Corrosion Behavior and Mechanism of 304 Stainless Steel Welded Joints in Simulated Freshwater Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results
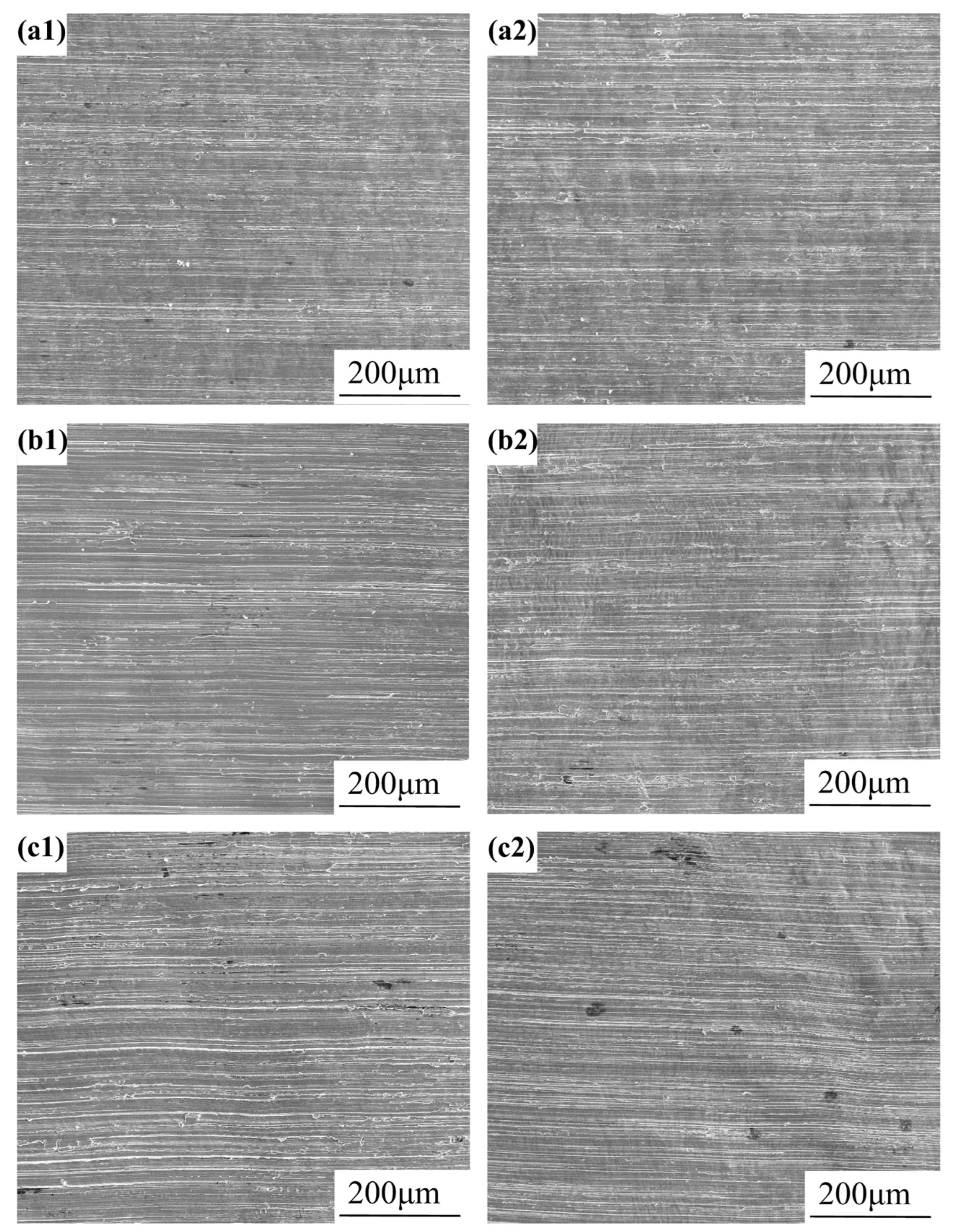
3.1. Metallographic Structure Observation
3.2. Corrosion Test Results
3.2.1. Dynamic Potential Reactivation Curve Test Results
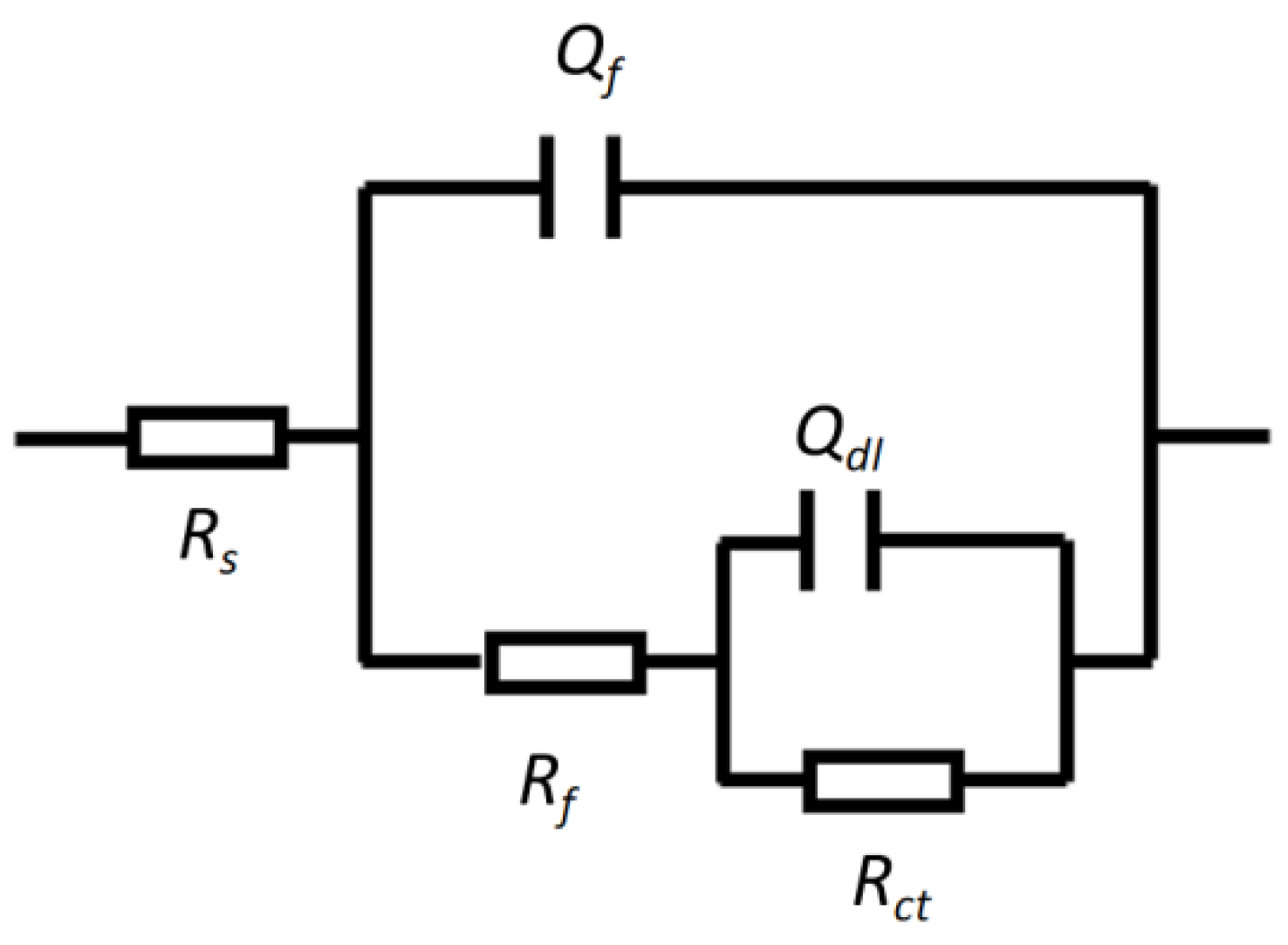
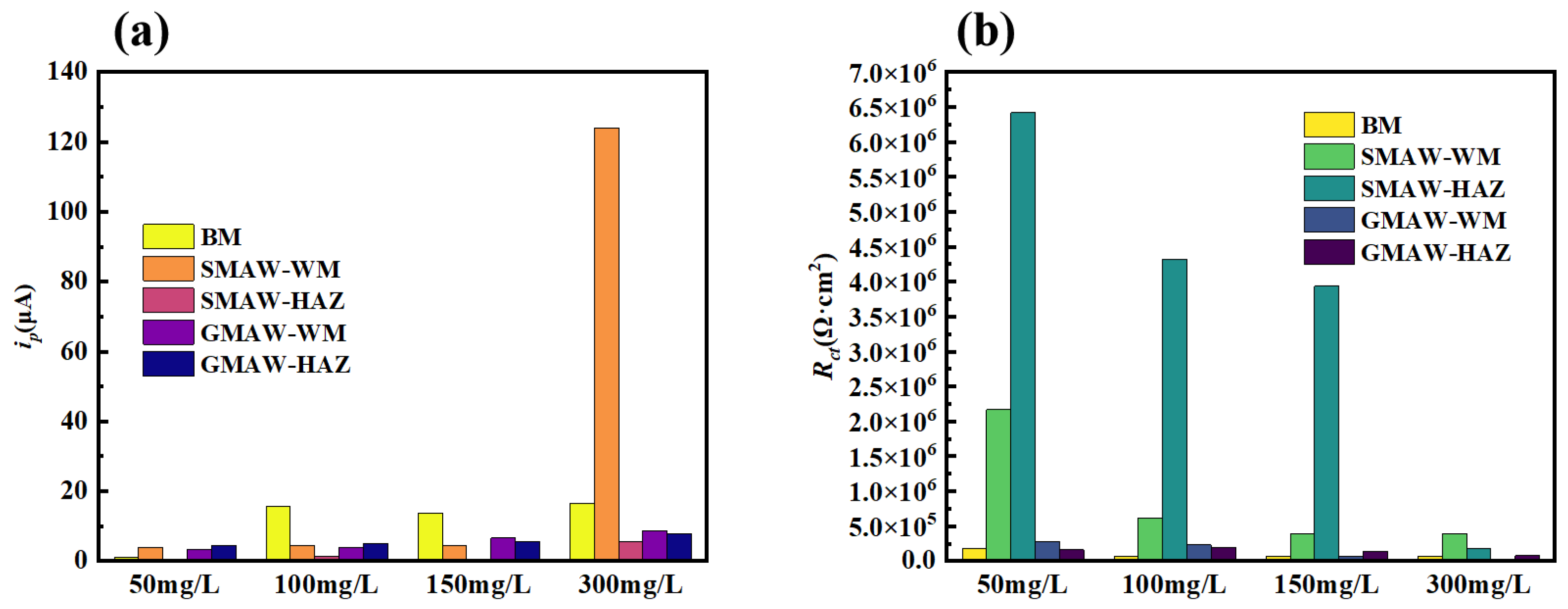
3.2.2. Electrochemical Test Results
3.2.3. Immersion Test Results
3.2.4. Stress Corrosion Performance Test Results
