Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
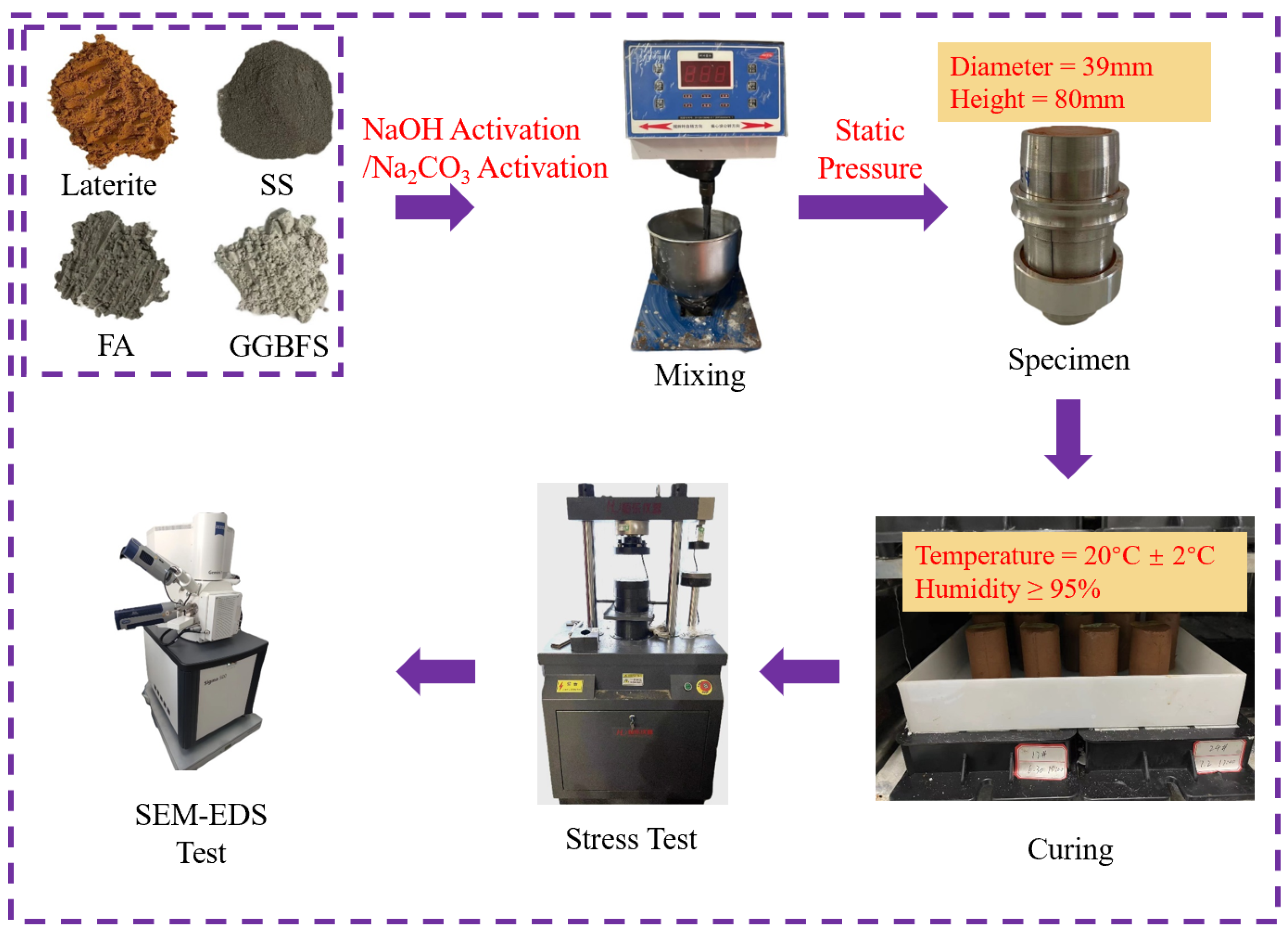
2.1. Materials
2.2. Specimen Preparation
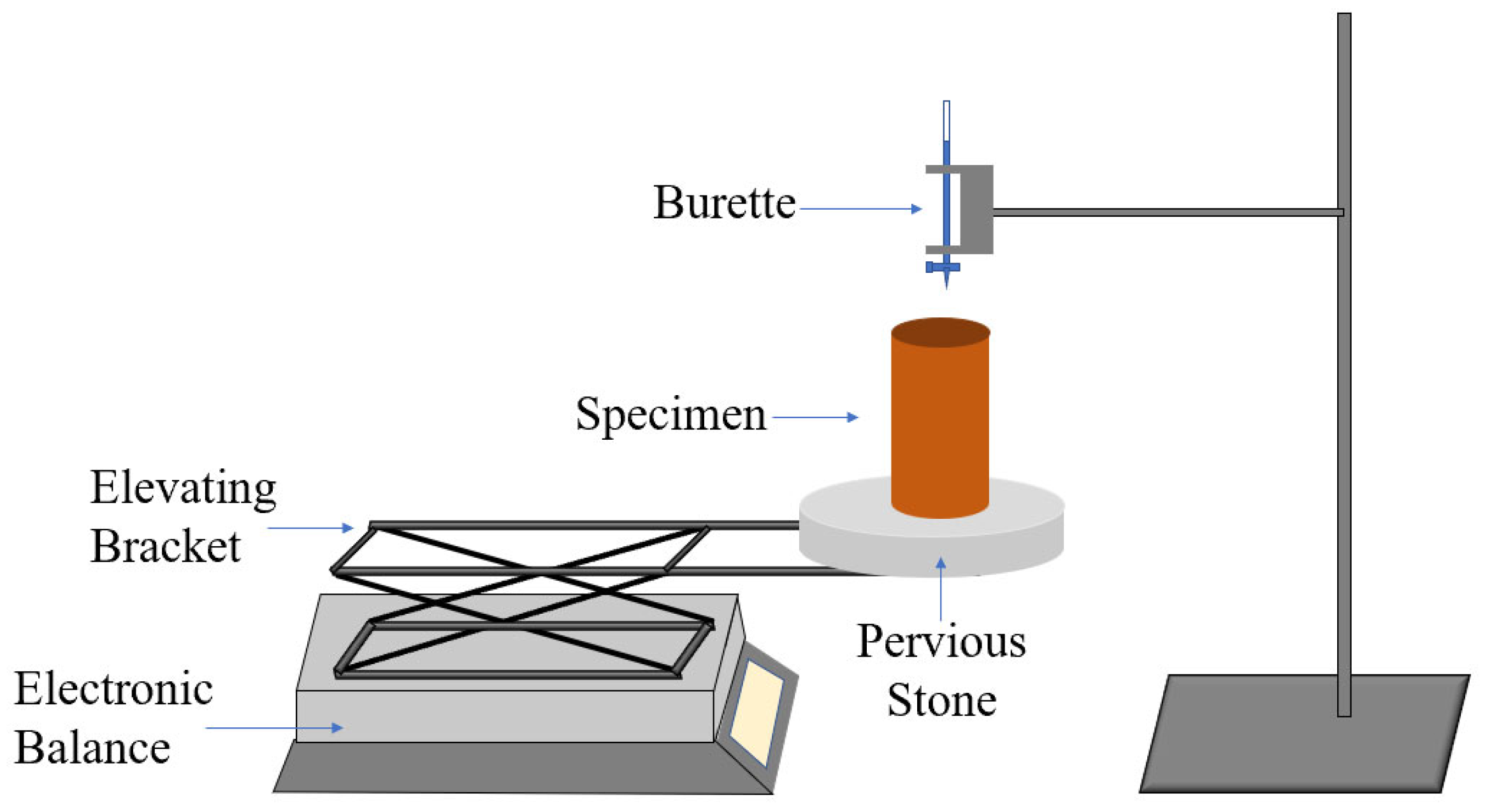
2.3. Test Methods
3. Results and Analysis
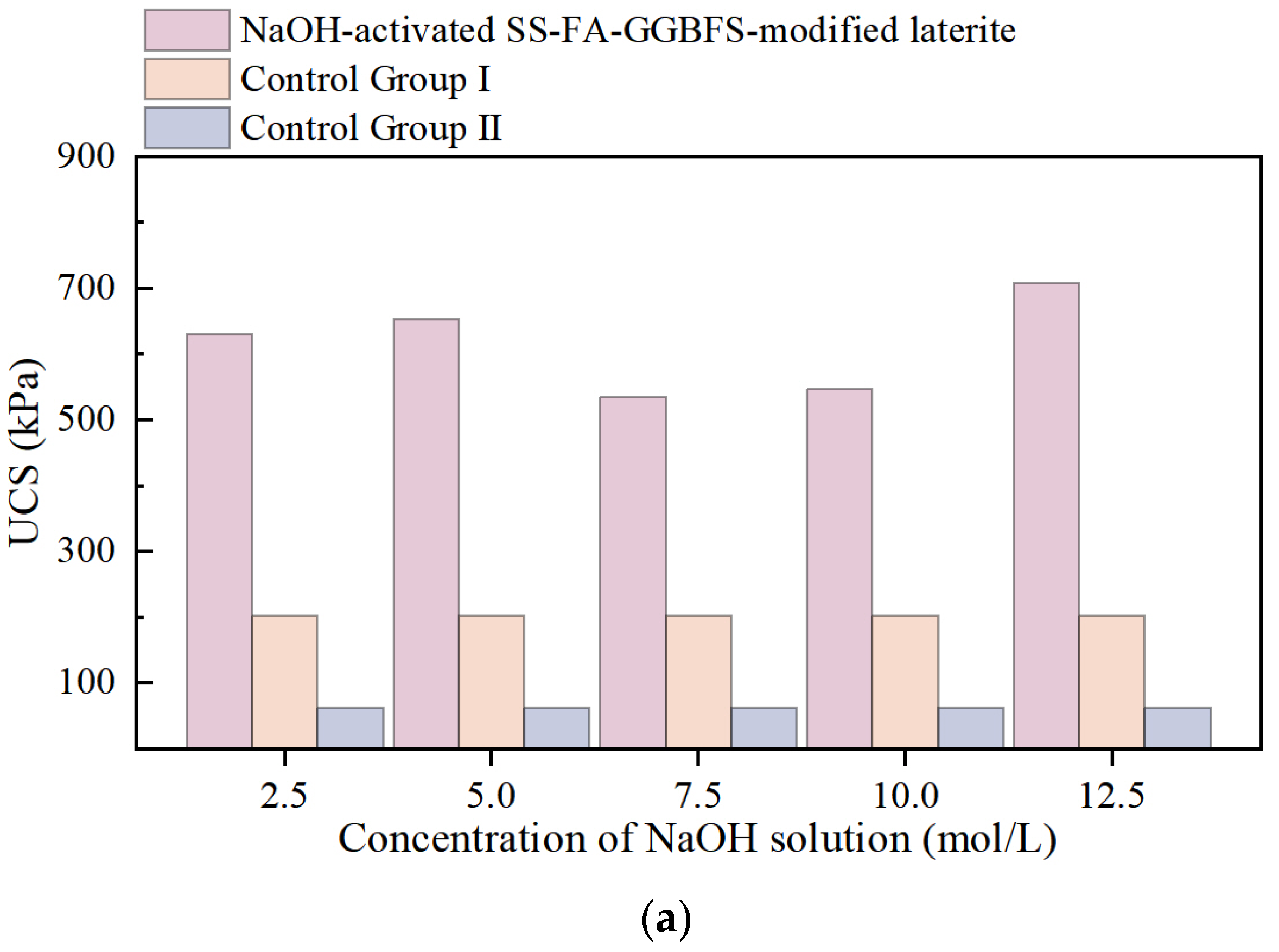
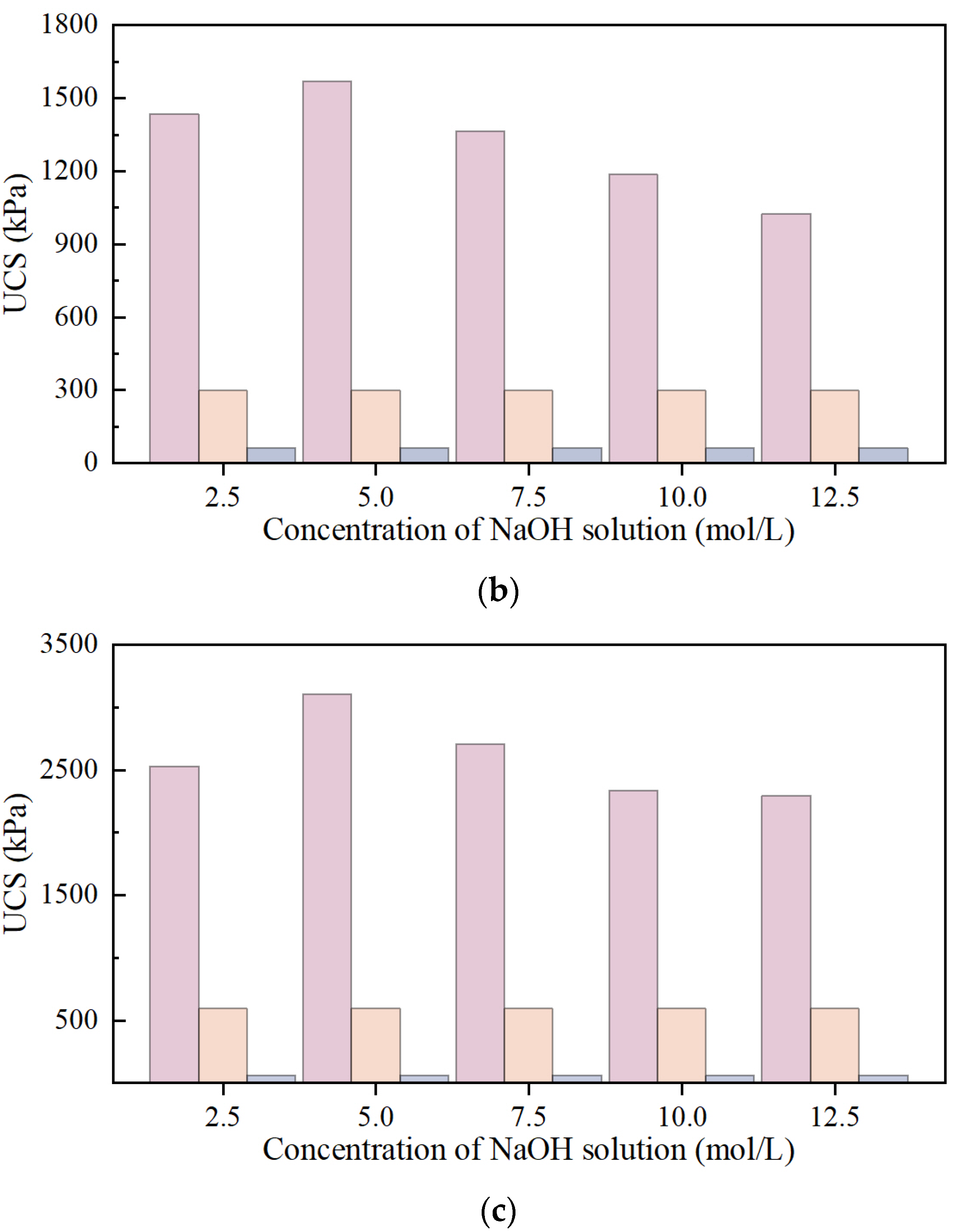
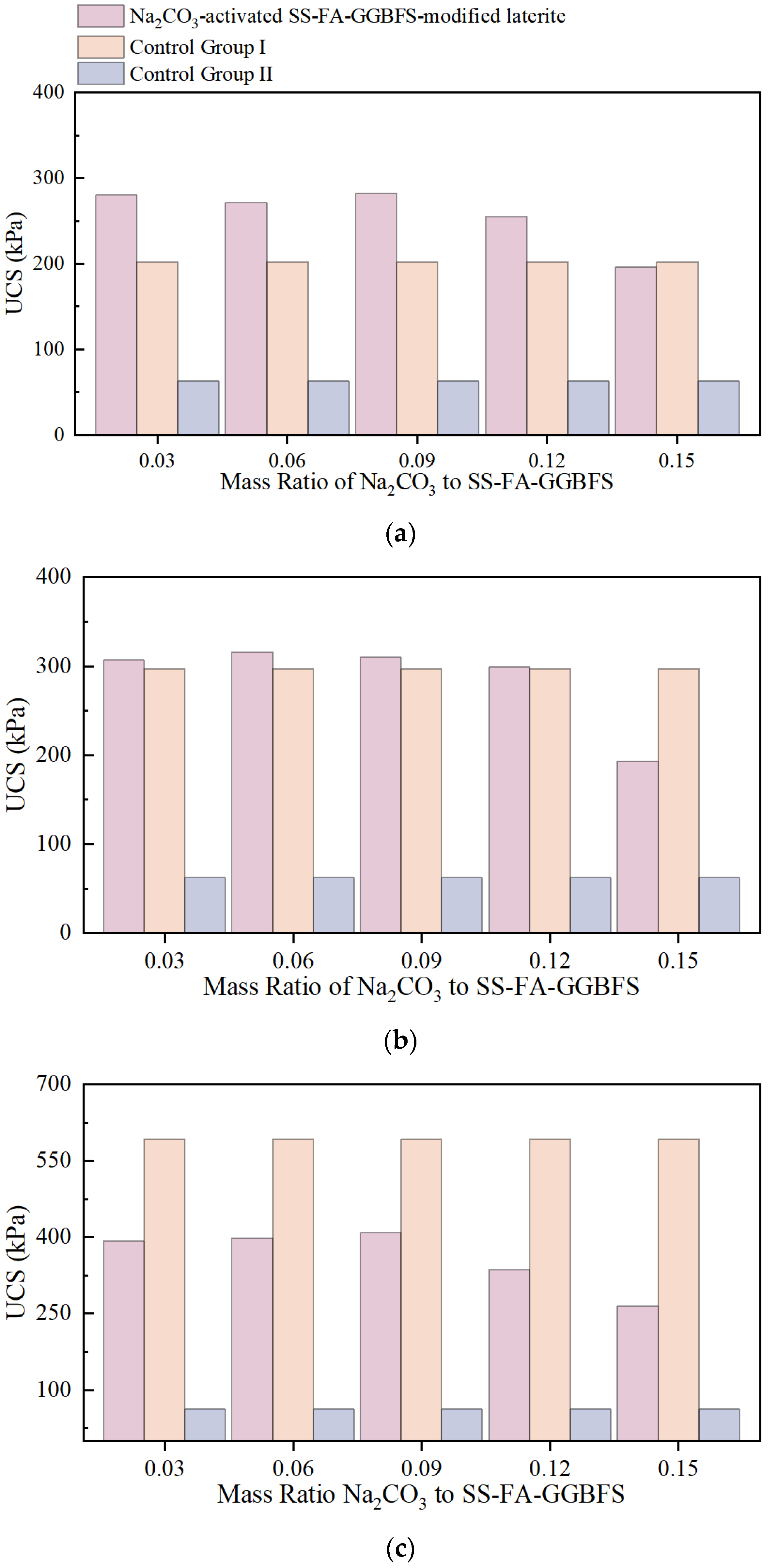
UCS of NaOH-Activated SS-FA-GGBFS-Modified Lateritic Clay Specimens and Na2CO3-Activated SS-FA-GGBFS-Modified Lateritic Clay Specimens
