The Growth Kinetic and Ultra High Hardness of CoCrFeNiTi High–Entropy Alloy by Mechanical Alloying and Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Phase Prediction
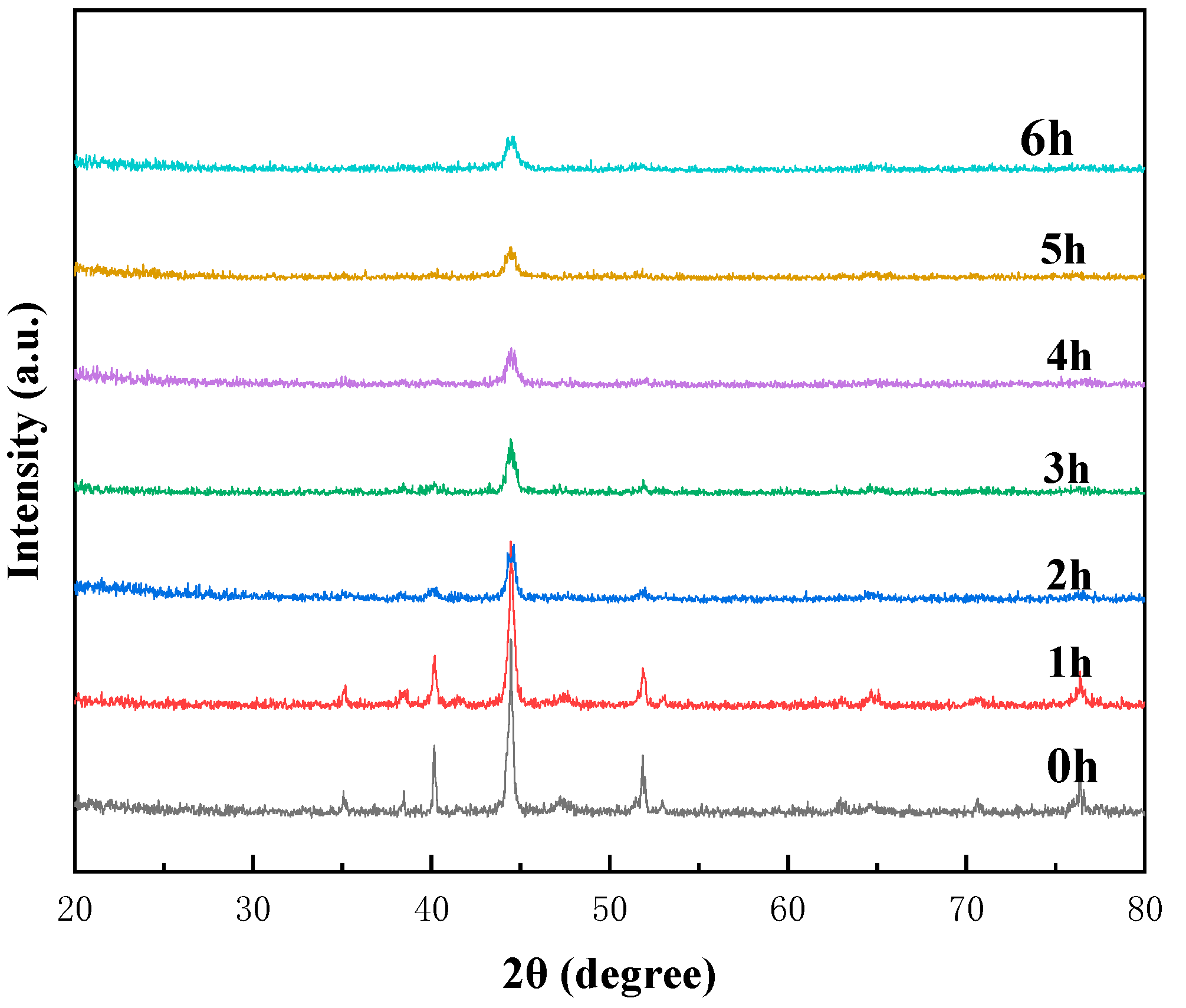
3.2. Microstructural and Phase Evolution
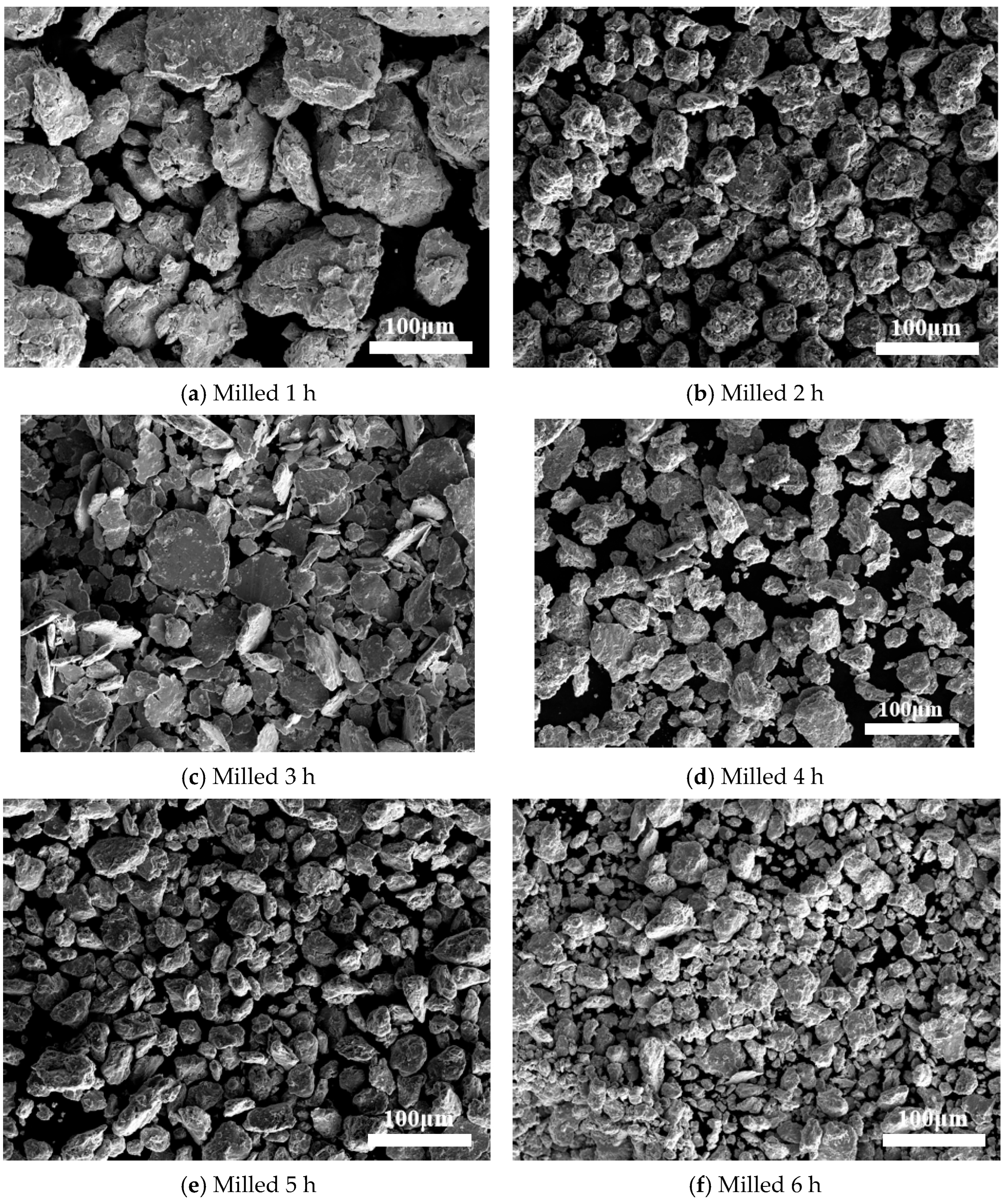
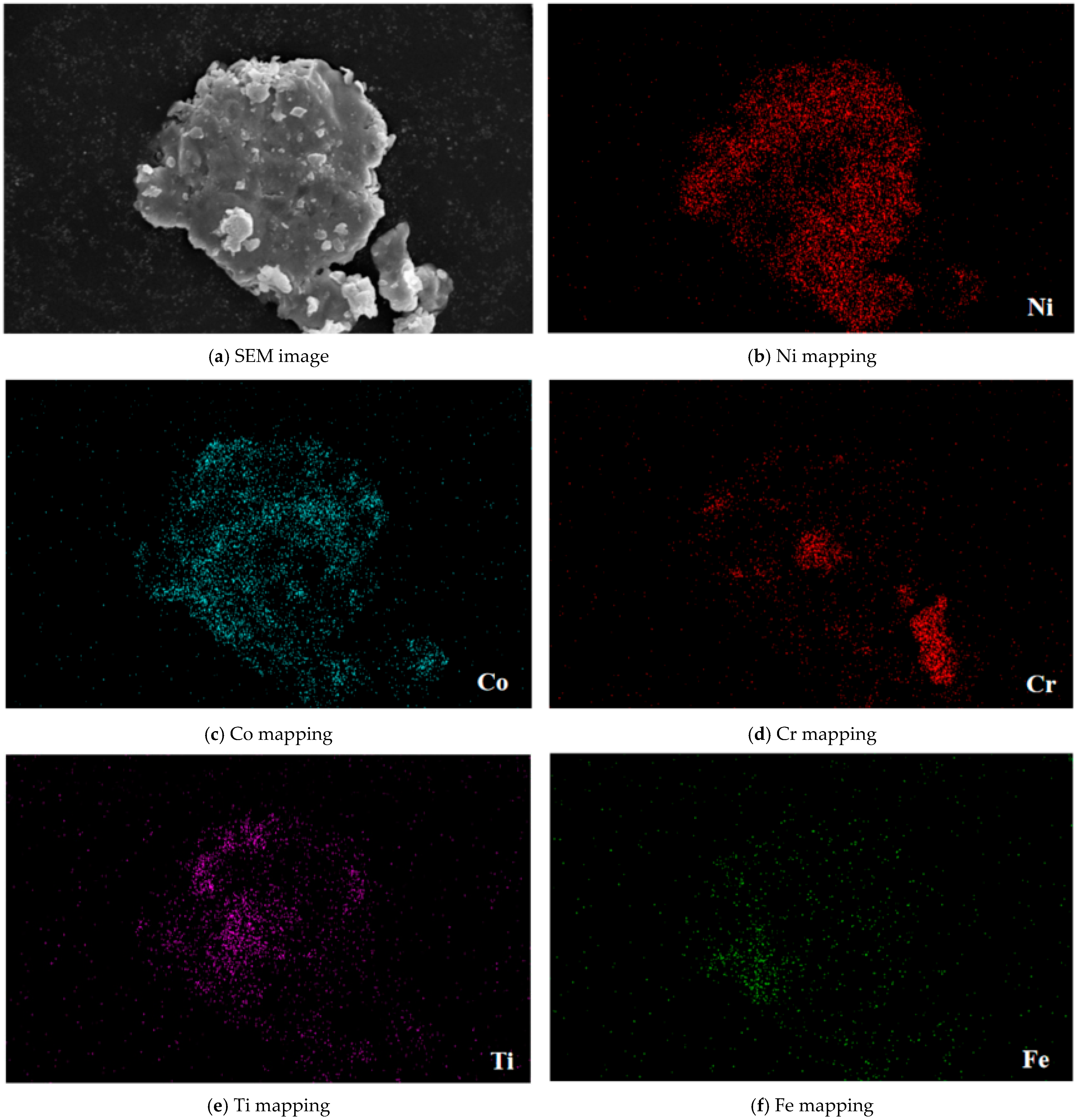
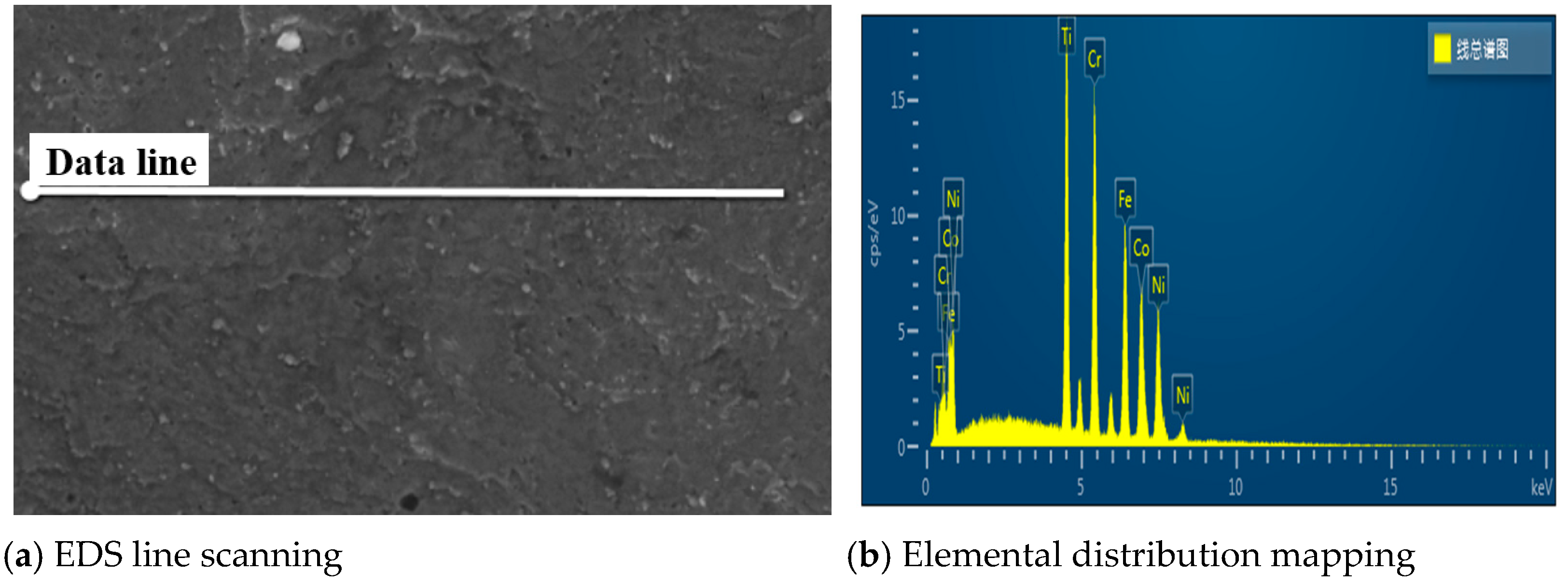
3.3. SEM Results
3.4. JMA Kinetic Method Calculations
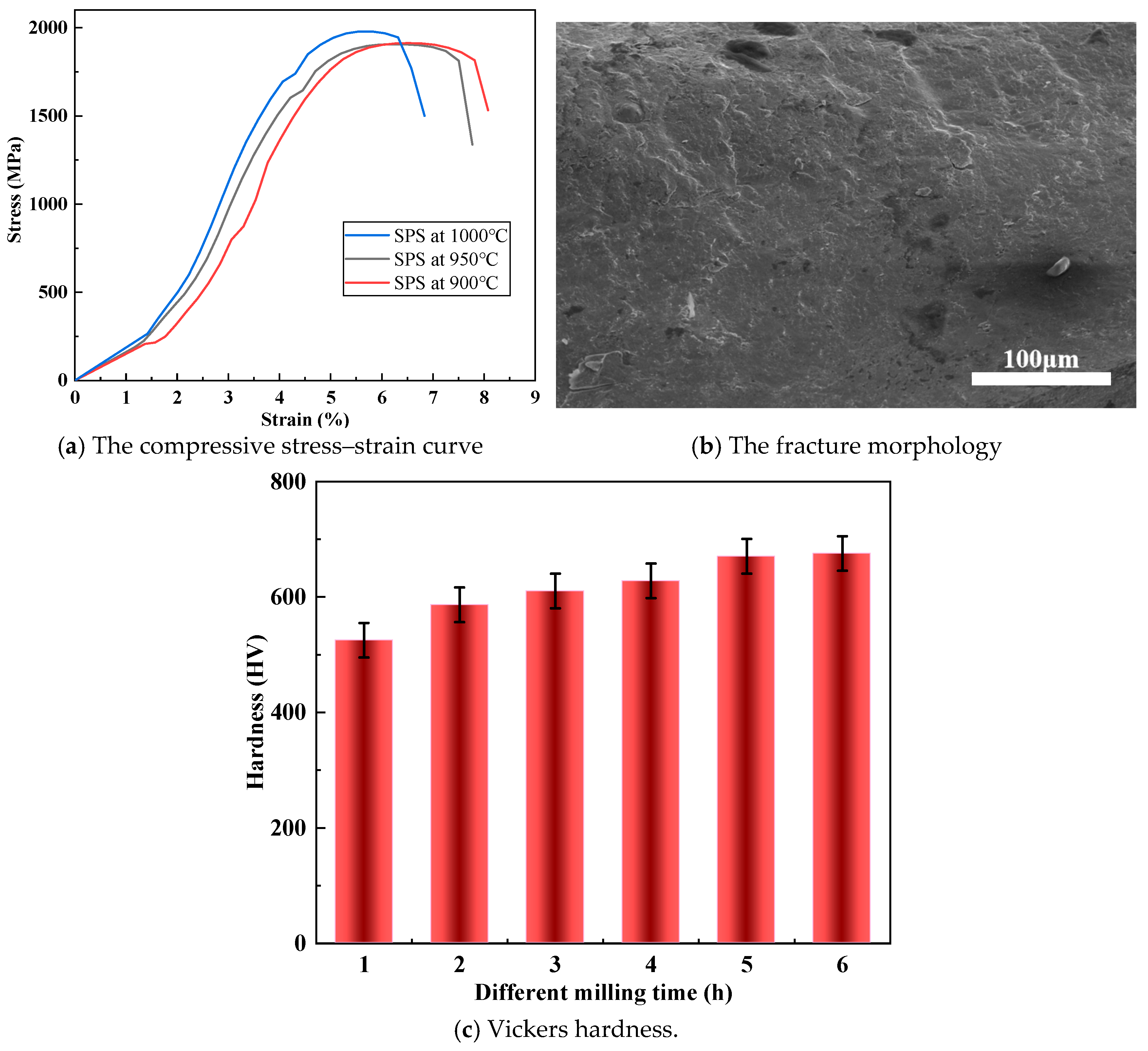
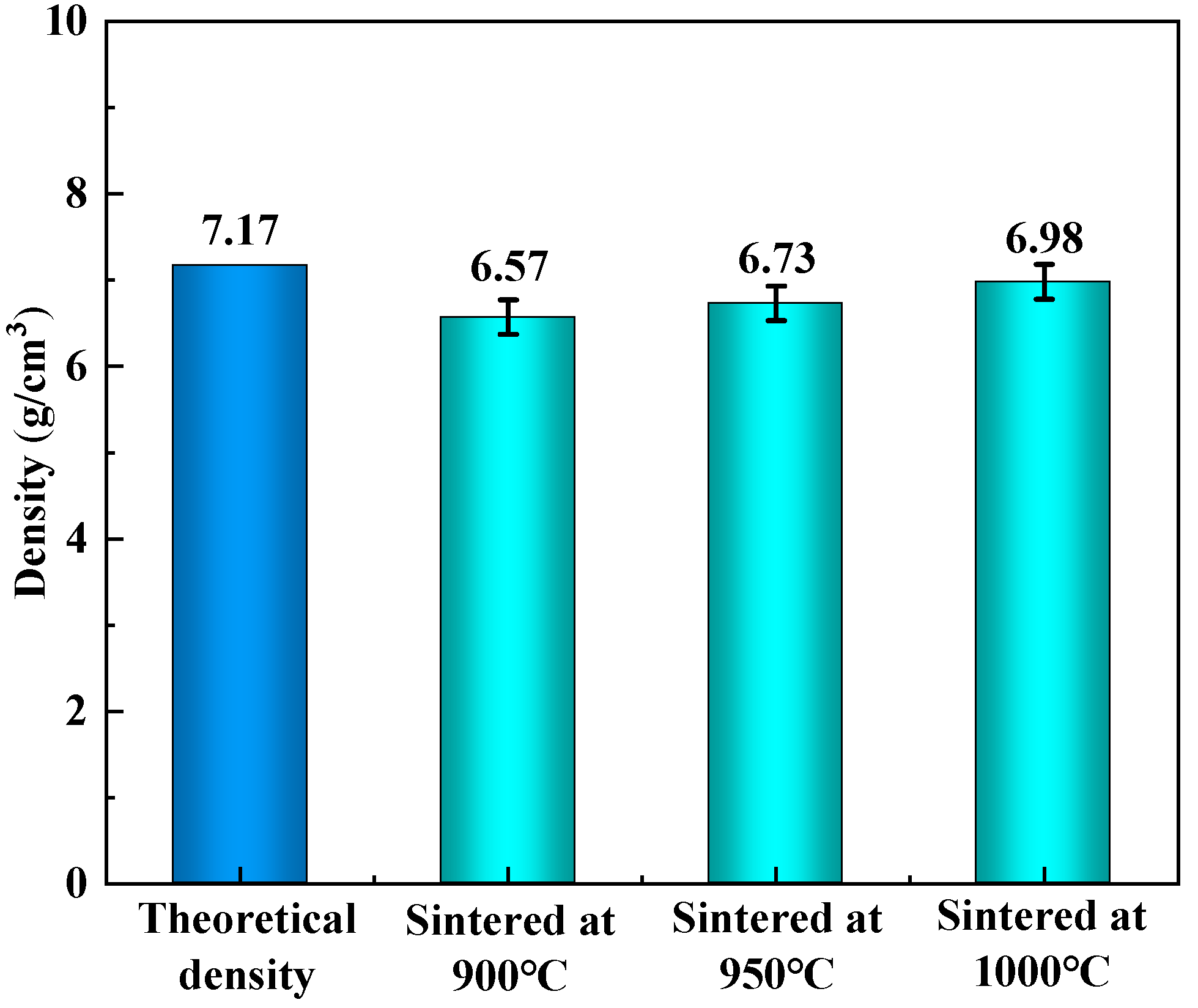
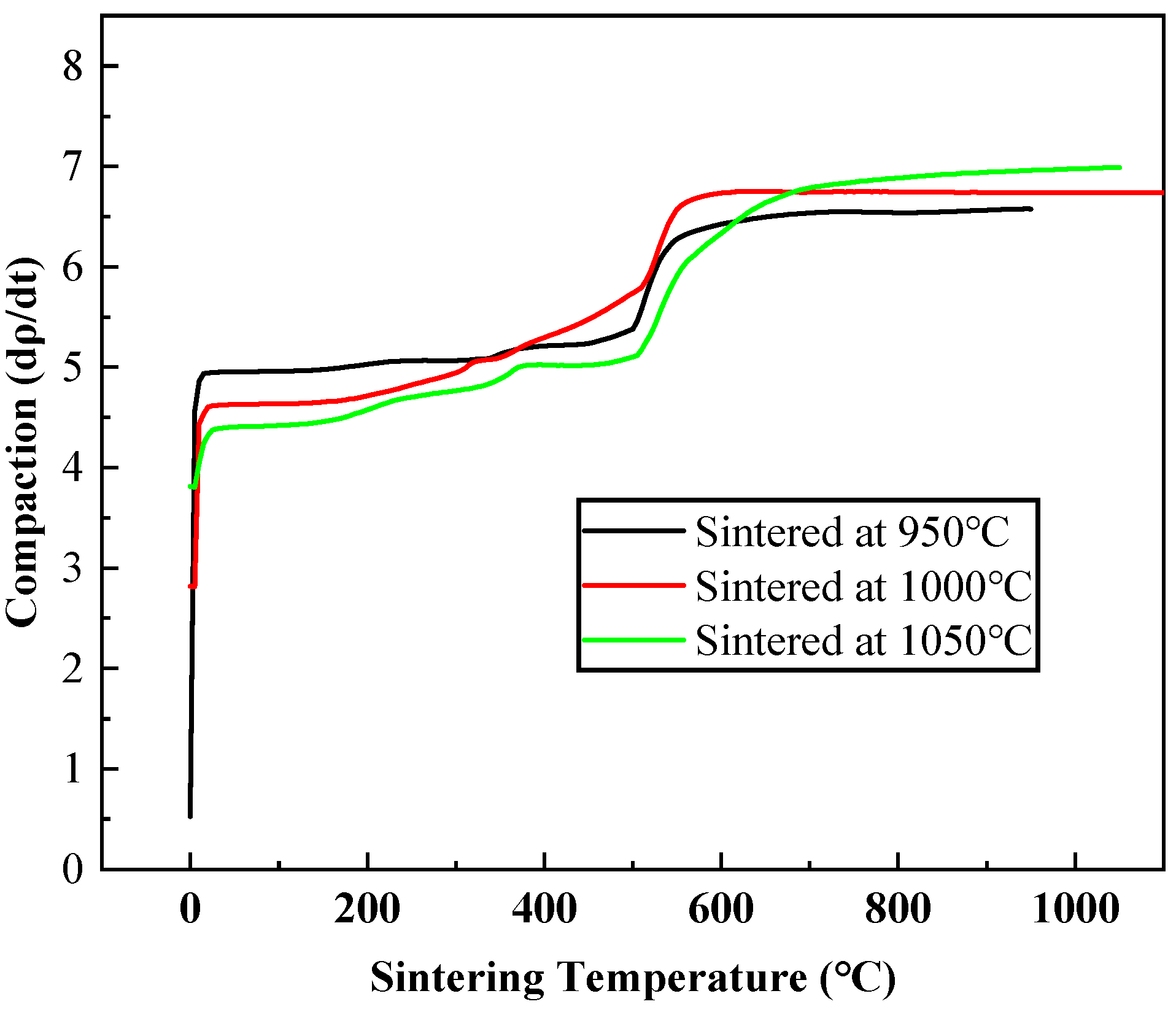
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high–entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar]
- Feng, L.; Wang, G.P.; Ma, K.; Yang, W.J.; An, G.S.; Li, W.S. Microstructure and properties of AlCoxCrFeNiCu high entropy alloy coating synthesized by cold spraying assisted induction remelting. Acta Metall. Sin. 2023, 59, 703–712. [Google Scholar]
- He, Q.F.; Ding, Z.Y.; Ye, Y.F.; Yang, Y. Design of High–Entropy Alloy: A Perspective from Nonideal Mixing. JOM 2017, 69, 2092–2098. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high–entropy alloy coatings on aluminum by laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar]