4. Discussion
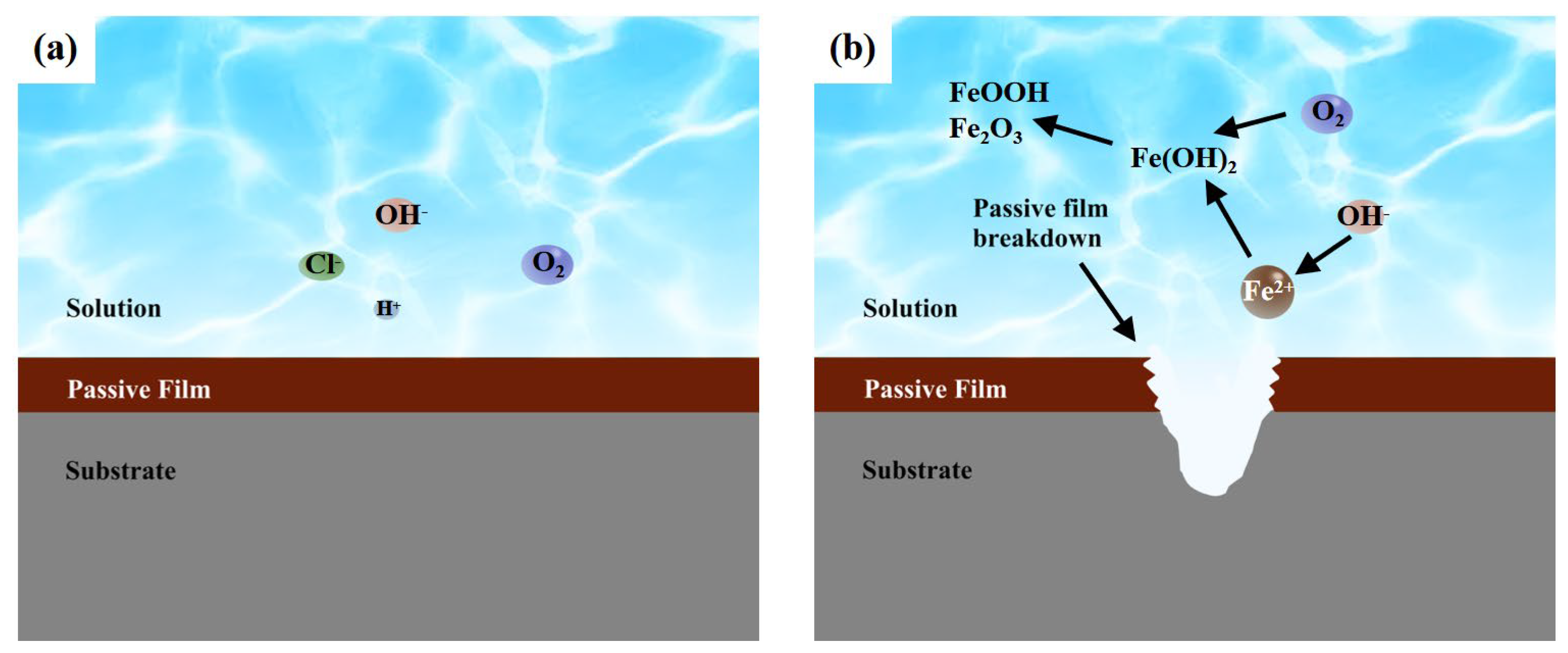
4.1. Corrosion Mechanism of 304 Stainless Steel Base Metal and Welded Joints in Environments with Different Cl− Concentrations
4.2. The Effect of the Welding Process on the Corrosion of the 304 Stainless Steel Base Metal and Welded Joints
4.3. The Effect of Stress on the 304 Stainless Steel BM and Welded Joints
5. Conclusions
- (1)
- The effect of chloride ions on the corrosion behavior of the 304 stainless steel substrate and welded joints is consistent at a concentration range of 50~300 mg/L. With the increase in the Cl− concentration, the corrosion resistance of the 304 stainless steel base metal and welded joints decreases.
- (2)
- All weldments showed a low intergranular corrosion (below 1%) susceptibility in the tested environment, with the HAZ exhibiting a higher DOS (0.196%, 0.0832%) than WM regions (0.4716%, 0.781). The DOS of the HAZ of the GMAW welded joints is about twice as much as that of the SMAW welded joints.
