Abstract
Developing efficient and durable electrocatalysts for the alkaline hydrogen evolution reaction (HER) is crucial for sustainable hydrogen production. Herein, we report a novel RuP2/Mn2P2O7 heterojunction anchored on a three-dimensional nitrogen and phosphorus co-doped porous carbon (RuP2/Mn2P2O7/NPC) framework as a high-performance HER catalyst, synthesized via a controlled pyrolysis–phosphidation strategy. The heterostructure achieves uniform dispersion of ultrafine RuP2/Mn2P2O7 heterojunctions with well-defined interfaces. Furthermore, phosphorus doping restructures the electronic configuration of Mn and Ru species at the RuP2/Mn2P2O7 heterointerface, enabling enhanced catalytic activity through the accelerated electron transfer and kinetics of the HER. This RuP2/Mn2P2O7/NPC catalyst exhibits exceptional HER activity with 1 M KOH, requiring only 69 mV of overpotential to deliver 10 mA·cm−2 and displaying a small Tafel slope of 69 mV·dec−1, rivaling commercial 20% Pt/C. Stability tests reveal negligible activity loss over 48 h, underscoring the robustness of the heterostructure. The RuP2/Mn2P2O7 heterojunction demonstrates markedly reduced overpotentials for the electrochemical HER process, highlighting its enhanced catalytic efficiency and improved cost-effectiveness compared to the conventional catalytic systems. This work establishes a strategy for designing a transition metal phosphide heterostructure through interfacial electronic modulation, offering broad implications for energy conversion technologies.
1. Introduction
Hydrogen energy has emerged as a global research focus in the ongoing energy transition, owing to its high density and clean combustion properties [1,2]. Among various hydrogen production technologies, water electrolysis has attracted considerable attention due to its efficiency and sustainability [3,4,5,6,7]. As a critical half-reaction in this process, the hydrogen evolution reaction (HER) plays a pivotal role, with the performance of HER catalysts directly influencing the overall efficiency and cost of hydrogen production [8,9,10,11]. Current studies have shown that platinum (Pt)-based catalysts exhibit outstanding HER activity in both acidic and alkaline electrolytes [12]. However, their high cost and limited natural reserves pose significant barriers to large-scale commercialization [13,14]. Consequently, the development of alternative catalysts based on transition metals with excellent catalytic performance has emerged as a critical area of research in the field of new energy materials [15,16].
Ruthenium (Ru)-based materials, with advantages such as high activity and low cost, are expected to become effective alternatives to Pt-based catalysts in alkaline HER [17,18,19]. However, in alkaline environments, Ru-based electrocatalysts frequently exhibit an electron-rich state, leading to strong adsorption of active hydrogen species (*H) and, consequently, hindering HER activity [20,21,22]. Transition metal phosphides (TMPs) have attracted considerable attention for the hydrogen evolution reaction (HER) under alkaline conditions, which can be attributed to their unique electronic structures and promising catalytic properties [23,24,25,26]. Recent studies have shown that the construction of metallic phosphide active sites can optimize the adsorption of reaction intermediates, thereby enhancing HER activity [27,28]. However, monometallic phosphide catalysts still suffer from several limitations, including a limited number of active sites, suboptimal electron transfer efficiency, and poor long-term stability [29]. To overcome these challenges, the construction of heterostructures has emerged as an effective strategy [30,31]. Through rationally integrating different metal phosphides, the complementary properties of each component can be exploited to achieve synergistic catalytic effects. Such heterostructures can increase both the number and diversity of active sites, while electronic interactions at the interface optimize charge distribution and facilitate charge transfer—thereby significantly enhancing overall catalytic performance [32]. Despite these advantages, ultrafine TMPs tend to agglomerate due to their high surface free energy [33]. Consequently, the controllable synthesis of stably dispersed TMPs nanoclusters remains a major challenge for efficient HER catalysis.
In this work, we fabricated a RuP2/Mn2P2O7 heterojunction supported on a three-dimensional nitrogen and phosphorus co-doped porous carbon (RuP2/Mn2P2O7/NPC) matrix. The three-dimensional-NPC not only provides excellent electrical conductivity and structural stability, but also offers abundant anchoring sites for the uniform dispersion of metal phosphides, thereby enhancing the utilization of active components. Electrochemical investigations confirmed that phosphorus doping and the formation of the RuP2/Mn2P2O7 heterojunction enhance HER performance via increasing the electrochemically active surface area (ECSA), requiring a low overpotential of 69 mV at 10 mA·cm−2 and exhibiting an ultra-low Tafel slope of 69 mV·dec−1 under alkaline conditions. Furthermore, precise control over the phosphorus precursor concentration enables the formation of the RuP2/Mn2P2O7 heterojunction, which facilitates optimized electron and proton transport pathways during the HER process, thereby significantly improving catalytic activity in alkaline media. Notably, the catalyst demonstrates HER performance comparable to that of commercial 20% Pt/C, along with excellent electrochemical stability. This work offers an effective synthetic strategy for the development of highly active and durable catalysts for alkaline HER applications.
2. Experimental Procedure
2.1. Chemicals
All chemical reagents were of analytical grade and used without further purification. Melamine (99%) was purchased from Energy Chemical (Nottinghamshire, UK). Ruthenium (III) chloride trihydrate (98%), manganese acetate (98%), and ammonium dihydrogen phosphate (AR) were purchased from Aladdin Chemical Co., Ltd. (Shanghai, China). Manganese acetate was purchased from Innochem (Pyeongtaek, Republic of Korea). The 5% Nafion-521 dispersion was purchased from Alfa Aesar (Waltham, MA, USA). Potassium hydroxide was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Nafion-117 membrane was purchased from DuPont (Wilmington, DE, USA). High-purity Ar gas (99.99%) was purchased from Beijing Millennium Capital Gas Co., Ltd. (Beijing, China).
2.2. Synthesis of RuP2/Mn2P2O7/NPC and Control Catalysts
The synthesis of catalysts was carried out through the direct pyrolysis of pre-organized precursors. For RuP2/Mn2P2O7/NPC, melamine foam (3 cm × 5 cm× 5 cm) was ultrasonically cleaned in ethanol and deionized water for 30 min. After drying for 12 h at 80 °C, the melamine foam substrate was immersed in an aqueous solution containing melamine powder, ruthenium trichloride trihydrate, and manganese acetate in a molar ratio of 1.1:1:1, followed by the addition of 100 mM ammonium dihydrogen phosphate. After stirring for 24 h, the impregnated melamine foam was dried at 80 °C until complete solvent evaporation was achieved. The obtained melamine foam was carbonized by heating at 500 °C for 2 h and then at 800 °C for 2 h with a heating rate of 3 °C/min under N2 atmosphere. The product was etched with 1 M HCl solution for 12 h at room temperature and dried to obtain the final RuP2/Mn2P2O7/NPC catalyst.
RuMn@NP50C and RuMn@NP150C were synthesized following the same procedure, except that the concentrations of ammonium dihydrogen phosphate were 50 and 150 mM, respectively. The synthesis of RuMn@NC followed the same procedure as that of RuP2/Mn2P2O7/NPC, except that no ammonium dihydrogen phosphate was added during the precursor preparation step. All other reagents, processing conditions, and pyrolysis steps remained unchanged. In addition, the single-metal catalysts (Mn@NP100C and Ru@NP100C) were prepared following the same procedure, except that only the corresponding metal precursor was added. The metal-free NP100C catalyst was synthesized following an identical procedure but without the addition of a metal precursor or ammonium dihydrogen phosphate solution.
2.3. Characterization
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were obtained via Hitachi S-4800 (JEOL, Tokyo, Japan) (at 30 kV) and JEM-2100F (JEOL, Tokyo, Japan) (at 300 kV), respectively. Powder X-ray diffraction (PXRD) patterns were collected on a RigakuD/max 2500 Pc using Cu Kα radiation (λ = 1.5406 Å) generated by a Cu X-ray tube operating at 40 kV and 40 mA. X-ray photoelectron spectroscopy (XPS) measurements were performed on a VG-ESCALAB250XI system (Thermo Scientific, Waltham, MA, USA). The particle sizes were measured and statistically analyzed using Nano Measurer 1.2.5 software based on the high-resolution TEM images.
