Microstructural Stability and High-Temperature Mechanical Behavior of Al–Ni–Zr Alloy Strengthened by L12-Al3Zr Precipitates
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Chemical Composition
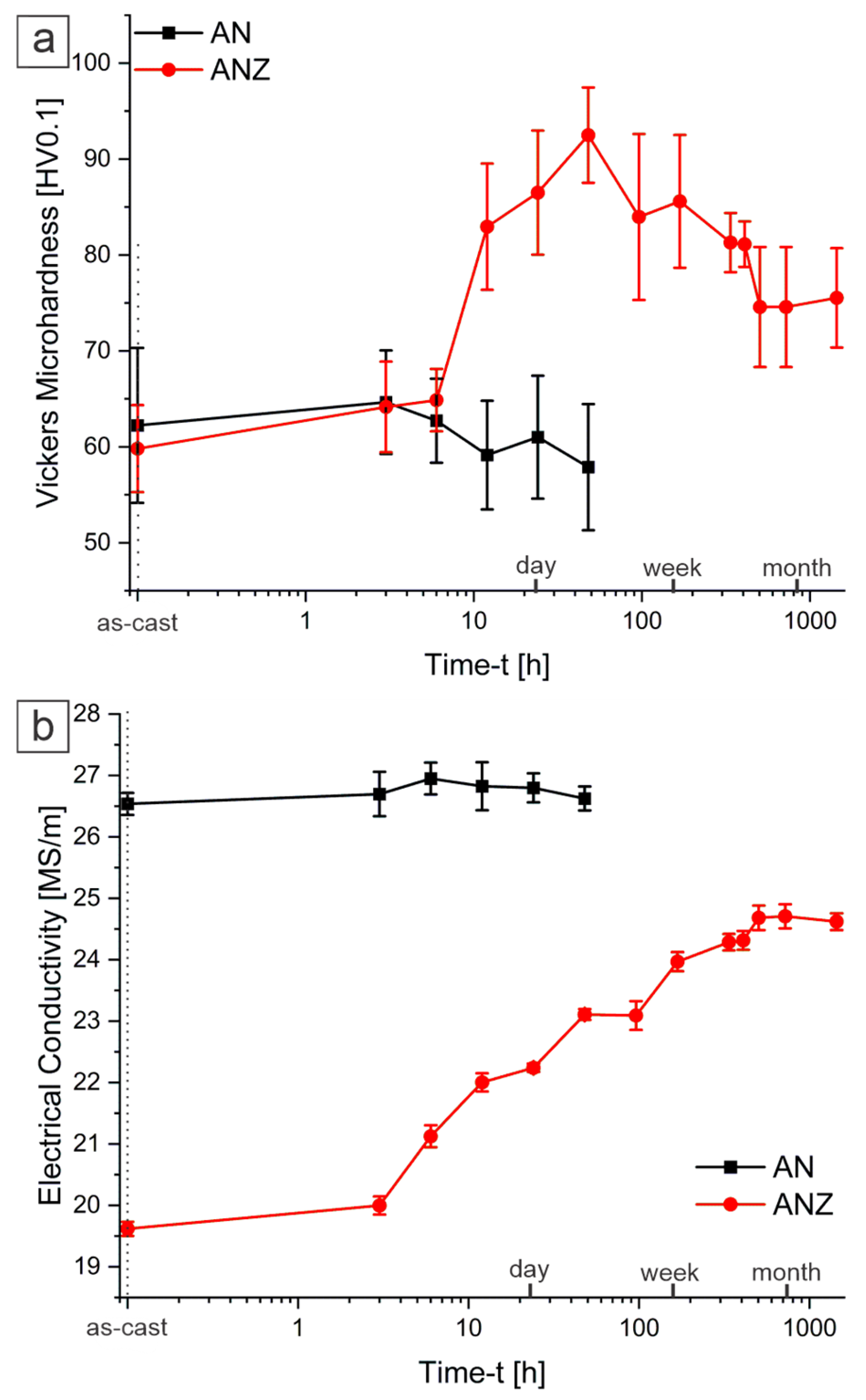
3.2. Microhardness and Electrical Conductivity
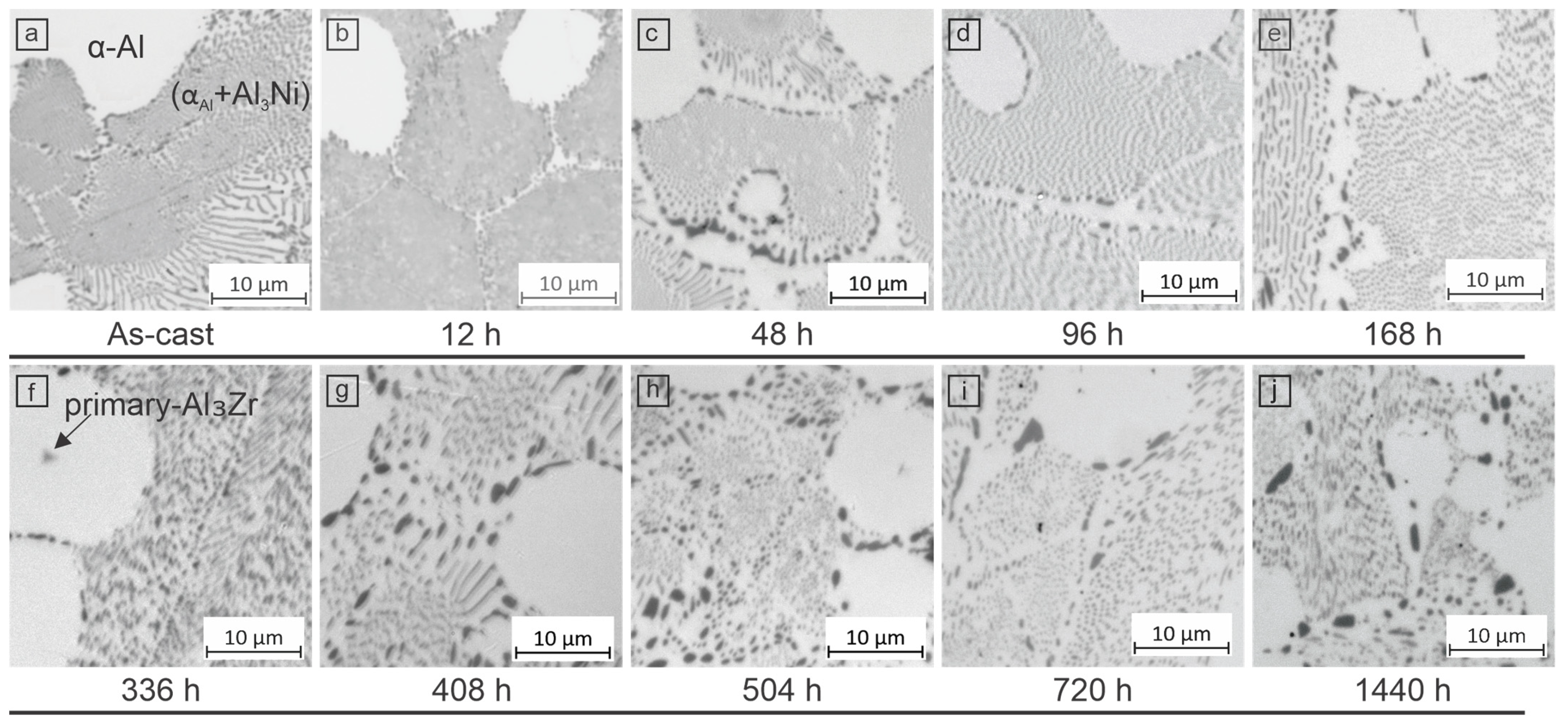
3.3. Microstructure
3.3.1. Optical Microscopy (LOM)
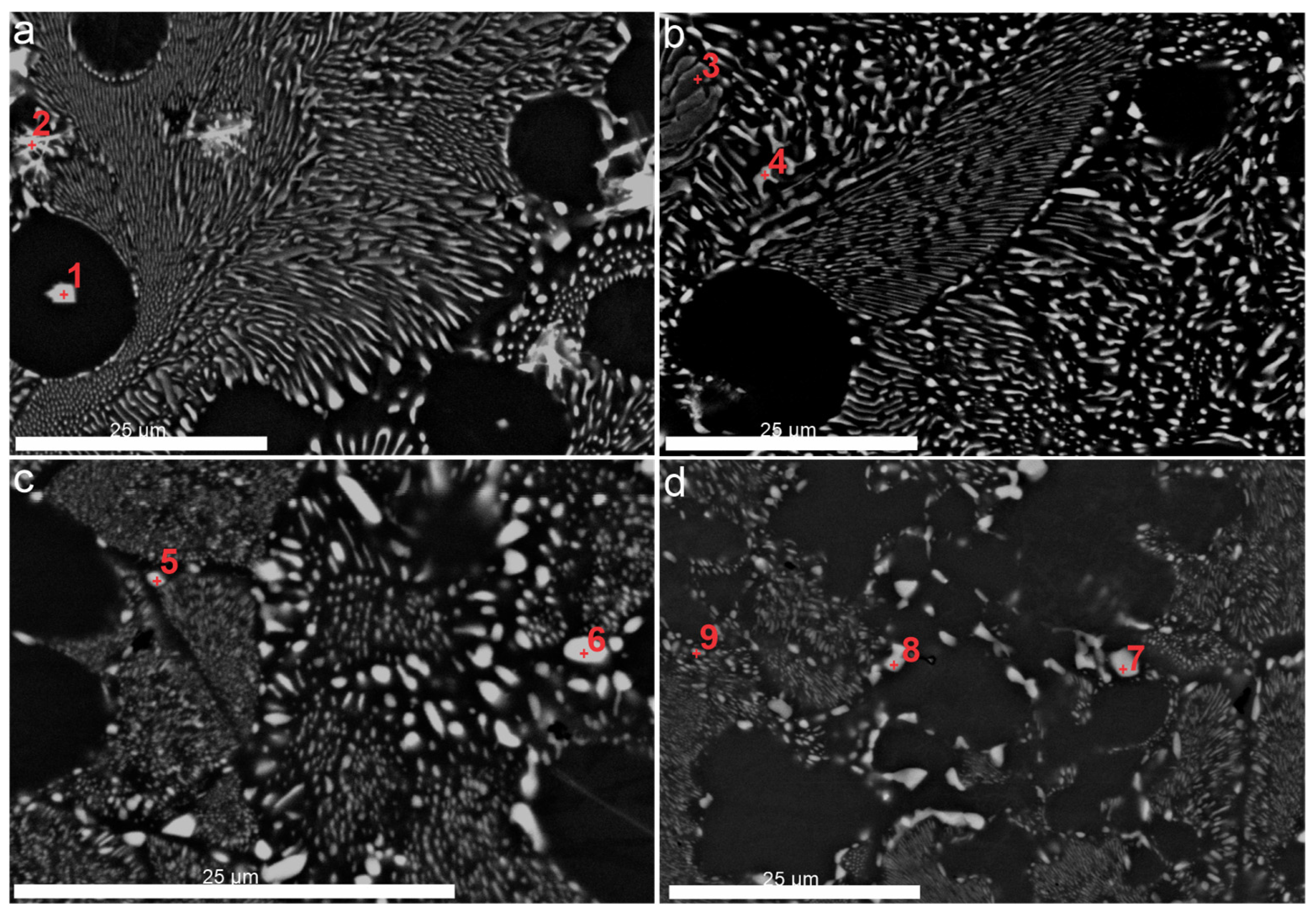