- (3)
- Both the BM and welded joints of the 304 stainless steel have a good corrosion resistance. The presence of different welding processes and stresses had little effect on the corrosion resistance of 304 stainless steel welded joints during the long-term immersion test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martins, C.M.B.; Moreira, J.L.; Martins, J.I. Corrosion in Water Supply Pipe Stainless Steel 304 and a Supply Line of Helium in Stainless Steel 316. Eng. Fail. Anal. 2014, 39, 65–71. [Google Scholar] [CrossRef]
- Alwin, B.; Lakshminarayanan, A.K.; Vasudevan, M.; Vasantharaja, P. Assessment of Stress Corrosion Cracking Resistance of Activated Tungsten Inert Gas-Welded Duplex Stainless Steel Joints. J. Mater. Eng Perform. 2017, 26, 5825–5836. [Google Scholar] [CrossRef]
- Sun, B.; Huang, X.; Pan, Y.; Yan, T.; Zhang, Y.; Sun, M.; Liu, Z.; Fan, L.; Li, X. A Comparative Study on the Passive Film and SCC Behavior of Ti-6Al-3Nb-2Zr-1Mo Alloy at Various Test Temperatures in Simulated Seawater. Corros. Sci. 2024, 233, 112066. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, K.H. Comparative Study on Girth Weld-Induced Residual Stresses between Austenitic and Duplex Stainless Steel Pipe Welds. Appl. Therm. Eng. 2014, 63, 140–150. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Lee, C.-G.; Yoon, H. Understanding the Relation between Pitting Corrosion Resistance and Phase Fraction of S32101 Duplex Stainless Steel. Corros. Sci. 2019, 149, 226–235. [Google Scholar] [CrossRef]
- Liu, P.; Liu, M.; Zheng, Q.; Xu, L.; Qiao, L.; Yan, Y. Study on the Failure Mechanism of the Heat-Affected Zone in 2205 Duplex Stainless Steel Weld-Joints Induced by Hydrogen. Corros. Sci. 2025, 244, 112657. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, B.; Wang, L.; Liu, Z.; Jiang, B.; Yang, W.; Deng, Y.; Li, X. Investigating the Correlation of Microstructure with Stress Corrosion Cracking of the High-Temperature Heat Treated 2205 Duplex Stainless Steel. J. Mater. Res. Technol. 2025, 35, 6280–6295. [Google Scholar] [CrossRef]
- Tang, W.; Chatzidakis, S.; Schrad, C.M.; Miller, R.G.; Howard, R. Study of Mechanical Properties, Microstructure, and Residual Stresses of AISI 304/304L Stainless Steel Submerged Arc Weld for Spent Fuel Dry Storage Systems. Metals 2024, 14, 262. [Google Scholar] [CrossRef]
- Kyung-Man, M.; Myung-Hoon, L. Corrosion Characteristics of Welding Zones by Laser and TIG Welding of 304 Stainless Steel. Corros. Sci. Technol. 2010, 6, 394–399. [Google Scholar]
- Ryl, J.; Wysocka, J.; Darowicki, K. Determination of causes of accelerated local corrosion of austenitic steels in water supply systems. Constr. Build. Mater. 2014, 64, 246–252. [Google Scholar] [CrossRef]
- Garcia, C.; Martin, F.; De Tiedra, P.; Blanco, Y.; Lopez, M. Pitting Corrosion of Welded Joints of Austenitic Stainless Steels Studied by Using an Electrochemical Minicell. Corros. Sci. 2008, 50, 1184–1194. [Google Scholar] [CrossRef]
- Garcia, C.; De Tiedra, M.P.; Blanco, Y.; Martin, O.; Martin, F. Intergranular Corrosion of Welded Joints of Austenitic Stainless Steels Studied by Using an Electrochemical Minicell. Corros. Sci. 2008, 50, 2390–2397. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Q.; Li, Y. Characterization of Microstructure, Mechanical Properties and Corrosion Resistance of Dissimilar Welded Joint between 2205 Duplex Stainless Steel and 16MnR. Mater. Des. 2011, 32, 831–837. [Google Scholar] [CrossRef]
- Bansod, A.V.; Patil, A.P.; Moon, A.P.; Shukla, S. Microstructural and Electrochemical Evaluation of Fusion Welded Low-Nickel and 304 SS at Different Heat Input. J. Mater. Eng. Perform. 2017, 26, 5847–5863. [Google Scholar] [CrossRef]
- Gucwa, M.; Winczek, J.; Giza, K.; Wieczorek, P.; Makles, K. The Effect of Welding Methods on the Corrosion Resistance of 304 Stainless Steel Joints. Acta Phys. Pol. A 2019, 135, 232–235. [Google Scholar] [CrossRef]
- Ge, G.; Tang, S.; Hu, J.; Huo, Y.; Qi, C.; Zhang, K.; Liu, Y.; Li, J. Influence of Arc Energy on the Microstructure and Corrosion Behavior of ER2209 Duplex Stainless Steel Deposited on Q345B Low-Alloy Steel by GMAW. Mater. Today Commun. 2024, 41, 110778. [Google Scholar] [CrossRef]
- Cho, D.M.; Park, J.S.; Hong, S.G.; Kim, S.J. Corrosion Behaviors According to the Welding Process of Superduplex Stainless Steel Welded Tubes: Gas Tungsten Arc Welding vs. Laser Beam Welding. Corros. Sci. 2023, 216, 111108. [Google Scholar] [CrossRef]
- Verma, J.; Taiwade, R.V.; Khatirkar, R.K.; Sapate, S.G.; Gaikwad, A.D. Microstructure, Mechanical and Intergranular Corrosion Behavior of Dissimilar DSS 2205 and ASS 316L Shielded Metal Arc Welds. Trans. Indian Inst. Met. 2017, 70, 225–237. [Google Scholar] [CrossRef]
- Jegdić, B.V.; Bobić, B.M.; Radojković, B.M.; Alić, B. Influence of the Welding Current Intensity and Nitrogen Content on the Corrosion Resistance of Austenitic Stainless Steels. Mater. Corros. 2018, 69, 1758–1769. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Raja, V.S.; Tewari, R.; Dey, G.K. Low Temperature Thermal Ageing Embrittlement of Austenitic Stainless Steel Welds and Its Electrochemical Assessment. Corros. Sci. 2012, 54, 278–290. [Google Scholar] [CrossRef]
- Ma, G.; Ling, X. Effect of Temperature and Chloride Content on the Stress Corrosion Cracking Susceptibility of 304 Stainless Steel Welded Joints Treated by Ultrasonic Impact Treatment. J. Press. Vessel Technol. 2013, 135, 051501. [Google Scholar] [CrossRef]
- Gao, S.; Dong, C.; Luo, H.; Xiao, K.; Li, X. Electrochemical Behavior and Nonlinear Mott-Schottky Characterization of a Stainless Steel Passive Film. Anal. Lett. 2014, 47, 1162–1181. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking Chloride Attack on the Passive Film of Metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16545-2015; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China: Beijing, China, 2015.