2.4. Electrocatalytic Measurement
The electrochemical hydrogen evolution reaction performance of RuP2/Mn2P2O7/NPC was evaluated in a customized H-type cell under ambient conditions using a three-electrode configuration. The system consisted of a carbon fiber paper working electrode, a platinum foil counter electrode (1 cm × 1 cm), and a saturated Hg/Hg2Cl2 (SCE) reference electrode. Electrochemical measurements were recorded using a CHI730e workstation (Shanghai Chenhua Instrument Co., Ltd., Shanghai, China). The catalyst dispersion was prepared by dispersing 2 mg of catalyst powder in a mixture of 220 µL isopropyl alcohol, 720 µL deionized water, and 60 µL Nafion-521 solution, followed by sonication for 1 h. The resulting catalyst ink was drop-cast onto either a glassy carbon electrode or a carbon paper electrode (1 × 1 cm2). The electrolyte solutions were tested in 1 M KOH. Each compartment of the H-type cell contained 45 mL of electrolyte, which was purged with high-purity Argon for 15 min before measurements. Linear sweep voltammetry (LSV) was performed at a scan rate of 10 mV/s, which was employed to directly determine the overpotential (η) and the reaction kinetics of the HER. The electrochemical active surface area (ECSA), a critical indicator of the density of active sites, was calculated using the following equation:
where the Cdl is the double-layer capacitance, and Csp is the specific capacitance per unit area-typically ranging from 40 μF·cm−2.
3. Results and Discussion
3.1. Synthesis and Characterization of RuP2/Mn2P2O7/NPC Catalyst
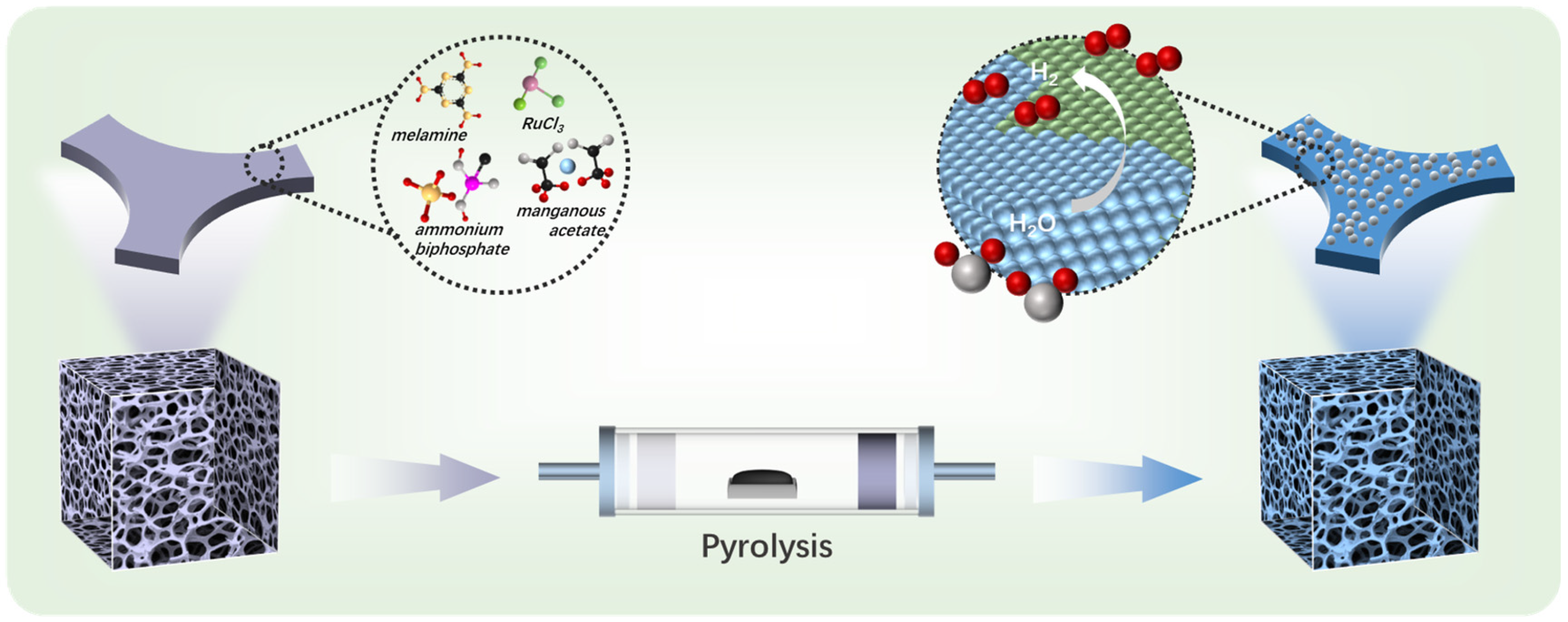
The RuP2/Mn2P2O7 heterojunction catalyst was synthesized through a high-temperature pyrolysis–phosphidation strategy using a melamine foam precursor pre-adsorbed with ruthenium and manganese ions. The precursor mixture consisted of melamine powder, ruthenium trichloride trihydrate, manganese acetate, ammonium dihydrogen phosphate, and melamine foam. The melamine powder served as the polymerization precursor, metal salts provided metal ions, the ammonium dihydrogen phosphate acted as a phosphorus source, and the melamine foam (MF) functioned as a template for creating hierarchical porous structures. Melamine foam, a lightweight porous material synthesized from melamine resin with a three-dimensional interconnected macroporous architecture and micrometer-scale surface asperities, demonstrates promising potential as a catalyst carrier capable of adsorbing significant quantities of metal precursors, making it promising for the synthesis of hierarchical porous structures. As illustrated in Scheme 1, the process began with melamine foam adsorbing metal ions, ammonium dihydrogen phosphate, and melamine at room temperature, forming RuxMny(PO4)Z/MF precursors. As the pyrolysis temperature increases, RuxMny(PO4)Z/MF precursors undergo further carbonization, ultimately forming a highly graphitized nitrogen-doped carbon framework loaded with a RuP2/Mn2P2O7 heterojunction under 800 °C. The final RuP2/Mn2P2O7/NPC catalyst with a hierarchical porous structure was obtained after HCl etching to remove the metal salts. The control catalysts (RuMn@NP50C, RuMn@NP150C, Mn@NP100C, Ru@NP100C, and NP100C) were synthesized following the same procedure with different precursors. By systematically varying the concentration of ammonium dihydrogen phosphate during synthesis (50, 100, and 150 mM), we found that only at 100 mM does the RuP2/Mn2P2O7 heterojunction form successfully. Lower concentrations lead to the incomplete transformation of Ru and Mn species, while higher concentrations promote the formation of amorphous or disordered phosphate phases that disrupt the crystalline heterostructure. This behavior likely arises from the balance of precursor stoichiometry, pyrolysis kinetics, and spatial confinement within the N,P-doped carbon matrix, which collectively influence phase selectivity and crystallinity [34,35,36,37].
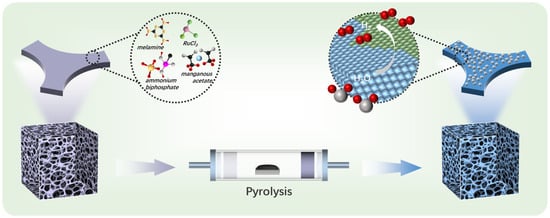
Scheme 1.
Schematic illustration of the preparation of RuP2/Mn2P2O7/NPC via a high-temperature pyrolysis–phosphidation strategy.