- Wei, X.; Zhang, L.; Zhang, F.; Zhang, C.; Jia, Q.; Sun, K.; Duan, D.; Jiang, H.; Li, G. Effect of carbon addition on the microstructure and corrosion resistance of the CoCrFeNi high–entropy alloy. Corros. Sci. 2024, 231, 111965. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Rehman, E.U.; Ullah, S.; Atif, M.; Tariq, A. A review on laser cladding of high–entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high–entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, R.; Xiao, B.; Zhang, Z.; Li, S.; Qiao, J.; Bai, S.; Zhang, Y.; Liaw, P.K. A review on the dynamic–mechanical behaviors of high–entropy alloys. Prog. Mater. Sci. 2023, 135, 101090. [Google Scholar] [CrossRef]
- Wang, H.; Koyama, M.; Hojo, T.; Akiyama, E. Hydrogen embrittlement and associated surface crack growth in fine–grained equiatomic CoCrFeMnNi high–entropy alloys with different annealing temperatures evaluated by tensile testing under in situ hydrogen charging. Int. J. Hydrogen Energy 2021, 46, 33028–33038. [Google Scholar] [CrossRef]
- Fu, G.; Liu, X.; Yi, X.; Zhang, S.; Cao, X.; Meng, X.; Gao, Z.; Wang, H. Development of High–Entropy Shape–Memory Alloys: A Review. Metals 2023, 13, 1279. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Wei, J.