3.3.2. Scanning Electron Microscopy (SEM)
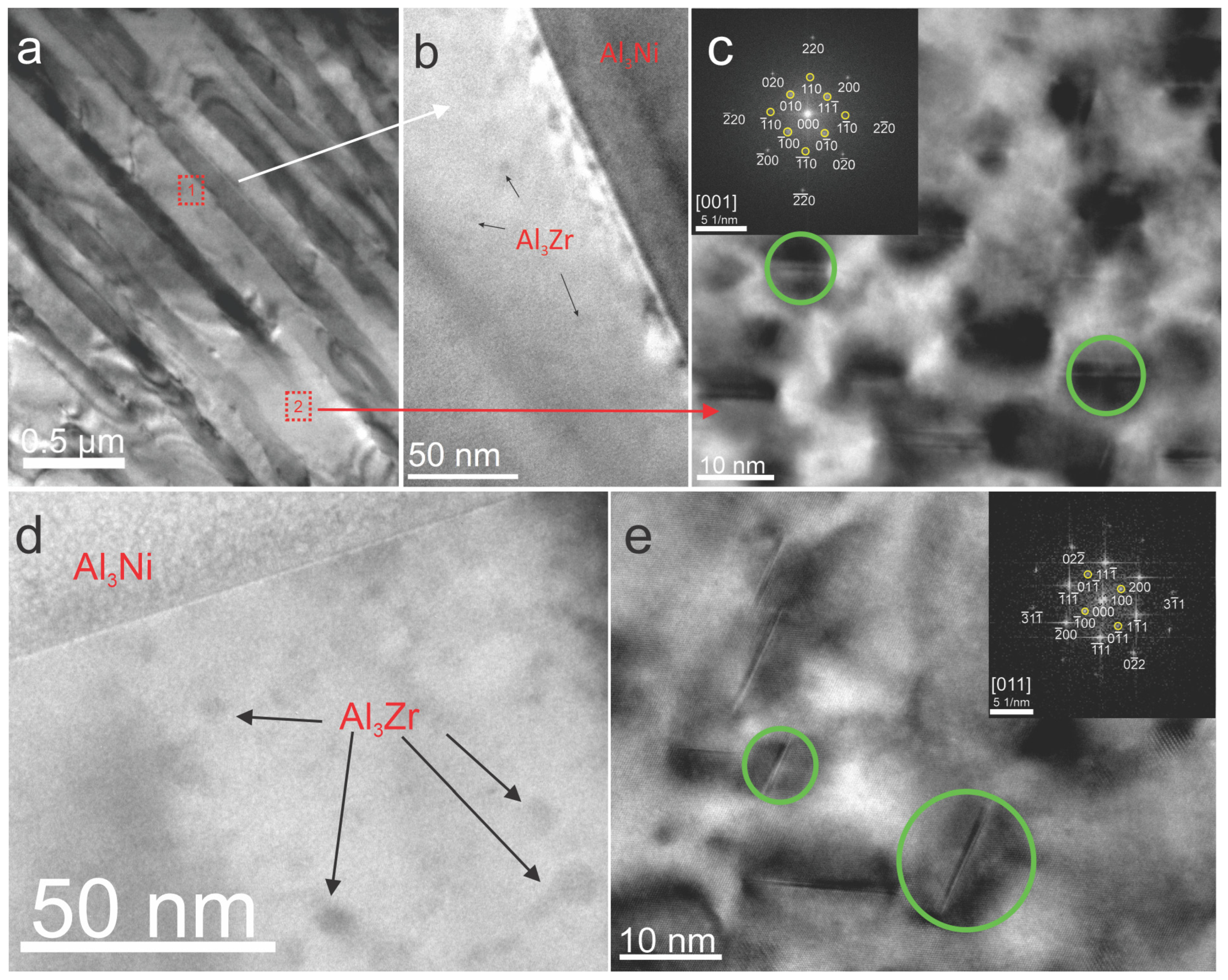
3.3.3. Transmission Electron Microscopy (TEM)
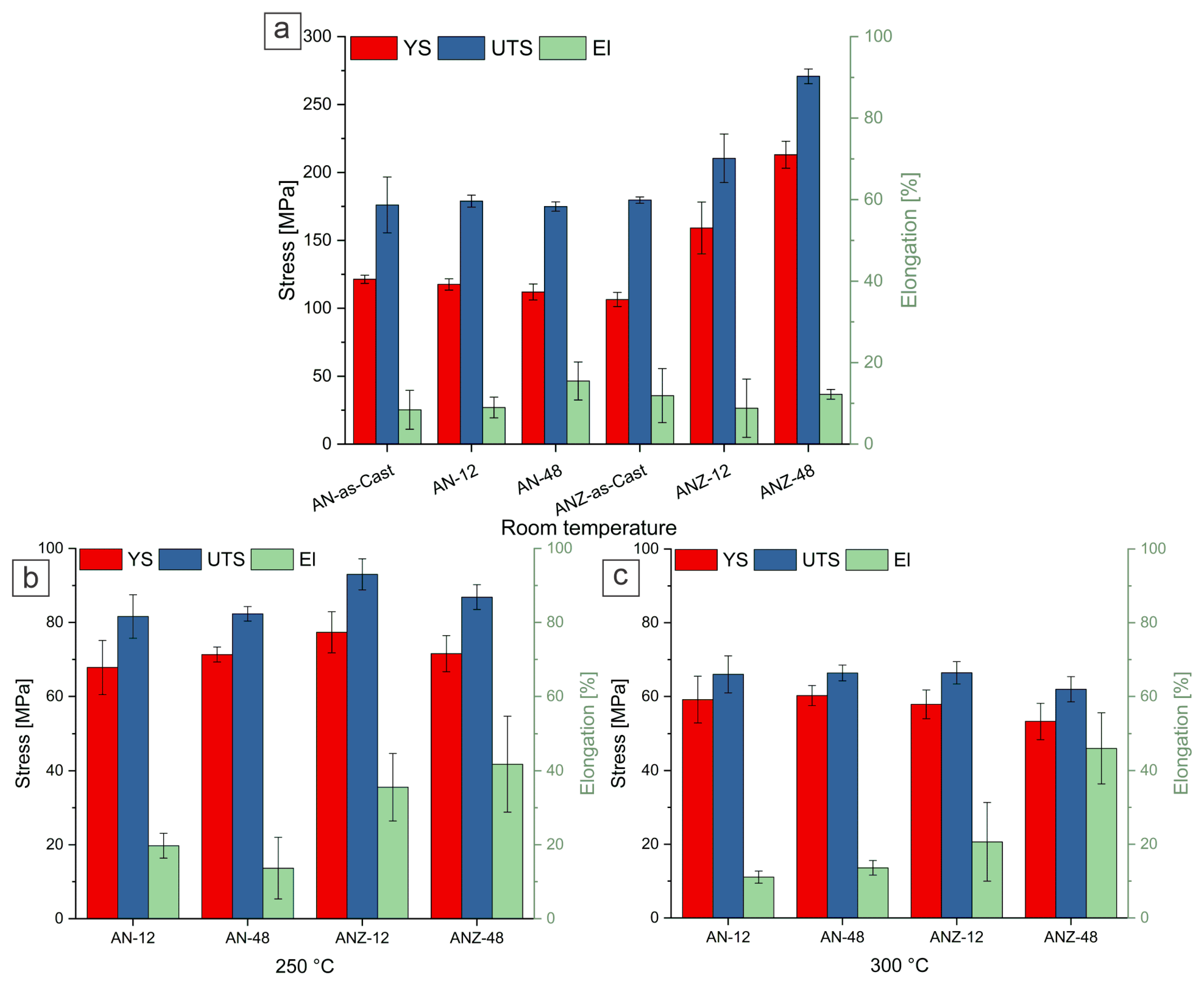
3.4. Tensile Tests