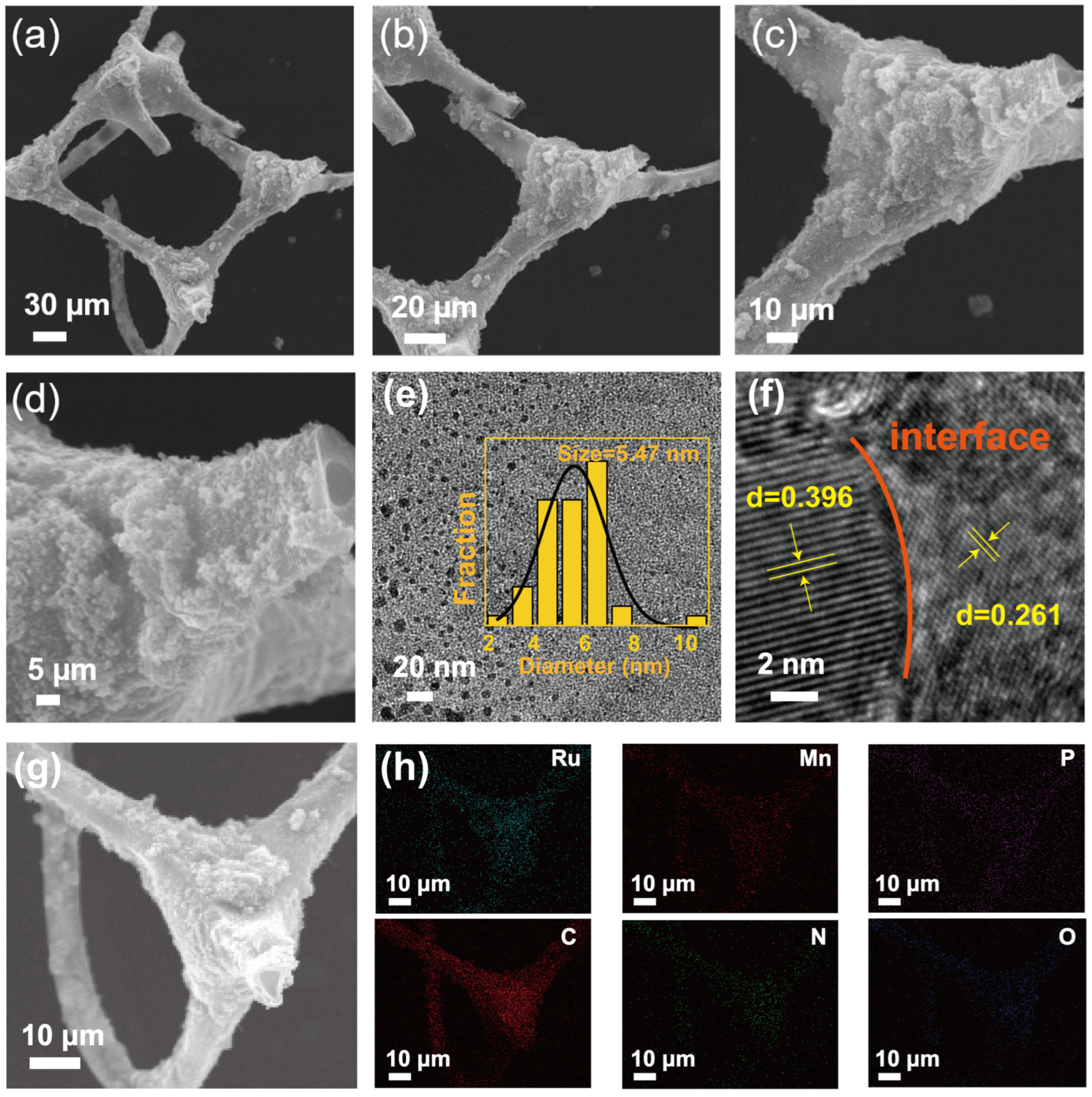
The SEM images reveal that RuP2/Mn2P2O7/NPC possesses a three-dimensional nitrogen-doped porous carbon framework. As shown in Figure 1a–d, RuP2/Mn2P2O7 was uniformly dispersed on the porous carbon framework, and the MF maintained the three-dimensional porous structure after high-temperature heat treatment. The highly porous architecture facilitates efficient mass transport, promotes rapid electrolyte penetration, and enables effective gas bubble dissipation during HER processes. To facilitate an in-depth examination of the morphological properties, high-resolution transmission electron microscopy (HRTEM) was carried out to further characterize the microstructure of the RuP2/Mn2P2O7/NPC. Specifically, the particle size distribution presented in Figure 1e was obtained by analyzing more than 100 nanoparticles from randomly selected regions of high-resolution TEM images using Nano Measurer 1.2.5 software. RuP2/Mn2P2O7 nanoparticles with an average size of 5.47 nm were generated in the RuP2/Mn2P2O7/NPC, and they were highly uniformly distributed on the nitrogen-doped porous carbon framework (insert graph of Figure 1e). The precision fabrication of highly dispersed RuP2/Mn2P2O7 nanoclusters benefited from the coordination confinement of P atoms enabling the isolation of the ruthenium and manganese precursors, effectively confining their migration and agglomeration during pyrolysis. Moreover, Figure 1f distinctly reveals the coexistence of two well-defined phases along with their interfacial region. More specifically, the HRTEM analysis quantitatively confirmed the lattice spacings of 0.396 nm and 0.261 nm in the RuP2/Mn2P2O7/NPC composite, which were unambiguously assigned to the (110) plane of RuP2 (PDF#71-0167) and the (130) plane of Mn2P2O7 (PDF#77-1244), respectively. This crystallographic evidence not only validates the phase composition but also provides direct visualization of the heterointerface between the RuP2 and Mn2P2O7 components. In addition, the SEM image and corresponding energy-dispersive X-ray spectroscopy (EDS) elemental mapping images confirm the homogeneous distribution of Ru, Mn, P, C, N, and O elements throughout the RuP2/Mn2P2O7/NPC catalyst (Figure 1g,h). These characterization results collectively demonstrate that the RuP2/Mn2P2O7/NPC catalyst exhibits a three-dimensional porous framework and RuP2/Mn2P2O7 heterojunction active sites.
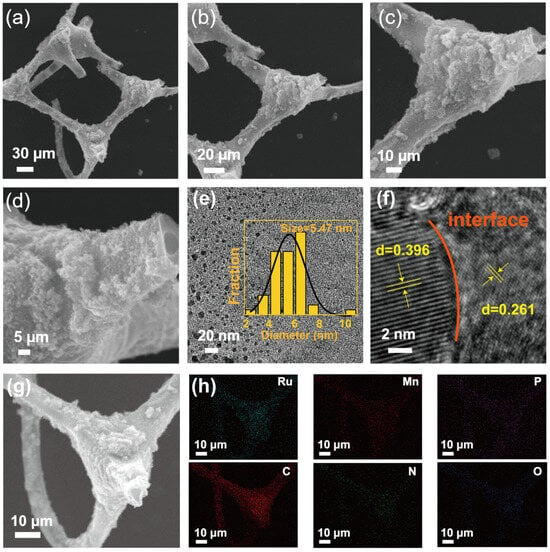
Figure 1.
(a–d) Scanning electron microscope (SEM) images of RuP2/Mn2P2O7/NPC at different magnification. (e) High-resolution transmission electron microscopy (HRTEM) image of RuP2/Mn2P2O7/NPC (insert graph showing metal particle size). (f) Lattice spacing of metallic elements. (g) SEM and (h) energy-dispersive spectroscopy (EDS) elemental mapping images of RuP2/Mn2P2O7/NPC (Ru: cyan, Mn: pink, P: purple, C: red, N: green, O: blue).
