Storage of Titanium Dental Implants in Ozone Nanobubble Water Retards Biological Aging and Enhances Osseointegration: An In Vivo Study
Abstract
1. Introduction
- (1)
- There is no difference in the degree of early osseointegration between titanium implants stored in NBW3 and those stored under ambient conditions.
- (2)
- Storage in NBW3 does not influence bone-to-implant contact (BIC) or peri-implant bone formation.
- (3)
- The preservation of surface hydrophilicity via NBW3 does not affect the biological performance of titanium implants in vivo.
2. Materials and Methods
2.1. Ozone Nanobubble Water
2.2. Titanium Implant Bodies
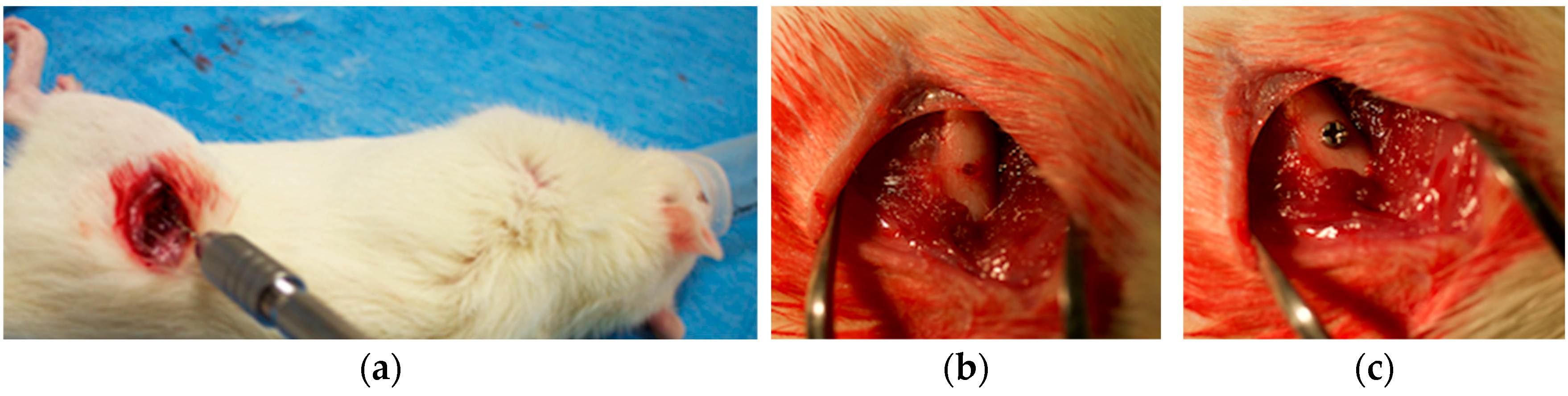
2.3. In Vivo Implantation in Sprague Dawley Rats
2.4. Mechanical Analysis
2.4.1. Removal Torque Measurement
2.4.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Analysis
2.5. Histological Preparation and Analysis
2.5.1. Sample Processing
2.5.2. Soft X-Ray Imaging
2.5.3. Histological Staining and Bone-to-Implant Contact (BIC) Analysis
2.6. Statistical Analysis
3. Results
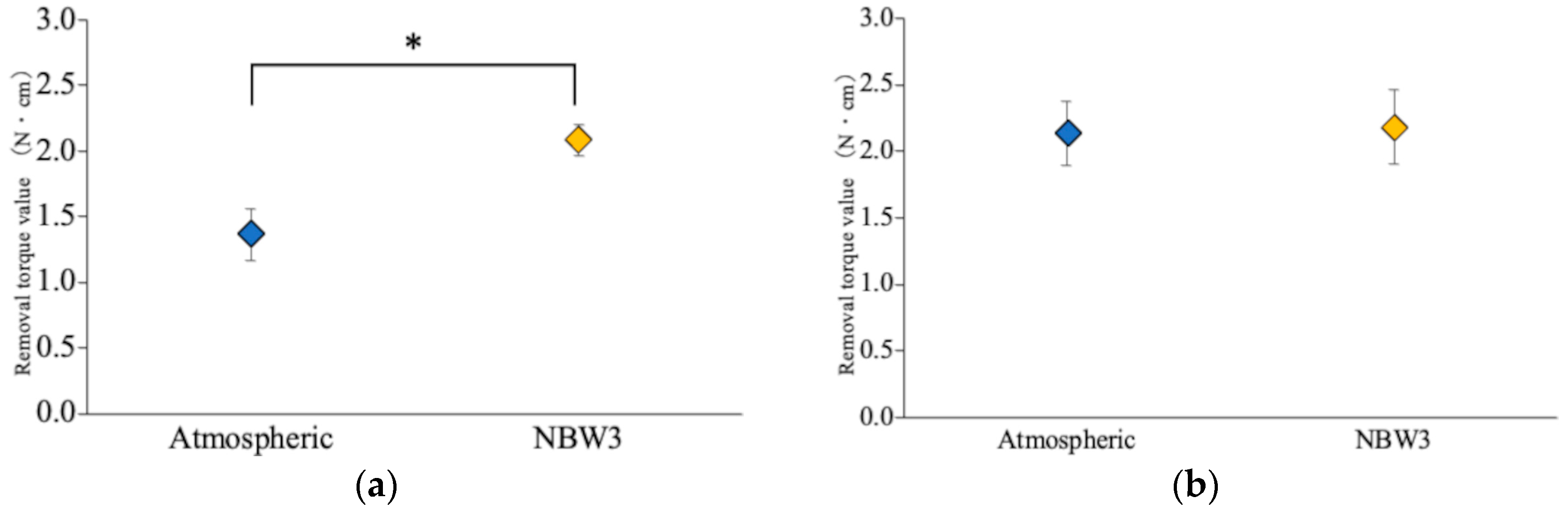
3.1. Removal Torque Analysis
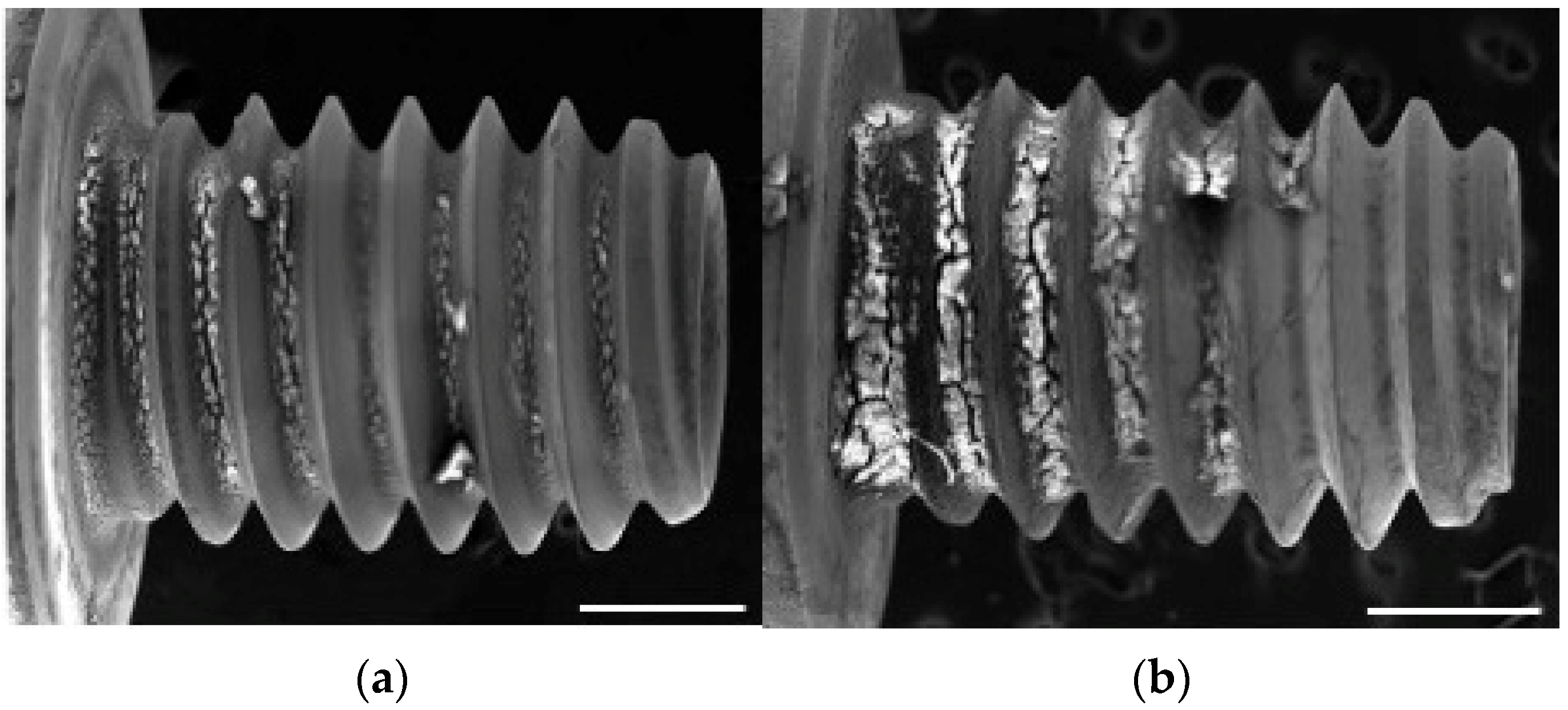
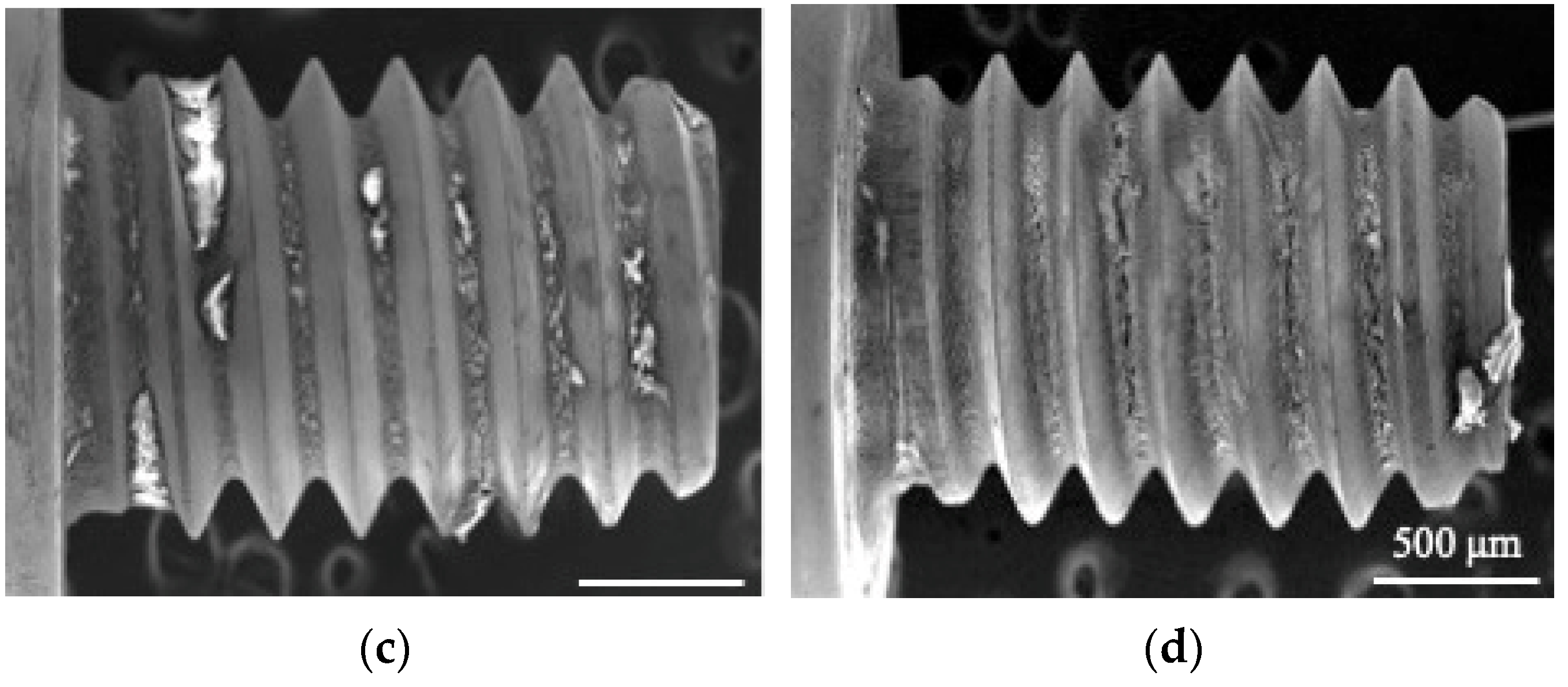
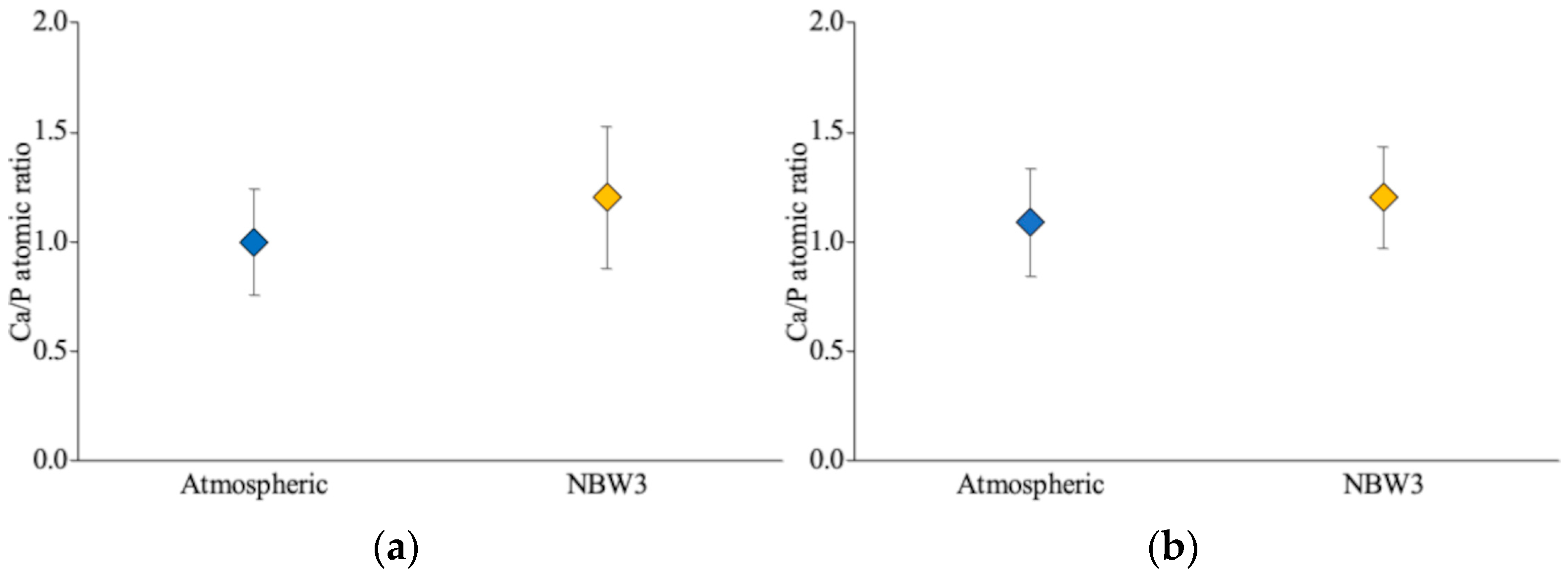
3.2. SEM and EDX Observations
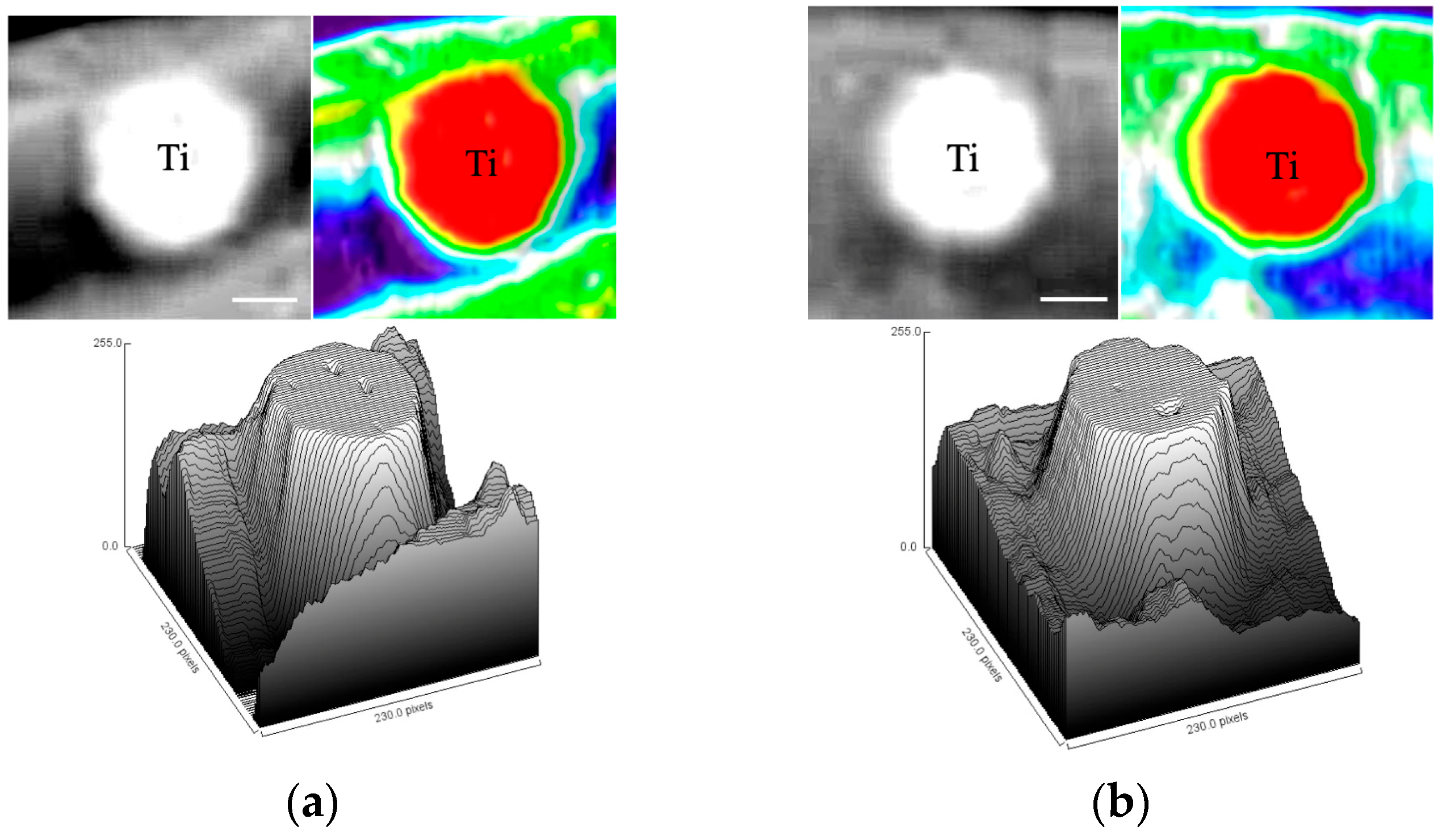
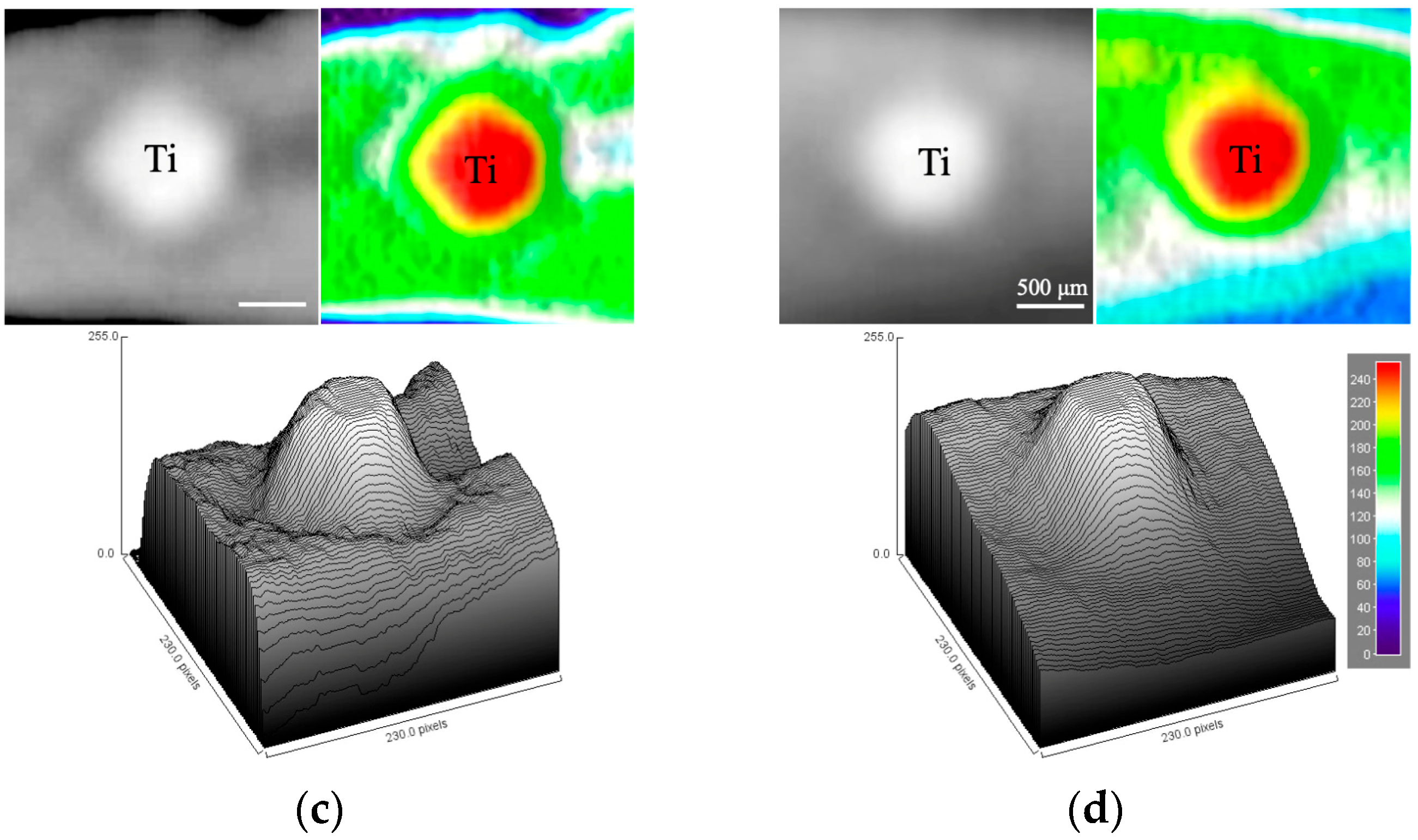
3.3. Soft X-Ray Analysis
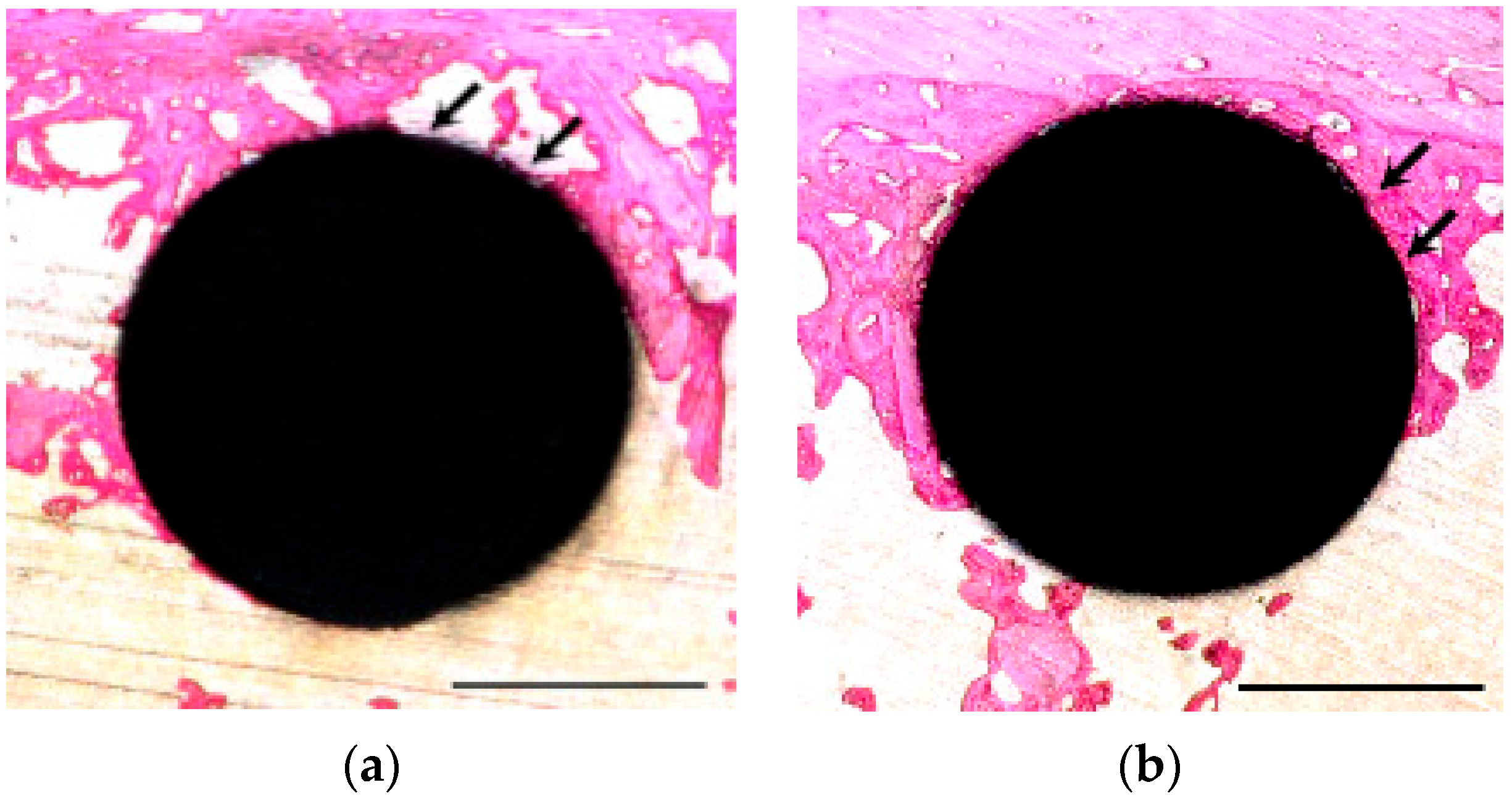
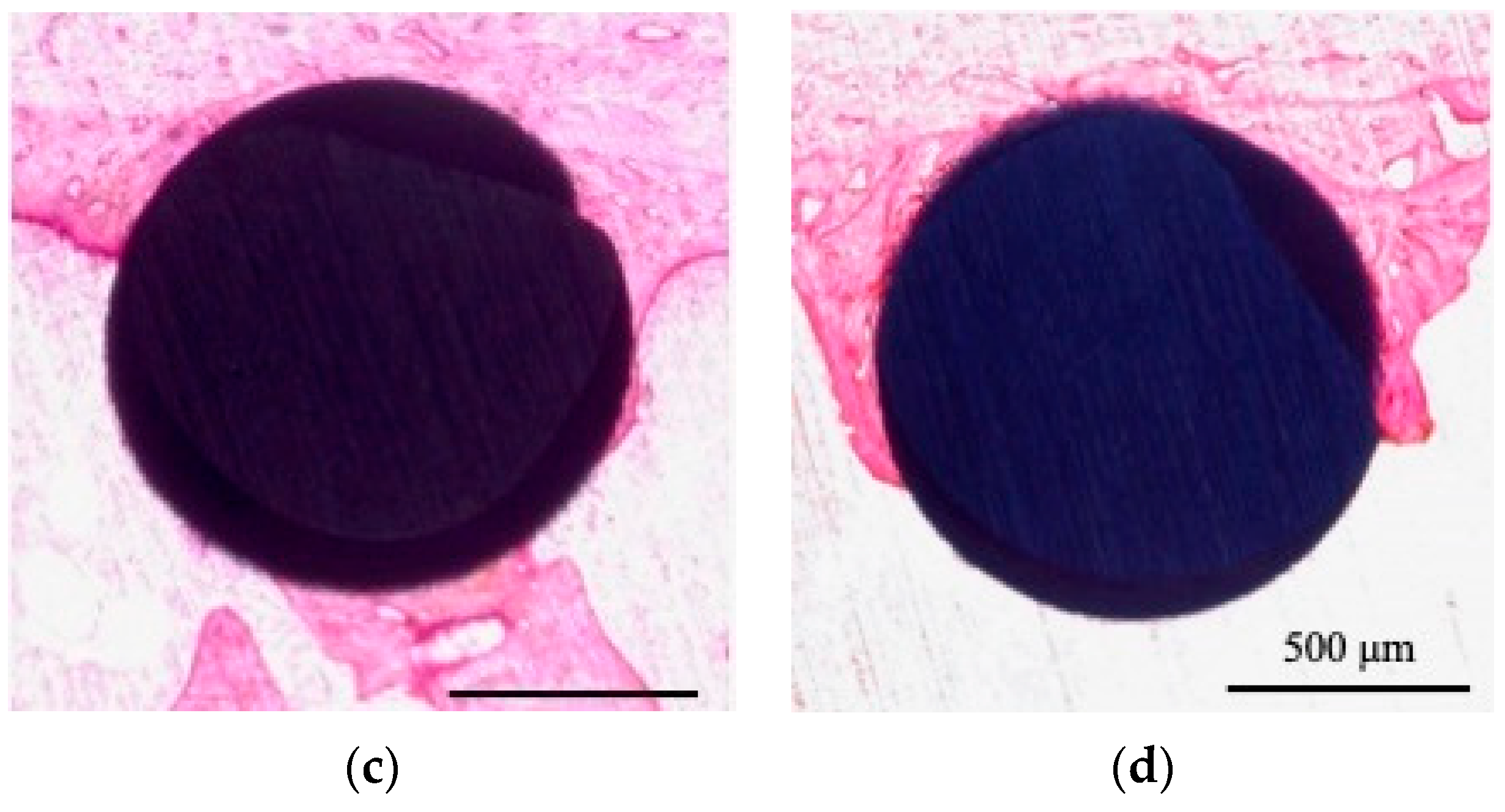
