Optimizing the Thermal Treatment of Mining-Waste-Amended Clays for Ceramic Aggregates in Pavement Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
| Material | Clay (%) | Silt (%) | Sand (%) |
| ineSandy soil (A1) | a11 | a12 | a13 |
| Waste (A2) | a21 | a22 | a23 |
| Clayey soil (A3) | a31 | a32 | a33 |
2.3. Physical Characterization
2.4. Mineralogical Characterization
2.5. Sample Preparation and Calcination
2.6. -Treton Parameter
- T = impact loss (%), also referred to as the -Treton value;
- = initial mass of the sample (g);
- = mass of material retained on the 1.7 mm sieve after impact (g).
3. Results and Discussion
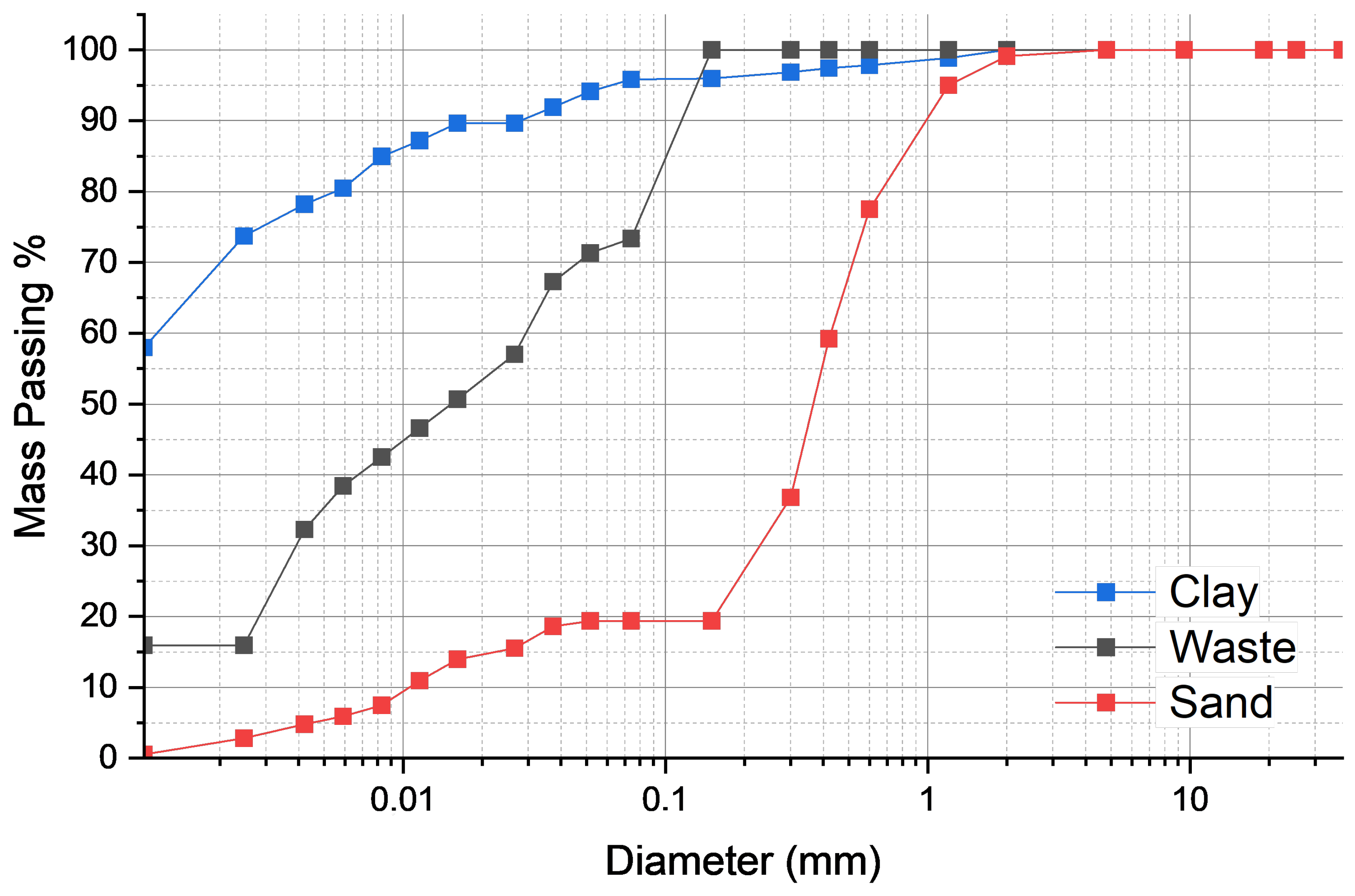
3.1. Physical Characterization
3.2. Winkler Classification
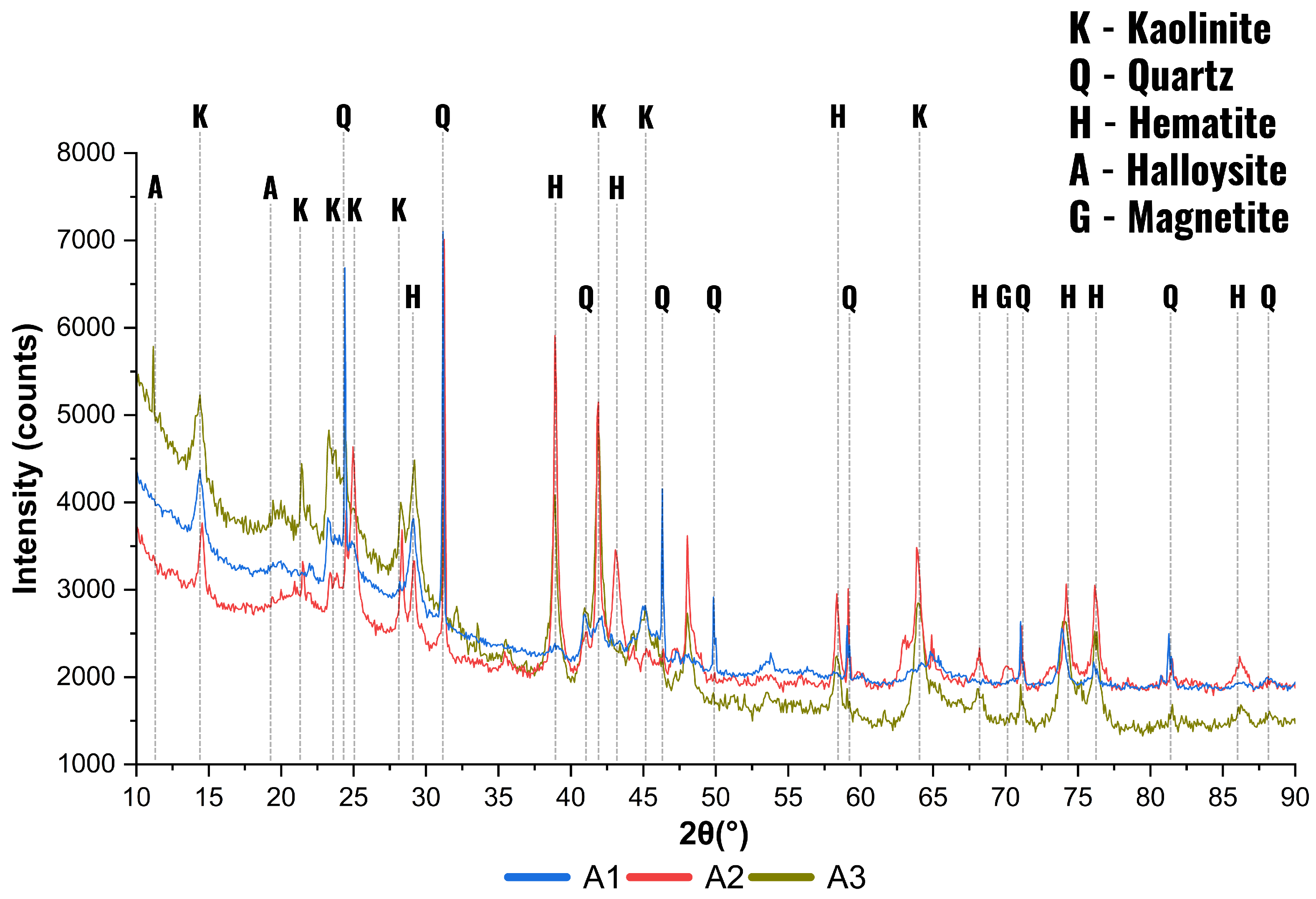
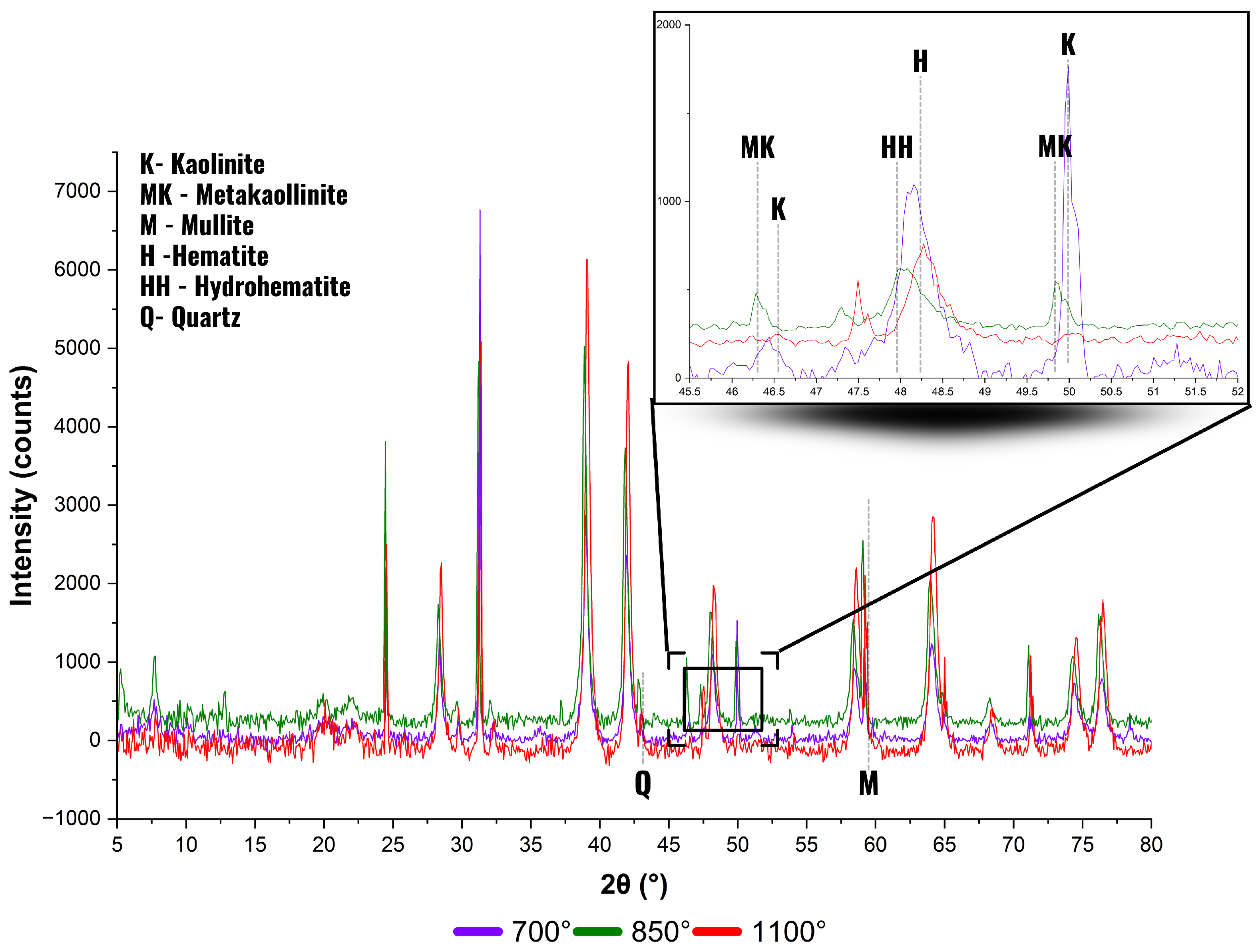
