CO2 and UV Laser-Induced Graphene Based on Polymer Transformation: Advanced Characterizations by 2D Raman Mapping Combined with Microscopy Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. LIG Sample Preparation
2.2. LIG Characterizations
2.2.1. Surface Scanning Confocal Raman Spectroscopy
2.2.2. Confocal Laser Scanning Microscopy
2.2.3. Water Contact Angle
2.2.4. Atomic Force Microscope
3. Results
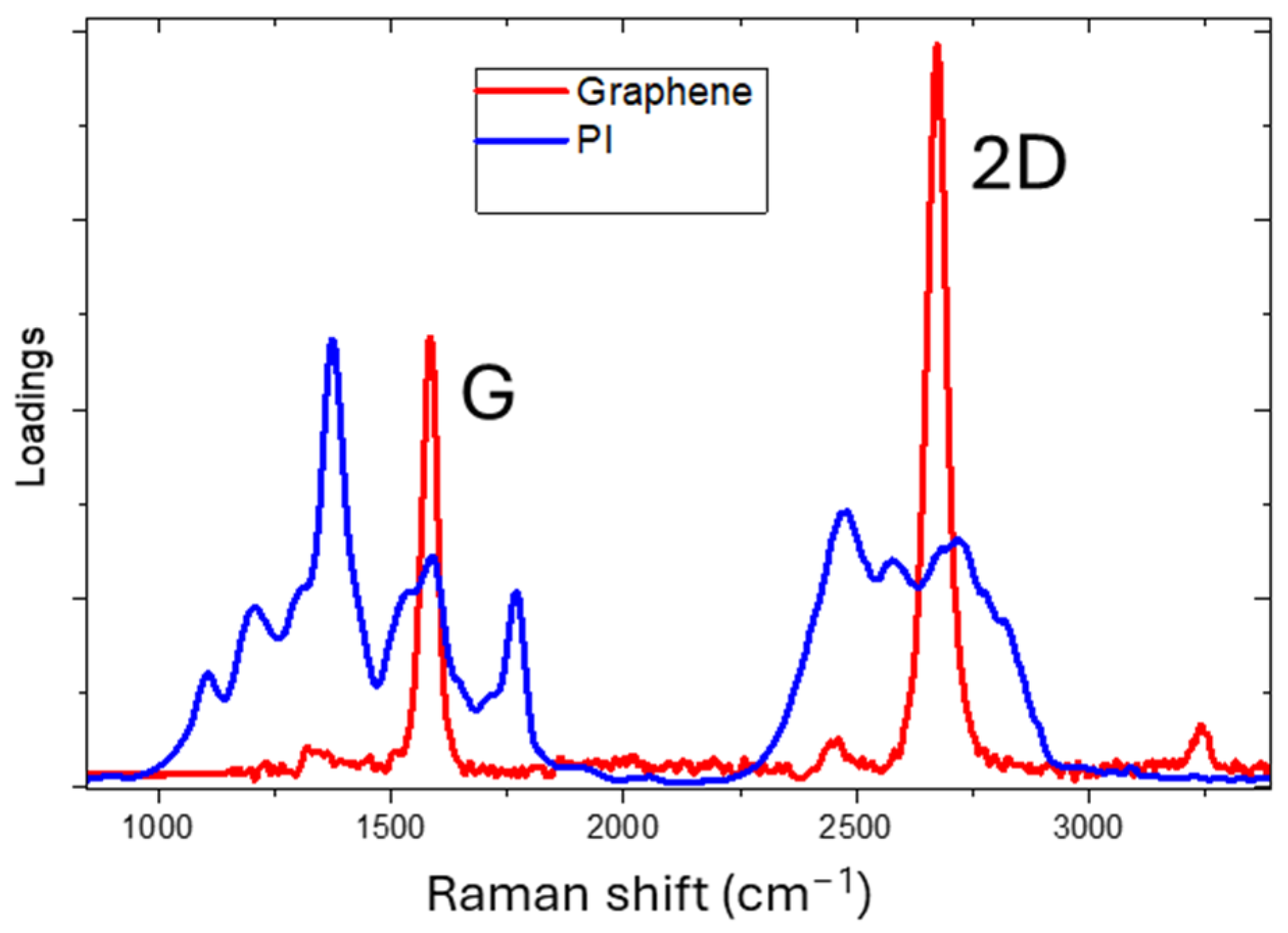
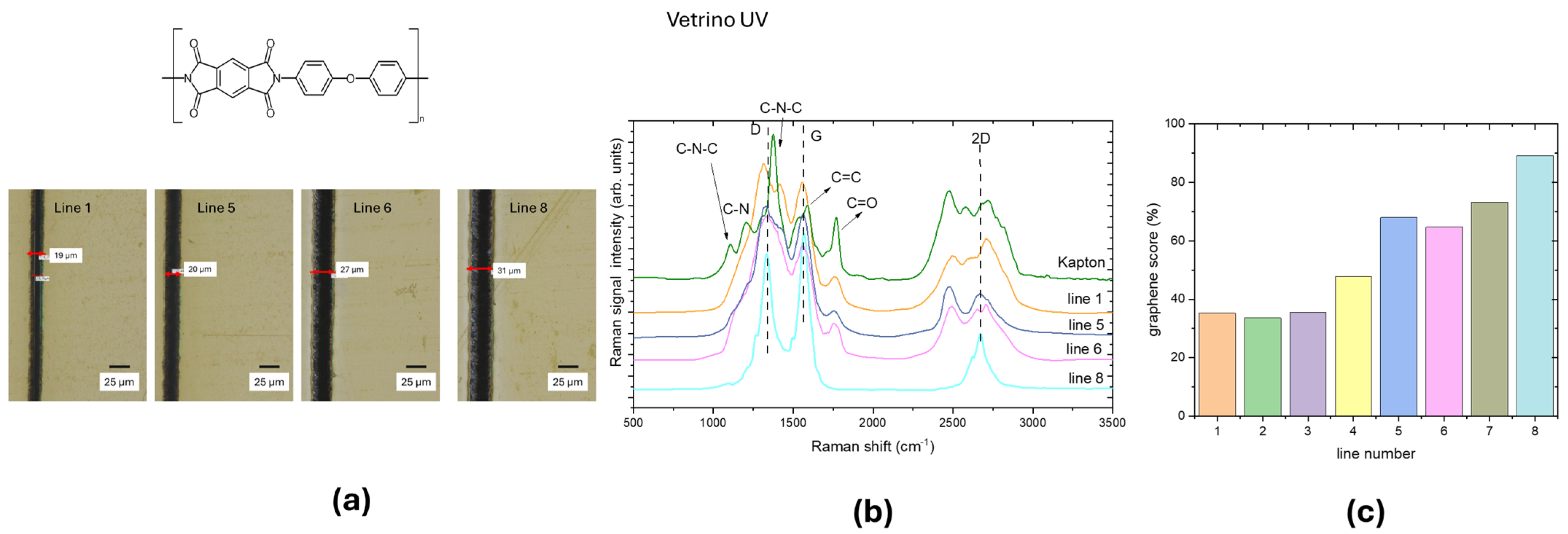
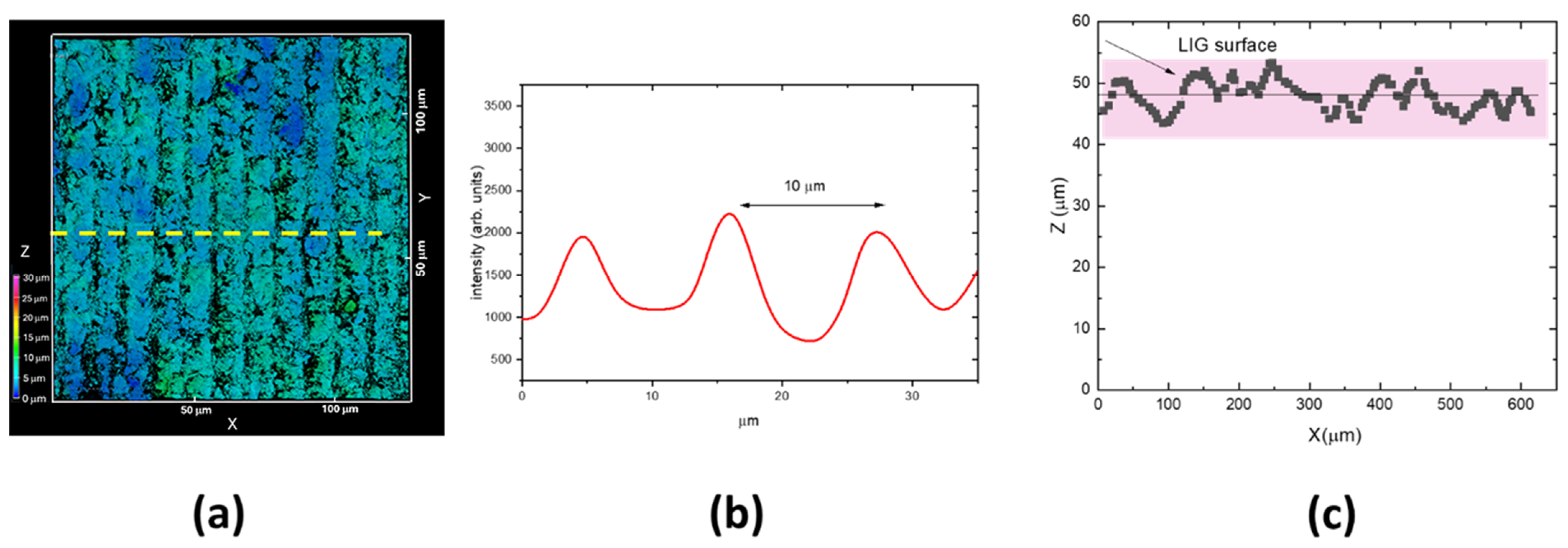
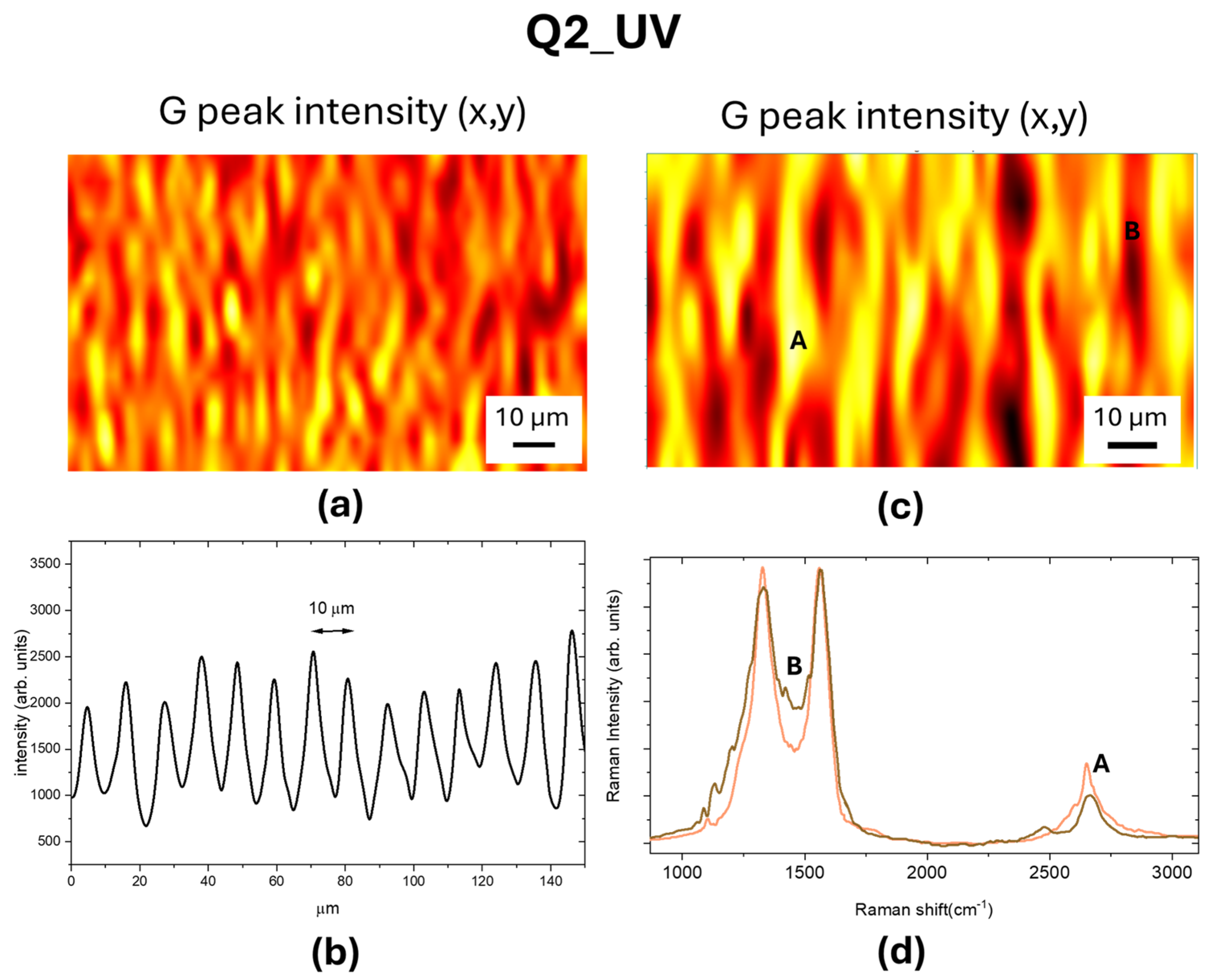
3.1. UV Laser Irradiation
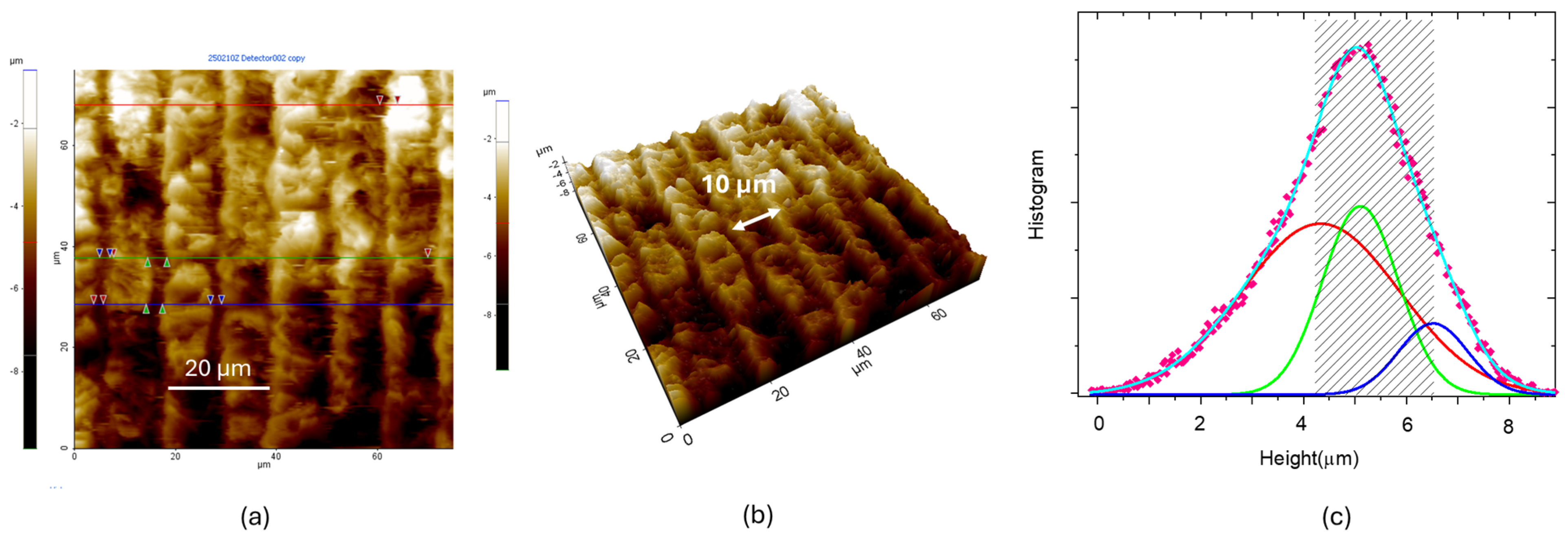
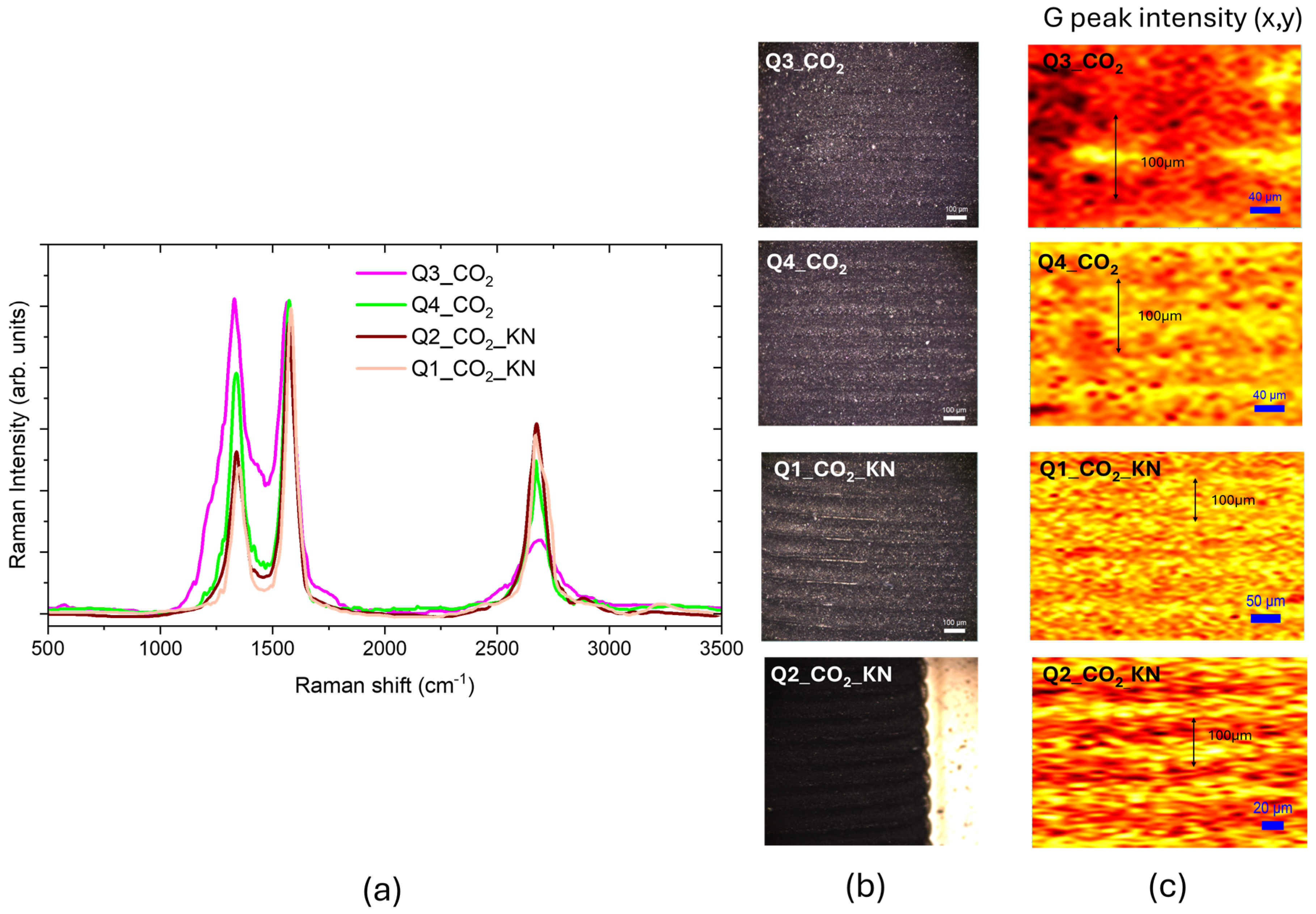
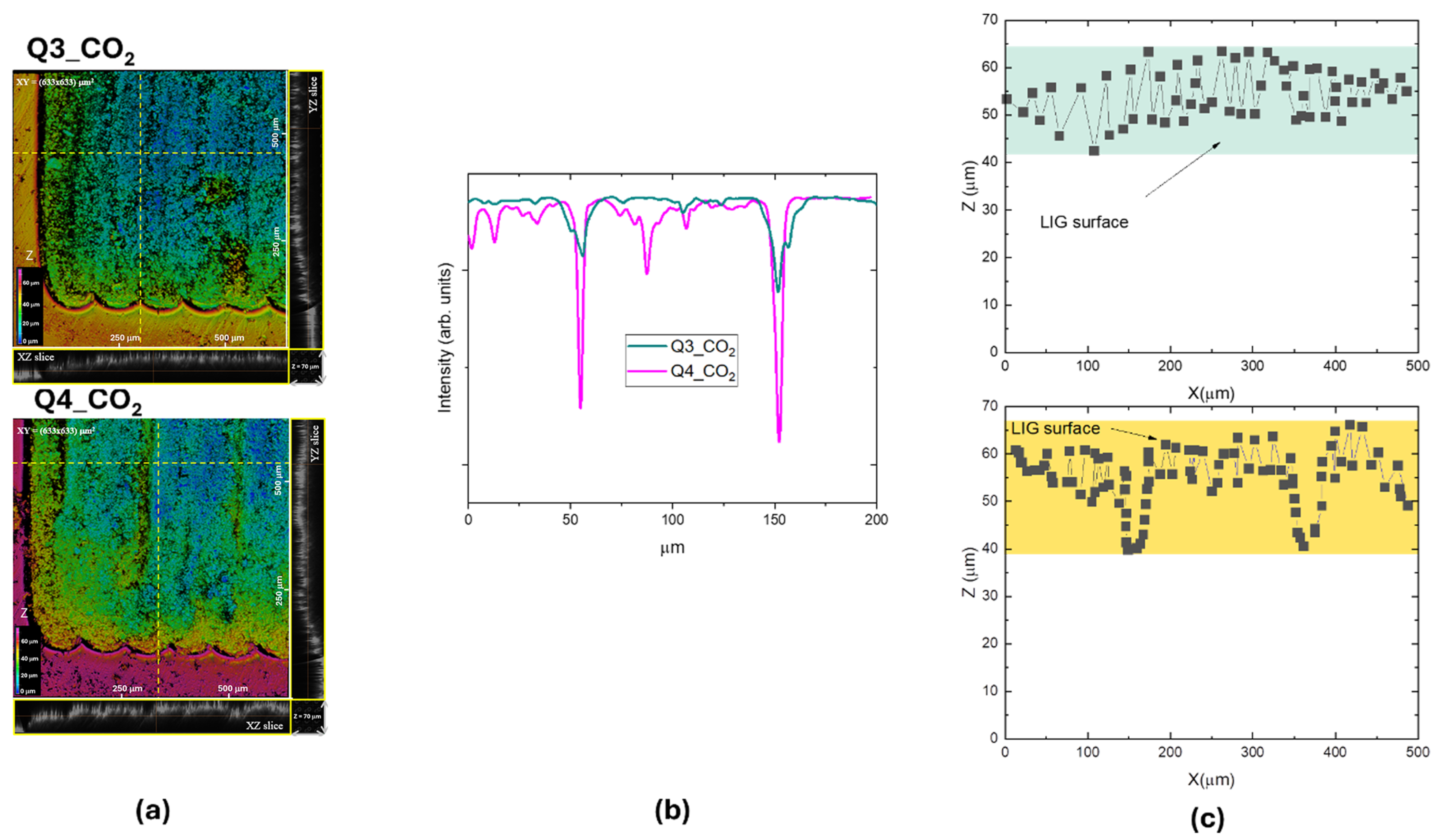
