Mineral-Forming Effect of the Joint Participation of Natural Infusible Calcium Silicate and Dust-like Silica in Ceramic Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Kaolins
2.1.2. Clays
2.1.3. Feldspars
2.1.4. Quartz Sands
2.1.5. Marshalite
2.1.6. Wollastonite
2.2. Preparation of Ceramic Masses, Molding of Samples
2.3. Heat Treatment of Semi-Finished Products
- the first period occurs with a temperature rise to 800–830 °C. During this period, very little sintering of the material is achieved. Within the range from 20 °C to 800 °C, the temperature rises at a rate of 150–200 °C per hour with a holding time of 550–600 °C;
- the second period begins with 800–850 °C and ends with a temperature of 1000–1080 °C. It is characterized by a slow rise in temperature of 30–50 °C per hour;
- in the third period, the temperature rises to 1350 °C at a rate of 60–100 °C per hour and ends with a 2–3 h hold at the final temperature (1350 °C);
- the fourth period is cooling, the temperature is reduced to 800–700 °C at a high speed (200–250 °C/hour), and then the cooling rate decreases.
2.4. Determination of the Main Technical Indicators of Synthesized Samples of Electrical Porcelain, Studies of the Chemical and Mineralogical Composition and Microstructure
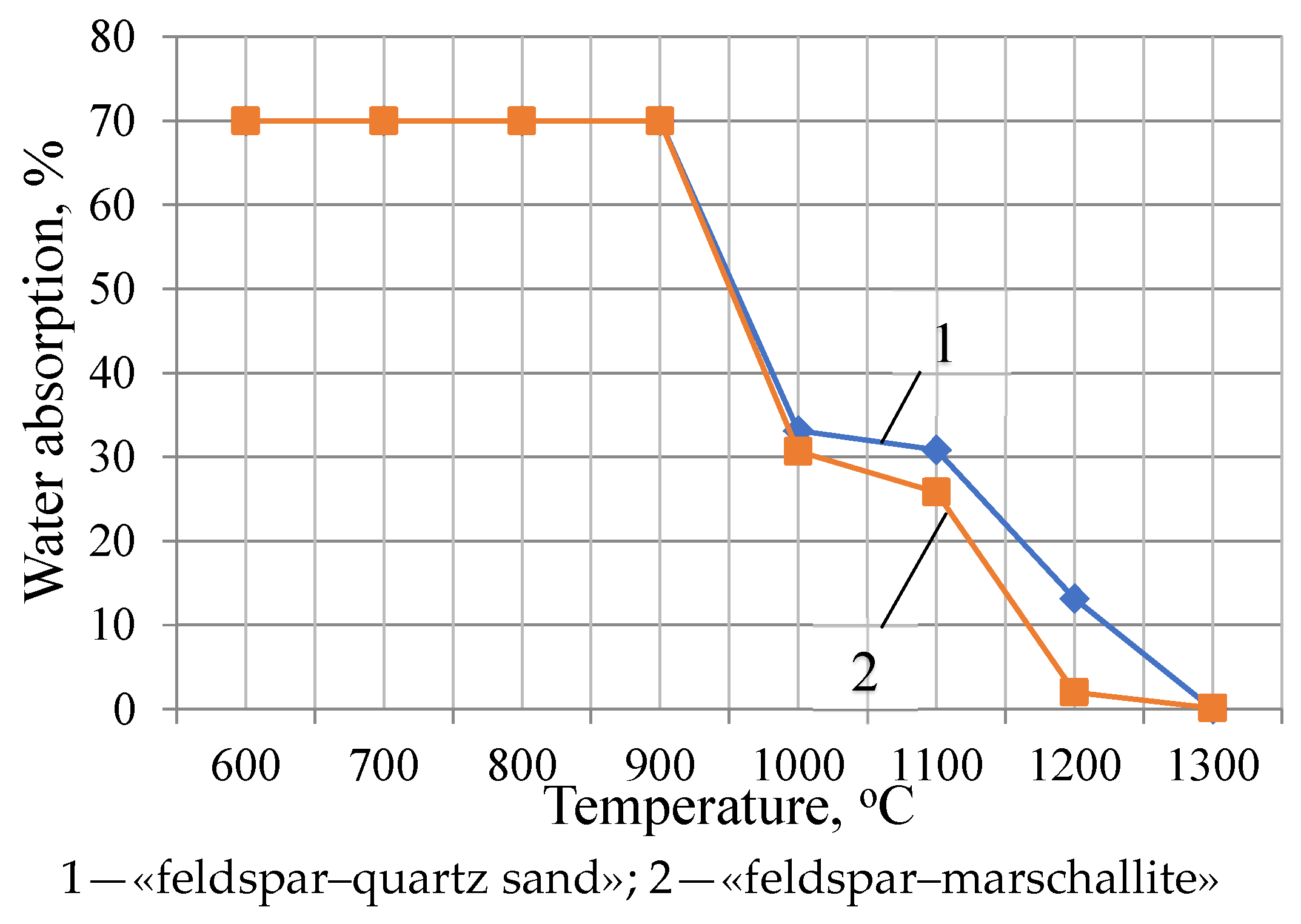
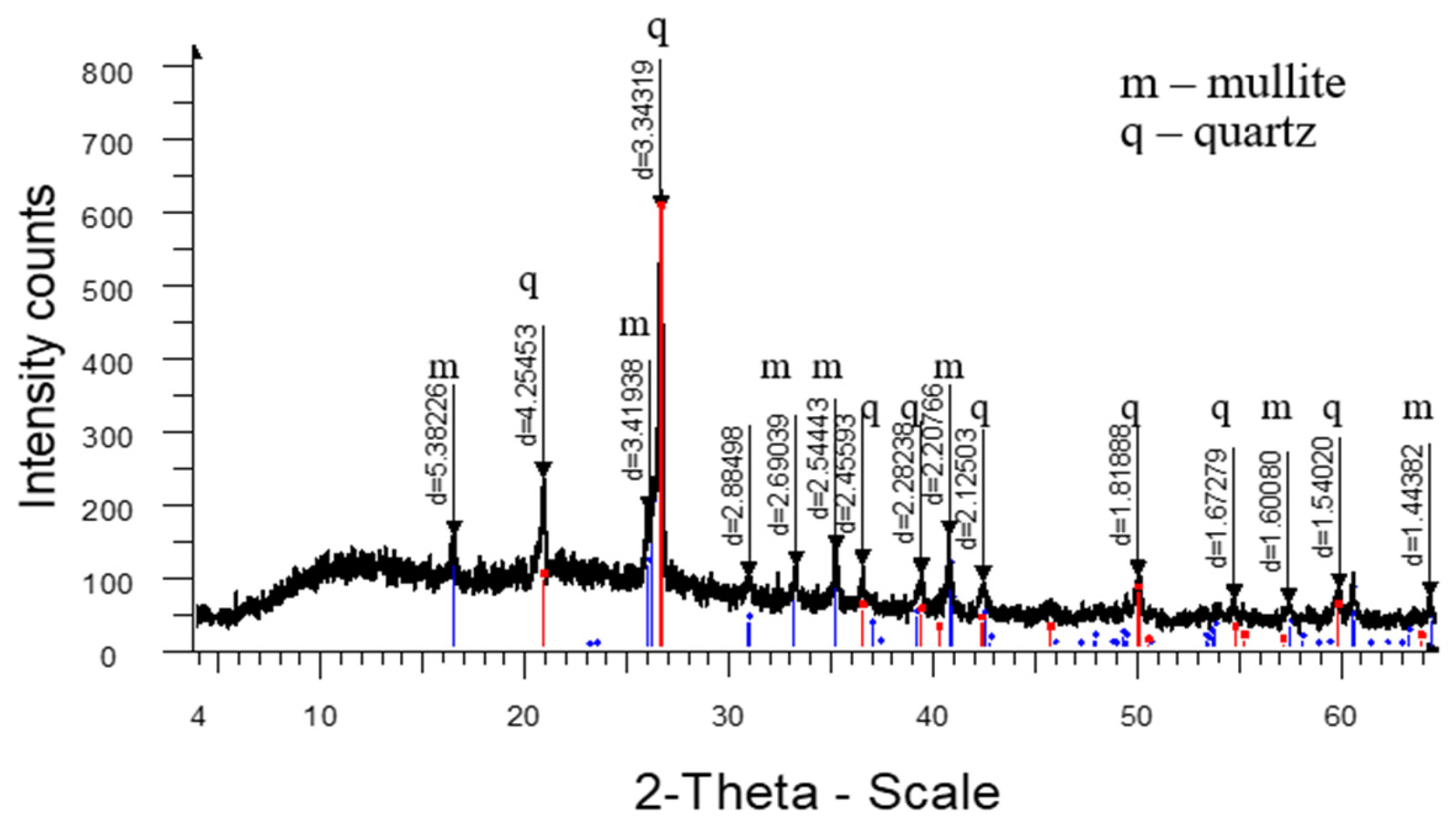
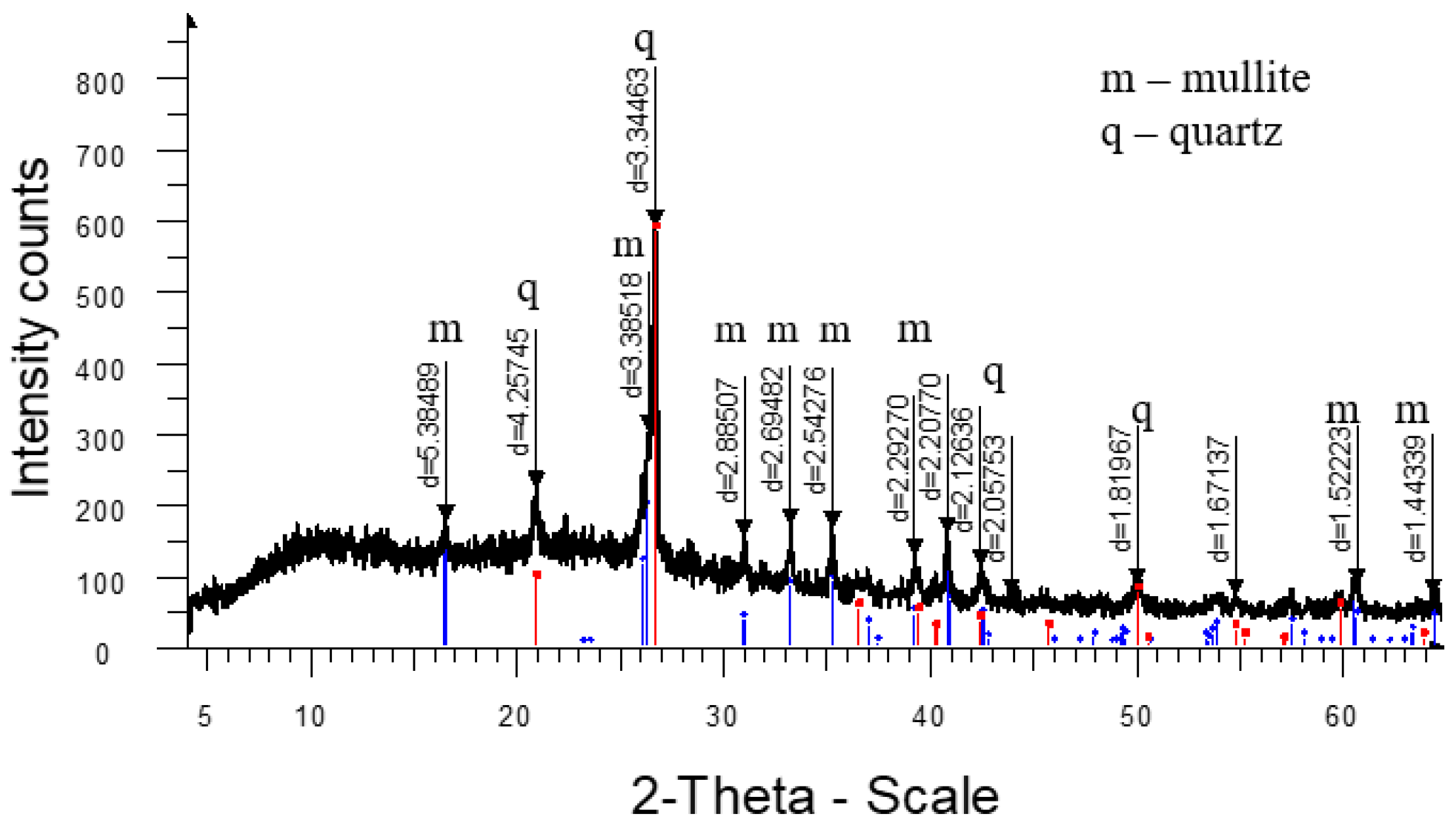
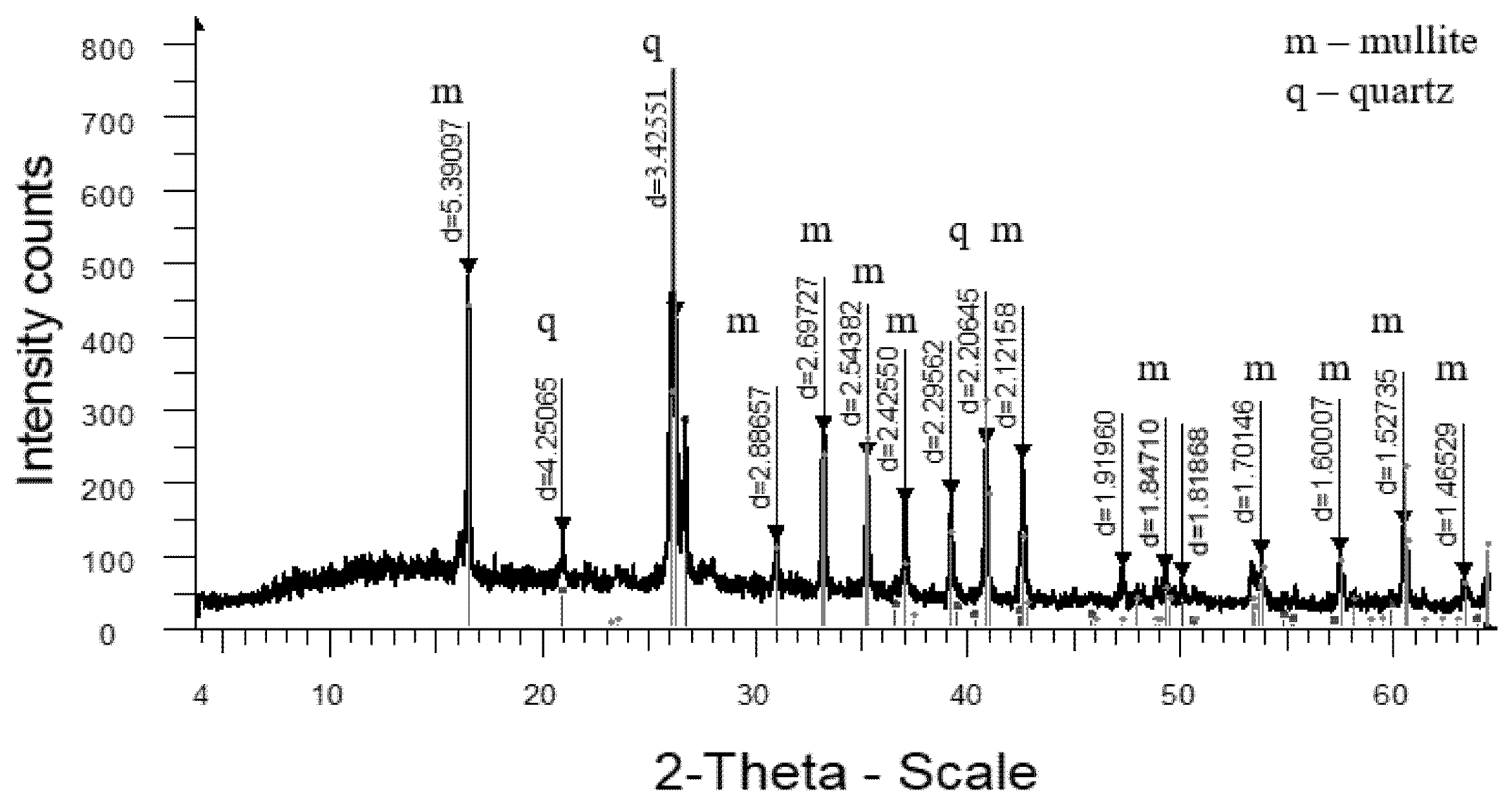
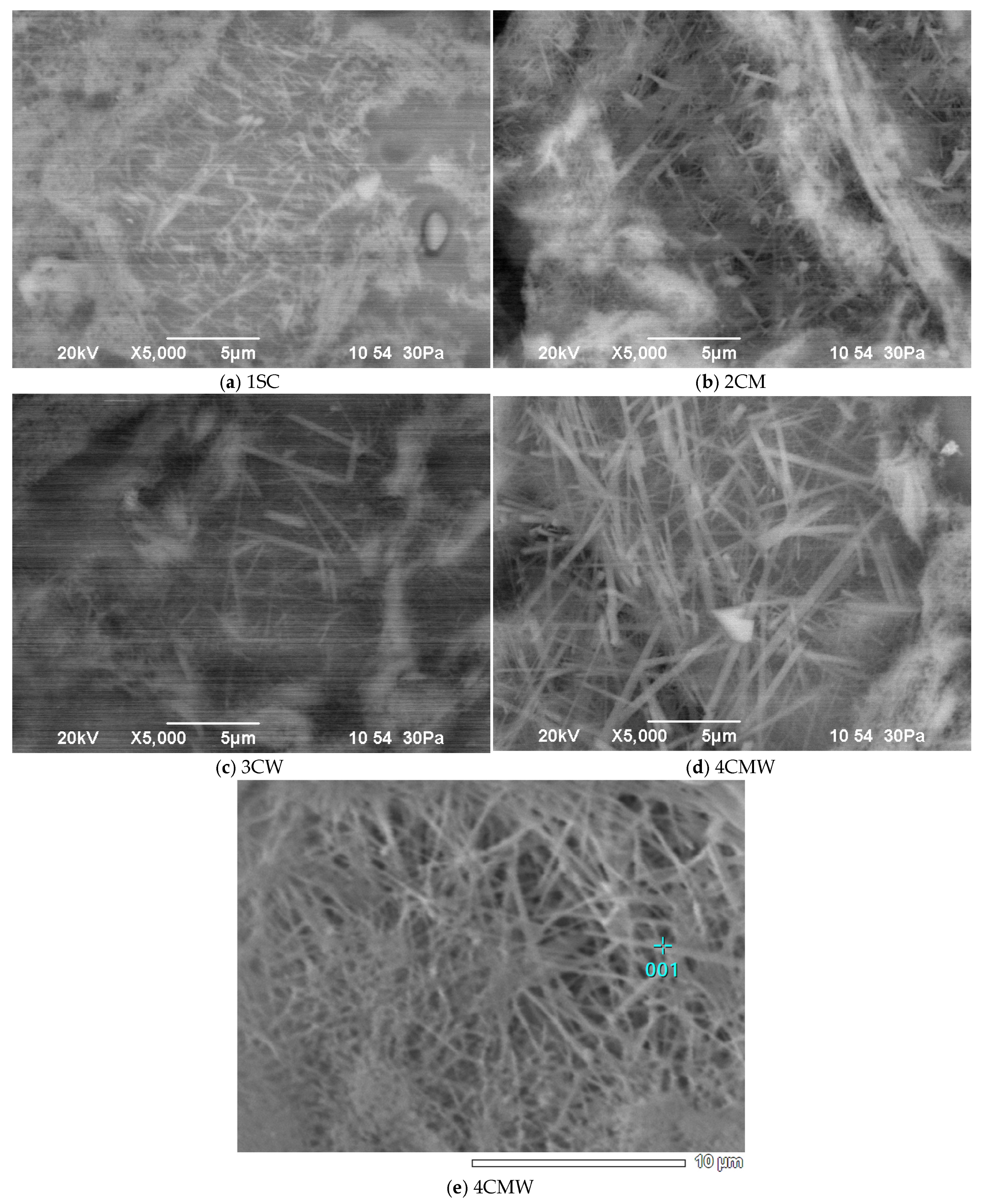
3. Results and Discussion
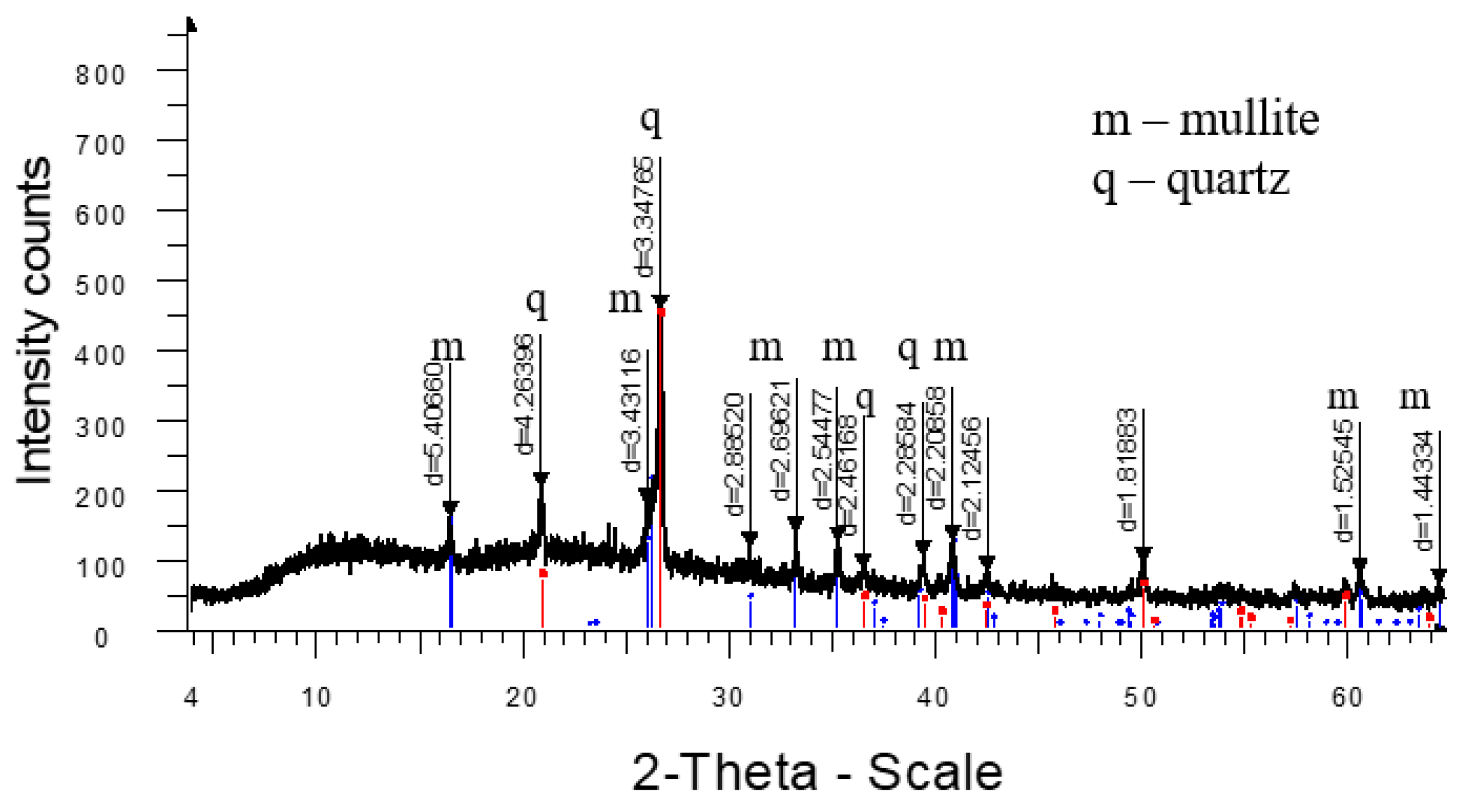

4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Augustinik, A.I. Ceramics; Stroyizdat: Leningrad, Russia, 1975; 592p. (In Russian) [Google Scholar]
- Maslennikova, G.N.; Kharitonov, F.Y.; Kostyukov, N.S.; Pirogov, K.S. Technology of Electroceramics; Energy: Moscow, Russia, 1974; 224p. (In Russian) [Google Scholar]
- Guzman, I.J. Chemical Technology of Ceramics; Building Materials: Moscow, Russia, 2003; 496p, ISBN 5-94026-004-7. (In Russian) [Google Scholar]
