Electrochemical and Kinetic Performance of Low-Cobalt and Cobalt-Free Rare-Earth AB5-Type Hydrogen Storage Alloys
Abstract
1. Introduction
2. Experimental Methods
2.1. Preparation of Alloys
2.2. Structural Characterization
2.3. Pressure–Composition–Temperature (PCT) Tests
2.4. Electrochemical Measurements
2.5. Electrochemical Kinetic Measurements
3. Results and Discussion
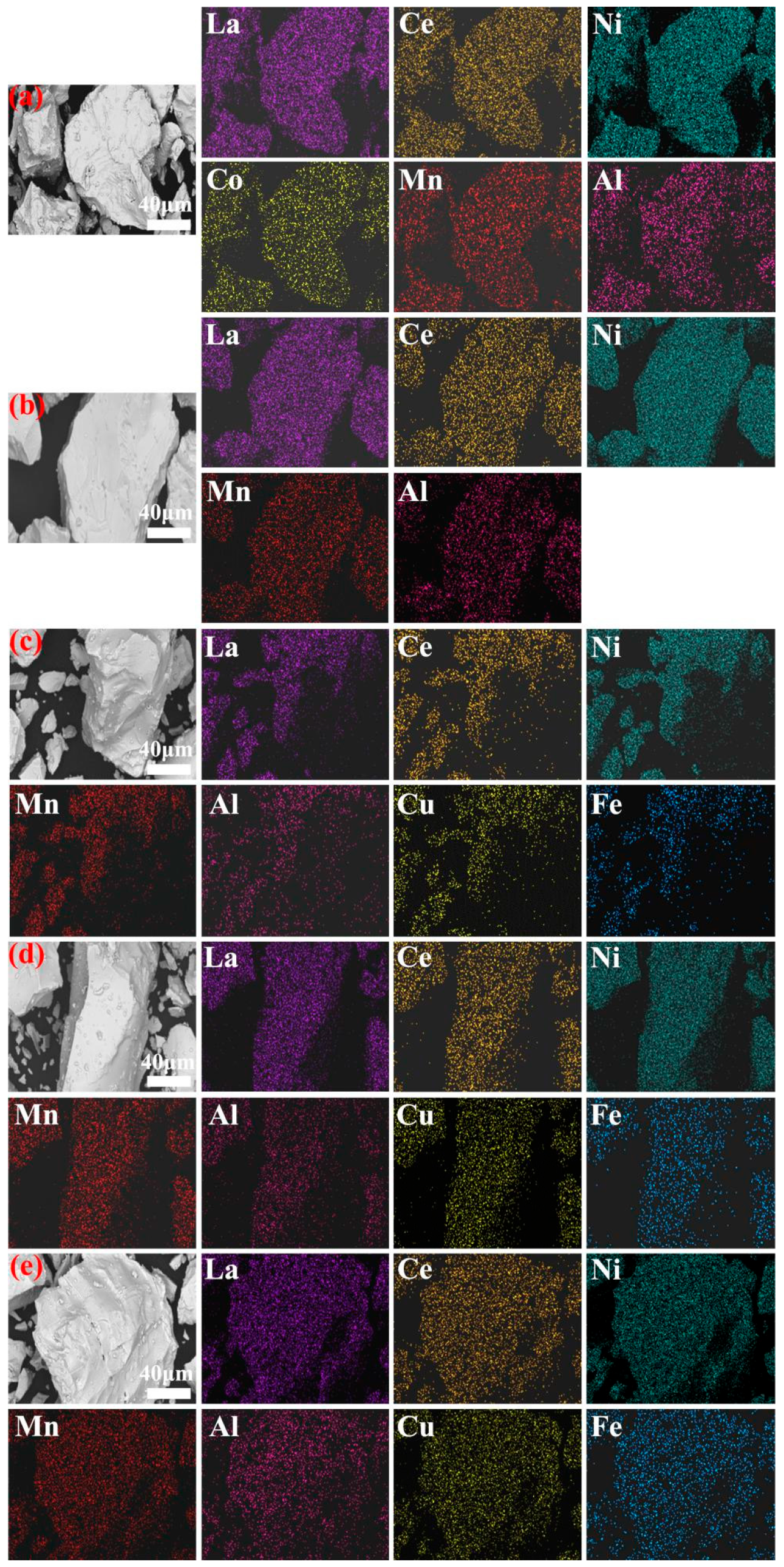
3.1. Crystal Structures
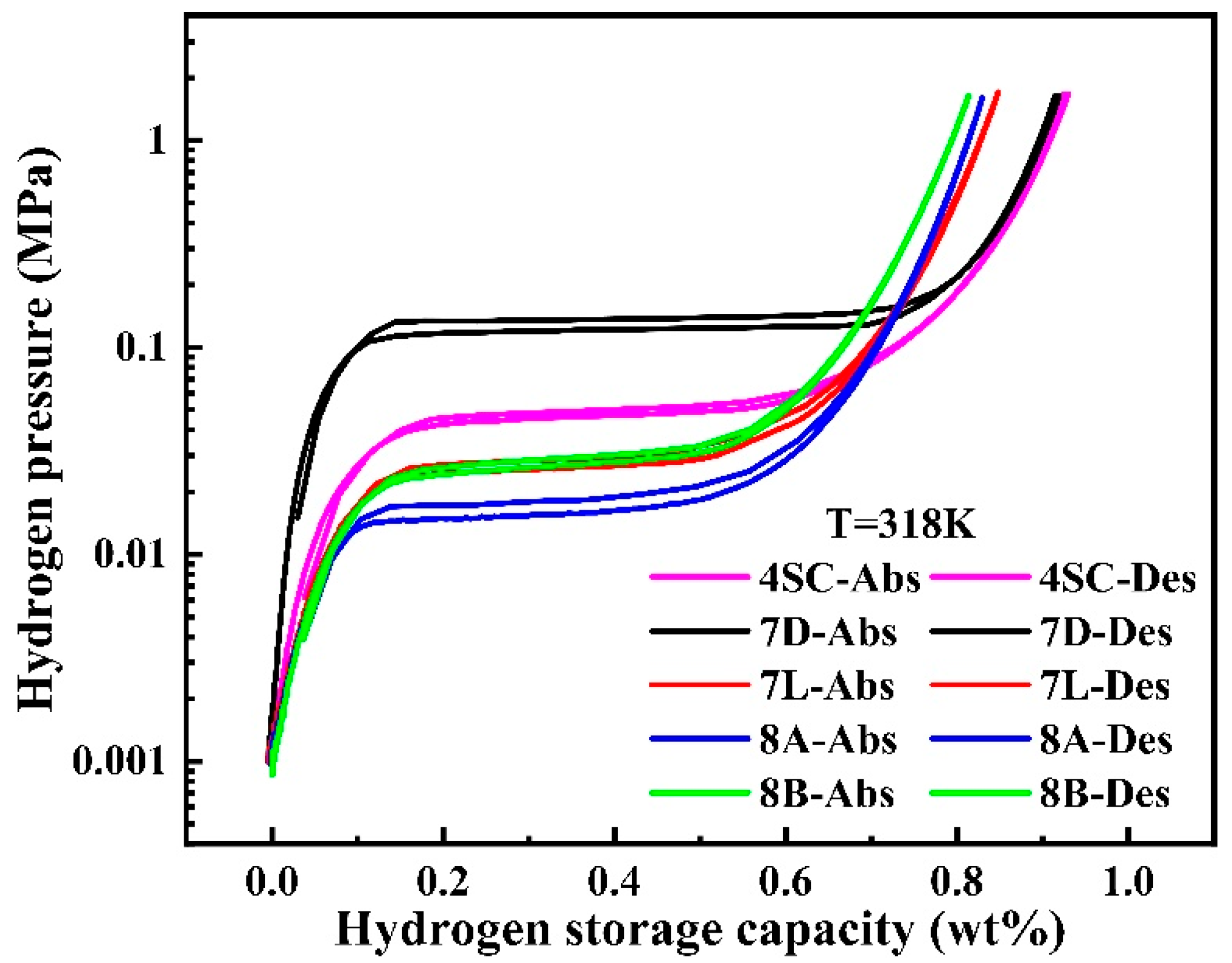
3.2. PCT Test
3.3. Electrochemical Performance
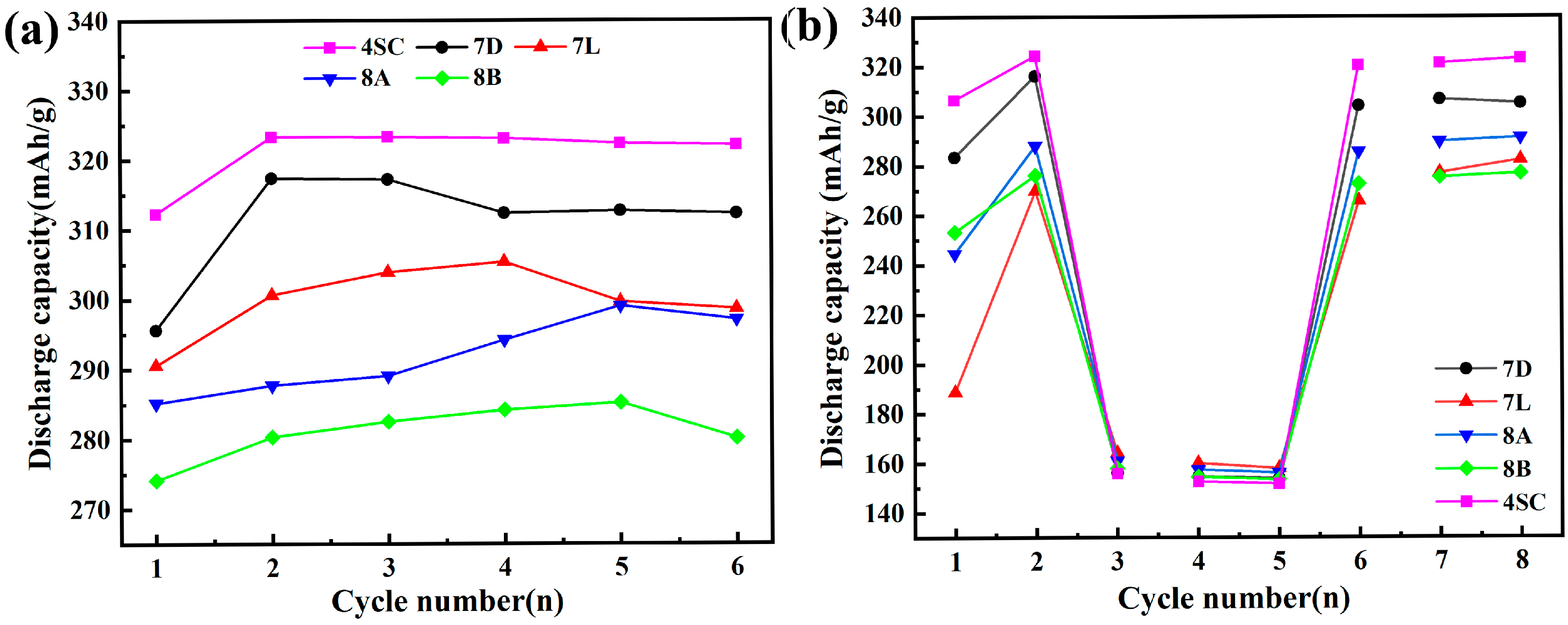
3.3.1. Maximum Discharge Capacity and Conventional Discharge Capacity
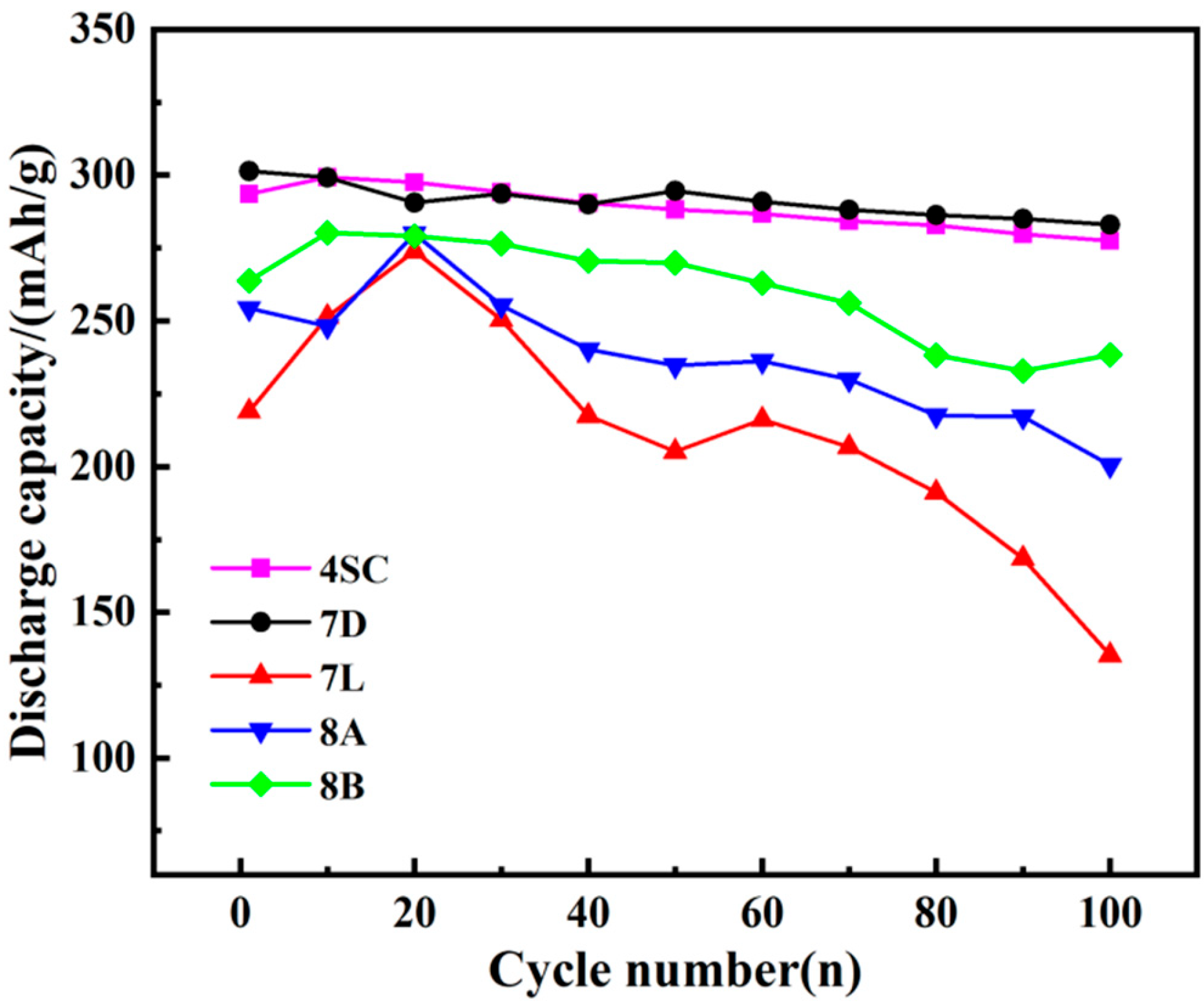
3.3.2. Cycle Stability
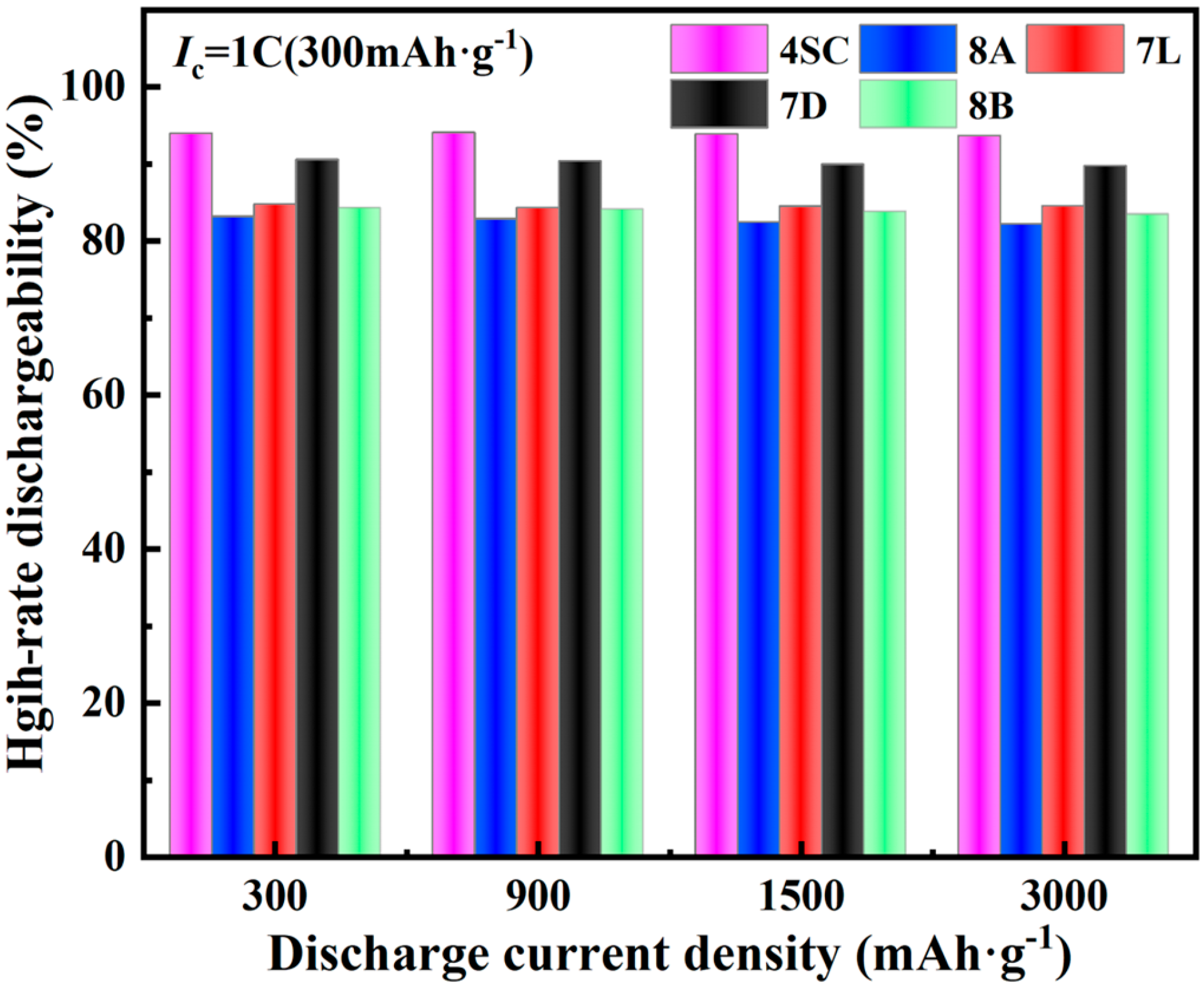
3.3.3. High-Rate Properties
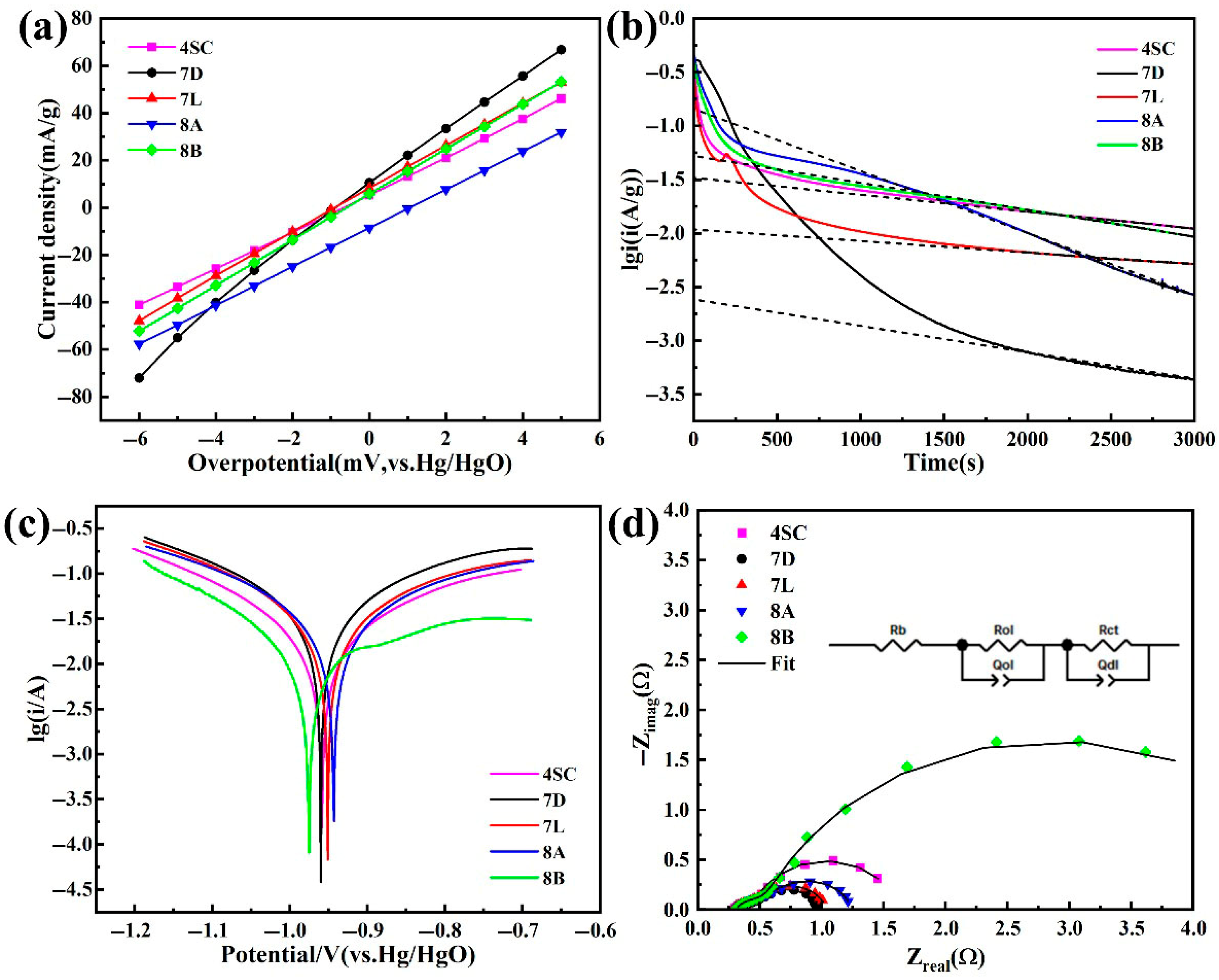
3.4. Electrochemical Reaction Kinetics
4. Conclusions
- (1)
- XRD and SEM analyses reveal that after doping with various elements, the metal hydride alloys retain the single-phase CaCu5-type LaNi5 structure, with elements uniformly distributed throughout the alloy matrix.
- (2)
- The electrochemical behavior of the metal hydride alloys was evaluated, revealing that alloys 4SC and 7D exhibit superior performance compared with the other three alloys. These two alloys attain their peak discharge capacities during the second or third cycle, with peak discharge capacities of 323.3 mAh g−1 and 317.4 mAh g−1, respectively. Their conventional discharge capacities are 323.1 mAh g−1 and 305.1 mAh g−1, respectively, and after 100 cycles, their capacity retention rates stay at 89.16% and 85.83%, respectively. Under a 3 C discharge rate, their HRD values are 94.1% and 90.3%, respectively.