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Kai, J.J.; et al. Multicomponent intermetallic nanoparticles and superb mechanical behaviors of complex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.; Abedi, H.R.; Zhang, Y. A review study on thermal stability of high entropy alloys: Normal/abnormal resistance of grain growth. J. Alloys Compd. 2023, 960, 170826. [Google Scholar] [CrossRef]
- Hua, D.; Xia, Q.; Wang, W.; Zhou, Q.; Li, S.; Qian, D.; Shi, J.; Wang, H. Atomistic insights into the deformation mechanism of a CoCrNi medium entropy alloy under nanoindentation. Int. J. Plast. 2021, 142, 102997. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, A.X.; Zhan, S.; Liu, C.-T.; Cao, S.C. Refractory high–entropy alloys: A focused review of preparation methods and properties. J. Mater. Sci. Technol. 2022, 142, 196–215. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high–entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, D.D.; Wang, S.Y. High–entropy alloys for electrocatalysis: Design, characterization and applications. Small 2022, 18, 2104339. [Google Scholar]
- Güler, S.; Alkan, E.D.; Alkan, M. Vacuum arc melted and heat treated AlCoCrFeNiTiX based high–entropy alloys: Thermodynamic and microstructural investigations. J. Alloys Compd. 2022, 903, 163901. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Jiang, H.; Wang, Z.; Cao, Z.; Guo, S.; Wang, T.; Li, T.; Liaw, P.K. Promising properties and future trend of eutectic high entropy alloys. Scr. Mater. 2020, 187, 202–209. [Google Scholar] [CrossRef]
- Braic, V.; Balaceanu, M.; Braic, M.; Vladescu, A.; Panseri, S.; Russo, A. Characterization of multi–principal–element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Behav. Biomed. Mater. 2012, 10, 197–205. [Google Scholar] [CrossRef]
- Nagase, T.; Rack, P.D.; Noh, J.H.; Egami, T. In–situ TEM observation of structural changes in nano–crystalline CoCrCuFeNi multicomponent high–entropy alloy (HEA) under fast electron irradiation by high voltage electron microscopy (HVEM). Intermetallics 2015, 59, 32–42. [Google Scholar] [CrossRef]
- Buravlev, I.; Shichalin, O.; Marmaza, P.; Kolodeznikov, E.; Dvornik, M.; Sakhnevich, A.; Buravleva, A.; Chuklinov, S.; Papynov, E. Microstructural evolution and mechanical behavior of WC–4wt.%TiC–3wt.%TaC–12wt.%Co refractory cermet consolidated by spark plasma sintering of mechanically activated powder mixtures. Adv. Powder Technol. 2024, 35, 104625. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Gorobtsov, P.Y.; Kuznetsov, N.T. Effect of nanocrystalline SiC addition on reactive SPS and oxidation resistance of Ta4HfC5 ceramics. Ceram. Int. 2022, 49, 9691–9701. [Google Scholar] [CrossRef]