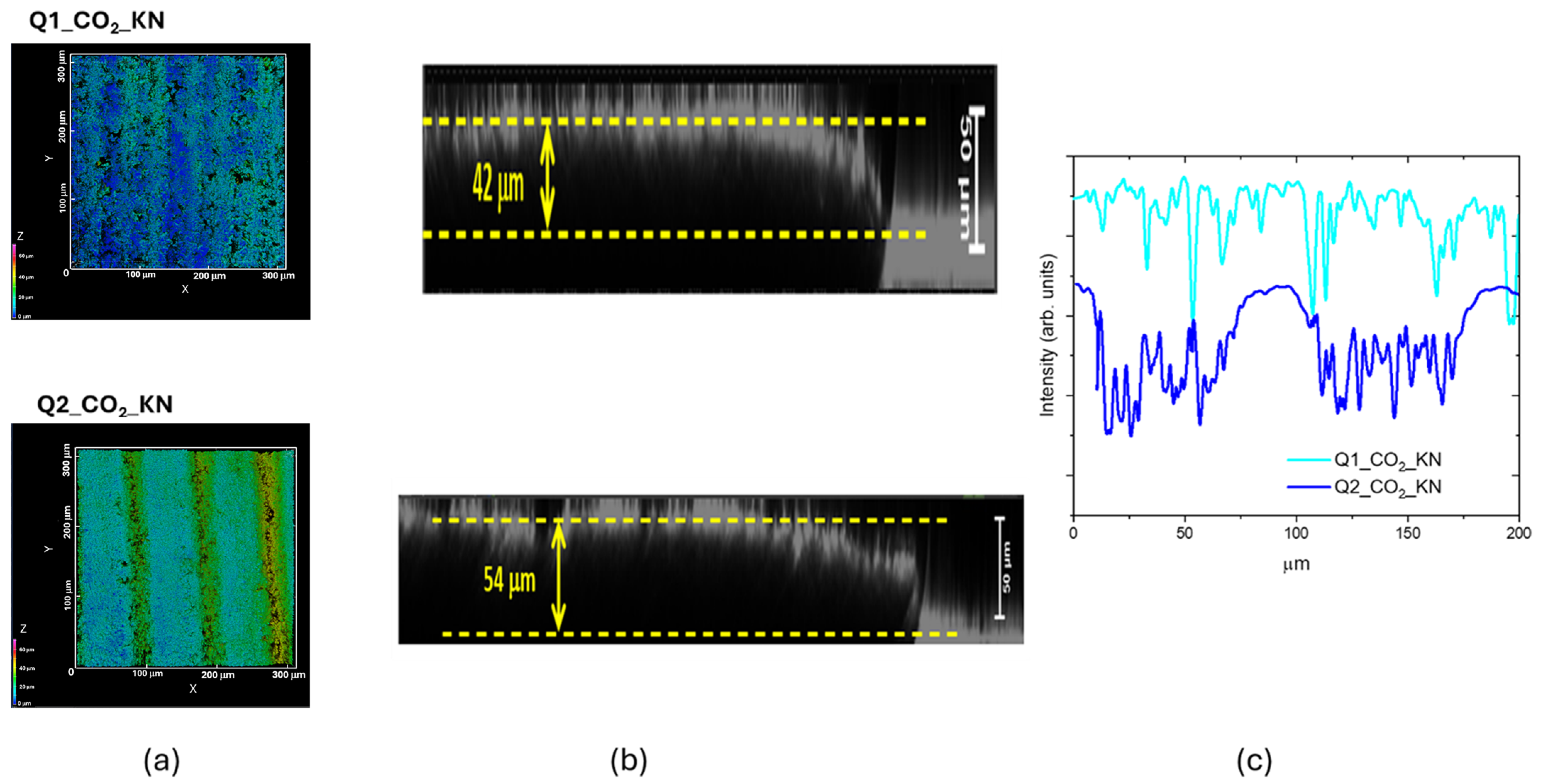
3.2. CO2 Laser Irradiation
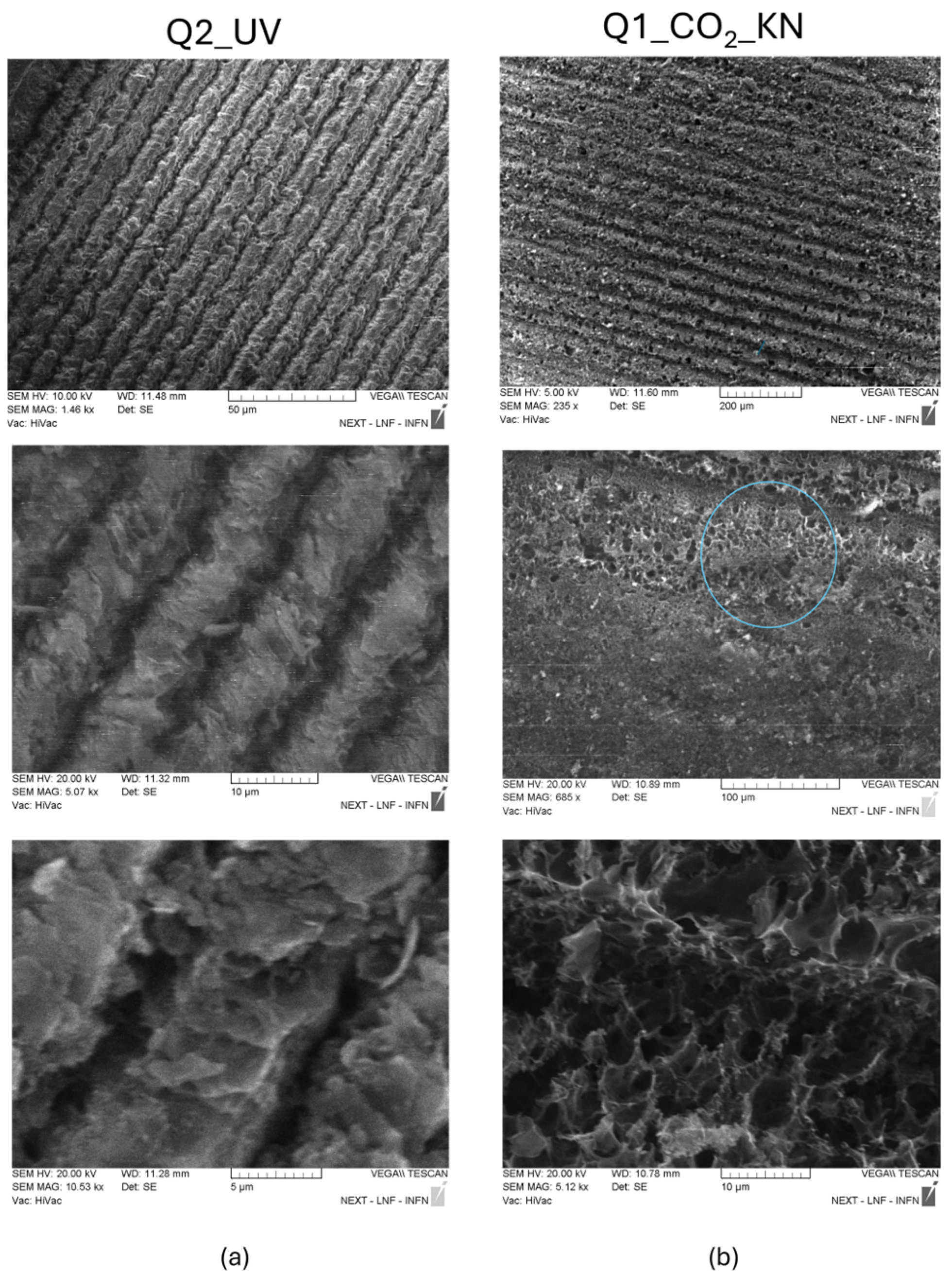
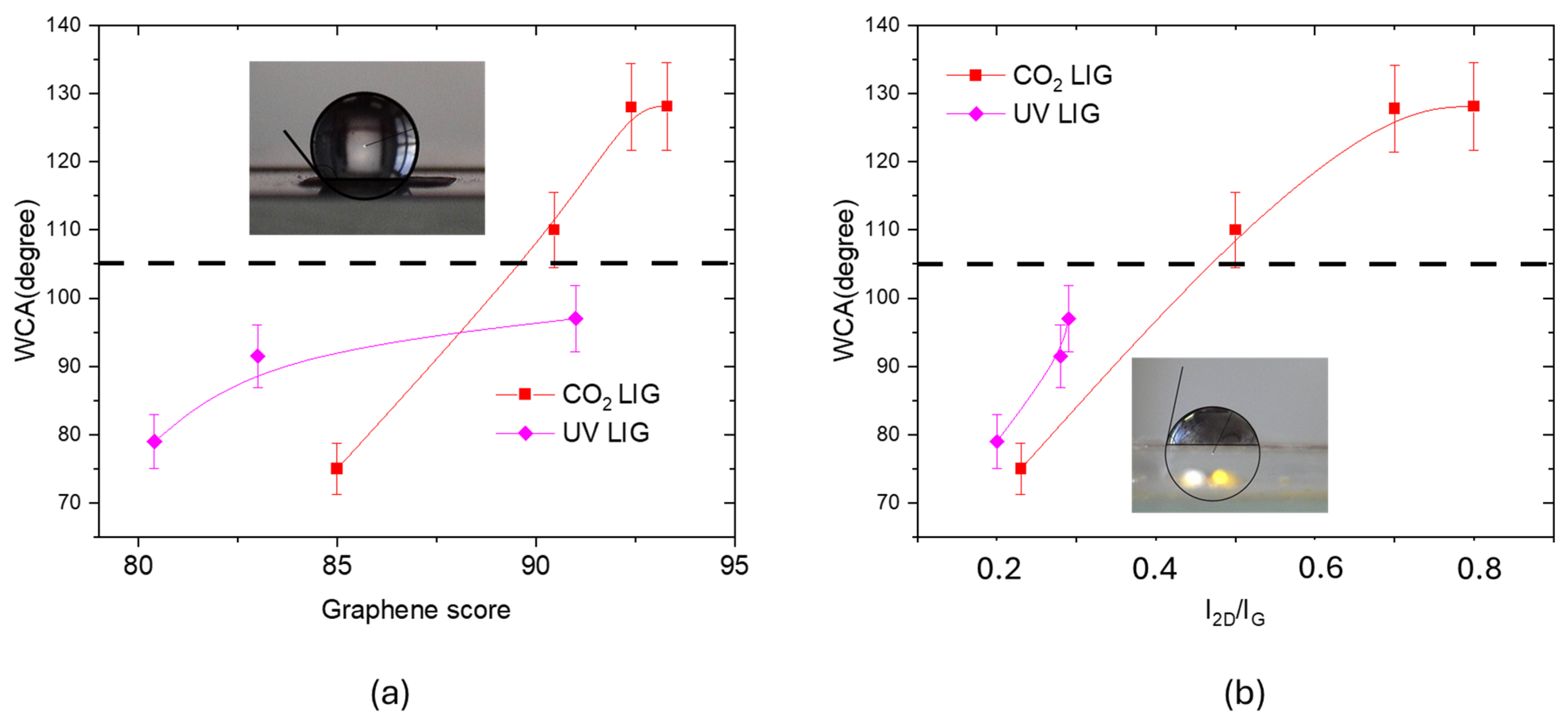
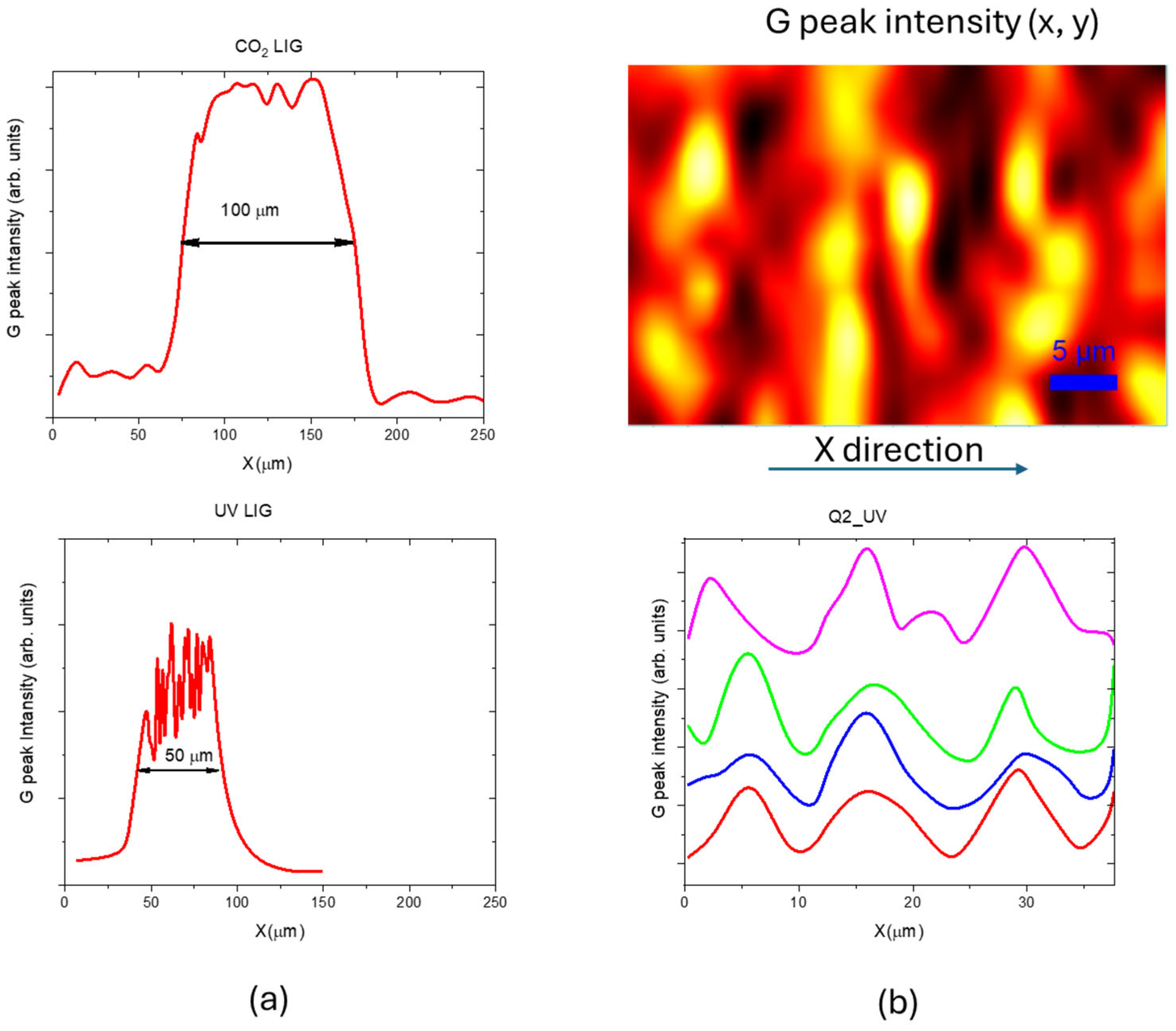
3.3. Morphological Comparison Between UV and CO2 Laser LIG Structures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Zhu, H.; Zhang, W.; Zhang, Z.; Lu, J.; Xu, K.; Liu, Y.; Saetang, V. A review of laser-induced graphene: From experimental and theoretical fabrication processes to emerging applications. Carbon 2014, 214, 118356. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene: From discovery to translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Zhou, S.; Shi, D.; Huang, Y.; Chen, X.; Gao, J.; Zhao, N.; Wong, C.P. UV Laser-Induced