Quenching Sensitivity Study of New High-Strength Aluminum Alloys Based on an Immersion End-Quenching and Step-Quenching Technique
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Material
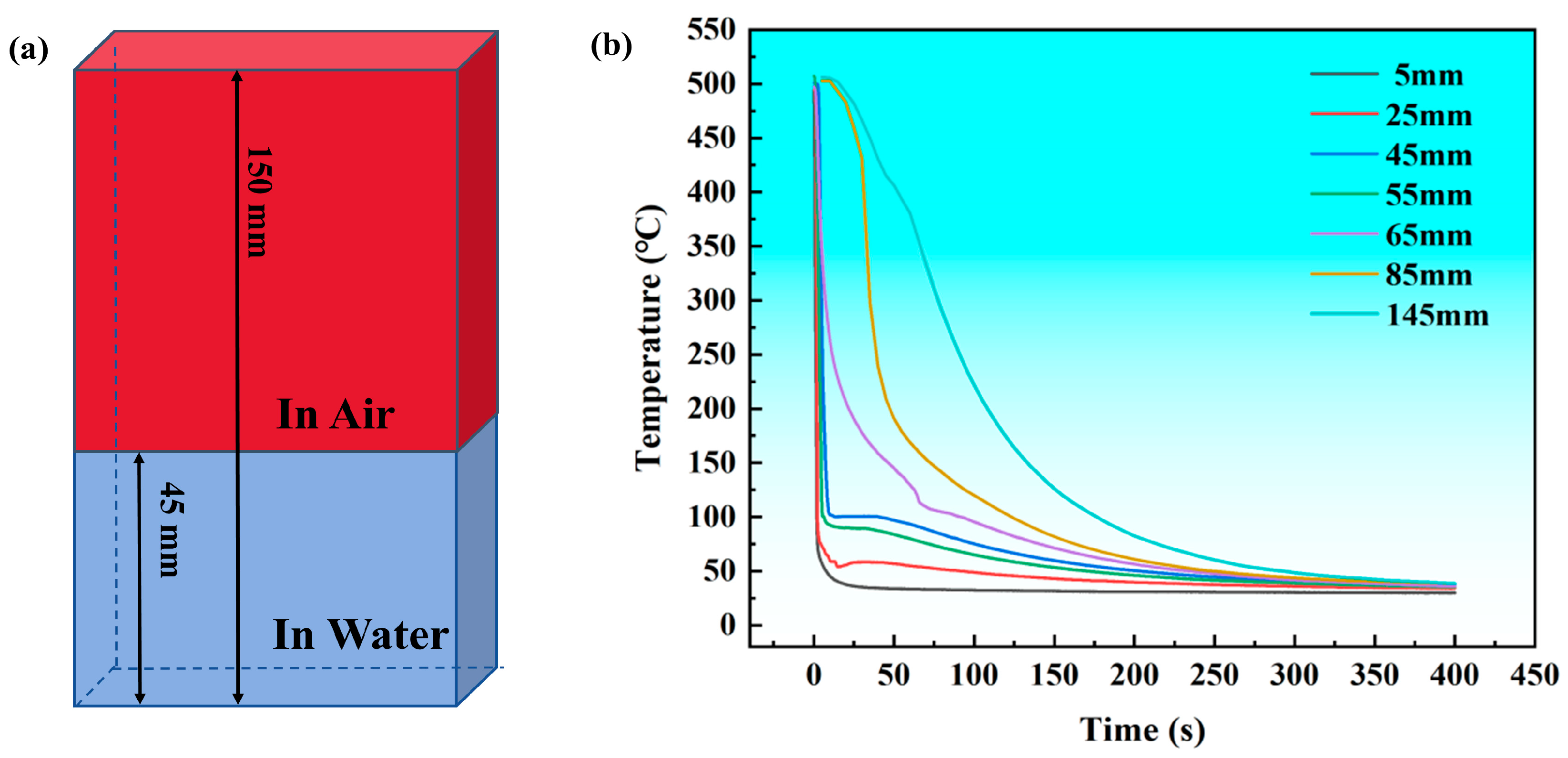
2.2. Immersion End-Quenching Experiment
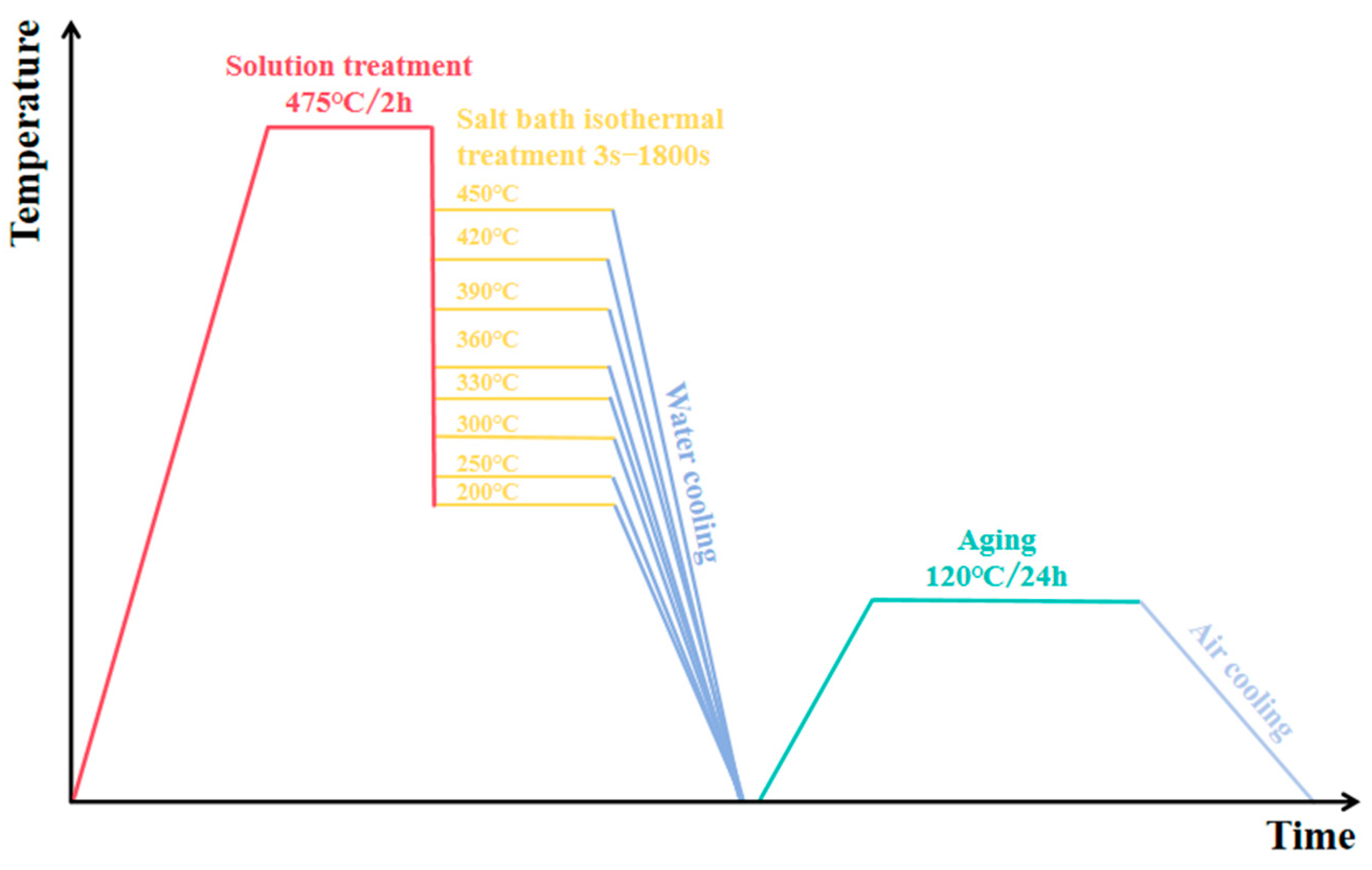
2.3. Step-Quenching Experiment
2.4. Hardness Measurement
2.5. Microstructure Analysis
3. Results
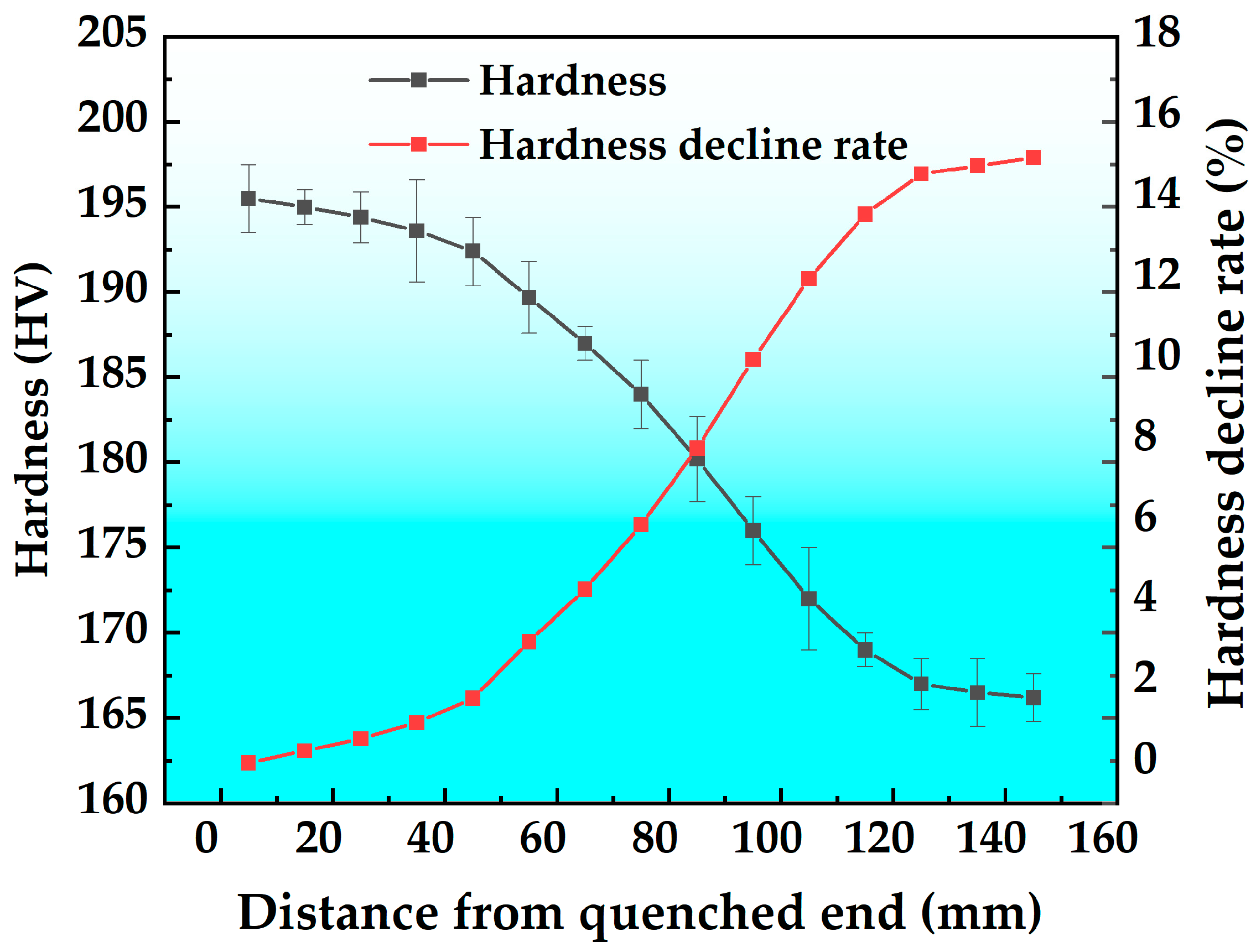
3.1. Immersion End-Quenching Experiment
3.2. Step-Quenching Experiment
4. Analysis and Discussion
5. Conclusions
- (1)
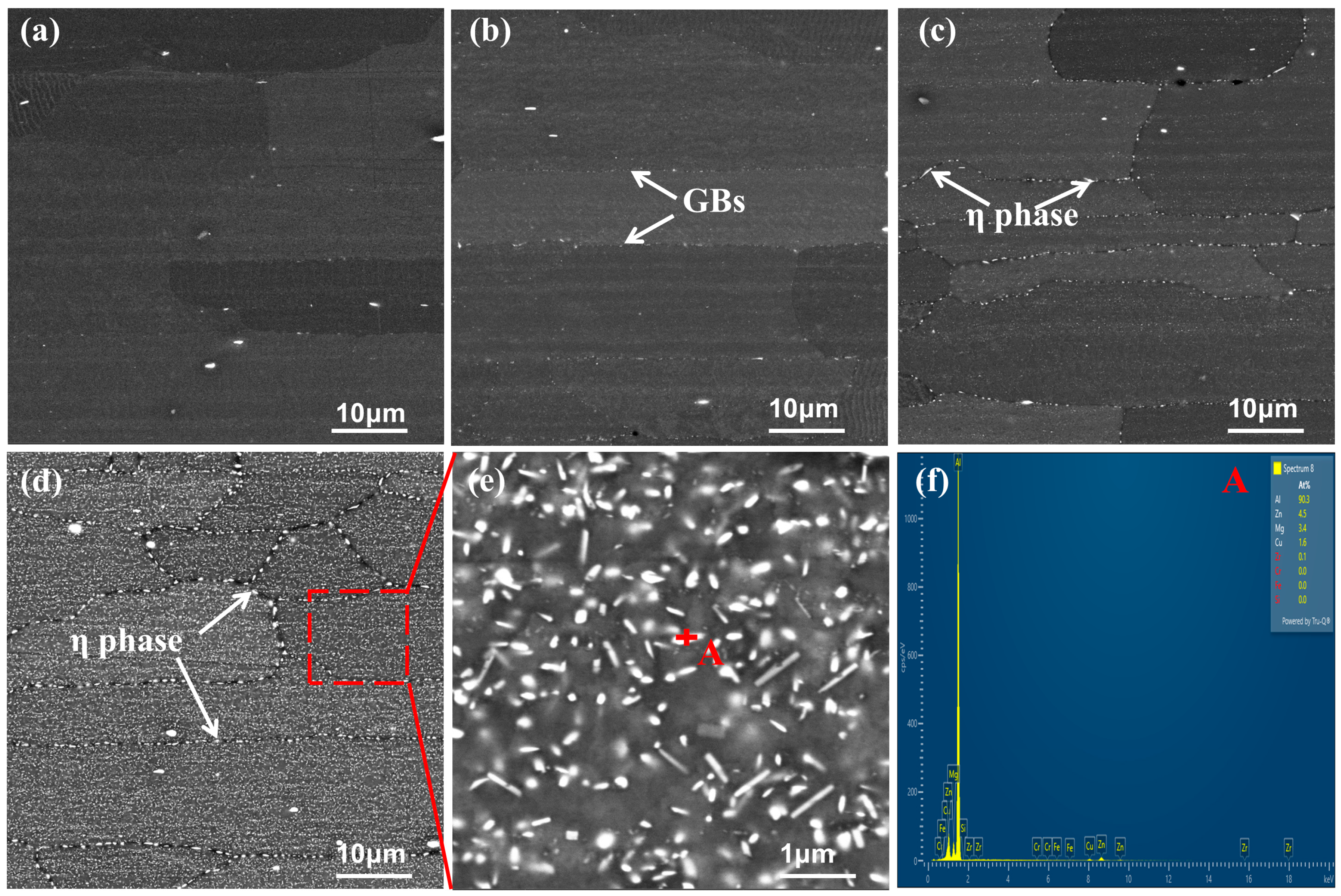
- When the cooling rate decreases from 402.5 °C/s to 3.6 °C/s, the hardness reduction rate is 15%. With decreasing cooling rate, the η phase precipitates at grain boundaries and within grains (on