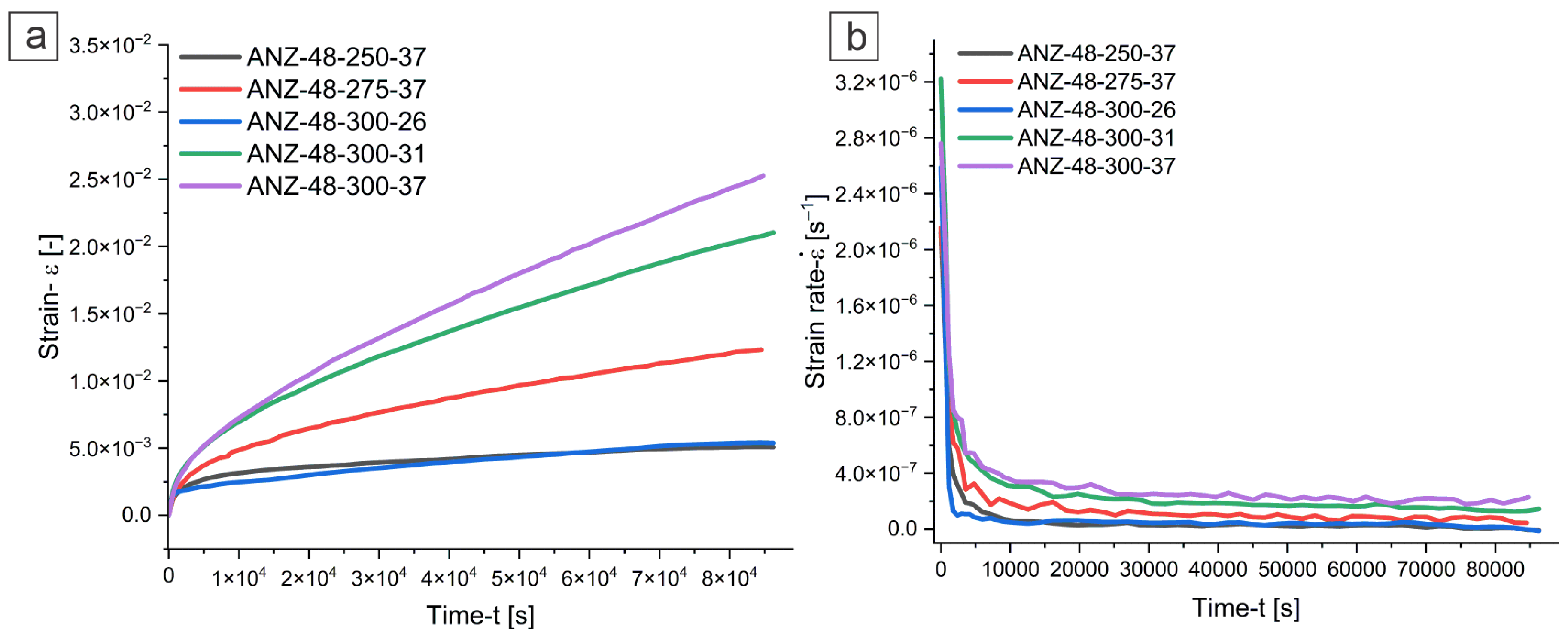
3.5. Accelerated Compressive Creep Test
4. Conclusions
- The introduction of 0.6 wt.% Zr into the eutectic Al–Ni system leads to an increased proportion of equiaxed αAl dendrites (~30%). Aging alloy at 350 °C leads to a peak in microhardness at 92 HV0.1 after 48 h. Extended aging results in the partial coarsening of the microstructure, with the microhardness stabilizing at 75 HV0.1 after 500 h.
