Unveiling the Influence of Activators on Stability and Pore Features of Foamed Concrete
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Sample Preparation
2.3. Testing Methods
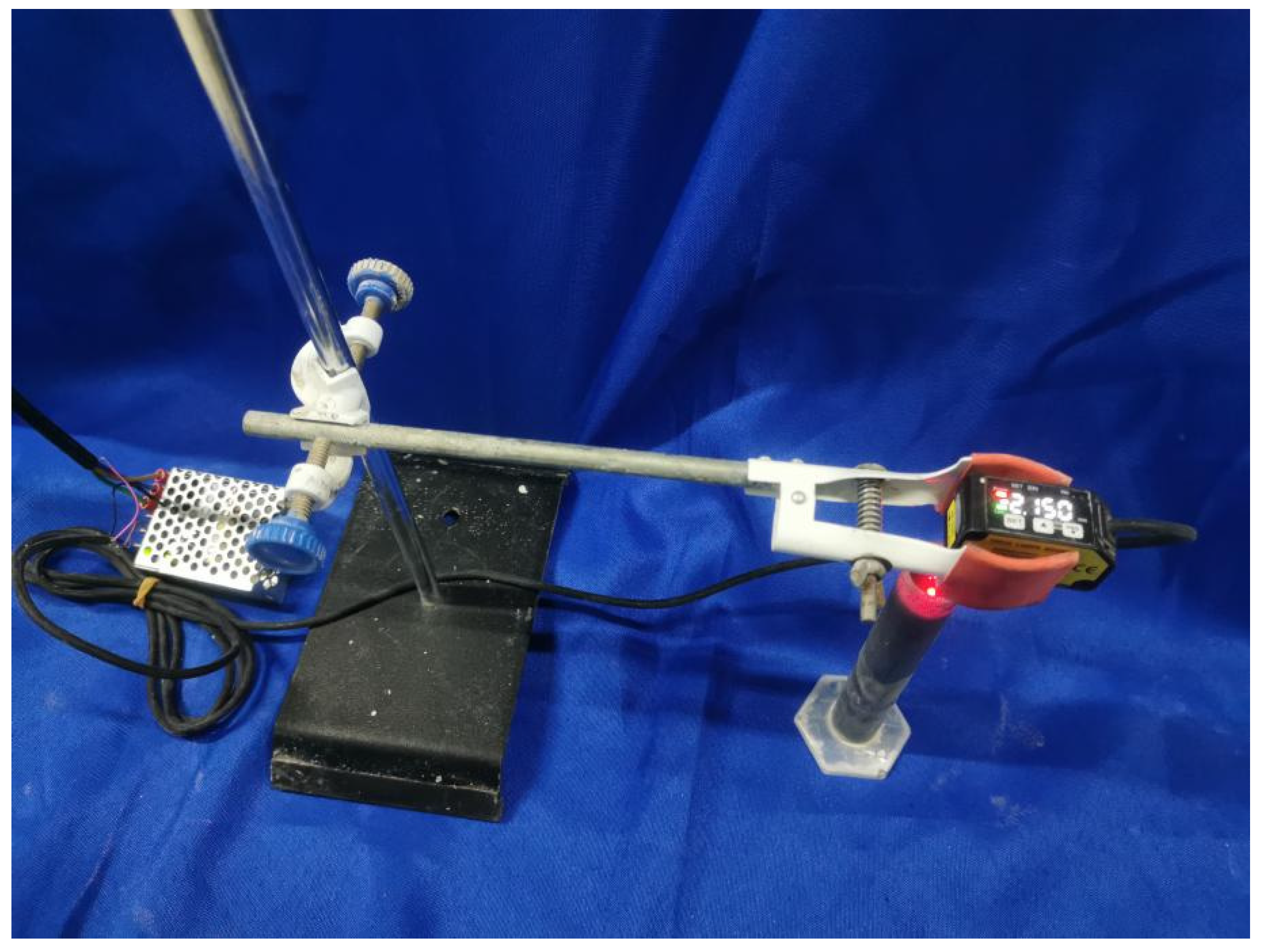
2.3.1. Yield Stress of Matrix
2.3.2. The Settlements
2.3.3. Setting Time
2.3.4. XRD Detection
2.3.5. SEM-EDS Testing
2.3.6. Bubble Evolution and Pore Structure
2.3.7. Hardened Foamed Concrete Properties
3. Results and Discussion
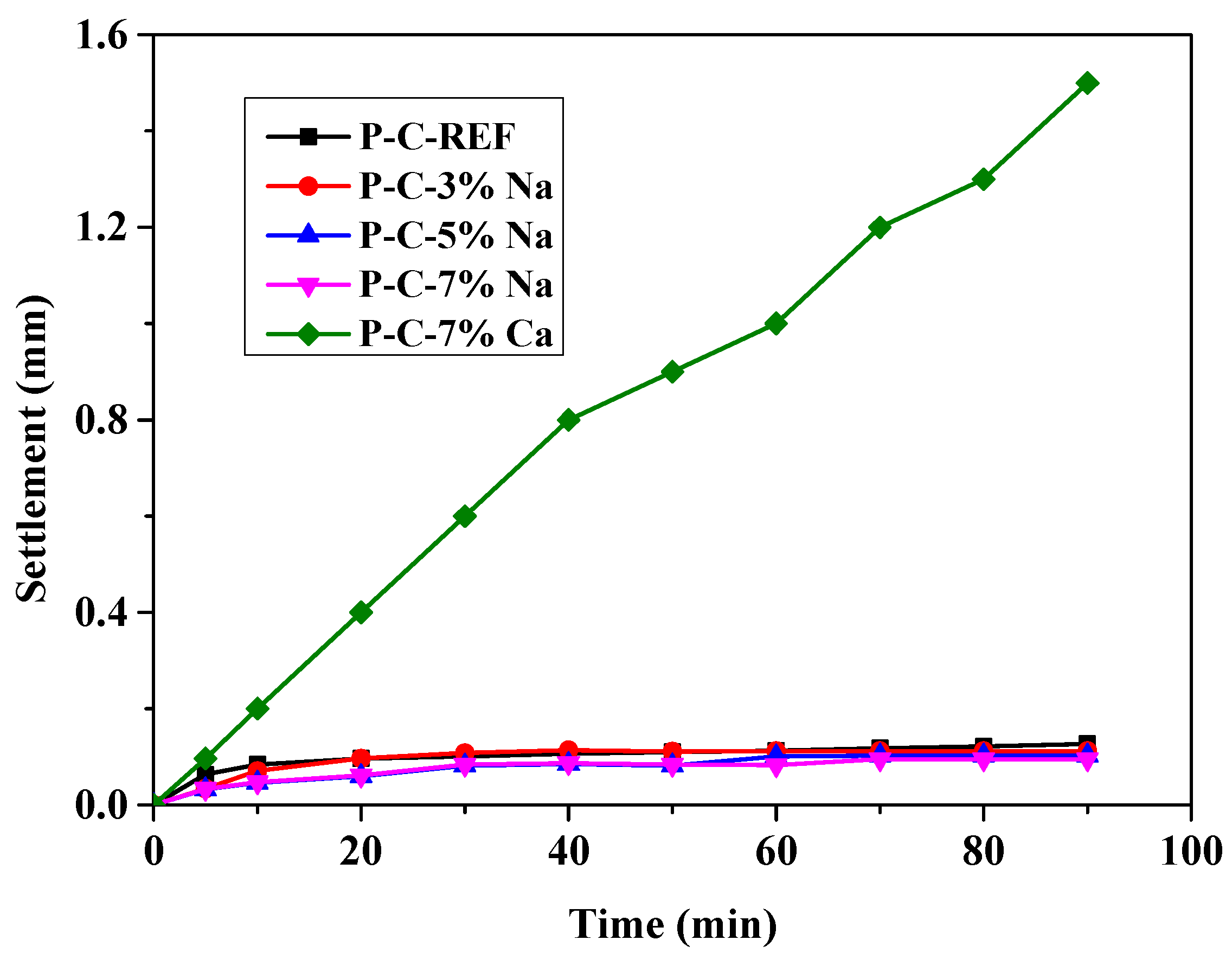
3.1. Effect of Activator on Matrix Stability
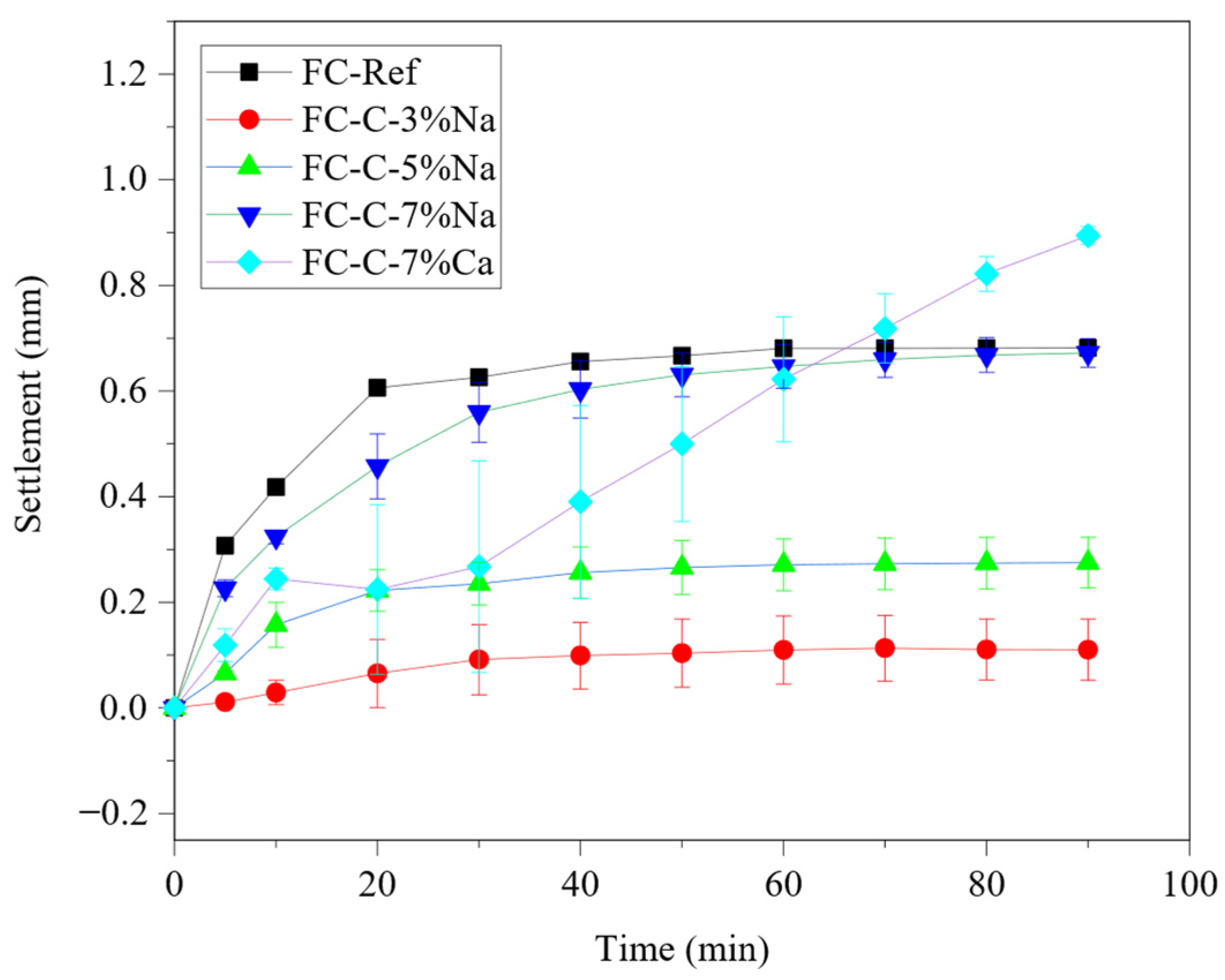
3.2. Effect of Activator on Stability of Foam Concrete
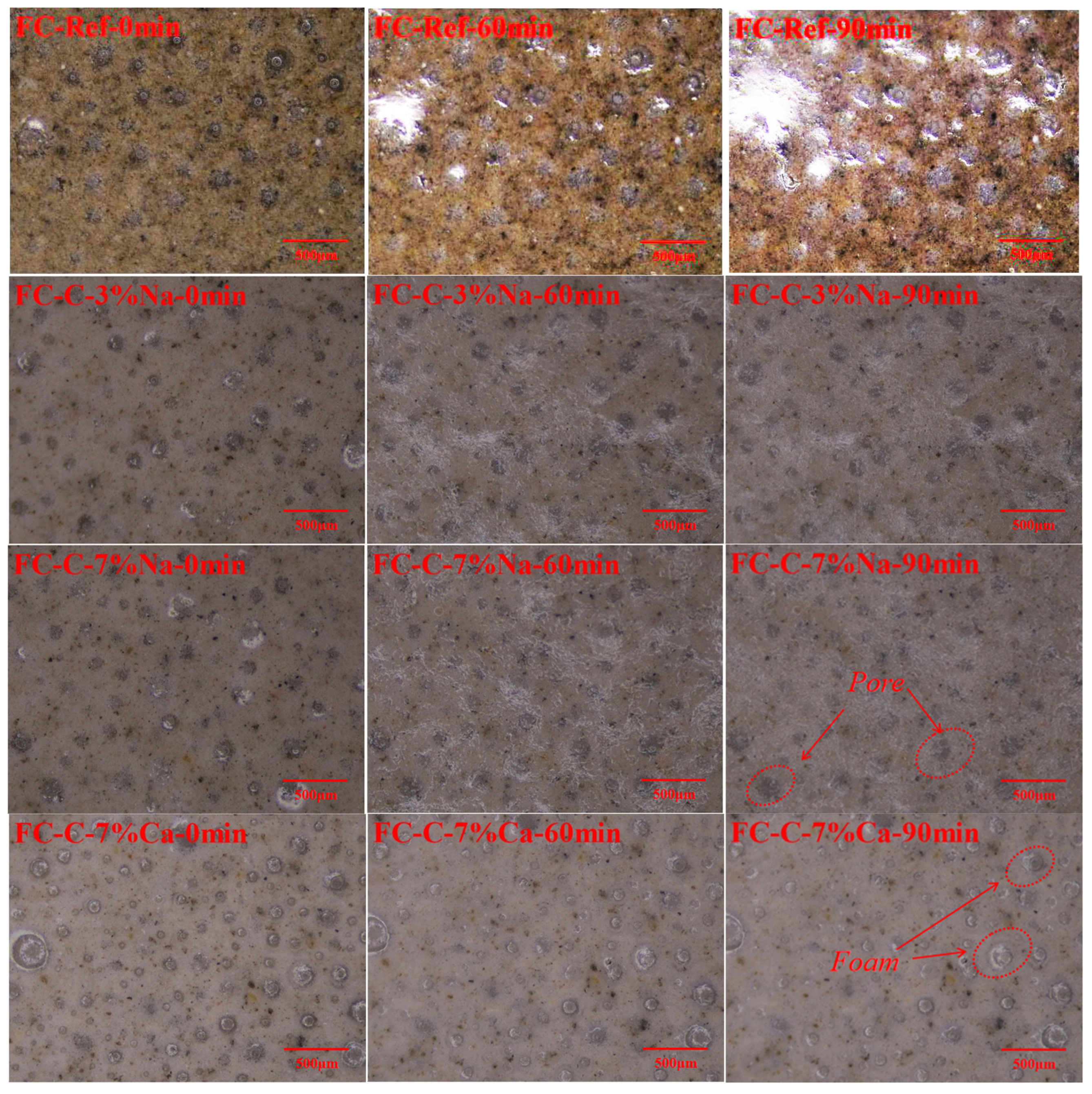
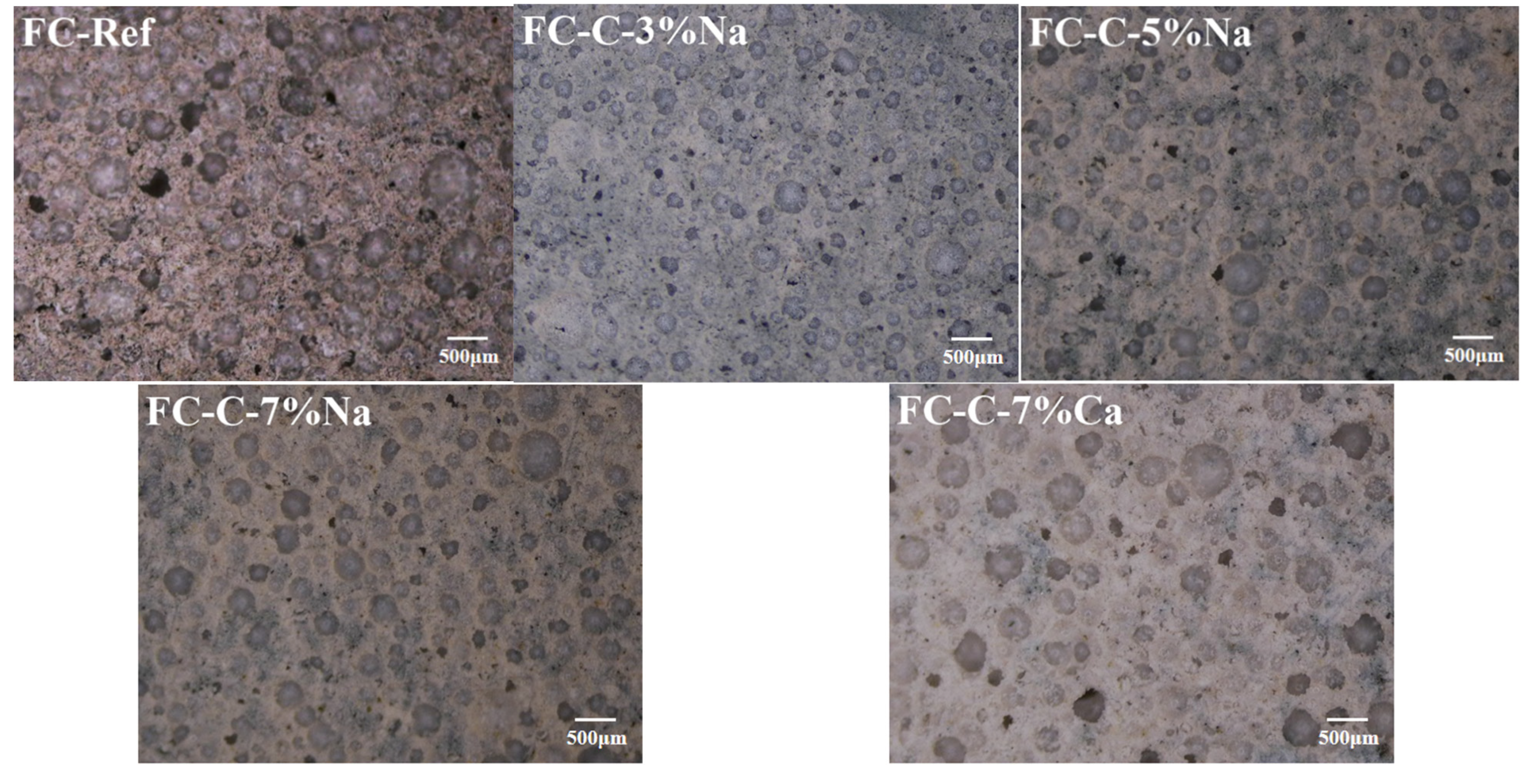
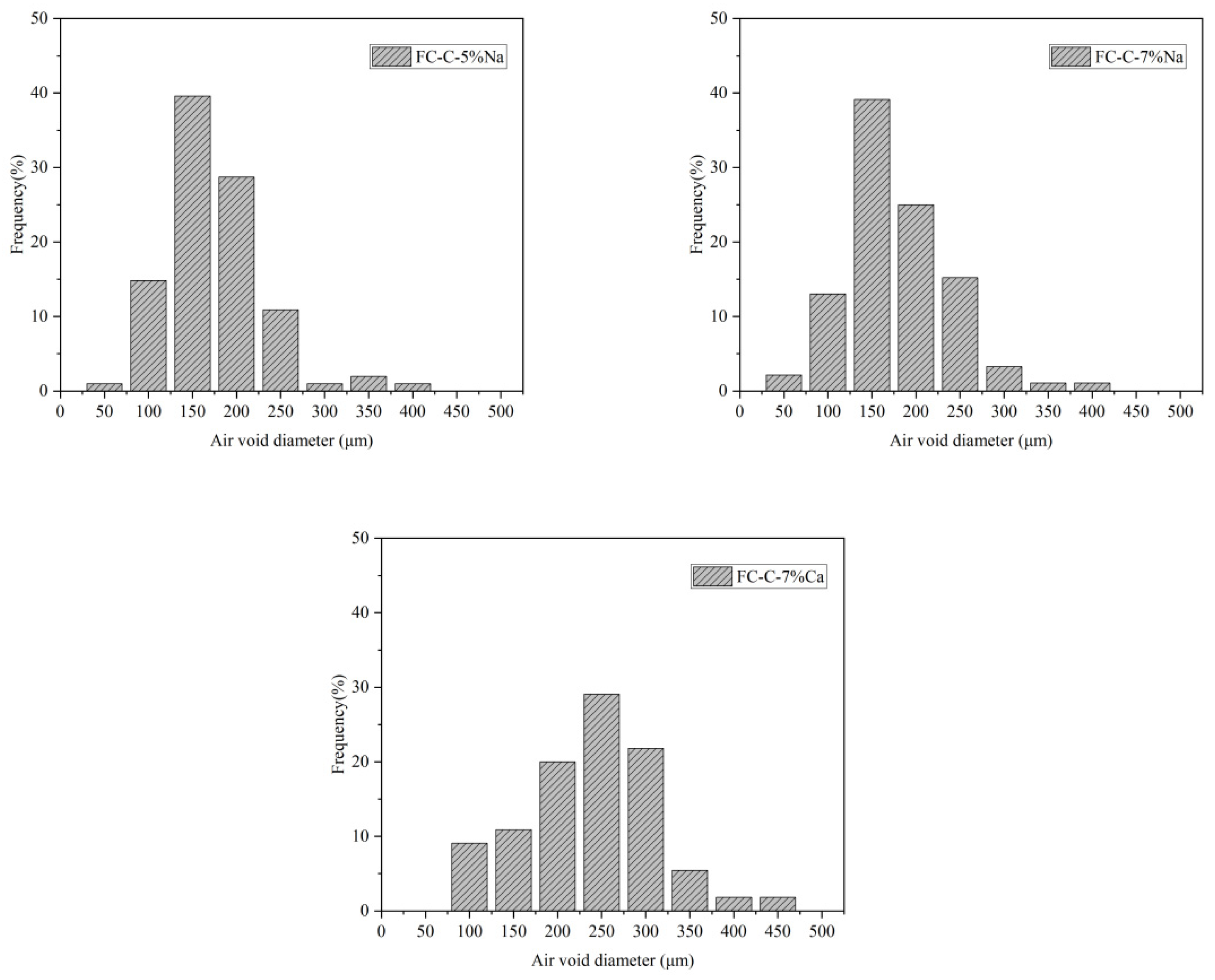
3.3. Foam Concrete Pore Structure
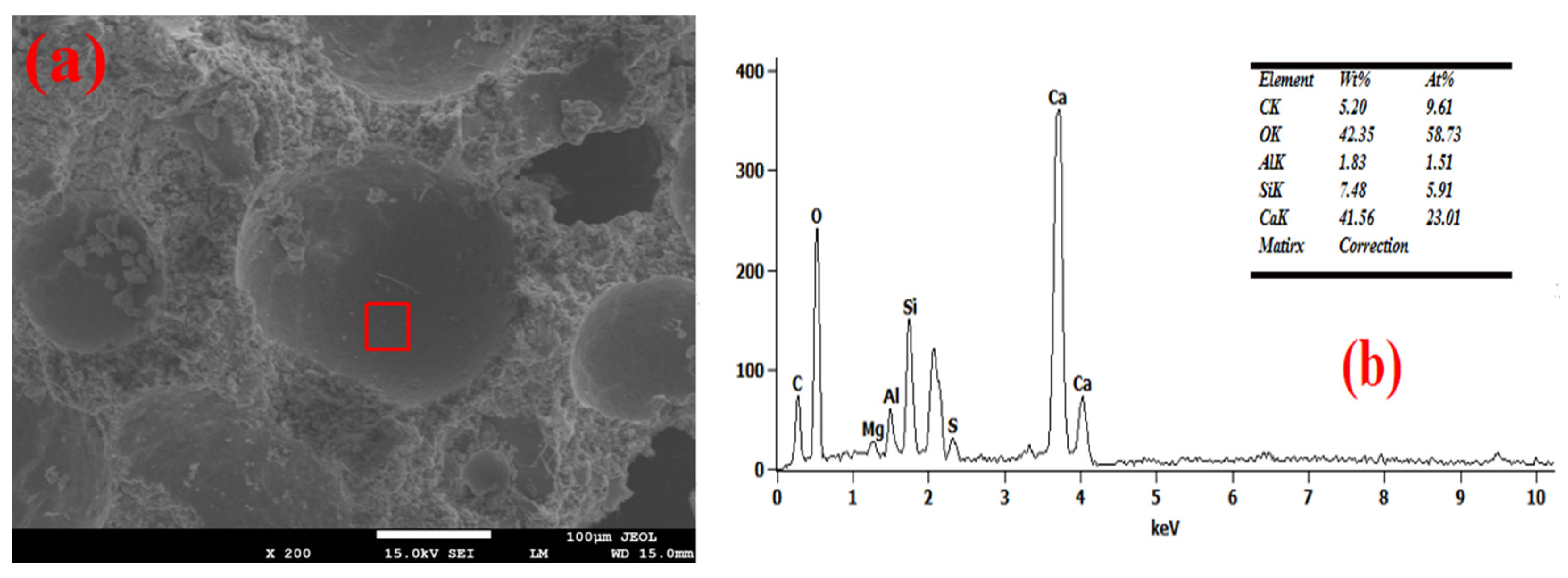
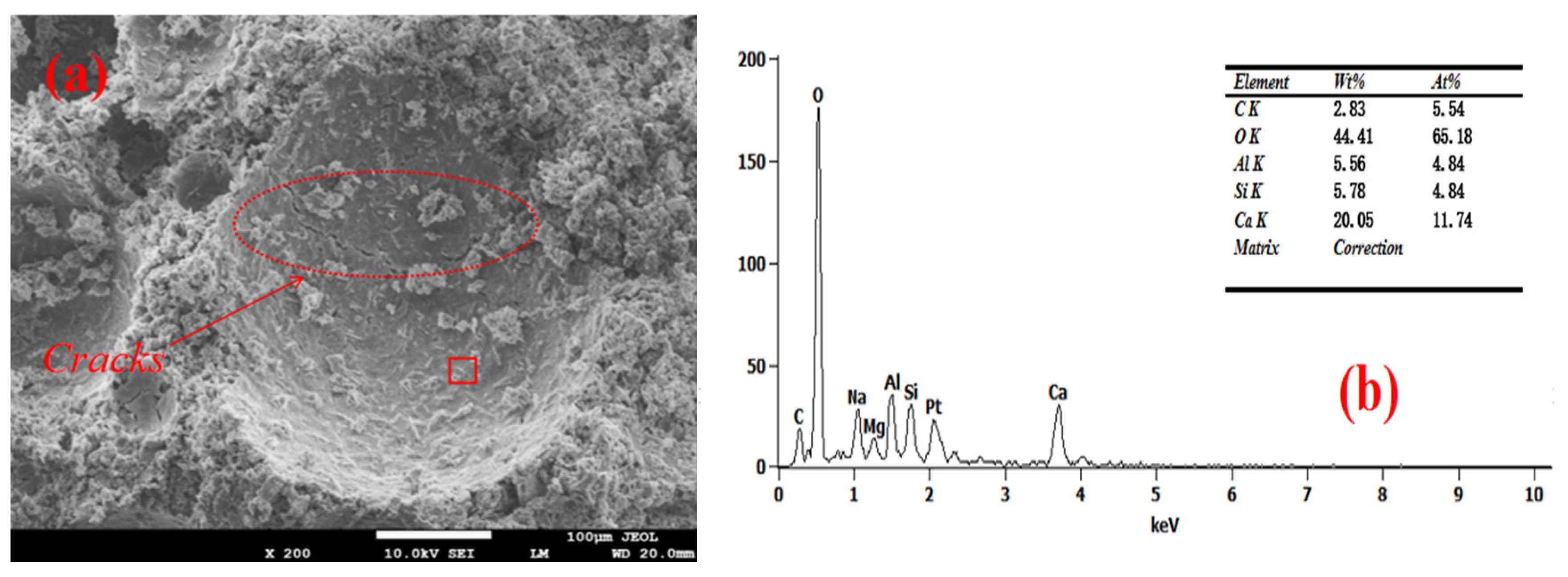
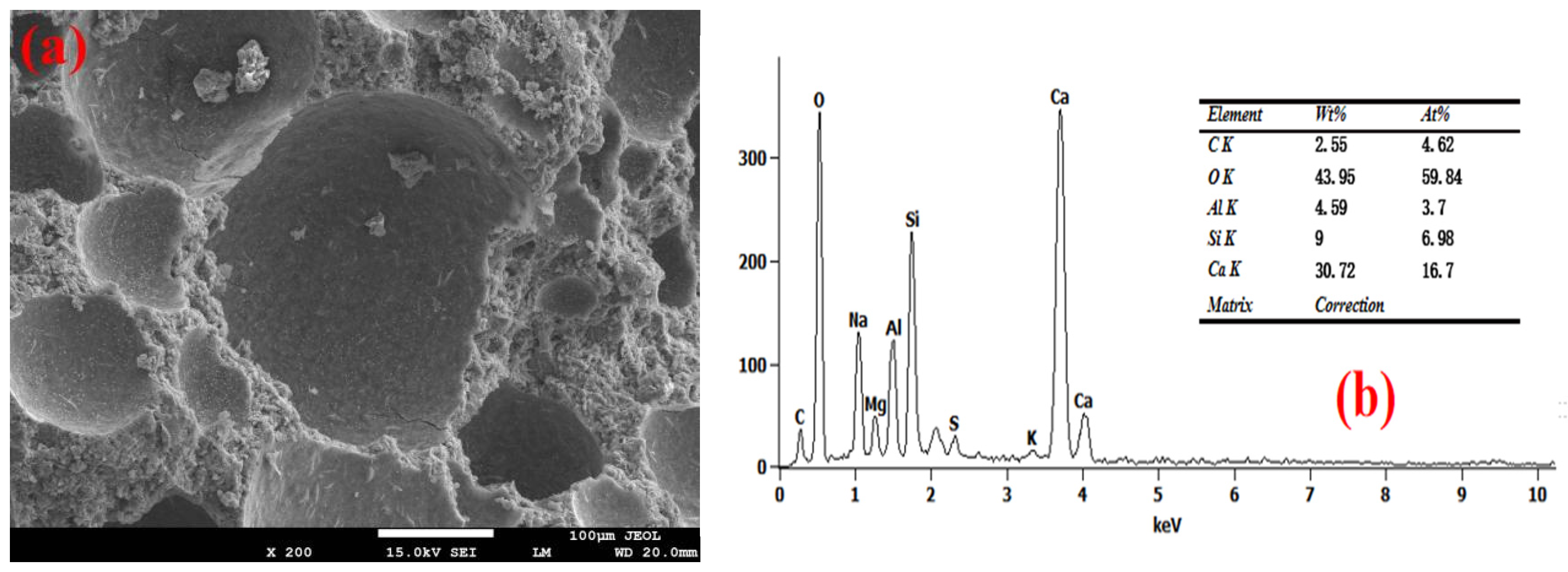
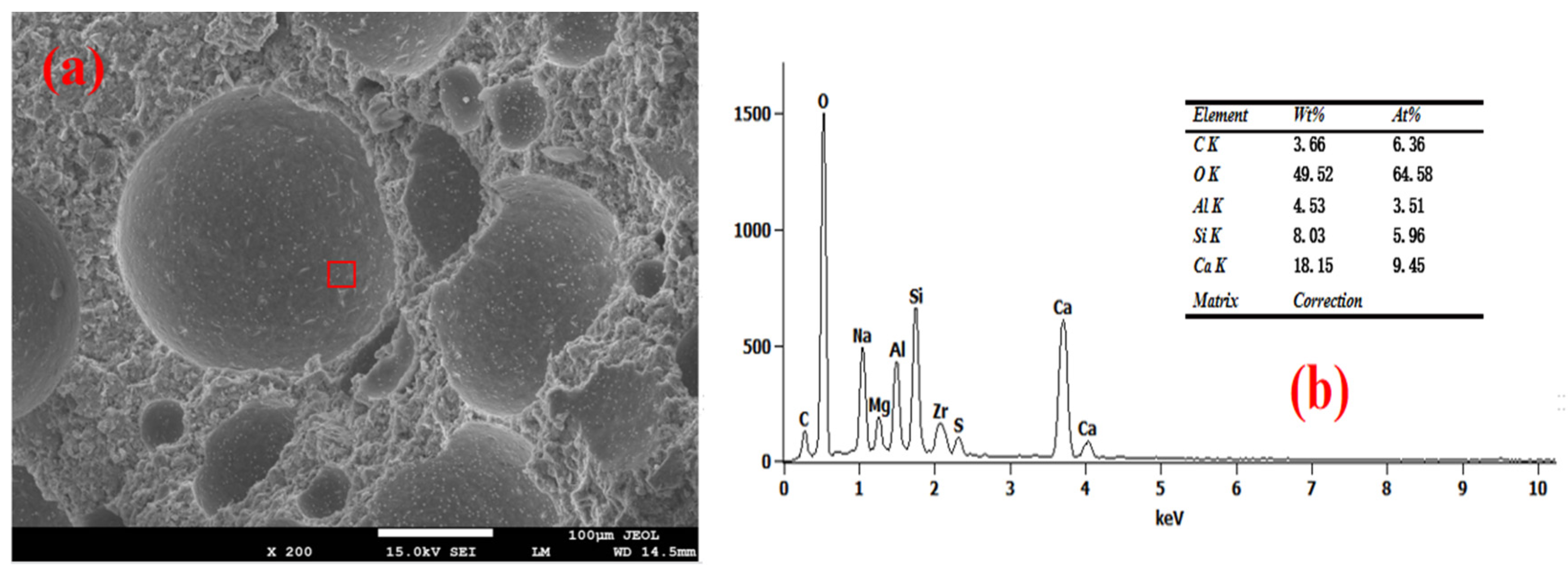
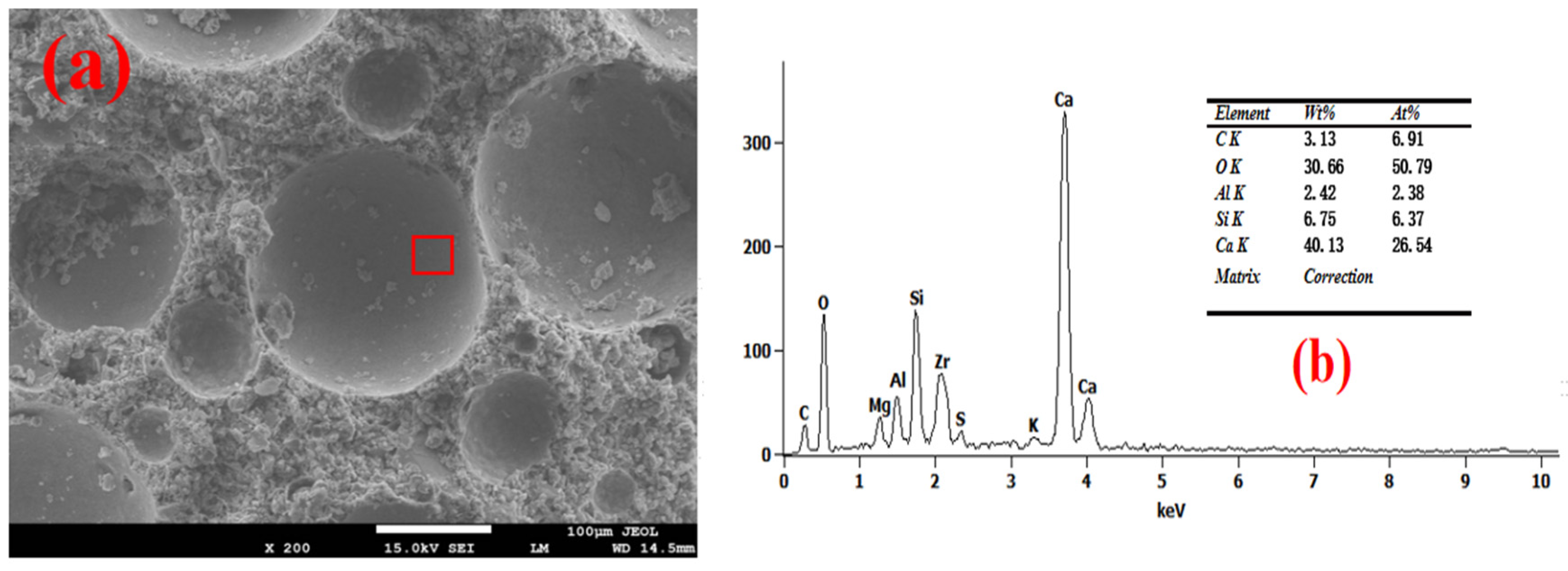
3.4. Pore Wall of Foam Concrete
3.5. Hydration Process of Foamed Concrete
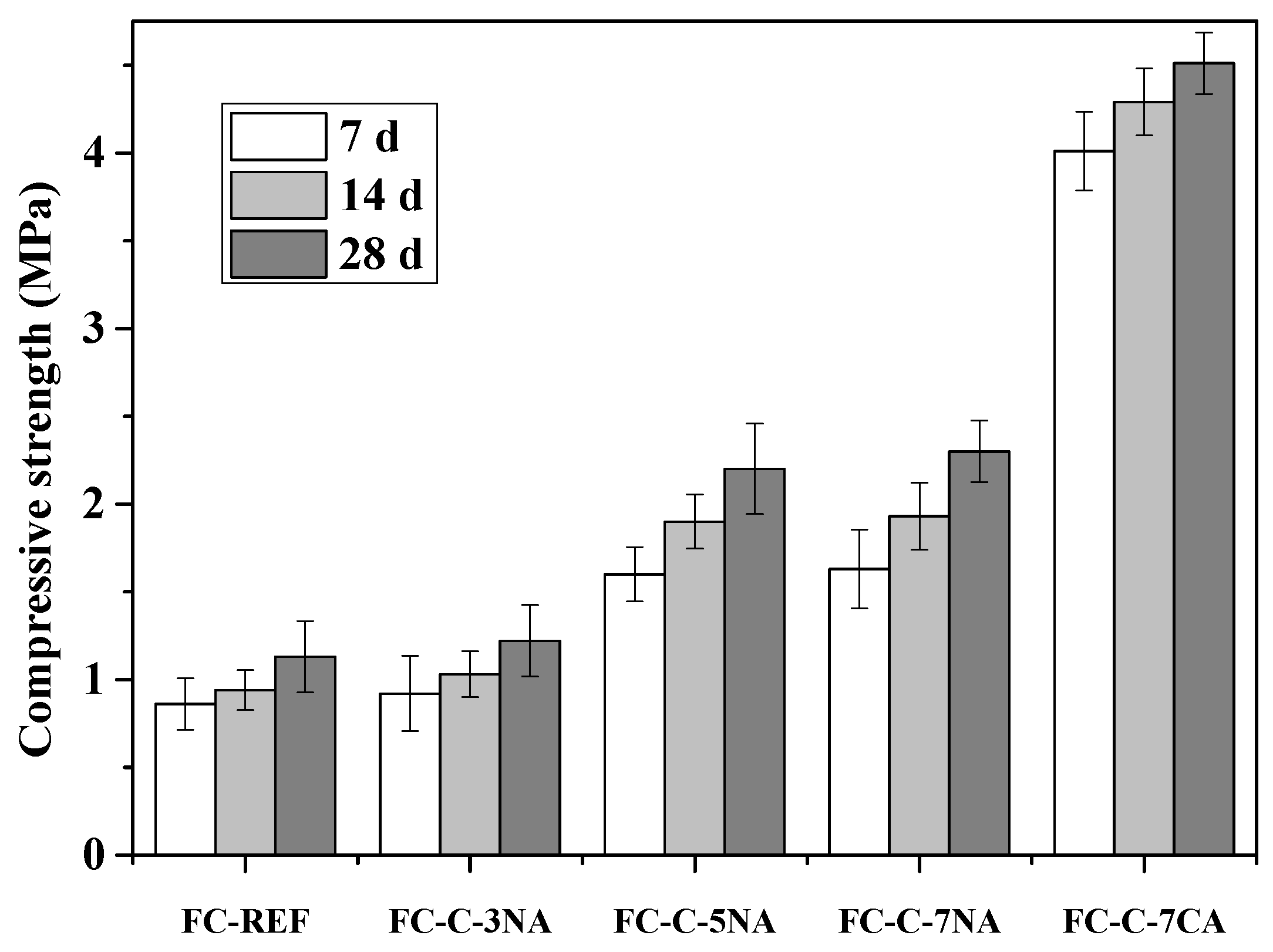
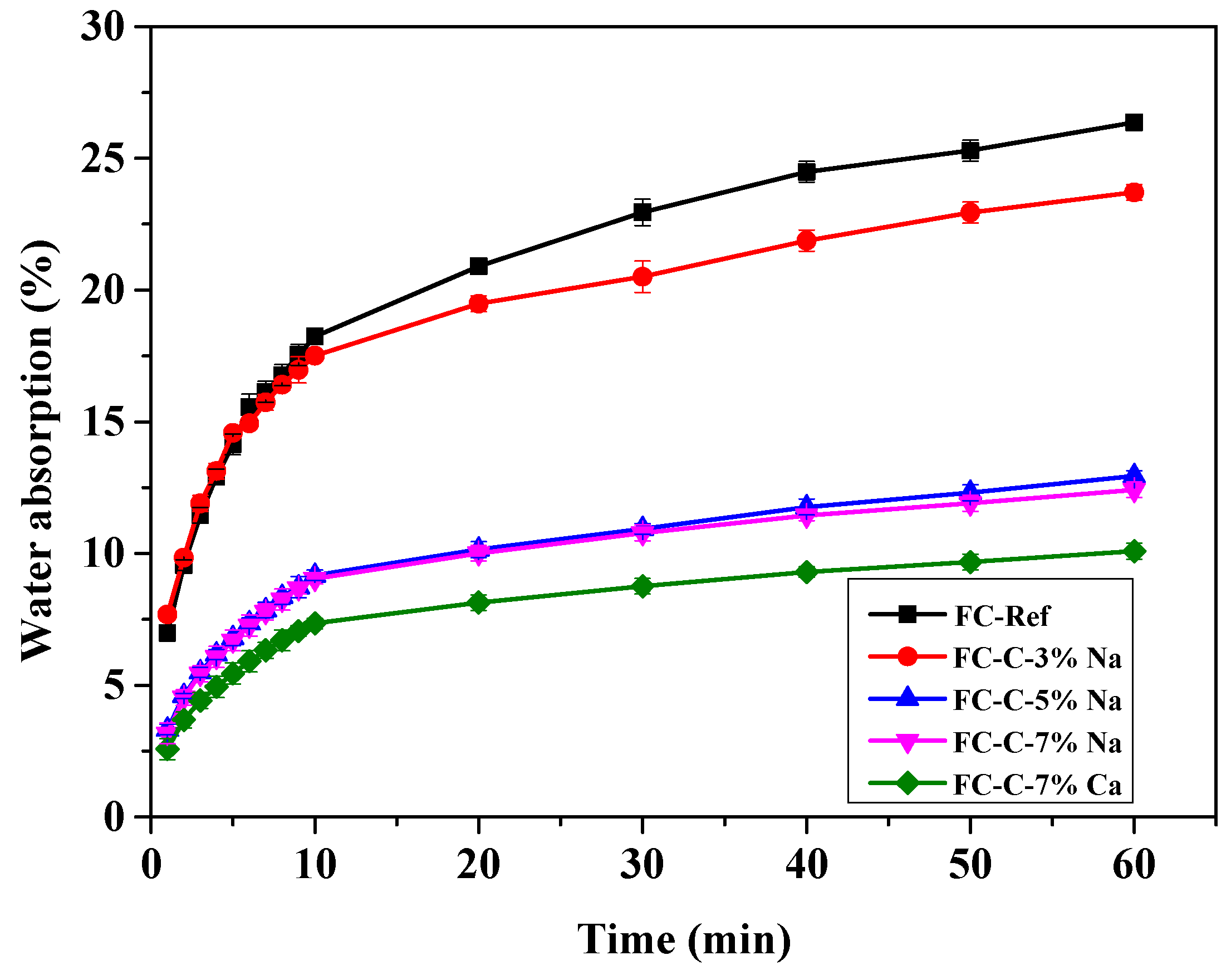
3.6. Compressive Strength and Water Absorption Rate
4. Conclusions
- The presence of sodium hydroxide can also enhance the hydration rate of the base mix and increase the density of the pore wall in foamed concrete, thus enhancing the compressive strength of foamed concrete.