- Sun, B.; Zuo, X.; Cheng, X.; Li, X. The Role of Chromium Content in the Long-Term Atmospheric Corrosion Process. Npj Mater. Degrad. 2020, 4, 37. [Google Scholar] [CrossRef]
- Kadoi, K.; Mari, K.; Hiroshige, I. Effect of Ferrite Morphology on Pitting Corrosion Resistance of Austenitic Stainless Steel Weld Metals. Corros. Sci. 2023, 221, 111356. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Q.; Pan, Y.; Liu, Z.; Du, C.; Li, X. Understanding the Non-Steady Electrochemical Mechanism on SCC of 304 SS under Applied Polarization Potentials. Corros. Sci. 2024, 227, 111686. [Google Scholar] [CrossRef]
- Jung, R.-H.; Tsuchiya, H.; Fujimoto, S. Growth Process of Passive Films on Austenitic Stainless Steels under Wet-Dry Cyclic Condition. ISIJ Int. 2012, 52, 1356–1361. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sun, A. An Electrochemical Impedance Spectroscopic Study of the Passive State on Alloy-22. Electrochim. Acta 2006, 51, 1767–1779. [Google Scholar] [CrossRef]
- Li, K.; Sun, L.; Cao, W.; Chen, S.; Chen, Z.; Wang, Y.; Li, W. Pitting Corrosion of 304 Stainless Steel in Secondary Water Supply System. Corros. Commun. 2022, 7, 43–50. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance Investigation of Thermally Formed Oxide Films on AISI 304L Stainless Steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Meng, S.; Yue, P.; Zhang, S.; Li, H.; Zhao, Y.; Hua, Y.; Zhang, T.; Wang, F. Synergistic Effects of CO2 and H2S on Stress Corrosion Cracking of Stainless Steel 254SMo in Extremely Aggressive Oilfield Environment. Corros. Commun. 2024, 16, 81–95. [Google Scholar] [CrossRef]
- Pereira, R.D.S.; Droppa, R.; de Oliveira, M.C.L.; Antunes, R.A. Effect of Milling Parameters on the Stability of the Passive Film of AISI 304 Stainless Steel. J. Mater. Eng. Perform. 2021, 30, 8131–8144. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, B.; Aday, X. Impact of Strain on the Corrosion Resistance of 304 Stainless Steel Welded Joints Using Electrochemical Methods and Numerical Modeling of Stress Corrosion. Int. J. Electrochem. Sci. 2025, 20, 100910. [Google Scholar] [CrossRef]
- Naskar, A.; Bhattacharyya, M.; Jana, S.; Darsell, J.; Raja, K.S.; Charit, I. Chloride-Induced Stress Corrosion Cracking of Friction Stir-Welded 304L Stainless Steel: Effect of Microstructure and Temperature. Crystals 2024, 14, 556. [Google Scholar] [CrossRef]