- Sulimenko, L.M. General Technology of Silicates; INFRA-M: Moscow, Russia, 2010; 336p, ISBN 978-5-16-003832-2. (In Russian) [Google Scholar]
- Maslennikova, G.N.; Konesheva, T.I. The effect of mineralizers on the sintering of porcelain masses. Glass Ceram. 1987, 4, 13–15. (In Russian) [Google Scholar]
- Moroz, I.I.; Komskaya, M.S.; Oleynikova, L.L. Reference Book of the Porcelain and Faience Industry; V. 2; Higher School: Moscow, Russia, 1989; 327p. (In Russian) [Google Scholar]
- Oladiji, A.O.; Borode, J.O.; Adewuyi, B. Development of porcelain insulators from locally sourced materials. USEP J. Res. Inf. Civ. Eng. 2010, 7, 47–58. [Google Scholar]
- Park, S.E.; Ngayakamo, B. Effect of firing temperature on triaxial electrical porcelain properties made from Tanzania locally sourced ceramic raw materials. Építöanyag-J. Silic. Based Compos. Mater. 2018, 70, 106–109. [Google Scholar] [CrossRef]
- Olupot, P.W.; Jonsson, S.; Byaruhanga, J.K. Development of Electrical Porcelain Insulators from Ceramic Minerals in Uganda. Mech. Prop. Perform. Eng. Ceram. Compos. IX 2014, 35, 115. [Google Scholar] [CrossRef]
- Nwachukwu, V.C.; Lawal, S.A. Investigating the Production Quality of Electrical Porcelain Insulators from Local Materials. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Conference on Engineering for Sustainable World, Ota, Nigeria, 9–13 July 2018; IOP Publishing Ltd.: Bristol, UK, 2018; Volume 413, pp. 1–8. [Google Scholar] [CrossRef]
- Dana, K.; Das, S.K. Differences in Densification Behaviour of K–and Na–Feldspar–Containing Porcelain Bodies. Thermochim. Acta 2003, 406, 199–206. [Google Scholar] [CrossRef]
- Noori, N.R.; Mamoory, R.S.; Mehraeen, S. Effect of Materials Design on Properties of Porcelain Insulators. Am. Ceram. Soc. Bull. 2017, 86, 9201–9203. [Google Scholar]
- Moyo, M.G.; Park, E. Ceramic Raw Materials in Tanzania—Structure and Properties for Electrical Insulation Application. Int. J. Eng. Res. Technol. 2014, 3, 1015–1020. [Google Scholar]
