Insights into the Crystal Structure and Magnetodielectric Properties of High-Energy Ball Milled Sr Substituted LaFeO3
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
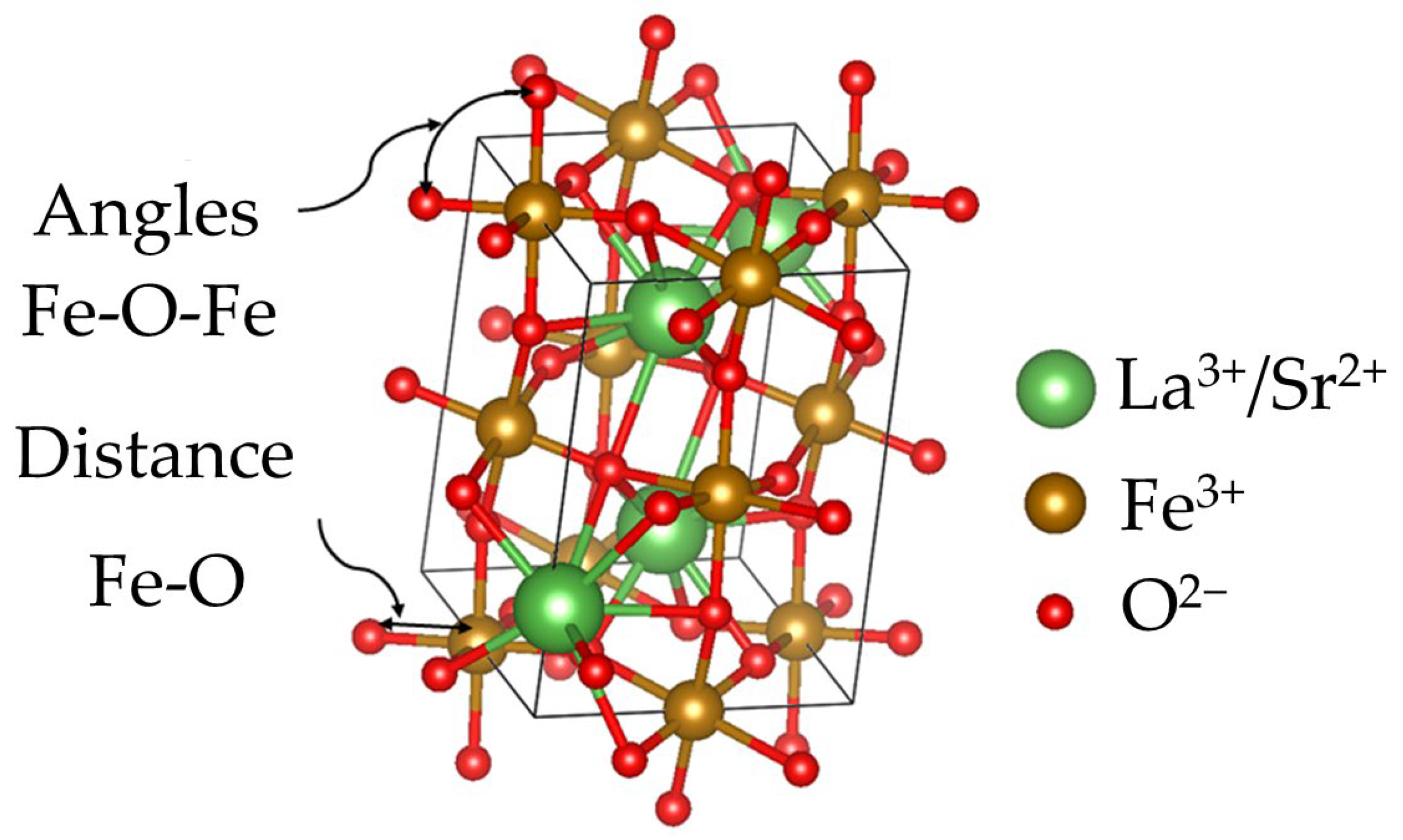
3.1. Crystal Structure
3.2. FT-IR Analysis
3.3. Surface Morphology Analysis
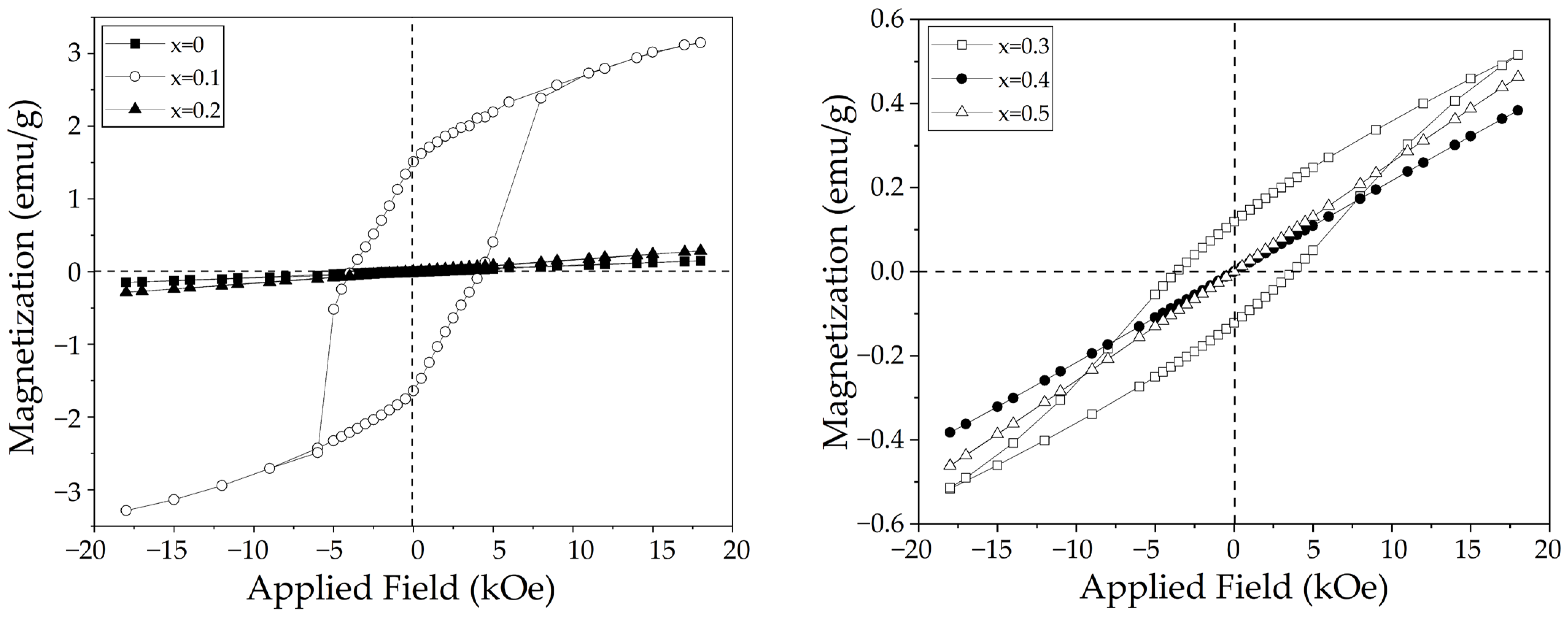
3.4. Magnetic Properties
3.5. Electric Properties
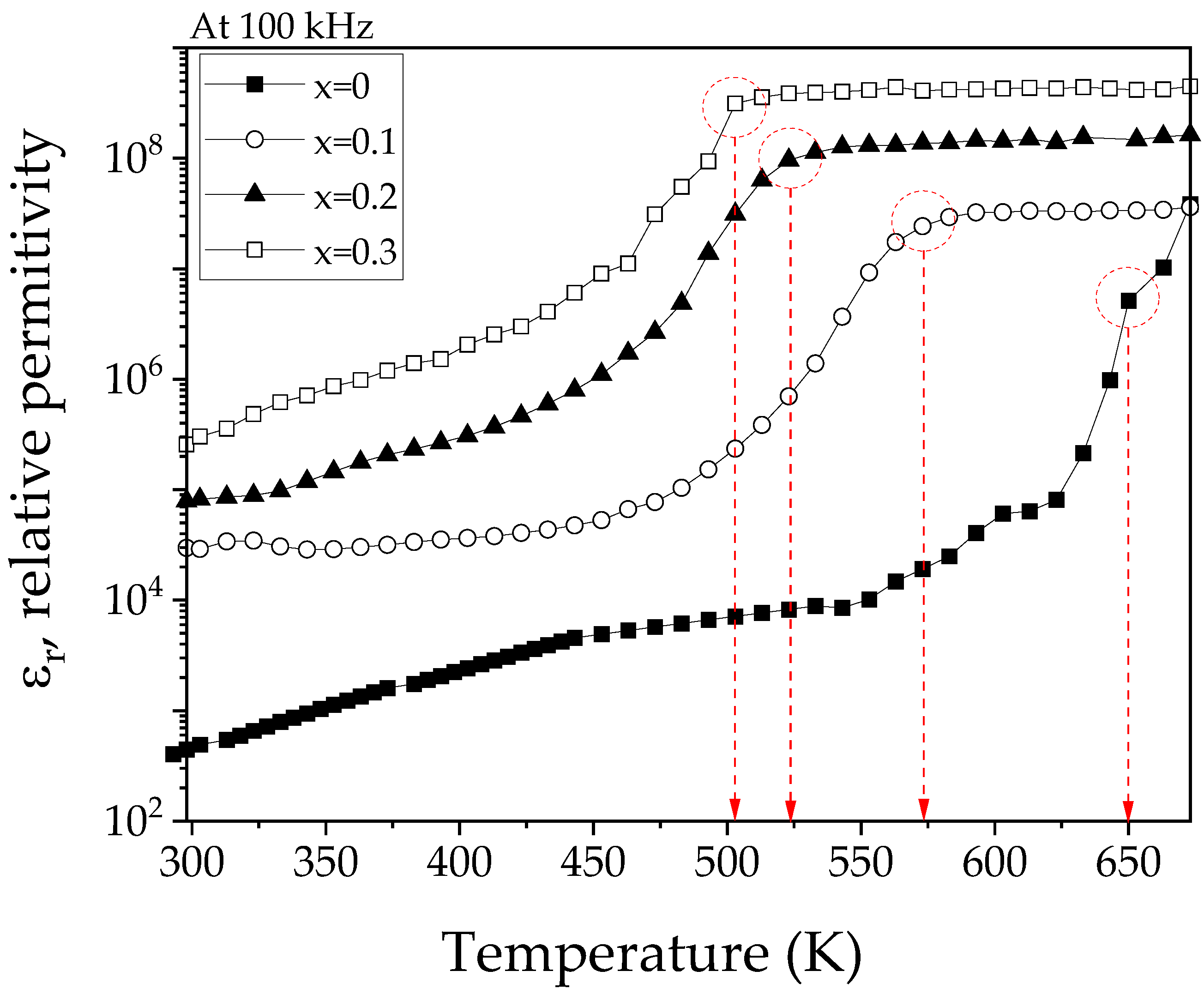
3.6. Dielectric Properties
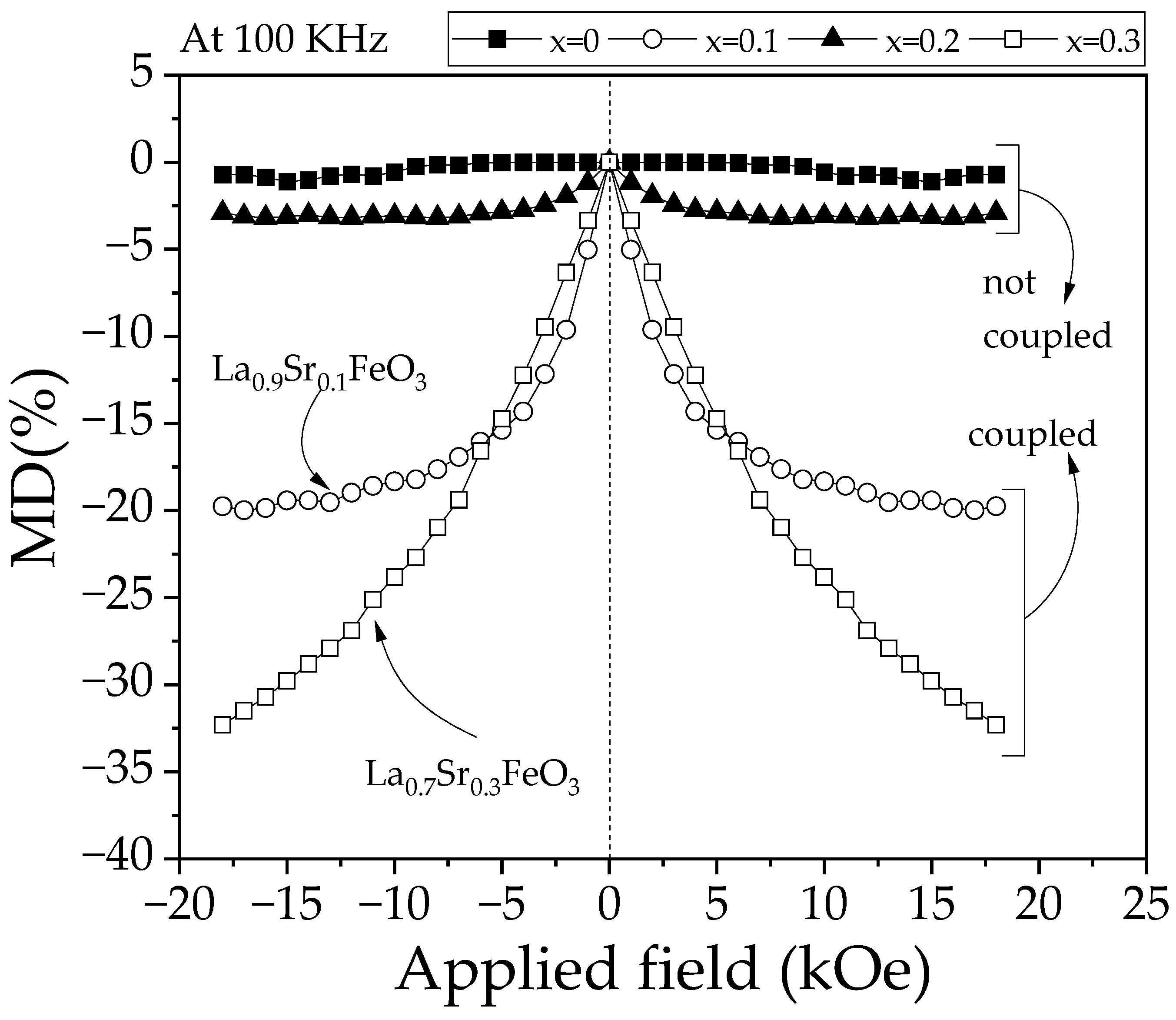
3.7. Magnetodielectric Coupling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowri, G.; Saravanan, R.; Srinivasan, N.; Karunya, K.; Jeyasheela, P.; Uthra, M. Probing the Effects of Al Dopant over the Structure and Charge-Related Optical, Magnetic, and Electrical Properties of Al3+-Doped LaFeO3 Bulk Multiferroic Materials. Chem. Pap. 2021, 75, 4337–4353. [Google Scholar] [CrossRef]
- Lee, K.; Hajra, S.; Sahu, M.; Kim, H.J. Colossal Dielectric Response, Multiferroic Properties, and Gas Sensing Characteristics of the Rare Earth Orthoferrite LaFeO3 Ceramics. J. Alloys Compd. 2021, 882, 160634. [Google Scholar] [CrossRef]
- Mahapatra, A.S.; Mitra, A.; Mallick, A.; Shaw, A.; Greneche, J.M.; Chakrabarti, P.K. Modulation of Magnetic and Dielectric Property of LaFeO3 by Simultaneous Doping with Ca2+ and Co2+-Ions. J. Alloys Compd. 2018, 743, 274–282. [Google Scholar] [CrossRef]
- Warnhus, I.; Vullum, P.; Holmestad, R.; Grande, T.; Wiik, K. Electronic Properties of Polycrystalline LaFeO3. Part I: Experimental Results and the Qualitative Role of Schottky Defects. Solid State Ion. 2005, 176, 2783–2790. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Naumovich, E.N.; Tikhonovich, V.N. Oxygen Permeability of LaFe1−xNixO3−δ Solid Solutions. Mater. Res. Bull. 1999, 34, 1311–1317. [Google Scholar] [CrossRef]