3.2. Structural Analysis of the Materials
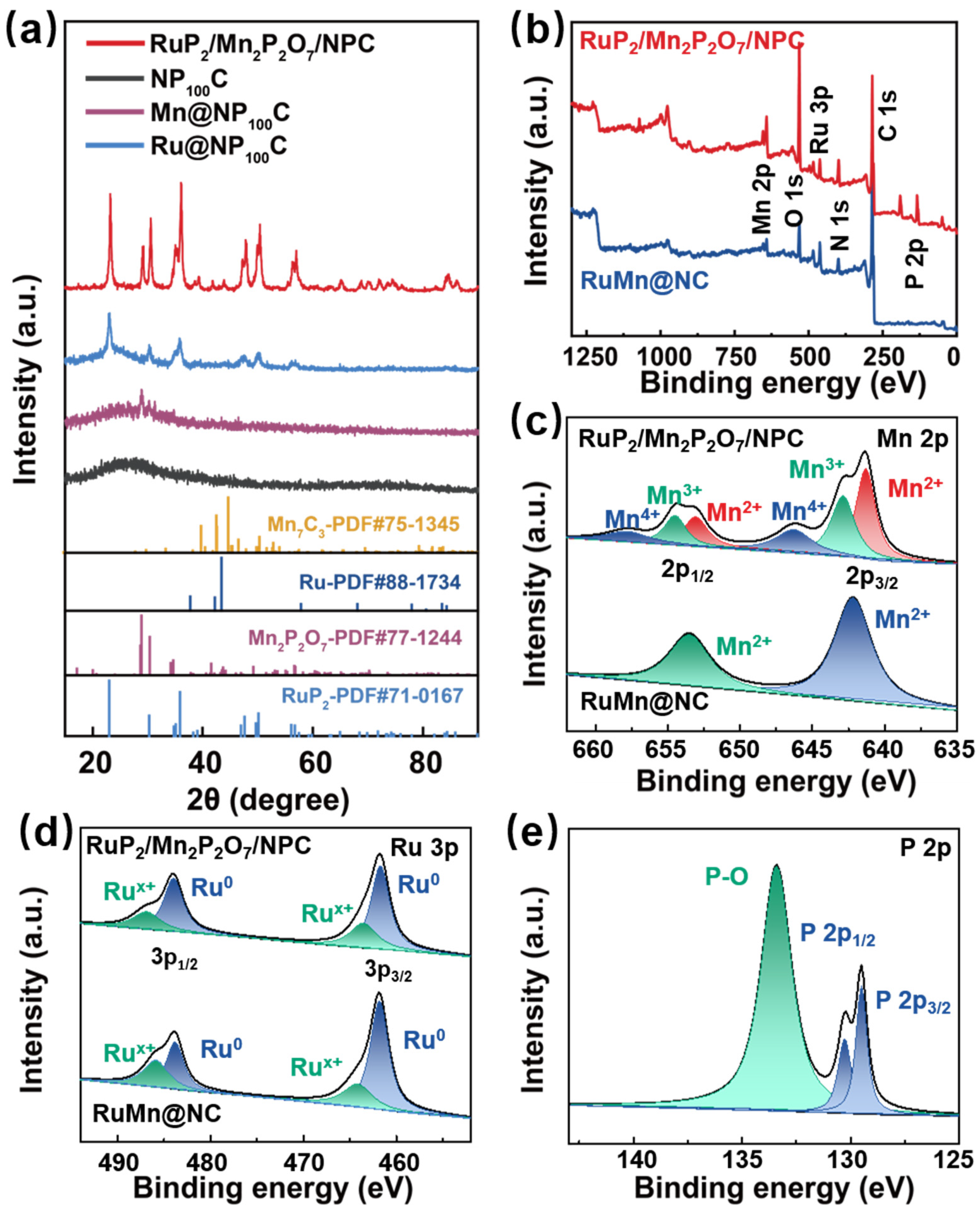
To investigate the structural information on and lattice parameters of the RuP2/Mn2P2O7/NPC catalysts, we conducted PXRD characterizations. As depicted in Figure 2a, the XRD pattern of the RuP2/Mn2P2O7/NPC exhibits several sharp peaks at 23.3°, 30.5°, 35.0°, 36.0°, 38.5°, 39.2°, 47.2°, 47.9°, 49.9°, 50.3°, 56.3°, 56.9°, 65.1°, 68.7°, 72.2°, 74.4°, 84.5°, and 86.3°, corresponding to the standard card (PDF#71-0167), demonstrating the successful preparation of RuP2 species. Additionally, the five distinct peaks at 0.2°, 29.1°, 41.7°, 43.8°, and 70.7°, which are attributed to the (001), (021), (131), (−311), and (−351) crystal planes of Mn2P2O7 (PDF#77-1244), indicate the existence of Mn2P2O7 species in the RuP2/Mn2P2O7/NPC. The absence of prominent Mn2P2O7 characteristic peaks in the PXRD patterns of both the RuMn@NP150C and the RuMn@NP50C confirms the absence of Mn2P2O7 species in both catalysts (Figure S1). These results demonstrate that the concentration of the phosphorus doping plays a critical role in determining the RuP2/Mn2P2O7 heterojunction of the catalyst. For the RuMn@NC sample, the PXRD analysis revealed a series of distinct diffraction peaks at 38.3°, 40.5°, 46.2°, 69.4°, and 86.2° (Figure S1). These reflections exhibit relatively strong intensity and sharp features, suggesting a good degree of crystallinity. While these peaks partially overlap with the characteristic planes of Mn7C3 (PDF#75-1345), a definitive phase attribution remains tentative due to possible phase mixing and structural complexity. Moreover, additional diffraction peaks appear at 42.2°, 57.8°, 78.0°, 83.5°, and 84.8°, which are close to but slightly right-shifted compared to the (002), (102), (103), (112), and (201) reflections of the metallic Ru (PDF#88-1734). This peak shift may indicate lattice distortion or subtle variations in local crystal fields, possibly arising from Mn incorporation or heteroatomic interactions within the structure. These results suggest that the RuMn@NC sample primarily contains crystalline Ru phases, with possible Mn-C species co-existing in a complex microstructure.
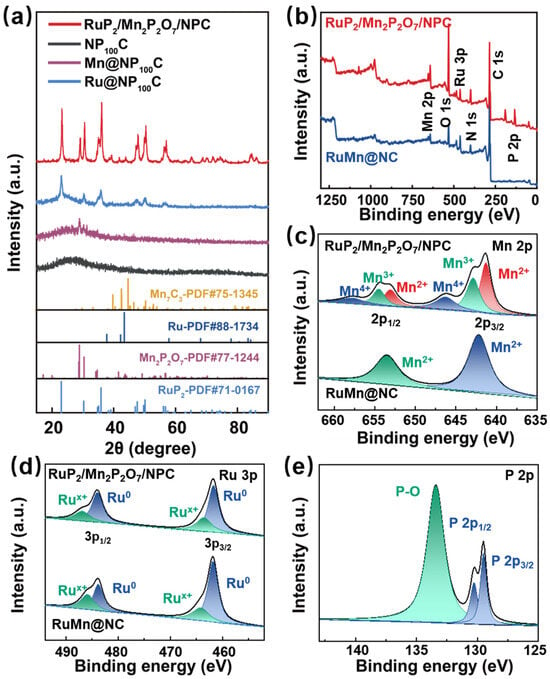
Figure 2.
(a) PXRD patterns of the obtained catalysts. (b) XPS spectra of RuP2/Mn2P2O7/NPC and RuMn@NC. (c) Mn 2p, (d) Ru 3p, and (e) P 2p XPS spectra of RuP2/Mn2P2O7/NPC.
Furthermore, the PXRD patterns of the Mn@NP100C and Ru@NP100C catalysts reveal different levels of crystallinity and phase purity. For the Mn@NP100C, only several weak and broad diffraction peaks were observed, which were tentatively attributed to the Mn2P2O7 species with relatively poor crystallinity, and their positions correspond to the standard Mn2P2O7 pattern (PDF#77-1244). In contrast, the PXRD pattern of the Ru@NP100C exhibits sharp and intense peaks that match well with the reference pattern of RuP2 (PDF#89-2743), confirming its high phase purity and crystallinity. This contrast further supports the successful construction of the RuP2/Mn2P2O7/NPC heterojunction. The presence of Ru during synthesis appears to promote the formation and crystallization of Mn2P2O7, likely due to localized thermal effects from exothermic phosphidation, enhanced lattice compatibility, and a modified redox environment. These synergistic effects contribute to the improved structural ordering and phase integration observed in the heterojunction system [38,39]. The PXRD pattern of the NP100C exhibits a broad diffraction feature centered around 23°, corresponding to the typical amorphous carbon structure, while no clear reflections associated with graphitic ordering were observed, suggesting a low degree of carbon framework ordering and the predominance of disordered carbon domains in the NP100C sample.