- (3)
- Kinetic performance analysis of the hydrogen storage alloys indicates that alloys 4SC and 7D exhibit outstanding performance, with I0 values of 302.97 mA g−1 and 317.70 mA g−1 and D values of 5.88 × 10−10 cm2 s−1 and 9.4 × 10−10 cm2 s−1, respectively. Cobalt-free alloy 7D demonstrates superior electrochemical kinetic performance compared with the other four alloys, providing a critical theoretical foundation for the development of cobalt-free industrial AB5-type hydrogen storage alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Zhang, Y.H.; Wang, X.L.; Lin, Y.F.; Qu, X.H. Research of Mm(NiMnAlCu) 4.9Co0.2 hydrogen storage alloys prepared by cast and rapidly quenched. J. Power Sources 2003, 124, 285–292. [Google Scholar] [CrossRef]
- Cocciantelli, J.M.; Bernard, P.; Fernandez, S.; Atkin, J. The influence of Co and various additives on the performance of MmNi4.3−xMn0.33Al0.4Cox hydrogen storage alloys and Ni/MH prismatic sealed cells. J. Alloys Compd. 1997, 253–254, 642–647. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.L.; Zhang, Y.H.; Wu, J.M.; Li, R.; Qu, X.H. Research of low-Co AB5 type rare-earth-based hydrogen storage alloy electrodes. J. Alloys Compd. 2003, 354, 310–314. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.L.; Zhang, Y.H.; Li, R.; Wu, J.M.; Qu, X.H. Research on electrochemical characteristics and microstructure of Mm(NiMnAl)4.9Co0.2 rapidly quenched alloy. J. Alloys Compd. 2003, 353, 278–282. [Google Scholar] [CrossRef]
