1. Introduction
Three-dimensional (3D) (bio)printing is a cutting-edge technology for the precise deposition of (bio)materials onto specified locations with a high resolution. This process involves fabricating objects layer by layer using a printer head, nozzle, or similar technology [
1]. The convergence of additive manufacturing with biomaterials has introduced new paradigms in the field of biotechnology. The development of advanced printing materials and 3D printing technologies capable of fabricating complex scaffold geometries has facilitated the integration of sensing elements, thereby opening new avenues for biosensor design and functionality [
2,
3]. Three-dimensional bioprinting now extends to critical applications, including embedding active biomolecular recognition elements into 3D printed objects for (bio)sensing [
2,
3,
4,
5], applications in tissue engineering [
6], wound healing [
6], and the production of various medical implants and models [
7]. The major challenges commonly encountered during printing processes with bio-based hydrogels are structural collapse and loss of shape fidelity, depending on the viscoelastic properties of the printing material [
8]. Rapidly solidifying “inks” are desirable for printing to prevent the deformation of the printed structure. An ideal hydrogel ink should flow through a nozzle during printing and retain its shape after printing and curing, which requires custom materials with tailored properties to be formulated for each application. Therefore, several hydrogels with varying viscosities have been adapted for 3D printing via microextrusion [
9].
Hydrogels, particularly those derived from natural polymers such as alginate, gelatin, and chitosan, are widely utilized in bioprinting because of their biocompatibility and ability in an aqueous environment, which is essential for cell viability and growth [
10,
11]. Hydrogels are hydrophilic, 3D polymeric networks characterized by high flexibility and capacity to absorb and hold substantial amounts of water while maintaining mechanical stability [
1,
12]. Physical hydrogels have several advantages, such as easy preparation and sol–gel transition [
12,
13]. However, such hydrogels often exhibit mechanical weaknesses in terms of gelation and printability, which limit their application in additive manufacturing processes. Therefore, significant efforts have been directed toward enhancing the mechanical properties of hydrogels, thereby improving their printability and functionality in tissue engineering. For instance, using a composite approach, alginate–gelatin composite bioinks, together with the incorporation of nanoparticles, have significantly improved mechanical strength, enhancing printability and cellular support in tissue engineering applications [
14].
There are numerous challenges associated with working with conventional hydrogels, such as dynamic rheological behavior under different printing conditions, which leads to a loss of printing fidelity and structural integrity post-printing [
15,
16]. To address such challenges, efforts such as the use of composite polymers and blending of inorganic nanoparticles with biopolymers are being made for structural and functional enhancement. In addition, the use of sacrificial copolymers and printing into sacrificial support baths, whereby the sacrificial copolymer and support bath are removed after printing, is being actively investigated [
17,
18]. Furthermore, tissue engineering applications that cultivate electrically excitable cells, such as neurons, skeletal myocytes, and cardiomyocytes, could benefit from enhanced cell adhesion, cell–cell communication, proliferation, and differentiation when the hydrogel is conductive [
19,
20]. However, a critical research question remains to be answered: how can we engineer a hydrogel that retains the biocompatibility, printability, and rheological properties of alginate while enhancing its electrical conductivity properties?
Research on conductive hydrogels is advancing because of their potential applications in various fields including bioelectronics, drug delivery systems, tissue engineering, and biosensors [
3]. Despite these advances, current hydrogel-based bioinks fail to fully support the cultivation and electrical stimulation of electrically excitable cells, such as neurons and cardiomyocytes, owing to their insufficient electrical conductivity. This limits the potential of 3D bioprinted tissues to mimic the native electroactive tissues. Therefore, continuous research efforts have been directed toward incorporating conductive materials into hydrogel bioinks by doping hydrogels with intrinsically conductive materials (ICMs) such as polypyrrole, poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) (PEDOT: PSS) [
21,
22], graphene oxide [
23], polythiophene, and nanoparticles. While the incorporation of ICMs into hydrogels has been explored and shown to enhance the electrical properties while maintaining the unique characteristics of the hydrogels, such as flexibility and biocompatibility [
23], conductive hydrogels prepared by doping ICMs present a significant setback: uncontrolled leaching of the conductive unit from the hydrogel owing to the non-covalent nature of the incorporation [
24]. Thus, we hypothesized that covalently binding the intrinsically conductive unit to the alginate polymer could lead to a stable 3D printable electrically conductive hydrogel.
Sodium alginate is an ideal biopolymer candidate because of its excellent biocompatibility, non-toxicity, and ability to rapidly form 3D hydrogels upon interaction with divalent cations [
25]. Furthermore, its thixotropic properties, including its shear-thinning behavior and viscosity recovery [
26], make it particularly suitable for 3D printing applications, either as a standalone material or in combination with other polymers.
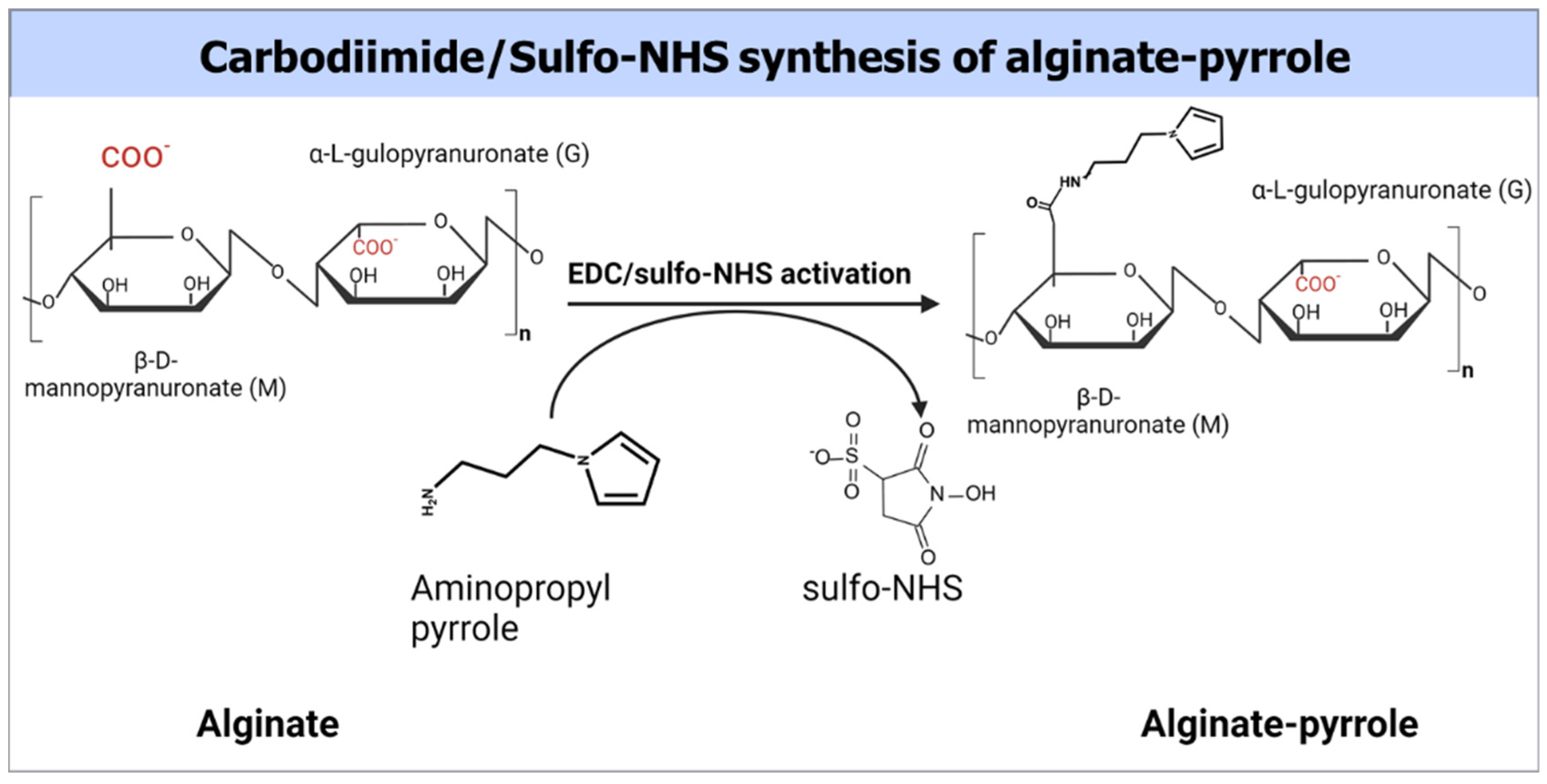
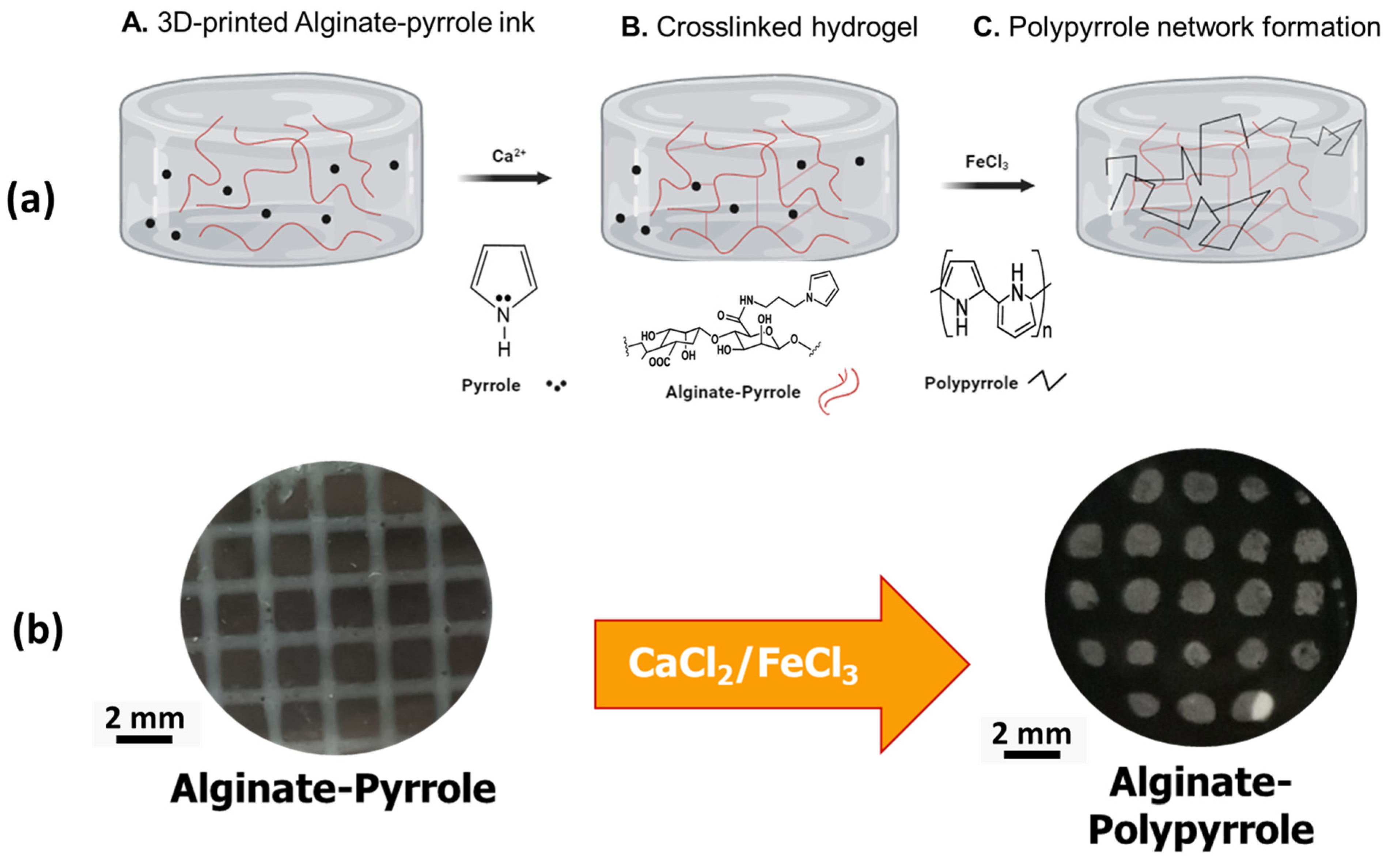
Despite their documented advantages, alginate hydrogels, like other biopolymer-based hydrogels, lack sufficient electrical conductivity, which limits their widespread application in bioelectronics, biosensors, and electroactive tissue scaffolds. Thus, we propose the integration of polypyrrole into alginate hydrogels to confer electrical properties to alginate while maintaining its rheological features, making it amenable to 3D bioprinting. This study introduces a new approach for creating an electrically conductive alginate-based composite hydrogel by covalently binding sodium alginate to a pyrrole monomer, from which conductive polypyrrole is chemically synthesized, either in situ or ex situ. Specifically, an N-substituted pyrrole derivative, aminopropyl pyrrole, was synthesized and conjugated with alginate using carbodiimide chemistry. The resulting alginate–pyrrole conjugate was characterized by its rheological properties, electrical conductivity, and 3D printability.
