The Synergistic Utilization of Glass Aggregates and Glass Powder on the Thermal and Mechanical Properties of Concrete
Abstract
1. Introduction
2. Material Characteristics and Experimental Program
2.1. Raw Materials
2.1.1. Cementitious Materials
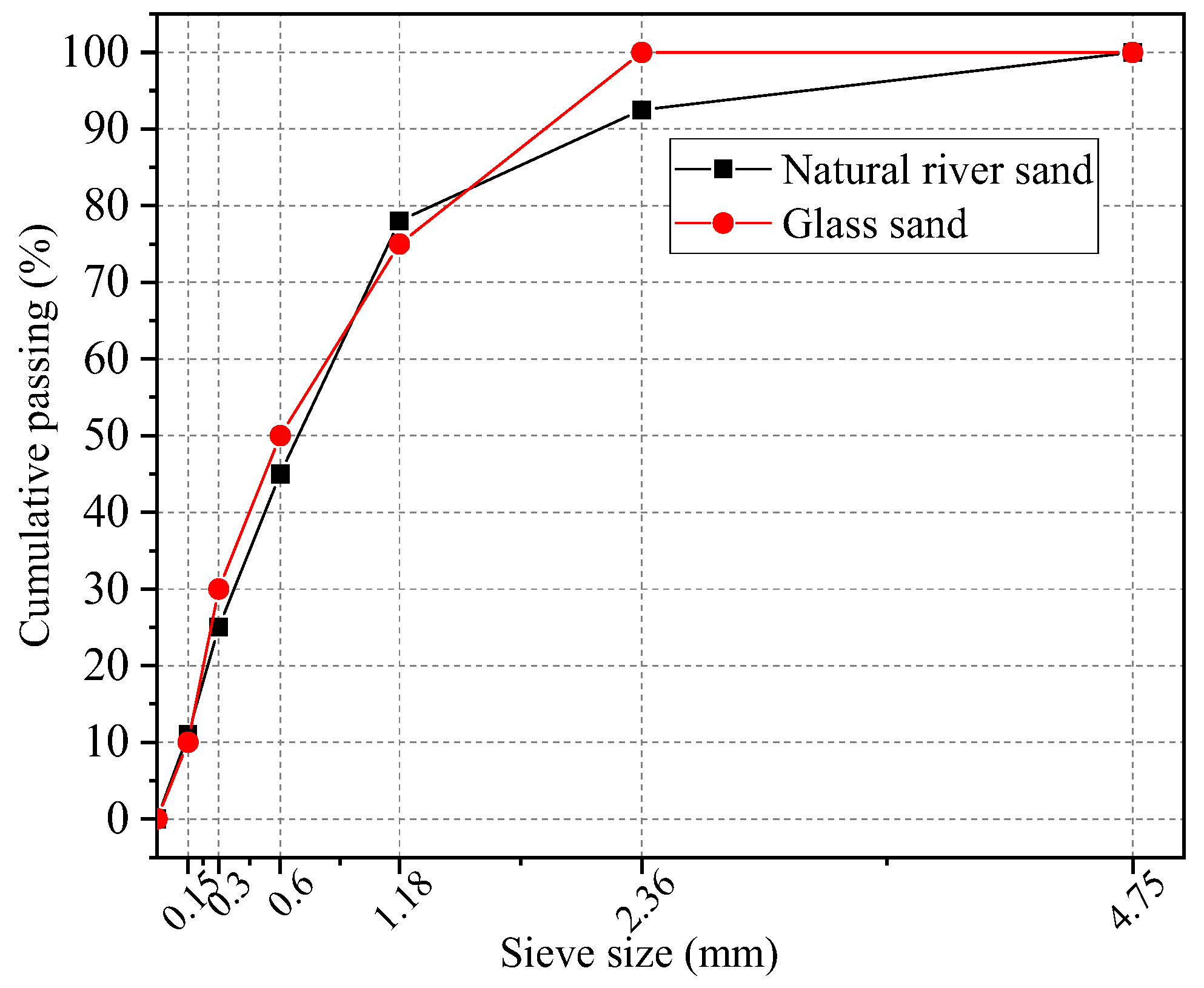