3.4. Histological Observation and BIC Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmedo-Gaya, M.V.; Manzano-Moreno, F.J.; Cañaveral-Cavero, E.; de Dios Luna-del Castillo, J.; Vallecillo-Capilla, M. Risk Factors Associated with Early Implant Failure: A 5-Year Retrospective Clinical Study. J. Prosthet. Dent. 2016, 115, 150–155. [Google Scholar] [CrossRef]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early Dental Implant Failure: Risk Factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Fasih, P.; Alborzi, S.; Nikzad, H.; Romoozi, E. Risk Factors Associated with Early Implant Failure: A Retrospective Review. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101749. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous Anchorage of Dental Prostheses. I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.J.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of Titanium Surface Roughness on Proliferation, Differentiation, and Protein Synthesis of Human Osteoblast-like Cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Badihi, L.; Rosen, G.; Steinberg, D.; Kohavi, D. Adsorption of Human Plasma Proteins to Modified Titanium Surfaces. Clin. Oral Implant. Res. 2007, 18, 630–638. [Google Scholar] [CrossRef]
- Kramer, P.R.; Janikkeith, A.; Cai, Z.; Ma, S.; Watanabe, I. Integrin Mediated Attachment of Periodontal Ligament to Titanium Surfaces. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 877–883. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Yoshinari, M.; Oda, Y.; Inoue, T.; Matsuzaka, K.; Shimono, M. Bone Response to Calcium Phosphate-Coated and Bisphosphonate-Immobilized Titanium Implants. Biomaterials 2002, 23, 2879–2885. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Kimura, Y.; Matsuzaka, K.; Yoshinari, M.; Inoue, T. Initial Attachment of Human Oral Keratinocytes Cultured on Zirconia or Titanium. Dent. Mater. J. 2012, 31, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The Effect of UV-Photofunctionalization on the Time-Related Bioactivity of Titanium and Chromium-Cobalt Alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Gentleman, M.M.; Ruud, J.A. Role of Hydroxyls in Oxide Wettability. Langmuir ACS J. Surf. Colloids 2010, 26, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Metiu, H.; Chrétien, S.; Hu, Z.; Li, B.; Sun, X. Chemistry of Lewis Acid–Base Pairs on Oxide Surfaces. J. Phys. Chem. C 2012, 116, 10439–10450. [Google Scholar] [CrossRef]
- Ogawa, T. Ultraviolet Photofunctionalization of Titanium Implants. Int. J. Oral Maxillofac. Implant. 2014, 29, e95–e102. [Google Scholar] [CrossRef]
- Rupp, F.; Scheideler, L.; Eichler, M.; Geis-Gerstorfer, J. Wetting Behavior of Dental Implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 1256–1266. [Google Scholar]
- Kawahara, D.; Ong, J.L.; Raikar, G.N.; Lucas, L.C.; Lemons, J.E.; Nakamura, M. Surface Characterization of Radio-Frequency Glow Discharged and Autoclaved Titanium Surfaces. Int. J. Oral Maxillofac. Implant. 1996, 11, 435–442. [Google Scholar]