Wright et al. showed the printability of alginate mixed with a pyrrole monomer, which was subsequently polymerized into polypyrrole, and reported uncontrolled leaching of polypyrrole and brittleness of the resulting hydrogel [
24]. Therefore, two strategies were explored in this study to incorporate polypyrrole into the alginate matrix: (1) oxidative polymerization of pyrrole directly within the 3D printed alginate–pyrrole network (in situ polymerization), referred to as alginate–PPy, and (2) pre-synthesis of polypyrrole nanoparticles (PPy-NP), which were subsequently blended with alginate (alginate@PPy-NP) prior to 3D printing. To the best of our knowledge, this is the first time that this approach has been employed to create an alginate–polypyrrole-based conductive hydrogel. The effects of these strategies on the printability, mechanical integrity, and electrical properties of hydrogels were examined. Notably, the electrical conductivity increased with the pyrrole content in both cases, and both types of alginate–polypyrrole were 3D printable, while alginate mixed with already-made polypyrrole nanoparticles displayed appreciably higher compressibility, without leaching out of the ICMs, highlighting the potential of this covalently functionalized alginate–pyrrole as an electrically conductive bioink.
2. Materials and Methods
2.1. Materials
All chemicals and reagents used in this study were of analytical grade and used without further purification, unless otherwise specified.
For alginate modification and hydrogel fabrication, sodium alginate (low viscosity, A2158), 1-ethyl-[3-(dimethyl amino)propyl]-3-ethyl carbodiimide HCl (EDC, E-1769), N-hydroxysulfosuccinimide (sulfo-NHS, 24510), 1-(2-cyanoethyl)-pyrrole, 2-[N-morpholino] ethane sulfonic acid (MES) buffer (M-8250), ammonium persulfate, phosphate-buffered saline (PBS) (P4417), sodium chloride, and calcium chloride (C-5426) were purchased from Sigma-Aldrich (Saint Louis, MO, USA). 3-(Aminopropyl) pyrrole was synthesized in-house as described in
Section 2.2.
For nanoparticle synthesis, the pyrrole monomer (8.07492, 98% w/v) was distilled using a Heidolph rotavapor (Schwabach, Germany) and stored under nitrogen/argon at −80 °C until use. Ammonium persulfate (APS, ≥98%) was used as the oxidant for polypyrrole synthesis. Lithium aluminum hydride (LiAlH4, 212792) was employed as the reduction step in the synthesis of 3-(aminopropyl)pyrrole. All the reagents were obtained from Sigma-Aldrich (Saint Louis, MO, USA).
For crosslinking and 3D printing, calcium chloride (150 mM) was used as the ionic crosslinker. Gelatin (Type A, bloom 300) was obtained from Sigma-Aldrich (USA) and used in the preparation of the support bath for the FRESH (Freeform Reversible Embedding of Suspended Hydrogels) technique. Dulbecco’s Modified Eagle’s medium (DMEM) was purchased from Biological Industries (Kibbutz Beit-Haemek, Israel).
For instrumentation and imaging: Deuterated chloroform (CDCl3,151823) and methanol-d4 for NMR spectroscopy were obtained from Sigma-Aldrich (USA). Acetone, methanol, isopropyl alcohol (IPA), and deionized water were used to clean the electrode surfaces and were purchased from Bio-Lab (Ashkelon, Israel).
2.2. Synthesis of Aminopropyl Pyrrole
A solution of 1-(2-cyanoethyl) pyrrole (0.02 mol) was added dropwise to anhydrous ether (15 mL) in a suspension of LiAlH
4 (0.05 mol) prepared in anhydrous ether (150 mL), refluxed for 10 h, and cooled. The excess hydride was quenched by successive addition of water (1.7 mL), a solution of 15% (
w/
v) NaOH (1.7 mL), and water (5.1 mL), and then heated at 40 °C for two h. The resulting solution was filtered through celite and the filtrate was evaporated to dryness. The synthesis of N-(3-aminopropyl)-pyrrole was confirmed by
1H NMR (400 MHz, CDCl
3) δ: 6.70 (s, 2H, H–α), 6.24 (s, 2H, H–β), 3.25 (t, J = 6.8 Hz, 2H, –CH
2–NH
2), 2.65 (t, J = 7.2 Hz, 2H, –CH
2–CH
2–NH
2), 1.85 (m, 2H, –CH
2–CH
2–CH
2–) (
Supplementary Materials Figure S1), FTIR, and mass (
Supplementary Materials, Figure S2) spectroscopies.
2.3. Synthesis of Alginate–Pyrrole
Na-alginate solution (2.5%
w/
v) was prepared by dissolving 500 mg of alginate in 20 mL of double-distilled water (DDW) with a resistivity of 18.2 MΩ and sterile filtered through a 0.22 µm filter (Stericup and Steritop EMD Millipore Corp., Billerica, MA, USA). To the filtered alginate solution, 852 mg of MES buffer and 400 mg of NaCl were added, and the mixture was stirred thoroughly. The pH of the solution was adjusted to 6.5. The carboxyl groups of sodium alginate were then activated using 1 mmol EDC and 0.5 mmol NHS to achieve a theoretical 50% molar modification relative to the total number of alginate carboxyl groups. The resulting solution was stirred for 3 h at room temperature. Subsequently, 480 mg (3 mmol) N-(3-aminopropyl) pyrrole was added to the activated alginate solution and stirred overnight at room temperature (
Figure 1). The resulting polymer composite was dialyzed against doubly distilled water using a 3.5 kDa MWCO membrane (Spectrum Lab Membrane Filtration Products, Inc., Irving, TX, USA), with the water changed twice a day for three days. The dialysate was then lyophilized. The extent of pyrrole incorporation was determined by UV-vis spectroscopy using a pedestal mode of NanoDrop ONE
C desktop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Briefly, the extent of alginate modification by
N-(3-aminopropyl)-pyrrole was determined by dissolving the alginate–pyrrole samples to produce a 0.01% (
w/
v) alginate–pyrrole solution and measuring the absorbance. The extent of alginate modification was determined from the calibration curve by measuring the absorbance of different amounts of
N-(3-aminopropyl)-pyrrole in a 0.01% (
w/
v) Na-alginate solution. A standard solution of Na-alginate at a concentration of 0.01% (
w/
v) was used as blank (
Supplementary Figure S5).
2.4. Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
Infrared spectra of the synthesized aminopropyl pyrrole and alginate–pyrrole were recorded using a Smart iTRTM Diamond ATR-FTIR sampling accessory and Nicolet 6700 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) to characterize the functional groups of the synthetic products. All measurements were performed using a built-in diamond-attenuated total-reflection (ATR) crystal. The FTIR spectra were acquired over the range 4000–650 cm−1 at a resolution of 4 cm−1 and represented an average of 36 scans. The spectrum of the clean, dry diamond ATR crystal in ambient atmosphere (air) was used as the background for infrared measurements. The presence of pyrrole, amine, and amide groups was assessed by identifying the characteristic C=C, C–N, and N–H vibrations for pyrrole, N-H and C-N bonds for amines, and C=O and N–H bonds for amides.