- Hu, W.K.; Lee, H.; Kim, D.M.; Jeon, S.W.; Lee, J.Y. Electrochemical behaviors of low-Co Mm-based alloys as MH electrodes. J. Alloys Compd. 1998, 268, 261–265. [Google Scholar] [CrossRef]
- Jiang, L.J.; Zhan, F.; Bao, D.Y.; Qing, G.G.; Li, Y.Q.; Wei, X.Y. Low cost AB5-type hydrogen storage alloys for a nickel-metal hydride battery. J. Alloys Compd. 1995, 231, 635–638. [Google Scholar] [CrossRef]
- Nahm, K.S.; Kim, W.Y.; Hong, S.P.; Lee, W.Y. The reaction kinetics of hydrogen storage in LaNi5. Int. J. Hydrogen Energy 1992, 17, 333–338. [Google Scholar] [CrossRef]
- Sakai, T.; Miyamura, H.; Kuriyama, N.; Kato, A.; Oguro, K.; Ishikawa, H. Metal hydride anodes for nickel-hydrogen secondary battery. J. Electrochem. Soc. 1990, 137, 795–799. [Google Scholar] [CrossRef]
- Pan, H.G.; Ma, J.X.; Wang, C.S.; Chen, C.P.; Wang, Q.D. Effect of Co content on the kinetic properties of the MlNi4.3−xCoxAl0.7 hydride electrodes. Electrochim. Acta 1999, 44, 3977–3987. [Google Scholar] [CrossRef]
- Pandey, S.K.; Srivastava, A.; Srivastava, O.N. Improvement in hydrogen storage capacity in through substitution of Ni by Fe. Int. J. Hydrogen Energy 2007, 32, 2461–2465. [Google Scholar] [CrossRef]
- Chartouni, D.; Meli, F.; Züttel, A.; Gross, K.; Schlapbach, L. The influence of cobalt on the electrochemical cycling stability of LaNi5-based hydride forming alloys. J. Alloys Compd. 1996, 241, 160–166. [Google Scholar] [CrossRef]
