Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment
Abstract
1. Introduction
- Elevated friction resistance may lead to a decrease in cruising speed [16];
- The recurrent dry-docking procedure generates substantial toxic waste, detrimental to the environment [24];
- Vessels transport invasive organisms globally, potentially resulting in the bio-invasion of non-native species in marine ecosystems devoid of natural enemies [25].
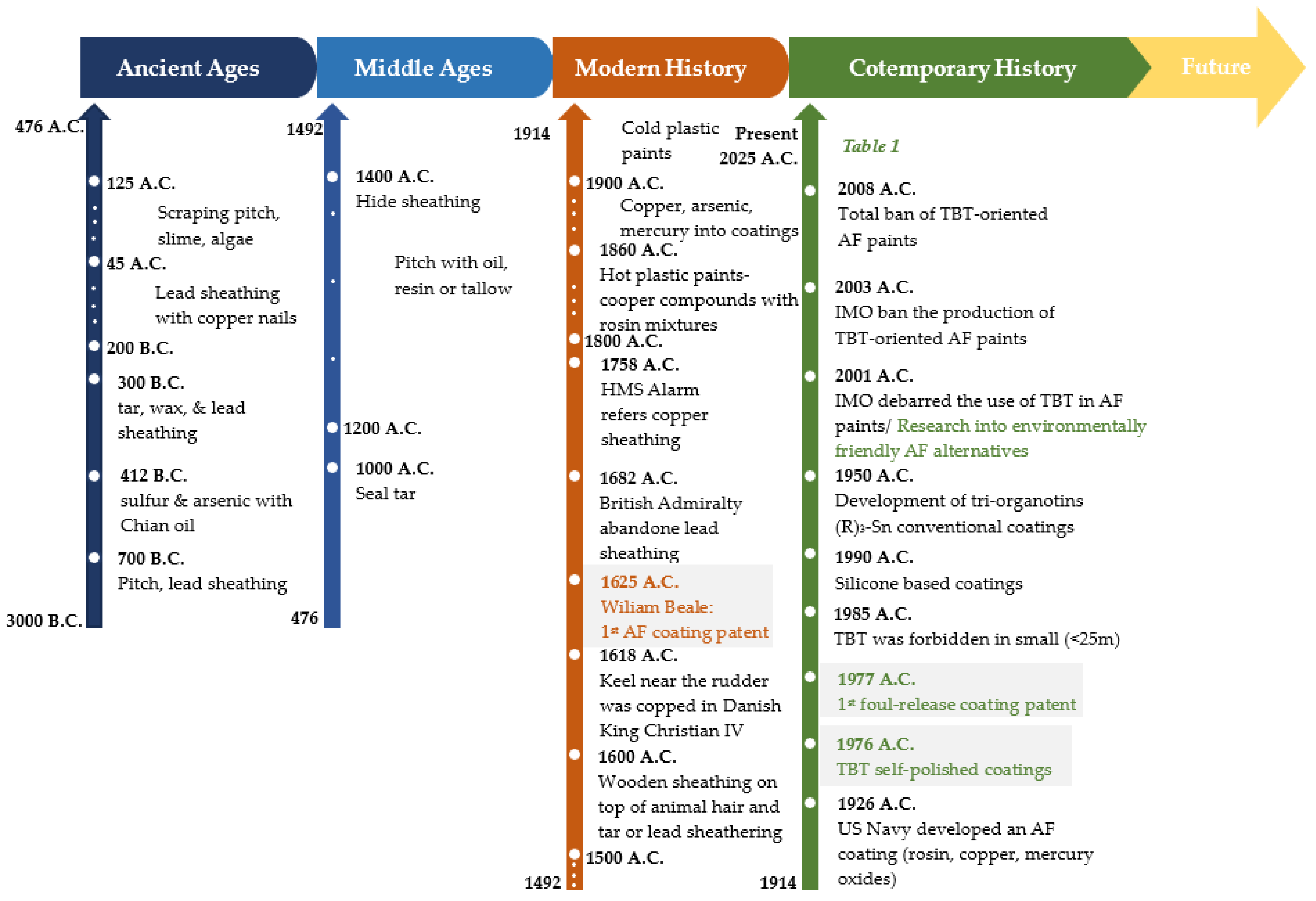
| Criterion | Self-Polishing Copolymers | Silicon/ Fluoropolymer Coatings | Biomimetic Surfaces | Robotic Hull Cleaning | UV/ Electromechanical Systems |
|---|---|---|---|---|---|
| Antifouling Efficacy |  |  |  |  (removes, not prevents) |  (still in development) |
| Environmental Compliance |  (low tox biocides) |  (no biocides) |  (no chemicals) |  |  |
| Durability/Mechanical Resistance |  |  (softer coatings) |  |  |  (vulnerable tech) |
| Ease of Application/Maintenance |  |  (requires special prep.) |  (experimental, costly) |  (operational only) |  (lab-scale) |
| Cost Effectiveness |  |  (high initial cost) |  (R&D phase) |  (high upkeep) |  (experimental) |
| Fuel Efficiency/ Drag Reduction |  |  (very smooth) |  |  (does not alert surface) |  |
| Smart/ Innovative Features |  |  (self-cleaning) |  (bio-inspired) |  (autonomous) |  (novel tech) |
| Best for | Cargo, tankers, general fleet | High-performance, eco-focused ships | Research, green shipping demos | All ship types during port | Future ships (concept/prototype) |
 = excellent;
= excellent;  = poor.
