Mechanistic Insights into Full Solid-Waste Activators for Enhancing the Performance of Blast Furnace Slag–Fly Ash Cementitious Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
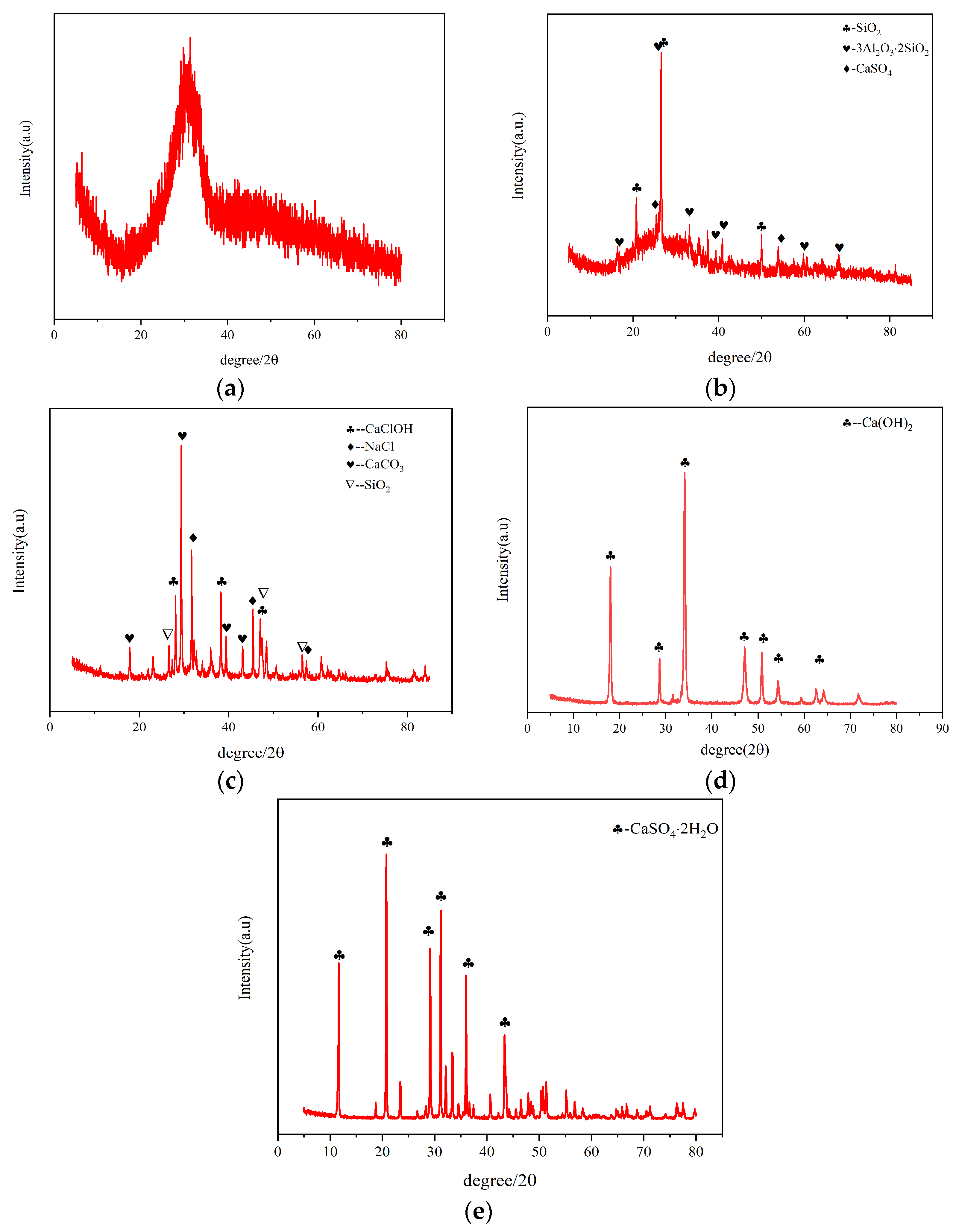
- GGBS (Figure 2a) displayed a broad hump between 20 and 40° 2θ, indicating predominant amorphous glass phases with high reactivity.
- FA (Figure 2b) contained crystalline quartz (SiO2) and mullite (3Al2O3·2SiO2) with secondary phases of anhydrite (CaSO4), accompanied by reactive glassy phases.
- SR (Figure 2c) primarily consisted of Calcite (CaCO3), Common salt (NaCl), and Calcium chloride (CaClOH) phases formed through complex precipitation processes involving Ca2+, Cl−, CO32−, and Na+ ions.
- CS showed a dominant Ca(OH)2 phase (91.66% CaO content).
- Gypsum was identified as dihydrate calcium sulfate (CaSO4·2H2O).
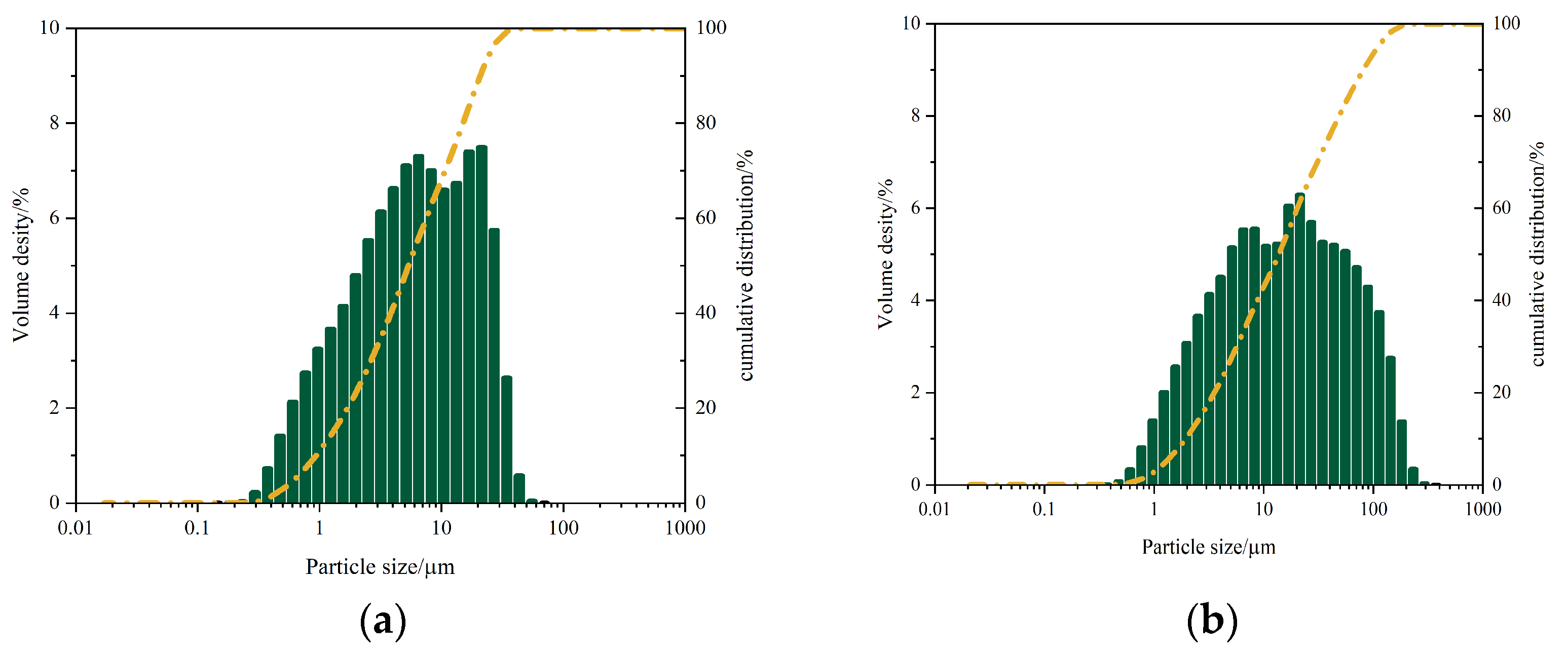
- GGBS: median diameter (D50) = 5.40 μm, mean diameter = 4.91 μm, mode diameter = 19.02 μm, SD = 0.49;
- FA: D50 = 13.74 μm, mean diameter = 12.89 μm, mode diameter = 19.02 μm, SD = 0.59.