dispersed particles), consuming a significant amount of solute atoms. This results in a decrease in the quantity and an increase in the size of aging precipitates. Additionally, distinct PFZs form around grain boundaries and η phase particles, ultimately leading to the reduction of hardness.
- (2)
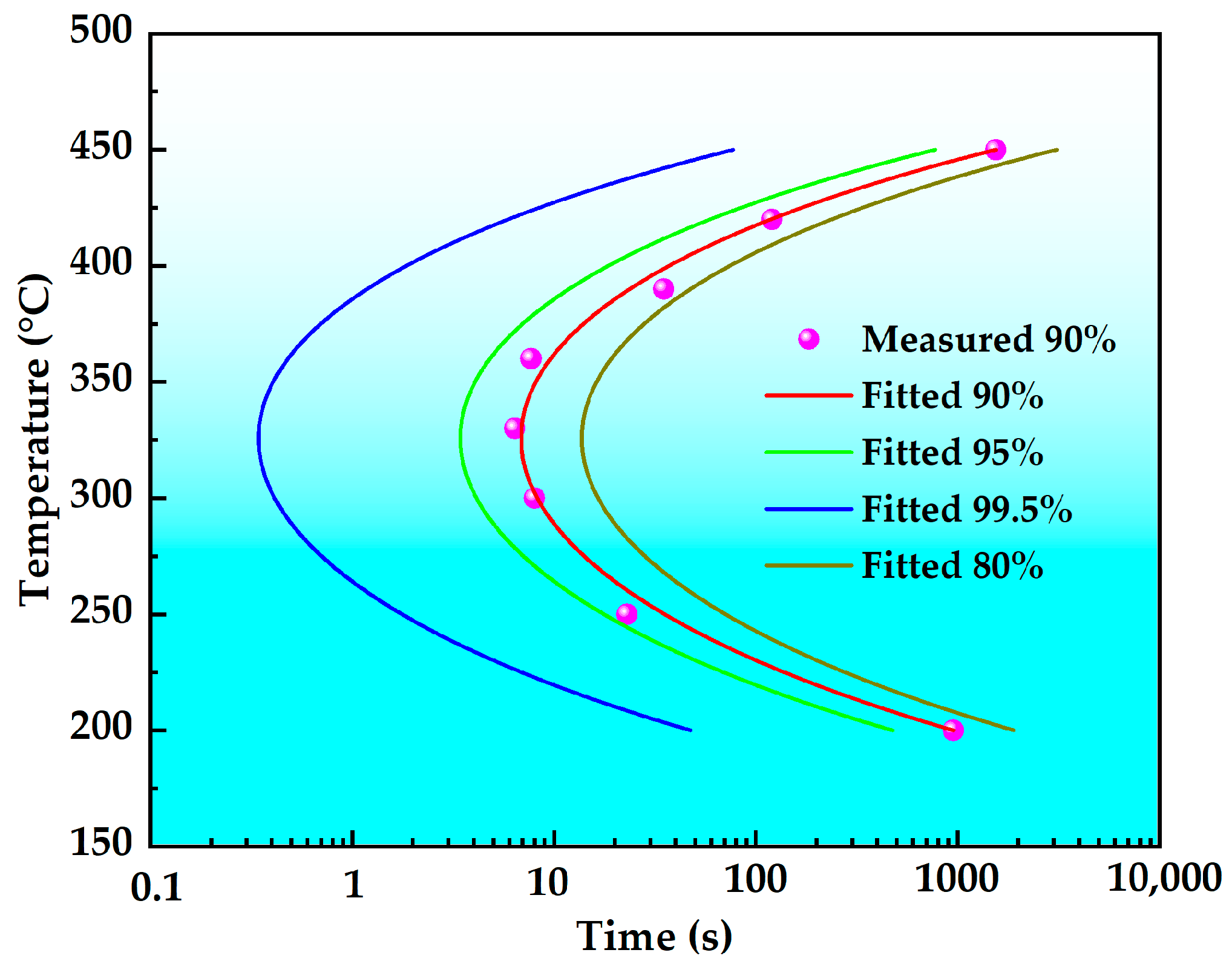
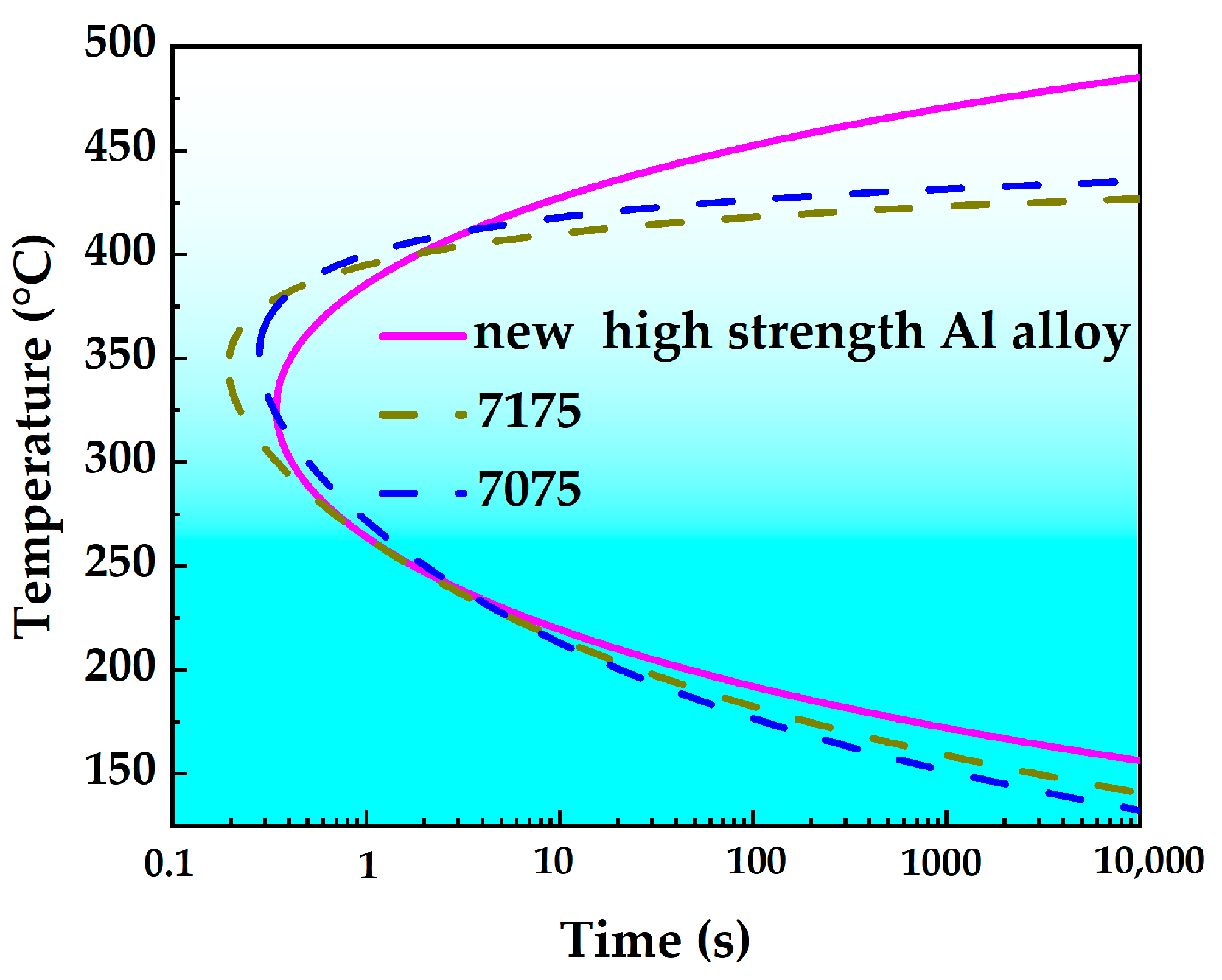
- The nose temperature of the TTP curve for this novel alloy is 325 °C, with a critical transformation time of 0.4 s. The temperature range corresponding to a 99.5% peak hardness reduction on the TTP curve after isothermal holding for 10 s is 219 °C to 427 °C. Comparative analysis reveals that the new high-strength aluminum alloy exhibits lower quench sensitivity than both 7075 and 7175 alloys. Consequently, this novel alloy achieves effective quench sensitivity reduction through optimized alloying element composition design.