- Vivet, S.; Joubert, J.M.; Knosp, B.; Percheron-Guégan, A. Effects of cobalt replacement by nickel, manganese, aluminium and iron on the crystallographic and electrochemical properties of AB5-type alloys. J. Alloys Compd. 2003, 356, 779–783. [Google Scholar] [CrossRef]
- Lei, Y.Q.; Zhang, S.K.; Lu, G.L.; Chen, L.X.; Wang, Q.D.; Wu, F. Influence of the material processing on the electrochemical properties of cobalt-free Ml(NiMnAlFe)5 alloy. J. Alloys Compd. 2002, 330, 861–865. [Google Scholar] [CrossRef]
- Khaldi, C.; Mathlouthi, H.; Lamloumi, J.; Percheron-Guégan, A. Electrochemical study of cobalt-free AB5-type hydrogen storage alloys. Int. J. Hydrogen Energy 2004, 29, 307–311. [Google Scholar] [CrossRef]
- Tliha, M.; Khaldi, C.; Mathlouthi, H.; Lamloumi, J.; Percheron-Guégan, A. Electrochemical investigation of the iron-containing and no iron-containing AB5-type negative electrodes. J. Alloys Compd. 2007, 440, 323–327. [Google Scholar] [CrossRef]
- Wei, X.D.; Liu, S.S.; Dong, H.; Zhang, P.; Liu, Y.N.; Zhu, J.W.; Yu, G. Microstructures and electrochemical properties of Co-free AB5-type hydrogen storage alloys through substitution of Ni by Fe. Electrochim. Acta 2007, 52, 2423–2428. [Google Scholar] [CrossRef]
- Tliha, M.; Khaldi, C.; Lamloumi, J. AC Impedance Behavior of LaNi3.55Mn0.4Al0.3Co0.6Fe0.15 Hydrogen-Storage Alloy: Effect of Surface Area. J. Mater. Eng. Perform. 2016, 25, 1578–1585. [Google Scholar] [CrossRef]