2.2. Mix Proportion Design
- (1).
- Phase I—Activator optimization:
- Baseline groups (BG-series) with GGBS as sole precursor;
- CS: 4/8/12 wt%;
- SR: 26/22/18 wt%;
- Constant GGBS content: 70 wt%.
- (2).
- Phase II—Binary precursor system:
- Optimal activator combination with FA substitution (10/20/30 wt%, OG-series).
- (3).
- Phase III—Sulfate activation:
- Gypsum substitution (4–10 wt%, SOG-series) in FA-containing system
2.3. Specimen Preparation
- (1).
- Precisely weigh constituents according to Table 2 proportions.
- (2).
- Sequentially add materials to the pre-wetted mortar mixer:
- Initial low-speed mixing (140 ± 5 rpm): 30 s binder–water blending;
- Standard sand incorporation during the second 30 s of low-speed mixing;
- High-speed mixing (285 ± 10 rpm): 30 s + 60 s after 90 s rest period.
- (3).
- Cast 40 × 40 × 160 mm prism specimens using two-layer placement.
- (4).
- Compact each layer with 60 vibrations on the standard jolting table.
- (5).
- Cure under controlled conditions (20 ± 2 °C, 95% RH) for 24 h prior to demolding.
2.4. Testing Protocols
- Strength development: Measure 3/7/28-day compressive strength per GB/T 17671 (ISO 679) [16];