- (3)
- Quench factor analysis is employed to predict the alloy hardness. The predicted values showed close agreement with experimental measurements, with a maximum deviation of approximately 5%. The smaller the quenching factor τ, the greater the hardness of the alloy after aging. By combining the relevant data of the TTP curve to optimize and control the quenching process of the alloy, the performance of the alloy can be improved. By integrating TTP curve data to optimize and control quenching rates, alloy performance is enhanced. Through developing novel high-strength aluminum alloys and optimizing their quenching processes, this research provides a theoretical basis for selecting hot forming–quenching integrated process parameters in automotive high-strength Al alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Liu, B.; Zhang, S. The Advancement of 7XXX Series Aluminum Alloys for Aircraft Structures: A Review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Rometsch, P.; Zhang, Y.; Knight, S. Heat Treatment of 7XXX Series Aluminum Alloys: Some Recent Developments. Trans. Nonferrous Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, K.; Ren, C.; Naseem, S. Precipitation Sequence and Hardening Effect in 7A85 Aluminum Alloy. J. Alloys Compd. 2021, 875, 159950. [Google Scholar] [CrossRef]
- Kvvssn, V.; Butt, M.; Laieghi, H.; Uddin, Z.; Salamci, E.; Kim, D.; Kizil, H. Recent progress in additive manufacturing of 7XXX aluminum alloys. Int. J. Adv. Manuf. Technol. 2025, 137, 4353–4399. [Google Scholar] [CrossRef]