- Shichalin, O.; Buravlev, I.; Papynov, E.; Buravleva, A.; Sakhnevich, V.; Dvornik, M.; Vlasova, N.; Gerasimenko, A.; Reva, V.; Yudakov, A. Comparative study of WC–based hard alloys fabrication via spark plasma sintering using Co, Fe, Ni, Cr, and Ti binders. Int. J. Refract. Met. Hard Mater. 2022, 102, 105725. [Google Scholar] [CrossRef]
- Zeraati, M.; Khayati, G.R.; Feizabad, M.H.K. Microstructure and Magnetic Characteristics of AlCrCuFeNiTi High–Entropy Alloy Prepared by Mechanical Alloying Followed by Spark Plasma Sintering. J. Mater. Eng. Perform. 2024, 34, 7965–7975. [Google Scholar] [CrossRef]
- Adabavazeh, Z.; Hosseini, S.; Karimzadeh, F.; Abbasi, M. Fabrication and performance assessment of CuFeNiMnTi high entropy alloy through mechanical alloying and spark plasma sintering. Mater. Today Commun. 2024, 42, 111124. [Google Scholar] [CrossRef]
- Yurchenko, N.; Shaysultanov, D.; Povolyaeva, E.; Moskovskikh, D.; Zherebtsov, S.; Stepanov, N. Structure and mechanical properties of low–density AlCrFeTiX (X = Co, Ni, Cu) high–entropy alloys produced by spark plasma sintering. J. Alloys Compd. 