- Swierczek, K.; Marzec, J.; Palubiak, D.; Zajac, W.; Molenda, J. LFN and LSCFN Perovskites—Structure and Transport Properties. Solid State Ion. 2006, 177, 1811–1817. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Yun, H.J.; Yoon, J.-W. Characterization and Magnetic Properties of LaFeO3 Nanofibers Synthesized by Electrospinning. J. Alloys Compd. 2014, 583, 320–324. [Google Scholar] [CrossRef]
- Acharya, S.; Mondal, J.; Ghosh, S.; Roy, S.K.; Chakrabarti, P.K. Multiferroic Behavior of Lanthanum Orthoferrite (LaFeO3). Mater. Lett. 2010, 64, 415–418. [Google Scholar] [CrossRef]
- Long, D.; Liu, J.; Chen, H.; Liu, P.; Zheng, K.; Zeng, Y.; Chen, X.; Li, S.; Lu, M. Electronegative Diversity Induced Localized Built-in Electric Field in a Single Phased MoSxSeyNz for Selectivity-Enhanced Visible Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2023, 330, 122625. [Google Scholar] [CrossRef]
- Rosales-González, O.; Olivares-Lugo, L.I.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Martínez-Luévanos, A.; Bolarín-Miró, A.M. Crystal Structure and Photocatalytic Properties of Bismuth-Doped Lanthanum Ferrite. J. Electron. Mater. 2023, 52, 7652–7666. [Google Scholar] [CrossRef]
- Andoulsi-Fezei, R.; Sdiri, N.; Horchani-Naifer, K.; Férid, M. Dielectric Properties of Calcium-Substituted Lanthanum Ferrite. J. Asian Ceram. Soc. 2020, 8, 94–105. [Google Scholar] [CrossRef]
- Berezhnaya, M.V.; Perov, N.S.; Almjasheva, O.V.; Mittova, V.O.; Nguyen, A.T.; Mittova, I.Y.; Druzhinina, L.V.; Alekhina, Y.A. Synthesis and Magnetic Properties of Barium-Doped Nanocrystal Lanthanum Orthoferrite. Russ. J. Gen. Chem. 2019, 89, 480–485. [Google Scholar] [CrossRef]
- Borgekov, D.B.; Kozlovskiy, A.L.; Shakirzyanov, R.I.; Zhumazhanova, A.T.; Zdorovets, M.V.; Shlimas, D.I. Properties of Perovskite-like Lanthanum Strontium Ferrite Ceramics with Variation in Lanthanum Concentration. Crystals 2022, 12, 1792. [Google Scholar] [CrossRef]
