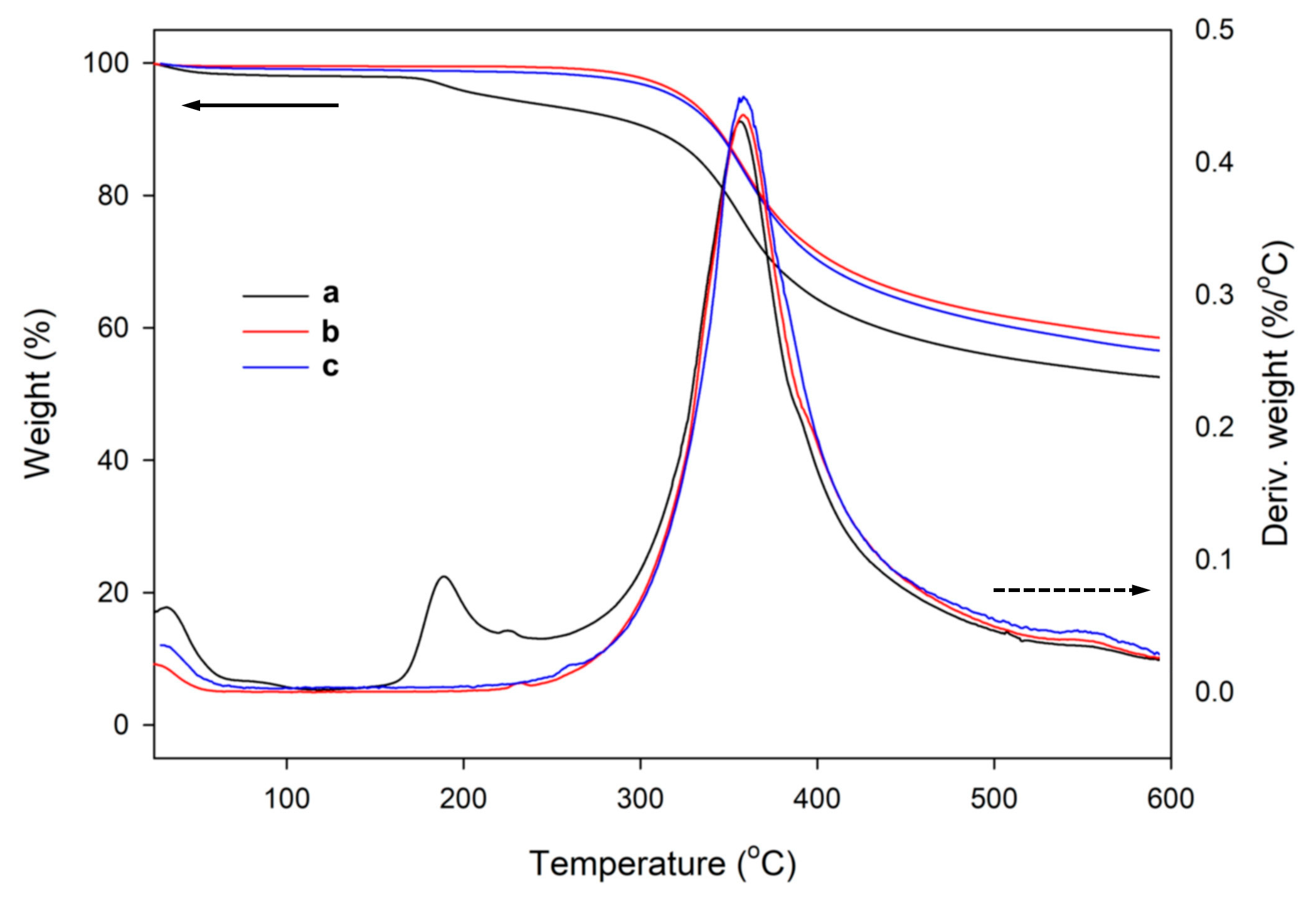
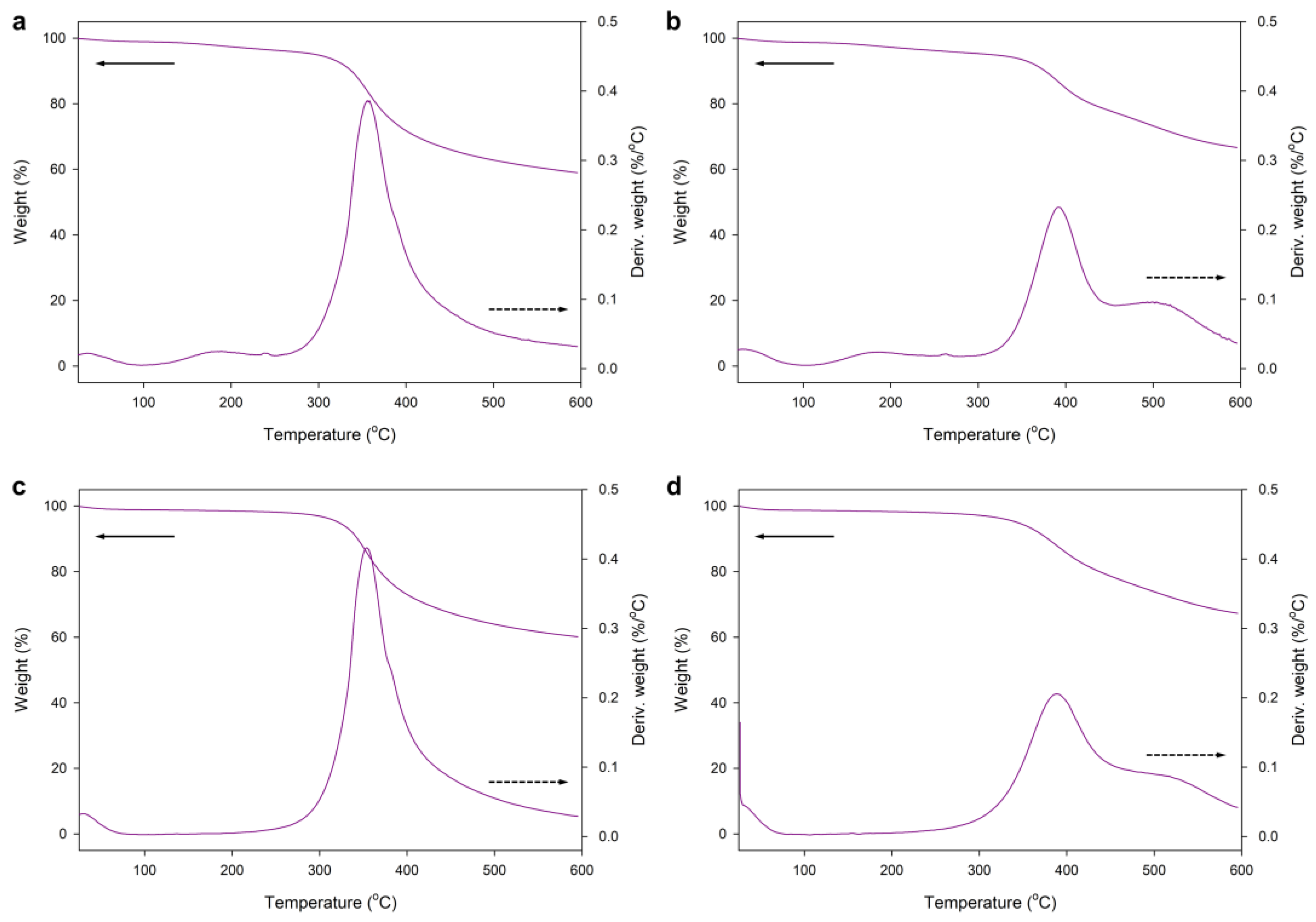
3.2.2. Thermal Stability of the Hybrid Silica
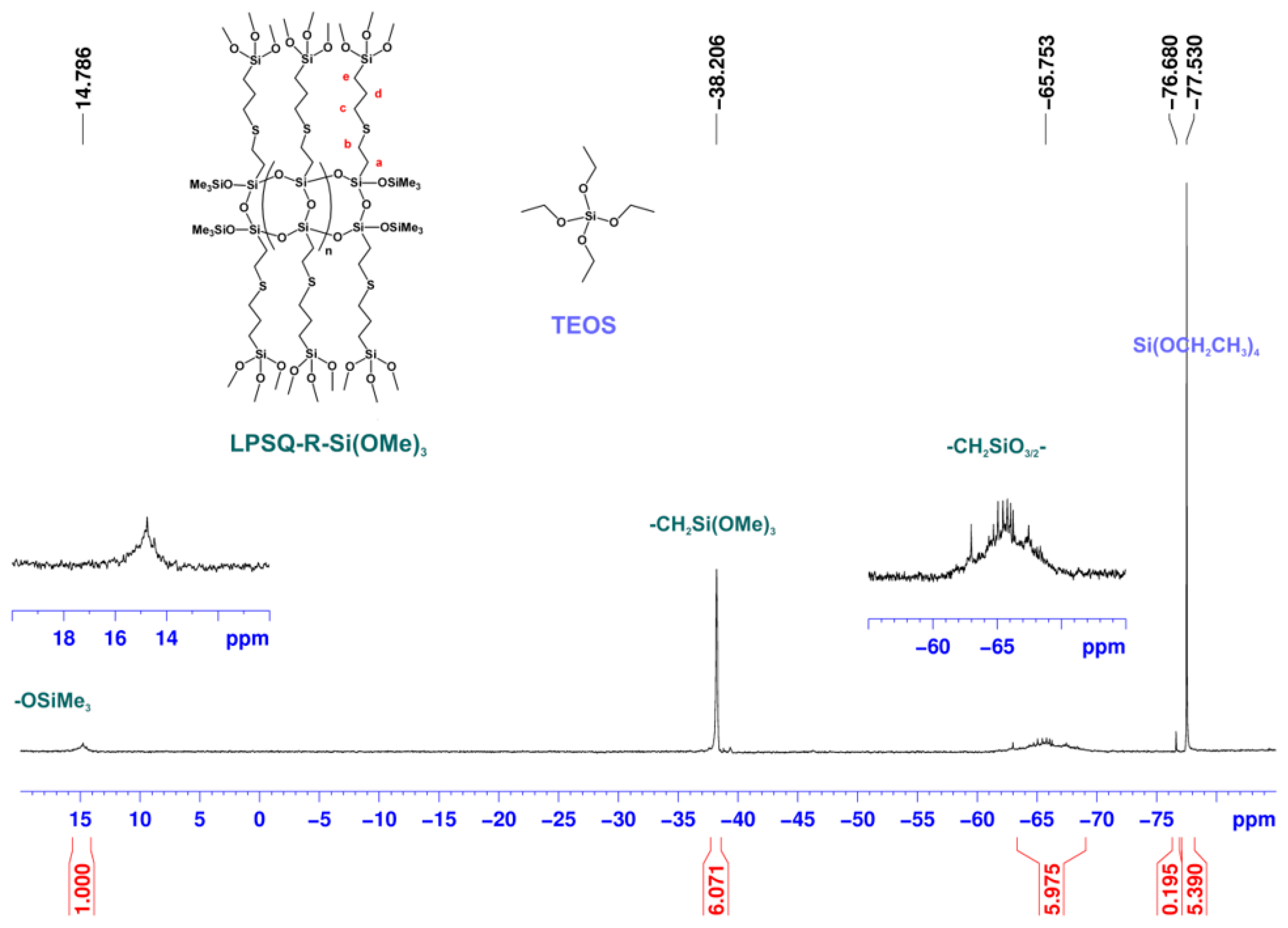
3.2.3. Studies on the Chemical Structure of Hybrid Silica with Solid State NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LPSQ | Linear polysilsesquioxanes |
| POSS | Polyhedral oligomeric silsesquioxanes |
| TEOS | Tetraethoxysilane |
| DMPA | 2,2-dimethoxy-2-phenylacetophenone |
| LPSQ-Vi | Linear poly(vinylsilsesquioxanes) |
| LPSQ-R-Si(OMe)3 | Linear polysilsesquioxanes functionalized with trimethoxysilyl groups |
| Zn(OAc)2 | Zinc acetate dihydrate |
| SS NMR | Solid-state nuclear magnetic resonance spectroscopy |
| HP Dec | High-power decoupling |
| TGA | Thermogravimetric analysis |
| DTA | Differential thermal analysis |
| Tmax | Maximum degradation temperature |
| SEM | Scanning electron microscopy |
| DPS | Particle-size distribution |
| D50 | Median particle size |
| SEM-EDS | Scanning electron microscopy equipped with energy dispersive spectroscopy |
| SEI | Secondary electron image |
| WAXS | Wide-angle X-ray scattering |
References
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol-Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel processing of silica nanoparticles and their applications. Adv. Coll. Interf. Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jon, K.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Mehdi, A.; Reye, C.; Corriu, R. From molecular chemistry to hybrid nanomaterials. Design and functionalization. Chem. Soc. Rev. 2011, 40, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materialsfor Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Wang, X.; Schröder, H.C.; Wang, K.; Kaandorp, J.A.; Müller, E.G. Genetic, biological and structural hierarchies during sponge spicule formation: From soft sol–gels to solid 3D silica composite structures. Soft Matter. 2012, 8, 9501–9518. [Google Scholar] [CrossRef]
- Al Ragib, A.; Chakma, R.; Wang, J.; Alanazi, Y.M.; El-Harbawi, M.; Arish, G.A.; Islam, T.; Siddique, M.A.B.; Islam, A.R.M.T.; Kormoker, T. The past to the current advances in the synthesis and applications of silica nanoparticles. Nano-Struct. Nano-Objects 2024, 40, 101395. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Coll. Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Anees-ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Transl. Res. 2022, 12, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Han, Y.-H.; Xia, H.-Y.; Wang, S.-B.; Chen, A.-Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Nogales, A.; Plaza-Palomo, G.; González-Larre, J.; Jiménez-Falcao, S.; Baeza, A. Silicasomes in Oncology: From Conventional Chemotherapy to Combined Immunotherapy. Molecules 2025, 30, 1257. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Gucci, F.; Cairns, G.; Chianella, I.; Leighton, G.J.T. Hollow Silica Nano and Micro Spheres with Polystyrene Templating: A Mini-Review. Materials 2022, 15, 8578. [Google Scholar] [CrossRef] [PubMed]
- Karimi, B.; Ganji, N.; Pourshiani, O.; Thiel, W.B. Periodic mesoporous organosilicas (PMOs): From synthesis strategies to applications. Progr. Mater. Sci. 2022, 125, 100896. [Google Scholar] [CrossRef]
- Kerkhofs, S.; Willhammar, T.; Van Den Noortgate, H.; Kirschhock, C.E.A.; Breynaert, E.; Van Tendeloo, G.; Bals, S.; Martens, J.A. Self-Assembly of Pluronic F127—Silica Spherical Core–Shell Nanoparticles in Cubic Close-Packed Structures. Chem. Mater. 2015, 27, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Yagci, M.B.; Ow-Yang, C.W.; Levent Demirel, A.L.; Mikó, A. Formation of mesoporous silica particles with hierarchical morphology. Micropor. Mesopor. Mater. 2020, 303, 110240. [Google Scholar] [CrossRef]
- Fermino, T.Z.; Awano, C.M.; Moreno, L.X.; Vollet, D.R.; de Vicente, F.S. Structure and thermal stability in hydrophobic Pluronic F127-modified silica aerogels. Micropor. Mesopor. Mater. 2018, 267, 242–248. [Google Scholar] [CrossRef]
- Paunikallio, T.; Suvanto, M.; Pakkanen, T.T. Grafting of 3-(trimethoxysilyl)propyl methacrylate onto polypropylene and use as a coupling agent in viscose fiber/polypropylene composites. React. Funct. Polym. 2008, 68, 797–808. [Google Scholar] [CrossRef]
- Cho, W.; Cha, B.J.; Lee, H.I.; Kim, J.M.; Char, K. Synthesis of porous silica with hierarchical structure directed by a silica precursor carrying a pore-generating cage. J. Mater. Chem. 2008, 18, 4971–4976. [Google Scholar] [CrossRef]
- Chang, C.; Wei, H.; Li, Q.; Yang, B.; Chen, N.; Zhou, J.-P.; Zhang, X.-Z.; Zhuo, R.-X. Construction of mixed micelle with cross-linked core and dual responsive shells. Polym. Chem. 2011, 2, 923–930. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Huang, K.; Gao, M. Synthesis and comparison of two poly (methyl methacrylate-b-3-(trimethoxysilyl)propyl methacrylate)/SiO2 hybrids by “grafting-to” approach. J. Coll. Interf. Sci. 2014, 433, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Jones, J.R.; Georgiou, T.K. Toward Hybrid Materials: Group Transfer Polymerization of 3-(Trimethoxysilyl)propyl Methacrylate. Macromol. Rapid. Commun. 2015, 36, 1806–1809. [Google Scholar] [CrossRef] [PubMed][Green Version]