= poor.2. Materials and Methods
2.1. Antifouling Coating
2.1.1. The Synthesis Procedure
2.1.2. Characterization of Nanocomposites
2.2. Panel Preparation
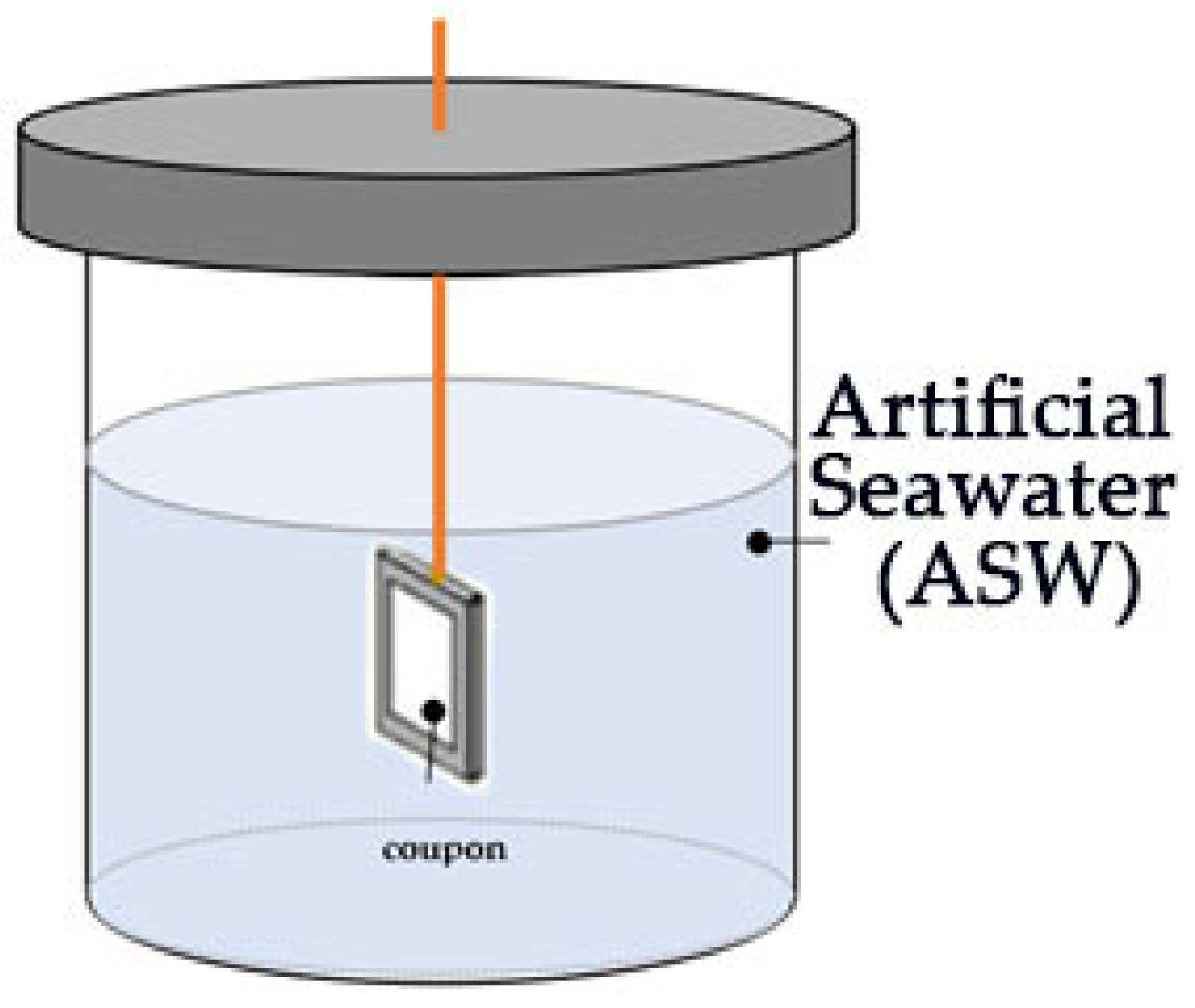
2.3. Laboratory Static Immersion Tests
2.4. In Situ Static Immersion Tests
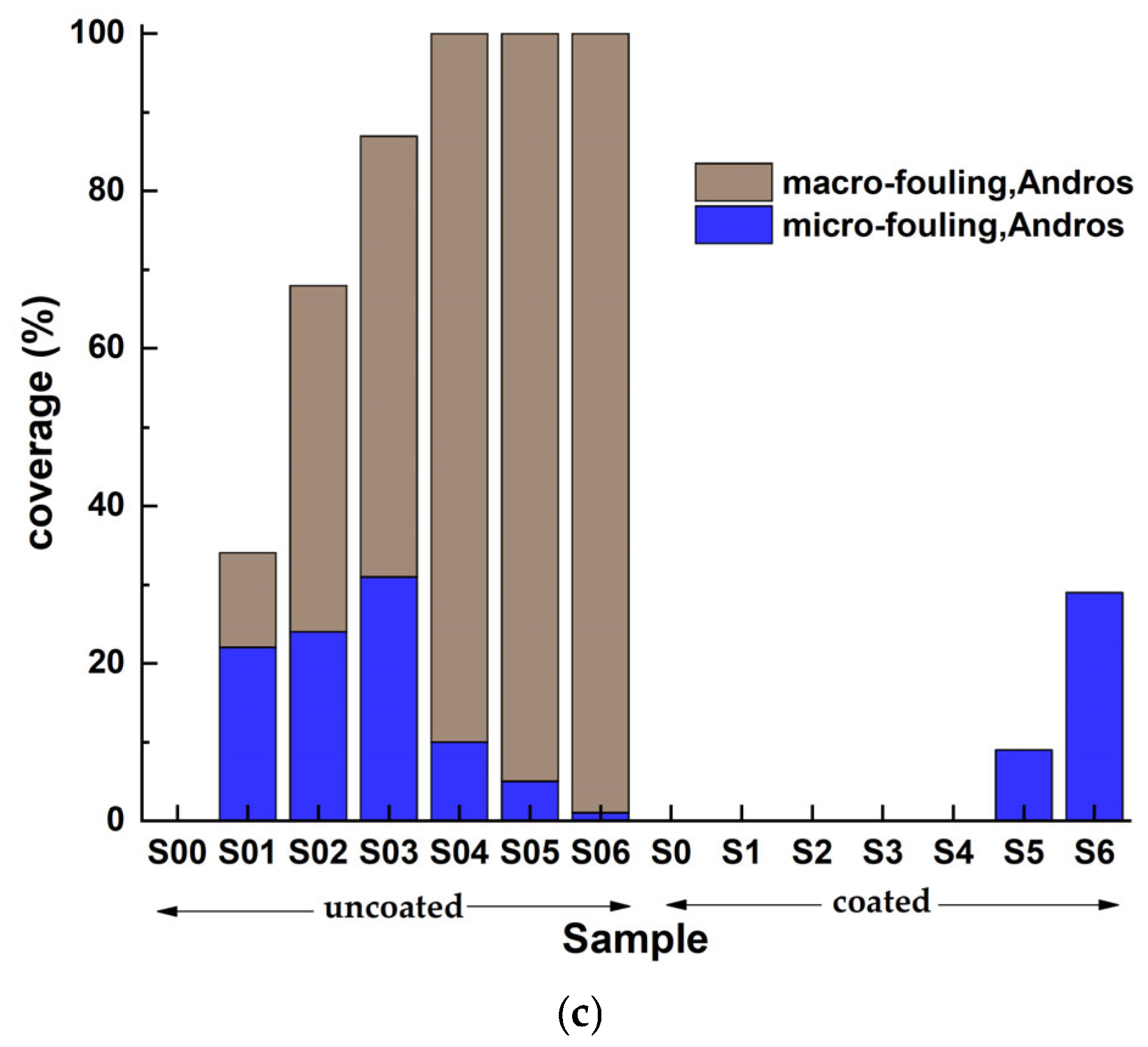
3. Results
3.1. Characterization of the Antifouling Nanocomposites
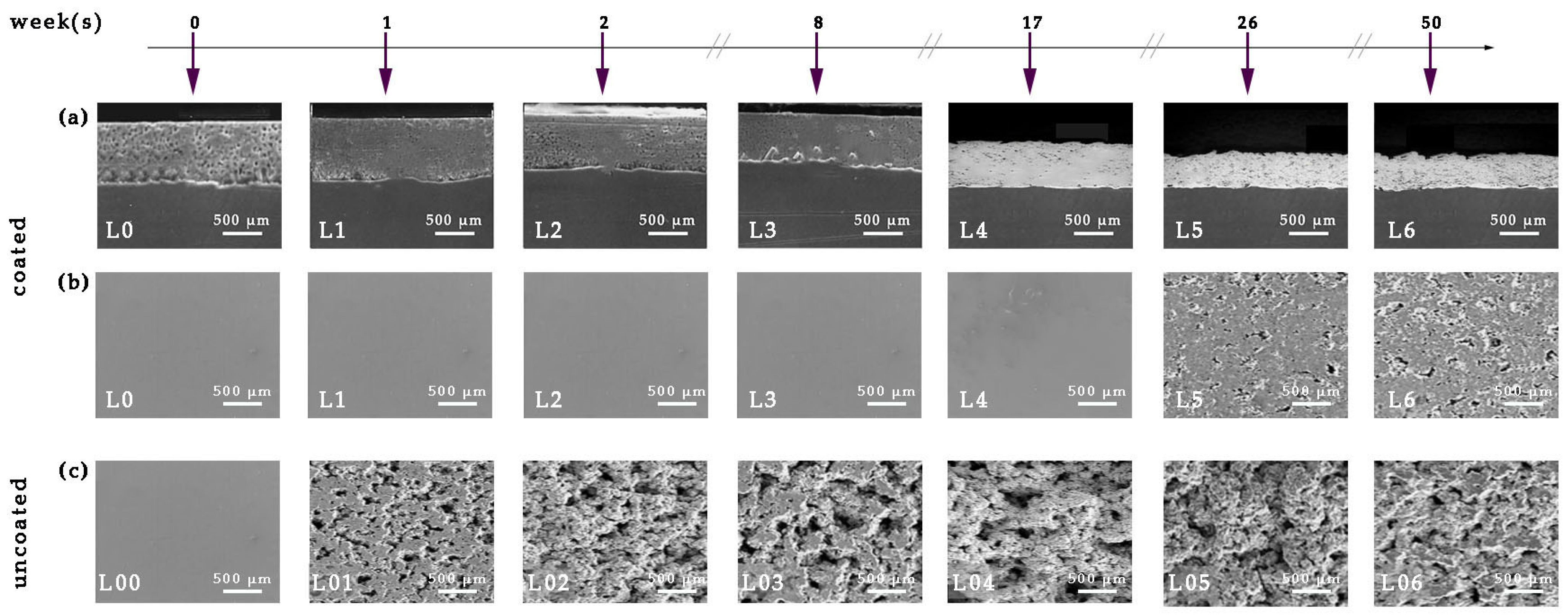
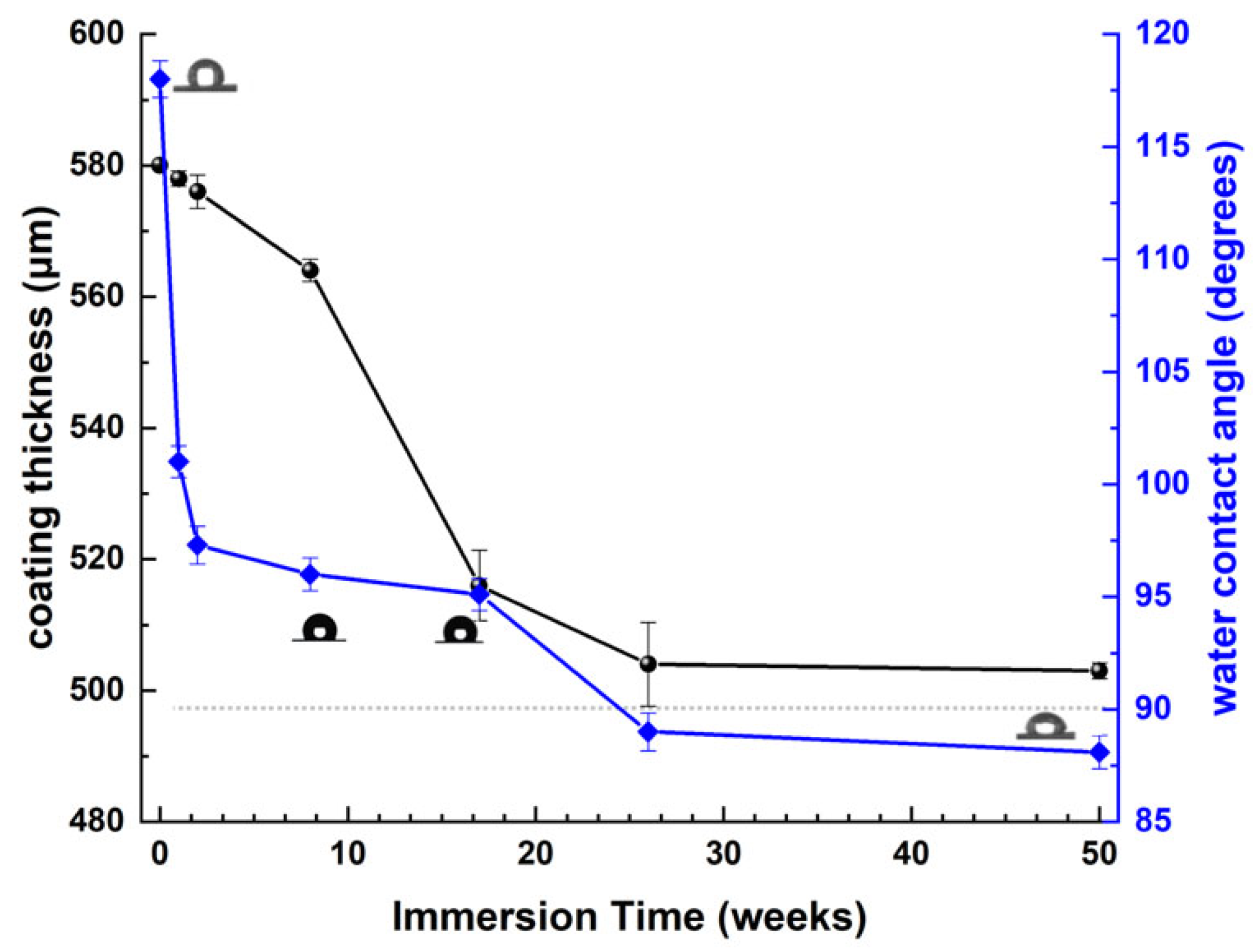
3.2. Corrosion Tests of Antifouling Coating
Static Immersion Tests
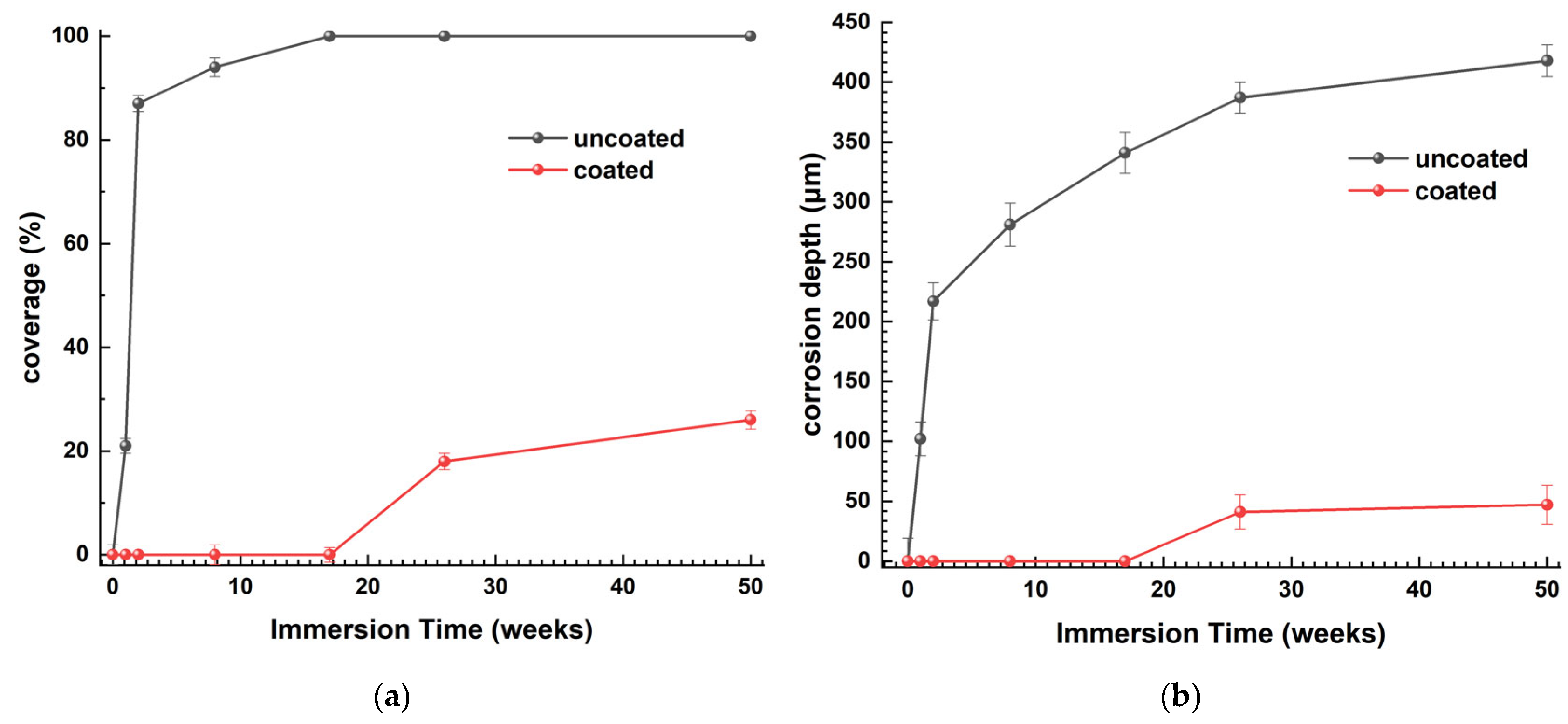
3.3. On-Site Immersion of Steels in Real Seawater
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | antifouling |
| TBT | tributyltin |
| CNTs | carbon nanotubes |
| PAni | polyaniline |
| NPs | nanoparticles |
| ASW | artificial seawater |
| SEM | scanning electron microscopy |
| FR | Foul Resistance |
| PDR | Physical Data Rating |
| OP | Overall Performance |
| CR | corrosion rate |
References
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling Technology—Past, Present and Future Steps towards Efficient and Environmentally Friendly Antifouling Coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine Paints: The Particular Case of Antifouling Paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Li, L.; Hong, H.; Cao, J.; Yang, Y. Progress in Marine Antifouling Coatings: Current Status and Prospects. Coatings 2023, 13, 1893. [Google Scholar] [CrossRef]
- Caruso, G. Microbial Colonization in Marine Environments: Overview of Current Knowledge and Emerging Research Topics. J. Mar. Sci. Eng. 2020, 8, 78. [Google Scholar] [CrossRef]
- Vinagre, P.A.; Simas, T.; Cruz, E.; Pinori, E.; Svenson, J. Marine Biofouling: A European Database for the Marine Renewable Energy Sector. J. Mar. Sci. Eng. 2020, 8, 495. [Google Scholar] [CrossRef]
- Clare, A.S.; Rittschof, D.; Gerhart, D.J.; Maki, J.S. Molecular Approaches to Nontoxic Antifouling. Invertebr. Reprod. Dev. 1992, 22, 67–76. [Google Scholar] [CrossRef]
- Vuong, P.; McKinley, A.; Kaur, P. Understanding Biofouling and Contaminant Accretion on Submerged Marine Structures. NPJ Mater. Degrad. 2023, 7, 50. [Google Scholar] [CrossRef]
- Cirillo, A.; Tomaiuolo, G.; Guido, S. Membrane Fouling Phenomena in Microfluidic Systems: From Technical Challenges to Scientific Opportunities. Micromachines 2021, 12, 820. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.J.; Babarro, J.M.F.; Lahoz, F.; Sansón, M.; Martín, V.S.; Norte, M.; Fernández, J.J. From Broad-Spectrum Biocides to Quorum Sensing Disruptors and Mussel Repellents: Antifouling Profile of Alkyl Triphenylphosphonium Salts. PLoS ONE 2015, 10, e0123652. [Google Scholar] [CrossRef]
- Weber, F.; Esmaeili, N. Marine Biofouling and the Role of Biocidal Coatings in Balancing Environmental Impacts. Biofouling 2023, 39, 661–681. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Thomas, P.J.; Thomas, P.; Greve, M.M. Antibiofouling Coatings for Marine Sensors: Progress and Perspectives on Materials, Methods, Impacts, and Field Trial Studies. ACS Sens. 2025, 10, 1600–1619. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, J.; Tian, L.; Gao, M.; Zhao, J.; Ren, L. Recent Advances in Emerging Integrated Antifouling and Anticorrosion Coatings. Mater. Des. 2022, 213, 110307. [Google Scholar] [CrossRef]