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
X-ray photoelectron spectroscopy (XPS) analysis was conducted on lyophilized samples of alginate–pyrrole and alginate using an “ESCALAB Xi+” instrument (Thermo Fisher Scientific, Waltham, MA, USA). The ionization energies of oxygen, nitrogen, and carbon present in the lyophilized samples were recorded as previously described for alginate [
27]. The analysis was performed at an ambient pressure of <1 × 10
−10 mbar. An aluminum Kα radiation source (1486.68 eV) was used for photoelectron emission at room temperature, and spectra were collected at an angle of 90° from the X-ray source. A low-energy electron flood gun was employed to minimize surface charging and measurements were performed with a spot diameter of 650 µm. Spectral analysis was performed using Avantage software version 6.6.0, provided by Thermo Fisher Scientific. High-resolution spectra of the C1s, O1s, and N1s peaks and a survey spectrum at a pass energy of 20 eV were obtained.
2.6. Thermogravimetric Analysis (TGA)
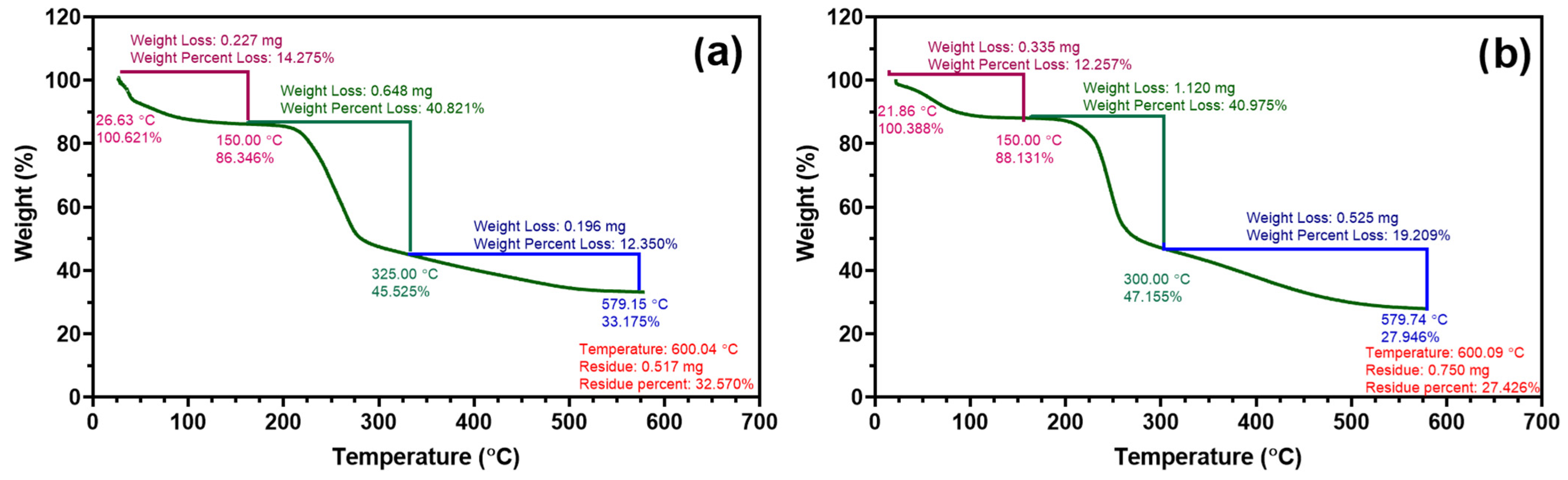
Thermogravimetric analysis (TGA) was conducted to assess the thermal stability of sodium alginate (Na-alginate) and the synthesized Na-alginate–pyrrole (alginate–PPy) composite. A known mass of lyophilized samples of sodium alginate (1.598 mg) and sodium alginate–pyrrole (2.745 mg) was accurately weighed in an open alumina crucible and analyzed using TA Instruments Q500 and Q50 thermogravimetric analyzers (New Castle, DE, USA). The samples were heated from 20 to 600 °C at a constant rate of 10 °C/min under a nitrogen atmosphere (flow rate: 60 mL/min). The resulting thermograms were used to compare the decomposition profiles and residual mass of the unmodified and functionalized alginate materials.
2.7. Chemical Synthesis of Polypyrrole Nanoparticles (PPy-NP)
Polypyrrole (PPy) was synthesized via chemical oxidative polymerization using ammonium persulfate (APS) as the oxidant with a monomer-to-oxidant molar ratio of 1:1.5. The final APS concentration was 212 mM and the reaction was conducted at 4 °C for 120 min. Briefly, 1.0 mL of pyrrole monomer (14.4 mmol) was added to 50 mL of ice-cold deionized water (DDW) under continuous stirring. Separately, 4.83 g APS (21.6 mmol) was dissolved in 10 mL DDW and added dropwise to the pyrrole solution. The total reaction volume was adjusted to 100 mL using DDW and the mixture was magnetically stirred (260 rpm). Aliquots of 5 mL were withdrawn at defined time intervals to monitor the polymerization progress via UV-Vis spectroscopy at 460 nm (
Supplementary Figure S4). Following polymerization, a black precipitate of PPy was collected by vacuum filtration, thoroughly washed with DDW and ethanol to remove residual monomers and oxidants, and dried under vacuum at 50 °C overnight.
Characterization of PPy-NPs
The surface morphology of the synthesized PPy was examined using field-emission scanning electron microscopy (FESEM, Thermo Fisher Verios 460 L, Waltham, MA, USA) at different magnifications. The samples were sputter-coated with gold and imaged at various magnifications to assess the surface features and particle aggregation.
Dynamic light scattering (DLS) analysis was carried out using an ALV/CGS-3 Compact Goniometer (Hessen, Germany) by dispersing 1 mg of dried PPy-NP powder in 1 mL of 1-methyl pyrrolidone and sonicating for 10 min in a bath sonicator (Bransonic 1210 Branson Heating Water Bath Ultrasonic Cleaner 1210R-MTH, Frankfurt am Main, Germany) to ensure uniform dispersion. The suspension was filtered through a 0.45 µm syringe filter to remove aggregates prior to measurement.
2.8. Preparation of Alginate–Pyrrole as Bioink
2.8.1. Alginate–Pyrrole/Pyrrole (Alginate–Py) as Bioink
To create an optimal ink formulation, different stoichiometric ratios of pyrrole in alginate were prepared (
Table 1) using double-distilled water (DDW) with a resistivity of 18.2 MΩ. To enhance the electrical conductivity, a defined amount of pyrrole monomer (14.125 M) was added to the alginate–pyrrole ink formulation prior to polymerization. This supplementation ensured a sufficient pyrrole concentration for in situ polymerization, as sub-threshold levels typically yield nonconductive oligomers. The dual inclusion of a covalently grafted pyrrole and a free monomer was designed to promote homogeneous polypyrrole formation throughout the hydrogel matrix. Lyophilized alginate–pyrrole and sodium alginate were separately dissolved in double-distilled water to a final concentration of 2.5% (
w/
v) under stirring for 2 h. Primary (partial) crosslinking of the alginate was achieved by mixing 1.2 mL of alginate–pyrrole, 1.2 mL of alginate, 0.3 mL of CaCl
2 (0.1 M), and 0.021 mL of pyrrole monomer (14.125 M), using a homogenizer to equally distribute the calcium ions throughout the solution (
Table 1). The mixture was then stirred at RT until further use. The partially crosslinked alginate–pyrrole/pyrrole was transferred to a sterile 3D printer syringe.
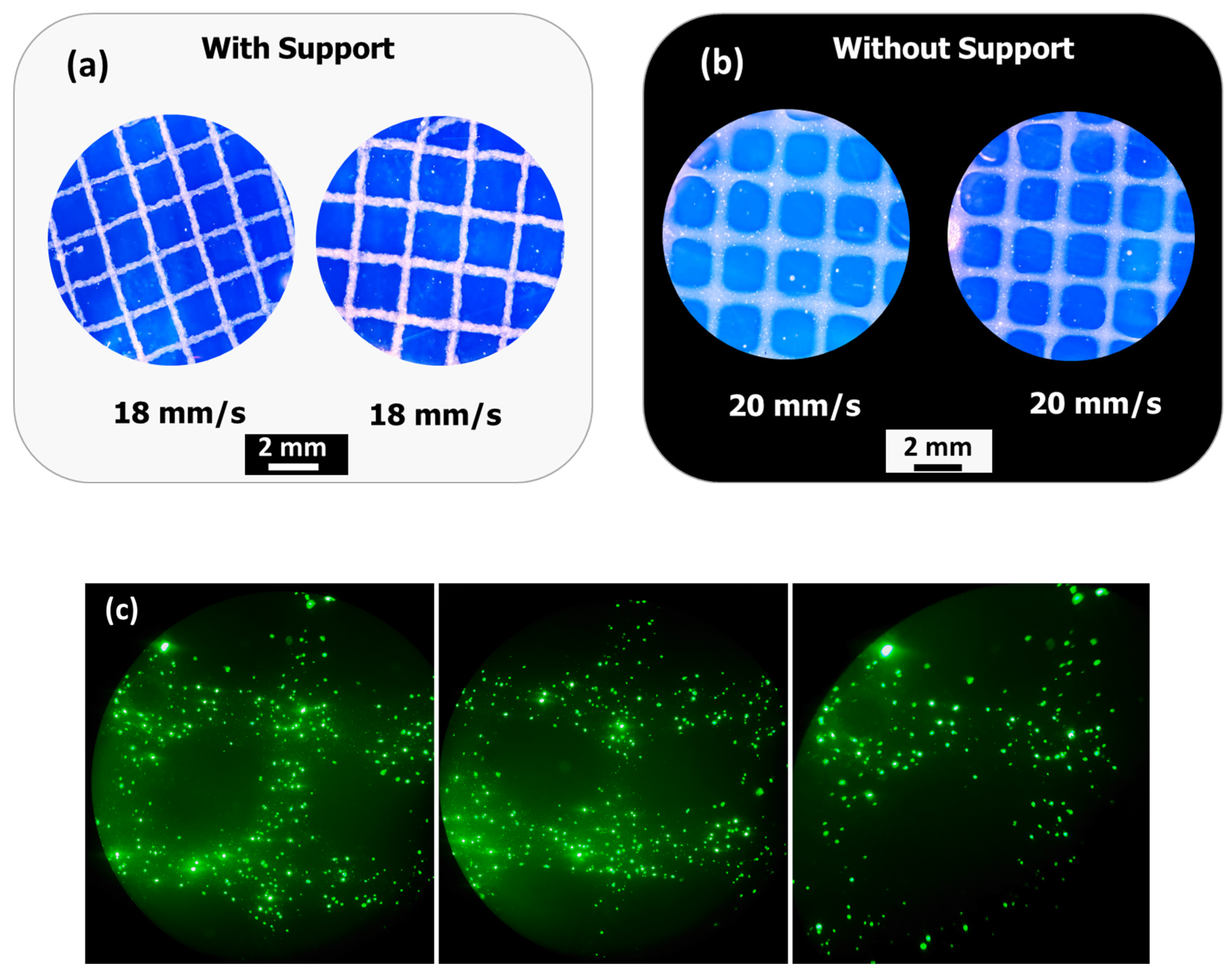
For the cell-laden bioink, 0.28 mL of Dulbecco’s Modified Eagle Medium (DMEM) containing 2.6 × 106 cells/mL of isolated GFP-expressing fibroblasts mixed with DMEM was loaded into an additional sterile syringe, which was immediately connected to the alginate–pyrrole/pyrrole-loaded syringe through a Luer-to-Luer connector. The solutions were mixed by gently pushing the pistons back and forth for 1 min until homogeneous mixing was achieved. The final bioink consisted of 2% alginate–pyrrole (w/v), 0.01 M CaCl2 (or 0.36% w/v calcium gluconate), and 2.6 × 106 cells/mL.