2.1.2. Aggregates
2.2. Concrete Mix Design
2.3. Specimen Preparation
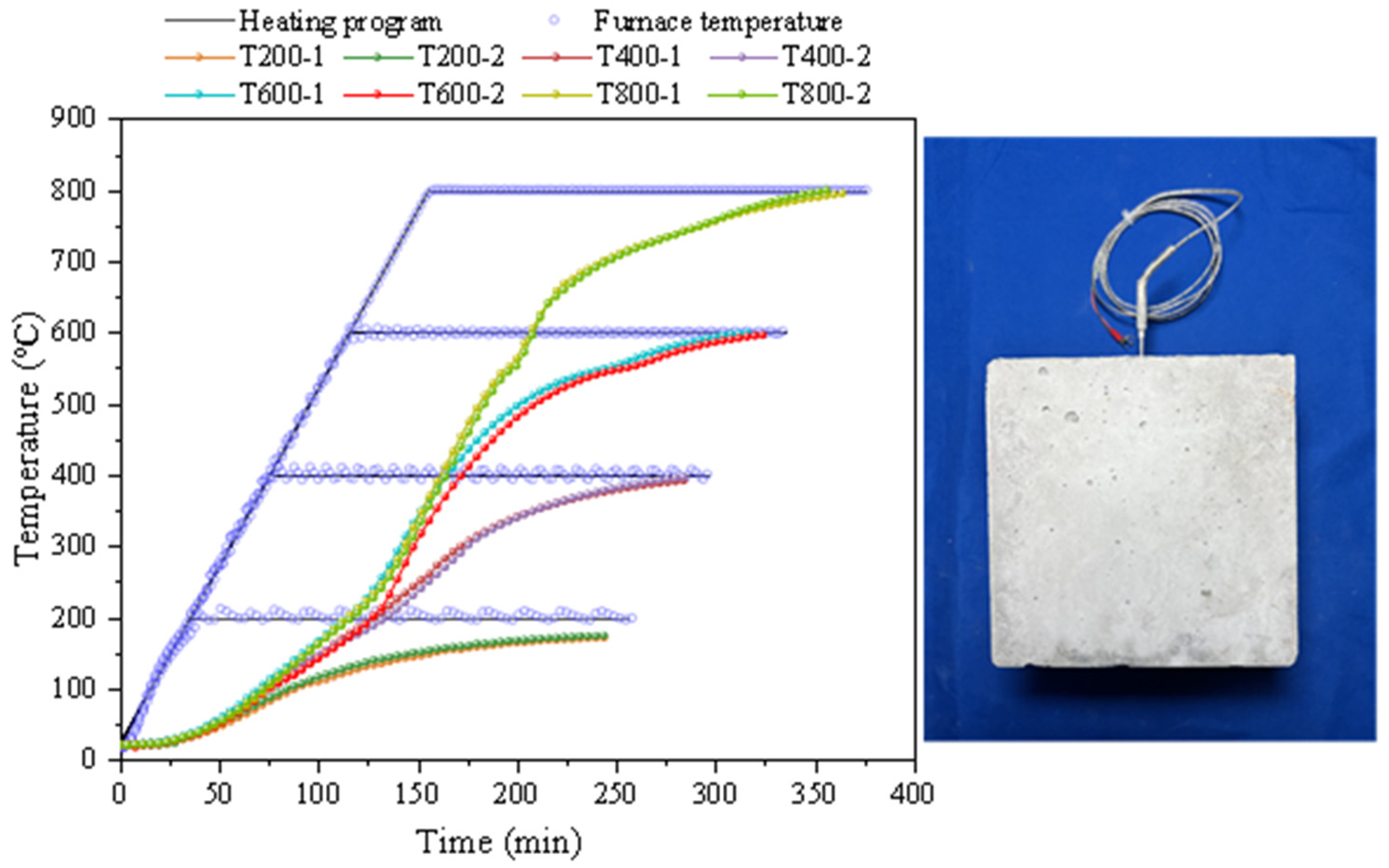
2.4. Heating Method
2.5. Test Methods
2.5.1. Mechanical Property Test
2.5.2. Expansion Test
2.5.3. Mass Loss Test
2.5.4. Thermal Conductivity Test
2.5.5. Specific Heat Test
2.5.6. Microstructure Observations