- Lataoui, R.; Triki, A.; Hcini, S.; Zemni, S.; Dhahri, J.; Kanoun, O. Structural, Morphological and Dielectric Analyses of La1−xSrxFeO3 Solid Solutions. Appl. Phys. A 2021, 127, 721. [Google Scholar] [CrossRef]
- Paiva, J.A.E.; Daza, P.C.C.; Rodrigues, F.A.; Ortiz-Mosquera, J.F.; Da Silva, C.R.M.; Montero Muñoz, M.; Meneses, R.A.M. Synthesis and Electrical Properties of Strontium-Doped Lanthanum Ferrite with Perovskite-Type Structure. Ceram. Int. 2020, 46, 18419–18427. [Google Scholar] [CrossRef]
- Ritzmann, A.M.; Muñoz-García, A.B.; Pavone, M.; Keith, J.A.; Carter, E.A. Ab Initio DFT+U Analysis of Oxygen Vacancy Formation and Migration in La1−xSrxFeO3−δ (x=0, 0.25, 0.50). Chem. Mater. 2013, 25, 3011–3019. [Google Scholar] [CrossRef]
- She, S.; Yu, J.; Tang, W.; Zhu, Y.; Chen, Y.; Sunarso, J.; Zhou, W.; Shao, Z. Systematic Study of Oxygen Evolution Activity and Stability on La1−xSrxFeO3−δ Perovskite Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 11715–11721. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al-Solami, F.; Al Angari, Y.M.; Awad, A.; Al-Juaid, A.A.; Saeed, A. Structural, Magnetic, and Electrical Characterization of Sr-Substituted LaFeO3 Perovskite Synthesized via Sucrose Auto-Combustion Route. J. Mater. Sci. Mater. Electron. 2020, 31, 3146–3158. [Google Scholar] [CrossRef]
- Gowri, G.; Saravanan, R.; Sasikumar, S.; Nandhakumar, M.; Ragasudha, R. Interatomic Chemical Bonding and Charge Correlation of Optical, Magnetic and Dielectric Properties of La1−xSrxFeO3 Multiferroics Synthesized by Solid- State Reaction Method. J. Mater. Sci. Mater. Electron. 2019, 30, 4409–4426. [Google Scholar] [CrossRef]
- Yang, F.; Yang, X.X.; Lin, Q.; Wang, R.J.; Yang, H.; He, Y. Microstructure and Magnetic Studies of La1−xSrxFeO3 Nano Particles Fabricated by the Citrate Sol-Gel Method. Mater. Sci. 2019, 25, 231–237. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, L.; Pan, S.; He, Y.; Tian, C.; Yu, J.; Zhou, H. Effects of Sr Doping on the Structure, Magnetic Properties and Microwave Absorption Properties of LaFeO3 Nanoparticles. Ceram. Int. 2020, 46, 27352–27361. [Google Scholar] [CrossRef]