2.8.2. Alginate@polypyrrole Nanoparticles (Alginate@PPy-NP) as Bioink
The possibility of blending PPy-NPs into a Ca-alginate hydrogel as a bioink (alginate@PPy-NPs) for 3D printing was explored. Different solutions of 3 mL (2% w/v) Na-alginate containing PPy-NP at final concentrations of 1, 0.33, and 0.17% w/v, corresponding to solutions with PPy-NP/alginate ratios of 8.3, 16.7, and 50% w/w, respectively, were prepared. The primary crosslinking was performed by dropwise addition of 0.05 M CaCl2 to reach a final concentration of 0.01 M and was stirred overnight and subsequently used for 3D printing or further processed for rheological testing and electrical conductivity testing.
2.9. Three-Dimensional Printing of Alginate–Pyrrole Conjugate and Alginate@polypyrrole Composites
Basic 3D models of the scaffold constructs were designed and spliced using Perfectory RP and software supplied by the Envision TEC 3D bioplotter. The designs were printed using a 3D Bioplotter Manufacturer Series system (EnvisionTEC GmbH, Marl, Germany) using VisualMachine BP interface software (V2.2; EnvisionTEC GmbH). Scaffolds were 11 mm × 11 × 2.5 mm with an inner strand distance of 2.25 mm and inner strand angles of 0° and 90°. The strands were dispensed layer-by-layer using pneumatic pressure in a piston-based system through 25-gauge blunt dispense needles (EFD Nordson, Wallisellen, Switzerland). The printing parameters were optimized in terms of the printing speed and pressure in the range of 10–22 mm per second and 0.5–1.0 bar, respectively.
Prior to bioprinting, as previously described [
18], the bioink was deposited into a sterilized 30 mL printer cylindrical cartridge sealed with a fitted plunger utilizing a Luer-to-Luer connector. The printer cartridge was subsequently sealed and centrifuged for 1 min at 150×
g to eliminate any remaining air bubbles. A sterile 25-gauge needle tip was attached to the cartridge and a cap connecting the print head to the barrel was affixed. The barrel was placed in the low-temperature head of the bioprinter (EnvisionTEC 3D-bioplotter Developer Series, Marl, Germany), set to 25 °C. The bioink was allowed to equilibrate to the printing head temperature for 30 min before bioprinting. The 3D printing was conducted by extrusion over the range of 0.5–0.8 bar and printing speed of 10–22 mm/s, followed by secondary crosslinking with 0.15 M CaCl
2. In addition, the possibility of printing using the freeform reversible embedding of suspended hydrogels (FRESH), as described by Hinton et al. [
17], was evaluated. The recipe and preparation of the FRESH can be found in
Supplementary Material Protocol S1.
To eliminate the gelatin support bath, bioprinted constructs were incubated for 1 h at 37 °C and 5% CO2. The constructs were then washed and incubated for 10 min at 37 °C in DMEM supplemented with 0.1 M CaCl2 to further crosslink the 3D-bioprinted constructs, after which the constructs were incubated in DMEM supplemented with 20% fetal bovine serum (FBS) in a new 12-well plate.
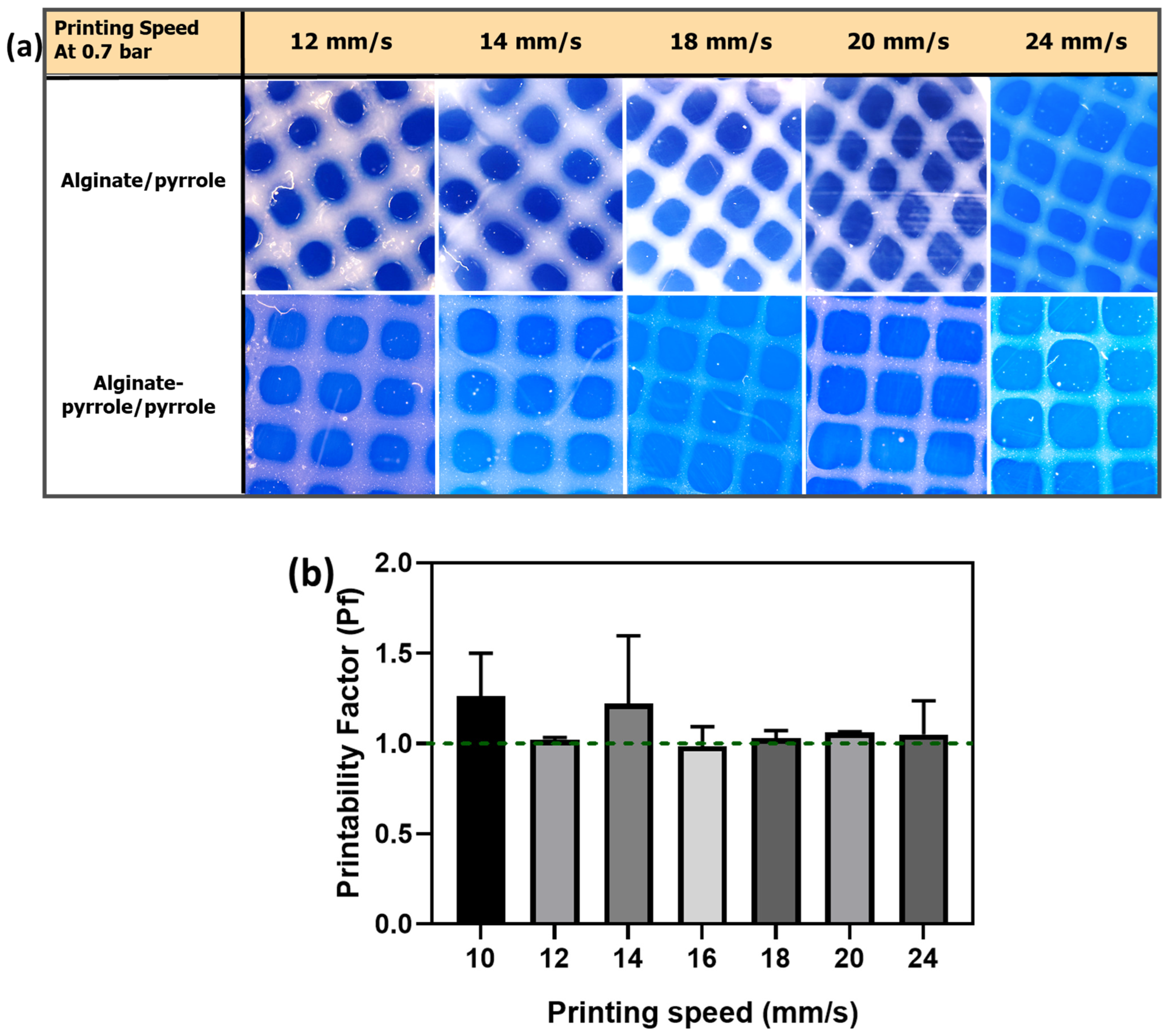
Semi-Quantification of Printability
The printing factor (Pf) of the calcium alginate–polypyrrole inks to achieve square-shaped pores was quantified from light microscopy images using ImageJ software (ImageJ 1.54f, Java 1.8.0._322 64-bit) by comparing the measured area of the printed pores to the theoretical design. This parameter, adapted from previous studies on grid-based constructs, provides a quantitative indication of pore shape fidelity and filament uniformity [
28]. The printability of the bioink was determined according to the method described by Ouyang et al. [
28], based on the understanding of the circularity (C) of an enclosed area. The circularity of an enclosed area is defined as
where L is the perimeter and A is the area.
The circles exhibit the highest circularity (
C = 1). The closer the value of C is to one, the closer the shape is to a circle. For a square shape, the circularity is
π/4 [
28]. Because the model designed in this study was square, the bioink printability (Pr) based on the square shape was defined according to the following equation [
28]:
In this study, the printability factor (Pf) is defined as
In this context, three statuses of Pf are defined as follows: Pf < 1 corresponds to under-gelation and typically rounded pore corners because of the fusion of layers and swelling; Pf = 1 corresponds to proper gelation with ideal square-shaped pores; and Pf > 1 corresponds to over-gelation, causing shrinkage of the construct [
29]. Therefore, a Pf value close to 1 indicates a high degree of shape retention.