- John, Ł.; Janeta, M.; Rajczakowska, M.; Ejfler, J.; Łydżba, D.; Szafert, S. Synthesis and microstructural properties of the scaffold based on a 3-(trimethoxysilyl)propyl methacrylate–POSS hybrid towards potential tissue engineering applications. RSC Adv. 2016, 6, 6037–66047. [Google Scholar] [CrossRef]
- Prozorova, G.; Kuznetsova, N.; Shaulina, L.; Bolgova, Y.; Trofimova, O.; Emel’yanov, A.; Pozdnyakov, A. Synthesis and sorption activity of novel cross-linked 1-vinyl-1,2,4-triazole–(trimethoxysilyl)methyl-2-methacrylate copolymers. J. Organomet. Chem. 2020, 916, 121273. [Google Scholar] [CrossRef]
- Maciejewska, M.; Rogulska, M. Porous Copolymers of 3-(Trimethoxysilyl)propyl Methacrylate with Trimethylpropane Trimethacrylate Preparation: Structural Characterization and Thermal Degradation. Materials 2024, 17, 4796. [Google Scholar] [CrossRef] [PubMed]
- Miksa, B.; Trzeciak, K.; Kaźmierski, S.; Rozanski, A.; Potrzebowski, M.; Rozga-Wijas, K.; Sobotta, L.; Ziabka, M.; Płódowska, M.; Szary, K. Nature-Inspired Synthesis of Yeast Capsule Replicas Encased with Silica-Vinyl Functionality: New Fluorescent Hollow Hybrid Microstructures. Molecules 2024, 29, 5363. [Google Scholar] [CrossRef] [PubMed]
- Paria, S.; Chaudhuri, R.G. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Wahba, M.A.; Khaled, R.K.; Dawy, M.; Moharam, M.E. Enhanced optical and antimicrobial activities of mono Zn and bimetallic (Zn, Co), (Zn, Pd) ions modified MCM-41: Structural and morphological investigation. J. Porous Mater. 2024, 31, 1915–1931. [Google Scholar] [CrossRef]
- Chia, S.L.; Leong, D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica encapsulation of ZnO nanoparticles reduces their toxicity for cumulus cell oocyte-complex expansion. Part. Fibre Toxicol. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Dhaffouli, A.; Holzinger, M.; Carinelli, S.; Barhoumi, H.; Salazar-Carballo, P.A. ZnO Doped Silica Nanoparticles (ZnO@SiO2) for Enhanced Electrochemical Detection of Cd2+ Ions in Real Samples. Sensors 2024, 24, 4179. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, M.; Amoee, S. Porous biomorphic silica@ZnO nanohybrids as the effective photocatalysts under visible light. Environm. Sci. Pollut. Res. 2022, 29, 49784–49795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Xiong, H.-M.; Ren, Q.-G.; Xia, Y.-Y.; Kong, J.-L. ZnO@silica core–shell nanoparticles with remarkable luminescence and stability in cell imaging. J. Mater. Chem. 2012, 22, 13159–13165. [Google Scholar] [CrossRef]