- Revathi, S.; Srimathi, R.; Kumar, A.; Kaur, J.; Yuvaraj, S.; Ubaidullah, M.; Sundararajan, M.; Prakash, C.; Kumar, A.; Shaikh, S.F.; et al. Monitoring the Effect of In3+ Doping on the Structural, Morphological, Optical, Vibrational, and Magnetic Properties of Perovskite LaFeO3 Nanoparticles. Inorg. Chem. Commun. 2024, 168, 112777. [Google Scholar] [CrossRef]
- Fan, J.; Ran, X.; Fu, H.; Sun, J.; An, X.; Yang, X. The Roles of Oxygen Vacancies in LaFeO3 Photocathodes in Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2024, 649, 159011. [Google Scholar] [CrossRef]
- Olivares-Lugo, L.I.; Sánchez-De Jesús, F.; Rosales-González, O.; Cortés-Escobedo, C.A.; Barba-Pingarrón, A.; Bolarín-Miró, A.M. Evidence of Magnetodielectric Coupling in Bismuth Doped Lanthanum Ferrite Obtained by High-Energy Ball Milling. Phys. B Condens. Matter 2022, 643, 414190. [Google Scholar] [CrossRef]
- Yin, X.-T.; Huang, H.; Xie, J.-L.; Dastan, D.; Li, J.; Liu, Y.; Tan, X.-M.; Gao, X.-C.; Shah, W.A.; Ma, X.-G. High-Performance Visible-Light Active Sr-Doped Porous LaFeO3 Semiconductor Prepared via Sol–Gel Method. Green. Chem. Lett. Rev. 2022, 15, 546–556. [Google Scholar] [CrossRef]
- Jennings, A.J.; Skinner, S.J.; Helgason, Ö. Structural Properties of LaxSr2−xFeO4 at High Temperature and under Reducing Conditions. J. Solid State Chem. 2003, 175, 207–217. [Google Scholar] [CrossRef]
- Kuo, L.; Roitzheim, C.; Valencia, H.; Mayer, J.; Möller, S.; Myung, S.; Finsterbusch, M.; Guillon, O.; Fattakhova-Rohlfing, D.; Kaghazchi, P. Doping-Induced Surface and Grain Boundary Effects in Ni-Rich Layered Cathode Materials. Small 2024, 20, 2307678. [Google Scholar] [CrossRef]