2.5.7. Pore Structure Test
3. Results and Analysis
3.1. Effect of Glass Materials on Concrete Performance
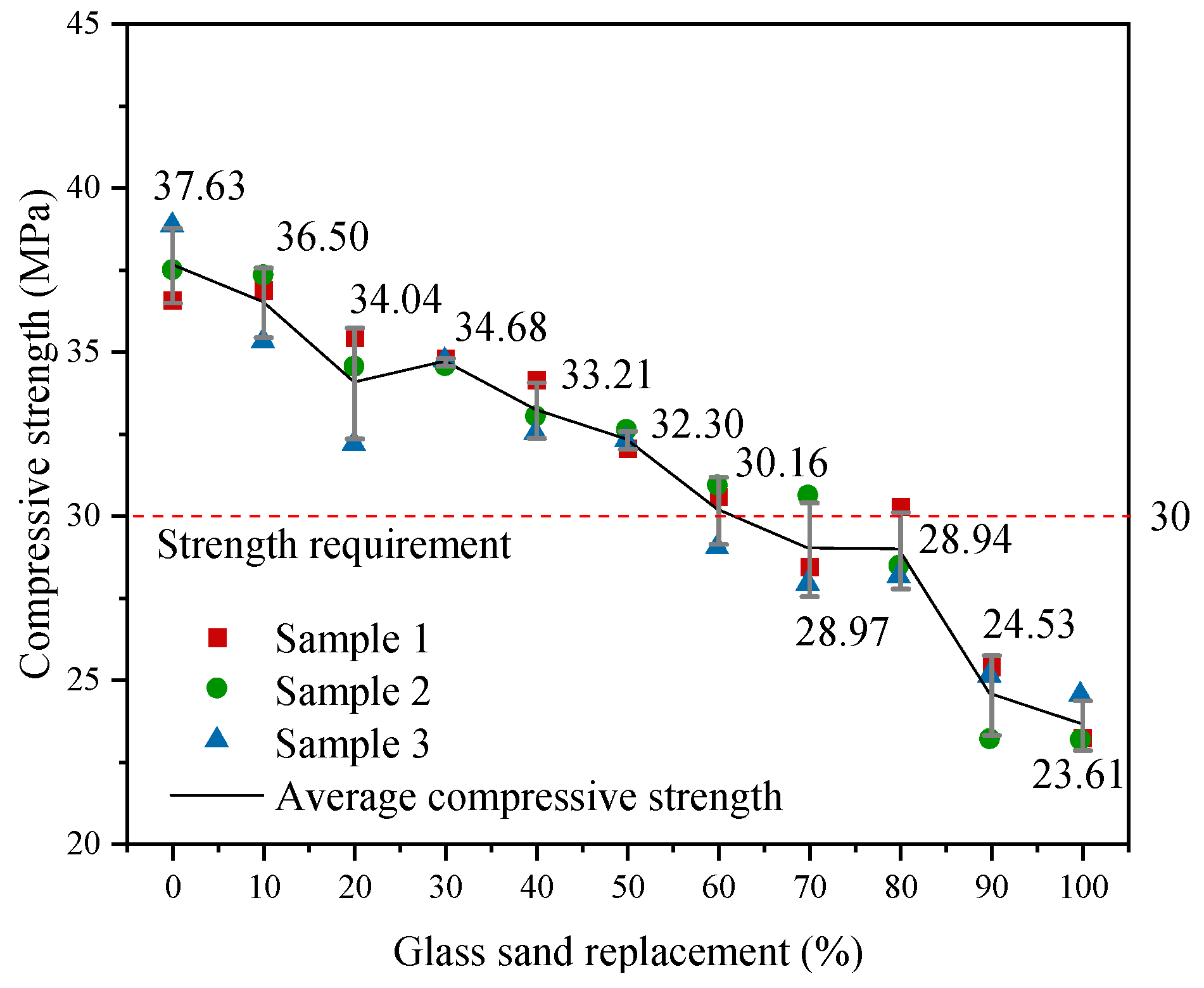
3.1.1. Compressive Strength
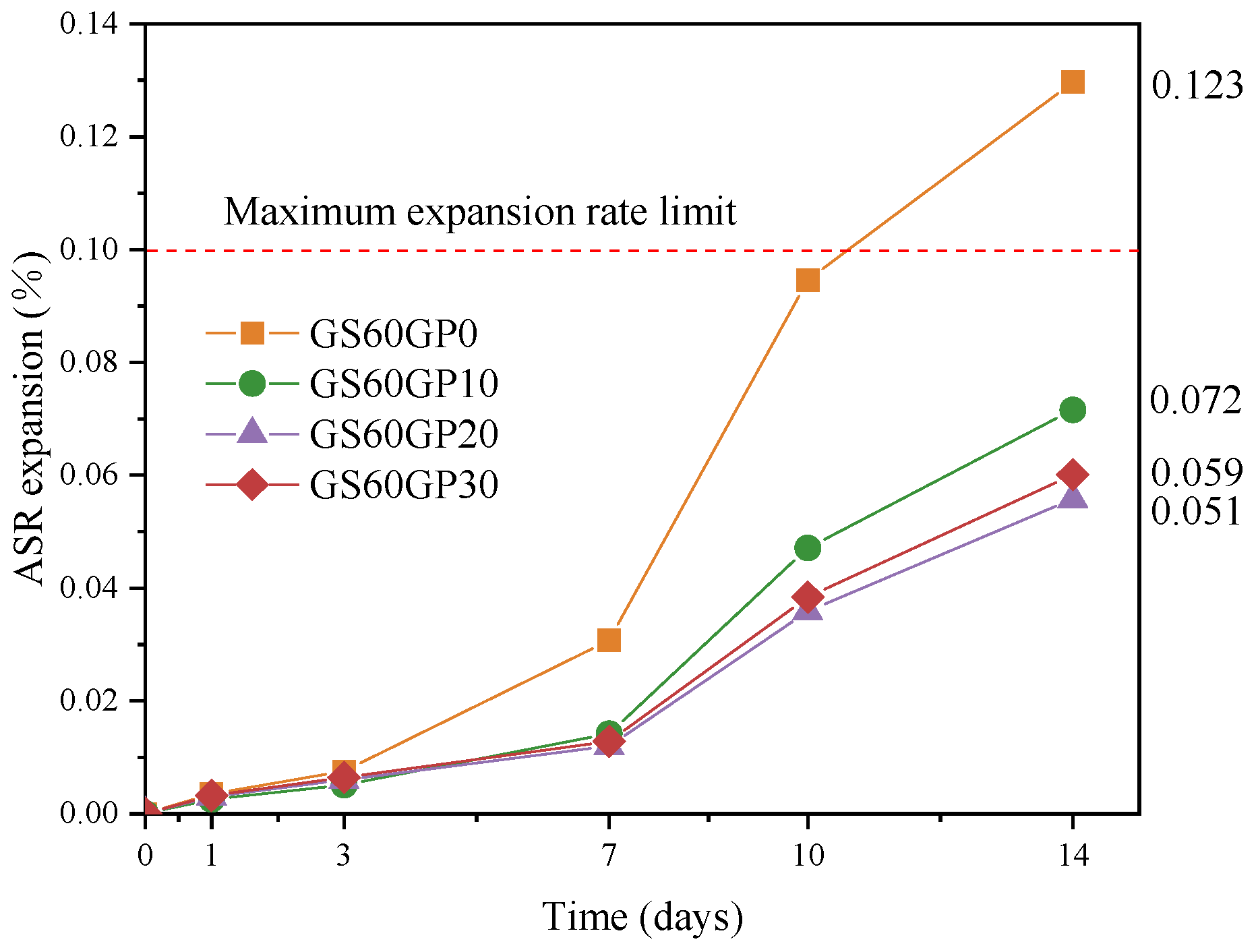
3.1.2. Alkali–Silica Reactivity of Concrete
3.2. Thermal Properties