2024, 1007, 176445. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chang, Y.-J.; Murakami, H.; Gorsse, S.; Yeh, A.-C. Designing high entropy superalloys for elevated temperature application. Scr. Mater. 2020, 187, 177–182. [Google Scholar] [CrossRef]
- Chen, D.; He, F.; Han, B.; Wu, Q.; Tong, Y.; Zhao, Y.; Wang, Z.; Wang, J.; Kai, J.-J. Synergistic effect of Ti and Al on L12–phase design in CoCrFeNi–based high entropy alloys. Intermetallics 2019, 110, 106476. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, L.; Yang, L.; Zhang, W.; Ji, G.; Zhou, X.; Curtin, W.; Chen, X.; Liaw, P.; Chen, S.; et al. High throughput synthesis enabled exploration of CoCrFeNi–based high entropy alloys. J. Mater. Sci. Technol. 2022, 110, 269–282. [Google Scholar] [CrossRef]
- Zhi, Q.; Tan, X.; Liu, Z.; Liu, Y.; Zhang, Q.; Chen, Y.; Li, M. Effect of Zr content on microstructure and mechanical properties of lightweight Al2NbTi3V2Zrx high entropy alloy. Micron 2021, 144, 103031. [Google Scholar] [CrossRef] [PubMed]


| Fe | Cr | Ti | Ni | Co | |
|---|---|---|---|---|---|
| Fe | - | −1 | −17 | −2 | −1 |
| Cr | −1 | - | −7 | −7 | −4 |
| Ti | −17 | −7 | − | −35 | −28 |
| Ni | −2 | −7 | −35 | - | 0 |