- Papadopoulos, N.D.; Vourna, P.; Falara, P.P.; Koutsaftiki, P.; Xafakis, S. Dual-Function Coatings to Protect Absorbent Surfaces from Fouling. AIMS Mater. Sci. 2023, 10, 981–1003. [Google Scholar] [CrossRef]
- Schultz, M.P.; Walker, J.M.; Steppe, C.N.; Flack, K.A. Impact of Diatomaceous Biofilms on the Frictional Drag of Fouling-Release Coatings. Biofouling 2015, 31, 759–773. [Google Scholar] [CrossRef]
- Schultz, M.P. Effects of Coating Roughness and Biofouling on Ship Resistance and Powering. Biofouling 2007, 23, 331–341. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Vourna, P.; Falara, P.; Vourna, P. A Modern Approach Towards Efficient Antifouling Coating Technologies. Nanotechnol. Adv. Mater. Sci. 2023, 6, 1–4. [Google Scholar] [CrossRef]
- Falara, P.P.; Papadopoulos, N.D.; Vourna, P. Microstructure and Performance of Antibiofouling Coatings on High-Strength Steel Substrates Immersed in the Marine Environment. Micro 2022, 2, 277–294. [Google Scholar] [CrossRef]
- Champ, M.A. A Review of Organotin Regulatory Strategies, Pending Actions, Related Costs and Benefits. Sci. Total Environ. 2000, 258, 21–71. [Google Scholar] [CrossRef]
- Paz-Villarraga, C.A.; Castro, Í.B.; Fillmann, G. Biocides in Antifouling Paint Formulations Currently Registered for Use. Environ. Sci. Pollut. Res. 2022, 29, 30090–30101. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent Progress in Marine Foul-Release Polymeric Nanocomposite Coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Degiuli, N.; Martić, I. An Investigation into the Effect of Hard Fouling on the Ship Resistance Using CFD. Appl. Ocean Res. 2020, 100, 102205. [Google Scholar] [CrossRef]
- Byrnes, T.A.; Dunn, R.J.K. Boating- and Shipping-Related Environmental Impacts and Example Management Measures: A Review. J. Mar. Sci. Eng. 2020, 8, 908. [Google Scholar] [CrossRef]
- Davidson, I.C.; Smith, G.; Ashton, G.V.; Ruiz, G.M.; Scianni, C. An Experimental Test of Stationary Lay-up Periods and Simulated Transit on Biofouling Accumulation and Transfer on Ships. Biofouling 2020, 36, 455–466. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Fan, Y.; Xu, D. Microbially Influenced Corrosion of Steel in Marine Environments: A Review from Mechanisms to Prevention. Microorganisms 2023, 11, 2299. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling Strategies: History and Regulation, Ecological Impacts and Mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Satasiya, G.; Kumar, M.A.; Ray, S. Biofouling Dynamics and Antifouling Innovations: Transitioning from Traditional Biocides to Nanotechnological Interventions. Environ. Res. 2025, 269, 120943. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-50323-2. [Google Scholar]
- Terlizzi, A.; Fraschetti, S.; Gianguzza, P.; Faimali, M.; Boero, F. Environmental Impact of Antifouling Technologies: State of the Art and Perspectives. Aquat. Conserv. 2001, 11, 311–317. [Google Scholar] [CrossRef]