- Nuthalapati, S.; Kee, K.E.; Pedapati, S.R.; Jumbri, K. A Review of Chloride Induced Stress Corrosion Cracking Characterization in Austenitic Stainless Steels Using Acoustic Emission Technique. Nucl. Eng. Technol. 2024, 56, 688–706. [Google Scholar] [CrossRef]

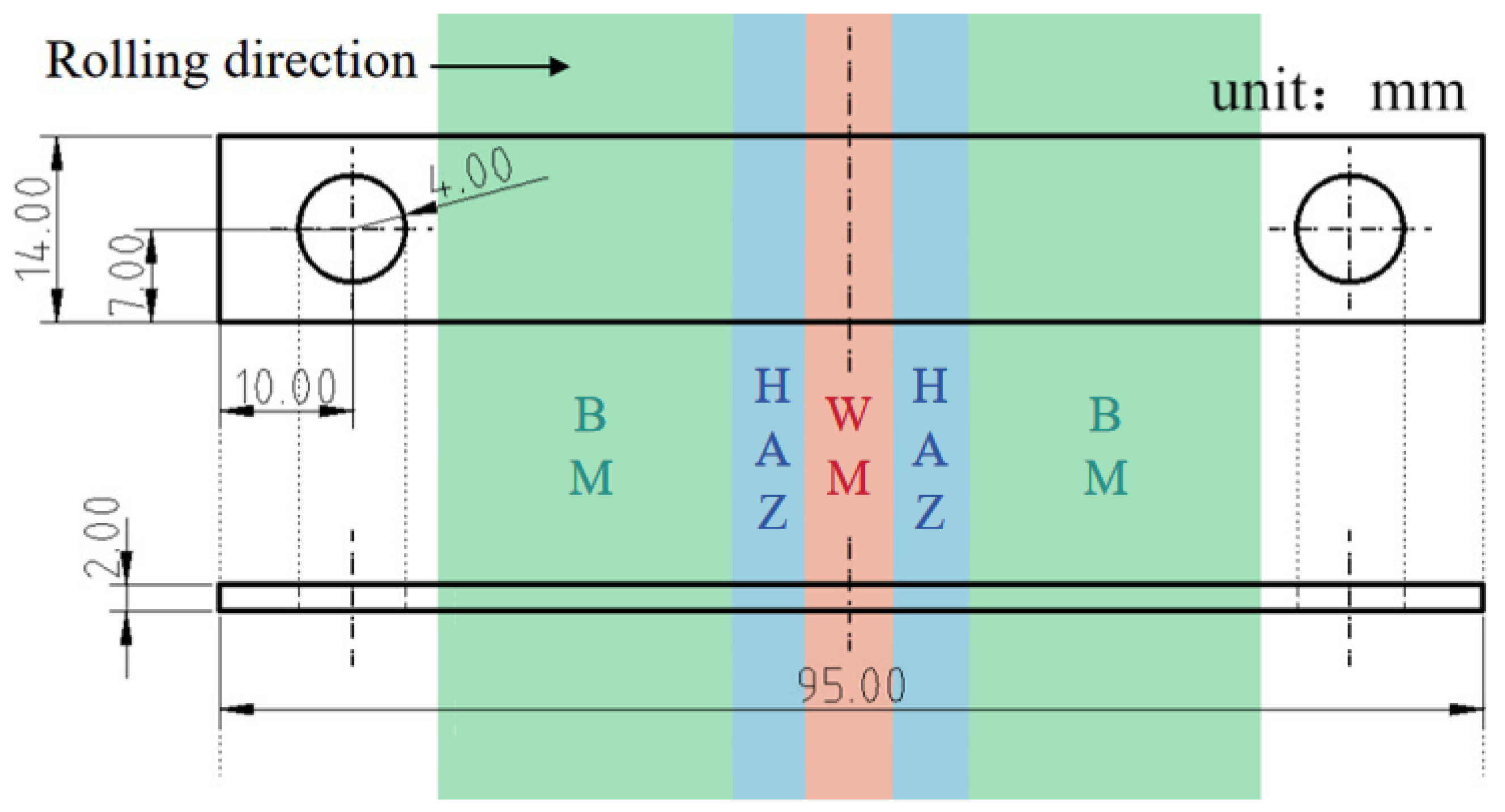
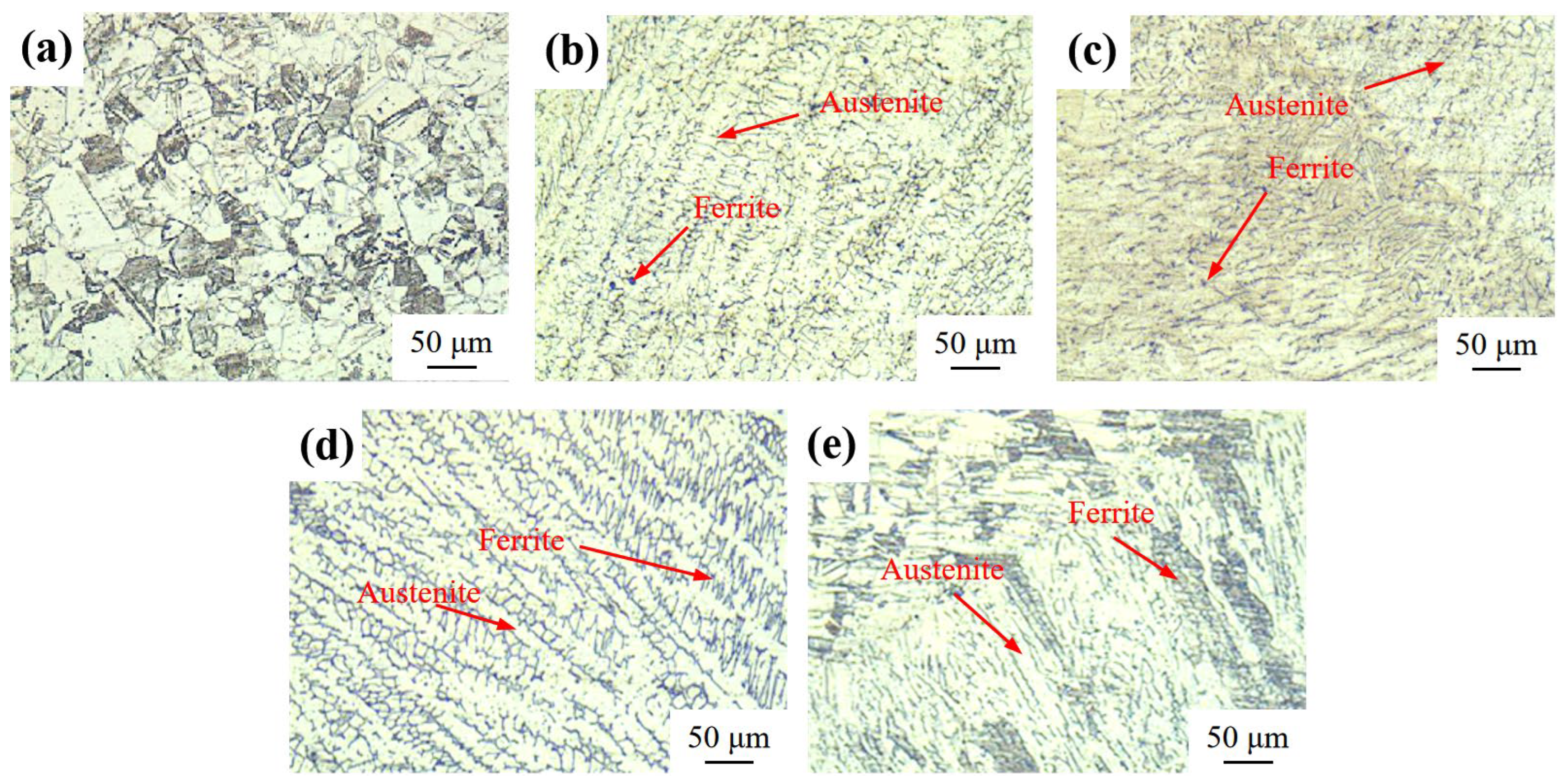
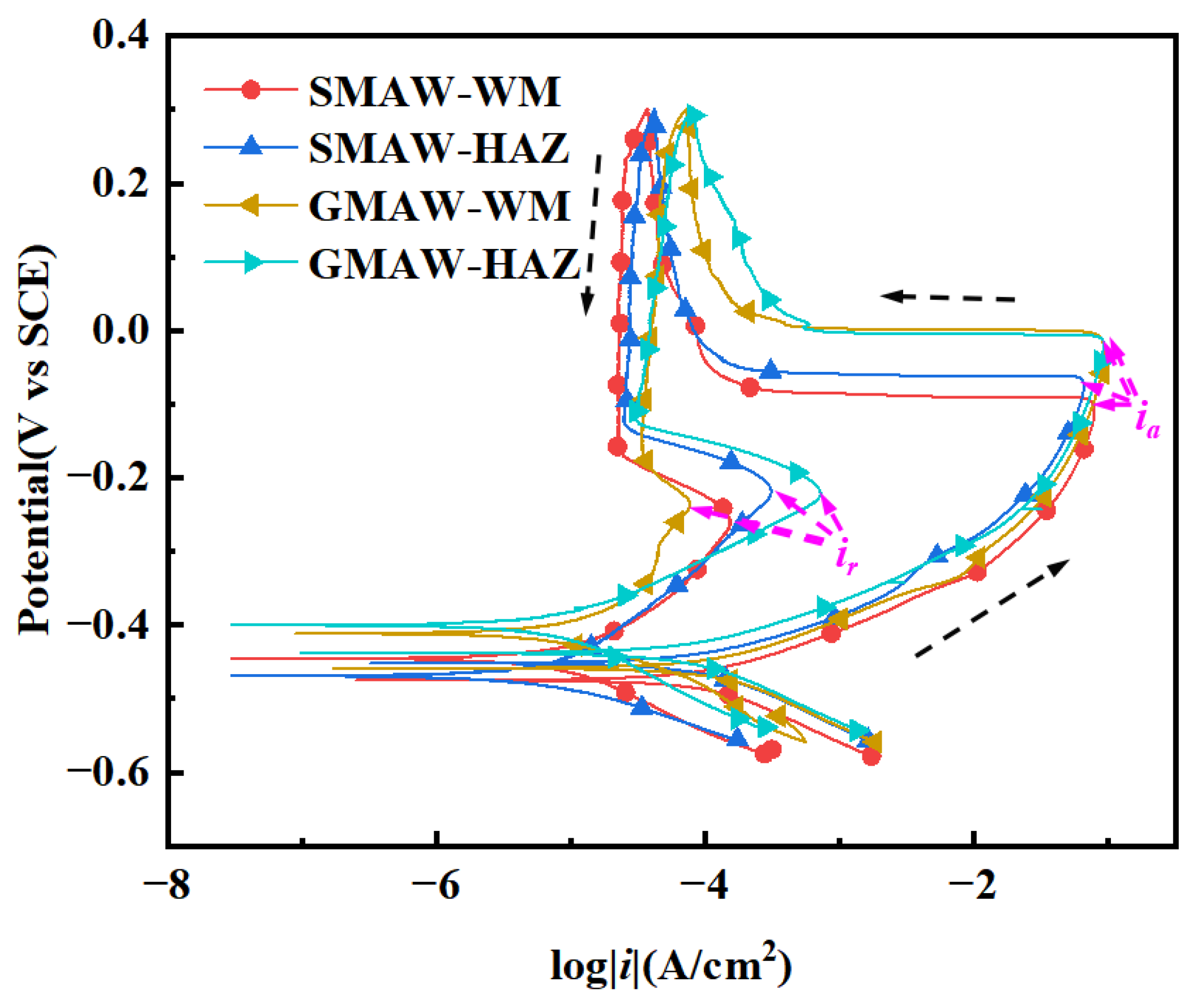
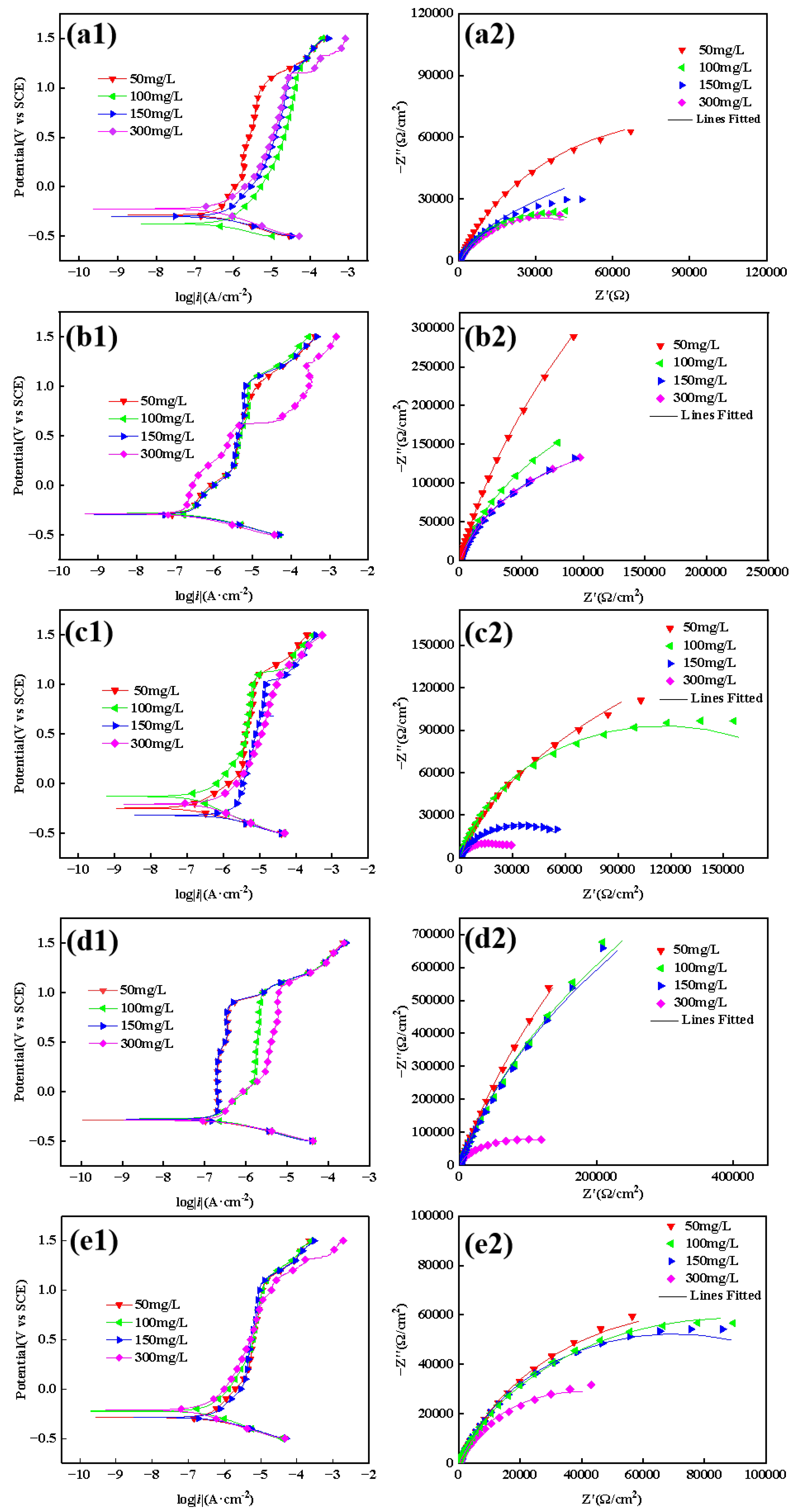
| C | Si | Mn | P | S | Cr | Ni | Mo | Cu | |
|---|---|---|---|---|---|---|---|---|---|
| A102 | 0.08 | 1.00 | 0.5–2.5 | 0.04 | 0.03 | 18.0–21.0 | 9.0–11.0 | 0.75 | 0.75 |
| ER308L | 0.03 | 0.60 | 1.80 | 0.015 | 0.008 | 20.0 | 10.0 | - | - |
| CaCl2 | MgCl2 | KCl | NaHCO3 | Na2SO4 | NaCl | |
|---|---|---|---|---|---|---|
| 50 | 29.31 | 37.99 | 6.00 | 262.54 | 193.31 | / |
| 100 | 29.31 | 37.99 | 6.00 | 262.54 | 192.31 | 82.39 |
| 150 | 29.31 | 37.99 | 6.00 | 262.54 | 192.31 | 165.67 |
| 300 | 29.31 | 37.99 | 6.00 | 262.54 | 192.31 | 412.95 |
| Welding Method | Microstructure | DOS (%) |
|---|---|---|
| 304-SMAW | WM | 0.196 |
| HAZ | 0.4716 | |
| 304-GMAW | WM | 0.0832 |
| HAZ | 0.781 |