- Tang, W.; Gai, Y.; Zheng, H. Deterioration of copper-containing mischmetal-nickel-based hydrogen absorption electrode materials. J. Alloys Compd. 1995, 224, 292–298. [Google Scholar] [CrossRef]
- Li, S.L.; Wang, P.; Chen, W.; Luo, G.; Hua, X.B.; Chen, D.M.; Yang, K. Study on hydrogen storage properties of LaNi3.8Al1.2−xMnx alloys. Int. J. Hydrogen Energy 2010, 35, 12391–12397. [Google Scholar] [CrossRef]
- Lototsky, M.V.; Yartys, V.A.; Marinin, V.S.; Lototsky, N.M. Modelling of phase equilibria in metal–hydrogen systems. J. Alloys Compd. 2003, 356, 27–31. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Park, C.N.; Oates, W.A. Hysteresis in solid state reactions. Prog. Solid State Chem. 1995, 23, 291–363. [Google Scholar] [CrossRef]
- Nishina, T.; Ura, H.; Uchida, I. Determination of the chemical diffusion coefficients in metal hydride particles with a microelectrode technique. J. Electrochem. Soc. 1997, 144, 1273–1277. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Swain, E., Ed.; University Graphics: New York, NY, USA, 2001; pp. 89–93. [Google Scholar]
- Zhou, W.H.; Tang, Z.Y.; Zhu, D.; Ma, Z.W.; Wu, C.L.; Huang, L.W.; Chen, Y.G. Low-temperature and instantaneous high-rate output performance of AB5-type hydrogen storage alloy with duplex surface hot-alkali treatment. J. Alloys Compd. 2017, 692, 364–374. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhou, H.Y.; Liu, P.; Yao, Q.R.; Deng, J.Q.; Wang, Z.M. Effect of Ce content on structure and electrochemical properties of La0.8–xCexPr0.1Nd0.1B5 (B = Ni, Co, Mn; 0 ≤ x ≤ 0.3) hydrogen storage alloys. Russ. J. Electrochem. 2016, 52, 169–173. [Google Scholar] [CrossRef]