- Amare, E.; Merga, A.; Ahmed, K.; Bekele, E.; Murthy, H. Fabrication of electrical porcelain insulator from ceramic raw materials of Oromia region, Ethiopia. Heliyon 2019, 5, e02327. [Google Scholar] [CrossRef]
- Dana, K.; Das, S.; Das, S.K. Effect of Substitution of Fly Ash for Quartz in Triaxial Kaolin–Quartz–Feldspar System. J. Eur. Ceram. Soc. 2004, 24, 3169–3175. [Google Scholar] [CrossRef]
- Díaz-Tato, L.; González-Carranza, Y.; Rodríguez, E.A.; López-Perales, J.F. Effect of binary raw materials replacement (quartz and feldspar) for porcelain chamotte on the electro-technical siliceous porcelain properties. Front. Mater. 2023, 10, 1322898. [Google Scholar] [CrossRef]
- Guedes, D.G.; Santana, L.N.d.L.; Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A.; da Costa, F.P. Sustainable ceramic materials manufactured fromceramic formulations containing quartzite scheelite tailings. Sustainability 2020, 12, 9417. [Google Scholar] [CrossRef]
- Correia, S.L.; Dienstmann, G.; Folgueras, M.V.; Segadaes, A.M. Effect of quartz sand replacement by agate rejects in triaxial porcelain. J. Hazard. Mater. 2009, 163, 315–322. [Google Scholar] [CrossRef]
- Brehane, B.; Tullu, A.M.; Zereffa, E.A.; Addis, T.; Wondemagegnehu, E.B. Sugarcane Bagasse ash substituent feldspar for the production of porcelain electrical insulators. Ceram. Int. 2023, 49, 7727–7736. [Google Scholar]
- Kawai, S. Preparation of mullite M. Yoshida from kaolin by dry grinding. J. Ceram. Soc. Jpn. 1990, 98, 363–369. [Google Scholar] [CrossRef]
- Brindley, J.W. New concept of the transformation sequence of kaolinite to mullite. J. Eur. Ceram. Soc. 1958, 181, 1333–1334. [Google Scholar] [CrossRef]
