Cleaner Production of Metallurgical-Grade Iron from High-Iron Bauxite Residue via Smelting Reduction: Thermodynamic Control, Industrial Application Potential, and Slag Utilization Strategy
Abstract
1. Introduction
2. Materials and Methods
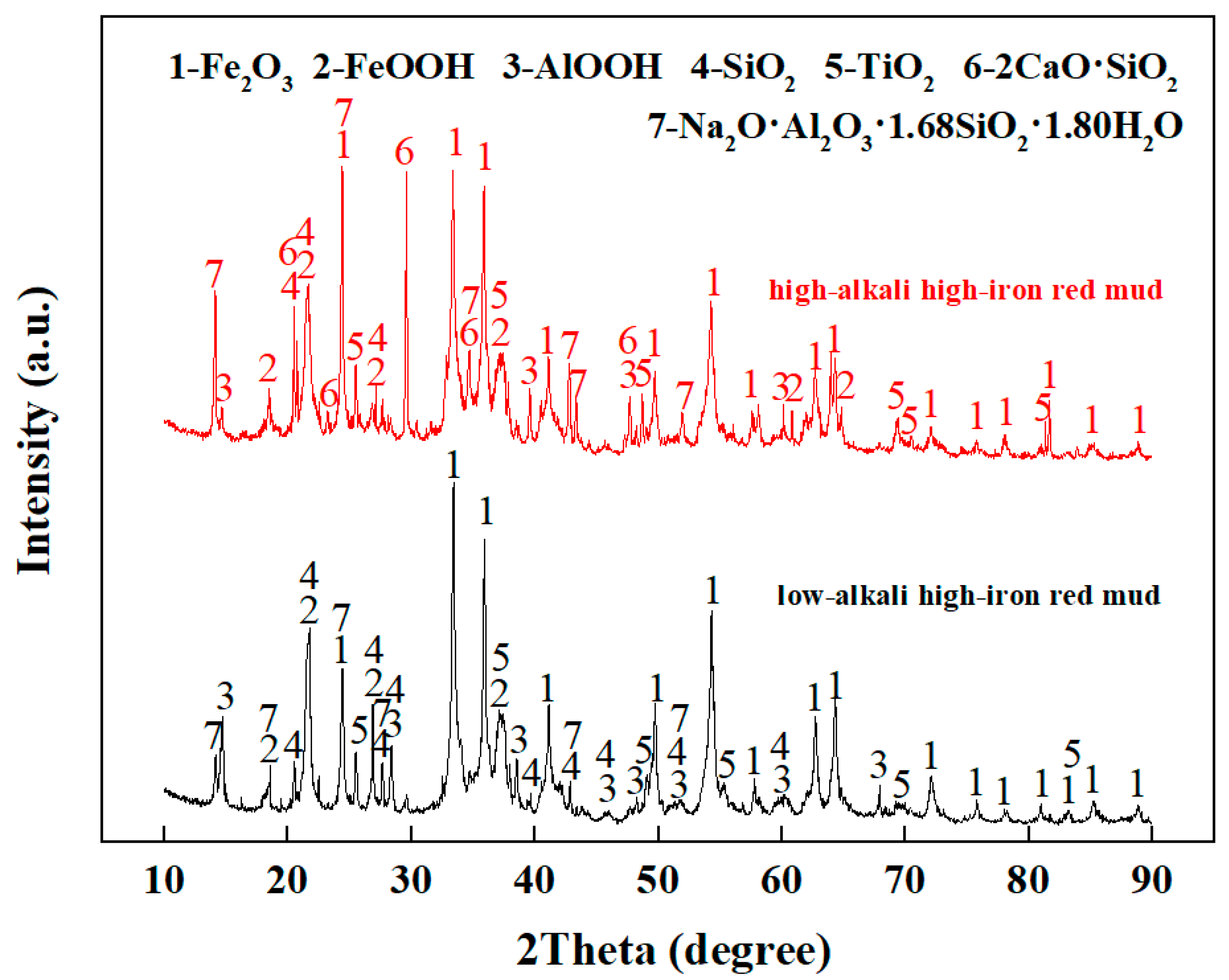
2.1. Raw Materials
2.2. Experimental Methods
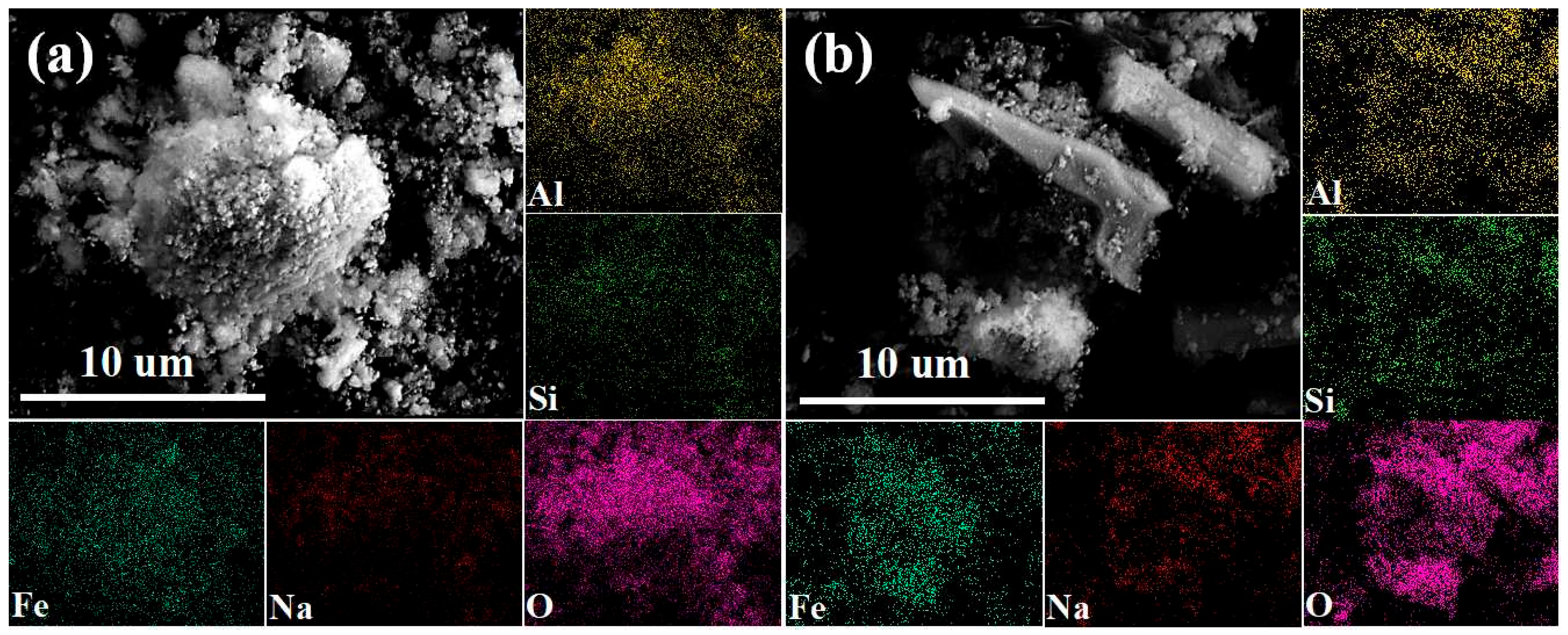
2.3. Analysis Methods