- Liao, B.; Lei, Y.Q.; Chen, L.X.; Lu, G.L.; Pan, H.G.; Wang, Q.D. A study on the structure and electrochemical properties of La2Mg(Ni0.95M0.05)9 (M = Co, Mn, Fe, Al, Cu, Sn) hydrogen storage electrode alloys. J. Alloys Compd. 2004, 376, 186–195. [Google Scholar] [CrossRef]
- Liu, J.J.; Li, K.; Cheng, H.H.; Yan, K.; Wang, Y.; Liu, Y.; Jin, H.M.; Zheng, Z. New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5-xAlx alloys. Int. J. Hydrogen Energy 2017, 42, 24904–24914. [Google Scholar] [CrossRef]
- Gao, M.X.; Zhang, S.C.; Miao, H.; Liu, Y.F.; Pan, H.G. Pulverization mechanism of the multiphase Ti-V-based hydrogen storage electrode alloy during charge/discharge cycling. J. Alloys Compd. 2010, 489, 552–557. [Google Scholar] [CrossRef]
- Ben-Moussa, M.; Abdellaoui, M.; Mathlouthi, H.; Lamloumi, J.; Percheron Guégan, A. Electrochemical Properties of the MmNi3.55Mn0.4Al0.3Co0.75−xFex(x = 0.55 and 0.75) Compounds. J. Alloys Compd. 2008, 458, 410–414. [Google Scholar] [CrossRef]
- Kong, L.; Chen, B.; Young, K.; Koch, J.; Chan, A.; Li, W. Effects of Al- and Mn-contents in the negative MH alloy on the self-discharge and long-term storage properties of Ni/MH battery. J. Power Sources 2012, 213, 128–139. [Google Scholar] [CrossRef]