To further investigate electronic interactions at the heterointerface of RuP2/Mn2P2O7/NPC, the chemical environment and bonding configurations of C, N, P, Ru, Mn, and O in the RuP2/Mn2P2O7/NPC catalysts were examined using XPS. For the comparison, the electronic structure of the RuMn@NC was employed. The full XPS survey spectra of the RuP2/Mn2P2O7/NPC and RuMn@NC (Figure 2b) reveal distinct elemental compositions at the surface. The RuP2/Mn2P2O7/NPC exhibits six characteristic elements (Mn, Ru, P, C, N, and O), while the RuMn@NC shows five primary elements (Mn, Ru, C, N, and O), with the absence of phosphorus being particularly notable. In the Mn 2p XPS spectra, the peaks of the Mn 2p3/2 were observed at 641.3 eV, 642.8 eV, and 646.2 eV, corresponding to Mn2+, Mn3+, and Mn4+ species for the RuP2/Mn2P2O7/NPC, indicating a higher oxidation state of Mn species in the RuP2/Mn2P2O7/NPC compared to the RuMn@NC (Mn2+) (Figure 2c). Additionally, in the RuP2/Mn2P2O7/NPC, the Ru 3p XPS analysis also indicates the existence of multiple valence states: the peaks located at binding energies of 461.7 eV and 483.9 eV are attributed to the Ru 3p3/2 and 3p1/2 orbitals, respectively, whereas the new peaks at 463.5 eV and 486.8 eV represent the presence of the Rux+ (0 < x < 4) species, suggesting that Ru not only exists in the zero-valence state, but also partially oxidizes to form a high-valence state. In contrast, the RuMn@NC shows two different binding energies for Ru species, Ru0 (461.8 eV) and Rux+ (464.1 eV), corresponding to the Ru 3p3/2 orbitals (Figure 2d). In the Ru 3p XPS spectra, the Rux+ species in the RuP2/Mn2P2O7/NPC shows a negative shift of 0.6 eV compared to the RuMn@NC, indicating the remodeling effect of the heterostructure on the electronic configuration. In the P 2p spectrum (Figure 2e), three distinct subpeaks were observed: 130.2 eV and 129.4 eV correspond to the 2p1/2 and 2p3/2 orbitals of P in the metal phosphide, respectively, whereas the broad peak at 133.4 eV was attributed to the presence of P-O bonding, confirming the formation of the P-O coordination structure and indirectly verifying the effective doping of the P element. These XPS results reveal that constructing a RuP2/Mn2P2O7 heterojunction serves as an effective strategy for manipulating the local electronic environment of Ru and Mn centers.
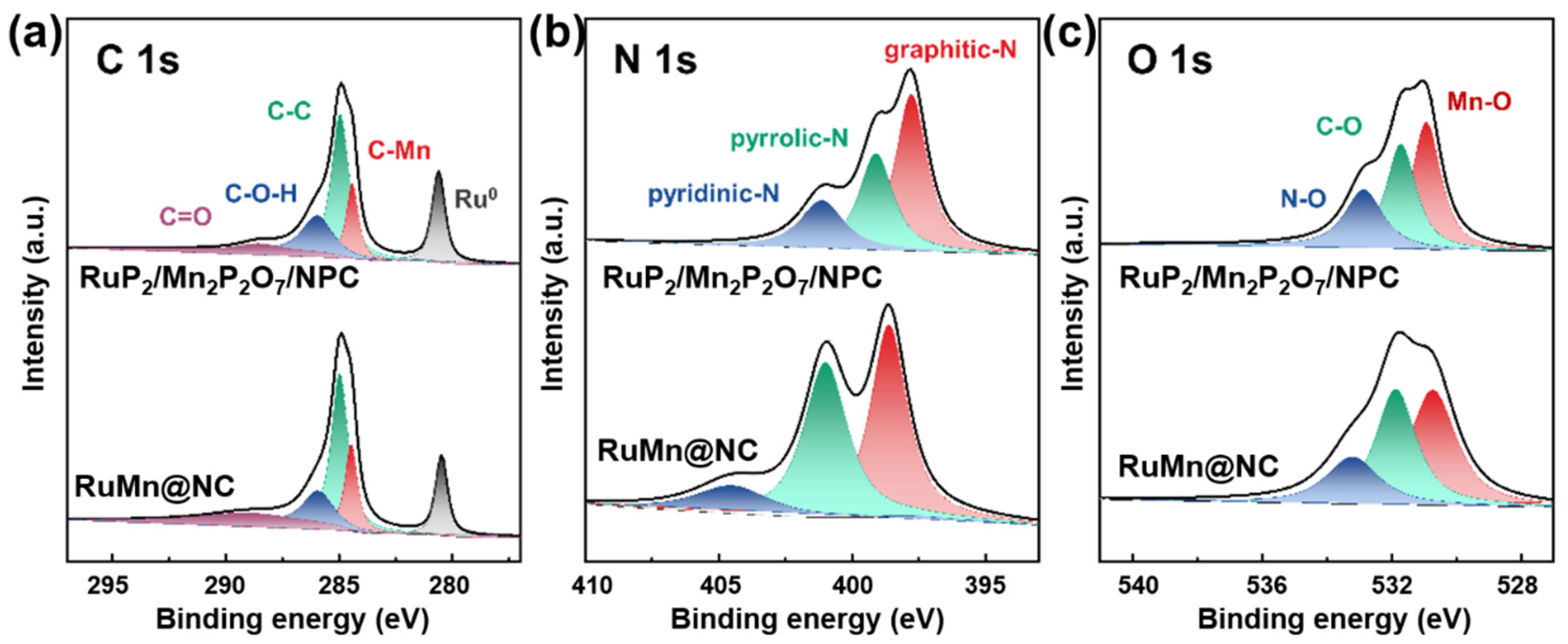
Furthermore, the XPS analysis of the C, N, and O elements confirmed the presence of interfacial electronic interactions between the carbon substrate and the metal components. In the C 1s spectra (Figure 3a) of the RuP2/Mn2P2O7/NPC, five distinct peaks were observed at 288.7 eV, 286.0 eV, 284.9 eV, and 284.4 eV, which were assigned to the C=O, C-O-H, C-C, and C-Mn (284.4 eV), and, notably, a peak at 280.6 eV, which was attributed to Ru-C interactions. These findings indicate strong electronic coupling between the metal species (Ru and Mn) and the carbon support. In the N 1s spectrum (Figure 3b), the RuP2/Mn2P2O7/NPC exhibited peaks corresponding to pyridinic-N (397.8 eV), pyrrolic-N (399.1 eV), and graphitic-N (401.1 eV). Compared to the RuMn@NC (in which these peaks appeared at 398.6, 401.0, and 404.6 eV, respectively), the binding energy in the RuP2/Mn2P2O7/NPC was significantly negatively shifted, which suggests that P-doping increases the electron density around N atoms, thereby enhancing the material’s overall electron transport capability. Nitrogen, primarily introduced from melamine precursors, regulates catalytic performance by modulating the carbon matrix’s electronic structure, thereby improving conductivity and charge transfer. Nitrogen species (e.g., pyridinic, pyrrolic, and graphitic N) also serve as coordination sites, stabilizing metal species and promoting dispersion. Additionally, nitrogen doping increases defect density and strengthens metal–carbon interactions, contributing to structural integrity and long-term durability. Furthermore, the O 1 s spectra (Figure 3c) further support the above conclusion, exhibiting three distinct peaks at 530.9 eV (Mn-O), 531.7 eV (C-O), and 532.9 eV (N-O). The appearance of the Mn-O peak serves as evidence for the formation of a stable Mn–O coordination bond, which enhances the structural integrity of the heterostructure. The elemental composition analysis of RuP2/Mn2P2O7/NPC and RuMn@NC is presented in Table 1. Comparative XPS studies demonstrate that phosphorus doping induces substantial modifications in the surface elemental distribution of the RuP2/Mn2P2O7/NPC compared to the undoped RuMn@NC. These findings highlight how controlled heterostructure engineering can effectively modulate active sites and electronic structures through coordinated structural and compositional adjustments. In summary, the RuP2/Mn2P2O7 heterojunction on carbon substrates demonstrates synergistic stability, with phosphorus doping effectively modulating the electronic configuration and strengthening interfacial interactions. These modifications collectively boost the catalytic performance of RuP2/Mn2P2O7/NPC.
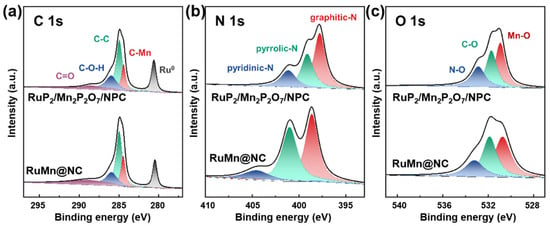
Figure 3.
(a) C 1s, (b) N 1s, and (c) O 1s XPS spectra of RuP2/Mn2P2O7/NPC and RuMn@NC.

Table 1.
Elemental composition of RuP2/Mn2P2O7/NPC and RuMn@NC.