3.3. Mineralogical Analysis
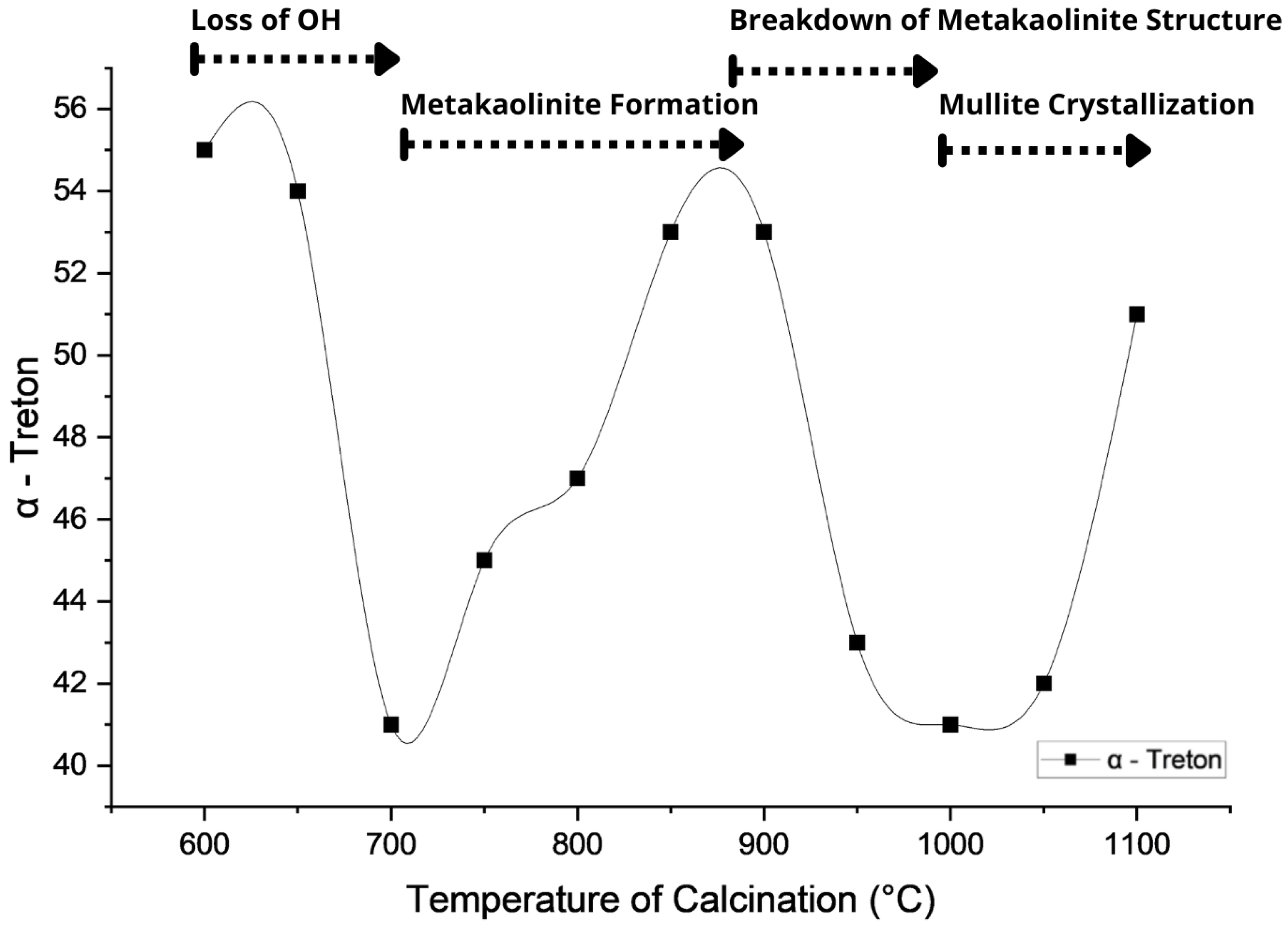
3.4. -Treton Parameter
- (i)
- 600–700 °C: A reduction in -Treton values is observed, attributed to the initial dehydroxylation of kaolinite, which weakens the lamellar structure and increases porosity. Recent findings by Fernandez and Snellings [59] demonstrate that this structural disruption temporarily reduces mechanical cohesion.