- Mehta, N.S.; Sahu, P.K.; Tripathi, P.; Pyare, R.; Majhi, M.R. Influence of alumina and silica addition on the physico-mechanical and dielectric behavior of ceramic porcelain insulator at high sintering temperature. Boletín Soc. Española Cerámica Vidrio 2018, 57, 151–159. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. (Eds.) Crystal Structures of Clay Minerals and Their X-ray Identification; The Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1980; 499p. [Google Scholar]
- Yesimov, B.O.; Rusnak, V.V.; Adyrbaeva, T.A.; Egorov, Y.u.V.; Anashkin, A.V.; Adyrbaev, B.O. Mineral Resources of the Turkestan Region for Cement, Ceramic and Glass Industries; Monograph, Alem Printing House: Shymkent, Kazakhstan, 2025; 352p. (In Russian) [Google Scholar]
- Vereshchagin, V.I.; Mogilevskaya, N.V.; Gorbachev, D.V. Low-fired porcelain with diopside and marshalite additions. Glass Ceram. 2013, 69, 401–404. [Google Scholar] [CrossRef]
- Katerinopoulou, A.; Schmith, J.H.; Piazolo, S.; Balić-Žunić, T. Full analysis of feldspar texture and crystal structure by combining X-ray and electron techniques. Am. Mineral. 2012, 98, 41–52. [Google Scholar] [CrossRef]
- Melchiades, F.; Boschi, A.; Alves, H. Effect of feldspar particle size on the porous microstructure and stain resistance of polished porcelain tiles. J. Eur. Ceram. Soc. 2012, 32, 2095–2102. [Google Scholar]
- Correia, S.L.; Oliveira, A.P.N.; Hotza, D.; Segadães, A.M. Properties of Triaxial Porcelain Bodies: Interpretation of Statistical Modeling. J. Am. Ceram. Soc. 2006, 89, 3356–3365. [Google Scholar] [CrossRef]
- Adyrbaev, B.O.; Darkhan, A.Z.; Yessimov, B.O.; Adyrbaeva, T.A.; Dubinina, E.S. Synthesis of ceramic granite based on domestic feldspar raw materials. In News of the National Academy of Sciences of the Republic of Kazakhstan; Geology and Engineering Sciences Series; National Academy of Sciences of the Republic of Kazakhstan: Almaty, Kazakhstan, 2024; Volume 6, pp. 6–18. [Google Scholar] [CrossRef]
- Oruru, B.; Ochen, W.; D’UJanga, F.M. Influence of residual stress on the mechanical behavior of ceramics with various quartz sizes. Sci. Afr. 2021, 11, e00648. [Google Scholar] [CrossRef]