- Gosavi, P.V.; Biniwale, R.B. Pure Phase LaFeO3 Perovskite with Improved Surface Area Synthesized Using Different Routes and Its Characterization. Mater. Chem. Phys. 2010, 119, 324–329. [Google Scholar] [CrossRef]
- Kaewpanha, M.; Suriwong, T.; Wamae, W.; Nunocha, P. Synthesis and Characterization of Sr-Doped LaFeO3 Perovskite by Sol-Gel Auto-Combustion Method. J. Phys. Conf. Ser. 2019, 1259, 012017. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-Type Lanthanum Ferrite Based Photocatalysts: Preparation, Properties, and Applications. J. Energy Chem. 2022, 66, 314–338. [Google Scholar] [CrossRef]
- Ghozza, M.H.; Yahia, I.S.; Hussien, M.S.A. Structure, Magnetic, and Photocatalysis of La0.7Sr0.3MO3 (M = Mn, Co, and Fe) Perovskite Nanoparticles: Novel Photocatalytic Materials. Environ. Sci. Pollut. Res. 2023, 30, 61106–61122. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Subohi, O.; Dayal, S.; Srivastava, S. Structural Studies and Exchange Bias Interaction in Sr+2 Doped LaFeO3 Synthesised via PVA Based Sol-Gel Method. Mater. Res. Bull. 2019, 120, 110593. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Soares, J.M.; Da Costa, J.A.P.; De Araújo, J.H.; Rodrigues, A.R.; Machado, F.L.A. Local Iron Ion Distribution and Magnetic Properties of the Perovskites La1−xSrxFeO3−γ. J. Magn. Magn. Mater. 2018, 466, 306–310. [Google Scholar] [CrossRef]
- Shen, Z.; Zhuang, Y.; Li, W.; Huang, X.; Oropeza, F.E.; Hensen, E.J.M.; Hofmann, J.P.; Cui, M.; Tadich, A.; Qi, D.; et al. Increased Activity in the Oxygen Evolution Reaction by Fe4+-Induced Hole States in Perovskite La1−xSrx FeO3. J. Mater. Chem. A 2020, 8, 4407–4415. [Google Scholar] [CrossRef]
- Qing, G.; Thompson, D.; Benamara, M.; Heske, C.; Greenlee, L.; Chen, J. Ambient-Pressure Ozone Treatment Enables Tuning of Oxygen Vacancy Concentration in the La1−xSrxFeO3−δ (0 ≤ x ≤ 1) Perovskite Oxides. Mater. Adv. 2022, 3, 8229–8240. [Google Scholar] [CrossRef]