- Zheng, G.; Popov, B.N.; White, R.E. Determination of Transport and Electrochemical Kinetic Parameters of Bare and Copper-Coated LaNi4.27Sn0.24 Electrodes in Alkaline Solution. J. Electrochem. Soc. 1996, 143, 834–839. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhong, C.L.; Ma, Z.W.; Yang, F.; Wu, Y.C.; Zhu, D.; Wu, C.L.; Chen, Y.G. Improvement in high-temperature performance of Co-free high-Fe AB5-type hydrogen storage alloys. Int. J. Hydrogen Energy 2012, 30, 361–366. [Google Scholar]
- Kuriyama, N.; Sakai, T.; Miyamura, H.; Uehara, I.; Ishikawa, H.; Iwasaki, T. Electrochemical Impedance and Deterioration Behavior of Metal Hydride Electrodes. J. Alloys Compd. 1993, 202, 183–197. [Google Scholar] [CrossRef]
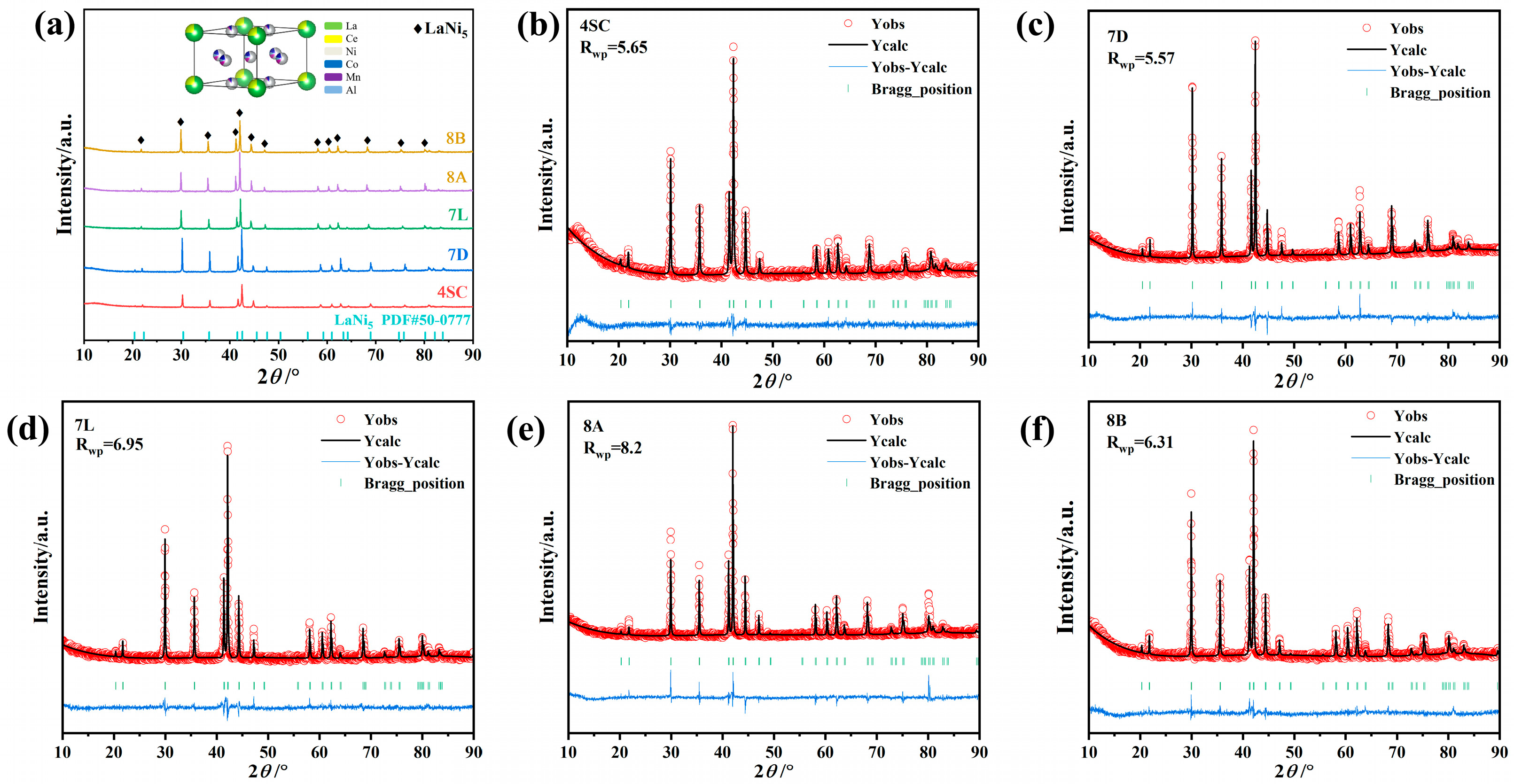
| Samples | Lattice Constants | Cell Volume (Å3) | ||
|---|---|---|---|---|
| a (Å) | c (Å) | c/a | ||
| 4SC | 5.0171 | 4.0512 | 0.8077 | 88.314 |
| 7D | 5.0048 | 4.0459 | 0.8084 | 87.764 |
| 7L | 5.0344 | 4.0871 | 0.8118 | 89.712 |
| 8A | 5.0601 | 4.0780 | 0.8059 | 90.426 |
| 8B | 5.0491 | 4.0797 | 0.8080 | 90.069 |
| Samples | H2 Storage Capacity (wt%) | H2 Absorption Plateau (MPa) | H2 Desorption Plateau (MPa) | Slope | Hysteresis |
|---|---|---|---|---|---|
| 4SC | 0.926 | 0.050 | 0.047 | 3.928 | 0.062 |
| 7D | 0.915 | 0.139 | 0.123 | 2.402 | 0.122 |
| 7L | 0.848 | 0.029 | 0.026 | 4.638 | 0.109 |
| 8A | 0.830 | 0.018 | 0.016 | 5.581 | 0.118 |
| 8B | 0.814 | 0.030 | 0.027 | 5.314 | 0.105 |
| Samples | Na a | Cmax (mAh g−1) | Cr,8 b (mAh g−1) | S100 c (%) |
|---|---|---|---|---|
| 298 K | ||||
| 4SC | 3 | 323.3 | 323.1 | 85.83 |
| 7D | 2 | 317.4 | 305.1 | 89.16 |
| 7L | 4 | 305.4 | 282.2 | 44.30 |
| 8A | 5 | 299.1 | 291.2 | 67.03 |