- The addition of excessive sodium hydroxide may introduce too much water and increase the settlement of foamed concrete, thus resulting in a coarse and uneven pore structure.
- The presence of 7% calcium hydroxide could enhance dense pore walls, thus increasing the compressive strength and reducing the water absorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wu, D.; Qin, S.; Shi, P.; Deng, R. Preparation of sulfoaluminate cementitious carbon dioxide foamed concrete: Application to coal mine insulation. Constr. Build. Mater. 2025, 490, 142449. [Google Scholar] [CrossRef]
- Tran, N.P.; Nguyen, T.N.; Ngo, T.D.; Le, P.K.; Le, T.A. Strategic progress in foam stabilisation towards high-performance foam concrete for building sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 375, 133939. [Google Scholar] [CrossRef]
- Gencel, O.; Balci, B.; Bayraktar, O.Y.; Nodehi, M.; Sarı, A.; Kaplan, G.; Hekimo, G.; Gholampour, A.; Benli, A.; Ozbakkaloglu, T. The effect of limestone and bottom ash sand with recycled fine aggregate in foam concrete. J. Build. Eng. 2022, 54, 104689. [Google Scholar] [CrossRef]
- Bayraktar, O.Y.; Kaplan, G.; Gencel, O.; Benli, A.; Sutcu, M. Physico-mechanical, durability and thermal properties of basalt fiber reinforced foamed concrete containing waste marble powder and slag. Constr. Build. Mater. 2021, 288, 123128. [Google Scholar] [CrossRef]