- Phase analysis: Rigaku D/max-A XRD (Tokyo, Japan) (Cu-Kα, 40 kV/40 mA, 5–85° 2θ, 2°/min);
- Thermal analysis: Shimadzu DTG-60 AH (Shanghai, China) (N2, 50 mL/min, 10 °C/min to 900 °C);
- Molecular characterization: Nicolet iS5 FTIR (Beijing, China) (400–4000 cm−1, KBr pellet);
- Microstructural observation: ZEISS Sigma300 SEM (Jena, Germany) (90 s Au-sputtered samples).
2.5. Statistical Analysis
3. Results and Discussion
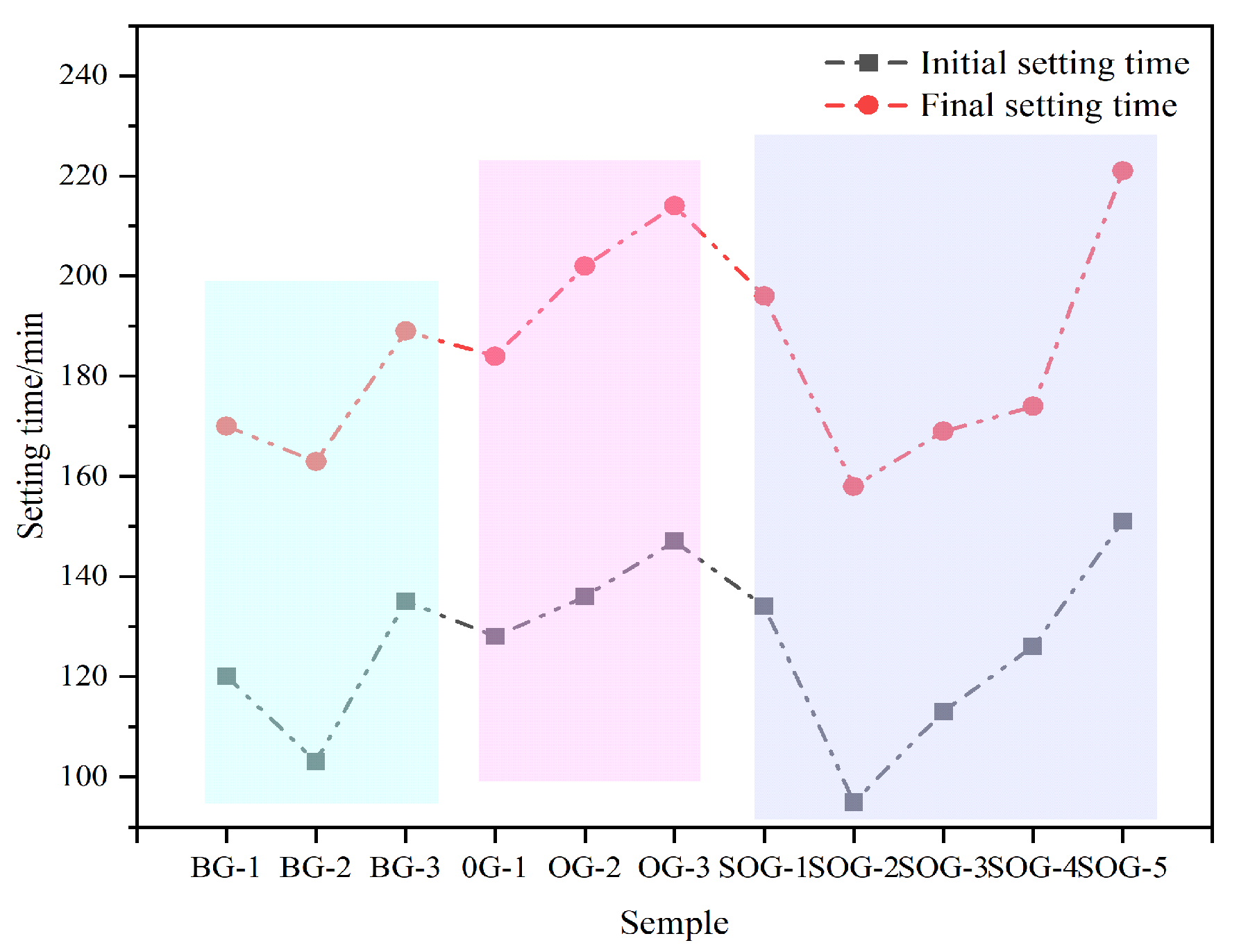