| 8B | 5 | 311.2 | 276.8 | 76.57 |
| Samples | ct (Ω) | I0 (mA/g) | D (×10−10 cm2/s) | corr (V) | icorr (A) |
|---|---|---|---|---|---|
| 4SC | 0.875 | 302.97 | 5.88 | −0.961 | 0.011 |
| 7D | 0.490 | 317.70 | 9.45 | −0.961 | 0.021 |
| 7L | 0.580 | 235.39 | 4.04 | −0.952 | 0.017 |
| 8A | 0.646 | 209.46 | 9.36 | −0.944 | 0.016 |
| 8B | 4.703 | 246.50 | 9.36 | −0.987 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zhang, F.; Ma, H.; Zhao, Y.; Wang, Y.; Wang, X.; Li, X.; Luo, Y.; Lu, B. Electrochemical and Kinetic Performance of Low-Cobalt and Cobalt-Free Rare-Earth AB5-Type Hydrogen Storage Alloys. Materials 2025, 18, 3317. https://doi.org/10.3390/ma18143317
Shen Y, Zhang F, Ma H, Zhao Y, Wang Y, Wang X, Li X, Luo Y, Lu B. Electrochemical and Kinetic Performance of Low-Cobalt and Cobalt-Free Rare-Earth AB5-Type Hydrogen Storage Alloys. Materials. 2025; 18(14):3317. https://doi.org/10.3390/ma18143317
Chicago/Turabian StyleShen, Yingying, Fengji Zhang, Hengyu Ma, Yun Zhao, Yong Wang, Xinfeng Wang, Xiuyan Li, Youcheng Luo, and Bingang Lu. 2025. "Electrochemical and Kinetic Performance of Low-Cobalt and Cobalt-Free Rare-Earth AB5-Type Hydrogen Storage Alloys" Materials 18, no. 14: 3317. https://doi.org/10.3390/ma18143317
APA StyleShen, Y., Zhang, F., Ma, H., Zhao, Y., Wang, Y., Wang, X., Li, X., Luo, Y., & Lu, B. (2025). Electrochemical and Kinetic Performance of Low-Cobalt and Cobalt-Free Rare-Earth AB5-Type Hydrogen Storage Alloys. Materials, 18(14), 3317. https://doi.org/10.3390/ma18143317