2.10. Rheological Characterization
The viscoelastic behavior of calcium alginate and calcium alginate–pyrrole hydrogels was assessed using a stress-controlled advanced rheometer (AR 2000; TA Instruments). A 40 mm diameter 3°58 steel cone geometry with a truncation gap height of 104 μm was used. The storage modulus (G′), loss modulus (G″), and complex viscosity (|η∗|) were recorded as a function of time through oscillatory measurements in a frequency range of 0.1–10 Hz, and the strain was determined to be in the linear viscoelastic region. In addition, the apparent viscosities (Pa·s) of the partially crosslinked hydrogels were assessed at shear rates between 0.1 and 100 s−1. Compressibility was measured using a rheometer (TA Instruments, model AR 2000) operated in flat-plate mode with a 40 mm diameter in an increasing normal force. Time and frequency sweep tests were conducted at a constant strain of 1%, which was within the linear viscoelastic region, as determined by preliminary strain amplitude tests. The applied stress during the time sweep measurements ranged between 0.5 and 1 Pa depending on the formulation, ensuring linear viscoelastic behavior throughout. Rheological measurements were performed with a sample size of n = 1 due to the large volume requirements of the rheometer and limited availability of material for each formulation.
Rheological Data Analysis
The steady-state flow behavior of the hydrogel formulations was analyzed by fitting the shear stress (τ) versus shear rate (γ˙) data to the Power Law model (Ostwald–de Waele equation) as follows:
where K is the consistency index (Pa·s
n) and n is the flow behavior index (dimensionless). Log–log linear regression was performed using OriginPro 2024 (OriginLab Corp., Northampton, MA, USA) to obtain the slope (n) and intercept (log K) of each material. The flow behavior index indicates the degree of shear thinning, with n < 1 confirming pseudoplastic behavior. The yield stress was approximated as the shear stress corresponding to the lowest non-zero shear rate.
For instance, the alginate control exhibited a shear-thinning index of n = 0.283 and a consistency index of K = 18.01 Pa·s
n, indicating strong shear-thinning behavior. The yield stress was estimated to be 13.24 Pa, corresponding to a shear rate of 0.1 s
−1. Complete rheological data can be found in the
Supplementary Materials.
2.11. Electrical Conductivity Characterization
Electrical measurements were performed using a sandwich configuration at room temperature. Partially crosslinked calcium alginate, alginate–Py (later polymerized to alginate–PPy after crosslinking), and alginate@PPy-NP hydrogels were individually cast into custom-made molds and fully crosslinked overnight by the addition of 150 mM calcium ions. Each hydrogel sample (volume = 1 cm3) was placed between two stainless-steel plates (1 × 1 cm2), with the bottom plate acting as the working electrode and the top plate serving as the contact electrode.
Prior to assembly, the stainless steel plates were sequentially cleaned by sonication in acetone, methanol, isopropyl alcohol (IPA), and deionized water (DDW) for 15 min each, followed by nitrogen drying. The assembled device was positioned in a probe station (JANIS ST-500, Woburn, MA, USA) and secured by applying a vertical pressure to the top probe.
Current–voltage (I–V) measurements were conducted using a Keithley 2635 Source-Meter Unit (Cleveland, OH, USA), with data acquisition controlled by a custom MATLAB program (version 23.2.0.2365128 (R2023b)). The measurements spanned a voltage range from −1 V to 1 V in 0.05 V increments. The resistance (R
b) was calculated from the linear slope of the I–V curve. The electrical conductivity (σ) was computed using the following equation:
where σ is the conductivity (µS/cm), L is the hydrogel thickness (m), A is the contact area (m
2), and R
b is the bulk resistance (Ω). The hydrogel dimensions were measured using a digital caliper, and all measurements were performed at room temperature under ambient conditions. All measurements were performed at room temperature, and a minimum of three replicates were tested per sample type for statistical validity.
2.12. Statistical Analysis
A two-way or one-way ANOVA, followed by a Tukey multiple comparisons test, was conducted using GraphPad Prism version 8.0.2 for Windows (GraphPad Software, San Diego, CA, USA,
www.graphpad.com, accessed on 26 November 2024).
4. Discussion
In the category of conducting polymers, polypyrrole (PPy) is widely used because of its low-cost preparation, excellent conductivity, and large surface area. PPy, a conductive polymer, facilitates the immobilization of various metallic nanoparticles on the surface of electrodes through π-π stacking, electrostatic interactions, or entrapment procedures [
42]. In this study, we synthesized an N-substituted pyrrole (aminopropyl pyrrole) and its covalent conjugation to calcium alginate, which could be polymerized in situ into a calcium alginate–polypyrrole (calcium alginate–PPy) conjugate. In addition, polypyrrole nanoparticles (PPy-NPs) were chemically synthesized and blended with sodium alginate to produce a stable conductive alginate–polypyrrole composite (alginate@PPy-NP), which was used independently together with calcium alginate–PPy as inks for extrusion-based 3D printing.
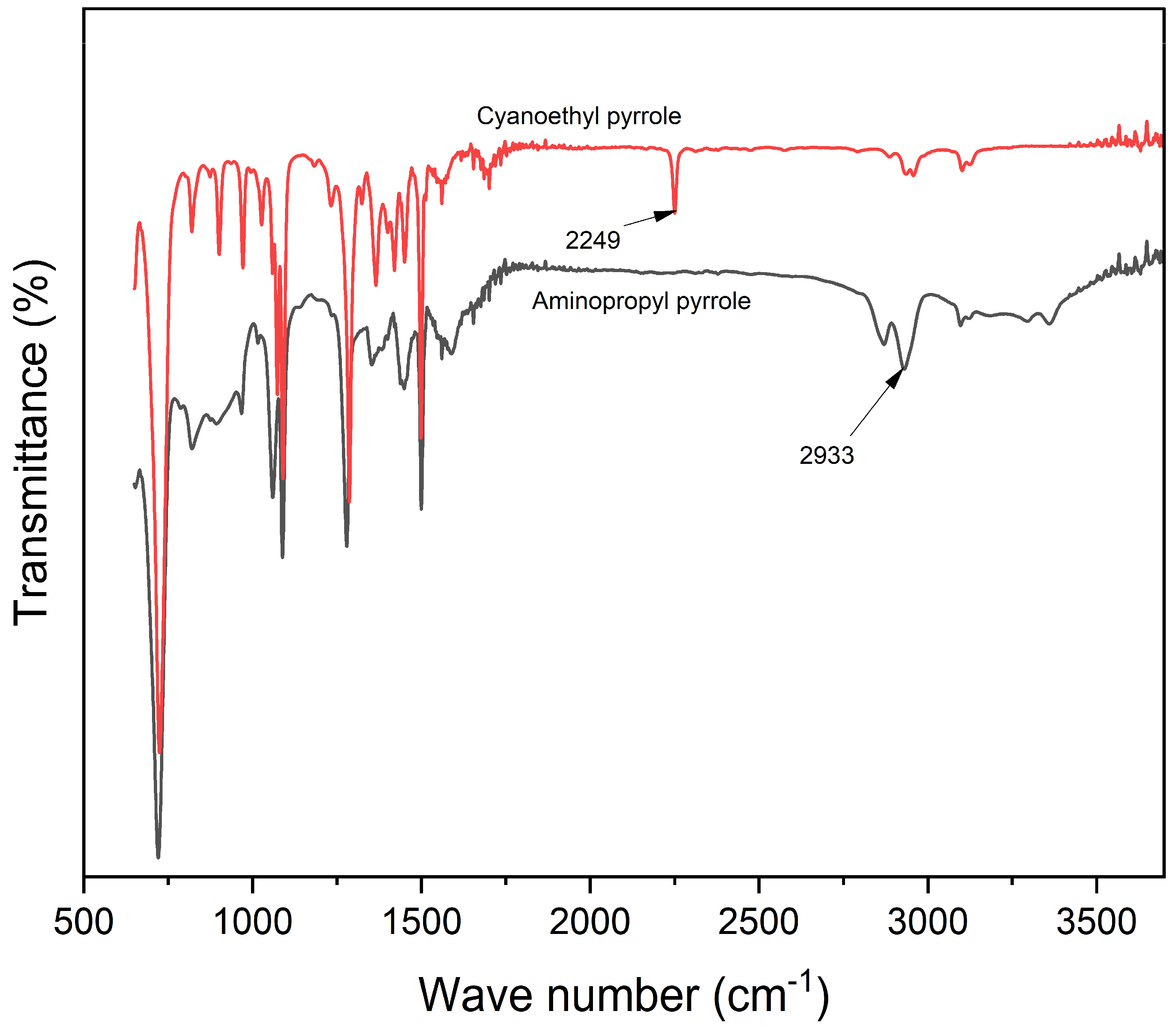
Spectroscopic analysis confirmed the successful incorporation of PPy into the alginate matrix via both in situ polymerization and nanoparticle embedding. FTIR, mass spectrometry, and NMR (
Supplementary Figures S1 and S2), confirmed the success of aminopropyl pyrrole synthesis, while X-ray photoelectron spectroscopy (XPS) revealed characteristic binding energies for C-C, C-O, and N-H and NH-C=O species, characteristic of amide bond formation (
Figure 3), and ATR-FTIR (
Supplementary Figure S3) confirmed the presence of polypyrrole and its interaction with the alginate backbone. From the existing literature, it is well-established that pyrrole presents different nitrogen species contributing to the XPS N 1s spectrum. Specifically, the binding energy associated with pyrrolic nitrogen (−NH) is typically observed around 400.4 eV [
39]. Polypyrrole nanoparticles (PPy-NPs) were synthesized via chemical oxidative polymerization using ammonium persulfate (APS) at 4 °C for 120 min. The particle size of PPy-NPs is strongly influenced by several synthesis parameters, including monomer concentration, oxidant-to-monomer molar ratio, temperature, and stirring speed. Under the specified reaction conditions (monomer-to-oxidant ratio of 1:1.5, 4 °C, magnetic stirring), the particle size was typically in the range of 40–200 nm, as reported in the literature [
43]. Mahmood et al. demonstrated that PPy-NPs synthesized via low-temperature oxidative polymerization could achieve sizes ranging from 85 to 300 nm, with the particle size being tunable by varying the reactant concentration [
43]. Additionally, Effati et al. [
44] reported the successful synthesis of PPy-NPs using a microfluidic system, highlighting that factors such as the oxidant-to-monomer molar ratio and reaction temperature significantly influence particle size and morphology [
44]. Furthermore, a comprehensive review by Hao et al. discussed the control of PPy nanoparticle size and morphology, emphasizing the roles of synthesis conditions and the use of stabilizing agents [
45]. These studies corroborate our reported size range of 40–300 nm for PPy-NPs synthesized under the specified conditions (monomer-to-oxidant ratio of 1:1.5, 4 °C, 260 rpm) and underscore the critical influence of the synthesis parameters on nanoparticle size control.