- Salman, M.M.; Nhabih, H.T. Influence of quartz replacement by Iraqi porcelanite and silica fume on the properties of porcelain products. Res. Eng. Struct. Mater. 2025, 11, 921–931. [Google Scholar] [CrossRef]
- Braganca, S.; Bergmann, C.; Hubner, H. Effect of quartz particle size on the strength of triaxial porcelain. J. Eur. Ceram. Soc. 2006, 26, 3761–3768. [Google Scholar] [CrossRef]
- Junior, A.D.N.; Hotza, D.; Soler, V.C.; Vilches, E.S. Effect of quartz particle size on the mechanical behavior of porcelain tile subjected to different cooling rates. J. Eur. Ceram. Soc. 2009, 29, 1039–1046. [Google Scholar]
- Boussouf, L.; Zehani, F.; Khenioui, Y.; Boutaoui, N. Effect of amount and size of quartz on mechanical and dielectric properties of electrical porcelain. Trans. Indian Ceram. Soc. 2018, 77, 132–137. [Google Scholar] [CrossRef]
- Bragança, S.R.; Bergmann, C.P. Waste glass in porcelain. Mater. Res. 2005, 8, 39–44. [Google Scholar] [CrossRef]
- Kasmamytov, N.K.; Kalenderov, A.Z. Effect of mineral additives on firing temperature properties of electrical porcelain (review). Eurasian Sci. Assoc. 2020, 59, 40–45. (In Russian) [Google Scholar]
- Alarson, J.; Guillem, C.; Guillem, M.C. Action of calcium carbonate as mineraliser of porcelain bodies. Interceram 1984, 4, 37–39. [Google Scholar]
- Kurczyk, H.G. Synthetic diopside and synthetic wollastonite new raw materials for ceramics. In Advanced Ceramic Processing; Faenza Publishing: Faenza, Italy, 1978; pp. 22–29. [Google Scholar]
- Vereshchagin, V.I.; Kurbanbaev, M.E.; Yesimov, B.O.; Root, L.O.; Mogilevskaya, N.V. Sintering processes, phase formation, structure formation and properties of electrical porcelain using marshalite and wollastonite additives. News Univ. Chem. Chem. Technol. 2024, 67, 87–98. [Google Scholar] [CrossRef]
- Turkmen, O. Effect of wollastonite addition on sintering of hard porcelain. Ceram. Int. 2015, 41, 5505–5512. [Google Scholar] [CrossRef]