- González-Suárez, B.W.; Pantoja-Espinoza, J.C.; Lardizábal-Gutierrez, D.; Flores, M.U.; Paraguay-Delgado, F. Effect of [Zn acetate]/[KOH] ratio on ZnO particles synthesis and photocatalytic Rhodamine B degradation. J. Mater. Res. Technol. 2024, 30, 8092–8107. [Google Scholar] [CrossRef]
- Abisha Meji, M.; Usha, D.; Harihara Sankar, M.; Ashwin, B.M. Synthesis of ZnO nanoparticles from zinc acetate dihydrate—An environmental friendly technique. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Guzmán-Carrillo, H.R.; Rivera-Muñoz, E.M.; Cayetano-Castro, N.; Herrera-Basurto, R.; Barquera-Bibiano, Z.; Mercader-Trejo, F.; Manzano-Ramírez, A. Facile control of ZnO nanostructures by varying molar concentration of zinc acetate. Mater. Res. Bull. 2017, 90, 138–144. [Google Scholar] [CrossRef]
- De Souza, D.A.; da Silva, P.H.d.A.; da Silva, F.P.; Romaguera-Barcelay, Y.; Ferreira, R.D.; Araujo Junior, E.A.; Nascimento, J.F.d.L.; da Costa, F.F.; Takeno, L.L.; Leyet Ruiz, Y.; et al. Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye. Colorants 2024, 3, 229–252. [Google Scholar] [CrossRef]
- Demir, M.M.; Muñoz-Espí, R.; Lieberwirth, I.; Wegner, G. Precipitation of monodisperse ZnO nanocrystalsvia acid-catalyzed esterification of zinc acetate. J. Mater. Chem. 2006, 16, 2940–2947. [Google Scholar] [CrossRef]
- Zhang, Y.; Muhammed, M. Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions: VI. Hydrolysis and hydroxo-complexes of Zn2+ at 298.15 K. Hydrometallurgy 2001, 60, 215–236. [Google Scholar] [CrossRef]
- Cousy, S.; Gorodylova, N.; Svoboda, L.; Zelenka, J. Influence of synthesis conditions over simonkolleite/ZnO precipitation. Chem. Pap. 2017, 71, 2325–2334. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci, R.J., Jr.; Scully, J.R. Advanced chemical stability diagrams to predict the formation of complex zinc compounds in a chloride environment. RSC Adv. 2019, 9, 19905–19916. [Google Scholar] [CrossRef] [PubMed]
- Moezzi, A.; Cortie, M.; McDonagh, A. Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide. Dalton Trans. 2016, 45, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Lechner, C.C.; Becker, C.F.W. Exploring the effect of native and artificial peptide modifications on silaffin induced silica precipitation. Chem. Sci. 2012, 3, 3500–3504. [Google Scholar] [CrossRef]
- Lechner, C.C.; Becker, C.F.W. Silaffins in Silica Biomineralization and Biomimetic Silica Precipitation. Mar. Drugs 2015, 13, 5297–5333. [Google Scholar] [CrossRef] [PubMed]