3. Thermodynamic Analysis
3.1. Calculation of Gibbs Free Energy Changes
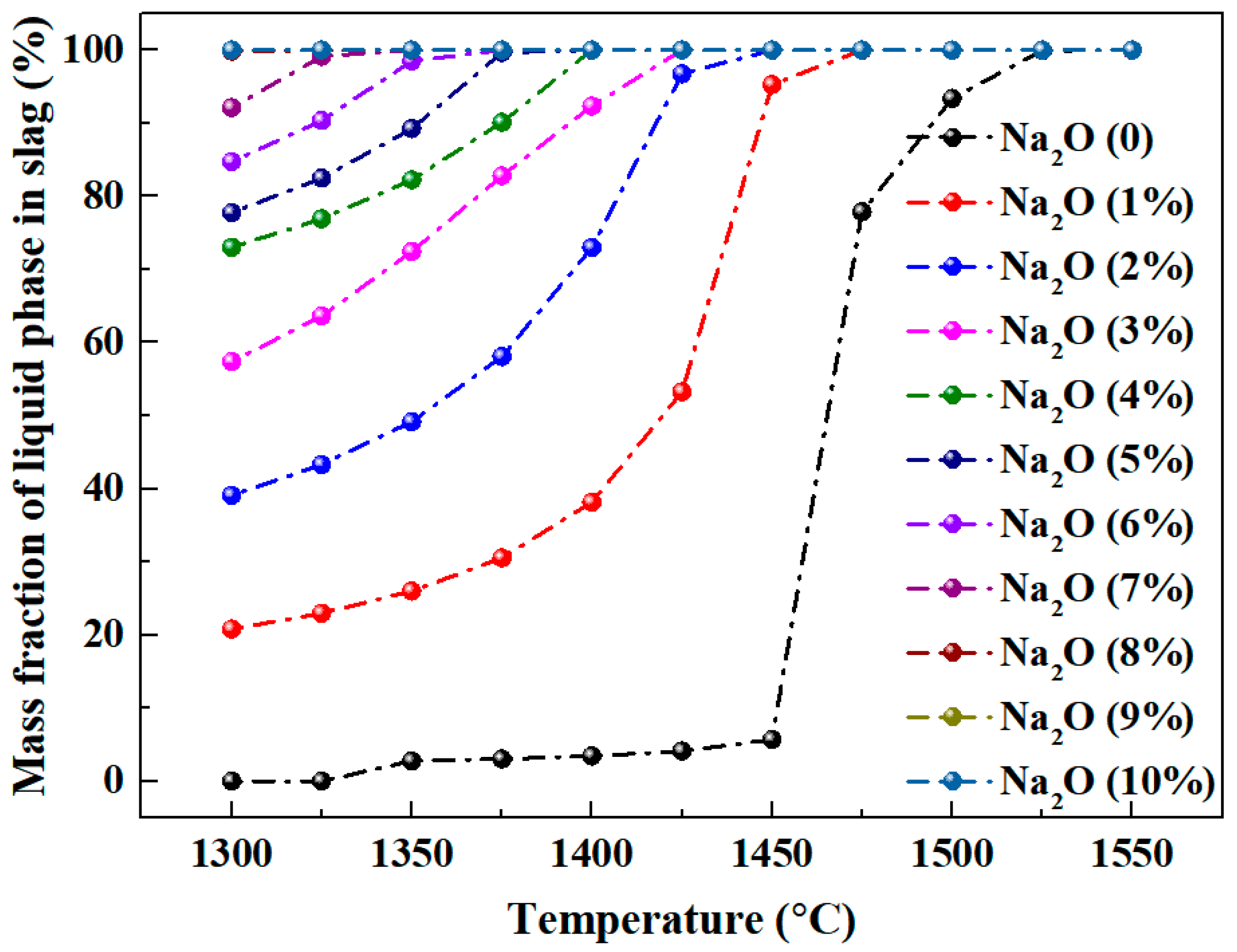
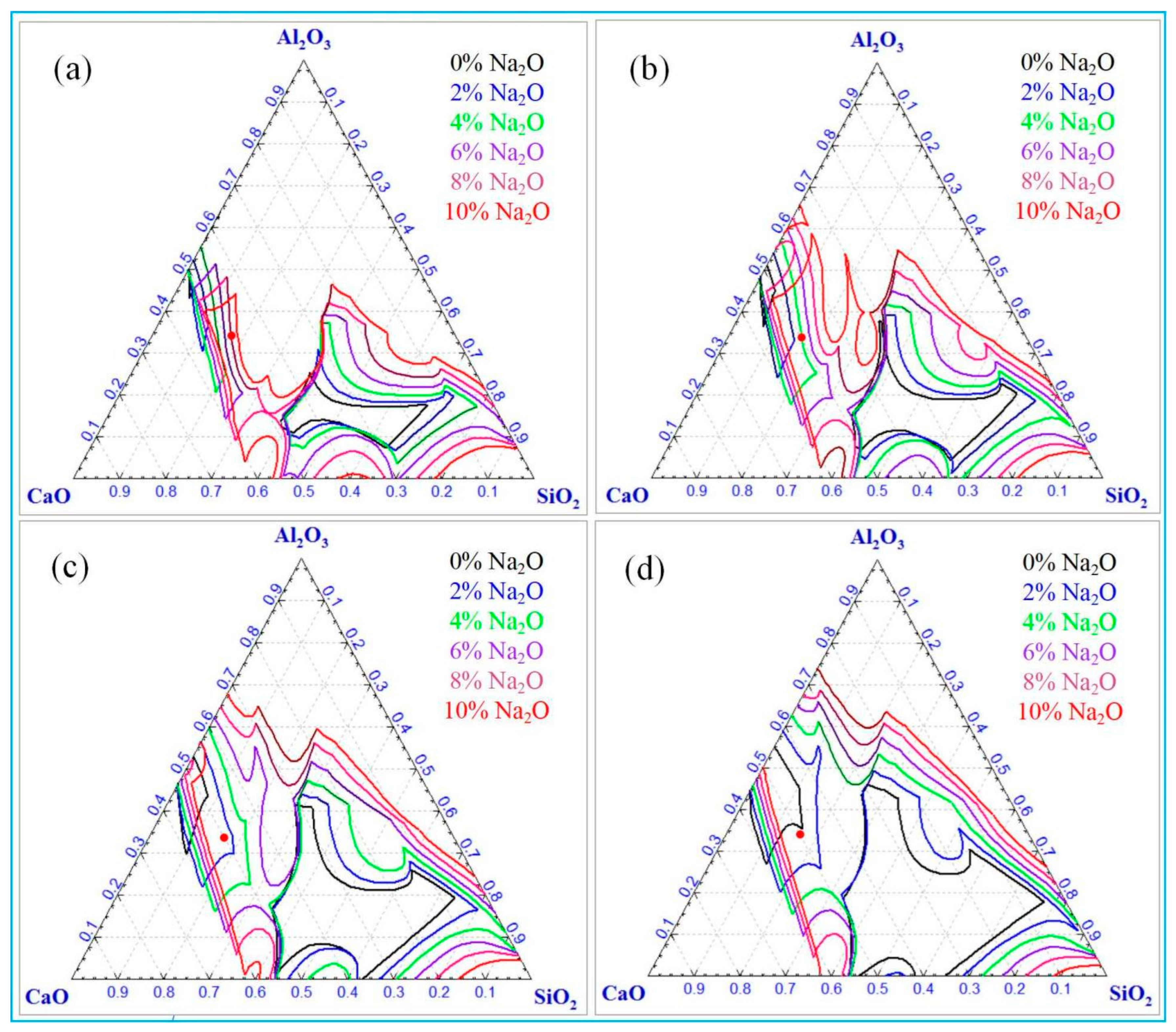
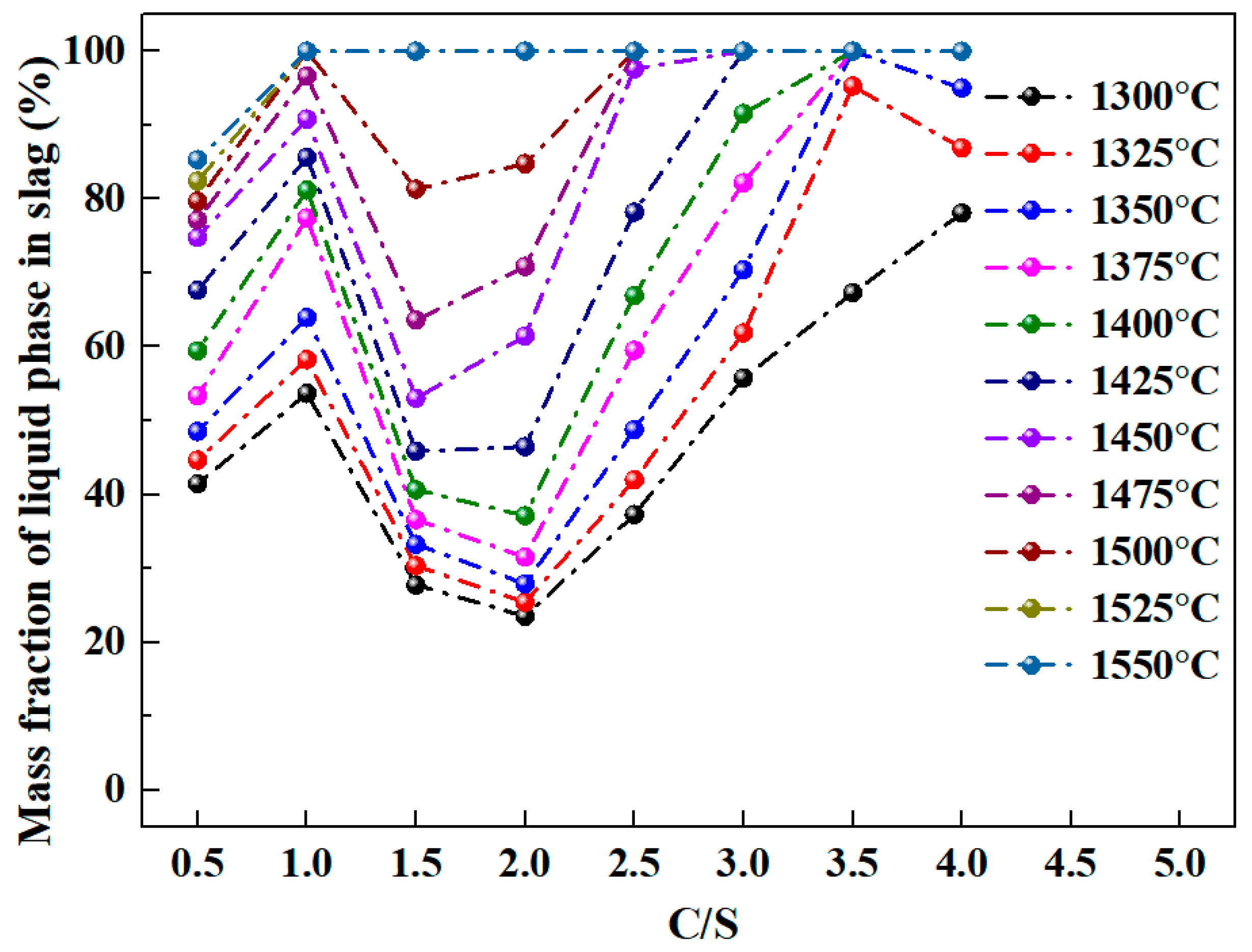
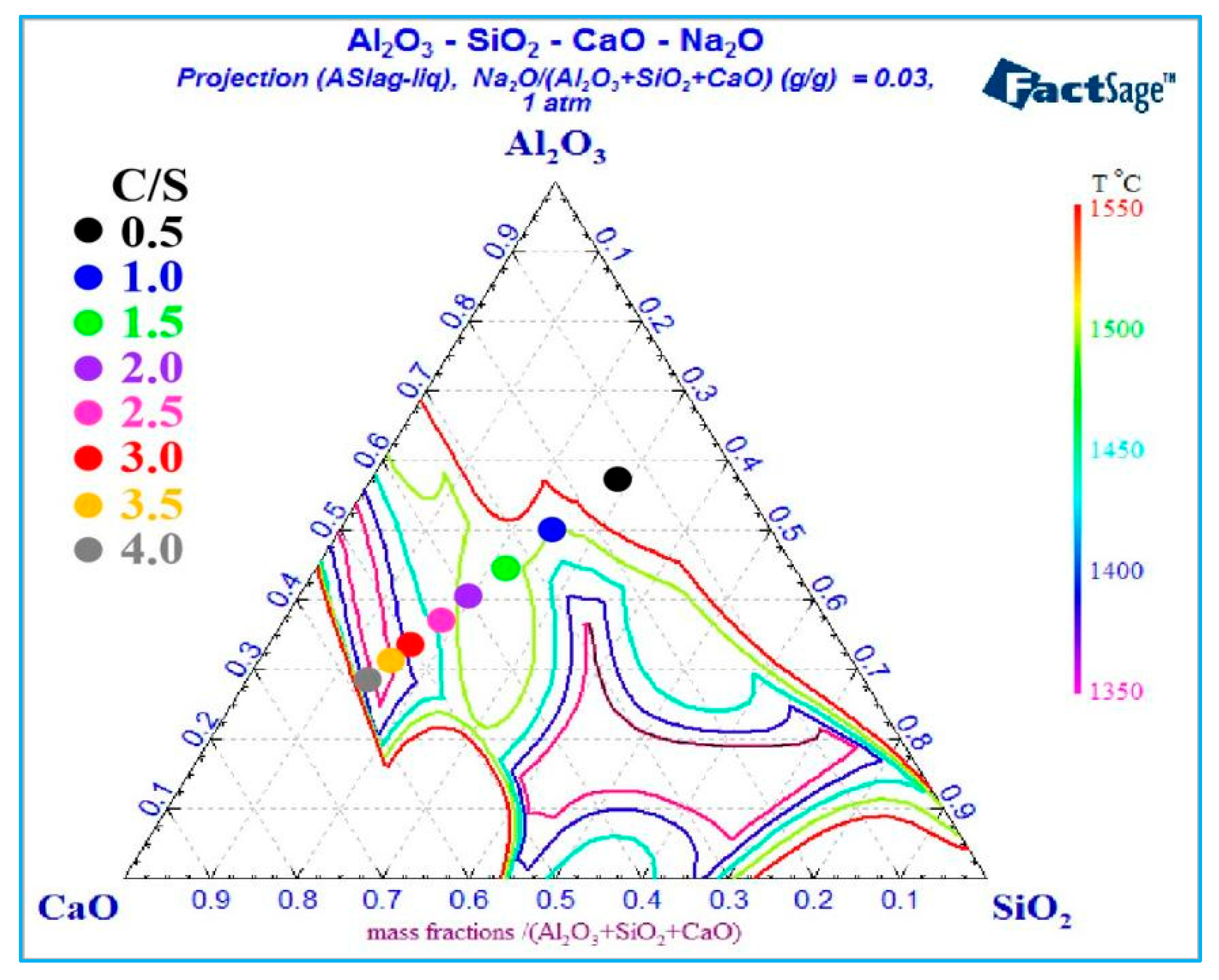
3.2. Calculation of Mass Fraction of Liquid Phase and Liquidus of Al2O3-SiO2-CaO-Na2O Slag System
3.2.1. Effect of Different Temperatures and Sodium Oxide Contents on Calculation Results