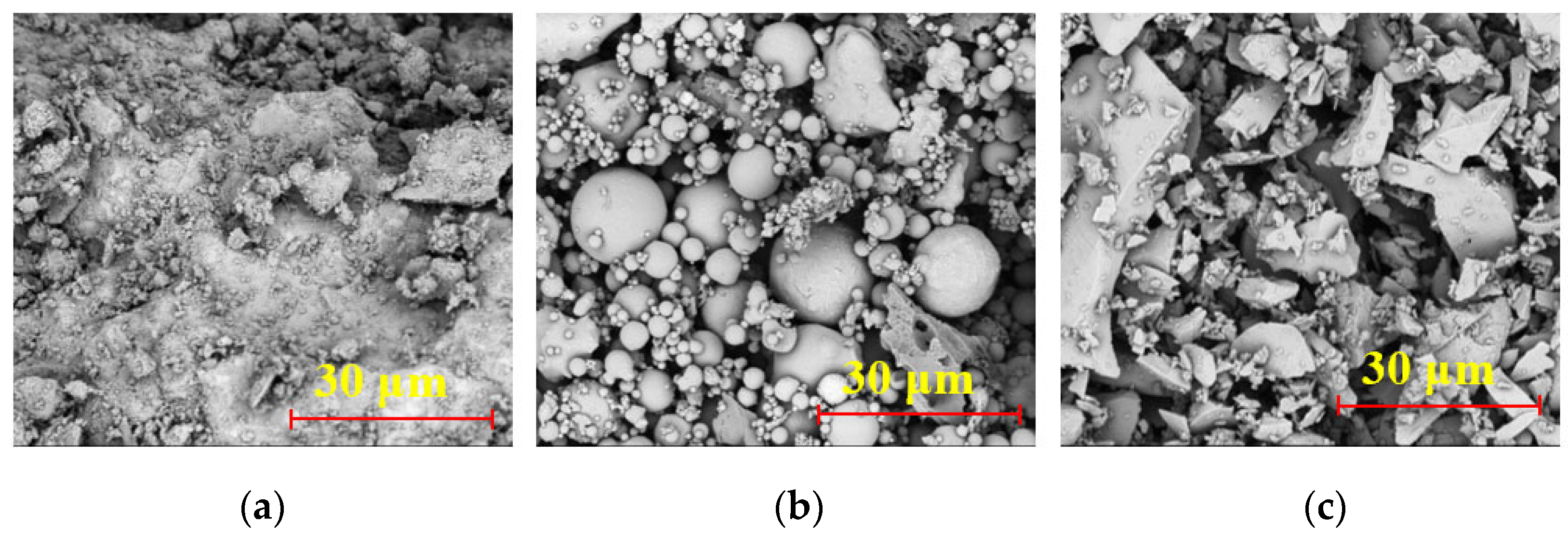
4. Microstructural and Elemental Characterization
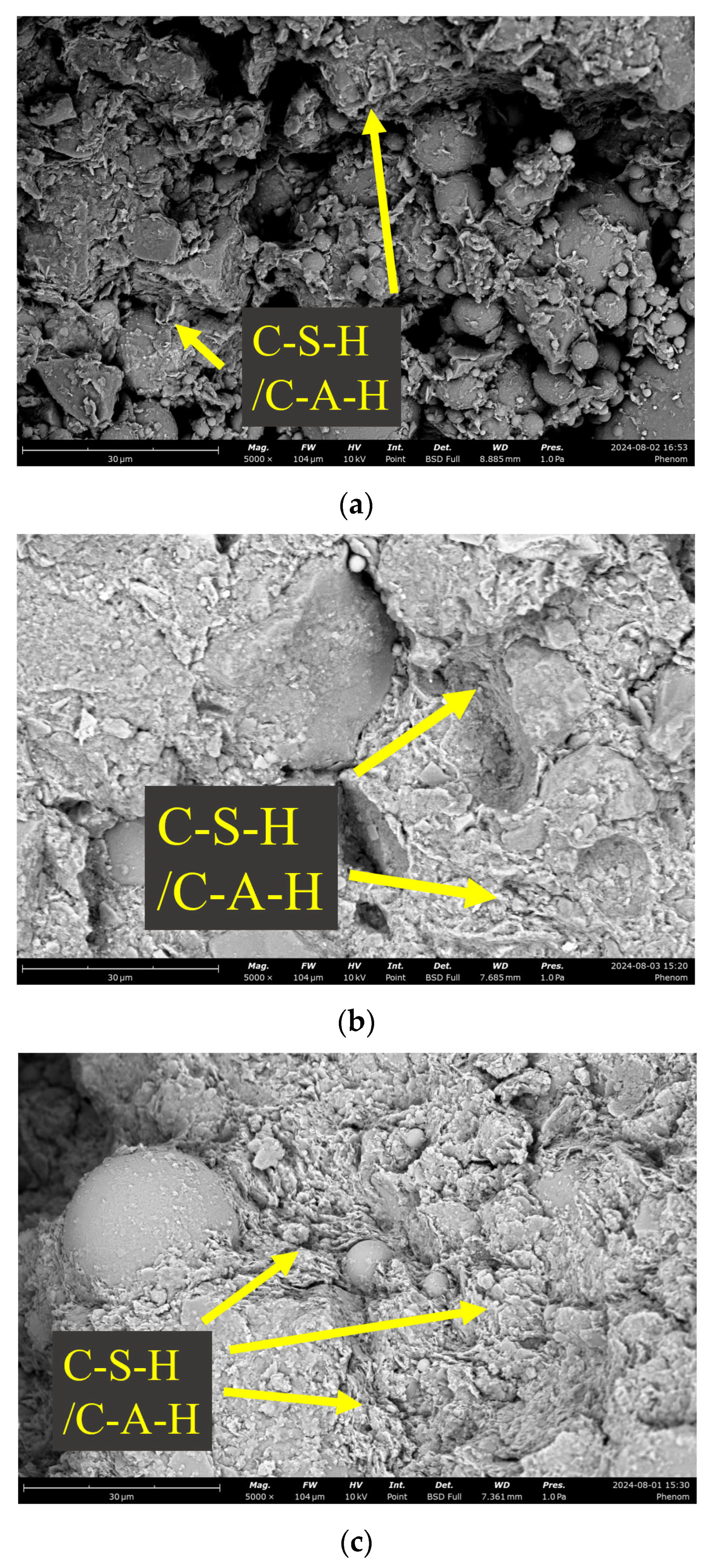
4.1. Comparative Analysis of Microstructure
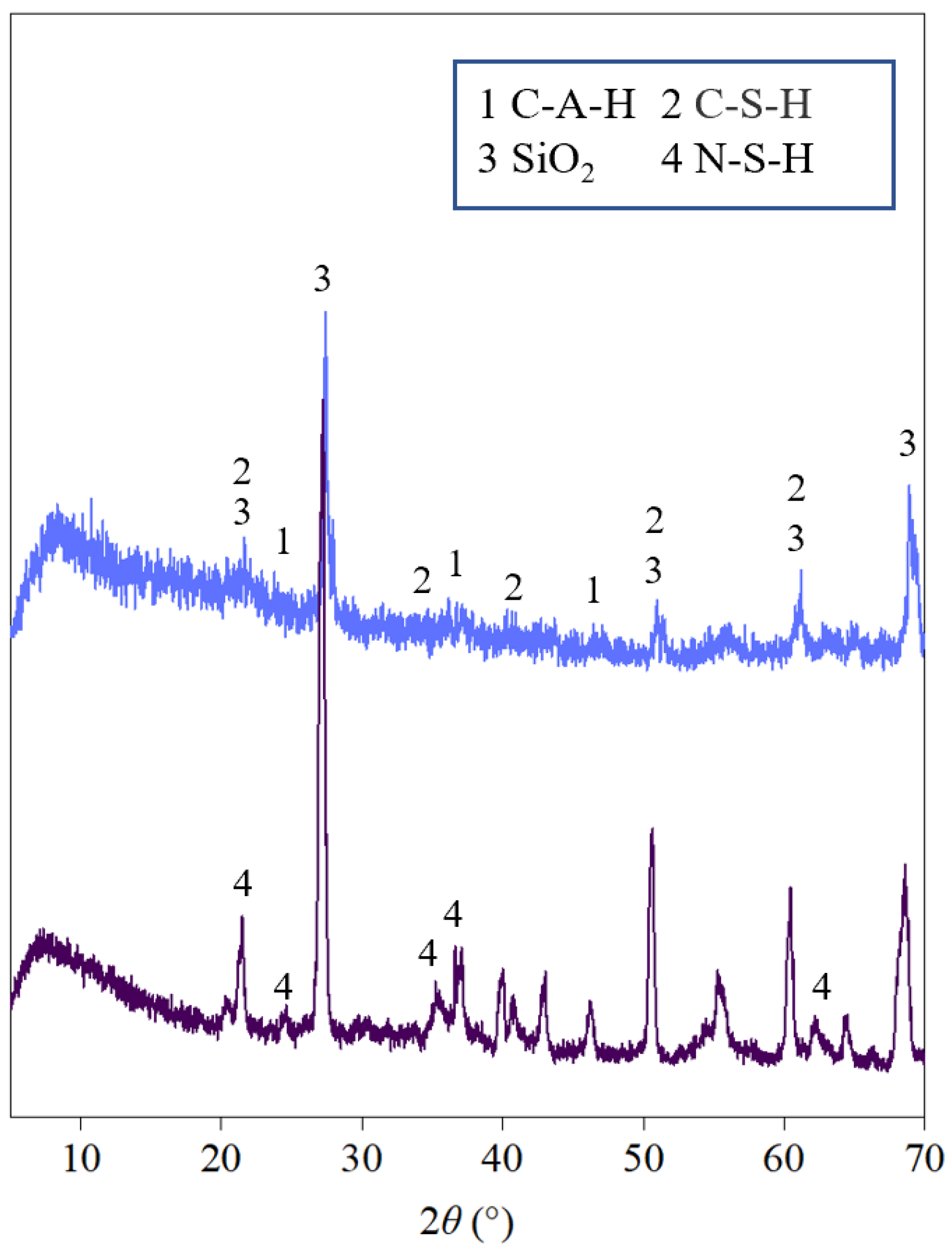
4.2. Elemental Characterization
4.3. Modification Mechanisms Solid Waste Composite and Alkali-Activated Solid Waste Composite Modified Lateritic Clay
5. Conclusions
- (1)