- Shibata, Y.; Hosaka, M.; Kawai, H.; Miyazaki, T. Glow Discharge Plasma Treatment of Titanium Plates Enhances Adhesion of Osteoblast-like Cells to the Plates through the Integrin-Mediated Mechanism. Int. J. Oral Maxillofac. Implant. 2002, 17, 771–777. [Google Scholar]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet Light-Mediated Photofunctionalization of Titanium to Promote Human Mesenchymal Stem Cell Migration, Attachment, Proliferation and Differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef]
- Akanuma, M.; Nakanishi, Y.; Yui, T.; Hirose, Y.; Kado, T.; Oh, H.; Ochi, M. Effect of TiN Coating on Pure Ti Abutments for Corrosion Resistance and Peri-Implant Tissue Cells. J. Hard Tissue Biol. 2024, 33, 113–124. [Google Scholar] [CrossRef]
- Furuichi, A.; Arakawa, S.; Mano, Y.; Morita, I.; Tachikawa, N.; Yamada, Y.; Kasugai, S. Comparative Analysis of Efficacy of Ozone Nano Bubble Water (NBW3) with Established Antimicrobials. Bactericidal Efficacy and Cellular Response. An in Vitro Study. J. Oral Tissue Eng. 2013, 10, 131–141. [Google Scholar] [CrossRef]
- Takahashi, M.; Ishikawa, H.; Asano, T.; Horibe, H. Effect of Microbubbles on Ozonized Water for Photoresist Removal. J. Phys. Chem. C 2012, 116, 12578–12583. [Google Scholar] [CrossRef]
- Hayakumo, S.; Arakawa, S.; Mano, Y.; Izumi, Y. Clinical and Microbiological Effects of Ozone Nano-Bubble Water Irrigation as an Adjunct to Mechanical Subgingival Debridement in Periodontitis Patients in a Randomized Controlled Trial. Clin. Oral Investig. 2013, 17, 379–388. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakazawa, M.; Nishimoto, T.; Odajima, M.; Shirai, Y.; Sugawa, S. Impact of Bulk Nanobubble Water on a TiO2 Solid Surface: A Case Study for Medical Implants. Langmuir 2024, 40, 25950–25956. [Google Scholar] [CrossRef]