- Strobl, J.; Kozak, F.; Kamalov, M.; Reichinger, D.; Kurzbach, D.; Becker, C.F.W. Understanding Self-Assembly of Silica-Precipitating Peptides to Control Silica Particle Morphology. Adv. Mater. 2023, 35, 2207586. [Google Scholar] [CrossRef] [PubMed]
- Torkelson, K.; Naser, N.Y.; Qi, X.; Li, Z.; Yang, W.; Pushpavanam, K.; Chen, C.-L.; Baneyx, F.; Pfaendtner, J. Rational Design of Novel Biomimetic Sequence-Defined Polymers for Mineralization Applications. Chem. Mater. 2024, 36, 786–794. [Google Scholar] [CrossRef]
- McCutchin, C.A.; Edgar, K.J.; Chen, C.-L.; Dove, P.M. Silica-Biomacromolecule Interactions: Toward a Mechanistic Understanding of Silicification. Biomacromolecules 2025, 26, 43–84. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Sumper, M. Biomimetic Patterning of Silica by Long-Chain Polyamines. Angewandte Chem. Int Ed. 2004, 43, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Min Sang Kwon, M.S. Recent progress in ladder-like polysilsesquioxane: Synthesis and applications. Mater. Chem. Front. 2024, 8, 2689–2726. [Google Scholar] [CrossRef]
- Kowalewska, A.; Majewska-Smolarek, K. Poly(vinylsilsesquioxanes) of ladder structure—Synthesis, modification and applications. Inorg. Chim. Acta 2025, 579, 122581. [Google Scholar] [CrossRef]
- Kowalewska, A.; Nowacka, M.; Tracz, A.; Makowski, T. Supramolecular self-assembly of linear oligosilsesquioxanes on mica-AFM surface imaging and hydrophilicity studies. Soft Matter. 2015, 11, 4818–4829. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, A.; Nowacka, M.; Makowski, T.; Michalski, A. Thermal stability of self-assembled surfaces and micropatterns made of ladder polysilsesquioxanes. Polymer 2016, 90, 147–155. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Makowski, T. Nanostructured surfaces by supramolecular self-assembly of linear oligosilsesquioxanes with biocompatible side groups. Beilstein J. Nanotechnol. 2015, 6, 2377–2387. [Google Scholar] [CrossRef] [PubMed]
- Herc, A.S.; Bojda, J.; Nowacka, M.; Lewiński, P.; Maniukiewicz, W.; Piorkowska, E.; Kowalewska, A. Crystallization, structure and properties of polylactide/ladder poly(silsesquioxane) blends. Polymer 2020, 201, 122563. [Google Scholar] [CrossRef]
- Herc, A.S.; Lewiński, P.; Kaźmierski, S.; Bojda, J.; Kowalewska, A. Hybrid SC-polylactide/poly(silsesquioxane) blends of improved thermal stability. Thermochim. Acta 2020, 687, 178592. [Google Scholar] [CrossRef]