- Song, Q.; Xu, S.; Zou, Y.; Niu, D.; Zhao, H.; Xia, J.; Bao, J.; Xue, S.; Tang, X. Enhancing the performance of multiple solid wastes foam concrete by foam stabilizer synergistic application and quantitative evaluation. Constr. Build. Mater. 2025, 489, 142298. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Z.; Liu, H.; Jiang, Y.; Wang, H. Drying shrinkage behavior of hybrid alkali activated cement (HAAC) mortars. Constr. Build. Mater. 2022, 316, 126068. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, Y.; Su, R.K.L. Novel high performance green calcined clay-based foam concrete. J. Build. Eng. 2025, 110, 113069. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Kashani, K.; Deventer, J.S.J.V. Alkali activated slag foams: The effect of the alkali reaction on foam characteristics. J. Clean. Prod. 2017, 147, 330–339. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA-GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- Badanoiu, A.I.; Al Saadi, T.H.A.; Stoleriu, S.; Voicu, G. Preparation and characterization of foamed geopolymers from waste glass and red mud. Constr. Build. Mater. 2015, 84, 284–293. [Google Scholar] [CrossRef]
- De Vargas, A.; De Gutierrez, R.; Castro, G. Study of geopolymeric binders of fly ash/metakaolin mixtures cured at room temperature. Key Eng. Mater. 2014, 600, 338–344. [Google Scholar] [CrossRef]