- Horikawa, H.; Yui, T.; Nakanishi, Y.; Hirose, Y.; Nezu, T.; Oh, H.; Ochi, M. Ozone Nanobubble Water Inhibits the Biological Aging and Maintains the Affinity of Titanium Disks: An in Vitro Study. J. Hard Tissue Biol. 2024, 33, 203–212. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-Mediated Mineral Deposition in Culture Is Dependent on Surface Microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding Peri-Implant Endosseous Healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Takahashi, M.; Chiba, K. Ozone Water and Method for Producing the Same. Japanese Patent JP2005-246293A, 15 September 2005. [Google Scholar]
- Nagasawa, M.; Takano, R.; Maeda, T.; Uoshima, K. Observation of the Bone Surrounding an Overloaded Implant in a Novel Rat Model. Int. J. Oral Maxillofac. Implant. 2013, 28, 109–116. [Google Scholar] [CrossRef]
- Takano, R.; Nagasawa, M.; Kitami, M.; Rosales Rocabado, J.M.; Kaku, M.; Stegaroiu, R.; Uoshima, K. Correlation Between Stress Distributions and Biological Reactions in Bone Surrounding Implants That Support Cantilevers in Supraocclusal Contact in Rats. Implant. Dent. 2016, 25, 204–213. [Google Scholar] [CrossRef]
- Naka, T.; Yokose, S. Application of Laser-Induced Bone Therapy by Carbon Dioxide Laser Irradiation in Implant Therapy. Int. J. Dent. 2012, 2012, 409496. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of Osteoblast Adhesion to UV-Photofunctionalized Titanium via an Electrostatic Mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; McMurray, R.J.; Biggs, M.J.P.; Kantawong, F.; Oreffo, R.O.C.; Dalby, M.J. Nanotopographical Control of Stem Cell Differentiation. J. Tissue Eng. 2010, 2010, 120623. [Google Scholar] [CrossRef]
- Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. [Google Scholar] [CrossRef]
- Shin, S.-I.; Min, H.-K.; Park, B.-H.; Kwon, Y.-H.; Park, J.-B.; Herr, Y.; Heo, S.-J.; Chung, J.-H. The Effect of Er:YAG Laser Irradiation on the Scanning Electron Microscopic Structure and Surface Roughness of Various Implant Surfaces: An in Vitro Study. Lasers Med. Sci. 2011, 26, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Wehner, C.; Laky, M.; Shokoohi-Tabrizi, H.A.; Behm, C.; Moritz, A.; Rausch-Fan, X.; Andrukhov, O. Effects of Er:YAG Laser Irradiation of Different Titanium Surfaces on Osteoblast Response. J. Mater. Sci. Mater. Med. 2021, 32, 22. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon Deposition Attenuates Osteoblast Activity on Titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef]
- Uchiyama, H.; Yamada, M.; Ishizaki, K.; Sakurai, K. Specific Ultraviolet-C Irradiation Energy for Functionalization of Titanium Surface to Increase Osteoblastic Cellular Attachment. J. Biomater. Appl. 2014, 28, 1419–1429. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Effects of Surface Wettability and Contact Time on Protein Adhesion to Biomaterial Surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Takeshita, F.; Inoue, T.; Yoshinari, M.; Shimono, M.; Suetsugu, T.; Tanaka, T. An Immunoelectron Microscopic Localization of Noncollagenous Bone Proteins (Osteocalcin and Osteopontin) at the Bone-Titanium Interface of Rat Tibiae. J. Biomed. Mater. Res. 1998, 41, 111–119. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Gilmore, C.P.; Cooper, E.; Leggett, G.J.; Downes, S. Protein Adsorption and Human Osteoblast-like Cell Attachment and Growth on Alkylthiol on Gold Self-Assembled Monolayers. J. Biomed. Mater. Res. 2002, 59, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is Healing Induced via a Mild Oxidative Stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Wu, X. Osseointegration Pharmacology. JDR Clin. Transl. Res. 2017, 2, 211–213. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis Method of Hydroxyapatite: A Review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Kohles, S.S.; Martinez, D.A. Elastic and Physicochemical Relationships within Cortical Bone. J. Biomed. Mater. Res. 2000, 49, 479–488. [Google Scholar] [CrossRef]
- Cho, M.-I.; Matsuda, N.; Lin, W.-L.; Moshier, A.; Ramakrishnan, P.R. In Vitro Formation of Mineralized Nodules by Periodontal Ligament Cells from the Rat. Calcif. Tissue Int. 1992, 50, 459–467. [Google Scholar] [CrossRef]
- Nauman, E.A.; Ebenstein, D.M.; Hughes, K.F.; Pruitt, L.; Halloran, B.P.; Bikle, D.D.; Keaveny, T.M. Mechanical and Chemical Characteristics of Mineral Produced by Basic Fibroblast Growth Factor-Treated Bone Marrow Stromal Cells in Vitro. Tissue Eng. 2002, 8, 931–939. [Google Scholar] [CrossRef]