- (ii)
- 700–900 °C: A progressive increase in mechanical performance is noted, linked to the formation of metakaolinite, a highly reactive amorphous aluminosilicate. According to Rasmussen et al. [60], this phase contributes to particle agglomeration and matrix densification in ceramic systems.
- (iii)
- 900–1000 °C: A decline in -Treton values occurs, associated with the collapse of metakaolinite and the emergence of spinel-type phases and amorphous silica. This behavior is consistent with the results of Duxson et al. [61], who relate this transformation to internal structural disorder and the porosity increase.
- (iv)
- Above 1000 °C: A slight recovery in the parameter is detected, attributed to the crystallization of mullite and cristobalite. These phases contribute to structural integrity and long-term mechanical strength, as reported by Pereira et al. [62].
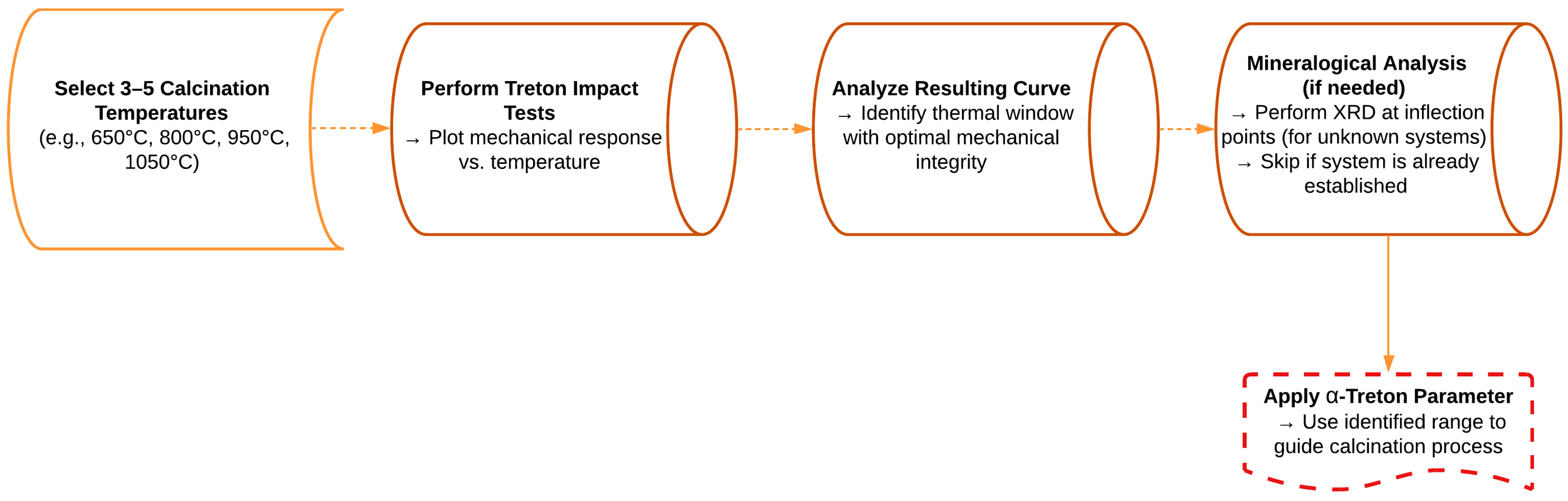
Application Strategy and Optimization Guidelines
4. Conclusions
- The proposed -Treton parameter proved to be a practical, sensitive, and low-cost proxy to evaluate the mechanical integrity of calcined aggregates. Its behavior aligned closely with the mineralogical changes typically observed in kaolinitic systems.
- The temperature range between 700 and 900 °C was identified as optimal for the formation of metakaolinite, corresponding to improved impact resistance. Outside this range, particularly above 900 °C, structural degradation and recrystallization processes reduce aggregate integrity.
- Although XRD analysis supported the interpretation of mineral phase evolution, the -Treton parameter alone may be sufficient for routine optimization of calcination in systems with known mineralogy. For heterogeneous or unexplored compositions, complementary mineralogical validation is recommended at key thermal inflection points.