- Cao, P.; Li, C.; Zhu, D.; Zhao, C.; Xiao, B.; Cao, C.; Xie, G. The Heterogeneous Precipitation Behavior of Al-Zn-Mg-Cu Alloy Was Studied by High-Throughput Experiment Based on End-Quenching Technology. Mater. Today Commun. 2024, 38, 107599. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, K.; Chen, S.; Zhang, X.; Fan, S.; Huang, L. Comparison of Hardenability and Over-Aging Precipitation Behaviour of Three 7xxx Aluminum Alloys. Mater. Sci. Technol. 2019, 35, 637–644. [Google Scholar] [CrossRef]
- Iskandar, M.; Reyes, D.; Gaxiola, Y.; Fudge, E.; Foyos, J.; Lee, E.W.; Kalu, P.; Garmestani, H.; Ogren, J.; Es-Saidet, O.S. On Identifying the Most Critical Step in the Sequence of Heat Treating Operations in a 7249 Aluminum Alloy. Eng. Fail. Anal. 2003, 10, 199–207. [Google Scholar] [CrossRef]
- Xie, P.; Chen, K.; Chen, S.; Ye, S.; Jiao, H.; Huang, L. Study on Quenching Sensitivity of 7097 Aluminum Alloy. Mater. Res. Express 2019, 7, 016505. [Google Scholar] [CrossRef]
- Yao, Y.; Euesden, R.; Burnett, T.; Shanthraj, P.; Prangnell, P. Effect of Cooling Rate on Quench-Induced Grain Boundary η-Phase Precipitation in 7xxx Aluminium Alloys. Metall. Mater. Trans. A 2025, 56, 1230–1251. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, C.; Ma, Z.; Jiang, H.; Huang, H. Hardness, Quench Sensitivity, and Electrical Conductivity of 7xxx Al Alloys with High Zn Concentrations. Sci. China Technol. Sci. 2020, 63, 953–959. [Google Scholar] [CrossRef]
- GB/T 3190-2008; Wrought Aluminium and Aluminium Alloys—Chemical Composition. Standardization Administration of China (SAC): Beijing, China, 2008.