- Kowalewska, A.; Herc, A.S.; Bojda, J.; Palusiak, M.; Markiewicz, E.; Ławniczak, P.; Nowacka, M.; Sołtysiak, J.; Różański, A.; Piorkowska, E. Supramolecular interactions involving fluoroaryl groups in hybrid blends of polylactide and ladder polysilsesquioxanes. Polym. Test. 2021, 94, 107033. [Google Scholar] [CrossRef]
- Nowacka, M.; Kowalewska, A.; Plażuk, D.; Makowski, T. Hybrid polysilsesquioxanes for fluorescence resonance energy transfer. Dyes Pigm. 2019, 170, 107622. [Google Scholar] [CrossRef]
- Nowacka, M.; Makowski, T.; Kowalewska, A. Hybrid fluorescent poly(Silsesquioxanes) with amide-and triazole-containing side groups for light harvesting and cation sensing. Materials 2020, 13, 4491. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, A.; Nowacka, M. Synthesis of ladder silsesquioxanes by in situ polycondensation of cyclic tetravinylsiloxanetetraols. Silicon 2015, 7, 133–146. [Google Scholar] [CrossRef]
- Herc, A.S.; Włodarska, M.; Nowacka, M.; Bojda, J.; Szymański, W.; Kowalewska, A. Supramolecular interactions between polylactide and model cyclosiloxanes with hydrogen bonding-capable functional groups. Express Polym. Lett. 2020, 14, 134–153. [Google Scholar] [CrossRef]
- Lan Zhao, L.; Zhu, G.; Zhang, D.; Di, Y.; Chen, Y.; Terasaki, O.; Qiu, S. Synthesis and Structural Identification of a Highly Ordered Mesoporous Organosilica with Large Cagelike Pores. J. Phys. Chem. B 2005, 109, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Rissing, C.; Son, D.Y. Thiol-ene chemistry of vinylsilanes. Main Group Chem. 2009, 8, 251–262. [Google Scholar] [CrossRef]
- Li, L.; Xue, L.; Feng, S.; Liu, H. Functionalization of monovinyl substituted octasilsesquioxane via photochemical thiol-ene reaction. Inorg. Chim. Acta 2013, 407, 269–273. [Google Scholar] [CrossRef]
- Xue, L.; Li, L.; Feng, S.; Liu, H. A facile route to multifunctional cage silsesquioxanes via the photochemical thiol-ene reaction. J. Organomet. Chem. 2015, 783, 49–54. [Google Scholar] [CrossRef]
- Rózga-Wijas, K.; Chojnowski, J. Synthesis of New Polyfunctional Cage Oligosilsesquioxanes and Cyclic Siloxanes by Thiol-ene Addition. J. Inorg. Organomet. Polym. 2012, 22, 588–594. [Google Scholar] [CrossRef]
- Przybylska, A.; Szymańska, A.; Maciejewski, H. A library of new organofunctional silanes obtained by thiol-(meth)acrylate Michael addition reaction. RSC Adv. 2023, 13, 14010–14017. [Google Scholar] [CrossRef] [PubMed]