3.1. The Influence of Multi-Source Solid-Waste Activation on the Standard Consistency Water Demand and Setting Time of the GGBS-FA System
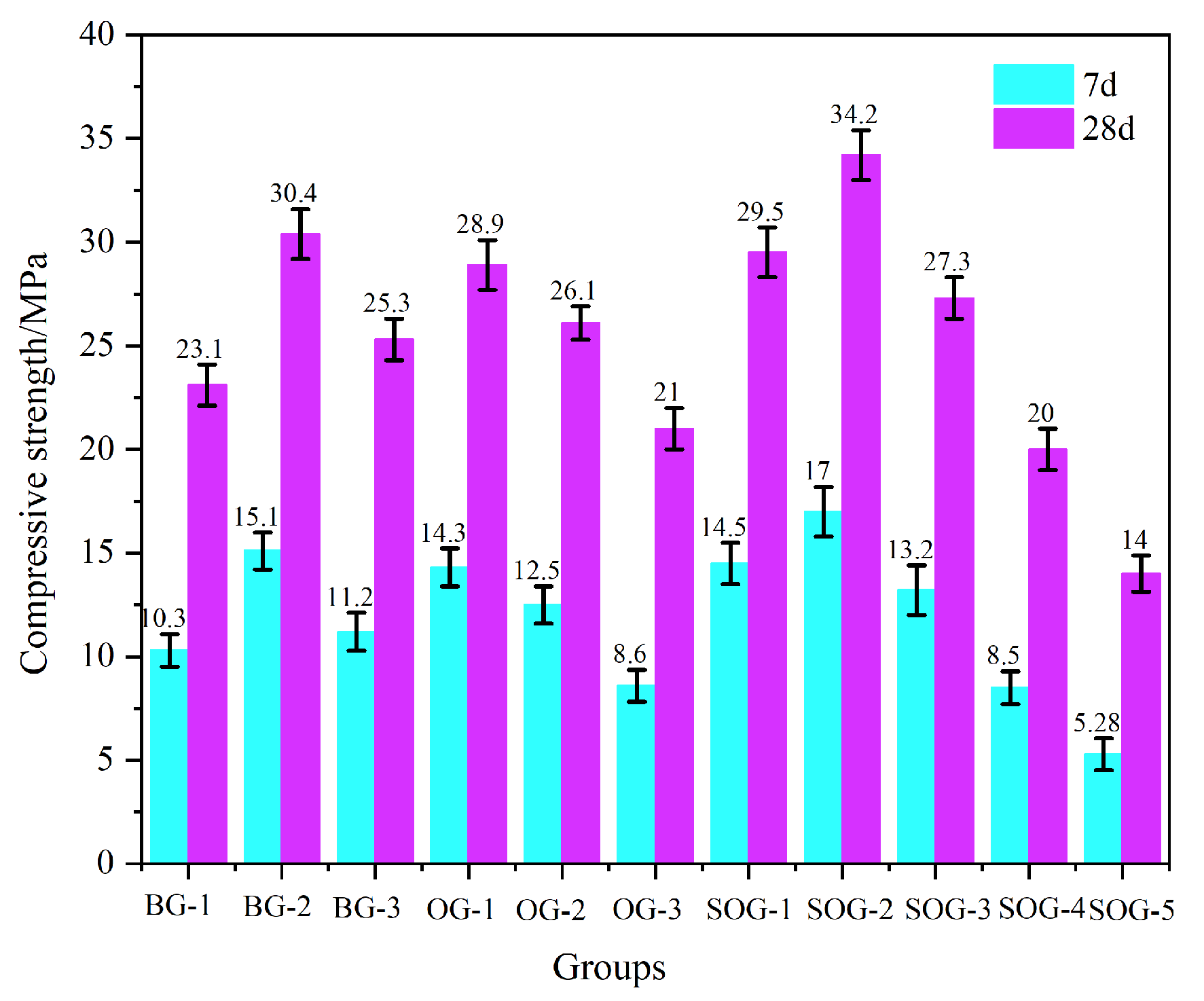
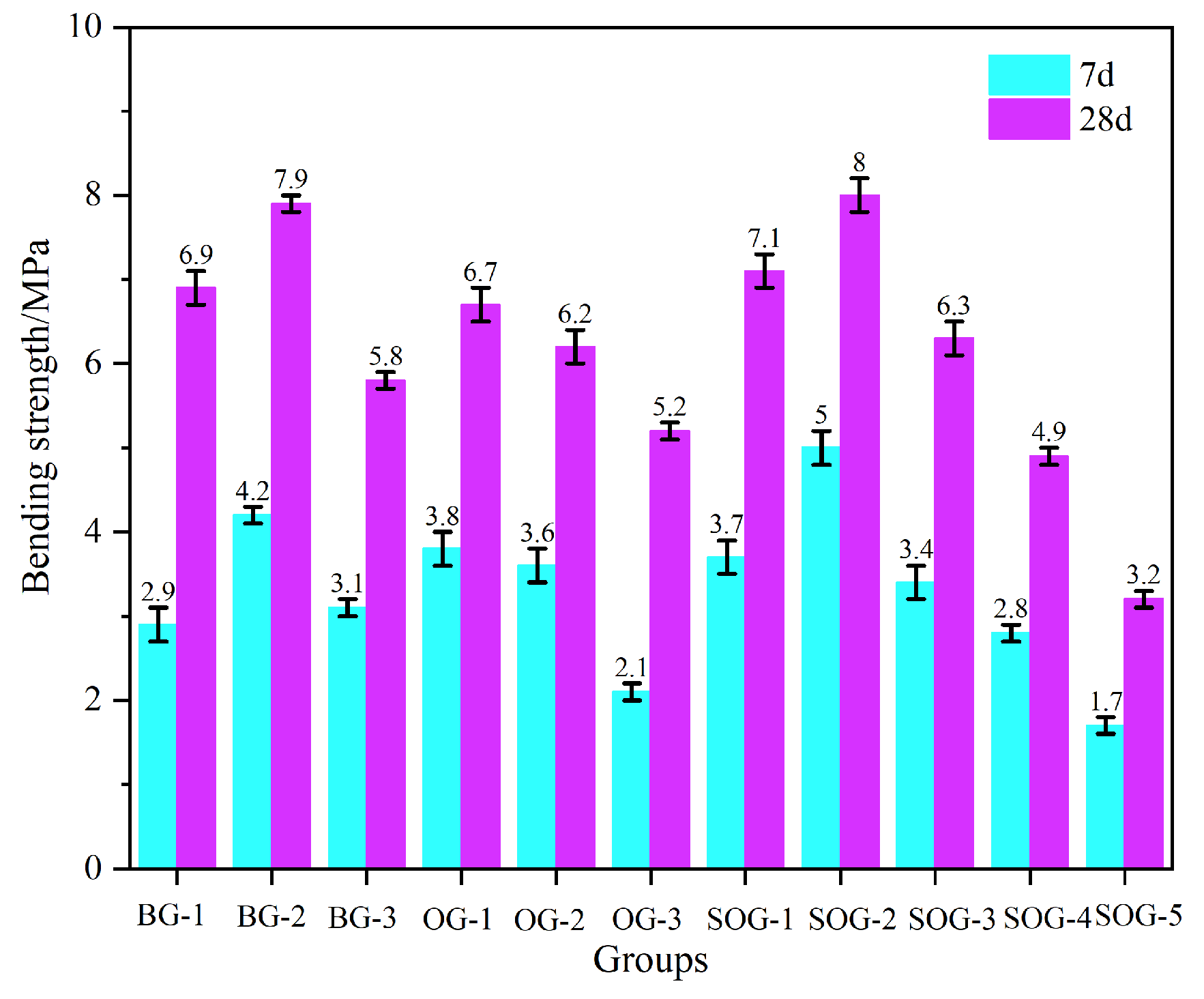
3.2. Effects of Multi-Source Solid-Waste Activation on Mechanical Properties of Slag–Fly Ash System
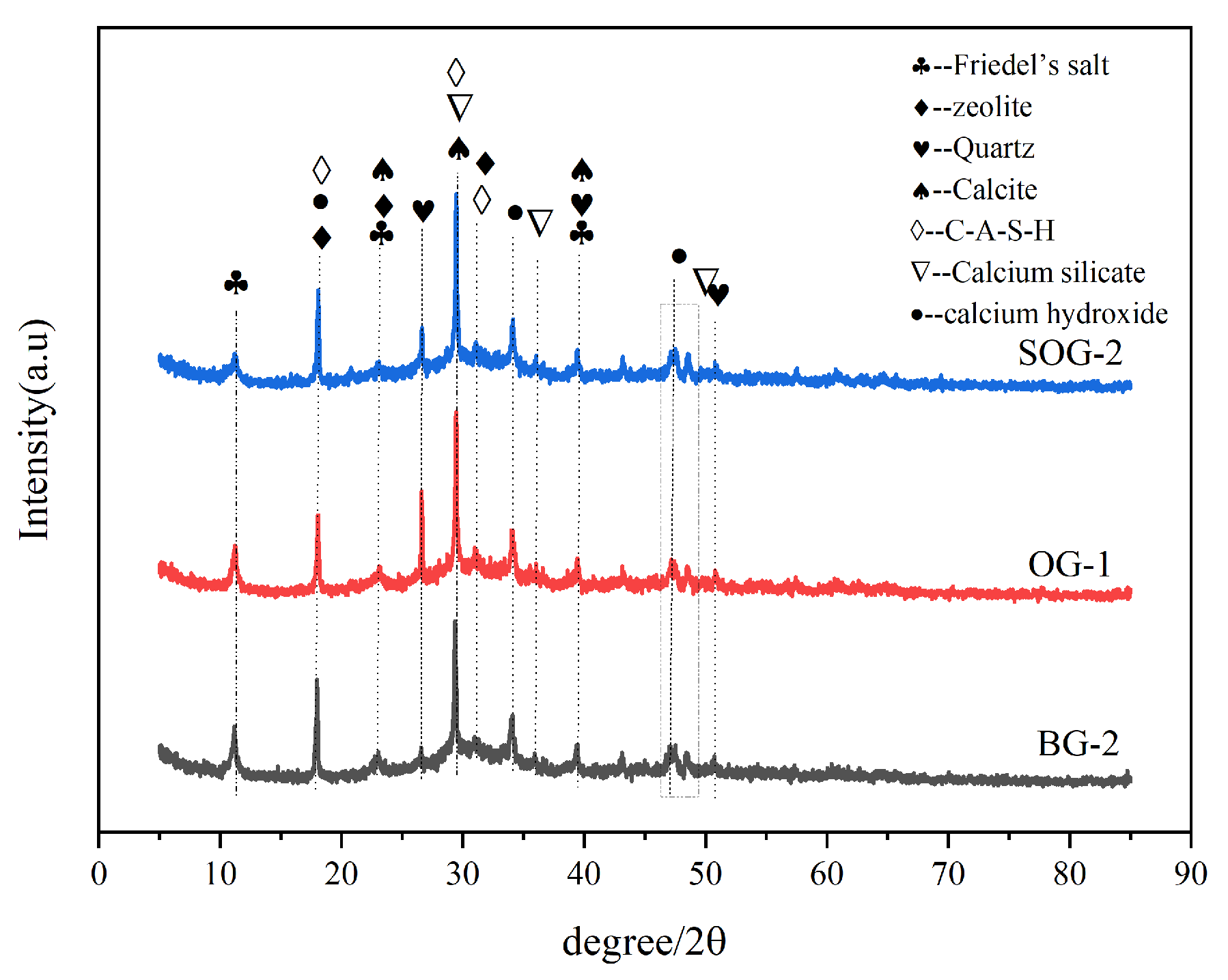
3.3. XRD Analysis
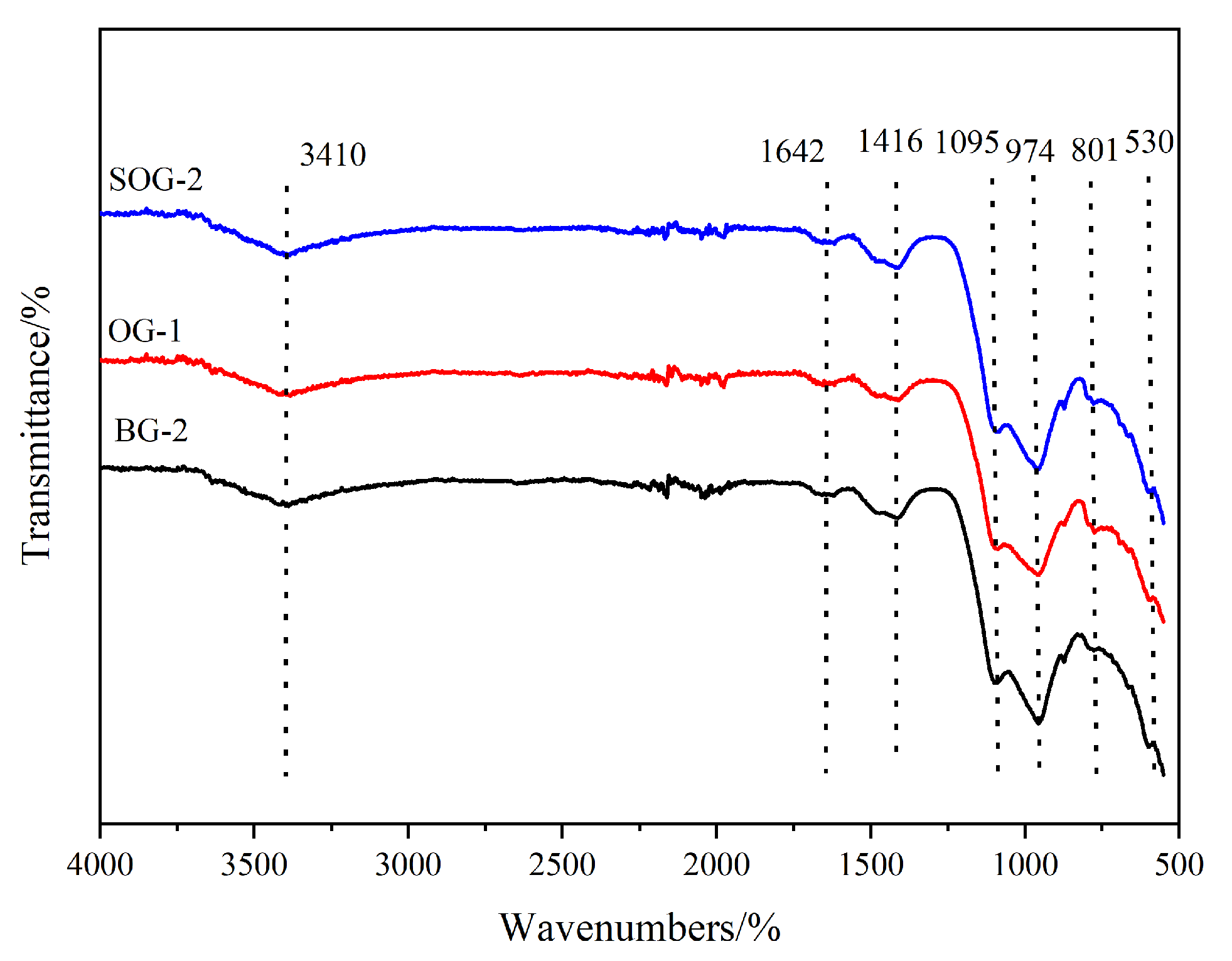
3.4. FTIR Analysis
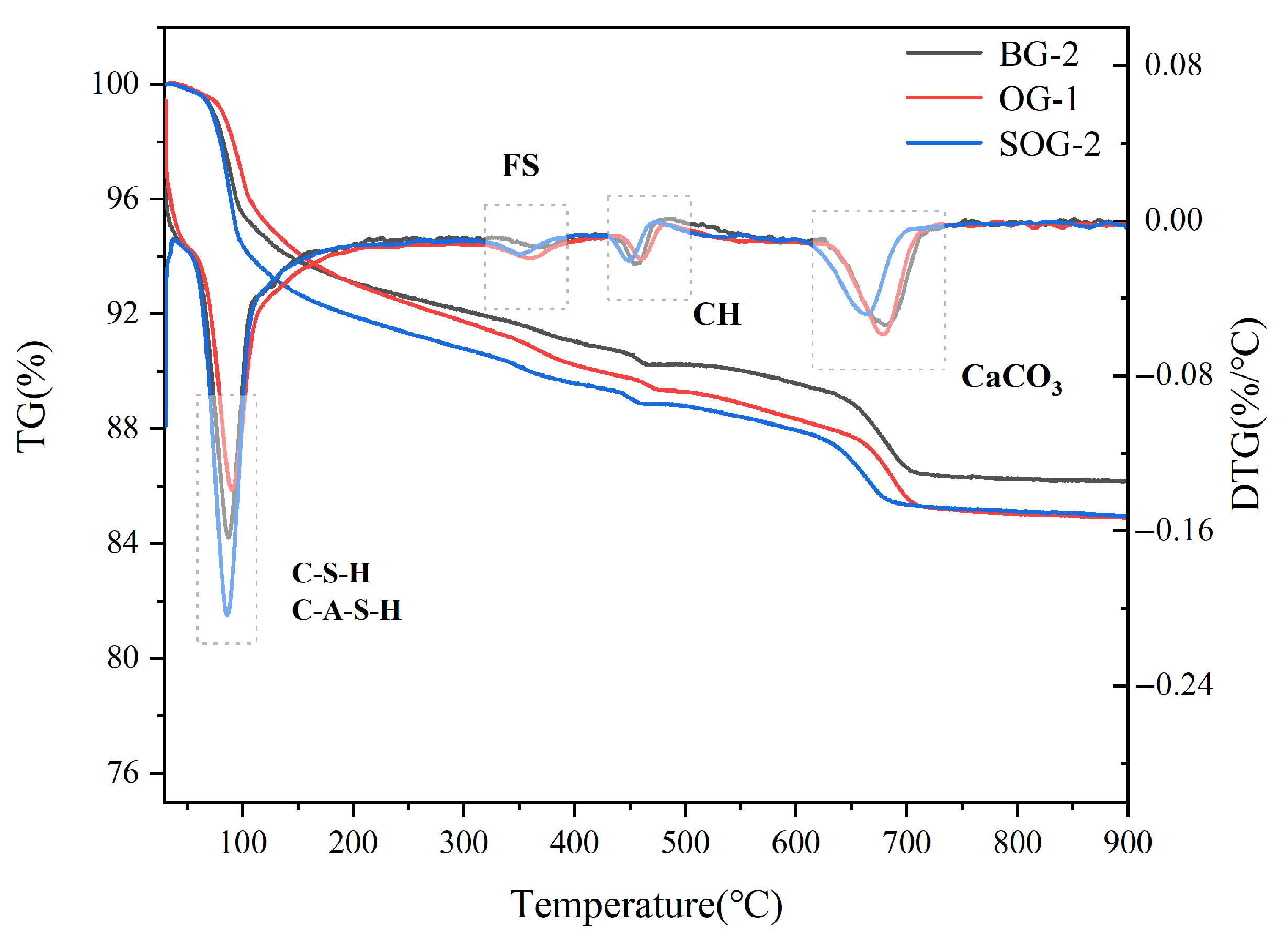
3.5. TG-DTG Analysis
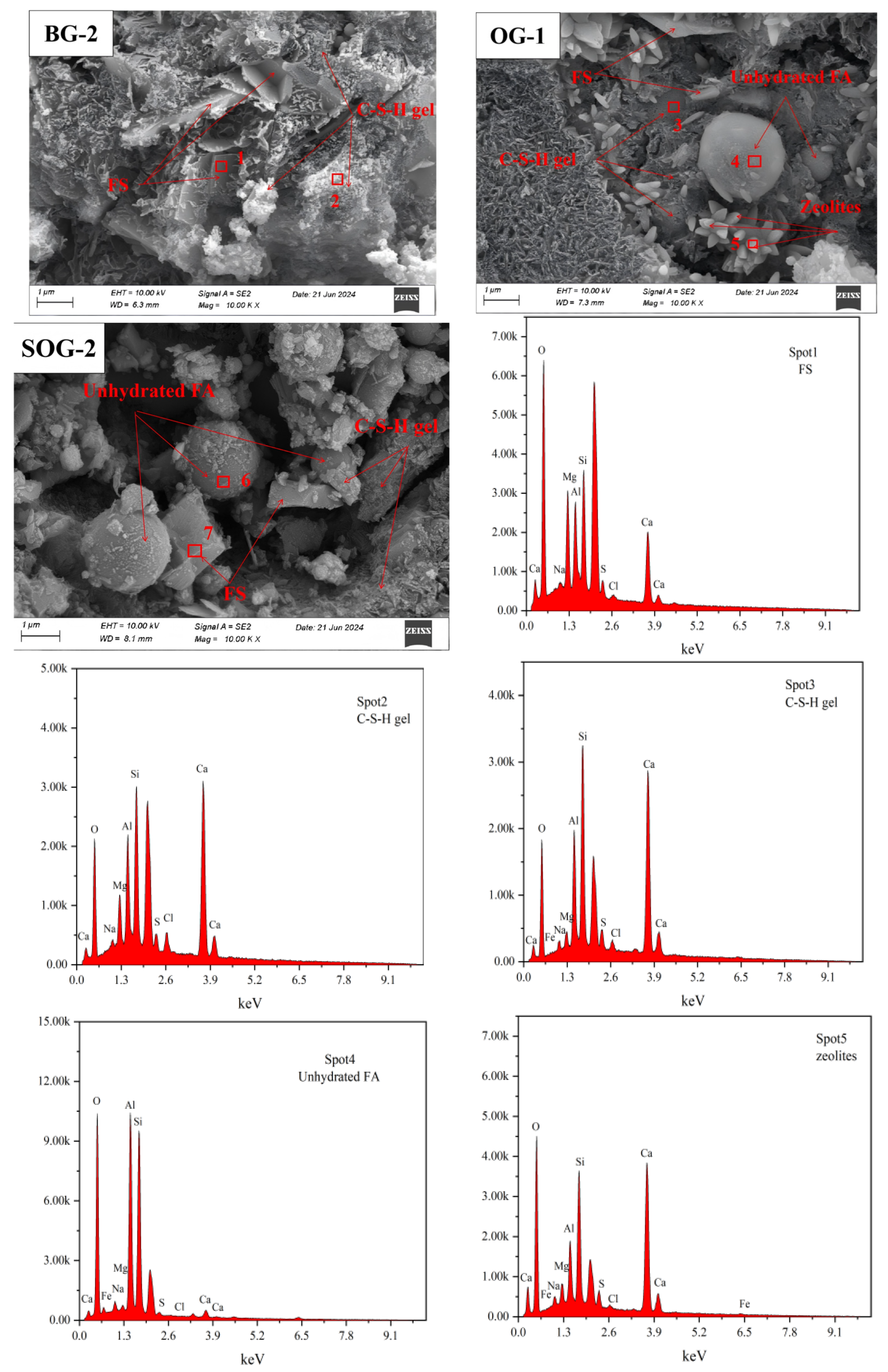
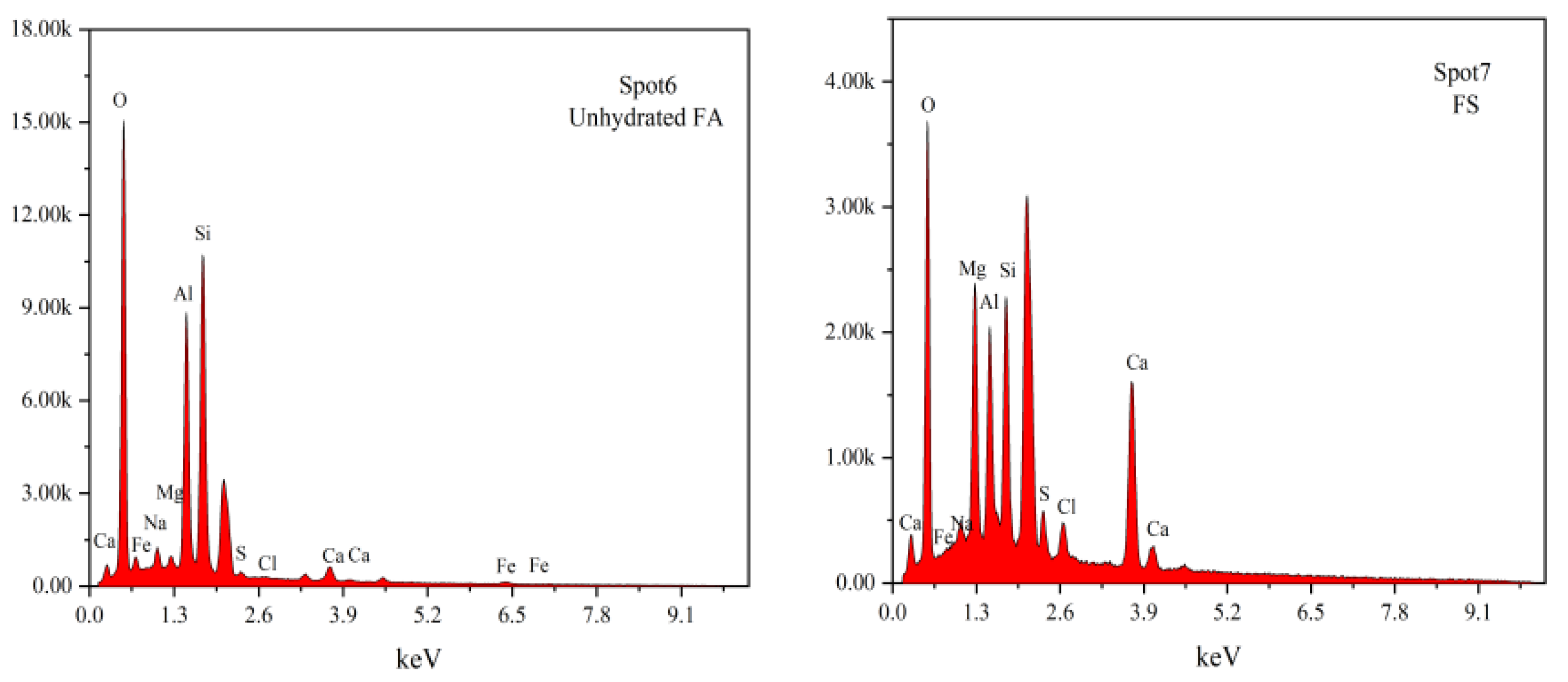
3.6. SEM-EDS Analysis
4. Conclusions
- (1)
- When the alkali residue and calcium carbide residue contents reached 22 wt% and 8 wt%, respectively, the highest activation efficiency for slag powder was achieved. The compressive strength reached 15.1 MPa at 7 days and 30.4 MPa at 28 days. Substituting 10 wt% slag powder with fly ash resulted in reduced compressive strength at both curing ages (7 d and 28 d). This decline is attributed to the significantly lower hydration activity of fly ash under ambient conditions compared to slag powder, which decelerates the overall hydration kinetics of the system.