- Li, H.; Tang, Y.; Zeng, Z.; Wang, F.; Sun, Y. Testing of Continuous Cooling Transformation Curve of Al-Zn-Mg-Cu Alloy. Chin. J. Nonferrous Met. 2008, 18, 1613–1621. [Google Scholar] [CrossRef]
- GB/T 3190-1996; Wrought Aluminium and Aluminium Alloys—Chemical Composition Limits. State Bureau of Technical Supervision: Beijing, China, 1996.
- Sujoy, K.; Diran, A. Effects of Mg and Cu Content on Quench Sensitivity of Al-Si-Mg Alloy. Int. J. Metalcast. 2016, 10, 138–146. [Google Scholar]
- Azam, B.; Shamseddin, M.; Zahra, L.; Bejan, H. Effect of Thermomechanical Treatment of Al-Zn-Mg-Cu with Minor Amount of Sc and Zr on the Mechanical Properties. Materials 2022, 15, 589. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Zhao, J. Quench Sensitivity of 7475 Aluminum Alloy Using End-Quenching Technique and TTP Diagrams. JOM 2021, 73, 1135–1143. [Google Scholar] [CrossRef]
- Wang, G.; Gu, Z.; Li, X.; Yu, G. Quench Sensitivity and Isothermal Transformation Behavior of 7075-H18 Aluminum Alloy Sheet. J. Mater. 2022, 50, 152–159. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Liu, S.; Deng, Y.; Zhang, X. Quenching Sensitivity and Heterogeneous Precipitation Behavior of AA7136 Alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3356–3369. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, Q.; Zhang, Y.; Liu, W.; Zhang, X.; Deng, Y. Investigation of Quench Sensitivity of High Strength Al-Zn-Mg-Cu Alloys by Time-Temperature-Properties Diagrams. Mater. Des. 2010, 31, 3116–3120. [Google Scholar] [CrossRef]
- Dolan, G.; Robinson, J. Residual Stress Reduction in 7175-T73, 6061-T6 and 2017A-T4 Aluminum Alloys Using Quench Factor Analysis. J. Mater. Process. Technol. 2004, 153, 346–351. [Google Scholar] [CrossRef]
- DIN 1725-1:1983-02; Aluminium Aloys; Wrought Alloys. Deutsches Institut für Normung (DIN): Berlin, Germany, 1983.
- GB/T 4340.3-2012; Metallic Materials—Vickers Hardness Test—Part 3: Calibration of Reference Blocks. Standardization Administration of China (SAC): Beijing, China, 2012.