Polyimide-to-Graphene Conversion: Modeling, Fabrication, and Application. Small Methods 2019, 3, 1900208. [Google Scholar] [CrossRef]
- Keshavarz, M.; Rezaul Haque Chowdhury, A.K.M.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Self-assembled N-doped Q-dot carbon nanostructures as a SERS-active biosensor with selective therapeutic functionality. Sens. Actuators B Chem. 2020, 323, 128703. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, J.; Wang, Z.; Song, W.; Cao, G. Recent advances in preparation and application of laser-induced graphene in energy storage devices. Mater. Today Energy 2020, 18, 100569. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Ismail, A.M.; Nasrallah, D.A.; El-Metwally, E.G. Modulation of the optoelectronic properties of polyimide (Kapton-H) films by gamma irradiation for laser attenuation and flexible space structures. Radiat. Phys. Chem. 2022, 194, 110026. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-Induced Graphene Strain Sensors Produced by Ultraviolet Irradiation of Polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and non-adiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Mallard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Botti, S.; Mezi, L.; Rufoloni, A.; Vannozzi, A.; Bollanti, S.; Flora, F. Extreme Ultraviolet Generation of Localized Defects in Single-Layer Graphene: Raman Mapping, Atomic Force Microscopy, and High-Resolution Scanning Electron Microscopy Analysis. ACS Appl. Electron. Mater. 2019, 1, 2560–2565. [Google Scholar] [CrossRef]