3.2.2. Effect of Different C/S and Temperature on Calculation Results
4. Results and Discussion
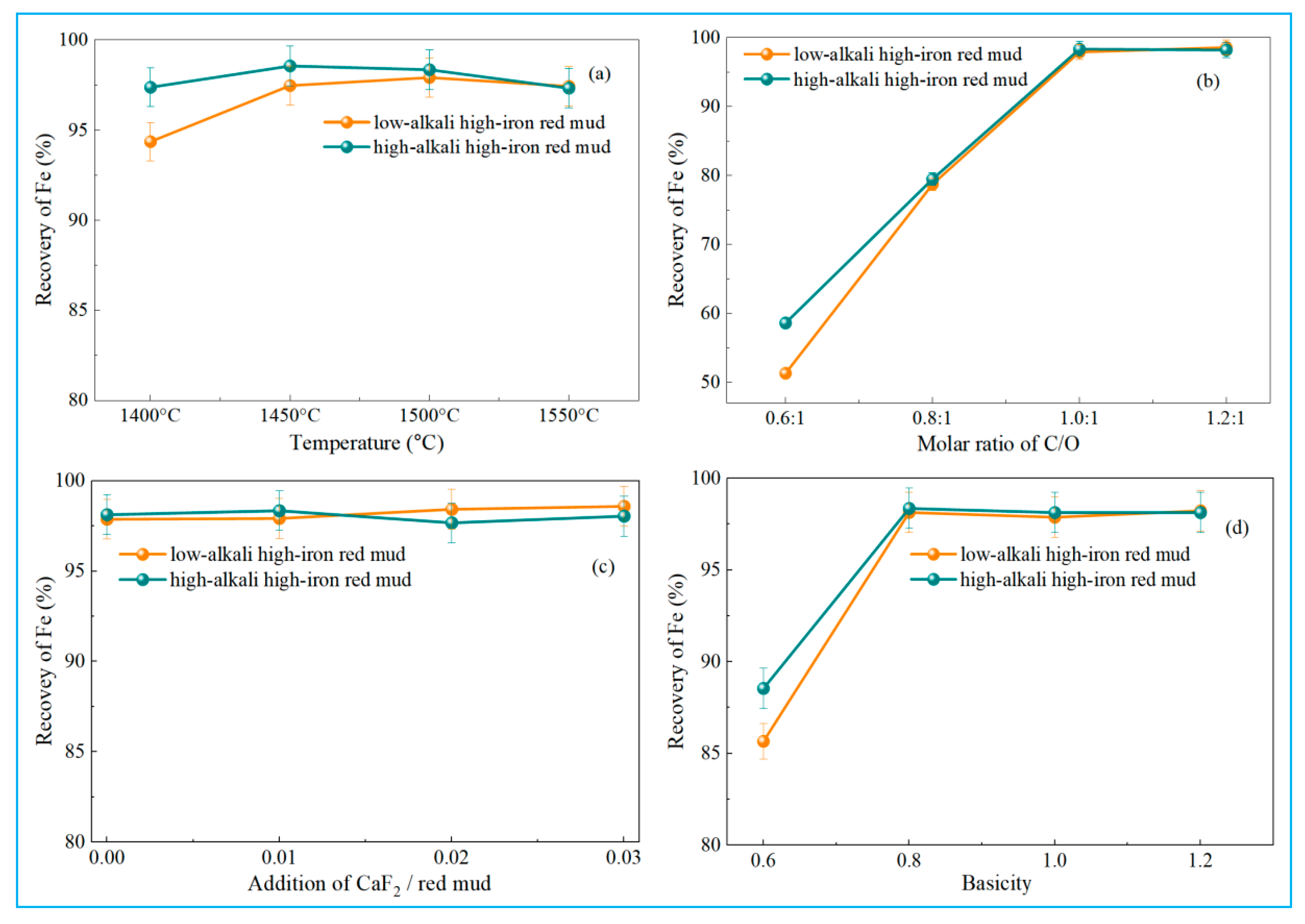
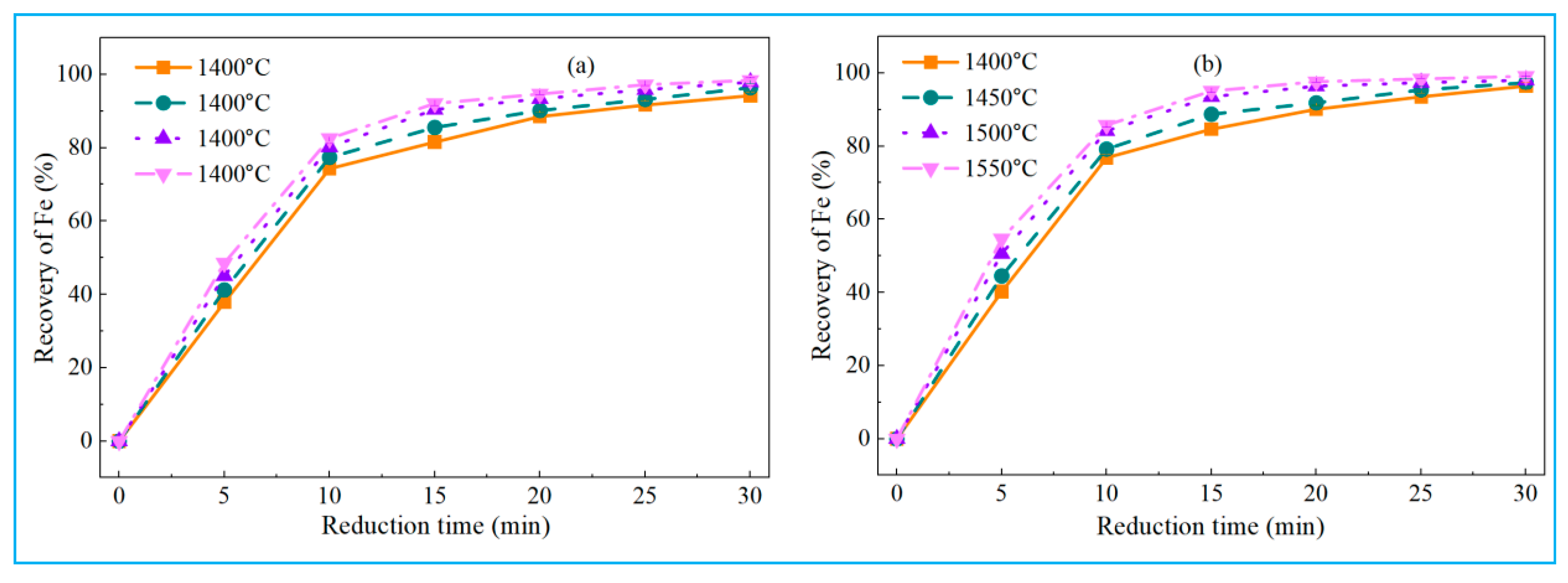
4.1. Reduction Behavior of High-Iron Red Mud Under Different Experimental Parameters in Smelting Reduction
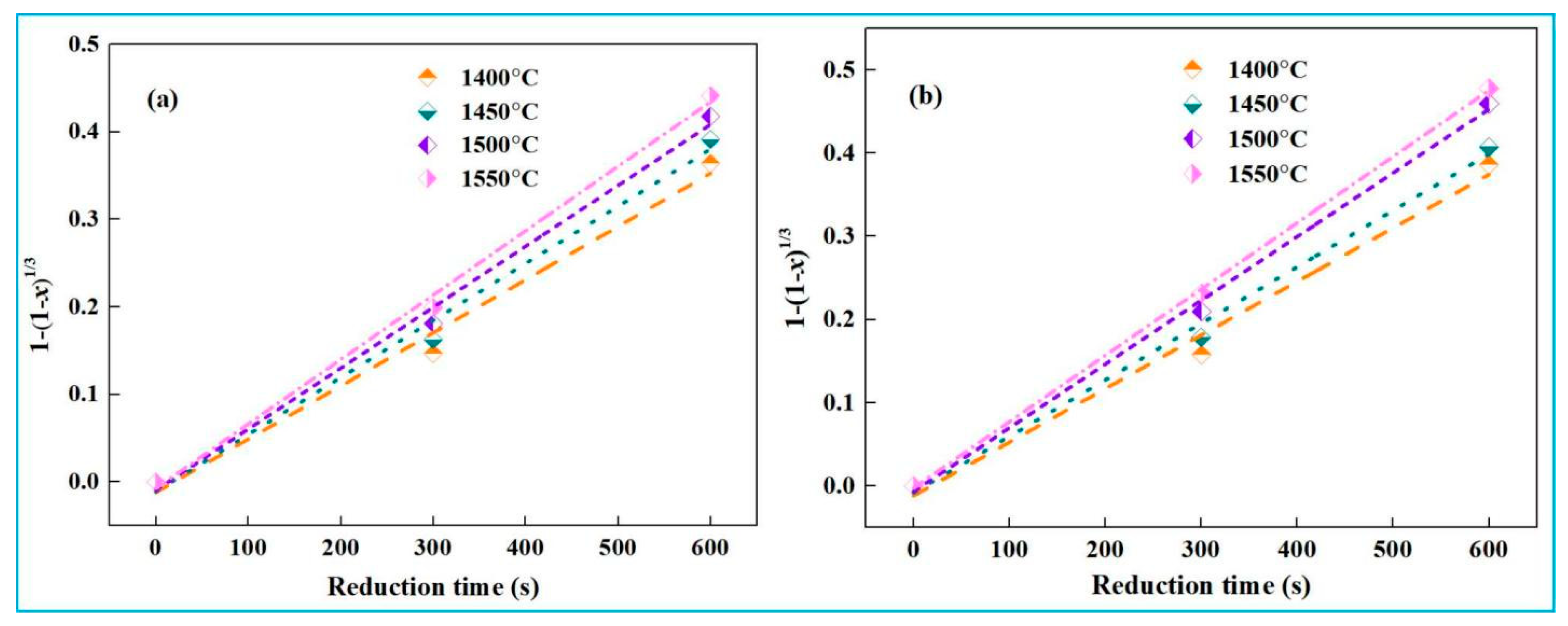
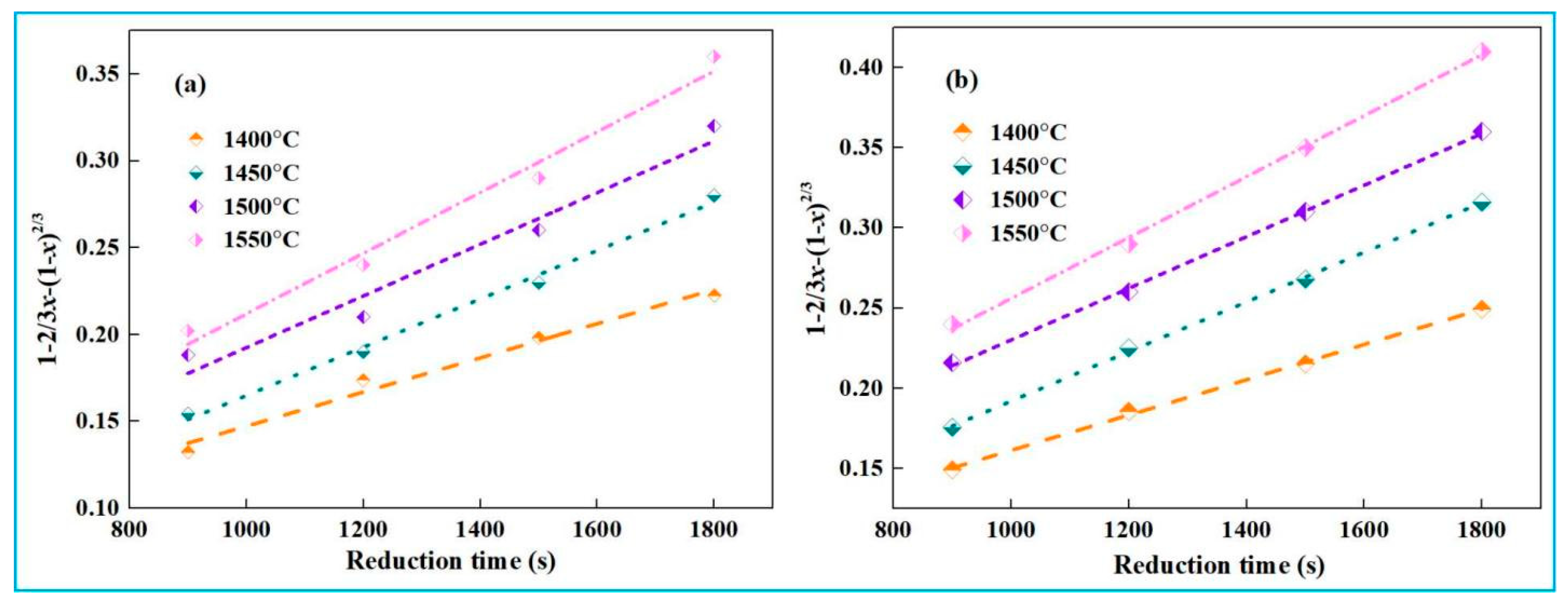
4.2. Kinetic Analysis of Recovery of Iron from High-Iron Red Mud During Smelting Reduction
- (1)
- Fe3+ and O2− ions diffuse to the molten-carbon interface.
- (2)
- At the molten-carbon interface, Fe3+ adsorbs on the carbon surface and reacts with carbon to form metallic iron. The lattice oxygen of iron oxides combines with carbon to form CO gas. Iron then carburizes to form Fe3C, followed by product desorption.
