Attachment of Human Epithelial Cells to an Anodized Titanium Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation and Surface Treatment
2.2. Surface Roughness and Surface Wettability
2.3. Cell Culture
2.4. Cell Proliferation Assay
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Immunofluorescence Observations and Morphology
2.7. Statistical Analysis
3. Results
3.1. Surface Roughness and Surface Wettability
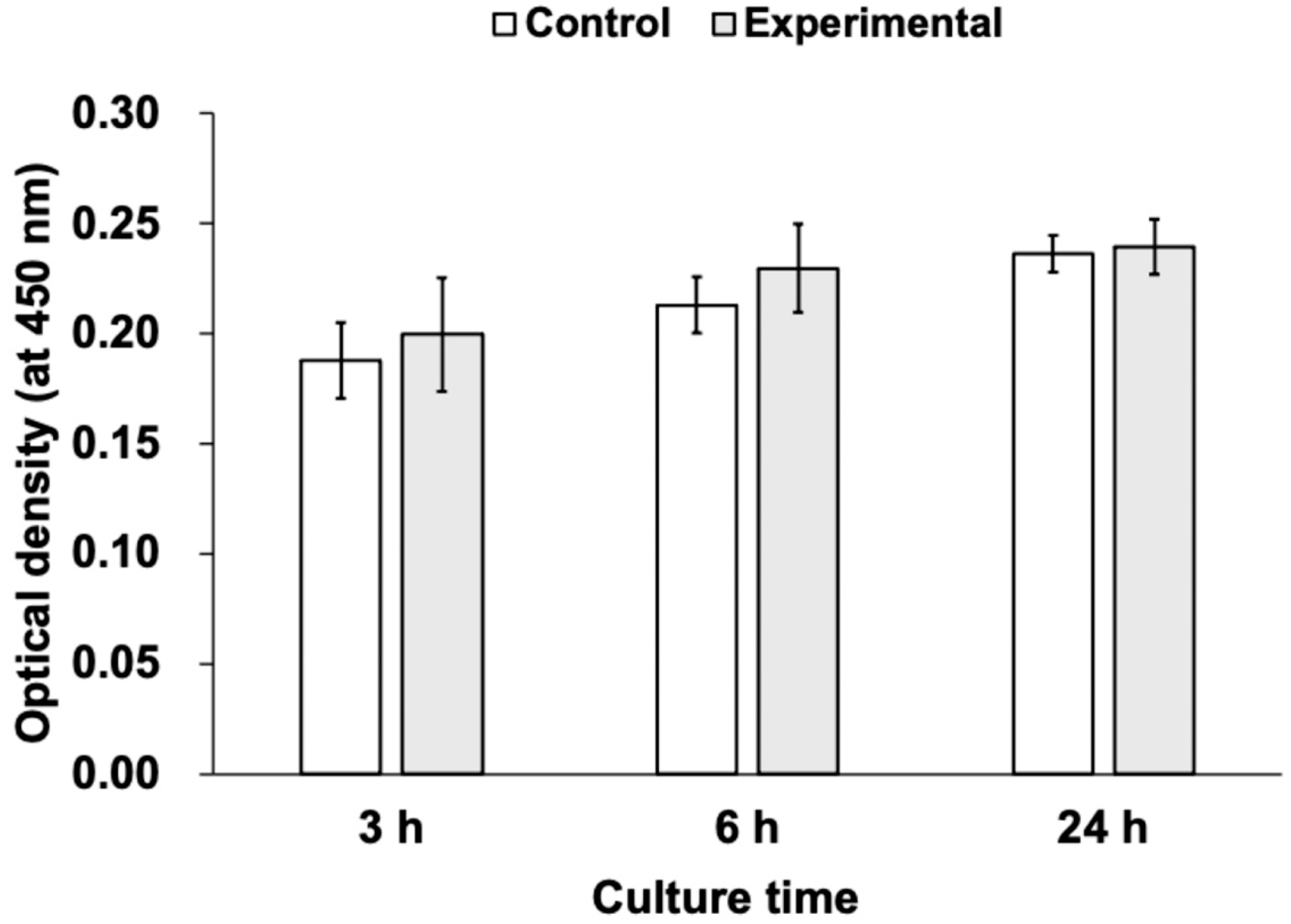
3.2. Cell Proliferation Assay
3.3. mRNA Expression (Quantitative RT-PCR)
3.4. Immunofluorescence Observations and Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| cDNA | complementary deoxyribonucleic acid |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle medium |
| EDTA | ethylenediaminetetraacetic acid |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| IgG | immunoglobulin G |
| mRNA | messenger ribonucleic acid |
| PBS | phosphate-buffered saline |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| Sa | arithmetic mean surface roughness |
| SD | standard deviation |
| UV | ultraviolet |
References
- Simão, B.S., Jr.; Costa, D.D.; Cristina, M.T.C.; Sotto-Maior, B.S.; Lana Devita, R.; de Carvalho, J.J.; da Silva Brum, I. Observational study on the success rate of osseointegration: A prospective analysis of 15,483 implants in a public health setting. BioMed 2022, 2, 422–430. [Google Scholar] [CrossRef]
- Borie, M.; Lecloux, G.; Bosshardt, D.; Barrantes, A.; Haugen, H.J.; Lambert, F.; Bacevic, M. Peri-implant soft tissue integration in humans—Influence of materials: A study protocol for a randomised controlled trial and a pilot study results. Contemp. Clin. Trials Commun. 2020, 19, 100643. [Google Scholar] [CrossRef]
- Elfarouk, M. Implant mucointegration, a key to enhance osteointegration. J. Clin. Med. Case Rep. Rev. 2022, 2, 1–4. [Google Scholar] [CrossRef]
- Nakhaei, K.; Ishijima, M.; Ikeda, T.; Ghassemi, A.; Saruta, J.; Ogawa, T. Ultraviolet light treatment of titanium enhances attachment, adhesion, and retention of human oral epithelial cells via decarbonization. Materials 2020, 14, 151. [Google Scholar] [CrossRef]
- Kobune, K.; Miura, T.; Sato, T.; Yotsuya, M.; Yoshinari, M. Influence of plasma and ultraviolet treatment of zirconia on initial attachment of human oral keratinocytes: Expressions of laminin γ2 and integrin β4. Dent. Mater. J. 2014, 33, 696–704. [Google Scholar] [CrossRef]
- Kimura, Y.; Matsuzaka, K.; Yoshinari, M.; Inoue, T. Initial attachment of human oral keratinocytes cultured on zirconia or titanium. Dent. Mater. J. 2012, 31, 346–353. [Google Scholar] [CrossRef]
- Akashi, Y.; Shimoo, Y.; Hashiguchi, H.; Nakajima, K.; Kokubun, K.; Matsuzaka, K. Effects of excimer laser treatment of zirconia disks on the adhesion of L929 fibroblasts. Materials 2022, 16, 115. [Google Scholar] [CrossRef]
- Razali, M.; Ngeow, W.C.; Omar, R.A.; Chai, W.L. An in-vitro analysis of peri-implant mucosal seal following photofunctionalization of zirconia abutment materials. Biomedicines. 2021, 9, 78. [Google Scholar] [CrossRef]
- Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of titanium, zirconia and modified PEEK after surface treatment using UV light or non-thermal plasma. Int. J. Mol. Sci. 2019, 20, 5596. [Google Scholar] [CrossRef]