- Omae, I. General Aspects of Tin-Free Antifouling Paints. Chem. Rev. 2003, 103, 3431–3448. [Google Scholar] [CrossRef]
- Wahl, M. Marine Epibiosis. I. Fouling and Antifouling: Some Basic Aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Canales, C.; Galarce, C.; Rubio, F.; Pineda, F.; Anguita, J.; Barros, R.; Parragué, M.; Daille, L.K.; Aguirre, J.; Armijo, F.; et al. Testing the Test: A Comparative Study of Marine Microbial Corrosion under Laboratory and Field Conditions. ACS Omega 2021, 6, 13496–13507. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, T.; Raorane, C.J.; Haldhar, R.; Asrafali, S.P.; Kim, S.-C. Development of Arbutin Based Sustainable Polybenzoxazine Resin for Antifouling and Anticorrosion of Low Carbon Steel. Prog. Org. Coat. 2022, 170, 106968. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Govindaraj, S.; Dhaipule, N.G.K.; Thirumalaisamy, N.; Anne, R.S.; Sublime, N.; Philip, J. Enhancing Microbiologically Influenced Corrosion Protection of Carbon Steels with Silanized Epoxy-Biocide Hybrid Coatings. Environ. Sci. Pollut. Res. 2024, 31, 13302–13326. [Google Scholar] [CrossRef]
- Eduok, U.; Suleiman, R.; Gittens, J.; Khaled, M.; Smith, T.J.; Akid, R.; El Ali, B.; Khalil, A. Anticorrosion/Antifouling Properties of Bacterial Spore-Loaded Sol–Gel Type Coating for Mild Steel in Saline Marine Condition: A Case of Thermophilic Strain of Bacillus Licheniformis. RSC Adv. 2015, 5, 93818–93830. [Google Scholar] [CrossRef]
- BFP Advanced Technologies. Electrically Anisotropic Antifouling Coatings. WO2024224120A1, 31 October 2024. [Google Scholar]
- Vourna, P.; Papadopoulos, N.D.; Falara, P.P.; Hristoforou, E. Barkhausen Noise Emission of Naval Steel: The Impact of Seawater Corrosion Coverage and Depth. NDT E Int. 2025, 151, 103319. [Google Scholar] [CrossRef]
- ASTM D1141; Standard Specifications for Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 1960; pp. 398–399.
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM: West Conshohocken, PA, USA, 2004.
- Media Cybernetics IMAGE-PRO® Discovery: The Enhanced Image Analysis Solution from the Image-Pro Family. Available online: https://www.digitalimagingsystems.co.uk/pdfs/image-pro-discovery.pdf (accessed on 16 June 2025).
- ASTM 3626; Test Method for Testing Antifouling Panels in Shallow Submergence. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D 6990; Practice for Evaluating Biofouling Resistance and Physical Performance of Marine Coating Systems. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- Hashemi Monfared, A.; Jamshidi, M. Synthesis of Polyaniline/Titanium Dioxide Nanocomposite (PAni/TiO2) and Its Application as Photocatalyst in Acrylic Pseudo Paint for Benzene Removal under UV/VIS Lights. Prog. Org. Coat. 2019, 136, 105257. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Kraljić Roković, M.; Žic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Deivanayaki, S.; Ponnuswamy, V.; Ashokan, S.; Jayamurugan, P.; Mariappan, R. Synthesis and Characterization of TiO2-Doped Polyaniline Nanocomposites by Chemical Oxidation Method. Mater. Sci. Semicond. Process. 2013, 16, 554–559. [Google Scholar] [CrossRef]