- Téllez-Tovar, X.J.; Aguirre-Espinosa, J.C.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Bolarín-Miró, A.M. Induction of Ferromagnetism in Multiferroic LaFeO3 by Doping with Ni2+. J. Mater. Sci. Mater. Electron. 2024, 35, 1406. [Google Scholar] [CrossRef]
- Pan, B.; Miao, H.; Liu, F.; Wu, M.; Yuan, J. Optimizing La1−xSrxFeO3−δ Electrodes for Symmetrical Reversible Solid Oxide Cells. Int. J. Hydrogen Energy 2023, 48, 11045–11057. [Google Scholar] [CrossRef]
- Bally, M.A.A.; Khan, F.A. Structural, Dielectric and Magnetic Properties of La0.55Sr0.45MnO3 Polycrystalline Perovskite. J. Magn. Magn. Mater. 2020, 509, 166897. [Google Scholar] [CrossRef]
- Fan, K.; Qin, H.; Wang, L.; Ju, L.; Hu, J. CO2 Gas Sensors Based on La1−xSrxFeO3 Nanocrystalline Powders. Sens. Actuators B Chem. 2013, 177, 265–269. [Google Scholar] [CrossRef]
- Alhadhrami, A.; Zeshan, M.; Farid, H.M.T. The Structural and Dielectric Properties of Lanthanum Substituted Strontium Based Spinel Ferrites Nano-Materials for High Frequency Device Applications. J. Taibah Univ. Sci. 2023, 17, 2236368. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Mahapatra, A.S.; Chakrabarti, P.K. Multiferroic Behavior, Enhanced Magnetization and Exchange Bias Effect of Zn Substituted Nanocrystalline LaFeO3 (La(1−x)ZnxFeO3, X = 0.10, and 0.30). J. Magn. Magn. Mater. 2013, 329, 133–141. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Kumari, S.; Vasudevan, R.K.; Dugu, S.; Das, P.T.; Puli, V.S.; Pradhan, D.K.; Kalinin, S.V.; Katiyar, R.S.; Rack, P.D.; et al. Room Temperature Multiferroicity and Magnetodielectric Coupling in 0–3 Composite Thin Films. J. Appl. Phys. 2020, 127, 194104. [Google Scholar] [CrossRef]