- A simplified experimental workflow based on the -Treton curve was proposed to streamline the process design across different raw material systems, providing a replicable framework for thermal optimization in ceramic aggregate production.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stempkowska, A.; Gawenda, T. Special Issue “Mineral Composite Materials Produced with Waste/Recycled Components”—Editorial Note and Critical Review of the Problems. Materials 2023, 16, 3911. [Google Scholar] [CrossRef]
- Nunes, J.; Teixeira, A.M.A.J.; Saraiva, R.M.D.d.C. Morphological characterization of Brazilian expanded clay lightweight aggregate using AIMS. Ambiente Construído 2021, 21, 213–227. (In Portuguese) [Google Scholar] [CrossRef]
- Olofinnade, O.; Ogara, J. Workability, strength, and microstructure of high strength sustainable concrete incorporating recycled clay brick aggregate and calcined clay. Clean. Eng. Technol. 2021, 3, 100123. [Google Scholar] [CrossRef]
- Hao, D.; Razak, R.; Kheimi, M.; Yahya, Z.; Abdullah, M.; Burduhos Nergis, D.; Fansuri, H.; Ediati, R.; Mohamed, R.; Abdullah, A. Artificial Lightweight Aggregates Made from Pozzolanic Material: A Review on the Method, Physical and Mechanical Properties, Thermal and Microstructure. Materials 2022, 15, 3929. [Google Scholar] [CrossRef]
- Sousa, J.; Anjos, M.; Neto, J.; Farias, E.; Branco, F.; Maia Pederneiras, C. Self-Compacting Concrete with Artificial Lightweight Aggregates from Sugarcane Ash and Calcined Scheelite Mining Waste. Appl. Sci. 2025, 15, 452. [Google Scholar] [CrossRef]
- Polivanov, H.; da Motta, L.M.G.; de Brito Fratte Modesto, F.; Barroso, E.V. Argilas Calcinadas para uso em Pavimentos rodoviáRios. Rev. Bras. Geol. Eng. Ambient. 2014, 4, 33–46. [Google Scholar]
- Cunha, M.J.A.; Silva, C.L.; Lima, C.A.P.; Frota, C.A. Efeito da temperatura no comportamento mecânico de misturas asfálticas com agregados sinterizados de argila calcinada (ASAC). Rev. Matéria 2018, 23, e11970. [Google Scholar] [CrossRef]
- Santos, M. Study of Synthetic Calcined Clay Aggregates Behavior for Use in Pavement Asphalt for Manaus. Master’s Thesis, University of Brasília, Brasília, Brazil, 2007. [Google Scholar]
- da Silva, C.; da Frota, H.; da Frota, C. Sintered Calcined Clay as an Alternative Coarse Aggregate for Asphalt Pavement Construction. Open J. Civ. Eng. 2015, 5, 281–288. [Google Scholar] [CrossRef]
- Cabrera, E.; Almenares, R.; Alujas, A. Assessment of the Pozzolanic Reactivity of Calcined Kaolinitic Clays by a Rapid Alkaline Solubility Test. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; Volume 16. [Google Scholar] [CrossRef]
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined Clay as Supplementary Cementitious Material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef]
- Pinheiro, V.; Alexandre, J.; Xavier, G.; Marvila, M.; Monteiro, S.; Azevedo, A. Methods for Evaluating Pozzolanic Reactivity in Calcined Clays: A Review. Materials 2023, 16, 4778. [Google Scholar] [CrossRef]
- ASTM C125-20; Standard Terminology Relating to Concrete and Concrete Aggregates. ASTM Standard: West Conshohocken, PA, USA, 2020; p. 9.
- Díaz, A.A.; Almenares Reyes, R.S.; Carratalá, F.A.; Martirena Hernández, J.F. Proposal of a methodology for the preliminary evaluation of kaolinitic clay deposits as a source of SCMs. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Martirena, F., Favier, A., Scrivener, K., Eds.; Springer: Dordrecht, The Netherlands, 2018; Volume 16. [Google Scholar] [CrossRef]
- Alujas, A.; Almenares, R.S.; Betancourt, S.; Leyva, C. Pozzolanic reactivity of low-grade kaolinitic clays: Influence of mineralogical composition. In Calcined Clays for Sustainable Concrete; RILEM Bookseries; Scrivener, K., Favier, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 10. [Google Scholar] [CrossRef]
- Schulze, S.E.; Rickert, J. Suitability of natural calcined clays as supplementary cementitious material. Cem. Concr. Compos. 2019, 95, 92–97. [Google Scholar] [CrossRef]