3.3. Electrochemical Performance of the Catalyst for HER
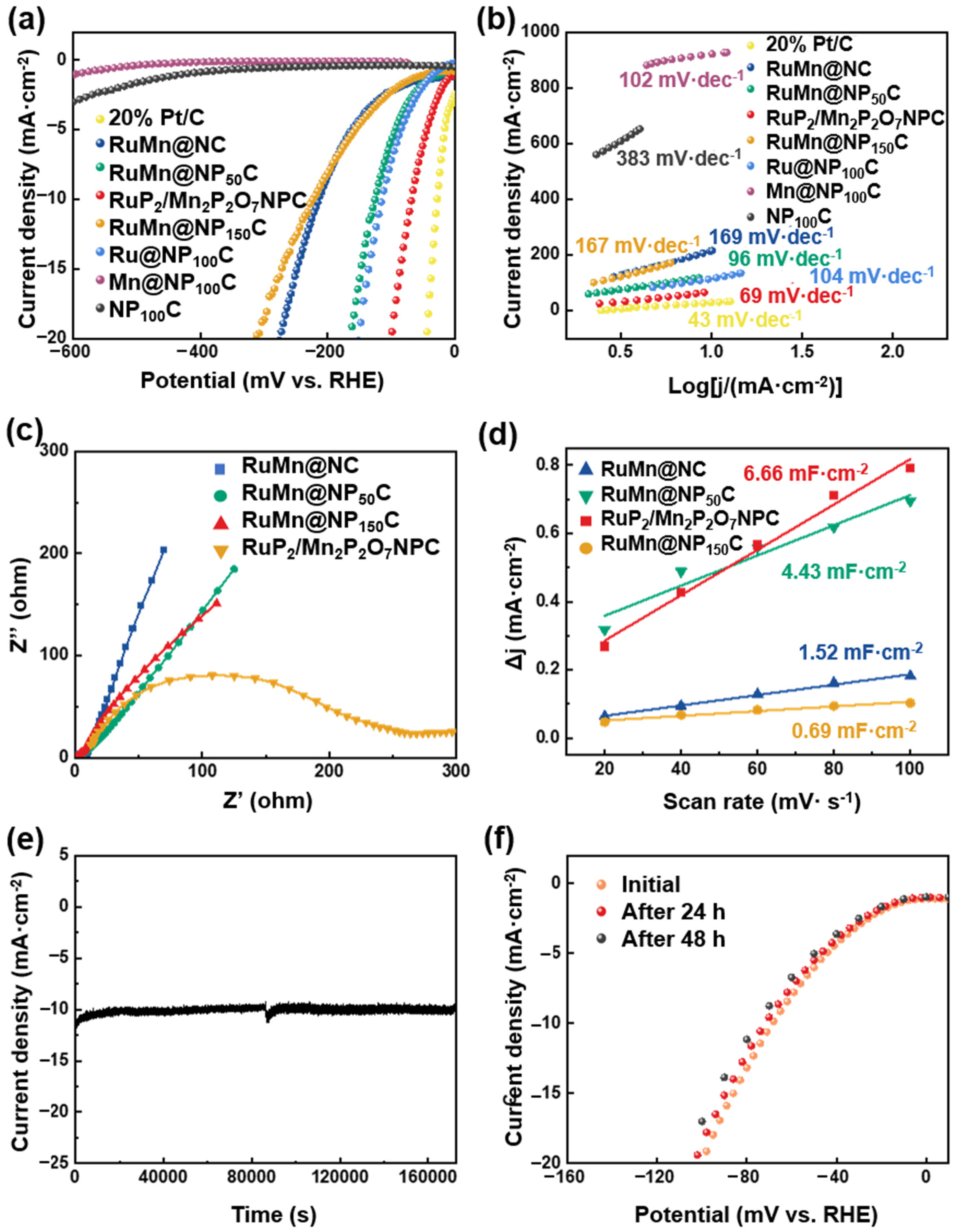
To evaluate the HER catalytic activity of the RuP2/Mn2P2O7/NPC, electrochemical experiments were conducted with 1 M KOH, employing a conventional three-electrode system. For comparison, several catalysts were measured, including RuMn@NC, RuMn@NP150C, RuMn@NP50C, Mn@NP100C, Ru@NP100C, NP100C, and commercial Pt/C (20 wt%). The catalyst ink was prepared by dispersing the synthesized powder in a mixture of water, isopropanol, and a small amount of Nafion binder. This ink was drop-cast onto a polished glassy carbon electrode, forming a uniform and adherent catalyst layer. Nafion serves as an effective adhesive, enhancing mechanical stability, while the porous carbon framework provides physical interlocking with the electrode. Electrochemical tests, including repeated voltammetric scans and chronoamperometry, demonstrated consistent current responses, confirming the catalyst layer’s robustness throughout the measurements. The HER performance of the prepared catalysts was first assessed using polarization curves with 95% iR compensation. As shown in Figure 4a, the RuP2/Mn2P2O7/NPC exhibits excellent HER activity, requiring a low overpotential of only 69 mV to reach a current density of 10 mA·cm−2, approaching the performance of 20 wt% Pt/C (30 mV), which was significantly lower than that of the single-metal phosphide catalysts, including Mn@NP100C, Ru@NP100C (115 mV), and NP100C, clearly indicating that both metal phosphide phases contributed active sites and that their synergistic interaction greatly enhanced the intrinsic HER performance. In addition, RuP2/Mn2P2O7/NPC demonstrates exceptional HER performance in terms of overpotential, higher than those of the previously reported non-noble metal catalysts (Table S1). Notably, RuP2/Mn2P2O7/NPC shows superior HER performance to that of non-phosphidated RuMn@NC (215 mV), RuMn@NP150C (224 mV), and RuMn@NP50C (126 mV), demonstrating that the constructed RuP2/Mn2P2O7 heterojunction serves as the dominant factor governing the enhanced HER catalytic activity. Furthermore, the Tafel slope analysis demonstrated significantly enhanced HER kinetics for RuP2/Mn2P2O7/NPC (69 mV·dec−1), exhibiting a reduction in Tafel slope of approximately 60–80% compared to the RuMn@NC (169 mV·dec−1), RuMn@NP50C (96 mV·dec−1), RuMn@NP150C (167 mV·dec−1), Ru@NP100C (104 mV·dec−1), Mn@NP100C (102 mV·dec−1), and NP100C (383 mV·dec−1) (Figure 4b). Additionally, an electrochemical impedance spectroscopy (EIS) analysis was carried out to evaluate the kinetic performance of the catalysts. As depicted in Figure 4c, the RuP2/Mn2P2O7/NPC exhibits a smaller charge transfer resistance than those of the RuMn@NC, RuMn@NP50C, and RuMn@NP150C, indicating faster charge transfer in RuP2/Mn2P2O7/NPC relative to the RuP2/Mn2P2O7 heterojunction. Electrochemical double-layer capacitance (Cdl) measurements (Figure 4d, Figures S2 and S3 and Table S2) in the non-Faradaic region revealed that RuP2/Mn2P2O7/NPC exhibits the highest Cdl value (6.66 mF·cm−2), representing a 438% increase over RuMn@NC (1.52 mF·cm−2), a 150% enhancement compared to RuMn@NP50C (4.43 mF·cm−2), a 965% improvement relative to RuMn@NP150C (0.69 mF·cm−2), and superiority to counter catalysts (Ru@NP100C: 3.58 mF·cm−2, Mn@NP100C: 2.32 mF·cm−2, and NP100C: 1.78: mF·cm−2), indicating its superior electrochemically active surface area. Long-term operational durability represents a fundamental requirement for implementing electrocatalytic materials in industrial applications. Hence, the electrochemical durability of the RuP2/Mn2P2O7/NPC was systematically evaluated through chronoamperometric measurements at a fixed potential under HER operation conditions. As illustrated in Figure 4e, the current density for the HER remained robust throughout the 48 h stability assessment under a constant potential. In addition, after the 48 h and 100 h i-t stability test, the LSV test on the RuP2/Mn2P2O7/NPC catalyst revealed that there was almost no decay at different current densities (Figure 4f and Figure S4). These results demonstrate that the synergistic interplay between RuP2 and Mn2P2O7 endows the heterostructure with both catalytically active sites and a robust structure.
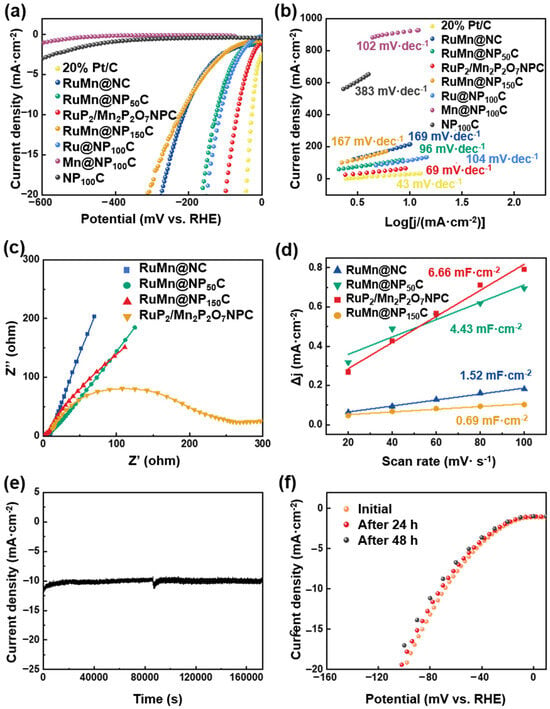
Figure 4.