- Carús, L.A.; de Souza, F.; Bragança, S.R. Use of wollastonite as a flux for bone china bodies. ISRN Ceram. 2012, 2012, 701821. [Google Scholar] [CrossRef]
- Kurbanbaev, M.E. High-Voltage Electrical Porcelain Based on Mineral Raw Materials of the Republic of Kazakhstan Using Marshalite and Wollastonite. Dissertation for the Degree of Candidate of Technical Sciences, National Research Tomsk Polytechnic University, Tomsk, Russia, 2021. (In Russian). [Google Scholar]
- Aitulova, Z.M.; Yessimov, B.O.; Adyrbaeva, T.A.; Dubinina, E.S.; Kurbanbayev, M.E. Synthesis of import-substituting blue ultramarine based on mineral raw materials from unique domestic deposits. In News of the National Academy of Sciences of the Republic of Kazakhstan; Geology and Engineering Sciences Series; National Academy of Sciences of the Republic of Kazakhstan: Almaty, Kazakhstan, 2025; Volume 1, pp. 6–18. [Google Scholar] [CrossRef]
- Kulinich, V.V.; Sagunov, V.G.; Uzhkenov, B.S.; Gulyaeva, N.Y.; Beiseev, O.B.; Vedernikov, N.N.; Antonenko, A.A. Deposits of mining raw materials in Kazakhstan. In Reference Book—Monograph. V.I. Almaty: Inform.—Analyst; Center for Geology, Ecology and Natural Resources of the Republic of Kazakhstan: Astana, Kazakhstan, 2000; 372p. (In Russian) [Google Scholar]
- Guzman, I.Y. (Ed.) Practical training in ceramics technology. In Textbook for Universities; Publishing House “Stroymaterialy”: Moscow, Russia, 2003; 336p. (In Russian) [Google Scholar]
- GOST 21216-2014; Clay Raw Materials. Test Methods. Standartinform: Moscow, Russia, 2015; 40p. (In Russian)
- GOST 24409-80; Ceramic Electrotechnical Materials. Methods of Testing. State Standard: Moscow, Russia, 1989; 30p. (In Russian)
- Ay, N.; Güngör, F. The effect of particle size of body components on the processing parameters of semi transparent porcelain. Ceram. Int. 2018, 44, 10611–10620. [Google Scholar] [CrossRef]