3.2.1. Thermal Conductivity
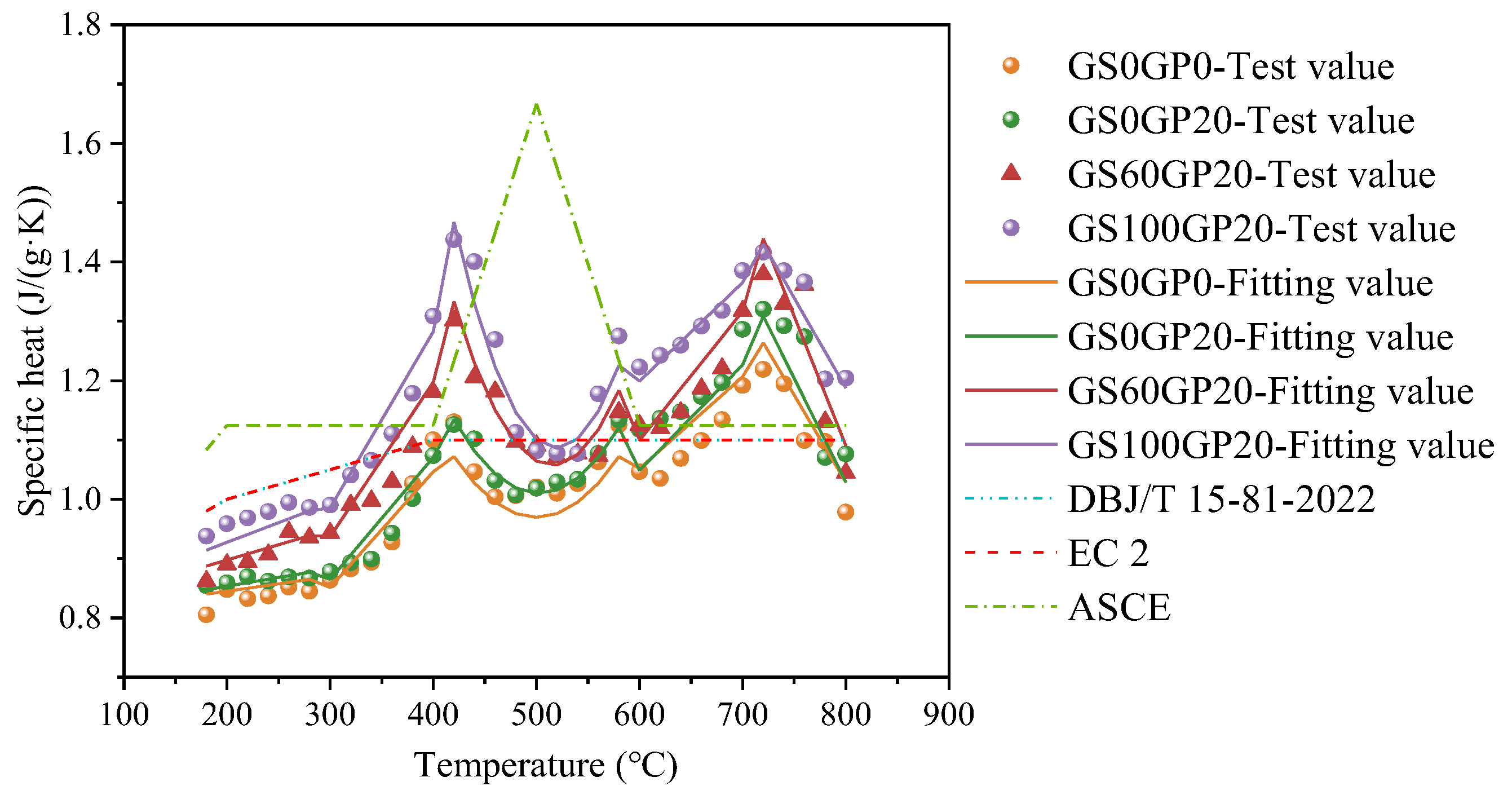
3.2.2. Specific Heat
3.3. High-Temperature Resistance
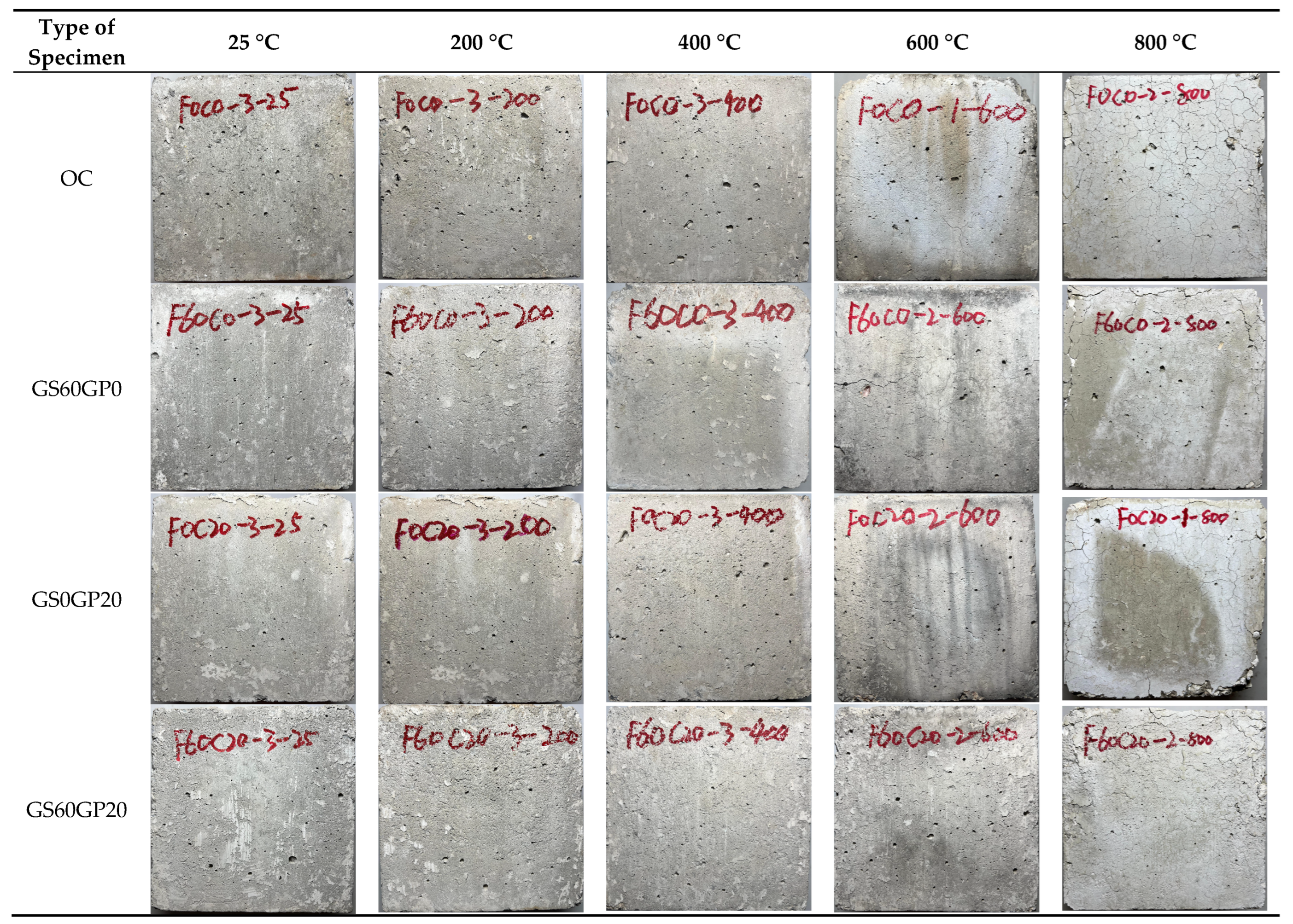
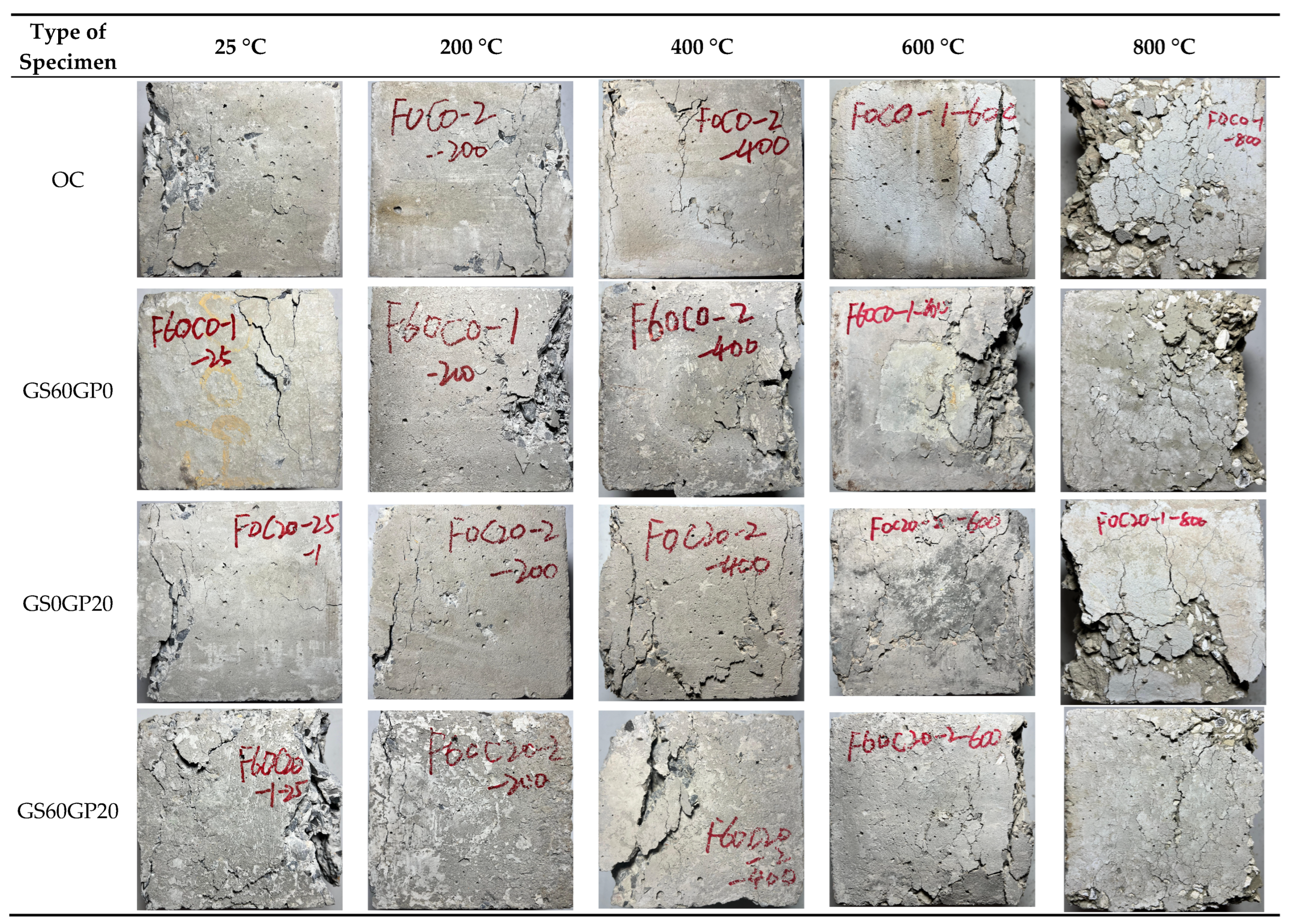
3.3.1. Apparent Characteristics
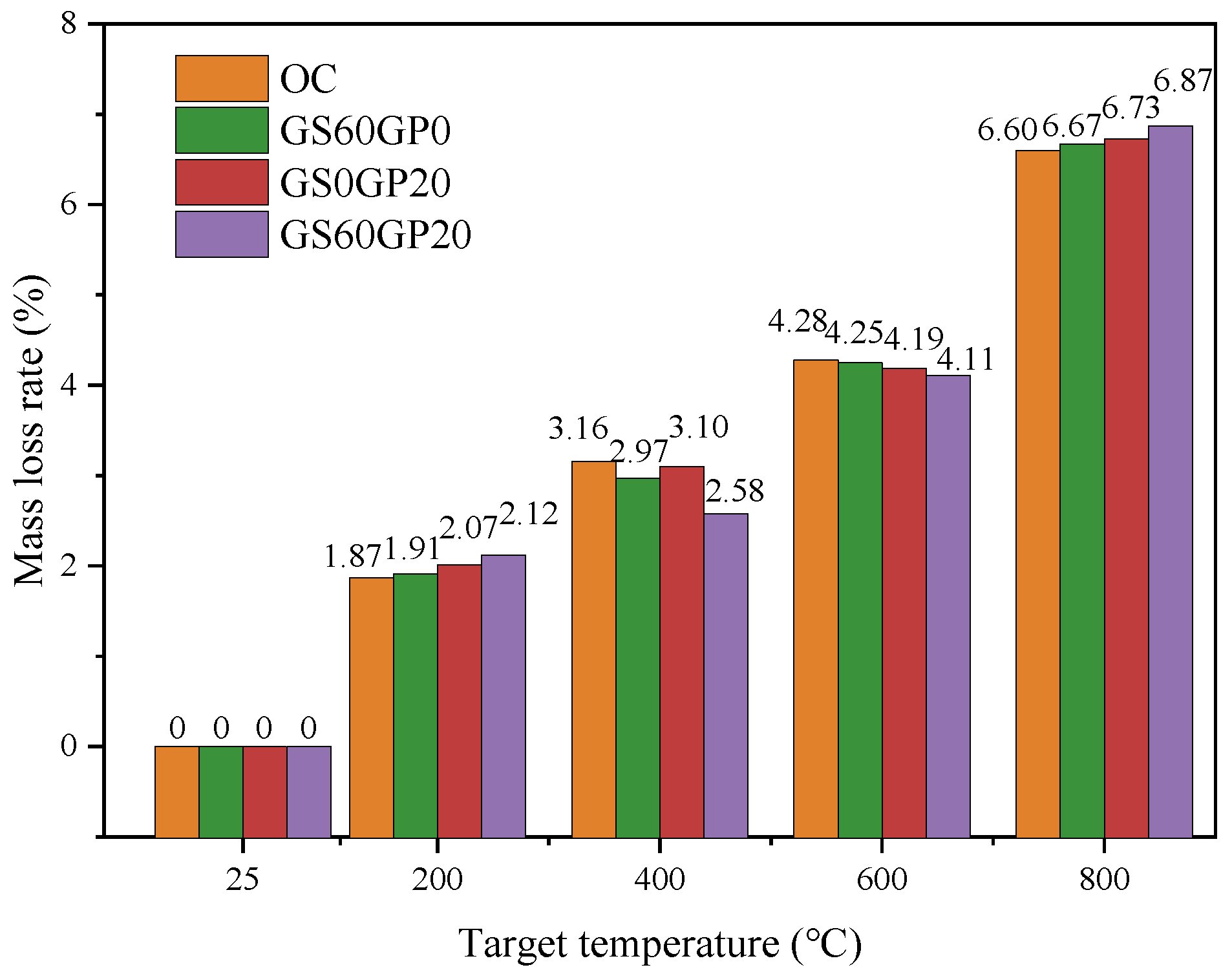