- The strength of NaOH-activated SS-FA-GGBFS-modified lateritic clay generally increases with curing age. For NaOH concentrations ranging from 2.5 to 12.5 mol/L, the strength first increases and then decreases with concentration, peaking at 5 mol/L. After 7+ days of curing, the 5 mol/L modified clay shows a strength several dozen times higher than unmodified clay under rainfall-induced water content changes, meeting the requirement for maintaining strength in precipitation.
- (2)
- The strength of lateritic clay modified by Na2CO3-activated SS-FA-GGBFS generally decreases with the increase in curing age. When the mass ratio of Na2CO3 to SS-FA-GGBFS ranges from 0.03 to 0.09, Na2CO3 can enhance the strength of the modified lateritic clay to a certain extent at the curing age of 3 days. However, with the extension of curing time, Na2CO3 has a negative effect on the strength of the modified lateritic clay.
- (3)
- The NaOH solution can dissolve the dense glassy phase on the surface of SS-FA-GGBFS, which is predominantly composed of silicon and aluminum oxides, thereby accelerating the dissolution and release of elements such as calcium, aluminum, and silicon from SS-FA-GGBFS.
- (4)
- Due to its weaker alkalinity, Na2CO3 has a less effective dissolution effect on the glassy phase of the SS-FA-GGBFS surface compared to NaOH. It primarily relies on the pore-filling effect of calcium carbonate crystals formed by the reaction between carbonate ions and free calcium oxide in SS-FA-GGBFS to improve the strength of the modified soil. However, over time, the continuous growth of calcium carbonate crystals generates mechanical stresses that disrupt the integrity of the modified soil, thereby reducing its strength.