- Jan, J.; Lee, S.; Chen, M.; Lee, S.; Lin, C.; Tzeng, Y. Effect of Homogenization and Zn/Mg Ratio on the Mechanical Properties, Stress Corrosion Cracking Resistance, and Quench Sensitivity of Al-Zn-Mg-2.5Cu (AA7095) Alloys. Adv. Eng. Mater. 2025, 27, 2402281. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, J.; Xiao, X.; Lin, H.; Wang, Y.; Zhen, Z. Quenching Sensitivity and Precipitation Kinetics of 2196 Alloy. Chin. J. Nonferrous Met. 2024, 34, 1475–1485. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Zhang, Y.; Liu, S.; Jiang, J.; Tang, J.; Ye, L.; Zhang, X. Effect of Hot Deformation on Microstructure and Quenching-Induced Precipitation Behavior of Al-Zn-Mg-Cu Alloy. Mater. Charact. 2021, 172, 110861. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, S.; Liu, S.; Deng, Y. Quench Sensitivity of Four Al-Zn-Mg-Cu Aluminum Alloys. Rare Met. Mater. Eng. 2017, 46, 3374–3381. [Google Scholar]
- Peng, Y.; Liu, C.; Wei, L.; Jiang, H.; Ge, Z. Quench Sensitivity and Microstructures of High-Zn-Content Al-Zn-Mg-Cu Alloys with Different Cu Contents and Sc Addition. Trans. Nonferrous Met. Soc. China 2021, 31, 24–35. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Xiong, B.; Zhang, Y.; Li, Z.; Yan, H.; Liu, H.; Huang, S. Quench Sensitivity of Novel Al-Zn-Mg-Cu Alloys Containing Different Cu Contents. Rare Met. 2020, 39, 1395–1401. [Google Scholar] [CrossRef]
- Bryant, A. The Effect of Composition upon the Quench-Sensitivity of Some Al-Zn-Mg Alloy. J. Inst. Met. 1966, 94, 94–99. [Google Scholar]
- Chen, H. Study on Quenching Sensitivity and Aging Precipitation Behavior of Al-Zn-Mg-Cu Alloy. Master’s Thesis, Central South University, Changsha, China, 2012. [Google Scholar]
- Qi, X. The Effect of Minor Sr, Zr on the Quench Sensitivity of 7085 Aluminum Alloy. Master’s Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Staley, J. Quench factor analysis of aluminium alloys. J. Mater. Sci. Technol. 1987, 3, 923–935. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Ou, Y.; Deng, Y. Quench Sensitivity of Ultrahigh Strength 7000 Series Aluminum Alloys. Trans. Nonferrous Met. Soc. China 2013, 23, 927–938. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; You, J.; Huang, Z.; Zhang, C.; Zhang, X. TTP Curve of 7055 Aluminum Alloy and Its Application. Trans. Nonferrous Met. Soc. China 2006, 16, 2034–2039. [Google Scholar] [CrossRef]




| Element | Zn | Mg | Cu | Cr | Zr | Ti | Al |
|---|---|---|---|---|---|---|---|
| Content | 5.80 | 2.40 | 1.40 | 0.08 | 0.08 | 0.05 | Bal. |
| Alloy | k2 (s) | k3 (J·mol−1) | k4 (K) | k5 (J·mol−1) | Ref. |
|---|---|---|---|---|---|
| This study alloy | 8.956 × 10−9 | 10,220.6 | 701.9 | 26,052.9 | |
| 7075 | 1.916 × 10−10 | 1183 | 815.8 | 114,500 | [17] |
| 7175 | 7.6 × 10−10 | 412 | 750 | 112,200 | [20] |
| Alloy | Critical Temperature Range (°C) | Nose Temperature (°C) | Transformation Time at Nose Temperature (s) |
|---|---|---|---|
| This study alloy | 219–427 (208) | 325 | 0.40 |
| 7075 | 213–428 (215) | 350 | 0.23 |
| 7175 | 209–406 (197) | 340 | 0.16 |
| Element | Zn | Mg | Cu | Cr | Fe | Si | Al |
|---|---|---|---|---|---|---|---|
| Content | 5.77 | 2.65 | 1.74 | 0.27 | 0.15 | 0.09 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, Q.; Qin, Y.; Cao, P.; Lin, S.; Lan, D.; Huang, W.; Zhou, W.; Chen, W. Quenching Sensitivity Study of New High-Strength Aluminum Alloys Based on an Immersion End-Quenching and Step-Quenching Technique. Materials 2025, 18, 3132. https://doi.org/10.3390/ma18133132
Li C, Chen Q, Qin Y, Cao P, Lin S, Lan D, Huang W, Zhou W, Chen W. Quenching Sensitivity Study of New High-Strength Aluminum Alloys Based on an Immersion End-Quenching and Step-Quenching Technique. Materials. 2025; 18(13):3132. https://doi.org/10.3390/ma18133132
Chicago/Turabian StyleLi, Chengbo, Qinyao Chen, Yiming Qin, Puli Cao, Shusheng Lin, Donghua Lan, Wenhui Huang, Wang Zhou, and Wengang Chen. 2025. "Quenching Sensitivity Study of New High-Strength Aluminum Alloys Based on an Immersion End-Quenching and Step-Quenching Technique" Materials 18, no. 13: 3132. https://doi.org/10.3390/ma18133132
APA StyleLi, C., Chen, Q., Qin, Y., Cao, P., Lin, S., Lan, D., Huang, W., Zhou, W., & Chen, W. (2025). Quenching Sensitivity Study of New High-Strength Aluminum Alloys Based on an Immersion End-Quenching and Step-Quenching Technique. Materials, 18(13), 3132. https://doi.org/10.3390/ma18133132