- Ghosh, A.; Trujillo, D.P.; Choi, H.; Nakhmanson, S.M.; Alpay, S.P.; Zhu, J.-X. Electronic and Magnetic Properties of Lanthanum and Strontium Doped Bismuth Ferrite: A First-Principles Study. Sci. Rep. 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Tan, G. Magnetodielectric Coupling Response in La-Modified M-Type Strontium Hexaferrite. Phys. Status Solidi (A) 2018, 215, 1800295. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, G. Antiferroelectric and Magnetodielectric Coupling Response of La0.2Sr0.7Fe12O19 Ceramics. arXiv 2018, arXiv:1803.00731. [Google Scholar] [CrossRef]

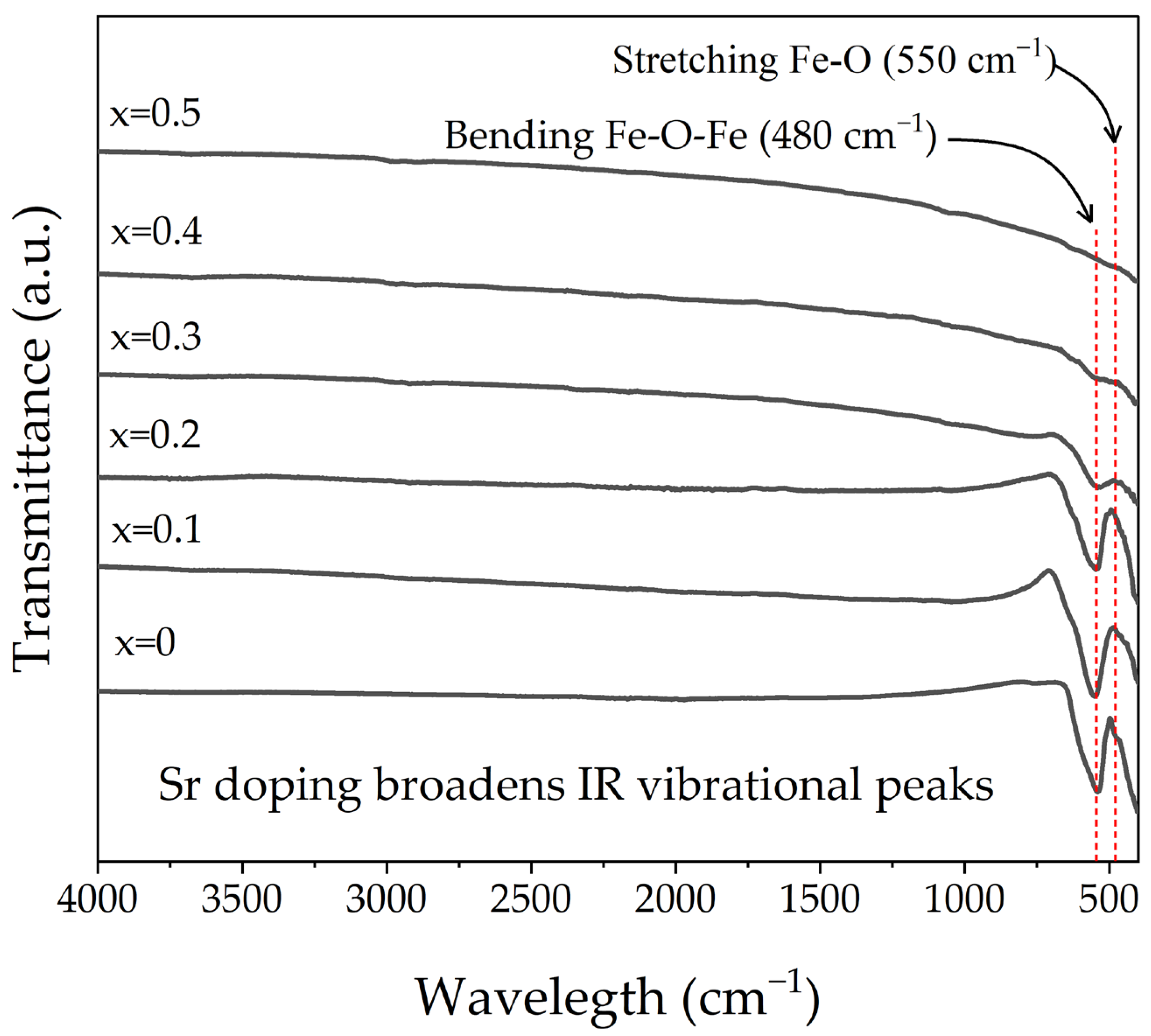


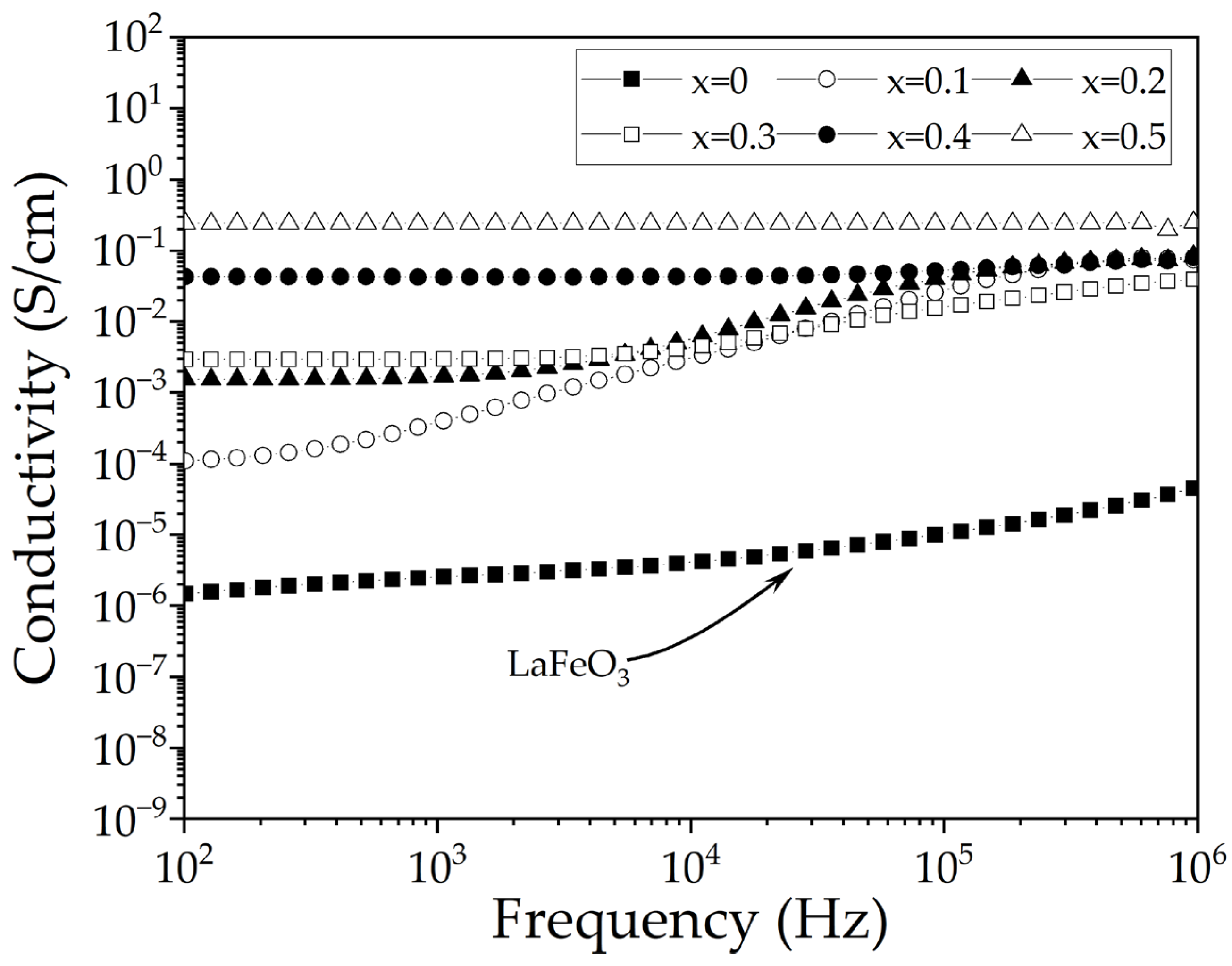


| Sr2+ Content (x, mol) | Phase S.G. | Phase wt.% | Lattice Parameters (Å) | Crystallite Size (nm) | Microstrain (×10−4) | Goodness of Fit | |||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | Rwp | χ2 | |||||
| 0 | LaFeO3 Pnma | 100 ±0.0 | 5.55 ±4 × 10−3 | 7.86 ±4 × 10−3 | 5.55 ±5 × 10−3 | 189.34 ±2.93 | 5.02 ±0.23 | 20.29 | 1.08 |
| 0.1 | LaFeO3 Pnma | 100 ±0.0 | 5.53 ±9 × 10−3 | 7.84 ±8 × 10−3 | 5.55 ±9 × 10−3 | 151.98 ±3.08 | 5.99 ±0.17 | 20.44 | 1.06 |
| 0.2 | LaFeO3 Pnma | 100 ±0.0 | 5.52 ±3 × 10−3 | 7.81 ±5 × 10−3 | 5.55 ±3 × 10−3 | 121.54 ±1.73 | 2.13 ±0.71 | 19.59 | 1.11 |
| 0.3 | LaFeO3 Pnma | 22.65 ±0.0 | 5.50 ±2 × 10−3 | 7.80 ±2 × 10−3 | 5.53 ±1 × 10−3 | 122.82 ±5.52 | 3.72 ±1.16 | 17.90 | 1.02 |
| La0.6Sr0.4FeO2.976 R-3c | 71.35 ±6.45 | 5.54 ±4 × 10−3 | - | 13.44 ±2 × 10−3 | 57.34 ±5.80 | 5.01 ±0.05 | |||
| La0.8Sr1.2FeO3.714 I4/mmm | 5.99 ±0.46 | 3.87 ±1 × 10−3 | - | 12.71 ±2 × 10−3 | 9.99 ±0.0 | 3.54 ±0.0 | |||
| 0.4 | La0.6Sr0.4FeO2.976 R-3c | 90.91 ±0.0 | 5.52 ±3 × 10−3 | - | 13.44 ±8 × 10−3 | 98.10 ±1.46 | 6.04 ±0.28 | 18.86 | 1.07 |
| La0.8Sr1.2FeO3.714 I4/mmm | 9.08 ±0.29 | 3.87 ±2 × 10−3 | - | 12.74 ±1 × 10−3 | 10.14 ±0.0 | 0.08 ±0.0 | |||
| 0.5 | La0.6Sr0.4FeO2.976 R-3c | 100 ±0.0 | 5.49 ±7 × 10−3 | - | 13.51 ±3 × 10−3 | 100.02 ±1.53 | 6.75 ±0.76 | 20.79 | 1.08 |
| Sr2+ Content (x, mol) | Element (at%) | |||
|---|---|---|---|---|
| La | Sr | Fe | O | |
| 0 | 20.23 | 0 | 22.78 | 56.99 |
| 0.1 | 16.38 | 4.39 | 20.83 | 58.39 |
| 0.2 | 15.47 | 6.10 | 21.62 | 56.81 |