3.3.2. Mass Loss Rate
3.3.3. Failure Modes
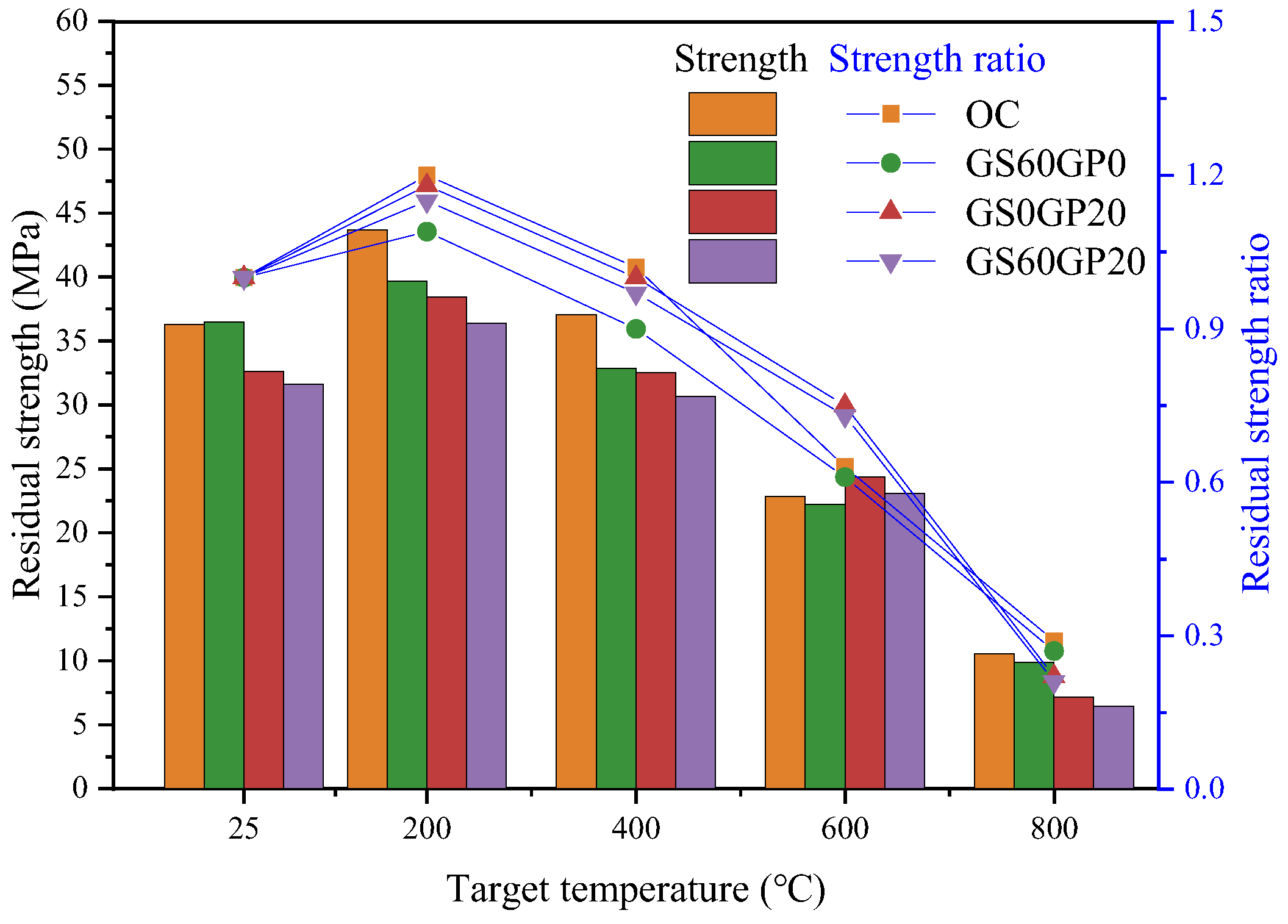
3.3.4. Residual Compressive Strength
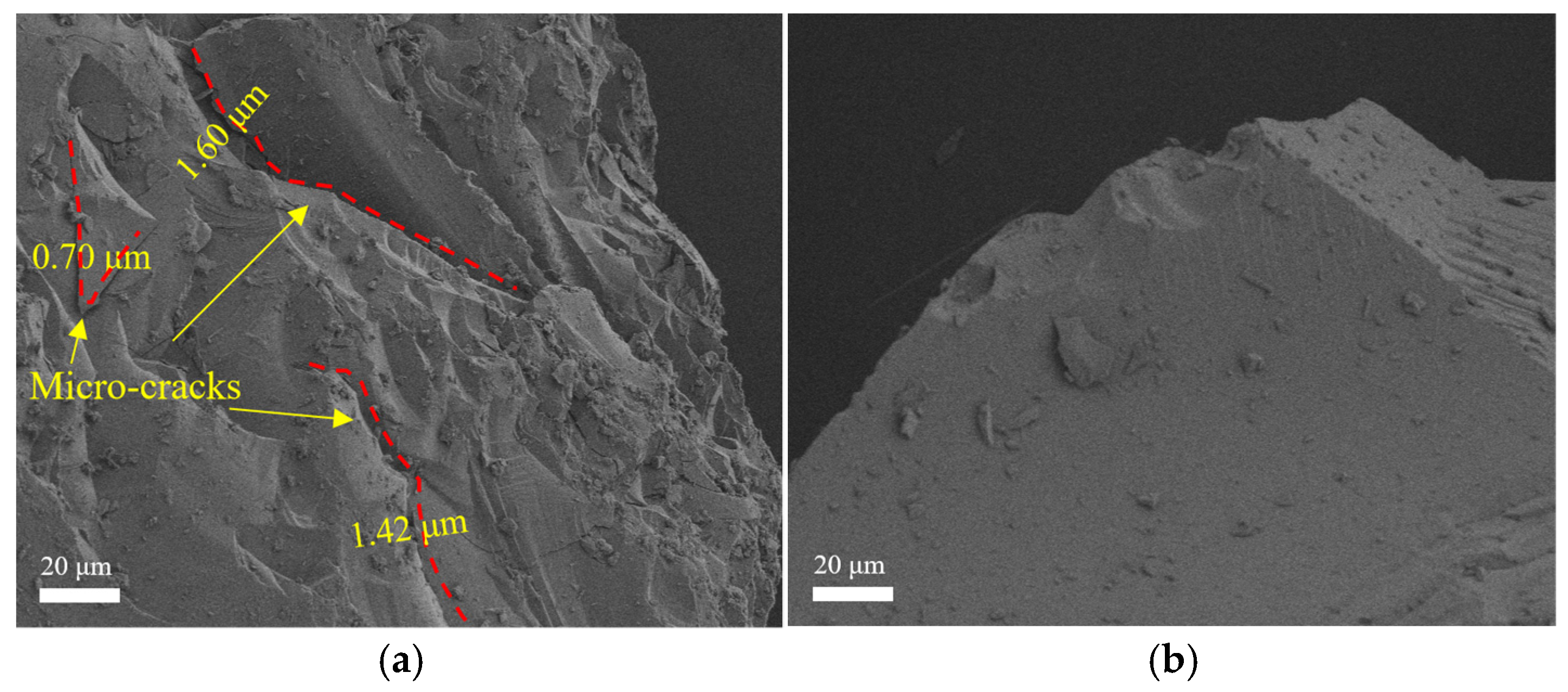
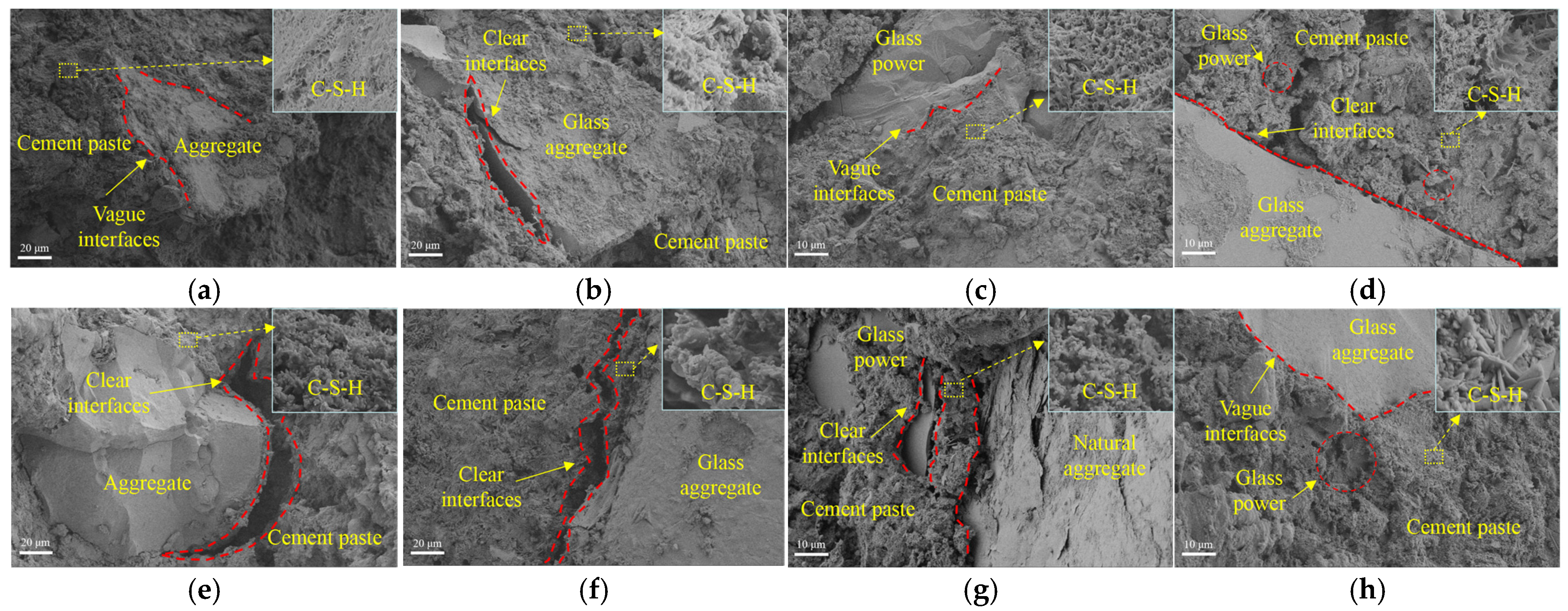
3.3.5. Microstructure Analysis
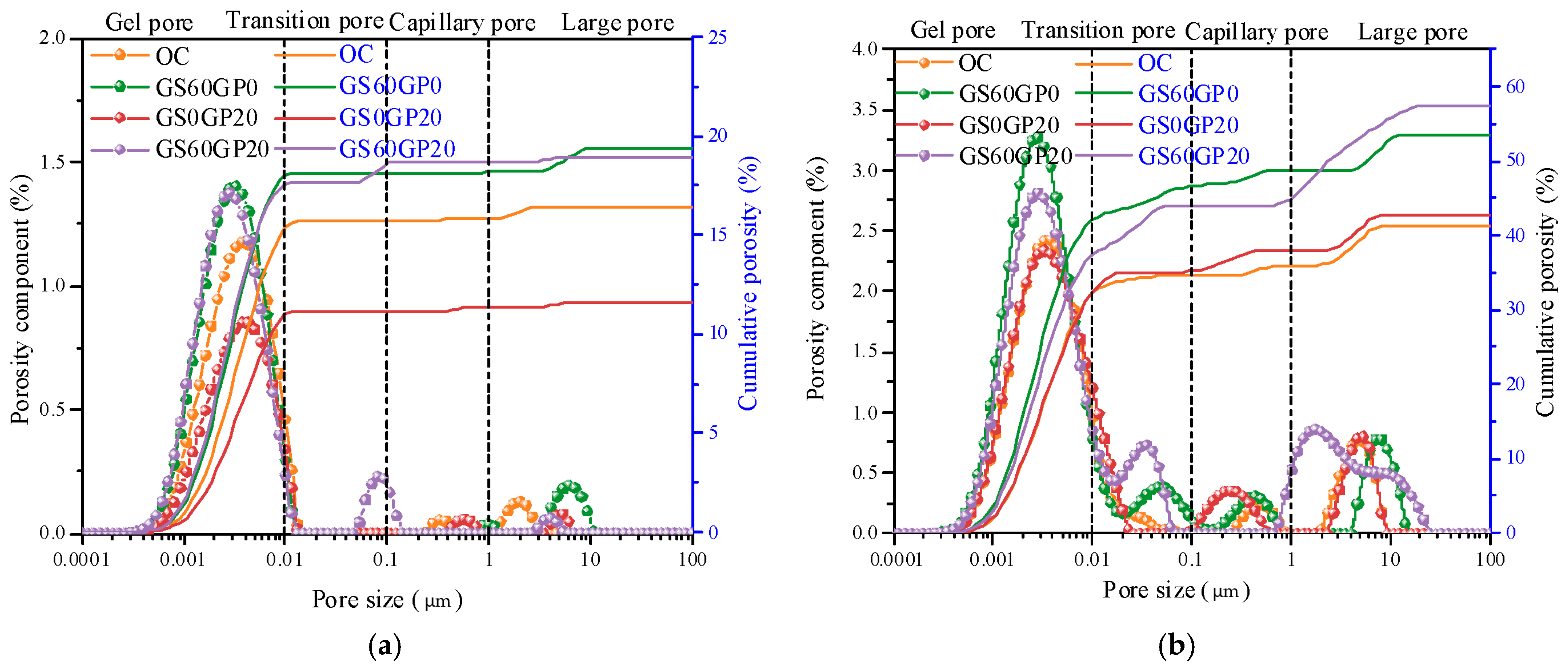
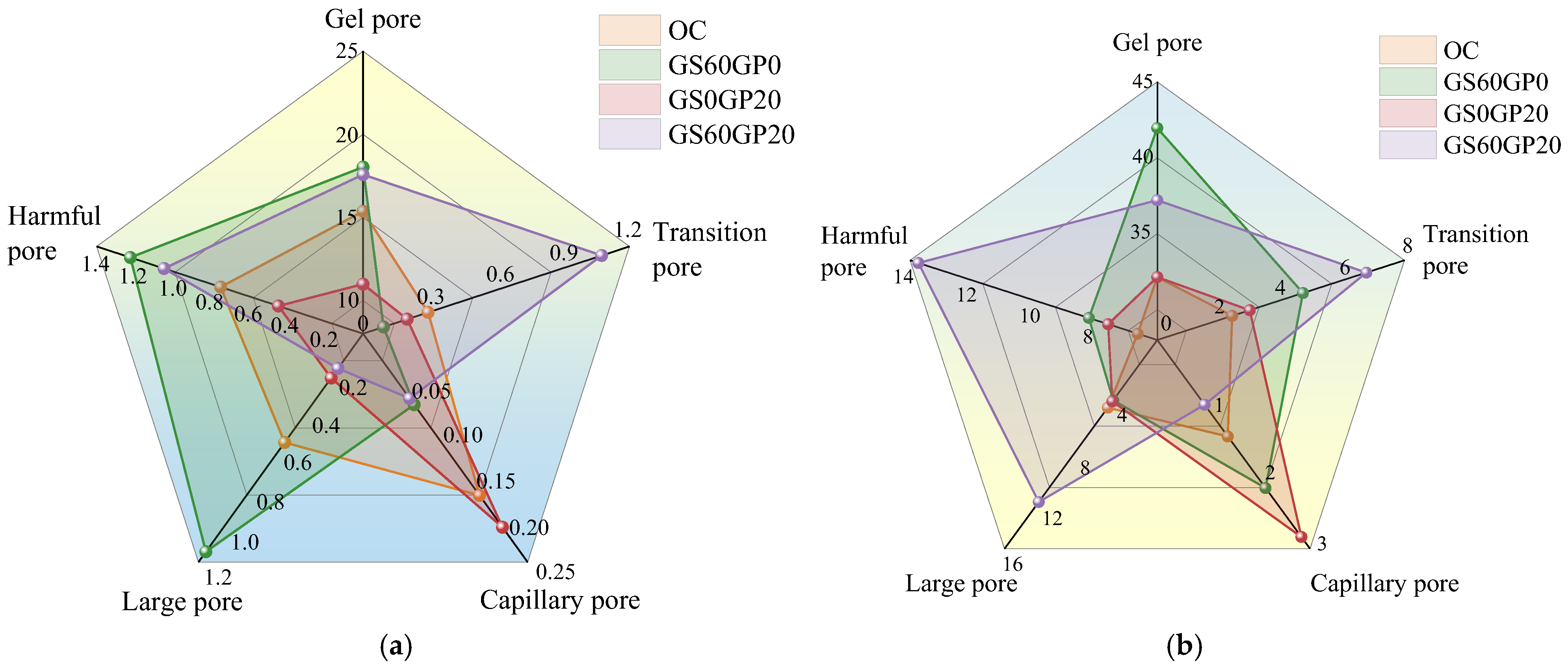
3.3.6. Pore Structure Analysis
4. Conclusions
- The pozzolanic reaction of glass powder significantly relieves the ASR expansion rate in waste glass concrete, which solves the drawbacks of single-blended glass concrete. The glass content can reach 17.79% of the total concrete mass.