- Ohira, O.; Isoyama, H.; Kobayashi, Y. Effect of Particle Size of Raw Materials on Densification and Pending Strength of Porcelain. J. Ceram. Soc. Jpn. 2000, 108, 921–925. [Google Scholar]
- Artamonov, V.V.; Alekseenko, Y.A.; Peddenezhnny, E.N. Effect of wollastonite additive on structural and physicomechanical characteristics of porcelain ceramics. In Modern Problems of Mechanical Engineering: Abstracts of Reports of the VI International Scientific and Technical Conference, Gomel, Belarus, 19–20 October 2006; Scientific Readings Dedicated to P. O. Sukhoi; Gomel State Technical University: Gomel, Belarus, 2006; pp. 63–64. [Google Scholar]
- Dyatlova, E.M.; Sergievich, O.A.; Ruba, M.A. Synthesis of wollastonite-containing ceramic materials for technical purposes for enterprises of the mechanical engineering industry. Refract. Tech. Ceram. 2019, 6, 31–40. (In Russian) [Google Scholar]
- Gotlib, Y.M.; Sokolova, A.G.; Gimranova, A.R. Study of the influence of wollastonite on the properties of ceramic products. Constr. Econ. 2023, 9, 149–152. (In Russian) [Google Scholar]
- Azarov, G.M.; Maiorova, E.V.; Oborina, M.A. Wollastonite raw materials and their applications (a review). Glass Ceram. 1995, 52, 237–240. [Google Scholar] [CrossRef]
- Negmatov, N.S.; Abdullaev, Z.H.Z. High-voltage electrical insulators using wollastonite. Glass Ceram. 2001, 11, 29–30. (In Russian) [Google Scholar]
- Andreeva, N.A.; Ordanyan, S.S. Technological Possibilities of Increasing the Strength of Porcelain. Refract. Tech. Ceram. 2002, 11, 2–6. (In Russian) [Google Scholar]
- Kryuchkov, Y.u.N. Features of sintering of ceramic materials. Glass Ceram. 2013, 11, 29–34. (In Russian) [Google Scholar]
- Kurbanbaev, M.E. Electrotechnical Porcelain Using Native Fine Silica-Containing Raw Materials and Wollastonites. Glass Ceram. 2020, 76, 468–473. [Google Scholar] [CrossRef]