- (3)
- Fe3C(l) and CO(g) overcome interfacial adsorption and leave the reaction interface.
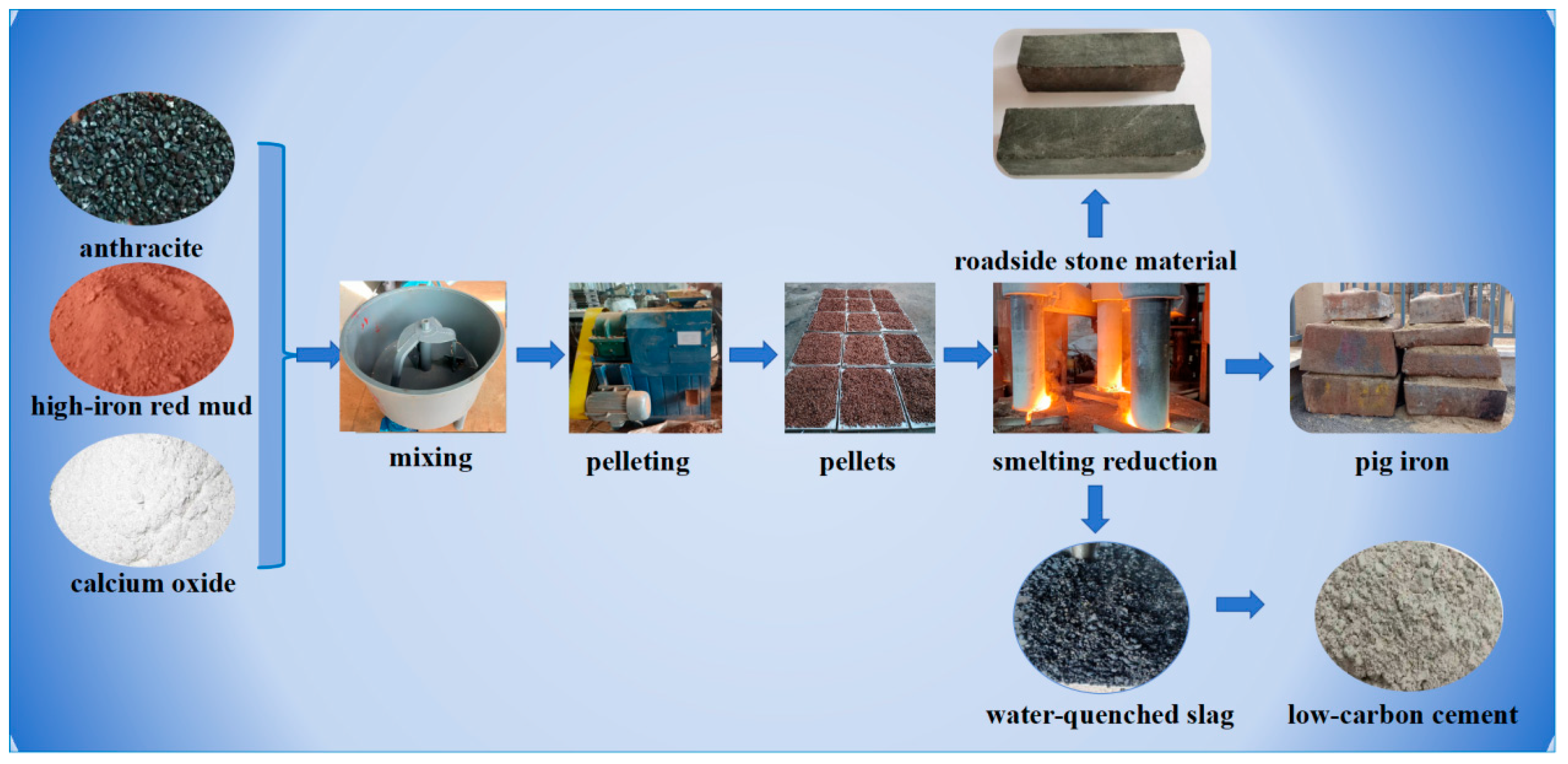
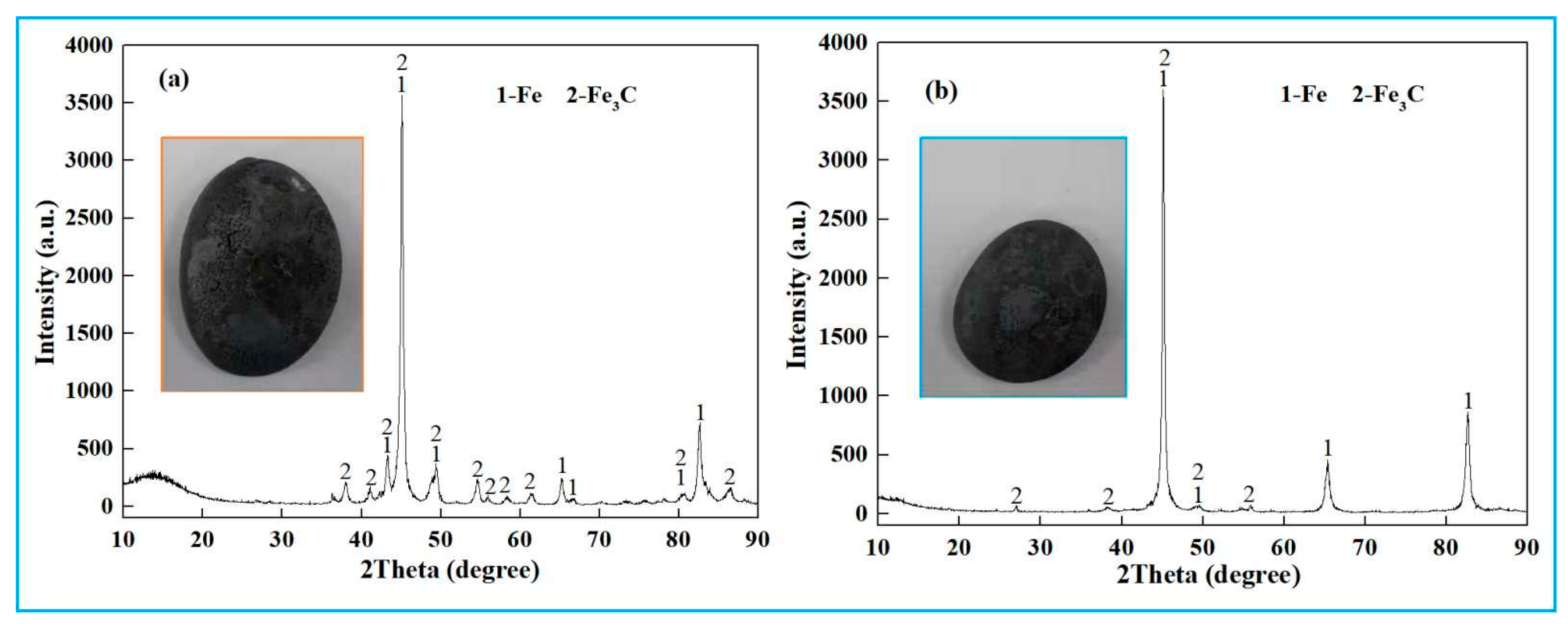
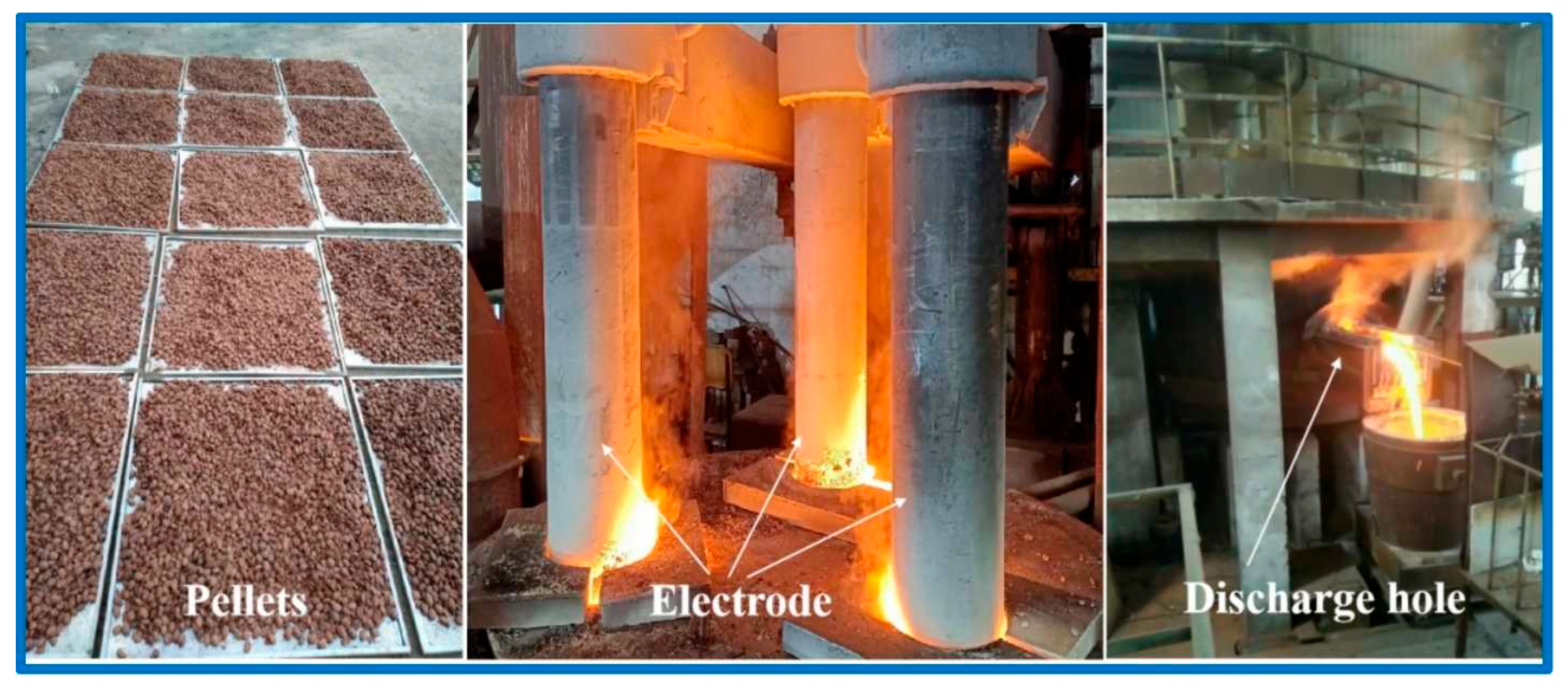
4.3. Pilot Scale Experiment of Smelting Reduction of High-Iron Red Mud
4.3.1. Smelting Reduction of High-Iron Red Mud
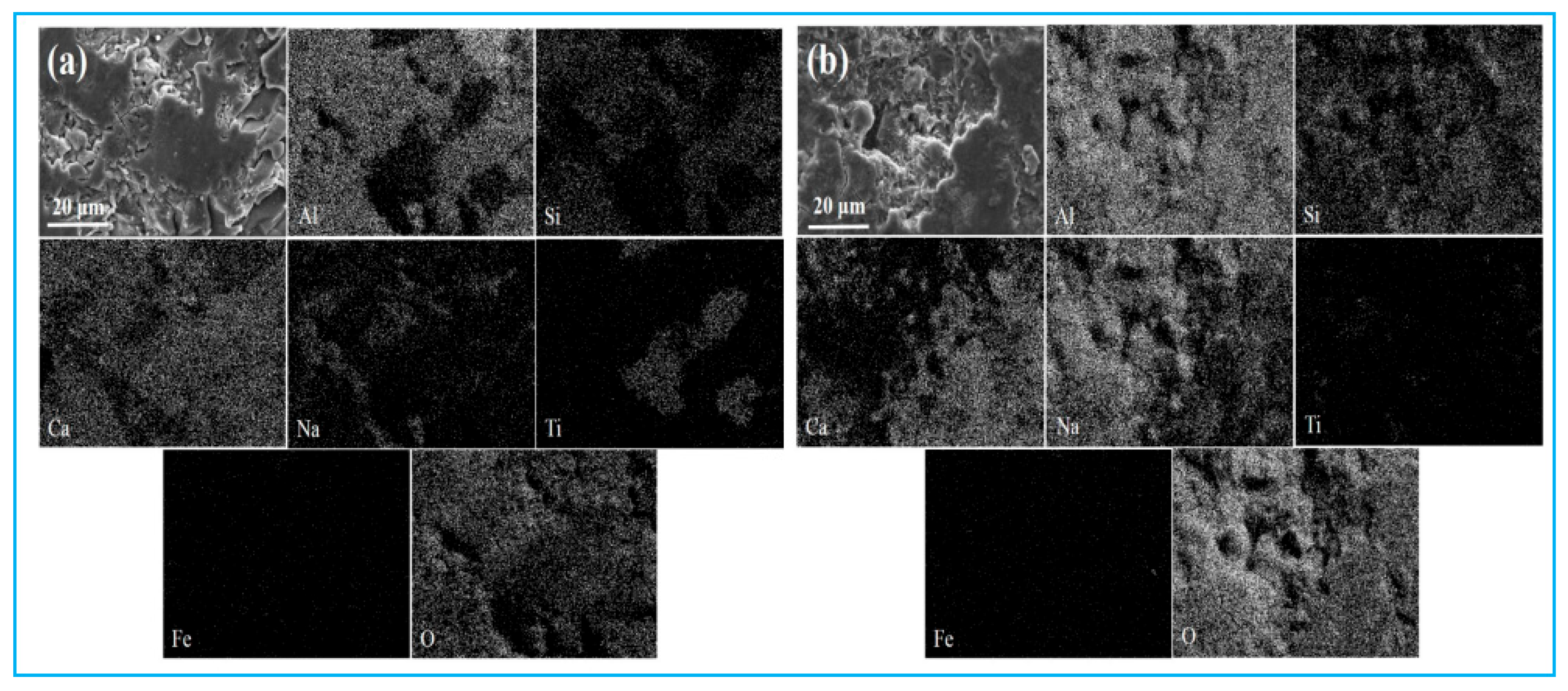
4.3.2. Utilization Strategy of Reduction Slag
5. Conclusions
- (1)
- Thermodynamic analysis indicates that during smelting reduction, alumina, silicon oxide, and titanium dioxide in high-iron red mud do not undergo reduction at 1300–1500 °C. Both hematite and sodium oxide are reducible, with hematite’s carbothermal reduction following the reaction Fe2O3 + 3C = 2Fe + 3CO.
- (2)
- For Al2O3-SiO2-CaO-Na2O slag, increasing temperature and sodium oxide content both enhance the liquid phase mass fraction. Higher sodium oxide content reduces slag viscosity. At constant temperature and A/S, the liquid phase mass fraction first increases, then decreases, and rises again with increasing C/S.
- (3)
- Optimizing reduction temperature, C/O ratio, and basicity significantly improves iron recovery from high-iron red mud, whereas CaF2 has minimal impact. Under optimal conditions, iron recoveries of low-alkali high-iron red mud and high-alkali high-iron red mud reached 98.14% and 98.36%, respectively. The reduced pig iron meets the L03 industrial standard for steel-making pig iron.
- (4)
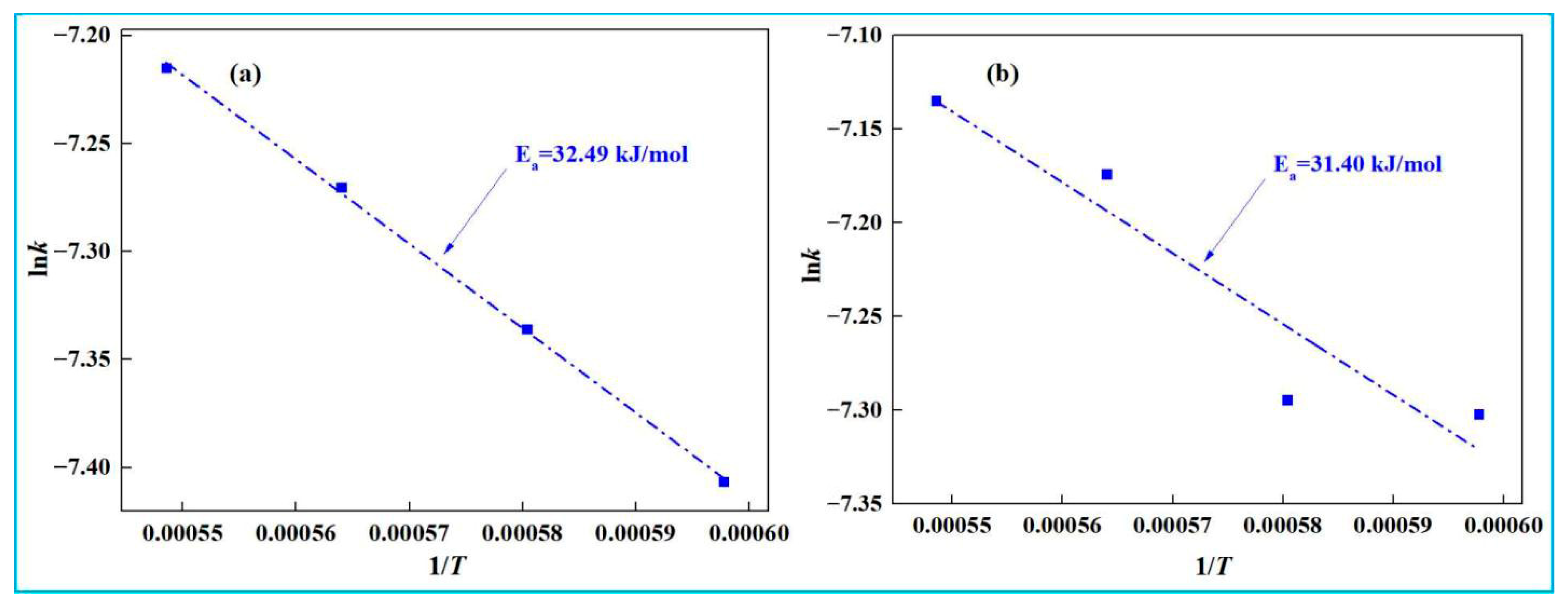
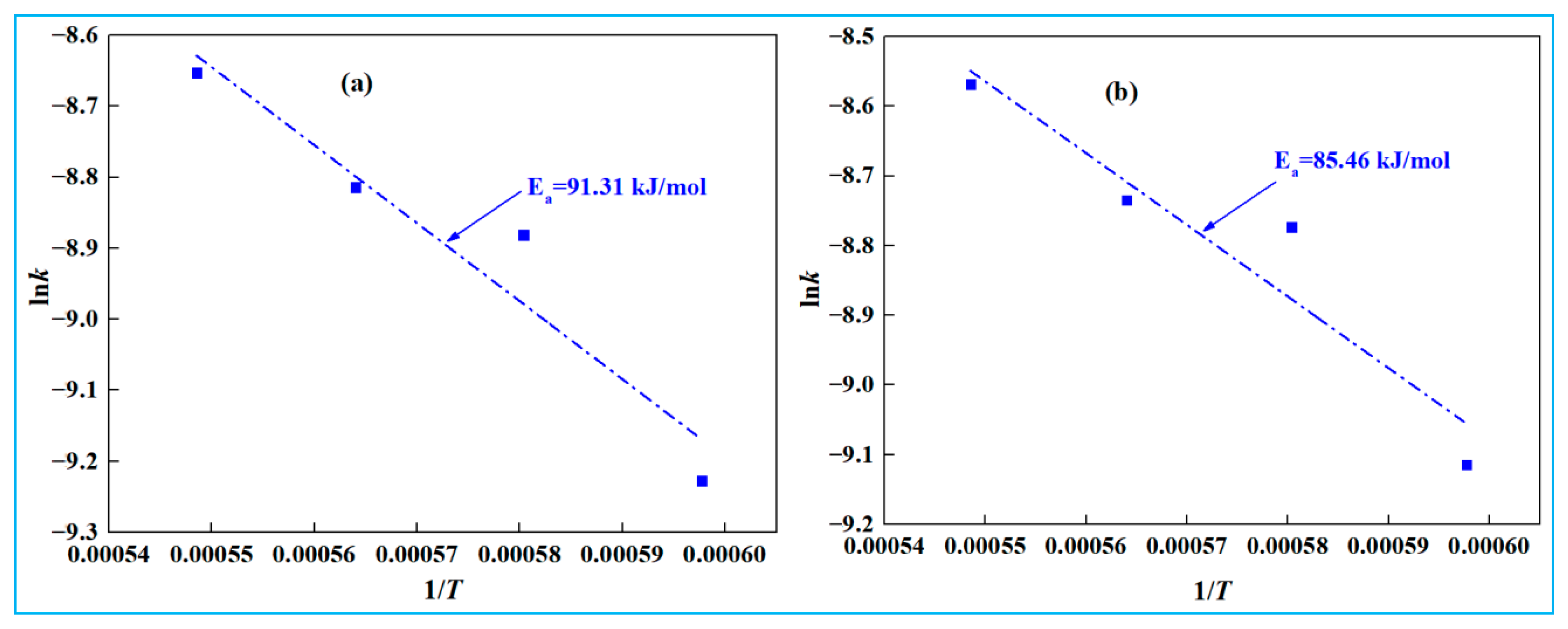
- Kinetic experiments show that the smelting reduction process of high-iron red mud is divided into two stages. In the initial stage (0–10 min), reaction kinetics is governed by interfacial chemical reaction, with apparent activation energies of 32.49 kJ·mol−1 (low-alkali) and 31.40 kJ·mol−1 (high-alkali), high-iron red mud. In the second stage (15–30 min), diffusion controls the process, with corresponding apparent activation energies of 91.31 kJ·mol−1 and 85.46 kJ·mol−1, respectively.
- (5)
- Pilot-scale reduction experiments confirm the high-value utilization potential of high-iron red mud for iron recovery. The reduced slag can be used as the roadside stone or cement clinker, with results providing theoretical guidance and technical support for its large-scale and high-value comprehensive utilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, H.X.; She, X.F.; You, X.M.; Zhang, G.Q.; Wang, J.S.; Xue, Q.G. Carbothermal reduction of red mud for iron extraction and sodium removal. High Temp. Mater. Process. 2022, 41, 161–171. [Google Scholar] [CrossRef]