- The thermal properties of CGC are closely related to the type of aggregate and the proportion of glass incorporated. Specifically, the thermal conductivity of CGC exhibits a linear correlation with temperature. The specific heat capacity shows three distinct peaks within the temperature range of 180 °C to 800 °C, which are attributed to chemical dehydration, quartz phase transitions, and the decarbonation of CaCO3, respectively.
- The temperature plays a predominant role in influencing the apparent characteristics and failure modes of concrete, while the impact of replacement methods is relatively minor. As the temperature increases, the concrete gradually transitions from light gray to dark gray, gray-yellow, and finally light white. When the temperature reaches above 600 °C, the decomposition of C-S-H in the concrete leads to the formation of pronounced cracks and spalling.
- The residual compressive strength ratios of CGC have a significant advantage at 600 °C (0.73), as the fine glass powder begins to melt and soften at this stage, enhancing its function as a binder. When the temperature reaches 800 °C, the cement matrix becomes brittle, and the decomposition of hydration products dominates concrete strength. At this time, the CGC residual intensity ratio is lower than that of OC.
- The smooth surface of large-size glass aggregates makes the aggregate interface more susceptible to high-temperature decomposition of calcium silicate, leading to interface cracks. The fine-grained glass powder exhibits stronger melting effects, significantly improving the residual strength ratio of concrete after exposure to 600 °C. At 800 °C, the edges of glass aggregates no longer have obvious angular features. However, the melting effect of glass cannot alleviate the decrease in concrete strength caused by low hydration product content.
- The inert glass powder reduces the degree of hydration in the concrete, thereby increasing the number of gel pores in CGC. However, the melting effect of glass can reduce concrete pore size, transforming large pores into capillary pores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.H.; Huang, R.; Wu, J.K.; Yang, C. Waste E-glass particles used in cementitious mixtures. Cem. Concr. Res. 2006, 36, 449–456. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, K. A review on the use of waste glasses in the production of cement and concrete. Resour. Conserv. Recycl. 2007, 52, 234–247. [Google Scholar] [CrossRef]
- Wang, H.Z.; Wen, B.; Xu, P.; Gao, G.Y.; Zhang, L.; Niu, D.T. Effect of CO2 curing on the strength and microstructure of composite waste glass concrete. Constr. Build. Mater. 2025, 463, 140042. [Google Scholar] [CrossRef]
- Ali-Boucetta, T.; Behim, M.; Cassagnabere, F.; Mouret, M.; Ayat, A.; Laifa, W. Durability of self-compacting concrete containing waste bottle glass and granulated slag. Constr. Build. Mater. 2021, 270, 121133. [Google Scholar] [CrossRef]
- de Castro, S.; de Brito, J. Evaluation of the durability of concrete made with crushed glass aggregates. J. Clean. Prod. 2013, 41, 7–14. [Google Scholar] [CrossRef]
- Pauzi, N.N.M.; Hamid, R.; Jamil, M.; Zain, M. The effect of melted-spherical and crushed CRT funnel glass waste as coarse aggregates on concrete performance. J. Build. Eng. 2021, 35, 102035. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C. Studies on expansion properties in mortar containing waste glass and fibers. Cem. Concr. Res. 2004, 34, 1145–1152. [Google Scholar] [CrossRef]
- Yan, R.; Yang, S.; Guo, M.Z.; Poon, C.S. Comparative evaluation of fire resistance of partition wall blocks prepared with waste materials. J. Clean. Prod. 2018, 182, 156–165. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Du, G.; Mao, Z.; Ma, Q.; Luo, Z.; Miao, Y.; Duan, Y. Properties of concrete with waste glass after exposure to elevated temperatures. J. Build. Eng. 2022, 57, 104822. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Using lithium nitrate and pozzolanic glass powder in concrete as ASR suppressors. Cem. Concr. Compos. 2008, 30, 497–505. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Effect of particle size on alkali–silica reaction in recycled glass mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Lee, G.; Poon, C.S.; Wong, Y.L.; Ling, T.C. Effects of recycled fine glass aggregates on the properties of dry–mixed concrete blocks. Constr. Build. Mater. 2013, 38, 638–643. [Google Scholar] [CrossRef]
- Oliveira, L.A.P.; Gomes, J.C.; Santos, P. Mechanical and durability properties of concrete with ground waste glass sand. In Proceedings of the 11DBMC International Conference on Durability of Building Materials and Components, Istanbul, Turkey, 11–14 May 2008. [Google Scholar]
- Esmaeili, J.; AL-Mwanes, A.O. A review: Properties of eco-friendly ultra-high-performance concrete incorporated with waste glass as a partial replacement for cement. Mater. Today Proc. 2021, 42, 1958–1965. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Lee, Y.; You, I.; Banthia, N.; Zi, G. Utilization of liquid crystal display (LCD) glass waste in concrete: A review. Cem. Concr. Compos. 2022, 130, 104542. [Google Scholar] [CrossRef]
- Belouadah, M.; Rahmouni, Z.E.A.; Tebbal, N. Effects of glass powder on the characteristics of concrete subjected to high temperatures. Adv. Concr. Constr. 2018, 6, 311. [Google Scholar]
- Lu, J.X.; Yan, X.; He, P.; Poon, C.S. Sustainable design of pervious concrete using waste glass and recycled concrete aggregate. J. Clean. Prod. 2019, 234, 1102–1112. [Google Scholar] [CrossRef]
- Flores-Ales, V.; Alducin-Ochoa, J.M.; Martín-del-Río, J.J.; Torres-Gonzalez, M.; Jimenez-Bayarri, V. Physical-mechanical behaviour and transformations at high temperature in a cement mortar with waste glass as aggregate. J. Build. Eng. 2020, 29, 101158. [Google Scholar] [CrossRef]
- Lu, J.X.; Poon, C.S. Use of waste glass in alkali activated cement mortar. Constr. Build. Mater. 2018, 160, 399–407. [Google Scholar] [CrossRef]
- Li, B.; Ling, T.C.; Yu, J.G.; Wu, J.; Chen, W. Cement pastes modified with recycled glass and supplementary cementitious materials: Properties at the ambient and high temperatures. J. Clean. Prod. 2019, 241, 118155. [Google Scholar] [CrossRef]
- Ashby, M.F. Materials and the Environment: Eco-Informed Material Choice; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Belebchouche, C.; Moussaceb, K.; Bensebti, S.E.; Aït-Mokhtar, A.; Hammoudi, A.; Czarnecki, S. Mechanical and microstructural properties of ordinary concrete with high additions of crushed glass. Materials 2021, 14, 1872. [Google Scholar] [CrossRef]
- ASTM C 1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2001.