- Shao, W.; Jamal, R.; Xu, F.; Ubul, A.; Abdiryim, T. The Effect of a Small Amount of Water on the Structure and Electrochemical Properties of Solid-State Synthesized Polyaniline. Materials 2012, 5, 1811–1825. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-Anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Jarvin, M.; Rosaline, D.R.; Gopalakrishnan, T.; Kamalam, M.B.R.; Foletto, E.L.; Dotto, G.L.; Inbanathan, S.S.R. Remarkable Photocatalytic Performances towards Pollutant Degradation under Sunlight and Enhanced Electrochemical Properties of TiO2/Polymer Nanohybrids. Environ. Sci. Pollut. Res. 2023, 30, 62832–62846. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, D.; Hu, J.; Xiao, G.; Wang, J.; Li, W.; Fu, X. Highly Efficient Photocatalytic Degradation of Organic Pollutants by PANI-Modified TiO2 Composite. J. Phys. Chem. C 2012, 116, 5764–5772. [Google Scholar] [CrossRef]
- Žic, M. The Influence of the PANI Structure on the Conductive Mechanism and on the Electrical Equivalent Circuit Analysis. J. Electroanal. Chem. 2009, 635, 29–38. [Google Scholar] [CrossRef]
- Stegarescu, A.; Cabrera, H.; Budasheva, H.; Soran, M.-L.; Lung, I.; Limosani, F.; Korte, D.; Amati, M.; Borodi, G.; Kacso, I.; et al. Synthesis and Characterization of MWCNT-COOH/Fe3O4 and CNT-COOH/Fe3O4/NiO Nanocomposites: Assessment of Adsorption and Photocatalytic Performance. Nanomaterials 2022, 12, 3008. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple Functionalization of Multi-Walled Carbon Nanotubes with Carboxyl and Amino Groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Abdullah, T.A.; Juzsakova, T.; Rasheed, R.T.; Salman, A.D.; Sebestyen, V.; Domokos, E.; Sluser, B.; Cretescu, I. Polystyrene-Fe3O4-MWCNTs Nanocomposites for Toluene Removal from Water. Materials 2021, 14, 5503. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, S.Y.; Wan, H.-Y.; Chen, Y.-T.; Yu, S.-K.; Wu, H.-C.; Yang, T.-I. Electrospun Hydrophobic Polyaniline/Silk Fibroin Electrochromic Nanofibers with Low Electrical Resistance. Polymers 2020, 12, 2102. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Song, Y.; Gong, Y.; Qi, M.; Hao, R. Fabrication of a High Water Flux Conductive MWCNTs/PVC Composite Membrane with Effective Electrically Enhanced Antifouling Behavior. Coatings 2021, 11, 1548. [Google Scholar] [CrossRef]
- Alterary, S.S.; Alyabes, R.M.; Alshahrani, A.A.; Al-Alshaikh, M.A. Unfunctionalized and Functionalized Multiwalled Carbon Nanotubes/Polyamide Nanocomposites as Selective-Layer Polysulfone Membranes. Polymers 2022, 14, 1544. [Google Scholar] [CrossRef]
- Malaret, F. Exact Calculation of Corrosion Rates by the Weight-Loss Method. Exp. Results 2022, 3, e13. [Google Scholar] [CrossRef]