- Krautwald, L.; Smeets, R.; Stolzer, C.; Rutkowski, R.; Guo, L.; Reitmeier, A.; Gosau, M.; Henningsen, A. Osseointegration of zirconia implants after UV-light or cold atmospheric plasma surface treatment in vivo. Materials 2022, 15, 496. [Google Scholar] [CrossRef]
- Brezavšček, M.; Fawzy, A.; Bächle, M.; Tuna, T.; Fischer, J.; Att, W. The effect of uv treatment on the osteoconductive capacity of zirconia-based materials. Materials 2016, 9, 958. [Google Scholar] [CrossRef]
- Guo, T.; Scimeca, J.; Ivanovski, S.; Verron, E.; Gulati, K. Enhanced corrosion resistance and local therapy from nano-engineered titanium dental implants. Pharmaceutics 2023, 15, 315. [Google Scholar] [CrossRef]
- Traver-Méndez, V.; Camps-Font, O.; Ventura, F.; Nicolau-Sansó, M.A.; Subirà-Pifarré, C.; Figueiredo, R.; Valmaseda-Castellón, E. In vitro characterization of an anodized surface of a dental implant collar and dental abutment on peri-implant cellular response. Materials 2023, 16, 6012. [Google Scholar] [CrossRef]
- Villaça-Carvalho, M.; De Araújo, J.; Beraldo, J.; Prado, R.; Moraes, M.; Júnior, L.; Codaro, E.; Acciari, H.; Machado, J.; Regone, N.; et al. Bioactivity of an experimental dental implant with anodized surface. J. Funct. Biomater. 2021, 12, 39. [Google Scholar] [CrossRef]
- Kim, M.; Park, K.; Choi, K.; Kim, S.; Kim, S.; Jeong, C.; Huh, J. Cell adhesion and in vivo osseointegration of sandblasted/acid etched/anodized dental implants. Int. J. Mol. Sci. 2015, 16, 10324–10336. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.J.; Kumar, P.T.S.; Han, P.; Ivanovski, S. Anodized anisotropic titanium surfaces for enhanced guidance of gingival fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110860. [Google Scholar] [CrossRef]
- Crenn, M.J.; Dubot, P.; Mimran, E.; Fromentin, O.; Lebon, N.; Peyre, P. Influence of Anodized Titanium Surfaces on the Behavior of Gingival Cells in Contact with: A Systematic Review of In Vitro Studies. Crystals. 2022, 12, 893. [Google Scholar] [CrossRef]
- Hall, J.; Neilands, J.; Davies, J.R.; Ekestubbe, A.; Friberg, B. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin. Implant. Dent. Relat. Res. 2019, 21 (Suppl. S1), 55–68. [Google Scholar] [CrossRef]
- Boukamp, P.; Popp, S.; Altmeyer, S.; Hülsen, A.; Fasching, C.; Cremer, T.; Fusenig, N.E. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCat. Genes Chromosom. Cancer 1997, 19, 201–214. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T. Anodization: A promising nano-modification technique of titanium implants for orthopedic applications. J. Nanosci. Nanotechnol. 2006, 6, 2682–2692. [Google Scholar] [CrossRef]
- Diamanti, M.; Curto, B.; Pedeferri, M. Interference colors of thin oxide layers on titanium. Color. Res. Appl. 2008, 33, 221–228. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Laurenti, M.; Zicola, E.; Munaron, L.; Rivolo, P.; Mandracci, P.; Carossa, S. Early Response of Fibroblasts and Epithelial Cells to Pink-Shaded Anodized Dental Implant Abutments: An In Vitro Study. Int. J. Oral. Maxillofac. Implant. 2018, 33, 571–579. [Google Scholar] [CrossRef]