- Ambrožić, G.; Markovic, M.K.; Peter, R.; Piltaver, I.K.; Badovinac, I.J.; Čakara, D.; Marković, D.; Knez, M. Building organosilica hybrid nanohemispheres via thiol-ene click reaction on alumina thin films deposited by atomic layer deposition (ALD). J. Coll. Interf. Sci. 2020, 560, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovskiy, I.; Temnikov, M.; Kononevich, Y.; Anisimov, A.; Drozdov, F.; Muzafarov, A. The Use of the Thiol-Ene Addition Click Reaction in the Chemistry of Organosilicon Compounds: An Alternative or a Supplement to the Classical Hydrosilylation? Polymers 2022, 14, 3079. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Przybylska, A.; Szymańska, A.; Dutkiewicz, A.; Maciejewski, H. Thiol-ene click reaction as an effective tool for the synthesis of PEG-functionalized alkoxysilanes-precursors of anti-fog coatings. Sci. Rep. 2023, 13, 21025. [Google Scholar] [CrossRef] [PubMed]
- Annenkov, V.V.; Danilovtseva, E.N.; Pal’shin, V.A.; Verkhozina, O.N.; Zelinskiy, S.N.; Maheswari Krishnan, U. Silicic acid condensation under the influence of water-soluble polymers: From biology to new materials. RSC Adv. 2017, 7, 20995–21027. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Inoue, T.; Hirai, T. Local Viscosity Analysis of Triblock Copolymer Micelle with Cyanine Dyes as a Fluorescent Probe. Langmuir 2010, 26, 17505–17512. [Google Scholar] [CrossRef] [PubMed]
- Basak, R.; Bandyopadhyay, R. Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Albano, J.M.R.; Grillo, D.; Facelli, J.C.; Ferraro, M.B.; Pickholz, M. Study of the Lamellar and Micellar Phases of Pluronic F127: A Molecular Dynamics Approach. Processes 2019, 7, 606. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Das, G.K.; Johanie, S.; Vernon, C.; Shaw, R. Characterization and crystal structure determination of zinc hydroxide chloride tetrahydrate Zn5(OH)8Cl2 4[(H2O)x(NH3)1–x]. J. Solid State Chem. 2020, 290, 121483. [Google Scholar] [CrossRef]
- Kowalewska, A.; Fortuniak, W.; Rózga-Wijas, K.; Handke, B. Thermolysis of new hybrid silsesquioxane-carbosilane materials. Thermochim. Acta 2009, 494, 45–53. [Google Scholar] [CrossRef]


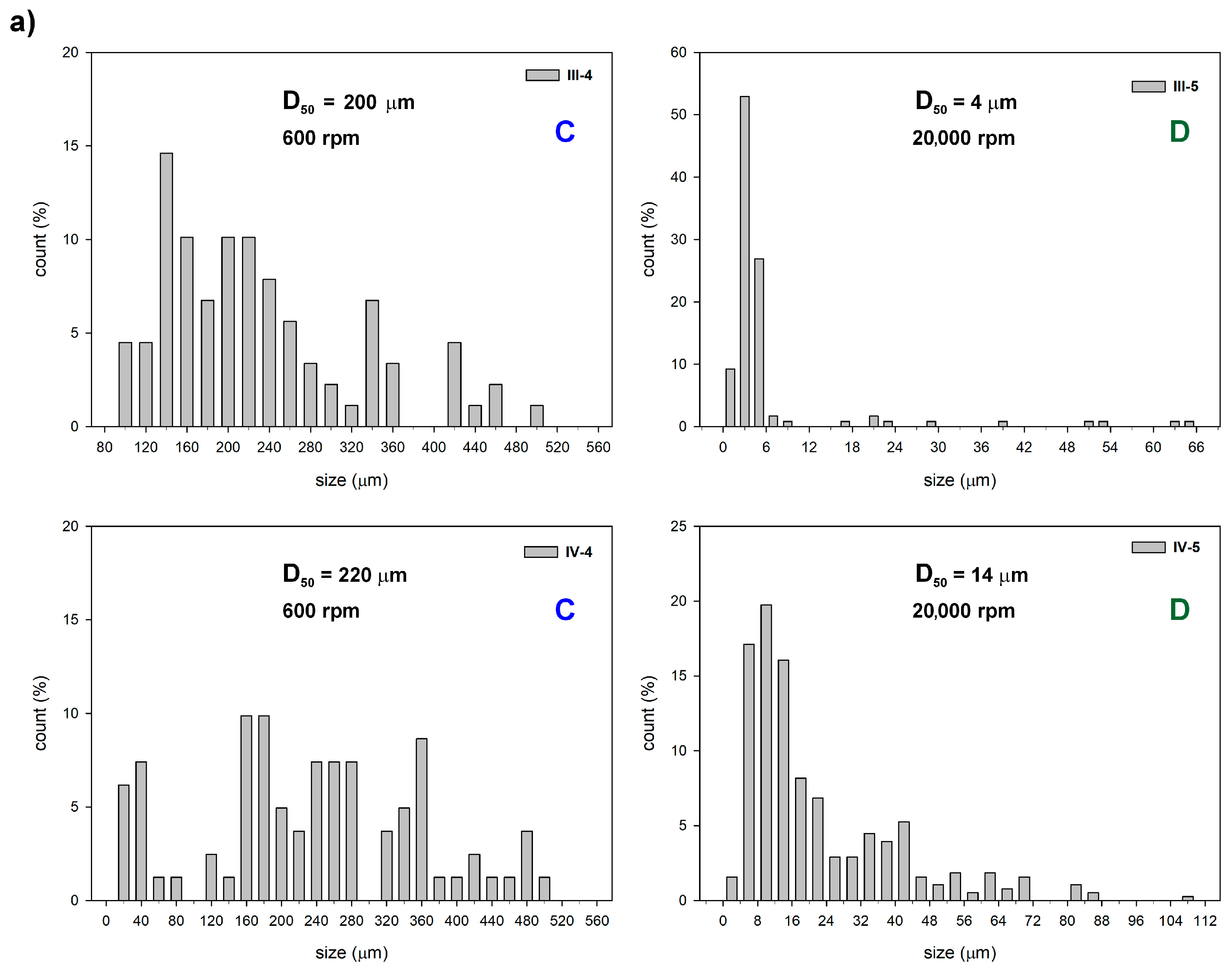
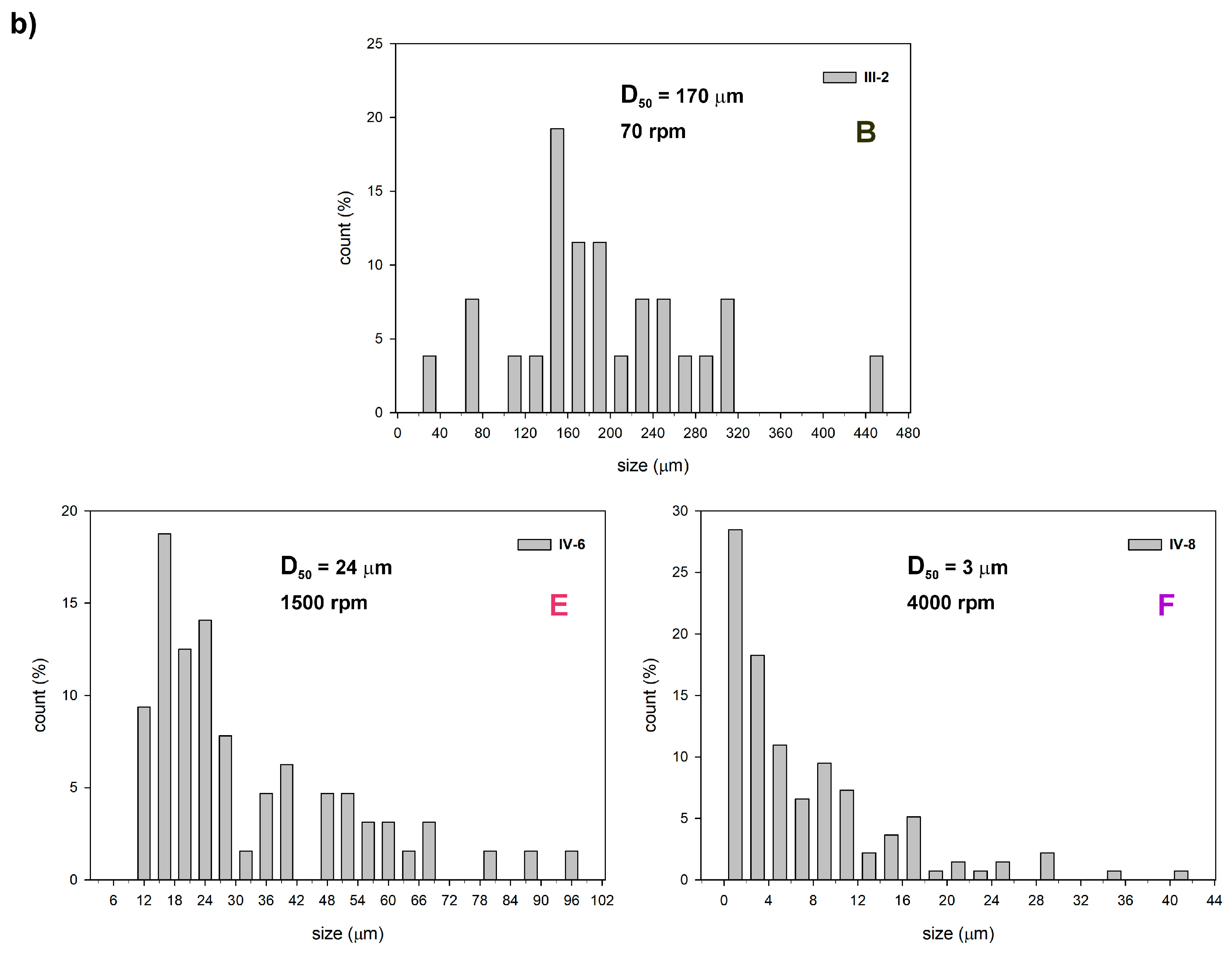
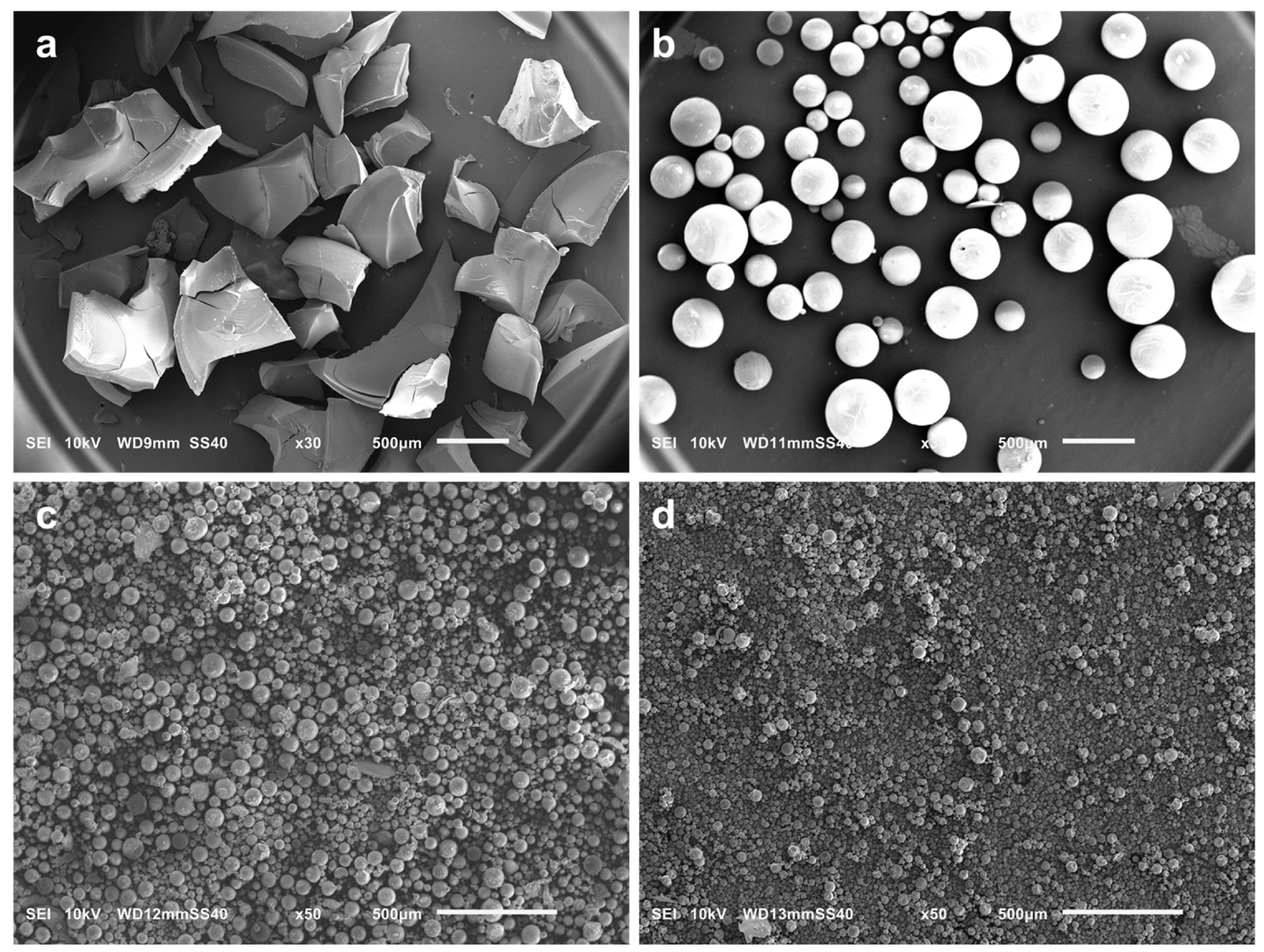
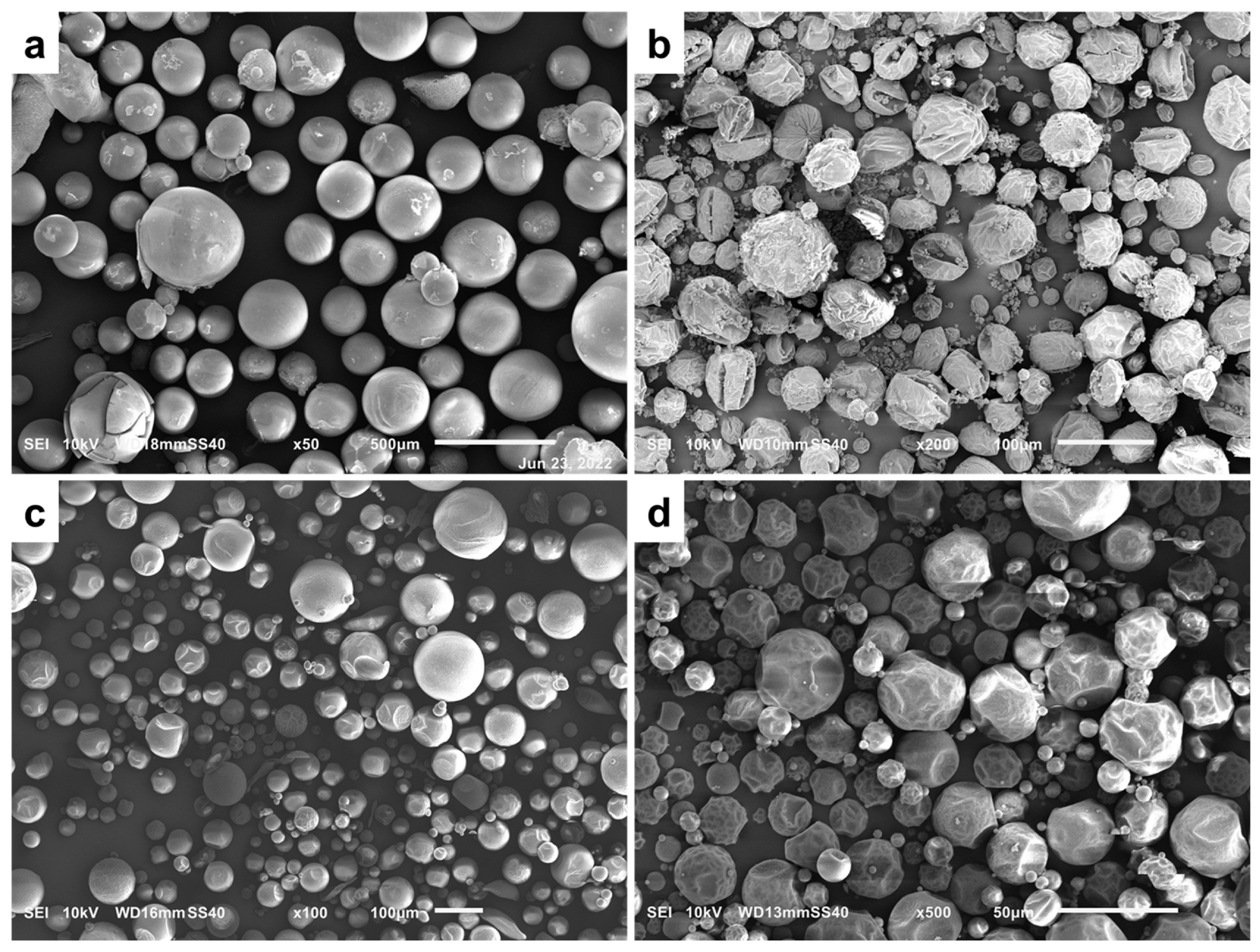

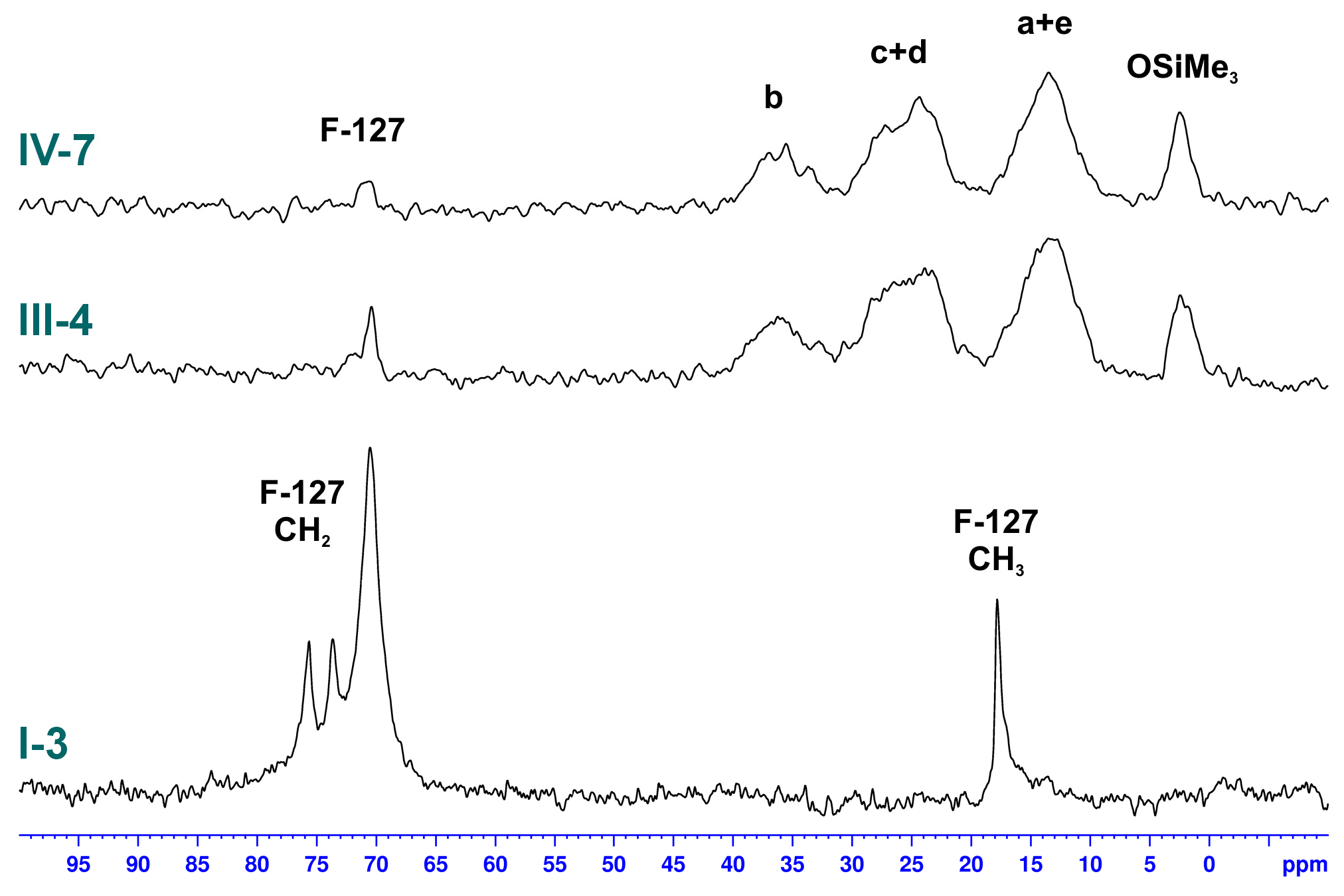

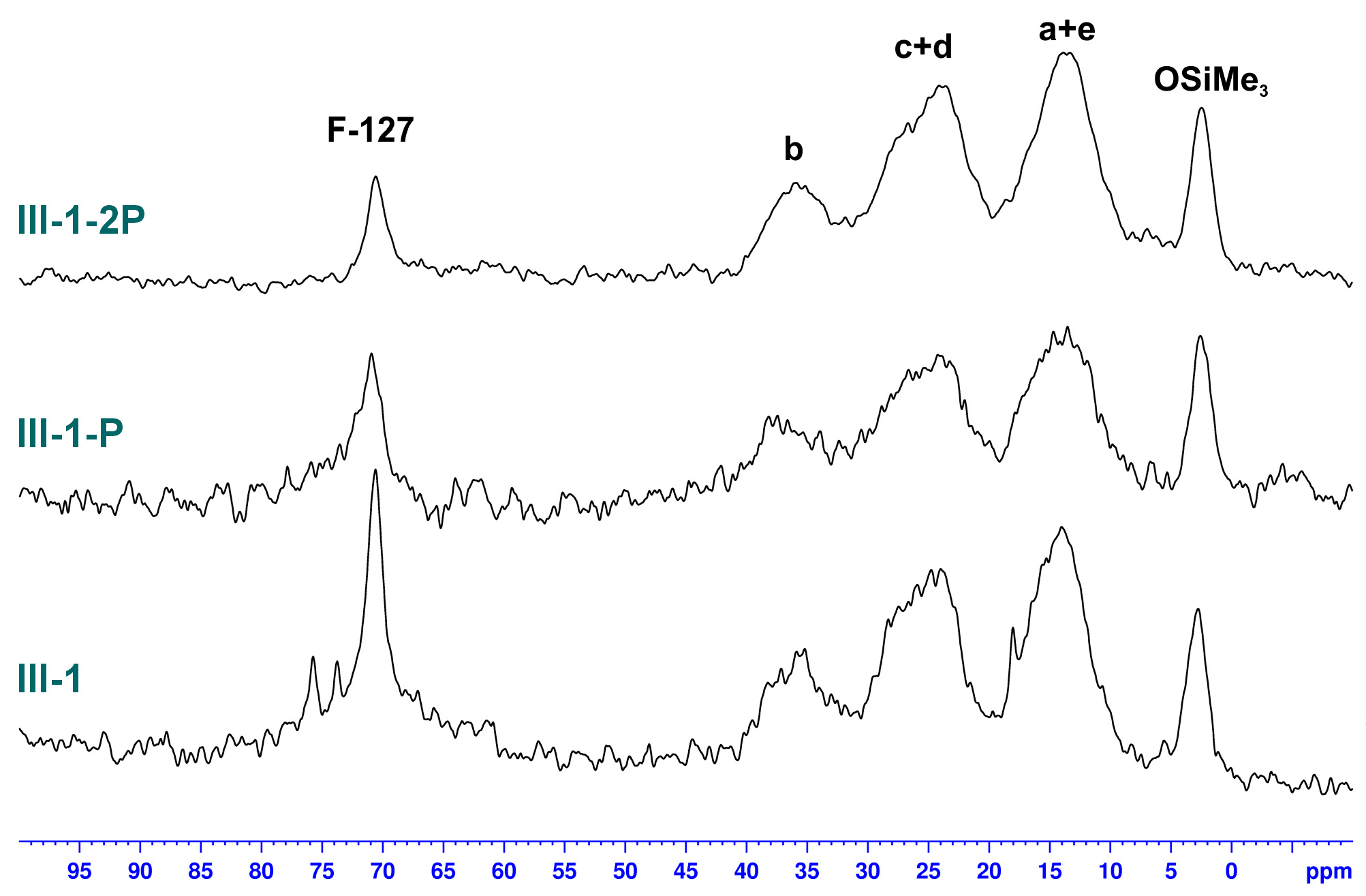
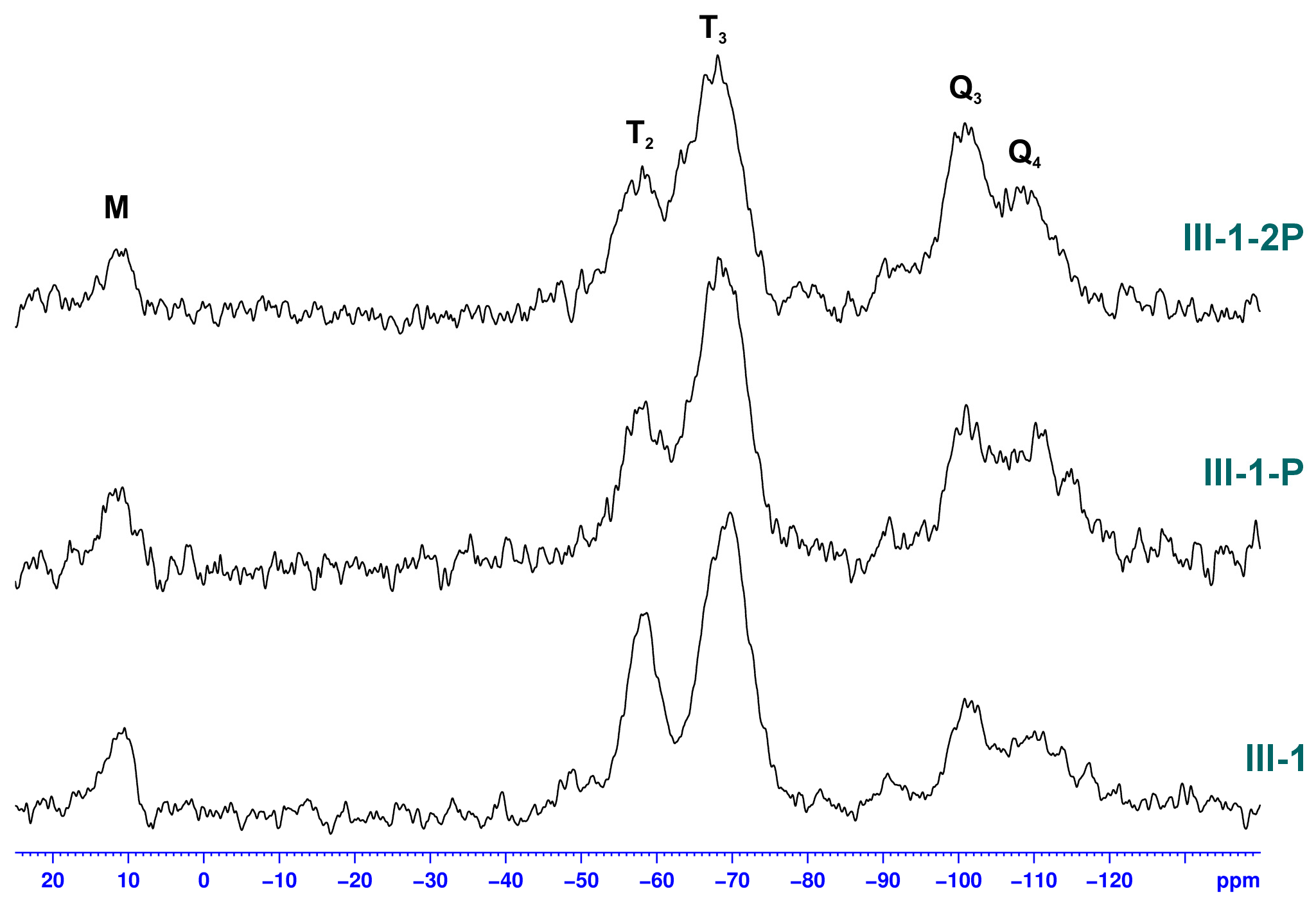
| Sample | -Si(OMe)3 [mmol] | TEOS [mmol] | Zn(OAc)2 [mmol] | F-127 [g] | X | Y [%] | W [wt%] | P [%] |
|---|---|---|---|---|---|---|---|---|
| I-1 | - | 8.82 | - | 0.92 | A | 50.9 | 63.48 | 18.97 |
| I-2 | - | 8.82 | - | 0.41 | B | 95.0 | 43.65 | 16.2 |