- (2)
- Replacing slag powder with desulfurization gypsum at 2 wt% increments initially increased then decreased compressive strength. The maximum strength (17 MPa at 7 d and 34.2 MPa at 28 d) occurred at 4 wt% desulfurization gypsum content. This enhancement stems from the dissolution of gypsum, which releases Ca2+ and SO42− ions, inducing a “sulfate activation” effect that accelerates hydration reactions in the precursor system.
- (3)
- XRD, FTIR, TG-DTG, and SEM-EDS analyses confirmed that the 28-day hydration products of BG-2, OG-1, and SOG-2 samples primarily consisted of FS, C-(A)-S-H gel, and zeolites. The quantities of FS and C-(A)-S-H gel followed the following order: SOG-2 > BG-2 > OG-1. Alkali residue and calcium carbide residue provided the necessary alkaline environment to promote slag hydration. Fly ash substitution (10 wt%) reduced hydration products due to its slower reaction kinetics, while subsequent 4 wt% desulfurization gypsum addition enhanced sulfate activation, increasing hydration product formation.
- (4)
- The results demonstrate the feasibility of using a fully solid-waste composite activator (alkali residue–calcium carbide residue–desulfurization gypsum) to replace conventional strong alkali chemicals for activating slag powder–fly ash systems. This approach enables the development of low-carbon cementitious materials based entirely on solid wastes, offering significant potential for advancing green building materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X. Analysis of the current situation and progress of comprehensive utilization of industrial solid waste in China. Resour. Sav. Environ. Protection. 2021, 2, 95–96. [Google Scholar]
- Wang, D.; Xu, D.; Zhang, Z.; Wang, J. The Evolution and Prospects of Cement-Based Cementitious Materials. J. Build. Mater. 2025, 1–12. (In Chinese) [Google Scholar]
- Ahmad, M.R.; Qian, L.P.; Fang, Y.; Wang, A.; Dai, J.G. A multiscale study on gel composition of hybrid alkali-activated materials partially utilizing air pollution control residue as an activator. Cem. Concr. Compos. 2023, 136, 104856. [Google Scholar] [CrossRef]
- Korniejenko, K.; Mikuła, J.; Brudny, K.; Aruova, L.; Zhakanov, A.; Jexembayeva, A.; Zhaksylykova, L. A Review of Industrial By-Product Utilization and Future Pathways of Circular Economy: Geopolymers as Modern Materials for Sustainable Building. Sustainability 2025, 17, 4536. [Google Scholar] [CrossRef]
- Xie, B. Characterization of Geopolymer Bi-Liquid Slurry Materials in Alkali-Excited Red Mud-Fly Ash Binary System; East China University of Science and Technology: Shanghai, China, 2022. [Google Scholar]
- Liu, Y.; Chen, X.; Wang, B.; Lu, N.; Xiao, X.; Luo, D. Preparation and strength mechanism of alkali-excited fly ash-slag-electrolytic slag-based aggregates. Bull. Chin. Ceram. Soc. 2023, 42, 1353. [Google Scholar]
- Cai, R.; Tian, Z.; Ye, H. Durability characteristics and quantification of ultra-high strength alkali-activated concrete. Cem. Concr. Compos. 2022, 134, 104743. [Google Scholar] [CrossRef]
- Xia, D.; Chen, R.; Cheng, J.; Tang, Y.; Xiao, Z.; Li, Z. Desert sand-high calcium fly ash-based alkali-activated mortar: Flowability, mechanical properties, and microscopic analysis. Constr. Build. Mater. 2023, 398, 131729. [Google Scholar] [CrossRef]
- An, S.; Wang, B.; Cheng, W.; Zhao, Q. Mechanism of action of calcium carbide slag to stimulate slag-fly ash composite cementitious materials. Silic. Bull. 2023, 42, 1333–1343. [Google Scholar]
- Gao, Y.; Meng, H.; Wan, H.; Hu, X.; Chen, C. Properties and microstructure of calcium carbide slag alkali-inspired slag/fly ash cementitious materials. J. Cent. South Univ. (Nat. Sci. Ed.) 2023, 54, 1739–1747. [Google Scholar]
- Luo, X.; Zhang, S.; Guo, R.; Jia, J.; Ma, F.; Kong, C. Effect of replacement of cement by calcium carbide slag as an alkali exciter on the properties and microstructure of cementitious materials made of persulfurized phosphorus gypsum. Mater. Herald 2023, 37, 298–304. [Google Scholar]
- Gao, Y.; Zhu, Z.; Meng, H.; Hu, X.; Li, Z. Synergistic enhancement mechanism of calcium carbide slag-desulfurization gypsum-steel slag modified fly ash geopolymer. J. Build. Mater. 2023, 26, 870–878. [Google Scholar]
- Sun, L. Preparation and Hydration Mechanism of Polymerized Materials from Aged Bayer Red Mud Bases. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2019. [Google Scholar]
- Hou, D.; Wu, D.; Wang, X.; Gao, S.; Yu, R.; Li, M.; Wang, P.; Wang, Y. Sustainable use of red mud in ultra-high performance concrete (UHPC): Design and performance evaluation. Cem. Concr. Compos. 2021, 115, 103862. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement and Concrete. China Standards Press: Beijing, China, 2017.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). China Standards Press: Beijing, China, 2021.