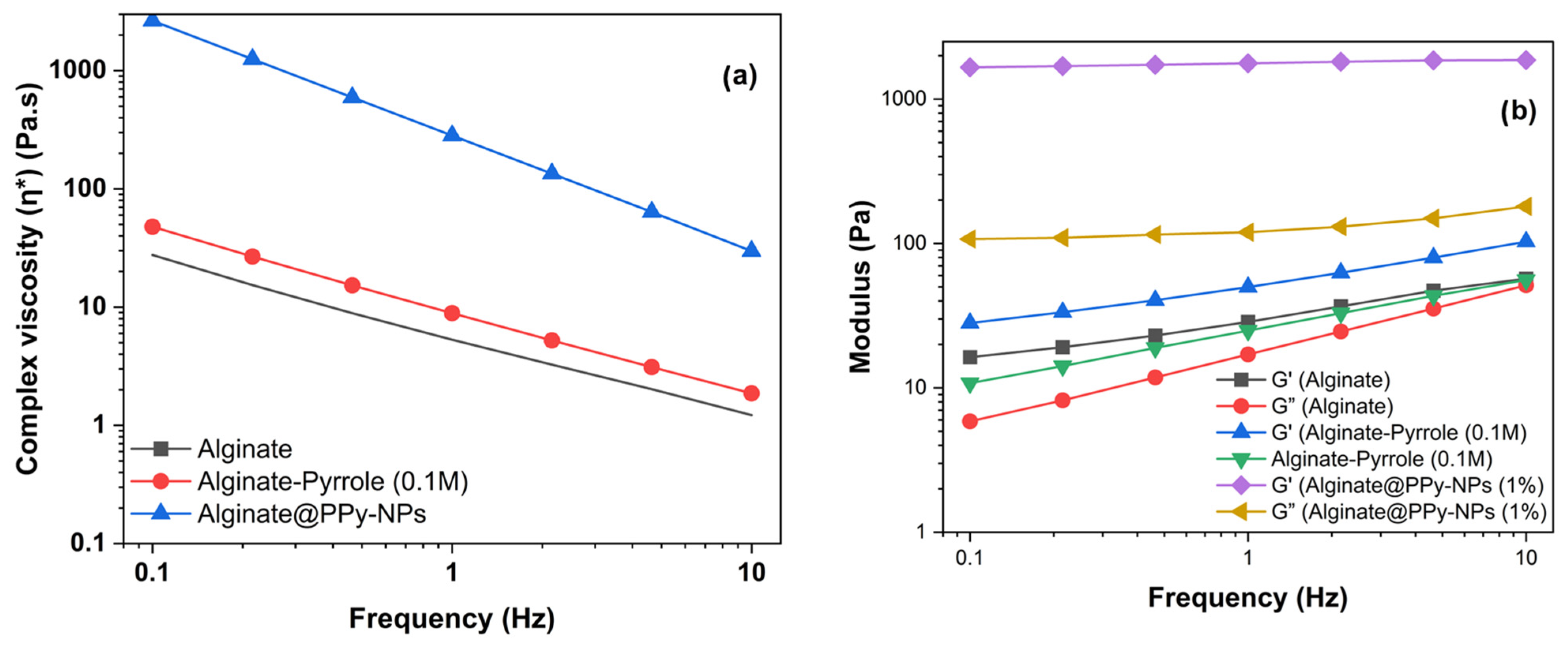
Rheological measurements demonstrated that both the alginate–PPy and alginate@PPy-NP systems exhibited time-dependent increases in the storage modulus (G′) and reductions in the complex viscosity (|η*|), indicating progressive gelation. The in situ polymerized calcium alginate–PPy system showed moderate improvements in viscoelasticity, whereas the alginate@PPy-NP system exhibited substantial enhancements, particularly at 1% (
w/
v) PPy-NP. A higher storage modulus (G′) indicates that the hydrogel possesses greater elasticity and structural integrity, which enables it to resist deformation and recover its original shape under mechanical stress. This characteristic is particularly important for applications that require mechanical stability and resilience, such as load-bearing or dynamic environments [
46]. G′ is a key parameter for evaluating the elastic behavior of hydrogels and their suitability for structural and functional applications. For instance, Abd El-Aziz et al. demonstrated that incorporating borosilicate glass into gelatin-based hydrogels significantly enhanced the compressive modulus, resulting in an elastic material with G’ that is markedly higher than the loss modulus (G″), thereby confirming its mechanical robustness and ability to maintain a self-standing structure [
47]. This characteristic is particularly beneficial in applications such as 3D printing and tissue scaffolding, where mechanical stability and resilience are necessary to support printing fidelity, cellular activity, and tissue regeneration [
48]. The ability of hydrogels to maintain their structural integrity under load is further emphasized by studies showing that higher G’ correlates with increased gel strength and reduced water absorbency under pressure [
48]. This concentration-dependent behavior suggests that the nanoparticles act as physical crosslinkers, promoting network densification and elastic recovery. Shear-thinning behavior was observed across all formulations, which is favorable for extrusion-based bioprinting.
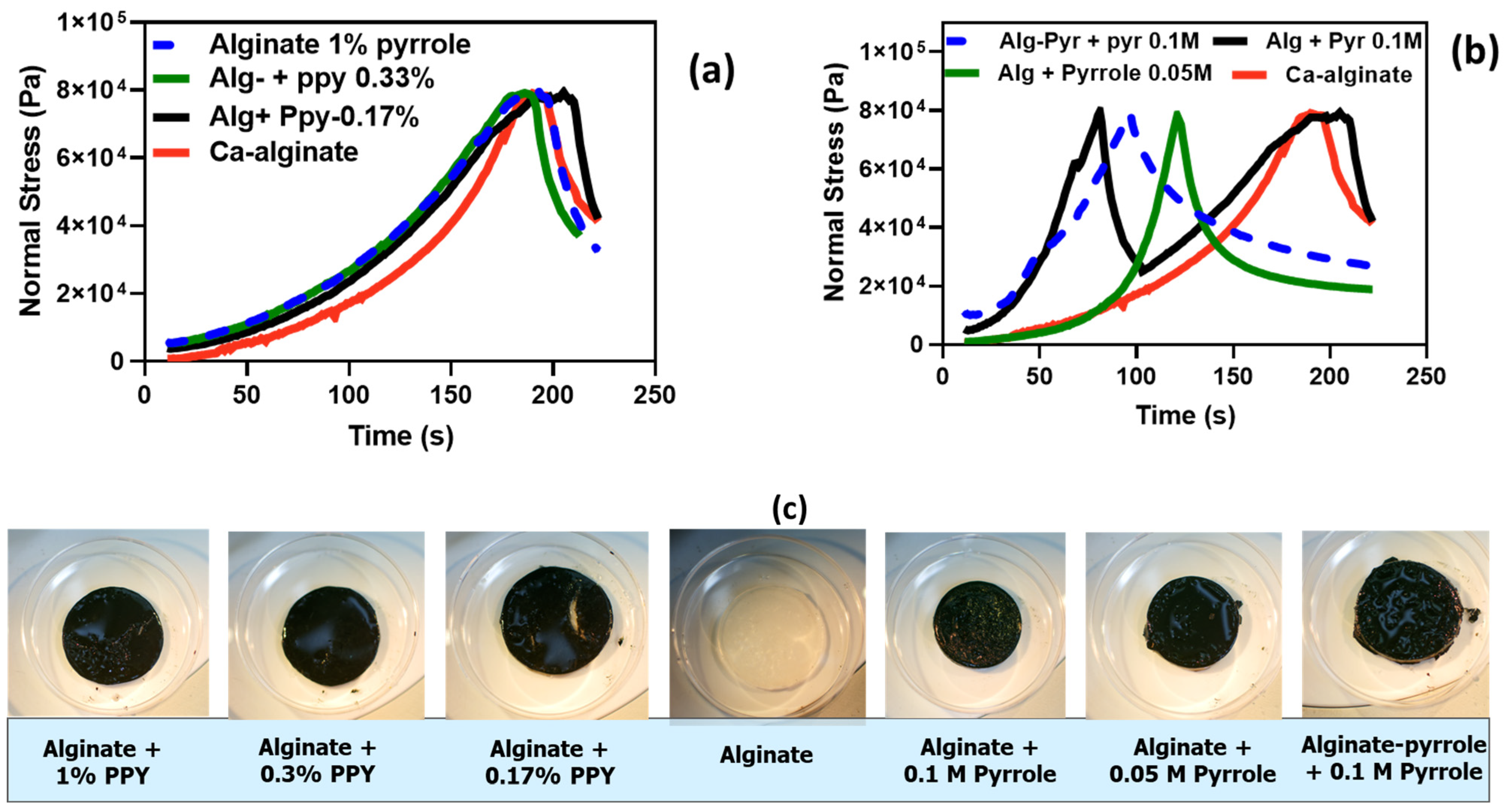
The mechanical analysis using compressibility tests further supported these rheological findings (
Figure 8). The Ca-alginate@PPy-NP hydrogels (
Figure 8a) exhibited a clear concentration-dependent increase in the compressive strength and deformation resistance, with the 1% NP variant achieving the highest mechanical robustness. In contrast, alginate–PPy hydrogels displayed symmetric stress–strain profiles, indicative of homogenous, yet brittle networks. Notably, the deformation pattern of alginate@PPy-NP extended beyond 180 s, whereas alginate–PPy (
Figure 8b) exhibited earlier yielding, reflecting differences in the network architecture. A higher crosslinking density generally leads to an increased compressive modulus, which enhances the ability of the hydrogel to resist deformation under the applied loads. As shown in
Figure 8, the compressive stress for both types of alginate–PPy (or alginate@PPy-NP) was 80 kPa, with a permanent deformation setting at different times. Calcium alginate@PPy-NP resulted in a more elastic composite hydrogel than the composite calcium alginate–polypyrrole. These results suggest potential nanoparticle-mediated reinforcement of the hydrogels, consistent with prior reports of enhanced compressive performance in nanocomposite hydrogels [
49,
50]. Conversely, the calcium alginate–PPy system (
Figure 8b) showed moderate improvements in mechanical integrity, with a single symmetric stress–strain peak that shifted slightly with increasing pyrrole content. Calcium alginate–PPy exhibited a sharp deformation pattern over a relatively short time (less than 100 s), whereas alginate@PPy-NP exhibited curve-like deformation behavior beyond 180 s. This is similar to the report by Wright et al. [
24], who reported that in situ-generated polypyrrole resulted in a brittle hydrogel film at certain concentrations.