- (5)
- The addition of NaOH solution accelerates the reaction of SS-FA-GGBFS blends, promoting the formation of C-S-H and C-A-H gels, which are primarily responsible for the strength enhancement of modified lateritic clay. However, an optimal NaOH concentration is critical; too high a concentration of NaOH solution impedes the dissolution efficiency of solid waste precursors and induces a phase transition from calcium-based (C-S-H/C-A-H) to sodium-based (N-S-H/N-A-H) hydration products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Q.-F.; Yu, H.-C.; Zeng, L.; Huang, Y.-X. Characterization of water retention behavior of cracked compacted lateritic soil exposed to wet-dry cycles. Bull. Eng. Geol. Environ. 2023, 82, 61. [Google Scholar] [CrossRef]
- Ma, H.; Zhuang, Y.; Chen, L.; Lv, M.; Xu, S. Experiments and Modeling of the Attenuation of the Dynamic Elastic Modulus of Saturated Red Clay under Cyclic Loading. Sustainability 2022, 15, 26. [Google Scholar] [CrossRef]
- Xue, K.; Ajmera, B.; Tiwari, B.; Hu, Y. Effect of long duration rainstorm on stability of Red-clay slopes. Geoenvironmental Disasters 2016, 3, 12. [Google Scholar] [CrossRef]
- Wu, N.; Ding, X.; Wen, Z.; Chen, G.; Meng, Z.; Lin, L.; Min, J. Contrasting Frontal and Warm-Sector Heavy Rainfalls over South China During the Early-Summer Rainy Season. Atmos. Res. 2020, 235, 104693. [Google Scholar] [CrossRef]
- Deng, Y.; Shao, Z.; Dang, C.; Huang, X.; Wu, W.; Zhuang, Q.; Ding, Q. Assessing Urban Wetlands Dynamics in Wuhan and Nanchang, China. Sci. Total Environ. 2023, 901, 165777. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, G.; Long, Z.; Yang, D.; Xu, Y. Dynamic characteristics of over consolidated remolded red clay in Southwest China: An experimental study. Bull. Eng. Geol. Environ. 2022, 81, 176. [Google Scholar] [CrossRef]
- Yang, L.; Kaisheng, C.; Mengfei, L.; Yingchao, W. Study on failure of red clay slopes with different gradients under dry and wet cycles. Bull. Eng. Geol. Environ. 2020, 79, 4609–4624. [Google Scholar] [CrossRef]
- Korzeniowski, W.; Poborska-Młynarska, K.; Skrzypkowski, K. The Idea of Recovery for Municipal Solid Waste Incineration (MSWI) Residues in Kłodawa Salt Mine S.A. Using Self-Solidifying Mixture Excavation Fills. Arch. Min. Sci. 2018, 63, 553–565. [Google Scholar]
- Zhang, H.; Zhu, W.; Bao, J.; Wei, D.; Shu, S. Pilot Study on in-Situ Solidification/Stabilization Technology of Sewage Sludge. Environ. Eng. 2014, 32, 101–104. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Cheng, L.-X. Study on general industrial solid waste and carbon reduction in China: Coupling coordination model, life cycle assessment and environmental safety control. Sustain. Chem. Pharm. 2024, 39, 101557. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, S.; Guo, D.; Su, L.; Xu, H. Synergetic Benefits of Pollution and Carbon Reduction from Fly Ash Resource Utilization—Based on the Life Cycle Perspective. Sci. Total Environ. 2023, 903, 166197. [Google Scholar] [CrossRef] [PubMed]
- Okoro, W.; Oyebisi, S. Mechanical and Durability Assessments of Steel Slag-Seashell Powder-Based Geopolymer Concrete. Heliyon 2023, 9, e13188. [Google Scholar] [CrossRef] [PubMed]
- Naresh, B.; Saravanan, M. Experimental Study of Replacement of Cement with Ground Granulated Blast Furnace Slag. Mater. Today Proc. 2022, 62, 3493–3496. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Chen, D.; An, S. Mechanical properties and microstructure evolution of alkali-activated GGBS-fly ash-steel slag ternary cements. Constr. Build. Mater. 2024, 444, 137727. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, W.; Zhao, X.; Bu, M.; Yuan, Q.; Niu, D. The microstructure and durability of fly ash-based geopolymer concrete: A review. Ceram. Int. 2021, 47, 29550–29566. [Google Scholar] [CrossRef]
- Wan, F.; Guo, Y.; Ge, K.; Zhuang, S.; Elghazouli, A.Y. Mechanical properties of sustainable engineered geopolymer composites with sodium carbonate activators. J. Build. Eng. 2025, 105, 112486. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, W.; Ye, J.; Chen, J.; Yan, F.; Ren, X.; Li, J. Mechanism of Na2CO3 on early properties of red mud-based alkali-activated cementitious materials. Constr. Build. Mater. 2024, 449, 138369. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.-W.; Li, L.; Wang, J.-X.; Shao, N.-N.; Wang, D.-M. Microstructure and phase evolution of alkali-activated steel slag during early age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Richardson, I.; Brough, A.; Groves, G.; Dobson, C. The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem. Concr. Res. 1994, 24, 813–829. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Zhang, X.; Li, M. Mechanical Properties and Microscopic Study of Steel Slag–Fly Ash-Solidified Loess under Alkaline Conditions. Appl. Sci. 2023, 13, 8737. [Google Scholar] [CrossRef]