| 0.3 | 11.89 | 7.66 | 20.87 | 59.57 |
| 0.4 | 10.74 | 10.75 | 20.48 | 58.04 |
| 0.5 | 8.48 | 14.51 | 19.91 | 57.10 |
| Sr2+ Content (x, mol) | Ms at 18 kOe (emu/g) | χm (×10−10 m3Kg−1) | Hc (kOe) | Mr (emu/g) | Magnetic Behavior |
|---|---|---|---|---|---|
| 0 | 0.14 | 1.04 ± 0.01 | - | - | AFM |
| 0.1 | 3.15 | - | 3.93 | 1.51 | FM |
| 0.2 | 0.28 | 0.20 ± 0.01 | - | - | AFM |
| 0.3 | 0.51 | - | 3.79 | 0.11 | FM |
| 0.4 | 0.38 | 0.27 ± 0.02 | - | - | AFM |
| 0.5 | 0.46 | 0.32 ± 0.03 | - | - | AFM |
| Sr+2 Content, x (mol) | Angle Fe-O-Fe (Degree) | Distance Fe-O (Å) |
|---|---|---|
| 0 | 157.89 | 2.00 |
| 0.1 | 153.71 | 2.03 |
| 0.2 | 180 | 1.95 |
| 0.3 | 180 | 1.92 |
| 0.4 | 180 | 1.93 |
| 0.5 | 174.44 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre-Espinosa, J.C.; Sánchez-De Jesús, F.; Cortés-Escobedo, C.A.; Bolarín-Miró, A.M. Insights into the Crystal Structure and Magnetodielectric Properties of High-Energy Ball Milled Sr Substituted LaFeO3. Materials 2025, 18, 3014. https://doi.org/10.3390/ma18133014
Aguirre-Espinosa JC, Sánchez-De Jesús F, Cortés-Escobedo CA, Bolarín-Miró AM. Insights into the Crystal Structure and Magnetodielectric Properties of High-Energy Ball Milled Sr Substituted LaFeO3. Materials. 2025; 18(13):3014. https://doi.org/10.3390/ma18133014
Chicago/Turabian StyleAguirre-Espinosa, Julio C., Félix Sánchez-De Jesús, Claudia A. Cortés-Escobedo, and Ana M. Bolarín-Miró. 2025. "Insights into the Crystal Structure and Magnetodielectric Properties of High-Energy Ball Milled Sr Substituted LaFeO3" Materials 18, no. 13: 3014. https://doi.org/10.3390/ma18133014
APA StyleAguirre-Espinosa, J. C., Sánchez-De Jesús, F., Cortés-Escobedo, C. A., & Bolarín-Miró, A. M. (2025). Insights into the Crystal Structure and Magnetodielectric Properties of High-Energy Ball Milled Sr Substituted LaFeO3. Materials, 18(13), 3014. https://doi.org/10.3390/ma18133014