| I-3 | - | 8.82 | - | 0.41 | C | 105.3 | 43.65 | 24.31 |
| I-4 | - | 4.48 | - | 0.41 | D | 49.3 | 60.41 | 23.02 |
| I-5 | - | 8.82 | - | 0.41 | D | 94.5 | 43.65 | 17.49 |
| II-1 | - | 8.82 | 4.56 | 0.41 | B | 59.4 | 29.65 | 14.09 |
| II-2 | - | 8.82 | 2.32 | 0.41 | E | 103.1 | 35.18 | 23.87 |
| II-3 | - | 8.82 | 2.32 | 0.41 | F | 39.0 | 35.18 | 12.16 |
| III-1 | 4.36 | 4.48 | - | 0.92 | A | 47.8 | 44.35 | 6.04 |
| III-2 | 4.36 | 2.24 | - | 0.41 | B | 68.7 | 28.67 | 4.05 |
| III-3 | 4.36 | 4.48 | - | 0.41 | B | 72.3 | 26.21 | 5.34 |
| III-4 | 4.36 | 4.48 | - | 0.41 | C | 64.0 | 26.21 | 3.81 |
| III-5 | 4.36 | 4.48 | - | 0.41 | D | 81.5 | 26.21 | 2.54 |
| IV-1 | 4.36 | 2.24 | 4.56 | 0.41 | B | 56.7 | 21.88 | 4.66 |
| IV-2 | 4.36 | 4.48 | 4.56 | 0.41 | B | 100.6 | 20.42 | 4.42 |
| IV-3 | 4.36 | 2.24 | 4.56 | 0.41 | C | 37.4 | 21.88 | 2.07 |
| IV-4 | 4.36 | 4.48 | 4.56 | 0.41 | C | 34.1 | 20.42 | 3.15 |
| IV-5 | 4.36 | 4.48 | 4.56 | 0.41 | D | 52.7 | 20.42 | 6.46 |
| IV-6 | 4.36 | 2.24 | 4.51 | 0.36 | E | 44.8 | 19.79 | 4.56 |
| IV-7 | 4.36 | 4.48 | 5.10 | 0.41 | E | 34.7 | 19.89 | 4.78 |
| IV-8 | 4.36 | 2.24 | 4.51 | 0.36 | F | 85.2 | 19.79 | 3.02 |
| IV-9 | 4.36 | 4.48 | 5.10 | 0.41 | F | 55.1 | 19.89 | 3.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalewska, A.; Majewska-Smolarek, K.; Herc, A.S.; Kaźmierski, S.; Bojda, J. Formation of Hybrid Spherical Silica Particles Using a Novel Alkoxy-Functional Polysilsesquioxane Macromonomer as a Precursor in an Acid-Catalyzed Sol-Gel Process. Materials 2025, 18, 3357. https://doi.org/10.3390/ma18143357
Kowalewska A, Majewska-Smolarek K, Herc AS, Kaźmierski S, Bojda J. Formation of Hybrid Spherical Silica Particles Using a Novel Alkoxy-Functional Polysilsesquioxane Macromonomer as a Precursor in an Acid-Catalyzed Sol-Gel Process. Materials. 2025; 18(14):3357. https://doi.org/10.3390/ma18143357
Chicago/Turabian StyleKowalewska, Anna, Kamila Majewska-Smolarek, Agata S. Herc, Sławomir Kaźmierski, and Joanna Bojda. 2025. "Formation of Hybrid Spherical Silica Particles Using a Novel Alkoxy-Functional Polysilsesquioxane Macromonomer as a Precursor in an Acid-Catalyzed Sol-Gel Process" Materials 18, no. 14: 3357. https://doi.org/10.3390/ma18143357
APA StyleKowalewska, A., Majewska-Smolarek, K., Herc, A. S., Kaźmierski, S., & Bojda, J. (2025). Formation of Hybrid Spherical Silica Particles Using a Novel Alkoxy-Functional Polysilsesquioxane Macromonomer as a Precursor in an Acid-Catalyzed Sol-Gel Process. Materials, 18(14), 3357. https://doi.org/10.3390/ma18143357