Conductive hydrogels can be engineered by incorporating conductive materials such as conductive polymers or nanoparticles into the hydrogel matrix. In this study, we demonstrated the possibility of improving the electrical conductivity of hydrogels through covalent the addition of a conductive polypyrrole polymer. To achieve a conductive hydrogel matrix, pyrrole monomers were added alongside pyrrole-functionalized sodium alginate to ensure a sufficient concentration for in situ oxidative polymerization. At sub-threshold concentrations, pyrrole tends to form short oligomers that lack the extended π-conjugation necessary for electrical conductivity [
51]. The system facilitated the development of a conductive network throughout the hydrogel by reaching the critical concentration required for polypyrrole chain formation. The presence of covalently grafted pyrrole units promotes the localized polymerization and anchoring of polypyrrole within the alginate matrix. Furthermore, the addition of chemically synthesized polypyrrole nanoparticles (PPy-NPs) provided an additional conductive phase and contributed to mechanical reinforcement. This dual-modification strategy was intentionally employed to combine a uniform network integration with enhanced conductivity and mechanical resilience.
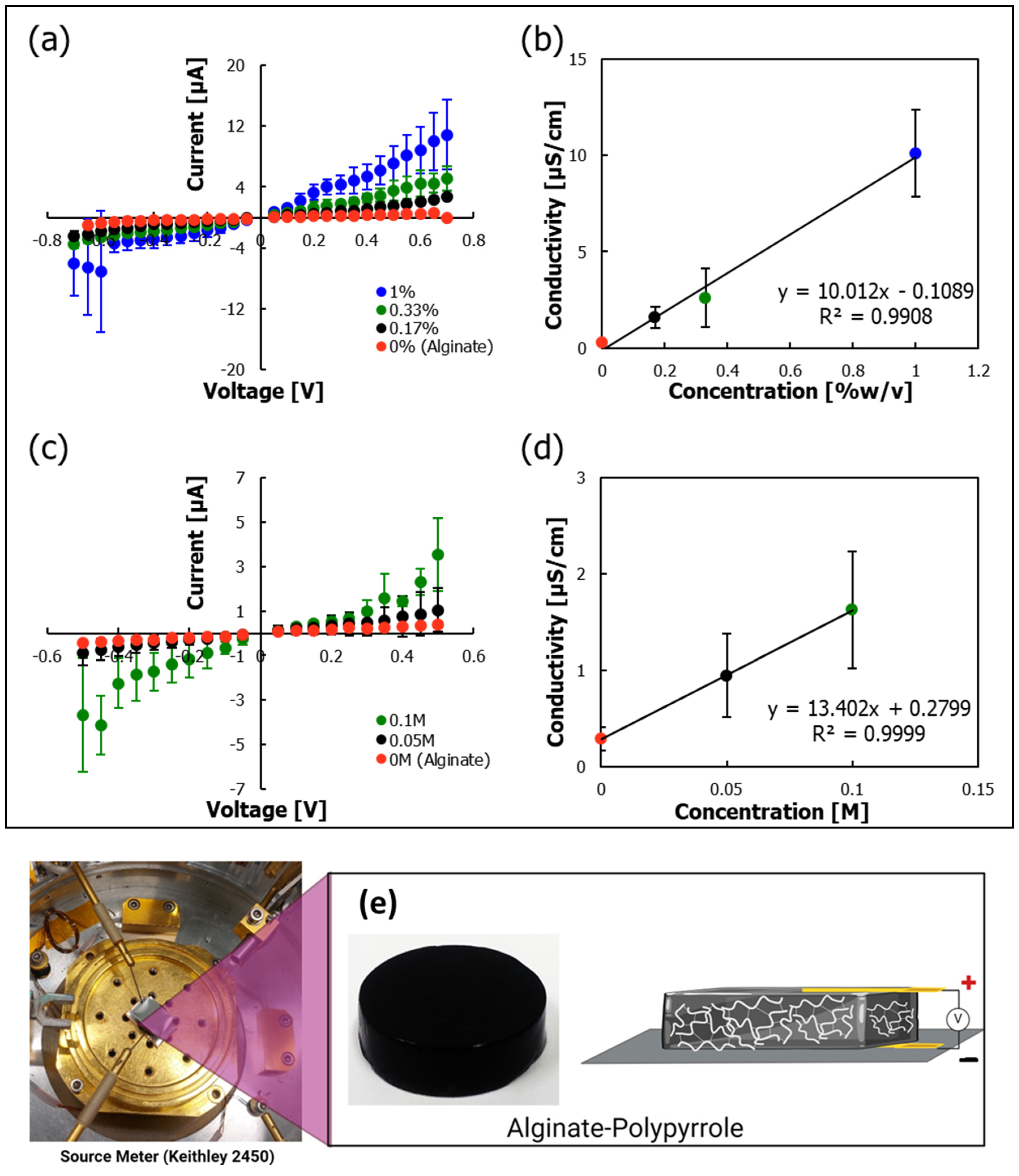
Electrical characterization showed that the integration of PPy imparted conductivity to the hydrogels, with a clear trend of increasing conductivity with increasing PPy content (
Figure 9). Different oxidants, such as hydrogen peroxide, ferric chloride, and ammonium persulfate (APS), have been used to synthesize polypyrrole from its monomer precursor. Ferric chloride and APS exhibited fast reaction rates (
Supplementary Figure S4). Because ferric ions, as multivalent cations, can interfere with sodium alginate crosslinking, APS was selected as the preferred oxidant to prepare polypyrrole [
52] because of its compatibility with calcium ion-based alginate crosslinking, avoiding interference from Fe
3⁺ ions. Polypyrrole-incorporated calcium–alginate hydrogels exhibited electrical conductivity that increased proportionally with polypyrrole content. The conductivity values obtained (
Figure 9) are within the range previously reported for conductive polysaccharide-based hydrogels. For example, Guo et al. [
53] reported that the electrical conductivity of pure chitosan (∼3.13 × 10
−8 S/cm) increased to 2.97 × 10
−5 S/cm upon the incorporation of 10% aniline as an intrinsically conducting component. In our system, crosslinked calcium alginate gels conducted electricity primarily through mobile ion transport, which was further enhanced by the introduction of a polypyrrole backbone featuring conjugated π-electron systems. This synergy results in a tunable and enhanced conductive profile, supporting the suitability of the hydrogel for electrically responsive tissue-engineering applications.