| Co | −1 | −4 | −28 | 0 | - |
| Parameter | Value |
|---|---|
| 6.15 | |
| −4.08 | |
| 15.53 | |
| VEC | 7.2 |
| Sample | Milling Time (h) | Crystal Size (nm) | |
|---|---|---|---|
| BCC | FCC | ||
| CoCrFeNiTi | 1 | 108 | 94 |
| 2 | 68 | 74 | |
| 3 | 58 | 66 | |
| 4 | 38 | 41 | |
| 5 | 34 | 38 | |
| Element | Concentration | k Ratio | wt% |
|---|---|---|---|
| Ti | 46.28 | 0.46283 | 20.04 |
| Cr | 51.33 | 0.51334 | 22.64 |
| Fe | 45.51 | 0.45513 | 20.88 |
| Co | 38.08 | 0.38085 | 18.09 |
| Ni | 39.64 | 0.39635 | 18.34 |
| Kinetics Model | Parameter | Plotted Function | |
|---|---|---|---|
| Chemical reaction | Maniple | Slope = kM | |
| Second–order | Slope = kS.O | ||
| Contracting cylinder | Slope = kC.C | ||
| Contracting sphere | Slope = kC.S | ||
| Diffusion model | Jander | Slope = kj | |
| Crank | Slope = kC | ||
| Dunwald–Wagner | Slope = kD,W | ||
| One–dimensional diffusion | Slope = kO.D.D | ||
| Nucleation–growth | Johnson–Mehl–Avrami | Slope = n, Intercept = nln[k] |
| n | Type of Nucleation | Geometries | |
|---|---|---|---|
| Interface–controlled growth | 1 | Exhausted rapidly | Plane |
| 2 | Exhausted rapidly | Cylinder | |
| 3 | Exhausted rapidly | Sphere | |
| 4 | Exhausted rapidly | Sphere | |