- Cancado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A.; Pimenta, M.A. Measuring the degree of stacking order in graphite by Raman spectroscopy. Carbon 2008, 46, 272–291. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectra of graphite. J. Chem. Phys. 1970, 53, 1126–1310. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman spectroscopy of graphene edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single-and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Diaspro, J.A. Confocal and Two-Photon Microscopy. In Foundations, Applications and Advances; Diaspro, A., Ed.; Wiley-Liss: New York, NY, USA, 2002. [Google Scholar]
- Blondelli, F.; Botti, S.; Bonfigli, F.; Toto, E.; Laurenzi, S.; Santonicola, M.G. Polyimide/graphene nanocomposites as antibacterial coatings for human exploration missions in space. In Proceedings of the 75th International Astronautical Congress (IAC), Milan, Italy, 14–18 October 2024. [Google Scholar]
- Sehnal, A.; Ogilvie, S.P.; Clifford, K.; Wood, H.J.; Graf, A.A.; Lee, F.; Tripathi, M.; Lynch, P.J.; Large, M.J.; Seyedin, S.; et al. Measuring the Surface Energy of Nanosheets by Emulsion Inversion. J. Phys. Chem. C 2024, 128, 17073–17080. [Google Scholar] [CrossRef] [PubMed]
- Dinani, H.S.; Reinbolt, T.; Zhang, G.; Zhao, G.; Gerald, R.E., II; Yan, Z.; Huang, J. Miniaturized Wearable Biosensors for Continuous Health Monitoring Fabricated Using the Femtosecond Laser-Induced Graphene Surface and Encapsulated Traces and Electrodes. ACS Sens. 2025, 10, 761–772. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, S.; Bonfigli, F.; Bruttomesso, A.; Micciulla, F.; Nigro, V.; Rufoloni, A.; Vannozzi, A. CO2 and UV Laser-Induced Graphene Based on Polymer Transformation: Advanced Characterizations by 2D Raman Mapping Combined with Microscopy Techniques. Materials 2025, 18, 3119. https://doi.org/10.3390/ma18133119
Botti S, Bonfigli F, Bruttomesso A, Micciulla F, Nigro V, Rufoloni A, Vannozzi A. CO2 and UV Laser-Induced Graphene Based on Polymer Transformation: Advanced Characterizations by 2D Raman Mapping Combined with Microscopy Techniques. Materials. 2025; 18(13):3119. https://doi.org/10.3390/ma18133119
Chicago/Turabian StyleBotti, Sabina, Francesca Bonfigli, Alessio Bruttomesso, Federico Micciulla, Valentina Nigro, Alessandro Rufoloni, and Angelo Vannozzi. 2025. "CO2 and UV Laser-Induced Graphene Based on Polymer Transformation: Advanced Characterizations by 2D Raman Mapping Combined with Microscopy Techniques" Materials 18, no. 13: 3119. https://doi.org/10.3390/ma18133119
APA StyleBotti, S., Bonfigli, F., Bruttomesso, A., Micciulla, F., Nigro, V., Rufoloni, A., & Vannozzi, A. (2025). CO2 and UV Laser-Induced Graphene Based on Polymer Transformation: Advanced Characterizations by 2D Raman Mapping Combined with Microscopy Techniques. Materials, 18(13), 3119. https://doi.org/10.3390/ma18133119