Electrocatalytic performance for HER in alkaline solution. (a) LSV polarization curves and (b) Tafel slope of RuP2/Mn2P2O7/NPC, RuMn@NC, RuMn@NP50C, RuMn@NP150C, Ru@NP100C, Mn@NP100C, NP100C, and 20% Pt/C. (c) EIS Nyquist plots of RuP2/Mn2P2O7/NPC, RuMn@NC, RuMn@NP50C, and RuMn@NP150C. (d) Cdl curves of RuMn@NC, RuMn@NP150C, RuMn@NP50C, and RuP2/Mn2P2O7/NPC catalysts. (e) i-t curve of RuP2/Mn2P2O7 catalyst stability test at 69 mV vs. RHE. (f) Comparison of polarization curves of RuP2/Mn2P2O7/NPC before and after 48 h.
4. Conclusions
In summary, we have developed a novel RuP2/Mn2P2O7 heterojunction integrated into a nitrogen and phosphorus co-doped porous carbon framework (RuP2/Mn2P2O7/NPC) as an efficient and durable electrocatalyst for the hydrogen evolution reaction (HER) in alkaline media. The rational design of this heterojunction is achieved through a controlled pyrolysis–phosphidation strategy, which promotes intimate interfacial contact and electronic coupling between RuP2 and Mn2P2O7 phases. This synergy effectively modulates the local electronic environment and accelerates charge transfer. As a result, RuP2/Mn2P2O7/NPC exhibits an overpotential of just 69 mV in order to reach 10 mA·cm−2, along with a Tafel slope as low as 69 mV·dec−1. Moreover, the catalyst demonstrates superior electrochemical stability, retaining its performance over 48 h of continuous operation. This work underscores the promise of transition metal phosphide heterojunctions for next-generation alkaline HER electrocatalysts and provides a generalizable strategy for interfacial engineering to enhance catalytic functionality in energy conversion applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18133065/s1. Figure S1. PXRD patterns of the counter catalysts. Figure S2. CV curves of (a) RuMn@NC, (b) RuMn@NP50C, (c) RuP2/Mn2P2O7/NPC, and (d) RuMn@NP150C catalysts. Figure S3. CV curves of (a) Ru@ NP100C, (b) Mn@NP100C, (c) NP100C, and (d) Cdl curves of Ru@ NP100C, Mn@NP100C, and NP100C catalysts. Figure S4. Stability test of RuP2/Mn2P2O7 catalyst at overpotential of 69 mV vs. RHE. Table S1. Performance comparison between RuP2/Mn2P2O7/NPC and recently reported catalysts. Table S2. Cdl comparison between RuP2/Mn2P2O7/NPC and counter catalysts.
Author Contributions
Writing and data curation, W.W.; investigation and data curation, W.G.; supervision and methodology, Z.L.; validation and methodology, C.Z.; validation and resources, A.L.; data curation, C.S.; review and editing and funding acquisition, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (No. 22478425).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Caihua Su was employed by the company Beijing Future Hydrogen Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, D.; Park, E.J.; Zhu, W.; Shi, Q.; Zhou, Y.; Tian, H.; Lin, Y.; Serov, A.; Zulevi, B.; Baca, E.D.; et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 2020, 5, 378–385. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, S.; Yan, L.; Feng, Y.; Zhang, Z.; Li, S.; Guo, X.; Li, T.; Li, H.; Zhuang, Z.; et al. 10,000-h-stable intermittent alkaline seawater electrolysis. Nature 2025, 639, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yu, L.; Huang, H.; Gao, C.; Huang, X.; Zhang, X.; Zhang, X.; Du, Z.; He, C. A molecular engineered strategy to remolding architecture of RuP2 nanoclusters for sustainable hydrogen evolution. Adv. Funct. Mater. 2024, 34, 2411111. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Luo, Y.; Cui, Z.; Yu, Q.; Gao, Z.; Zhang, Z.; Yang, F.; Kang, X.; Ge, S.; et al. Dual interfacial engineering of a Chevrel phase electrode material for stable hydrogen evolution at 2500 mA cm−2. Nat. Commun. 2022, 13, 6382. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Dastafkan, K.; Shen, Y.; Zhao, C.; Wang, M. Yttrium-doped NiMo-MoO2 heterostructure electrocatalysts for hydrogen production from alkaline seawater. Nat. Commun. 2025, 16, 773. [Google Scholar] [CrossRef]
- Xu, T.; Jiao, D.; Fan, J.; Dong, Y.; Jin, Z.; Zhang, L.; Zhang, W.; Zhao, J.; Zheng, W.; Cui, X. “Similar stacking”-inspired compressive strain of heterogeneous phosphide for efficient hydrogen evolution. Carbon Energy 2025, 7, e668. [Google Scholar] [CrossRef]
- Zhou, Z.; Su, Y.; Tan, H.; Wang, Y.; Huang, Q.; Wang, H.; Wang, J.; Kubo, M.; Ni, Z.; Kong, Y.; et al. Atomically dispersed Co-P moieties via direct thermal exfoliation for alkaline hydrogen electrosynthesis. J. Am. Chem. Soc. 2025, 147, 3994–4004. [Google Scholar] [CrossRef]
- Huang, L.; Yao, R.; Wang, X.; Sun, S.; Zhu, X.; Liu, X.; Kim, M.; Lian, J.; Liu, F.; Li, Y.; et al. In situ phosphating of Zn-doped bimetallic skeletons as a versatile electrocatalyst for water splitting. Energy Environ. Sci. 2022, 15, 2425–2434. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, S.; Zheng, X.; Liang, X.; Li, Z.; Gu, H.; Li, Z.; Wang, Y.; Liu, S.; Chen, W.; et al. Lattice strain and schottky junction dual regulation boosts ultrafine ruthenium nanoparticles anchored on a N-modified carbon catalyst for H2 production. J. Am. Chem. Soc. 2022, 144, 19619–19626. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Zhang, X.; Kong, W.; Peng, O.; Yang, Y.; Wang, S.; Cheng, J.; He, T.; Amini, A.; et al. Polyoxometalate-derived hexagonal molybdenum nitrides (MXenes) supported by boron, nitrogen codoped carbon nanotubes for efficient electrochemical hydrogen evolution from seawater. Adv. Funct. Mater. 2019, 29, 1805893. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuo, H.; Zhang, X.; Dai, X.; Yu, K.; Luan, C.; Yu, L.; Xiao, Y.; Li, J.; Wang, M.; et al. Synergistic effect between undercoordinated platinum atoms and defective nickel hydroxide on enhanced hydrogen evolution reaction in alkaline solution. Nano Energy 2018, 48, 590–599. [Google Scholar] [CrossRef]
- Zhou, L.; Wan, T.; Zhong, Y.; Liu, W.; Yu, L.; Li, T.; Sun, K.; Waterhouse, G.I.N.; Xu, H.; Kuang, Y.; et al. Ampere-level hydrogen generation via 1000 h stable seawater electrolysis catalyzed by Pt-cluster-loaded NiFeCo phosphide. Small 2024, 20, 2406076. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Wang, S.; Yao, S.; Ji, Y.; Li, J.; Wang, X.; Shi, L.; Wang, G.; Ren, W.; Wang, J.; et al. Built-in electric field in Ru/CoP bifunctional electrocatalyst enhances hydrazine-assisted water splitting. Adv. Mater. 2025, 2503182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Liu, Z.; Wang, Z.; Xie, F.; Yuan, X.; Tan, Y. Manipulating interfacial water via metallic Pt1Co6 sites on self-adaptive metal phosphides to enhance water electrolysis. Adv. Mater. 2025, 37, 2419644. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Jiang, W.; Zhang, S.; Zhu, J.; Wang, L.; Ou, H.; Zaman, S.; Tan, L.; Zhu, P.; et al. Engineering water molecules activation center on multisite electrocatalysts for enhanced CO2 methanation. J. Am. Chem. Soc. 2022, 144, 12807–12815. [Google Scholar] [CrossRef]