- Sousa-Cardoso, F.; Teixeira-Santos, R.; Mergulhão, F.J.M. Antifouling Performance of Carbon-Based Coatings for Marine Applications: A Systematic Review. Antibiotics 2022, 11, 1102. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of Nanomaterials in Antifouling: A Review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Esteves, D.S.; Melo, A.; Alves, S.; Durães, N.; Paiva, M.C.; Sequeiros, E.W. Magnetic Field-Assisted Orientation and Positioning of Magnetite for Flexible and Electrically Conductive Sensors. Micromachines 2025, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lee, H.S.; Lee, C.-G.; Park, S.-J.; Lee, J.; Jung, S.; Shin, G.-A. Application of PANI/TiO2 Composite for Photocatalytic Degradation of Contaminants from Aqueous Solution. Appl. Sci. 2020, 10, 6710. [Google Scholar] [CrossRef]
- Han, S.-J.; Park, J.-S. Understanding Membrane Fouling in Electrically Driven Energy Conversion Devices. Energies 2021, 14, 212. [Google Scholar] [CrossRef]
- Villardi De Oliveira, C.; Petitbois, J.; Faÿ, F.; Sanchette, F.; Schuster, F.; Alhussein, A.; Chaix-Pluchery, O.; Deschanvres, J.-L.; Jiménez, C. Marine Antibiofouling Properties of TiO2 and Ti-Cu-O Films Deposited by Aerosol-Assisted Chemical Vapor Deposition. Coatings 2020, 10, 779. [Google Scholar] [CrossRef]
- Yi, P.; Jia, H.; Yang, X.; Fan, Y.; Xu, S.; Li, J.; Lv, M.; Chang, Y. Anti-Biofouling Properties of TiO2 Coating with Coupled Effect of Photocatalysis and Microstructure. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130357. [Google Scholar] [CrossRef]
- Iesalnieks, M.; Eglītis, R.; Juhna, T.; Šmits, K.; Šutka, A. Photocatalytic Activity of TiO2 Coatings Obtained at Room Temperature on a Polymethyl Methacrylate Substrate. Int. J. Mol. Sci. 2022, 23, 12936. [Google Scholar] [CrossRef]
- Kordas, G. Novel Antifouling and Self-Healing Eco-Friendly Coatings for Marine Applications Enhancing the Performance of Commercial Marine Paints. In Engineering Failure Analysis; Thanapalan, K., Ed.; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78985-945-4. [Google Scholar]
- Chen, Q.; Zhang, Z.; Qi, Y. Construction of a Novel Environmentally-Friendly Long-Term Antifouling Coating with a Double-Layer Structure Regulated by Phenylmethyl Silicone Oil and Verification of the Static Antifouling Performance of the Coating. Surf. Interfaces 2025, 56, 105519. [Google Scholar] [CrossRef]










| Period | Technology | Advantages | Disadvantages |
|---|---|---|---|
| <1800 A.D. | Pitch, organic residues (oil), copper foil | Natural protection, easy application | Limited durability, environmentally unstable |
| 1800–1950 | Copper and lead-based marine paints | Effective, commercially available | Toxic to aquatic organisms |
| 1960–2000 | Paints with tributyltin (TBT) | Very high effectiveness | Very toxic Worldwide ban in 2008 |
| 2000–today | Self-polishing copolymers (SPCs) | Controlled release of biocides, stable performance | Still contain copper or other mild biocides |
| 2010–today | Silicone-based/fluoropolymer-based hydrophobic paints | Biocide free, very smooth surface, environmentally friendly | More precise, require high surface preparation |
| 2015–today | Biomimetic surfaces (e.g., SharkletTM, Aurora, CO, USA) | Chemical-free antifouling action | In development—cost, durability |