- Wen, J.L.; Yu, W.Z.; Yuan, H.T.; Chen, H.; Xiong, T.; Yang, F. A new strategy for extracting aluminum and iron from red mud via vacuum thermal reduction, alkali-leaching and magnetic separation. J. Clean. Prod. 2024, 485, 144409. [Google Scholar] [CrossRef]
- Bang, K.H.; Kang, Y.B. Recycling red mud to develop a competitive desulfurization flux for Kanbara Reactor (KR) desulfurization process. J. Hazard. Mater. 2022, 440, 129752. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, S.L.; Ma, S.H.; Zhang, Y. Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J. Hazard. Mater. 2011, 189, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.N.; Li, G.H.; Luo, J.; Ye, Q.; Liu, M.X.; Peng, Z.W.; Jiang, T. Enrichment of Sc2O3 and TiO2 from bauxite ore residues. J. Hazard. Mater. 2017, 331, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Lyu, F.; Hu, G.Y.; Tang, H.H.; Wang, L.; Sun, W.; Hu, Y.H.; Liu, R.Q. Dealkalization of bauxite residue through acid neutralization and its revegetation potential. JOM 2020, 72, 319–325. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. China Statistical Yearbook 2024. Available online: https://www.stats.gov.cn/sj/ndsj/2024/indexch.htm (accessed on 20 April 2025).
- Wang, M.F.; Liu, X.M. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Cui, Y.L.; Chen, J.N.; Zhang, Y.B.; Peng, D.P.; Huang, T.; Sun, C.W. pH-Dependent Leaching Characteristics of Major and Toxic Elements from Red Mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef]
- Lima, M.S.S.; Thives, L.P. Mechanical feasibility of using red mud as filler in asphalt mixtures to improve permanent deformation. Transportes 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Wang, S.H.; Jin, H.X.; Deng, Y.; Xiao, Y.D. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Liu, D.Y.; Wu, C.S. Stockpiling and comprehensive utilization of red mud research progress. Materials 2012, 5, 1232–1246. [Google Scholar] [CrossRef]
- Li, S.; Kang, Z.S.; Liu, W.C.; Lian, Y.C.; Yang, H.S. Reduction Behavior and Direct Reduction Kinetics of Red Mud-Biomass Composite Pellets. J. Sustain. Metall. 2021, 7, 126–135. [Google Scholar] [CrossRef]
- Jeremy, E.; Sanwani, E.; Chaerun, S.K.; Mubarok, M.Z. Exploring Bioflocculation: A Novel Approach for Iron Recovery from Red Mud-a Review. Miner. Process. Extr. Metall. Rev. 2025, 46, 415–435. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Zhang, T.A.; Li, X.F.; Chen, X. Investigation of the smelting reduction mechanism and of iron extraction from high-iron red mud. Mater. Res. Express 2020, 7, 126514. [Google Scholar] [CrossRef]
- Samal, S. Utilization of Red Mud as a Source for Metal Ions—A Review. Materials 2021, 14, 2211. [Google Scholar] [CrossRef]