| Cl− Concentration | BM Corrosion Rate (mm/a) | SMAW Corrosion Rate (mm/a) | GMAW Corrosion Rate (mm/a) |
|---|---|---|---|
| 100 mg/L | 0.00 | 0.00 | 0.00 |
| 300 mg/L | 1.13 × 10−3 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Wang, X.; Wei, S.; Chen, Z.; Wang, Z.; Li, M.; Liu, Z. Corrosion Behavior and Mechanism of 304 Stainless Steel Welded Joints in Simulated Freshwater Environment. Materials 2025, 18, 3074. https://doi.org/10.3390/ma18133074
Yu Y, Wang X, Wei S, Chen Z, Wang Z, Li M, Liu Z. Corrosion Behavior and Mechanism of 304 Stainless Steel Welded Joints in Simulated Freshwater Environment. Materials. 2025; 18(13):3074. https://doi.org/10.3390/ma18133074
Chicago/Turabian StyleYu, Yue, Xiayan Wang, Shilong Wei, Zengyao Chen, Zhanhua Wang, Mengnan Li, and Zhiyong Liu. 2025. "Corrosion Behavior and Mechanism of 304 Stainless Steel Welded Joints in Simulated Freshwater Environment" Materials 18, no. 13: 3074. https://doi.org/10.3390/ma18133074
APA StyleYu, Y., Wang, X., Wei, S., Chen, Z., Wang, Z., Li, M., & Liu, Z. (2025). Corrosion Behavior and Mechanism of 304 Stainless Steel Welded Joints in Simulated Freshwater Environment. Materials, 18(13), 3074. https://doi.org/10.3390/ma18133074