- Upon aging, coherent L12-Al3Zr precipitates with an average radius of r = 2.1 ± 1.6 nm were observed at peak aging conditions (350 °C, 48 h). Their high resistance to coarsening is evident after 30 days of aging, where the average radius of the precipitates increased only slightly to r = 3.1 ± 1.6 nm, while coherence with the matrix was maintained.
- The tensile tests of the peak-aged ANZ-48 sample revealed a significant increase in yield strength from 106 MPa to 213 MPa at room temperature. However, this increase is not retained at elevated temperatures (300 °C), where the yield strength drops to 53 MPa. The YS value is in a similar range to the alloy without Zr addition.
- Accelerated compressive creep tests indicated that the AN alloy exhibits a creep-dominated mechanism of pipe diffusion and limited viscous dislocation glide, whereas the ANZ alloy follows a power-law creep regime under the conditions tested.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arriaga-Benitez, R.I.; Pekguleryuz, M. Recent Progress in Creep-Resistant Aluminum Alloys for Diesel Engine Applications: A Review. Materials 2024, 17, 3076. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Makhlouf, M.M. The Al-Al3Ni Eutectic Reaction: Crystallography and Mechanism of Formation. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 3808–3812. [Google Scholar] [CrossRef]
- Weiss, D. Improved High-Temperature Aluminum Alloys Containing Cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Naumova, E.A.; Lukina, E.A.; Zavodov, A.V.; Khatkevich, V.M. High Strength Al–La, Al–Ce, and Al–Ni Eutectic Aluminum Alloys Obtained by High-Pressure Torsion. Materials 2021, 14, 6404. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Xiao, D.; Wu, M.; Huang, Y.; Liu, W. Enhanced Strength of Al-10Ce-3Mg-5Zn Heat-Resistant Alloy by Combining Extrusion and Heat Treatment. Materials 2025, 18, 1706. [Google Scholar] [CrossRef]
- Kozakevich, J.R.; Sediako, D.; Weiss, D.; Vogel, S.C. A Quantitative Phase Analysis by Neutron Diffraction of Conventional and Advanced Aluminum Alloys Thermally Conditioned for Elevated-Temperature Applications. Materials 2024, 17, 4311. [Google Scholar] [CrossRef]
- Doty, H.W.; El-Hadad, S.; Samuel, E.; Samuel, A.M.; Samuel, F.H. The Influence of Rare Earth Metals on the Microstructure and Mechanical Properties of 220 and 356.1 Alloys for Automotive Industry. Materials 2025, 18, 941. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Makhlouf, M.M. Rendering Wrought Aluminium Alloys Castable by Means of Minimum Composition Adjustments. Int. J. Cast Met. Res. 2017, 30, 231–243. [Google Scholar] [CrossRef]
- Carrara, A.P.; Kakitani, R.; Garcia, A.; Cheung, N. Effect of Cooling Rate on Microstructure and Microhardness of Hypereutectic Al–Ni Alloy. Arch. Civ. Mech. Eng. 2021, 21, 14. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal Stability of Aluminum-Nickel Binary Alloys Containing the Al-Al3Ni Eutectic. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2021, 52, 4342–4356. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kashyap, S.; Kim, D.H.; Chattopadhyay, K. Al Based Ultra-Fine Eutectic with High Room Temperature Plasticity and Elevated Temperature Strength. Mater. Sci. Eng. A 2015, 639, 359–369. [Google Scholar] [CrossRef]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for Developing Castable, Creep-Resistant Aluminum-Based Alloys—A Review. Int. J. Mater. Res. 2006, 97, 246–265. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, F.; Wang, Y.; Jin, Y.; Chang, X.; Chen, G. Microstructure and Mechanical Property of 6082 Aluminum Alloy via Sc and Zr Addition Combined with Squeeze Casting. Materials 2025, 18, 1988. [Google Scholar] [CrossRef] [PubMed]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Precipitation Evolution in Al-Zr and Al-Zr-Ti Alloys during Isothermal Aging at 375–425 °C. Acta Mater. 2008, 56, 114–127. [Google Scholar] [CrossRef]
- Fan, Y.; Makhlouf, M.M. The Effect of Introducing the Al-Ni Eutectic Composition into Al-Zr-V Alloys on Microstructure and Tensile Properties. Mater. Sci. Eng. A 2016, 654, 228–235. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Nanoscale Structural Evolution of Al3Sc Precipitates in Al(Sc) Alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef]
- Booth-Morrison, C.; Dunand, D.C.; Seidman, D.N. Coarsening Resistance at 400 °C of Precipitation-Strengthened Al-Zr-Sc-Er Alloys. Acta Mater. 2011, 59, 7029–7042. [Google Scholar] [CrossRef]
- Elasheri, A.; Elgallad, E.M.; Parson, N.; Chen, X.G. Evolution of Zr-Bearing Dispersoids during Homogenization and Their Effects on Hot Deformation and Recrystallization Resistance in Al-0.8%Mg-1.0%Si Alloy. J. Mater. Eng. Perform. 2021, 30, 7851–7862. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, G.; Chen, X.G. Effects of Two-Step Homogenization on Precipitation Behavior of Al3Zr Dispersoids and Recrystallization Resistance in 7150 Aluminum Alloy. Mater. Charact. 2015, 102, 122–130. [Google Scholar] [CrossRef]
- Booth-Morrison, C.; Seidman, D.N.; Dunand, D.C. Effect of Er Additions on Ambient and High-Temperature Strength of Precipitation-Strengthened Al-Zr-Sc-Si Alloys. Acta Mater. 2012, 60, 3643–3654. [Google Scholar] [CrossRef]
- Gao, Y.H.; Kuang, J.; Zhang, J.Y.; Liu, G.; Sun, J. Tailoring Precipitation Strategy to Optimize Microstructural Evolution, Aging Hardening and Creep Resistance in an Al–Cu–Sc Alloy by Isochronal Aging. Mater. Sci. Eng. A 2020, 795, 139943. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Toinin, J.P.; Michi, R.A.; Pandee, P.; Dunand, D.C.; Limmaneevichitr, C. Strengthening Mechanisms in Al–Ni–Sc Alloys Containing Al3Ni Microfibers and Al3Sc Nanoprecipitates. Acta Mater. 2019, 164, 334–346. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Pandee, P.; Patakham, U.; Dunand, D.C.; Limmaneevichitr, C. Effects of Zr Additions on Structure and Microhardness Evolution of Eutectic Al-6ni Alloy. In Light Metals 2019; Minerals, Metals and Materials Series; Springer: Cham, Switzerland, 2019; pp. 373–377. [Google Scholar]
- Belov, N.A. Aluminium Casting Alloys with High Content of Zirconium. Mater. Sci. Forum 1996, 217–222, 293–298. [Google Scholar] [CrossRef]
- Šmalc, J.; Zaky, A.; Markoli, B.; Šturm, R. The Impact of Small Zr Addition to Al–Ni Cast Alloy for Elevated Temperature Applications. J. Mater. Res. Technol. 2024, 32, 1928–1936. [Google Scholar] [CrossRef]
- ASTM E8M-04; Standard Test Methods for Tension Testing of Metallic Materials [Metric] (Withdrawn 2008). ASTM International: West Conshohocken, PA, USA, 2010.