- Yang, K.H.; Lee, K.H.; Song, J.K.; Gong, M.H. Properties and sustainability of alkali-activated slag foamed concrete. J. Clean. Prod. 2014, 68, 226–233. [Google Scholar] [CrossRef]
- Bilim, C.; Atiş, C.D. Alkali activation of mortars containing different replacement levels of ground granulated blast furnace slag. Constr. Build. Mater. 2012, 28, 708–712. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Sharp, J.H. Effect of temperature on the hydration of the main clinker phases in portland cements: Part I, neat cements. Cem. Concr. Res. 1998, 28, 1245–1257. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H. The Pore Characteristics of Geopolymer Foam Concrete and Their Impact on the Compressive Strength and Modulus. Front. Mater. 2016, 3, 38. [Google Scholar] [CrossRef]
- Zheng, W.; Zou, M.; Wang, Y. Literature review of alkali-activated cementitious materials. J. Build. Struct. 2019, 40, 28–39. [Google Scholar]
- El-Yamany, H.E.; El-Salamawy, M.A.; El-Assal, N.T. Microstructure and mechanical properties of alkali-activated slag mortar modified with latex. Constr. Build. Mater. 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Shi, C.; Jimenez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Variation in hybrid cements over time. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, Y.; Guo, J.; Zhang, Y.; Sun, G. Effects of foaming agent type on the workability, drying shrinkage, frost resistance and pore distribution of foamed concrete. Constr. Build. Mater. 2018, 186, 833–839. [Google Scholar] [CrossRef]
- Hilal, A.A.; Thom, N.H.; Dawson, A.R. On entrained pore size distribution of foamed concrete. Constr. Build. Mater. 2015, 75, 227–233. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S. Sanjayan, Effect of yield stress development on the foam-stability of aerated geopolymer concrete. Cem. Concr. Res. 2020, 138, 106233. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Z.; Liu, C.; Zhang, C.; Zhang, Y. Unveiling the role of Portland cement and fly ash in pore formation and its influence on properties of hybrid alkali-activated foamed concrete. Constr. Build. Mater. 2024, 411, 134336. [Google Scholar]
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, C.; Chen, C.; Zhang, Y. Effect of superabsorbent polymer on the foam-stability of foamed concrete. Cem. Concr. Compos. 2022, 127, 104398. [Google Scholar]
- Zhang, X.; Zhang, X.; Liu, J.; Zhu, K.; Zheng, Z.; Dong, Y. Study on properties and hydration mechanism of green alkali-activation lithium slag composite cementitious material. Constr. Build. Mater. 2025, 473, 140966. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B. Study on the influence of nano-silica substitution of reactive Si on the properties and hydration process of alkali-activated cementitious materials paste. Constr. Build. Mater. 2024, 443, 137759. [Google Scholar] [CrossRef]