- Fan, X.; Li, Z.; Zhang, W.; Jin, H.; Liu, J.; Xing, F.; Tang, L. New applications of municipal solid waste incineration bottom ash (MSWIBA) and calcined clay in construction: Preparation and use of an eco-friendly artificial aggregate. Constr. Build. Mater. 2023, 387, 131629. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 37, 303–318. [Google Scholar]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar]
- Cardinaud, G.; Rozière, E.; Martinage, O.; Loukili, A.; Barnes-Davin, L.; Paris, M.; Deneele, D. Calcined clay—Limestone cements: Hydration processes with high and low-grade kaolinite clays. Constr. Build. Mater. 2021, 277, 122271. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M. Performance of Calcined Impure Kaolinitic Clay as a Partial Substitute for Portland Cement Concrete: A Review. J. Compos. Sci. 2025, 9, 145. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N.; Sharbaf, M. Alkali-activated natural pozzolan/slag mortars: A parametric study. Constr. Build. Mater. 2018, 164, 625–643. [Google Scholar]
- Barbosa, V.; Marques, M.; Guimarães, A. Mineralogical characterization of a soil in Acre for the production of artificial calcined clay aggregates. Matéria 2018, 23, e12181. [Google Scholar]
- Friber, M.; Guimarães, A.; Martins, C.; Soares, J. Study of the Mining Waste in the Production of Calcined Aggregate for Use in Pavement. Minerals 2023, 13, 1543. [Google Scholar] [CrossRef]
- Cabral, G.d.L.L.; da Motta, L.M.G.; Lopes, L.A.S.; Vieira, A. Calcined Clay Aggregate: A Feasible Alternative for Brazilian Road Construction. In Proceedings of the 4th Eurasphalt and Eurobitume Congress, Copenhagen, Denmark, 21–23 May 2008; 9p. [Google Scholar]
- de Souza Campelo, N.; da Silva Campos, A.M.L.; Aragão, A.F. Comparative analysis of asphalt concrete mixtures employing pebbles and synthetic coarse aggregate of calcined clay in the Amazon region. Int. J. Pavement Eng. 2017, 20, 507–518. [Google Scholar] [CrossRef]
- Batista, F.d.S. Physical and Mechanistic Characterization of Calcined Clay Aggregates Produced from Fine Soils of BR-163/PA. Ph.D. Thesis, Military Institute of Engineering, Rio de Janeiro, Brazil, 2004. [Google Scholar]
- Usanga, I.N.; Okafor, F.O.; Ikeagwuani, C.C. Analysis of performance parameters based on surface free energy to evaluate moisture susceptibility of asphalt mixtures modified with calcined marl dust. Int. J. Pavement Eng. 2024, 25, 2301453. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Zhang, Z.; Dai, G.; Huo, Y.; Zhao, Y. Performance Analysis of Industrial-Waste-Based Artificial Aggregates: CO2 Uptake and Applications in Bituminous Pavement. Buildings 2023, 13, 2823. [Google Scholar] [CrossRef]
- Almeida, J.; Ribeiro, A.; Silva, A.S.; Faria, P. Overview of mining residues incorporation in construction materials and barriers for full-scale application. J. Build. Eng. 2020, 29, 101215. [Google Scholar] [CrossRef]
- Danish, A.; Totiç, E.; Bayram, M.; Sütçü, M.; Gencel, O.; Erdoğmuş, E.; Ozbakkaloglu, T. Assessment of Mineralogical Characteristics of Clays and the Effect of Waste Materials on Their Index Properties for the Production of Bricks. Materials 2022, 15, 8908. [Google Scholar] [CrossRef]
- Thejas, H.K.; Hossiney, N. Alkali-activated bricks made with mining waste iron ore tailings. Case Stud. Constr. Mater. 2022, 16, e00973. [Google Scholar] [CrossRef]
- Guimarães, A.; Arêdes, M.; Castro, C.; Coelho, L.; Monteiro, S. Evaluation of the Mechanical Behavior of Asphaltic Mixtures Utilizing Waste of the Processing of Iron Ore. Mining 2024, 4, 889–903. [Google Scholar] [CrossRef]
- Galhardo, D.; Guimarães, A.; Martins, C.; Narciso, M.; Monteiro, S.; Coelho, L. Influence of Iron Mining Waste Addition as a Sustainable Alternative on the Resilient and Physical Properties of Soils for Pavement Design. Sustainability 2024, 16, 10211. [Google Scholar] [CrossRef]
- Coelho, L.; Guimarães, A.; Alves Moreira, C.; dos Santos, G.; Monteiro, S.; da Silveira, P. Feasibility of Using Ferronickel Slag as a Sustainable Alternative Aggregate in Hot Mix Asphalt. Sustainability 2024, 16, 8642. [Google Scholar] [CrossRef]