| 2020–today | Robotic cleaners (π.χ. HullWiper (Dubai, United Arab Emirates), ECOsubsea (Torangsvåg, Norway)) | No chemicals, repeated use | Requires frequent application—limitations in ports |
| 2020–today | UV illumination/electrochemical methods | Fully non-chemical solutions | Experimental stage |
| Sample Notation for Laboratory Tests | Sample Notation for In Situ Tests | Immersion Time (Weeks) | |
|---|---|---|---|
| Uncoated samples | L00 | S00 | 0 |
| L01 | S01 | 1 | |
| L02 | S02 | 2 | |
| L03 | S03 | 8 | |
| L04 | S04 | 17 | |
| L05 | S05 | 26 | |
| L06 | S06 | 50 | |
| Coated samples | L0 | S0 | 0 |
| L1 | S1 | 1 | |
| L2 | S2 | 2 | |
| L3 | S3 | 8 | |
| L4 | S4 | 17 | |
| L5 | S5 | 26 | |
| L6 | S6 | 50 |
| Peak’s Number | Peak’s Wavenumber | Identification of Peak | Ref. |
|---|---|---|---|
| 1 | 3407 cm−1 | N–H stretching bands of PAni | [44] |
| 2 | 3230 cm−1 | C–H stretching bands PAni | [44] |
| 3 | 1567 cm−1 | C=C stretching vibrations of quinoid ring of PAni | [45] |
| 4 | 1487 cm−1 | C=C stretching vibrations of benzenoid ring of PAni | [45] |
| 5 | 1298 cm−1 | C–N stretching modes of the benzenoid ring of PAni | [46] |
| 6 | 1246 cm−1 | C–N stretching modes of the benzenoid ring of PAni | [46] |
| 7 | 816 cm−1 | C–H out of plane bending vibrations of PAni | [47] |
| 8 | 1141 cm−1 | C–H in plane bending vibrations of PAni | [48] |
| 9 | 647 cm−1 | Ti–O–Ti stretching mode of anatase (TiO2) | [49] |
| Peak’s Number | Peak’s Wavenumber | Identification of Peak | Ref. |
|---|---|---|---|
| 1 | 1620 cm−1 | aromatic C=C stretching vibrations | [52] |
| 2 | 1400 cm−1 | carboxylic functionalized group | [53] |
| 3 | 597 cm−1 | Fe–O–Fe stretching vibrations | [54] |
| Sample Notation | Immersion Time t (Weeks) | Initial Weight wi (g) | Final Weight wf (g) | Weight Change W = wi − wf (g) | Weight Change (w/wi) × 100 (%) | Corrosion Rate CR (mm/Year) |
|---|---|---|---|---|---|---|
| L00 | 0 | 126.425 ± 0.004 | 126.425 ± 0.006 | 0.000 ± 0.007 | 0.000 | - |
| L01 | 1 | 126.267 ± 0.007 | 121.158 ± 0.004 | 5.109 ± 0.008 | 4.046 ± 0.002 | 21.401 ± 0.263 |
| L02 | 2 | 126.419 ± 0.009 | 119.259 ± 0.007 | 7.160 ± 0.011 | 5.664 ± 0.002 | 14.996 ± 0.186 |
| L03 | 8 | 126.697 ± 0.008 | 108.369 ± 0.005 | 18.328 ± 0.009 | 14.466 ± 0.001 | 9.597 ± 0.038 |
| L04 | 17 | 126.694 ± 0.007 | 101.236 ± 0.009 | 25.458 ± 0.011 | 20.094 ± 0.001 | 6.273 ± 0.022 |
| L05 | 26 | 126.412 ± 0.009 | 99.269 ± 0.007 | 27.143 ± 0.011 | 21.472 ± 0.000 | 4.373 ± 0.014 |
| L06 | 50 | 126.159 ± 0.008 | 98.369 ± 0.003 | 27.790 ± 0.009 | 22.028 ± 0.000 | 2.328 ± 0.006 |
| L0 | 0 | 132.759 ± 0.008 | 132.759 ± 0.008 | 0.000 | 0.000 | - |
| L1 | 1 | 132.459 ± 0.004 | 132.459 ± 0.009 | 0.000 | 0.000 | 0.000 |
| L2 | 2 | 132.691 ± 0.000 | 132.690 ± 0.000 | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.002 ± 0.000 |
| L3 | 8 | 132.698 ± 0.000 | 132.696 ± 0.000 | 0.002 ± 0.000 | 0.002 ± 0.000 | 0.001 ± 0.000 |
| L4 | 17 | 132.459 ± 0.000 | 132.449 ± 0.000 | 0.010 ± 0.000 | 0.008 ± 0.000 | 0.002 ± 0.000 |