- Milleret, V.; Tugulu, S.; Schlottig, F.; Hall, H. Alkali Treatment of Microrough Titanium Surfaces Affects Macrophage/Monocyte Adhesion, Platelet Activation and Architecture of Blood Clot Formation. Eur. Cells Mater. 2011, 21, 430–444, discussion 444. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early Wound Healing around Endosseous Implants: A Review of the Literature. Int. J. Oral Maxillofac. Implant. 2005, 20, 425–431. [Google Scholar]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horikawa, H.; Yui, T.; Nakanishi, Y.; Hirose, Y.; Kado, T.; Nezu, T.; Oh, H.; Ochi, M. Storage of Titanium Dental Implants in Ozone Nanobubble Water Retards Biological Aging and Enhances Osseointegration: An In Vivo Study. Materials 2025, 18, 3156. https://doi.org/10.3390/ma18133156
Horikawa H, Yui T, Nakanishi Y, Hirose Y, Kado T, Nezu T, Oh H, Ochi M. Storage of Titanium Dental Implants in Ozone Nanobubble Water Retards Biological Aging and Enhances Osseointegration: An In Vivo Study. Materials. 2025; 18(13):3156. https://doi.org/10.3390/ma18133156
Chicago/Turabian StyleHorikawa, Hidehiro, Tomoo Yui, Yasuhiro Nakanishi, Yukito Hirose, Takashi Kado, Takashi Nezu, Hourei Oh, and Morio Ochi. 2025. "Storage of Titanium Dental Implants in Ozone Nanobubble Water Retards Biological Aging and Enhances Osseointegration: An In Vivo Study" Materials 18, no. 13: 3156. https://doi.org/10.3390/ma18133156
APA StyleHorikawa, H., Yui, T., Nakanishi, Y., Hirose, Y., Kado, T., Nezu, T., Oh, H., & Ochi, M. (2025). Storage of Titanium Dental Implants in Ozone Nanobubble Water Retards Biological Aging and Enhances Osseointegration: An In Vivo Study. Materials, 18(13), 3156. https://doi.org/10.3390/ma18133156