- Lv, Z.; Zhang, Y.; Liu, Z.; Peng, Z.; Wang, D. Study of the properties of red mud-based foamed geopolymers. Silic. Bull. 2023, 42, 3624–3632. [Google Scholar]
- You, H. Experimental Study on the Durability of Gravel Stabilized by Red Mud-Based Cementitious Materials. Master’s Thesis, Shandong University, Jinan, China, 2023. (In Chinese). [Google Scholar]
- Guo, Q.; Du, L.; Hua, L.; Gao, M.; Liu, R.; Ai, G. Performance study of alkali-excited slag-steel slag composite cementitious system. New Build. Mater. 2024, 51, 108–113. [Google Scholar]
- Yang, J.; Zeng, J.; He, X.; Zhang, Y.; Su, Y.; Tan, H. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag. Constr. Build. Mater. 2022, 330, 127218. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y. Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater. 2020, 238, 117713. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Li, X. Study on the Effect of Activated MgO on the Hydration and Compensation of Drying Shrinkage Properties of Alkali-stimulated Fly Ash-Slag. Master’s Thesis, Hebei University, Wuhan, China, 2023. [Google Scholar]
- Gao, Y.; Meng, H.; Leng, Z.; Pu, T.; Long, G.; Duan, K. Properties and microstructure of calcium carbide slag-desulfurization gypsum composite excitation filling material. J. Civ. Environ. Eng. 2023, 45, 99–106, (In English and Chinese). [Google Scholar]
- Guo, W.; Wang, S.; Xu, Z.; Zhang, Z.; Zhang, C.; Bai, Y.; Zhao, Q. Mechanical performance and microstructure improvement of soda residue–carbide slag–ground granulated blast furnace slag binder by optimizing its preparation process and curing method. Constr. Build. Mater. 2021, 302, 124403. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Bai, Y.; Zhao, G.; Sang, Z.; Zhao, Q. Development and characterization of a new multi-strength level binder system using soda residue-carbide slag as composite activator. Constr. Build. Mater. 2021, 291, 123367. [Google Scholar] [CrossRef]
- Kumar, S.; Maradani, L.S.R.; Mohapatra, A.K.; Pradhan, B. Effect of mix parameters on chloride content, sulfate ion concentration, and microstructure of geopolymer concrete. Constr. Build. Mater. 2024, 435, 136864. [Google Scholar] [CrossRef]
- Lodeiro, I.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Xu, D.; Ni, W.; Wang, Q.; Xu, C.; Jiang, Y. Preparation of clinker-free concrete with alkali slag composite cementitious materials. J. Harbin Inst. Technol. 2020, 52, 151–160. [Google Scholar]
- Wang, Q.; Li, J.; Yao, G.; Zhu, X.; Hu, S.; Qiu, J.; Chen, P.; Lyu, X. Characterization of the mechanical properties and microcosmic mechanism of Portland cement prepared with soda residue. Constr. Build. Mater. 2020, 241, 117994. [Google Scholar] [CrossRef]
- Abdalqader, A.F.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef]
- Zhao, G. Durability and Micro-Mechanism of Alkali Slag-Electric Slag-Inspired Mineral Powder-Fly Ash Cementitious Material System. Master’s Thesis, Yanshan University, Qinhuangdao, China, 2022. [Google Scholar]
- Wei, E. Preparation and Transformation Mechanism of Zeolite (NaA/NaX/SOD) Microspheres with Different Geopolymerization and Their Adsorption Properties on Pb2+. Master’s Thesis, Guangxi University, Nanning, China, 2022. [Google Scholar]
- Liu, X.; Zhang, N.; Yao, Y.; Sun, H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Emminger, H.Y.; Ladner, L.; Agudo, R.C. Comparative study of the early stages of crystallization of calcium silicate hydrate (C-S-H) and calcium aluminate silicate hydrate (C-A-S-H). Cem. Concr. Res. 2025, 193, 107873. [Google Scholar] [CrossRef]
- Ye, K.; Dasari, A.; Hooper, T.J.N. Structural characterization and fire performance of geopolymer-glass fiber composite panels. Constr. Build. Mater. 2023, 400, 132633. [Google Scholar] [CrossRef]
- Wu, H.; Shen, A.; Zhang, J. Quasi-static and dynamic mechanical properties of concrete containing cellulose fiber: Mechanism and effects. Constr. Build. Mater. 2025, 483, 141701. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Provis, J.L.; Cizer, Ö.; Ye, G. Autogenous shrinkage of alkali-activated slag: A critical review. Cem. Concr. Res. 2023, 172, 107244. [Google Scholar] [CrossRef]
- Thomas, R.J.; Ye, H.; Radlińska, A.; Peethamparan, S. Alkali-activated slag concrete: A closer look at sustainable alternatives to portland cement. Concr. Int. 2016, 38, 33–38. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mitigation strategies in alkali-activated slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Xue, L.; Ni, Z.; Zhou, Z.; Zhang, Z.; Xiong, H.; Wang, H.; Zhuge, X.; Liu, H. Effects of desulfurized gypsum on shrinkage behavior of alkali-activated slag (AAS) and hybrid alkali-activated cement (HAC). Case Stud. Constr. Mater. 2025, 22, e04320. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Wu, M. Development of green binder systems based on flue gas desulfurization gypsum and fly ash incorporating slag or steel slag powders. Constr. Build. Mater. 2020, 265, 120275. [Google Scholar] [CrossRef]


| Material | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | K2O | SO3 | MnO | Na2O | Cl− | Other | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GGBS | 32.96 | 31.43 | 18.92 | 0.22 | 10.20 | 1.31 | 0.44 | 2.55 | 0.49 | 1.09 | 0.12 | 0.04 | 0.23 |
| FA | 9.58 | 44.82 | 28.24 | 5.39 | 1.68 | 0.98 | 1.68 | 1.74 | 0.09 | 3.30 | 0.21 | 0.38 | 1.93 |
| SR | 55.86 | 11.18 | 1.85 | 0.74 | 3.6 | - | 0.21 | 1.06 | - | 4.18 | 18.58 | 0.15 | 2.43 |
| CS | 91.62 | 5.12 | 1.99 | 0.27 | - | 0.05 | - | 0.76 | - | - | - | 0.06 | 0.11 |
| Gypsum | 34.04 | 3.64 | 1.98 | 0.60 | 1.19 | 0.07 | 0.26 | 39.37 | 0.02 | 0.25 | 0.13 | 0.08 | 18.53 |
| ID | SR (wt%) | CS (wt%) | GGBS (wt%) | FA (wt%) | Gypsum (wt%) | W/B |
|---|---|---|---|---|---|---|
| BG-1 | 26 | 4 | 70 | - | - | 0.5 |
| BG-2 | 22 | 8 | 70 | - | - | |
| BG-3 | 18 | 12 | 70 | - | - | |
| OG-1 | 22 | 8 | 60 | 10 | ||
| OG-2 | 22 | 8 | 50 | 20 | ||
| OG-3 | 22 | 8 | 50 | 30 | ||
| SOG-1 | 22 | 8 | 58 | 10 | 2 | |
| SOG-2 | 22 | 8 | 56 | 10 | 4 | |
| SOG-3 | 22 | 8 | 54 | 10 | 6 | |
| SOG-4 | 22 | 8 | 52 | 10 | 8 | |
| SOG-5 | 22 | 8 | 50 | 10 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Y.; Wei, D.; Wu, X.; Wang, Y. Mechanistic Insights into Full Solid-Waste Activators for Enhancing the Performance of Blast Furnace Slag–Fly Ash Cementitious Composites. Materials 2025, 18, 3275. https://doi.org/10.3390/ma18143275
Zhang H, Li Y, Wei D, Wu X, Wang Y. Mechanistic Insights into Full Solid-Waste Activators for Enhancing the Performance of Blast Furnace Slag–Fly Ash Cementitious Composites. Materials. 2025; 18(14):3275. https://doi.org/10.3390/ma18143275
Chicago/Turabian StyleZhang, Huiying, Yongchun Li, Dingbang Wei, Xu Wu, and Yapeng Wang. 2025. "Mechanistic Insights into Full Solid-Waste Activators for Enhancing the Performance of Blast Furnace Slag–Fly Ash Cementitious Composites" Materials 18, no. 14: 3275. https://doi.org/10.3390/ma18143275
APA StyleZhang, H., Li, Y., Wei, D., Wu, X., & Wang, Y. (2025). Mechanistic Insights into Full Solid-Waste Activators for Enhancing the Performance of Blast Furnace Slag–Fly Ash Cementitious Composites. Materials, 18(14), 3275. https://doi.org/10.3390/ma18143275