| L5 | 26 | 132.784 ± 0.002 | 131.525 ± 0.003 | 1.259 ± 0.004 | 0.948 ± 0.003 | 0.203 ± 0.007 |
| L6 | 50 | 132.745 ± 0.001 | 130.476 ± 0.004 | 2.269 ± 0.004 | 1.709 ± 0.002 | 0.190 ± 0.005 |
| Locations | Parameters | S0 | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|---|
| Rafina | FR (%) | 100 | 100 | 99 | 99 | 99 | 99 | 99 |
| PDR (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| OP (%) | 100 | 100 | 99 | 99 | 99 | 99 | 99 | |
| Kalamata | FR (%) | 100 | 100 | 100 | 99 | 99 | 99 | 99 |
| PDR (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| OP (%) | 100 | 100 | 99 | 99 | 99 | 99 | 99 | |
| Andros | FR (%) | 100 | 100 | 99 | 99 | 99 | 99 | 99 |
| PDR (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| OP (%) | 100 | 100 | 99 | 99 | 99 | 99 | 99 |
| Material/System | Key Properties | Antifouling Mechanism | Advantages |
|---|---|---|---|
| PAni | Conductive | Disrupts biofoulant cell walls through redox cycling | Corrosion protection |
| TiO2 (anatase) | Photocatalytic | Generates reactive oxygen species (OH•−, O2•−) | |
| MWCNTs | Conductive | Anti-adhesion | Mechanical strength, electrical conductivity |
| Fe3O4 | Magnetic | Antibacterial | |
| PAni/TiO2 | Conductive + photocatalytic | reactive oxygen species generation | enhanced corrosion resistance |
| MWCNTs–Fe3O4 | Conductive + magnetic | Anti-adhesion | enhanced corrosion resistance |
| PAni–TiO2–MWCNTs–Fe3O4 | All-in-one system | Physical barrier | Broad-spectrum antifouling, corrosion resistance, mechanical strength |
| Antifouling Coating | Location | Conditions | Performance |
|---|---|---|---|
| Cooper biocide release | Rafina | In situ | 9-month coverage = 48% |
| Laboratory | 3.5% NaCl | maximum CR = 0.340 mm/y | |
| Self-polishing copolymer | Rafina | In situ | 9-month coverage = 92% |
| Laboratory | 3.5% NaCl | maximum CR = 1.600 mm/y | |
| Gradual polishing paint | Rafina | In situ | 9-month coverage = 92% |
| Laboratory | 3.5% NaCl | maximum CR = 2.600 mm/y | |
| This work | Rafina | In situ | 9-month coverage = 30% |
| Laboratory | ASW | maximum CR = 0.203 mm/y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vourna, P.; Falara, P.P.; Hristoforou, E.V.; Papadopoulos, N.D. Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment. Materials 2025, 18, 3095. https://doi.org/10.3390/ma18133095
Vourna P, Falara PP, Hristoforou EV, Papadopoulos ND. Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment. Materials. 2025; 18(13):3095. https://doi.org/10.3390/ma18133095
Chicago/Turabian StyleVourna, Polyxeni, Pinelopi P. Falara, Evangelos V. Hristoforou, and Nikolaos D. Papadopoulos. 2025. "Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment" Materials 18, no. 13: 3095. https://doi.org/10.3390/ma18133095
APA StyleVourna, P., Falara, P. P., Hristoforou, E. V., & Papadopoulos, N. D. (2025). Corrosion and Antifouling Behavior of a New Biocide-Free Antifouling Paint for Ship Hulls Under Both Artificially Simulated and Natural Marine Environment. Materials, 18(13), 3095. https://doi.org/10.3390/ma18133095