- Roussel, N.; Stefani, C.; Leroy, R. From mini-cone test to Abrams cone test: Measurement of cement-based materials yield342stress using slump tests. Cem. Concr. Res. 2005, 35, 817–822. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, Z.; Jia, Z.; Liu, C.; Ma, L.; Liu, Z. Effect of formic acid as an accelerator on foam-stability, compressive strength, and pore size distribution of foam concrete. J. Build. Eng. 2023, 66, 105923. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Delgado-Plana, P.; García-Díaz, A.; Bueno-Rodríguez, S.; Quesada, D.E. Influence of NaOH molarity and Portland cement addition on performance of alkali activated cements based in silicomanganese slags. Constr. Build. Mater. 2023, 407, 133544. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, M.; Yang, X.; Guo, X.; Jiang, Y.; Huang, S.; Zhu, F. Property of bauxite residue-activated cementitious materials and its engineering road application: A comprehensive review. Chin. J. Nonferrous Met. 2023, 33, 3421–3439. [Google Scholar]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Hamdan, S.; van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Ramírez, D.A.; GutiErrez, R.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, J.; Fu, H.; Liu, Q.; Li, Y.; Ma, X.; Zhang, P. The coupling impact of NaOH and NaNO3 on hydration process, products and Sr2+ adsorption ability of the major phases in Portland cement. Constr. Build. Mater. 2025, 459, 139813. [Google Scholar] [CrossRef]
- She, W.; Du, Y.; Miao, C.; Liu, J.; Zhao, G.; Jiang, J.; Zhang, Y. Application of organic- and nanoparticle-modified foams in foamed concrete: Reinforcement and stabilization mechanisms. Cem. Concr. Res. 2018, 106, 12–22. [Google Scholar] [CrossRef]



| CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | MgO | SO3 | LOI | K2O | |
|---|---|---|---|---|---|---|---|---|---|
| P O. 42.5 | 51.42 | 24.99 | 8.26 | 4.03 | 0.11 | 3.71 | 2.51 | 4.32 | 0.65 |
| GBFS | 35.58 | 35.10 | 16.32 | 0.69 | 9.32 | 1.17 | 0.41 | 0.49 | 0.92 |
| Mix | Target Density (kg/m3) | OP (kg) | GBFS (kg) | Water (kg) | NaOH (kg) | Ca(OH)2 (kg) | Actual Foam (m3) |
|---|---|---|---|---|---|---|---|
| FC-Ref | 800 | - | 498.13 | 249.07 | 14.94 | - | 0.57 |
| FC-C-3Na | 800 | 99.63 | 398.50 | 249.07 | 14.94 | - | 0.57 |
| FC-C-5Na | 800 | 99.63 | 398.50 | 249.07 | 24.91 | - | 0.57 |
| FC-C-7Na | 800 | 99.63 | 398.50 | 249.07 | 34.87 | - | 0.56 |
| FC-C-7Ca | 800 | 99.63 | 398.50 | 249.07 | - | 34.87 | 0.56 |
| Time (min) | Spread (mm) | Yield Stress (Pa) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-Ref | P-C-3Na | P-C-5%Na | P-C-7%Na | P-C-7%Ca | P-Ref | P-C-3%Na | P-C-5%Na | P-C-7%Na | P-C-7%Ca | |