- Liu, Z.B.; Li, H.X. Metallurgical process for valuable elements recovery from red mud-A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Gerven, T.V. Recovery of Rare Earths and Other Valuable Metals from Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Niu, A.Y.; Lin, C.X. Trends in research on characterization, treatment and valorization of hazardous red mud: A systematic review. J. Environ. Manag. 2024, 351, 119660. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Microwave reduction of red mud for recovery of iron values. J. Sustain. Metall. 2018, 4, 427–436. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Kar, M.K.; Safarian, J.; Kowalczuk, P.B. An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods. Metals 2023, 13, 946. [Google Scholar] [CrossRef]
- Xie, L.; Hao, J.; Hu, C.J.; Zhang, H.Q. Study on Magnetization Roasting Kinetics of High-Iron and Low-Silicon Red Mud. Materials 2023, 16, 6178. [Google Scholar] [CrossRef] [PubMed]
- Zinoveev, D.; Grudinsky, P.; Zakunov, A.; Semenov, A.; Panova, M.; Valeev, D.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Influence of Na2CO3 and K2CO3 addition on iron grain growth during carbothermic reduction of red mud. Metals 2019, 9, 1313. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.X.; He, F.Y.; Gao, P.; Yuan, S. Characteristic, hazard and iron recovery technology of red mud—A critical review. J. Hazard. Mater. 2021, 420, 126542. [Google Scholar] [CrossRef]
- Vasyunina, N.V.; Dubova, I.V.; Druzhinin, K.E.; Gilmanshina, T.R. Ferrum extraction in cast iron via reduction smelting of red mud. CIS Iron Steel Rev. 2023, 25, 115–121. [Google Scholar] [CrossRef]
- Guo, Y.H.; Gao, J.J.; Xu, H.J.; Zhao, K.; Shi, X.F. Nuggets Production by Direct Reduction of High Iron Red Mud. J. Iron Steel Res. Int. 2013, 20, 24–27. [Google Scholar] [CrossRef]
- Zhang, T.A.; Wang, Y.X.; Wang, K.; Dou, Z.H.; Lv, G.Z.; Liu, Y.; Zhao, Q.Y.; Niu, L.P.; Zhang, Z.M.; Han, J.B.; et al. A Method for Iron Extraction from High-Iron Red Mud and Production of Cement with Reduction Slag. Patent CN109913604B, 26 February 2021. [Google Scholar]
- Grahita, L.; Zulhan, Z.; Hidayat, T. High Temperature Reduction of Bauxite Residue. Mater. Proc. 2021, 5, 128. [Google Scholar]
- Mombelli, D.; Barella, S.; Gruttadauria, A.; Mapelli, C. Iron Recovery from Bauxite Tailings Red Mud by Thermal Reduction with Blast Furnace Sludge. Appl. Sci. 2019, 9, 4902. [Google Scholar] [CrossRef]
- YB/T 5296-2011; Pig Iron for Steelmaking. Ministry of Industry and Information Technology of the People’s Republic of China: Beijing, China, 2011.