Our findings align with those of previous studies, demonstrating the role of conductive polymers in enhancing the functional performance of hydrogel networks. Wright et al. [
24] reported that the in situ polymerization of polypyrrole within calcium alginate yielded brittle films with limited mechanical resilience. By contrast, our nanoparticle-blended approach (alginate@PPy-NP) achieved significantly improved elasticity, mechanical robustness, and compressive stability. Furthermore, the conductivity values observed in our composite system, particularly at higher polypyrrole concentrations, are comparable to or even exceed those reported for similar systems. For instance, hyaluronic acid hydrogels modified with polypyrrole have demonstrated electrical conductivities of approximately 7.3 mS/cm [
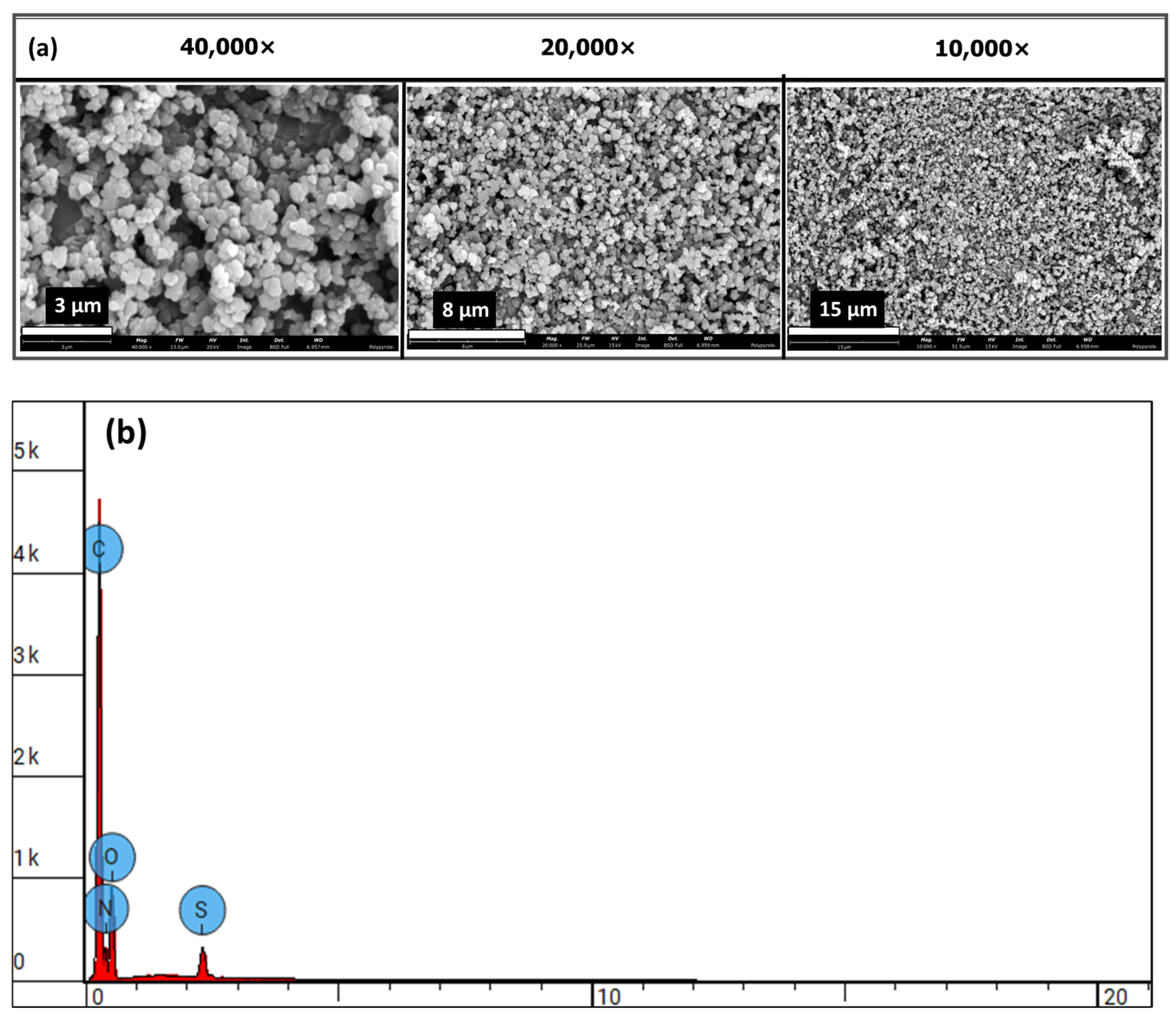
54], highlighting the effectiveness of our dual-modification strategy. Collectively, these comparisons underscore the versatility of our approach for engineering electroconductive hydrogels with customizable electrical and mechanical properties. Scanning electron microscopy (SEM) confirmed the nanoscale morphology (0.29182 ± 0.06076 µm), revealing uniform spherical particles (
Figure 5a) with rough surfaces, stable integration within the alginate matrix, and elemental distribution (
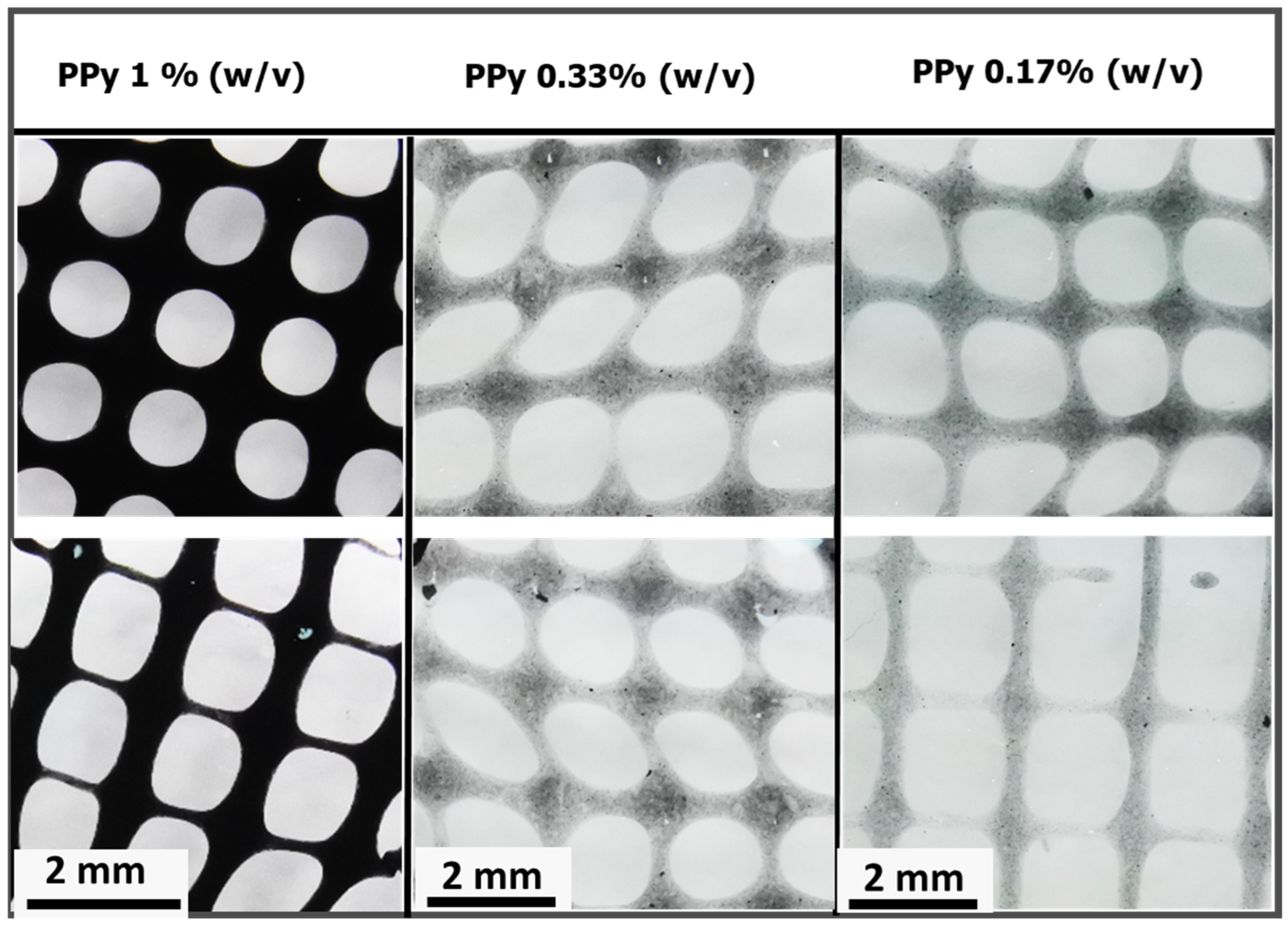
Figure 5b). The influence of these nanoparticles is evident in extrusion-based 3D printing and enhanced mechanical properties. The calcium alginate@PPy-NP constructs exhibited concentration-dependent improvement in print fidelity. At 1% PPy-NP, the constructs showed relatively well-defined pores and improved grid uniformity compared to lower concentrations (
Figure 13), although some filament spreading and edge blurring were still observed, particularly at the filament intersections. In contrast, constructs printed with 0.33% and 0.17% PPy-NPs doping displayed poor resolution, with visible pore collapse and filament merging, emphasizing the importance of nanoparticle concentration in maintaining structural stability. Printability was further enhanced by primary ionic crosslinking with 10 mM Ca
2⁺, which increased ink viscosity and improved extrusion stability. Additionally, printing within a support bath preserved the grid geometry even at higher speeds, demonstrating the robustness of the bioink formulation under varying extrusion conditions and its suitability for diverse tissue engineering applications.
To assess the planar print precision, the printing factor (Pf) was calculated using ImageJ by comparing the measured pore area with the designed geometry. This semi-quantitative metric, adapted from previously established methods, reflects the roundness and uniformity of printed filaments. A Pf value closer to 1 indicates better pore shape fidelity [
28,
29]. Although Pf provides valuable insight into in-plane accuracy, it does not directly assess the vertical resolution or interlayer adhesion. Although not quantitatively evaluated in this study, consistent filament stacking and minimal delamination were observed, particularly for the 1% PPy-NP constructs. These observations suggest good layer-to-layer cohesion. Future work will incorporate z-resolution analysis and mechanical testing of interlayer adhesion to fully characterize the 3D print fidelity and mechanical integration of multilayered constructs.
Taken together, the integration of polypyrrole into calcium alginate via covalent polymerization or nanoparticle dispersion significantly enhanced the physicochemical and functional properties of the resulting electrically conductive hydrogels. These modifications improve rheological behavior, mechanical integrity, electrical conductivity, and print fidelity, all of which are critical parameters for bioprinting applications. The ability to tailor these properties through pyrrole concentration and formulation methods makes these bioinks promising candidates for extrusion-based tissue engineering, particularly in scenarios requiring both mechanical robustness and electrical activity, such as neural or bone tissue regeneration.
5. Conclusions
This study aimed to develop and characterize electrically conductive alginate-based hydrogels by covalently integrating polypyrrole through EDC/NHS chemistry and incorporating chemically synthesized polypyrrole nanoparticles, with the goal of enhancing their suitability as bioinks for 3D bioprinting and tissue engineering applications. The resultant alginate–polypyrrole (alginate–PPy) and alginate@PPy-NP hydrogels exhibited tunable viscoelasticity, improved compressive strength, and enhanced electrical conductivity, all of which are critical for maintaining shape fidelity during extrusion and for supporting cell-laden constructs.
Among the two systems, calcium alginate@PPy-NP provided greater mechanical robustness and print resolution, whereas calcium alginate–PPy offered a more uniform gel matrix appropriate for applications requiring soft, cell-encapsulating hydrogels. Both systems demonstrated shear-thinning behavior and maintained printable characteristics under various extrusion conditions.
However, this study has certain limitations. The long-term stability and biocompatibility of the conductive hydrogels under physiological conditions were not assessed in this study and warrant further investigation. Additionally, the influence of electrical conductivity on specific cellular responses and tissue regeneration outcomes remains to be explored in depth.
Future research should focus on the in vitro and in vivo biological performances of these conductive hydrogels, including cell viability, proliferation, and differentiation. Expanding the system to include stimuli-responsive or drug-releasing functionalities could further enhance its utility in regenerative medicine, especially for electrically active tissues, such as nerves and cardiac muscles.