- Gu, Y.; Xi, B.J.; Wei, R.C.; Fu, Q.; Qain, Y.T.; Xiong, S. Sponge assembled by graphene nanocages with double active sites to accelerate alkaline HER kinetics. Nano Lett. 2020, 20, 8375–8383. [Google Scholar] [CrossRef]
- Bi, M.; Zhang, Y.; Jiang, X.H.; Sun, J.; Wang, X.; Zhu, J.; Fu, Y. Ruthenium-induced activation of molybdenum-cobalt phosphide for high-efficiency water splitting. Adv. Funct. Mater. 2024, 34, 2309330. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, Y.; Huangfu, Z.; Chen, L.; Cao, J.; Yang, X.; Mai, W.; Wang, Z. Noble metal nanoparticles anchored on transition metal phosphides for effective pH-universal hydrogen evolution. Adv. Sci. 2025, 37, 2504462. [Google Scholar] [CrossRef]
- Wang, C.; Guo, W.; Chen, T.; Lu, W.; Song, Z.; Yan, C.; Feng, Y.; Gao, F.; Zhang, X.; Rao, Y.; et al. Advanced noble-metal/transition-metal/metal-free electrocatalysts for hydrogen evolution reaction in water-electrolysis for hydrogen production. Coordin. Chem. Rev. 2024, 514, 215899. [Google Scholar] [CrossRef]
- An, W.; Lu, W.; Ma, L.; Li, W.; Yan, C.; Prova, U.H.; Wang, C.; Huang, G. Heterojunction regulates the function of coral like NiCoP for efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 35953–35961. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, H.; Chen, T.; Zhang, J.; Zhang, S.; Chen, L.; Pang, H.; Huang, Z. MOF derived phosphide nanocubes with internal heterojunction: A study powered by single entity electrochemistry. Nano Lett. 2025, 25, 4921–4929. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; He, S.; Li, B.; Wang, K.; Zhou, Z.; Li, J.; Wang, T.; Du, Z.; Ai, W.; Huang, W. Cascade reaction enables heterointerfaces-enriched nanoarrays for ampere-level hydrogen production. Angew. Chem. Int. Ed. 2025, 64, e202422393. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wu, Y.; Wan, X.; Gao, J.; Wen, D. Engineering the electronic structure of FeP with rare earth elements to enhance the electrocatalytic hydrogen evolution performance. J. Mater. Chem. A 2023, 11, 18126–18134. [Google Scholar] [CrossRef]
- Jin, M.T.; Zhang, X.; Shi, R.; Lian, Q.; Niu, S.Z.; Peng, O.W.; Wang, Q.; Cheng, C. Hierarchical CoP@Ni2P catalysts for pH-universal hydrogen evolution at high current density. Appl. Catal. B Environ. 2021, 296, 120350. [Google Scholar] [CrossRef]
- Niu, H.; Yan, Y.; Jiang, S.; Liu, T.; Sun, T.; Zhou, W.; Guo, L.; Li, J. Interfaces decrease the alkaline hydrogen-evolution kinetics energy barrier on NiCoP/Ti3C2TX MXene. ACS Nano 2022, 16, 11049–11058. [Google Scholar] [CrossRef]
- Wang, K.; He, S.; Li, B.; Du, H.; Wang, T.; Du, Z.; Xie, L.; Ai, W. Relaying alkaline hydrogen evolution over locally amorphous Ni/Co-based phosphides constructed by diffusion-limited phase-transition. Appl. Catal. B Environ. Energy 2023, 339, 123136. [Google Scholar] [CrossRef]
- Wang, F.; Ju, L.; Wu, B.; Li, S.; Peng, J.; Chen, Y.; Sendeku, M.G.; Wang, K.; Cai, Y.; Yi, J.; et al. Effect of intrinsic ferroelectric phase transition on hydrogen evolution electrocatalysis. Angew. Chem. Int. Ed. 2024, 63, e202402033. [Google Scholar] [CrossRef]
- Wang, X.; Le, J.; Fei, Y.; Gao, R.; Jing, M.; Yuan, W.; Li, C. Self-assembled ultrasmall mixed Co-W phosphide nanoparticles on pristine graphene with remarkable synergistic effects as highly efficient electrocatalysts for hydrogen evolution. J. Mater. Chem. A 2022, 10, 7694–7704. [Google Scholar] [CrossRef]
- Feng, D.; Wang, P.; Ma, B.; Zhao, X.; Chen, Y. MoN@NiO core-shell heterostructure nanorods array for highly efficient electrocatalytic hydrogen evolution reaction. Appl. Catal. B Environ. 2025, 374, 125373. [Google Scholar] [CrossRef]
- Yang, H.; Guo, P.; Wang, R.; Chen, Z.; Xu, H.; Pan, H.; Sun, D.; Fang, F.; Wu, R. Sequential phase conversion-induced phosphides heteronanorod arrays for superior hydrogen evolution performance to Pt in wide pH media. Adv. Mater. 2022, 34, 2107548. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, J.; Yang, E.; Qi, S.; Tian, H.; Chen, J.; Li, J.; Lou, Y.; Guo, Y. Work-function-induced electron rearrangement of in-plane FeP@CoP heterojunction enhances all pH range and alkaline seawater hydrogen evolution reaction. Nano Energy 2023, 114, 108601. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Guo, S.; Xin, X.; Wang, Y.; Huang, W.; Wang, M.; Yang, B.; Sobrido, A.J.; Ghasemi, J.B.; et al. Gradient heating epitaxial growth gives well lattice-matched Mo2C-Mo2N heterointerfaces that boost both electrocatalytic hydrogen evolution and water vapor splitting. Angew. Chem. Int. Ed. 2022, 61, e202209703. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Z.; Gu, S.; Liu, Y.; Deng, Y.; Li, Y.; Xiao, Z.; Liu, K.; Wu, Z.; Wang, L. The cobalt-based metal organic frameworks array derived CoFeNi-layered double hydroxides anode and CoP/FeNi2P heterojunction cathode for ampere-level seawater overall splitting. J. Colloid Interf. Sci. 2024, 676, 52–60. [Google Scholar] [CrossRef]
- Xu, H.; Wei, J.; Zhang, K.; Yukihide, S.; Du, Y. Hierarchical NiMo phosphide nanosheets strongly anchored on carbon nanotubes as robust electrocatalysts for overall water splitting. ACS Appl. Mater. Interfaces 2018, 10, 29647–29655. [Google Scholar] [CrossRef]
- Cai, J.; Song, Y.; Zang, Y.; Niu, S.; Wu, Y.; Xie, Y.; Zheng, X.; Liu, Y.; Lin, Y.; Qian, Y. N-induced lattice contraction generally boosts the hydrogen evolution catalysis of P-rich metal phosphides. Sci. Adv. 2020, 6, eaaw8113. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, D.; Zhang, P.; Hui, X.; Zhang, Z.; Wang, R.; Wang, C.; Ge, X.; Liu, X.; Li, Y.; et al. P-block element modulated 1 T phase MoS2 with Ru lattice grafting for high-performance Li | |O2 batteries. Nat. Commun. 2025, 16, 1453. [Google Scholar] [CrossRef]
- Shi, S.; Sun, C.; Yin, X.; Shen, L.; Shi, Q.; Zhao, K.; Zhao, Y.; Zhang, J. FeP quantum dots confined in carbon-nanotube-grafted P-doped carbon octahedra for high-rate sodium storage and full-cell applications. Adv. Funct. Mater. 2020, 30, 1909283. [Google Scholar] [CrossRef]
- Zhang, Q.; Ti, Z.; Zhu, Y.; Zhang, Y.; Cao, Y.; Li, S.; Wang, M.; Li, D.; Zou, B.; Hou, Y.; et al. Achieving ultralow lattice thermal conductivity and high thermoelectric performance in GeTe alloys via introducing Cu2Te nanocrystals and resonant level doping. ACS Nano 2021, 15, 19345–19356. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Chen, L.; Yang, X.; Guo, H.; Wang, Z.; Yuan, M.; Yan, X.; Yang, J.; Li, X.; et al. Ultrawide bandgap diamond/ε-Ga2O3 heterojunction pn diodes with breakdown voltages over 3 kV. Nano Lett. 2025, 25, 537–544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).