- Shibata, Y.; Suzuki, D.; Omori, S.; Tanaka, R.; Murakami, A.; Kataoka, Y.; Baba, K.; Kamijo, R.; Miyazaki, T. The characteristics of in vitro biological activity of titanium surfaces anodically oxidized in chloride solutions. Biomaterials 2010, 3, 8546–8555. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, L.; Kuang, Y.; Zhou, Z.; Li, X. Highly ordered nanotube-like microstructure on titanium dental implant surface fabricated via anodization enhanced cell adhesion and migration of human gingival fibroblasts. Int. J. Nanomed. 2024, 19, 2469–2485. [Google Scholar] [CrossRef]
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of modified titanium abutment surface on peri-implant soft tissue behavior: A systematic review of in vitro studies. Int. J. Oral. Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef]
- Hohenester, E. Structural biology of laminins. Essays Biochem. 2019, 63, 285–295. [Google Scholar]
- Timpl, R.; Brown, J. The laminins. Matrix Biol. 1994, 4, 275–281. [Google Scholar] [CrossRef]
- Engel, J. Laminins and other strange proteins. Biochemistry 1992, 44, 10643–10651. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2020, 89, 1619–1626. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef]
- Van Der Flier, A.; Sonnenberg, A. Function and interactions of integrins. Cell Tissue Res. 2001, 305, 285–298. [Google Scholar] [CrossRef]
- Ogawa, T.; Tsubota, Y.; Hashimoto, J.; Kariya, Y.; Miyazaki, K. The short arm of laminin gamma2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin beta4 chain. Mol. Biol. Cell 2007, 5, 1621–1633. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Choi, C.K.; Horwitz, A.R. Integrins in cell migration--the actin connection. J. Cell Sci. 2009, 122, 199–206. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Dworan, J.; Aellos, F.; Grauer, J.A.; Fabbri, G.; Harder, K.G.; Boccardo, S.; Cuevas, P.L.; Dawid, I.; Vicini, M.; Helms, J.A. Dynamics of Mucosal Integration of Machined versus Anodized Titanium Implants. J. Dent. Res. 2025, 104, 270–279. [Google Scholar] [CrossRef]





| Titanium Disc | Surface Roughness (Sa) [μm] |
|---|---|
| Control | 0.1830 ± 0.0226 |
| Experimental | 0.1968 ± 0.0117 |
| Titanium Disc | Contact Angle [Degree] |
|---|---|
| Control | 72.3 ± 4.9 |
| Experimental | 36.2 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akashi, Y.; Hashiguchi, H.; Yamaoka, Y.; Nakajima, K.; Kokubun, K.; Shimoo, Y.; Matsuzaka, K. Attachment of Human Epithelial Cells to an Anodized Titanium Surface. Materials 2025, 18, 3305. https://doi.org/10.3390/ma18143305
Akashi Y, Hashiguchi H, Yamaoka Y, Nakajima K, Kokubun K, Shimoo Y, Matsuzaka K. Attachment of Human Epithelial Cells to an Anodized Titanium Surface. Materials. 2025; 18(14):3305. https://doi.org/10.3390/ma18143305
Chicago/Turabian StyleAkashi, Yoshihiko, Hayato Hashiguchi, Yoshitaka Yamaoka, Kei Nakajima, Katsutoshi Kokubun, Yoshiaki Shimoo, and Kenichi Matsuzaka. 2025. "Attachment of Human Epithelial Cells to an Anodized Titanium Surface" Materials 18, no. 14: 3305. https://doi.org/10.3390/ma18143305
APA StyleAkashi, Y., Hashiguchi, H., Yamaoka, Y., Nakajima, K., Kokubun, K., Shimoo, Y., & Matsuzaka, K. (2025). Attachment of Human Epithelial Cells to an Anodized Titanium Surface. Materials, 18(14), 3305. https://doi.org/10.3390/ma18143305