- Michi, R.A.; Toinin, J.P.; Seidman, D.N.; Dunand, D.C. Ambient- and Elevated-Temperature Strengthening by Al3Zr-Nanoprecipitates and Al3Ni-Microfibers in a Cast Al-2.9Ni-0.11Zr-0.02Si-0.005Er (at.%) Alloy. Mater. Sci. Eng. A 2019, 759, 78–89. [Google Scholar] [CrossRef]
- Belov, N.; Zolotorevskij, V.S.; Goto, S.; Alabin, A.N.; Istomin-kastrovskij, V.V.; Mishin, V.I. Effect of Zirconium on Liquidus and Hardening of Al-6% Ni Casting Alloy. In Proceedings of the Materials Forum, Brisbane, Australia, 2–5 August 2004; Volume 28, pp. 533–538. [Google Scholar]
- Kakitani, R.; Reyes, R.V.; Garcia, A.; Spinelli, J.E.; Cheung, N. Relationship between Spacing of Eutectic Colonies and Tensile Properties of Transient Directionally Solidified Al-Ni Eutectic Alloy. J. Alloys Compd. 2018, 733, 59–68. [Google Scholar] [CrossRef]
- Khvan, A.V.; Eskin, D.G.; Starodub, K.F.; Dinsdale, A.T.; Wang, F.; Fang, C.; Cheverikin, V.V.; Gorshenkov, M.V. New Insights into Solidification and Phase Equilibria in the Al-Al3Zr System: Theoretical and Experimental Investigations. J. Alloys Compd. 2018, 743, 626–638. [Google Scholar] [CrossRef]
- Pandey, P.; Makineni, S.K.; Gault, B.; Chattopadhyay, K. On the Origin of a Remarkable Increase in the Strength and Stability of an Al Rich Al-Ni Eutectic Alloy by Zr Addition. Acta Mater. 2019, 170, 205–217. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Makhlouf, M.M. Alternatives to the Al–Si Eutectic System in Aluminum Casting Alloys. Int. J. Met. 2016, 10, 342–347. [Google Scholar] [CrossRef]
- Frost, H.J.; Ashby, M.F. Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics; Franklin Book Company: Elkins Park, PA, USA, 1982. [Google Scholar]
- Himbeault, D.D.; Cahoon, J.R. Creep Regimes for Directionally Solidified Al-Al3Ni Eutectic Composite. Metall. Trans. A 1993, 24, 2721–2730. [Google Scholar] [CrossRef]
- Kassner, M.E. New Developments in Understanding Harper–Dorn, Five-Power Law Creep and Power-Law Breakdown. Metals 2020, 10, 1284. [Google Scholar] [CrossRef]


| Expected Composition | Ni | Zr | Fe | Al | Designation |
|---|---|---|---|---|---|
| Al-6.1Ni | 6.41 ± 0.14 | 0.00 | 0.14 ± 0.02 | bal. | AN |
| Al-6.1Ni-0.6Zr | 6.28 ± 0.01 | 0.56 ± 0.1 | 0.11 ± 0.05 | bal. | ANZ |
| Spectrum Label | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Al | 77.4 | 75.9 | 91.1 | 84.0 | 82.9 | 68.7 | 72.3 | 73.5 | 83.2 |
| Ni | 0.8 | 7.2 | 8.5 | 15.5 | 15.3 | 31.2 | 27.5 | 26.3 | 16.6 |
| Fe | - | - | 0.1 | 0.2 | 1.8 | 0.1 | 0.2 | 0.2 | 0.2 |
| Zr | 21.6 | 16.4 | 0.3 | 0.3 | - | - | - | - | - |
| Si | 0.2 | 0.5 | - | - | - | - | - | - | - |
| Temperature T [°C] | Stress σ [MPa] | [s−1] | ||
|---|---|---|---|---|
| AN | ANZ-12 | ANZ-48 | ||
| 250 | 37 | 6.57 × 10−9 | 6.30 × 10−9 | 1.15 × 10−8 |
| 275 | 37 | 8.21 × 10−9 | 3.70 × 10−8 | 7.22 × 10−8 |
| 300 | 26 | 1.53 × 10−8 | 2.34 × 10−8 | 1.79 × 10−8 |
| 300 | 31 | 1.09 × 10−8 | 4.85 × 10−8 | 1.39 × 10−7 |
| 300 | 37 | 3.79 × 10−8 | 2.28 × 10−7 | 2.00 × 10−7 |
| Qc-Activation creep energy [kJ] | 86.2 | 178.8 | 142.9 | |
| n-stress exponent | 3 | 6 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šmalc, J.; Zaky, A.; Markoli, B.; Šturm, R. Microstructural Stability and High-Temperature Mechanical Behavior of Al–Ni–Zr Alloy Strengthened by L12-Al3Zr Precipitates. Materials 2025, 18, 3068. https://doi.org/10.3390/ma18133068
Šmalc J, Zaky A, Markoli B, Šturm R. Microstructural Stability and High-Temperature Mechanical Behavior of Al–Ni–Zr Alloy Strengthened by L12-Al3Zr Precipitates. Materials. 2025; 18(13):3068. https://doi.org/10.3390/ma18133068
Chicago/Turabian StyleŠmalc, Jan, Adam Zaky, Boštjan Markoli, and Roman Šturm. 2025. "Microstructural Stability and High-Temperature Mechanical Behavior of Al–Ni–Zr Alloy Strengthened by L12-Al3Zr Precipitates" Materials 18, no. 13: 3068. https://doi.org/10.3390/ma18133068
APA StyleŠmalc, J., Zaky, A., Markoli, B., & Šturm, R. (2025). Microstructural Stability and High-Temperature Mechanical Behavior of Al–Ni–Zr Alloy Strengthened by L12-Al3Zr Precipitates. Materials, 18(13), 3068. https://doi.org/10.3390/ma18133068