- Ono, H.; Masui, T.; Mori, H. Dephosphorization Kinetics and Reaction Region in Hot Metal during Lime Injection with Oxygen. Trans. Iron Steel Inst. Jpn. 1985, 25, 133–141. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Park, J.H.; Min, D.J. A Study on the Effect of Na2O on the Viscosity for Ironmaking Slags. Steel Res. Int. 2010, 81, 17–24. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y.; Duan, X.Q. Restraining Sodium Volatilization in the Ferric Bauxite Direct Reduction System. Minerals 2016, 6, 31. [Google Scholar] [CrossRef]
- Hua, Y.X. Introduction to Metallurgical Process Dynamics; Metallurgical Industry Press: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Qu, Y.X.; Yang, Y.X.; Zou, Z.S.; Zeilstra, C.; Meijer, K.; Boom, R. Reduction Kinetics of Fine Hematite Ore Particles with a High Temperature Drop Tube Furnace. ISIJ Int. 2015, 55, 952–960. [Google Scholar] [CrossRef]
- Wang, H.; She, X.F.; Zhao, Q.Q.; Xue, Q.G.; Wang, J.S. Production of lron Nuggets Using lron-rich Red Mud by Direct Reduction. Chin. J. Process Eng. 2012, 12, 816–821. (In Chinese) [Google Scholar]
- Wang, Y.X.; Zhang, T.A.; Lyu, G.Z.; Guo, F.F.; Zhang, W.G.; Zhang, Y.H. Recovery of alkali and alumina from bauxite residue (red mud) and complete reuse of the treated residue. J. Clean. Prod. 2018, 188, 456–465. [Google Scholar] [CrossRef]




| Compositions | Al2O3 | SiO2 | Fe2O3 | TiO2 | Na2O | CaO | S | P | LOI | A/S |
|---|---|---|---|---|---|---|---|---|---|---|
| low-alkali high-iron red mud | 17.01 | 6.11 | 53.71 | 6.49 | 3.02 | 0.67 | 0.11 | 0.13 | 11.92 | 2.78 |
| high-alkali high-iron red mud | 17.74 | 10.13 | 41.63 | 8.45 | 6.48 | 1.60 | 0.16 | 0.12 | 12.56 | 1.75 |
| Reaction | ΔGθ (kJ/mol) | Number | ||
|---|---|---|---|---|
| T = 1300 °C | T = 1400 °C | T = 1500 °C | ||
| Al2O3 + 3C = 2Al + 3CO | 421.89 | 363.67 | 305.67 | (2) |
| SiO2 + 2C = Si + 2CO | 132.17 | 98.24 | 61.83 | (3) |
| TiO2 + 2C = Ti + 2CO | 160.97 | 126.30 | 91.75 | (4) |
| Na2O + C = 2Na + CO | −37.32 | −53.86 | −70.24 | (5) |
| 3Fe2O3 + C = 2Fe3O4 + CO | −235.48 | −258.24 | −280.87 | (6) |
| 3Fe2O3 + 0.5C = 2Fe3O4 + 0.5CO2 | −183.62 | −197.87 | −212.04 | (7) |
| 3Fe2O3 + CO = 2Fe3O4 + CO2 | −131.75 | −137.51 | −143.21 | (8) |
| Fe3O4 + C = 3FeO + CO | −115.13 | −134.93 | −158.15 | (9) |
| Fe3O4 + 0.5C = 3FeO + 0.5CO2 | −63.26 | −74.56 | −89.32 | (10) |
| Fe3O4 + CO = 3FeO + CO2 | −11.40 | −14.20 | −20.49 | (11) |
| FeO + C = Fe + CO | −88.80 | −103.40 | −116.86 | (12) |
| FeO + 0.5C = Fe + 0.5CO2 | −36.93 | −43.04 | −48.04 | (13) |
| FeO + CO = Fe + CO2 | 14.94 | 17.33 | 20.79 | (14) |
| 3Fe + C = Fe3C | −5.16 | −6.01 | −6.55 | (15) |
| Component | Fe | C | Al | Si | Mn | P | S |
|---|---|---|---|---|---|---|---|
| reduced metal from low-alkali high-iron red mud | 94.33 | 4.32 | <0.01 | 0.32 | 0.022 | 0.18 | 0.025 |
| reduced metal from high-alkali high-iron red mud | 94.68 | 4.19 | <0.01 | 0.16 | 0.038 | 0.12 | 0.024 |
| iron nugget reduced from high-iron red mud in reference [28] | 96.52 | 3.09 | 0.051 | 0.013 | 0.076 | 0.091 | |
| L03 industrial standard for steel-making pig iron (YB/T 5296-2011) [32] | ≥3.5 | - | ≤0.35 | ≤0.40 | >0.10~0.15 | ≤0.030 |
| Component | Al2O3 | SiO2 | CaO | Na2O | TiO2 | TFe |
|---|---|---|---|---|---|---|
| Reduction slag of low-alkali high-iron red mud | 33.92 | 11.71 | 34.11 | 3.06 | 6.74 | 0.95 |
| Reduction slag of high-alkali high-iron red mud | 36.04 | 15.81 | 33.20 | 6.72 | 8.19 | 0.74 |
| Component | Fe | C | Al | Si | Mn | P | S |
|---|---|---|---|---|---|---|---|
| reduced metal from high-alkali high-iron red mud | 94.27 | 4.21 | <0.01 | 0.08 | 0.025 | 0.13 | 0.02 |
| L03 industrial standard for steel-making pig iron (YB/T 5296-2011) [32] | ≥3.5 | - | ≤0.35 | ≤0.40 | >0.10~0.15 | ≤0.03 |
| Test Item | Compressive Strength | Flexural Strength |
|---|---|---|
| Result | 102 MPa | 12.5 MPa |
| Doping Amount of Water Quenched Slag in Cement Samples | SSA | R7 | H7 | R28 | H28 |
|---|---|---|---|---|---|
| 30% | 418 | 35.8 | 89 | 48.4 | 93 |
| 492 | 39.9 | 98 | 50.3 | 96 | |
| 538 | 42.7 | 106 | 57.7 | 112 | |
| 50% | 418 | 29.3 | 74 | 47.8 | 93 |
| 492 | 33.8 | 84 | 49.7 | 97 | |
| 538 | 39.9 | 99 | 50.6 | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Zhang, T.-A.; Dou, Z.-H.; Liu, Y.; Lv, G.-Z. Cleaner Production of Metallurgical-Grade Iron from High-Iron Bauxite Residue via Smelting Reduction: Thermodynamic Control, Industrial Application Potential, and Slag Utilization Strategy. Materials 2025, 18, 3288. https://doi.org/10.3390/ma18143288
Wang K, Zhang T-A, Dou Z-H, Liu Y, Lv G-Z. Cleaner Production of Metallurgical-Grade Iron from High-Iron Bauxite Residue via Smelting Reduction: Thermodynamic Control, Industrial Application Potential, and Slag Utilization Strategy. Materials. 2025; 18(14):3288. https://doi.org/10.3390/ma18143288
Chicago/Turabian StyleWang, Kun, Ting-An Zhang, Zhi-He Dou, Yan Liu, and Guo-Zhi Lv. 2025. "Cleaner Production of Metallurgical-Grade Iron from High-Iron Bauxite Residue via Smelting Reduction: Thermodynamic Control, Industrial Application Potential, and Slag Utilization Strategy" Materials 18, no. 14: 3288. https://doi.org/10.3390/ma18143288
APA StyleWang, K., Zhang, T.-A., Dou, Z.-H., Liu, Y., & Lv, G.-Z. (2025). Cleaner Production of Metallurgical-Grade Iron from High-Iron Bauxite Residue via Smelting Reduction: Thermodynamic Control, Industrial Application Potential, and Slag Utilization Strategy. Materials, 18(14), 3288. https://doi.org/10.3390/ma18143288