| 0 | 245 | 178 | 191 | 209 | 151 | 13.66 | 65.58 | 46.20 | 29.51 | >100 |
| 10 | 215 | 167 | 184 | 192 | 143 | 26.25 | 90.22 | 55.68 | 45.10 | >100 |
| 30 | 198 | 150 | 166 | 175 | 134 | 39.62 | >100 | 93.17 | 71.70 | >100 |
| 60 | 192 | 126 | 152 | 155 | 120 | 46.20 | >100 | >100 | >100 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Wang, S.; Ma, L.; Wang, T.; Zhou, M.; Hu, Z.; Wang, Z. Unveiling the Influence of Activators on Stability and Pore Features of Foamed Concrete. Materials 2025, 18, 3320. https://doi.org/10.3390/ma18143320
Xiong Y, Wang S, Ma L, Wang T, Zhou M, Hu Z, Wang Z. Unveiling the Influence of Activators on Stability and Pore Features of Foamed Concrete. Materials. 2025; 18(14):3320. https://doi.org/10.3390/ma18143320
Chicago/Turabian StyleXiong, Yuanliang, Shiquan Wang, Liguo Ma, Tingcong Wang, Manling Zhou, Zhongshuai Hu, and Zhenyu Wang. 2025. "Unveiling the Influence of Activators on Stability and Pore Features of Foamed Concrete" Materials 18, no. 14: 3320. https://doi.org/10.3390/ma18143320
APA StyleXiong, Y., Wang, S., Ma, L., Wang, T., Zhou, M., Hu, Z., & Wang, Z. (2025). Unveiling the Influence of Activators on Stability and Pore Features of Foamed Concrete. Materials, 18(14), 3320. https://doi.org/10.3390/ma18143320