- Coelho, L.; dos Santos, W.; Guimarães, A.; Monteiro, S. Influence of the Bailey Gradation Method on the Mechanical Behavior of Asphalt Mixture Containing Steel Slag as an Alternative Aggregate. Buildings 2024, 14, 3942. [Google Scholar] [CrossRef]
- Mondem, N.; Balunaini, U. Manufacturing Artificial Aggregates from Overburden Coal Mine Waste and Their Properties for Pavement Applications. J. Mater. Civ. Eng. 2024, 36. [Google Scholar] [CrossRef]
- Cabral, E.; Sá, R.; Vieira, R.; Vasconcelos, R. Utilization of ceramic masses in the production of synthetic calcined clay aggregate for use in concrete. Ceramics 2008, 54, 404–410. (In Portuguese) [Google Scholar] [CrossRef]
- Monteiro, S.; Vieira, C. Influence of firing temperature on the ceramic properties of clays from Campos dos Goytacazes, Brazil. Appl. Clay Sci. 2004, 27, 229–234. [Google Scholar] [CrossRef]
- Amaral, L.; Vieira, C.; Monteiro, S. Formulation of Ceramic Body to Produce Roofing Tiles Using Winkler Diagram. In Characterization of Minerals, Metals, and Materials 2016; Ikhmayies, S.J., Li, B., Carpenter, J.S., Hwang, J.-Y., Monteiro, S.N., Li, J., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; Springer: Cham, Switzerland, 2016; pp. 217–225. [Google Scholar]
- Mendes, B.C.; Pedroti, L.G.; Fontes, M.P.; Ribeiro, J.C.L.; Vieira, C.M.; Pacheco, A.A.; de Azevedo, A.R. Technical and environmental assessment of the incorporation of iron ore tailings in construction clay bricks. Constr. Build. Mater. 2019, 227, 116669. [Google Scholar] [CrossRef]
- Behera, K.; Bose, B.P.; Mondal, M.K. Production of Construction Bricks Using Iron Ore Tailings and Clay. In Waste Management and Resource Efficiency; Ghosh, S., Ed.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Bonomo, B.R.; Lucas, A.C.L.; Silva, L.S.; Lopes, M.M.; Lima, G.E. Effect of the Incorporation of Bauxite and Iron Ore Tailings on the Properties of Clay Bricks. In Characterization of Minerals, Metals, and Materials 2021; Li, J., Zhang, M., Li, B., Monteiro, S.N., Ikhmayies, S.J., Kalay, Y.E., Hwang, J.-Y., Escobedo-Diaz, J.P., Carpenter, J.S., Brown, A.D., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Cabral, G. Production methodology and use of calcined clay aggregates for paving (In Portuguese). Dissertação de Mestrado, Instituto Militar de Engenharia, Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Pracidelli, S.; Melchiades, F.G. Importance of particle size distribution in ceramic bodies for red ceramics. Cerâmica Ind. 1997, 2, 31–35. (In Portuguese) [Google Scholar]
- Associação Brasileira de Normas Técnicas (ABNT). NBR 7180: Solo—Granular Analysis; Technical report; ABNT: Rio de Janeiro, Brazil, 1984. (In Portuguese) [Google Scholar]
- NBR 7181; Soil—Particle Size Analysis by Sedimentation. Brazilian Association of Technical Standards (ABNT): Rio de Janeiro, Brazil, 1984. (In Portuguese)
- Departamento Nacional de Estradas de Rodagem (DNER). ME 093/94—Determination of Real Density; Technical report; DNER: Rio de Janeiro, Brazil, 1994. (In Portuguese)
- NBR 6457; Soil—Moisture Content. Brazilian Association of Technical Standards (ABNT): Rio de Janeiro, Brazil, 2016. (In Portuguese)
- NBR 6459; Soil—Determination of Liquid Limit. Brazilian Association of Technical Standards (ABNT): Rio de Janeiro, Brazil, 1984. (In Portuguese)
- Gebremariam, A.T. CFD Modelling and Experimental Testing of Thermal Calcination of Kaolinite Rich Clay Particles: An Effort towards Green Concrete. Ph.D. Thesis, Department of Energy Technology, Aalborg University, Aalborg, Denmark, 2015. [Google Scholar]
- ME 399/99; Agregados—Aggregates—Determination of Impact Loss Using the Treton Apparatus. Departamento Nacional de Estradas de Rodagem (DNER): Rio de Janeiro, Brazil, 1999. (In Portuguese)
- Vieira, C.M.F.; Soares, T.M.; Monteiro, S.N. Ceramic bodies for roof tiles: Characteristics and firing behavior. Cerâmica 2003, 49, 245–250. (In Portuguese) [Google Scholar] [CrossRef]
- Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins. Am. Mineral. 2005, 90, 1473–1499. [Google Scholar] [CrossRef]