- Ren, J.; Chen, H.; Sun, T.; Song, H.; Wang, M. Mechanical Properties of Combined FA/GGBFS Geopolymer Concrete Beams after Elevated Temperature Exposure. Adv. Mater. Sci. Eng. 2017, 2017, 6854043. [Google Scholar] [CrossRef]
- Qi, W.; Duan, G.; Han, Y.; Zhao, Q.; Huang, Y.; Zhu, W.; Pang, H.; Zhang, J. Comparison of mechanical properties and microstructure of GGBS-based cementitious materials activated by different combined alkaline wastes. Constr. Build. Mater. 2024, 422, 135784. [Google Scholar] [CrossRef]
- Nath, S.; Kumar, S. Role of alkali concentration on reaction kinetics of fly ash geopolymerization. J. Non-Cryst. Solids 2019, 505, 241–251. [Google Scholar] [CrossRef]
- Jiang, M.; Li, F.; Zhou, L.; Ning, J.; Zhang, Z. Effects of Sodium Carbonate, Sodium Hydroxide and Water Glass Composite Activation on Properties of Geopolymer Cementitious Materials. Bull. Chin. Ceram. Soc. 2024, 43, 929. [Google Scholar] [CrossRef]
- Zhan, M.-J.; Xia, L.; Zhan, L.; Wang, Y. Recognition of Changes in Air and Soil Temperatures at a Station Typical of China’s Subtropical Monsoon Region (1961–2018). Adv. Meteorol. 2019, 2019, 6927045. [Google Scholar] [CrossRef]
- ASTM D2487-11; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D 4318; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4832; Standard Test Method for Preparation and Testing of Controlled Low Strength Material (CLSM) Test Cylinders. ASTM International: West Conshohocken, PA, USA, 2016.
- Mohamed, O.A.; Najm, O.; Zuaiter, H.A.; Saleem, S.K.; Ivak, S.; Al-Aribe, K. Effect of activator concentration on setting time, workability and compressive strength of sustainable concrete with alkali-activated slag binder. Mater. Today Proc. 2024, 4, 103. [Google Scholar] [CrossRef]
- Risdanareni, P.; Schollbach, K.; Wang, J.; De Belie, N. The effect of NaOH concentration on the mechanical and physical properties of alkali activated fly ash-based artificial lightweight aggregate. Constr. Build. Mater. 2020, 259, 119832. [Google Scholar] [CrossRef]
- Wang, Q.; Kang, S.; Wu, L.; Tang, N.; Zhang, Q. Molecular Simulation of N-A-S-H and C-A-S-H in Geopolymer Cementitious System. J. Build. Mater. 2020, 23, 184–191. [Google Scholar] [CrossRef]





| Oxides | Composition (wt.%) | |||
|---|---|---|---|---|
| Lateritic Clay | SS | FA | GGBS | |
| Calcium oxide | 0.74 | 41.22 | 3.60 | 59.31 |
| Silicon dioxide | 52.99 | 6.32 | 35.71 | 16.31 |
| Aluminum oxide | 18.78 | 2.88 | 37.34 | 10.24 |
| Ferric oxide | 13.48 | 22.44 | 9.86 | 1.46 |
| Magnesium oxide | 1.04 | 5.68 | 0.46 | 5.63 |
| Sulphate oxide | 0 | 0.59 | - | - |
| Potassium oxide | 5.06 | 0.33 | 1.66 | 0.57 |
| Manganese oxide | 0.17 | 3.80 | 0.09 | 1.00 |
| LOI (Loss on ignition) | 4.97 | 12.77 | 2.05 | - |
| Proportioning Scheme | Type of Activator | Activator Form | Solution Concentration (mol/L) | Mass of Activator Added per 100 g SS-FA-GGBFS (g) |
|---|---|---|---|---|
| 1 | NaOH | Solution | 2.5 | 38 |
| 2 | 5 | |||
| 3 | 7.5 | |||
| 4 | 10 | |||
| 5 | 12.5 | |||
| 6 | Na2CO3 | Solid | - | 3 |
| 7 | 6 | |||
| 8 | 9 | |||
| 9 | 12 | |||
| 10 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, W.; Yue, B.; Luo, Z.; Zhu, S.; Li, L.; Yang, H.; Luo, B. Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag. Materials 2025, 18, 3307. https://doi.org/10.3390/ma18143307
Qiao W, Yue B, Luo Z, Zhu S, Li L, Yang H, Luo B. Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag. Materials. 2025; 18(14):3307. https://doi.org/10.3390/ma18143307
Chicago/Turabian StyleQiao, Wei, Bing Yue, Zhihua Luo, Shengli Zhu, Lei Li, Heng Yang, and Biao Luo. 2025. "Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag" Materials 18, no. 14: 3307. https://doi.org/10.3390/ma18143307
APA StyleQiao, W., Yue, B., Luo, Z., Zhu, S., Li, L., Yang, H., & Luo, B. (2025). Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag. Materials, 18(14), 3307. https://doi.org/10.3390/ma18143307