| Diffusion–controlled growth | 1.2 | Exhausted rapidly | Plane |
| 1 | Exhausted rapidly | Cylinder | |
| 3.2 | Exhausted rapidly | Sphere | |
| 5.2 | Exhausted rapidly | Sphere |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, T.; Liu, M.; Yang, C.; Wang, X.; Wang, J. The Growth Kinetic and Ultra High Hardness of CoCrFeNiTi High–Entropy Alloy by Mechanical Alloying and Spark Plasma Sintering. Materials 2025, 18, 3242. https://doi.org/10.3390/ma18143242
Qu T, Liu M, Yang C, Wang X, Wang J. The Growth Kinetic and Ultra High Hardness of CoCrFeNiTi High–Entropy Alloy by Mechanical Alloying and Spark Plasma Sintering. Materials. 2025; 18(14):3242. https://doi.org/10.3390/ma18143242
Chicago/Turabian StyleQu, Tiejun, Mingpu Liu, Chuanhua Yang, Xin Wang, and Junfa Wang. 2025. "The Growth Kinetic and Ultra High Hardness of CoCrFeNiTi High–Entropy Alloy by Mechanical Alloying and Spark Plasma Sintering" Materials 18, no. 14: 3242. https://doi.org/10.3390/ma18143242
APA StyleQu, T., Liu, M., Yang, C., Wang, X., & Wang, J. (2025). The Growth Kinetic and Ultra High Hardness of CoCrFeNiTi High–Entropy Alloy by Mechanical Alloying and Spark Plasma Sintering. Materials, 18(14), 3242. https://doi.org/10.3390/ma18143242