- Silva, A.C.; Papini, R.M. Phosphorus removal from iron ore: A review on the strategies and their limitations. REM Rev. Esc. Minas 2015, 68, 331–335. [Google Scholar] [CrossRef]
- Varajão, A.F.D.C.; Rodrigues, S.; da Silva, L.F.; de Mello, C.L.; Souza, M.E. Lateritization as a process of formation of the aluminous iron ores. Appl. Earth Sci. 2001, 110, 32–38. [Google Scholar]
- Fernandez, J.; Snellings, R. Experimental investigation of soak and flash calcination on kaolinitic clays. J. Mater. Chem. A 2024, 12, 1122–1135. [Google Scholar]
- Martirena Hernández, J.F.; Antoni, M.; Oquendo-Machado, Y.; Borrajo-Perez, R.; Alujas-Diaz, A.; Almenares-Reyes, R. Impact of calcination technology on properties of calcined clays. RILEM Tech. Lett. 2023, 8, 190–197. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part I—Physical evolution. J. Non-Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Pereira, L.F.; Silva, D.M.; Souza, A.P. Synthesis of high-performance mullite ceramics from kaolinitic clays. J. Eur. Ceram. Soc. 2023, 43, 5812–5821. [Google Scholar]
- Sabir, B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Souza Santos, P. Science and Technology of Clays, 2nd ed.; Editora Edgard Blücher Ltd.: São Paulo, Brazil, 1989; Volume 1. (In Portuguese) [Google Scholar]
- Santos, H.S.; Kiyohara, P.; Coelho, A.C.V.; Santos, P.S. Electron microscopy study of the transformations during firing of Brazilian high-alumina clays. Cerâmica 2006, 52, 125–137. [Google Scholar] [CrossRef]

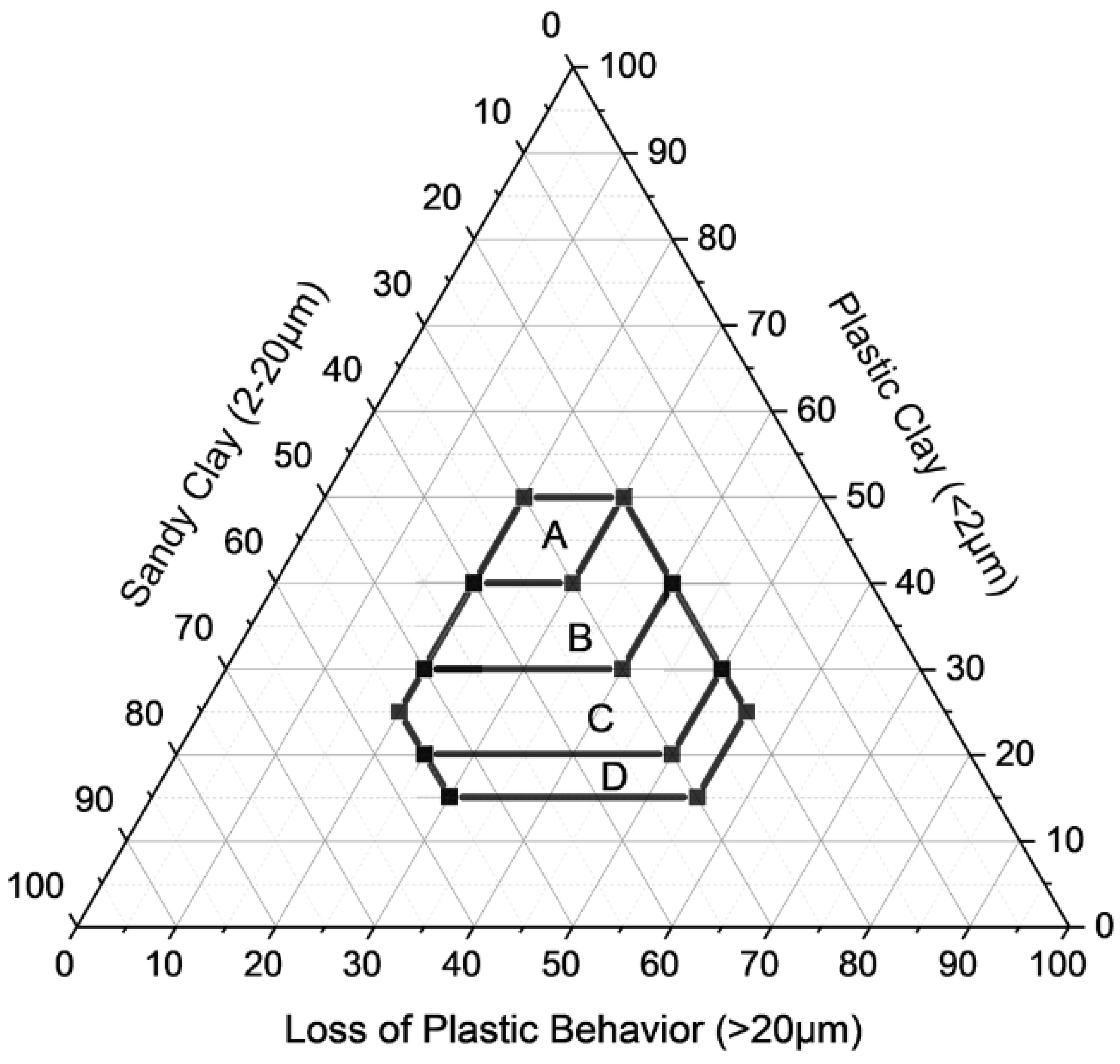



| Region | Clay (<2 μm) | Silt (2–20 μm) | Sand (>20 μm) |
|---|---|---|---|
| A—Quality ceramics | 40–50% | 20–40% | 20–30% |
| B—Roof tiles and covers | 30–40% | 20–50% | 20–40% |
| C—Perforated bricks | 20–30% | 20–55% | 20–50% |
| D—Solid bricks | 15–20% | 20–55% | 20–55% |
| Test | A1 | A2 | A3 |
|---|---|---|---|
| Specific gravity | 2.69 | 3.36 | 2.77 |
| Liquid limit (%) | 43.4 | 43.9 | 44.2 |
| Plastic limit (%) | 34.1 | 31.0 | 31.3 |
| Plasticity index (%) | 9.3 | 12.9 | 12.9 |
| Material | d10 (mm) | d50 (mm) | d90 (mm) | Dmean (mm) |
|---|---|---|---|---|
| A1 | 0.0020 | 0.0020 | 0.0500 | 0.0162 |
| A2 | 0.0020 | 0.0085 | 0.2000 | 0.0518 |
| A3 | 0.0500 | 0.1600 | 0.5000 | 0.2376 |
| Temperature Range (°C) | Main Phase Transformation | Structural Nature | Impact on Mechanical Resistance |
|---|---|---|---|
| 600–700 | Initial kaolinite dehydroxylation | Partially disordered | Decrease—temporary weakening due to structural breakdown |
| 700–900 | Formation of metakaolinite | Amorphous and cohesive | Increase—enhanced impact resistance due to structural reorganization |
| 900–1000 | Metakaolinite breakdown; spinel and amorphous silica | Unstable and disrupted | Decrease—reduced integrity due to phase disintegration |
| >1000 | Onset of mullite crystallization | Crystalline and dense | Moderate recovery—formation of mechanically robust phases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narciso, M.M.; Coelho, L.M.; Monteiro, S.N.; Guimarães, A.C.R. Optimizing the Thermal Treatment of Mining-Waste-Amended Clays for Ceramic Aggregates in Pavement Applications. Materials 2025, 18, 3180. https://doi.org/10.3390/ma18133180
Narciso MM, Coelho LM, Monteiro SN, Guimarães ACR. Optimizing the Thermal Treatment of Mining-Waste-Amended Clays for Ceramic Aggregates in Pavement Applications. Materials. 2025; 18(13):3180. https://doi.org/10.3390/ma18133180
Chicago/Turabian StyleNarciso, Murilo Miguel, Lisley Madeira Coelho, Sergio Neves Monteiro, and Antônio Carlos Rodrigues Guimarães. 2025. "Optimizing the Thermal Treatment of Mining-Waste-Amended Clays for Ceramic Aggregates in Pavement Applications" Materials 18, no. 13: 3180. https://doi.org/10.3390/ma18133180
APA StyleNarciso, M. M., Coelho, L. M., Monteiro, S. N., & Guimarães, A. C. R. (2025). Optimizing the Thermal Treatment of Mining-Waste-Amended Clays for Ceramic Aggregates in Pavement Applications. Materials, 18(13), 3180. https://doi.org/10.3390/ma18133180