- GB/T50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Standard Press: Beijing, China, 2019.
- GB/T 10297-2015; Test Method for Thermal Conductivity of Nonmetal Solid 335 Materials-Hot-Wire Method. China Standard Press: Beijing, China, 2015.
- ASTM E 1269-11; Standard Test Method for Determining Specifc Heat Capacity by Differential 321 Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2011.
- GB 50010-2020; Code for Design of Concrete Structures. China Standard Press: Beijing, China, 2020.
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Properties of concrete contains mixed colour waste recycled glass as sand and cement replacement. Constr. Build. Mater. 2008, 22, 713–720. [Google Scholar] [CrossRef]
- Sharifi, Y.; Afshoon, I.; Firoozjaei, Z.; Momeni, A. Utilization of waste glass micro-particles in producing self-consolidating concrete mixtures. Int. J. Concr. Struct. Mater. 2016, 10, 337–353. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete. Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Durgun, M.Y.; Sevinç, A.H. High temperature resistance of concretes with GGBFS, waste glass powder, and colemanite ore wastes after different cooling conditions. Constr. Build. Mater. 2019, 196, 66–81. [Google Scholar] [CrossRef]
- Patil, S.S.; Patil, Y.D. Effect of high temperature on the strength of mortar containing glass as a supplementary cementitious material: An experimental study. Mater. Today Proc. 2022, 65, 1511–1515. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C.; Kim, J.H. Studies on mechanical properties of concrete containing waste glass aggregate. Cem. Concr. Res. 2004, 34, 2181–2189. [Google Scholar] [CrossRef]
- ASTM C1567-11; Standard Test Method for Determining the Potential Alkali Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar Bar Method). ASTM International: West Conshohocken, PA, USA, 2011.
- ASCE. Structural Fire Protection; American Society of Civil Engineers: New York, NY, USA, 1992. [Google Scholar]
- Kodur, V.; Khaliq, W. Effect of temperature on thermal properties of different types of high-strength concrete. J. Mater. Civ. Eng. 2011, 23, 793–801. [Google Scholar] [CrossRef]
- Razafinjato, R.N.; Beaucour, A.L.; Hebert, R.L.; Ledesert, B.; Bodet, R.; Noumowe, A. High temperature behaviour of a wide petrographic range of siliceous and calcareous aggregates for concretes. Constr. Build. Mater. 2016, 123, 261–273. [Google Scholar] [CrossRef]
- EN 1992-1-2:2004; Design of Concrete Structures-Part 1-2: General Rules-Structural Fire Design. European Standard: Pilsen, Czech Republic, 2004.
- DBJ/T 15-81-2022; Code for Fire Resistance Design of Concrete Structures in Buildings. China Architecture and Building Press: Beijing, China, 2022.
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Sabeur, H.; Colina, H. Effect of heating–cooling cycles on transient creep strain of high performance, high strength and ordinary concrete under service and accidental conditions. Mater. Struct. 2015, 48, 1561–1579. [Google Scholar] [CrossRef]
- Boukhelkhal, A. Assessment of the behavior under conventional and high temperatures of eco-friendly self-compacting mortar containing glass and ceramic powders. Arab. J. Sci. Eng. 2022, 47, 4821–4831. [Google Scholar] [CrossRef]
- Lu, J.X.; Zhou, Y.; He, P.; Wang, S.; Shen, P.; Poon, C.S. Sustainable reuse of waste glass and incinerated sewage sludge ash in insulating building products: Functional and durability assessment. J. Clean. Prod. 2019, 236, 117635. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Z.; Peng, G.; Chen, L.; Huang, D.-G.; Li, N. Pore structure related triaxial mechanical response and strength criterion of basalt fibre-reinforced coral aggregate concrete. J. Cent. South Univ. 2023, 30, 1325–1344. [Google Scholar] [CrossRef]
- Huang, J.; Niu, D.; Wu, H.; Lv, Y.; Fu, Q. The industrial SO2-induced corrosion: Investigation of the corrosion onset. Cem. Concr. Compos. 2024, 150, 105535. [Google Scholar] [CrossRef]
- Wu, Z.; Lian, H. High Performance Concrete; Chinese Railway Press: Beijing, China, 1999; pp. 22–25. [Google Scholar]




| Material | Constituents (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | MgO | SO3 | Na2O | K2O | |
| Glass | 72.86 | 11.58 | 1.84 | 0.84 | 1.92 | 0.34 | 10.10 | 0.45 |
| Material | Apparent Density (kg/m3) | Fineness Modulus | Water Absorption (%) |
|---|---|---|---|
| Natural river sand | 2530 | 2.55 | 2.01 |
| Glass sand | 2410 | 2.35 | 0.32 |
| Type of Concrete | Water | Cement | Glass Powder | Sand | Glass Sand | Coarse Aggregate |
|---|---|---|---|---|---|---|
| OC | 175 | 343 | 0 | 621 | 0 | 1261 |
| GS0GP20 | 175 | 274.4 | 68.6 | 621 | 0 | 1261 |
| GS10GP20 | 175 | 274.4 | 68.6 | 558.9 | 59.2 | 1261 |
| GS20GP20 | 175 | 274.4 | 68.6 | 496.8 | 118.4 | 1261 |
| GS30GP20 | 175 | 274.4 | 68.6 | 434.7 | 177.6 | 1261 |
| GS40GP20 | 175 | 274.4 | 68.6 | 372.6 | 236.8 | 1261 |
| GS50GP20 | 175 | 274.4 | 68.6 | 310.5 | 296 | 1261 |
| GS60GP20 | 175 | 274.4 | 68.6 | 248.4 | 355.2 | 1261 |
| GS70GP20 | 175 | 274.4 | 68.6 | 186.3 | 414.4 | 1261 |
| GS80GP20 | 175 | 274.4 | 68.6 | 124.2 | 473.6 | 1261 |
| GS90GP20 | 175 | 274.4 | 68.6 | 62.1 | 532.8 | 1261 |
| GS100GP20 | 175 | 274.4 | 68.6 | 0 | 592 | 1261 |
| GS60GP0 | 175 | 343 | 0 | 248.4 | 355.2 | 1261 |
| GS60GP10 | 175 | 308.7 | 34.3 | 248.4 | 355.2 | 1261 |
| GS60GP30 | 175 | 240.1 | 102.9 | 248.4 | 355.2 | 1261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, B.; Wang, H.; Gao, G.; Zhang, L.; Yu, Z.; Wang, Z. The Synergistic Utilization of Glass Aggregates and Glass Powder on the Thermal and Mechanical Properties of Concrete. Materials 2025, 18, 2405. https://doi.org/10.3390/ma18102405
Wen B, Wang H, Gao G, Zhang L, Yu Z, Wang Z. The Synergistic Utilization of Glass Aggregates and Glass Powder on the Thermal and Mechanical Properties of Concrete. Materials. 2025; 18(10):2405. https://doi.org/10.3390/ma18102405
Chicago/Turabian StyleWen, Bo, Huaizheng Wang, Guanyi Gao, Lu Zhang, Zhengyao Yu, and Zhihao Wang. 2025. "The Synergistic Utilization of Glass Aggregates and Glass Powder on the Thermal and Mechanical Properties of Concrete" Materials 18, no. 10: 2405. https://doi.org/10.3390/ma18102405
APA StyleWen, B., Wang, H., Gao, G., Zhang, L., Yu, Z., & Wang, Z. (2025). The Synergistic Utilization of Glass Aggregates and Glass Powder on the Thermal and Mechanical Properties of Concrete. Materials, 18(10), 2405. https://doi.org/10.3390/ma18102405