- Kurbanbayev, M.E.; Vereshchagin, V.I.; Yesimov, B.O.; Adyrbaeva, T.A. Mineral raw materials selection and electrotechnical use porcelain synthesis. In News of the National Academy of Sciences of the Republic of Kazakhstan, Series of Geology and Technical Sciences; National Academy of Sciences of the Republic of Kazakhstan: Almaty, Kazakhstan, 2019; Volume 4, pp. 238–245. [Google Scholar] [CrossRef]
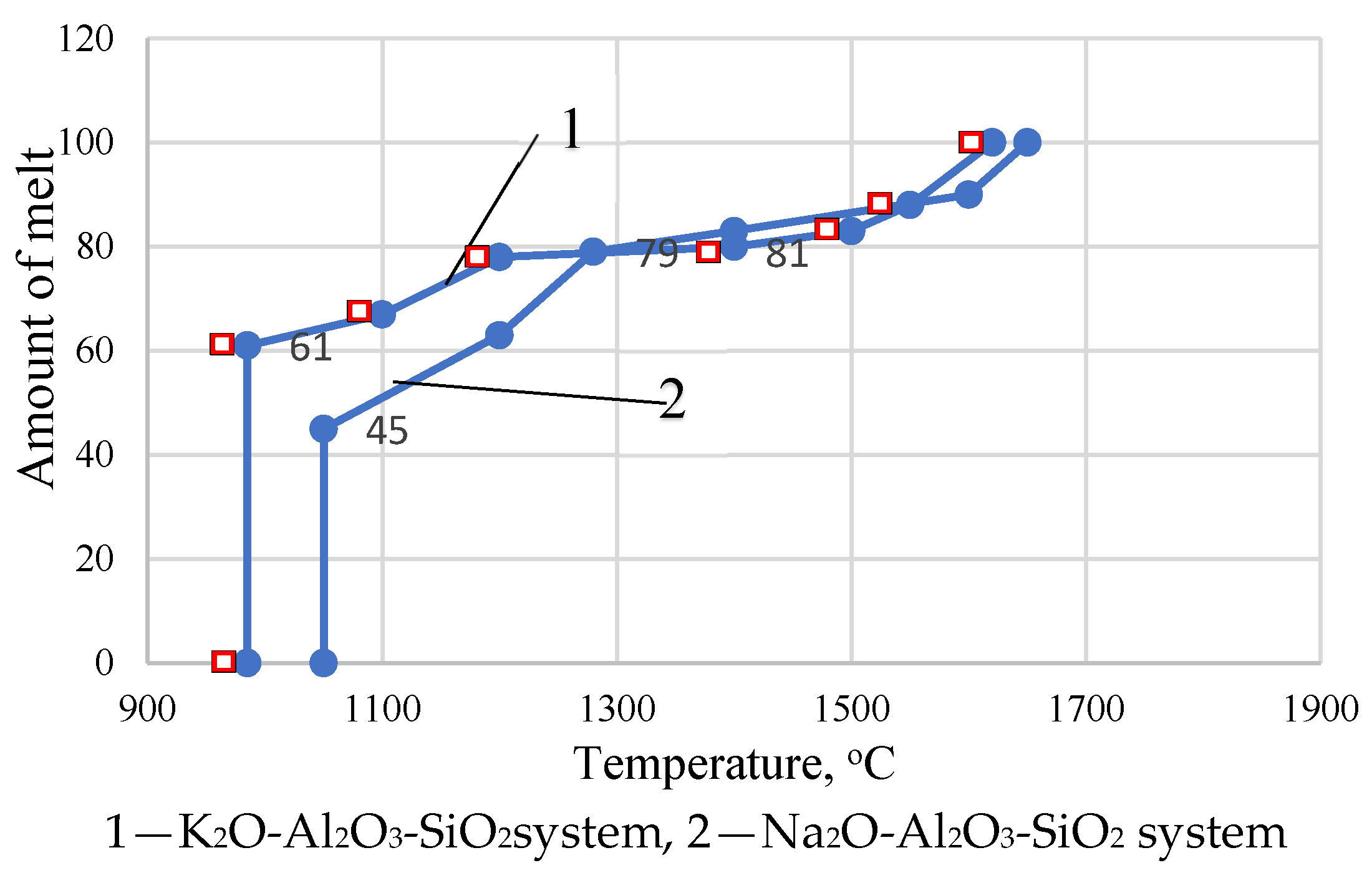


| System | Composition, Mass. % | Temperature, °C | Amount of Melt, % | ||||
|---|---|---|---|---|---|---|---|
| Na2O | K2O | Al2O3 | SiO2 | At the Eutectic Point | Sum in Mass | ||
| K2O-SiO2 | - | 26.4 | - | 73.6 | 767 | 6.55 | 6.55 |
| Na2O-SiO2 | 26.1 | - | - | 73.9 | 793 | 4.07 | 10.62 |
| Al2O3-SiO2 | - | - | 5.5 | 94.5 | 1585 | - | - |
| Properties | State Standard Requirements | Compositions | |||
|---|---|---|---|---|---|
| 1SC | 2CM | 3CW | 4CMW | ||
| Burning temperature, °C | - | 1340 ± 10 | 1290 ± 10 | 1300 ± 10 | 1270 ± 10 |
| Water absorption, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Shrinkage, % | - | 15.5 | 15.7 | 15.1 | 16.1 |
| Density, g/cm3 | 2.3 | 2.39 | 2.48 | 2.45 | 2.53 |
| Bending strength, MPa | 60 | 63.3 | 72.8 | 75.9 | 81.7 |
| Electrical strength, 50 Hz, kV/mm | 25 | 26.5 | 28.2 | 31.8 | 34.2 |
| Thermal resistance, K | 160 | 165 | 191 | 173 | 202 |
| Interval of sintered state, °C | - | 30–40 | 70–80 | 30–40 | 70–80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbanbayev, M.Y.; Yessimov, B.O.; Vereshchagin, V.I.; Adyrbayeva, T.A.; Dubinina, Y.S. Mineral-Forming Effect of the Joint Participation of Natural Infusible Calcium Silicate and Dust-like Silica in Ceramic Compositions. Materials 2025, 18, 2991. https://doi.org/10.3390/ma18132991
Kurbanbayev MY, Yessimov BO, Vereshchagin VI, Adyrbayeva TA, Dubinina YS. Mineral-Forming Effect of the Joint Participation of Natural Infusible Calcium Silicate and Dust-like Silica in Ceramic Compositions. Materials. 2025; 18(13):2991. https://doi.org/10.3390/ma18132991
Chicago/Turabian StyleKurbanbayev, Mukhtar Yendibayevich, Begen Omarovich Yessimov, Vladimir Ivanovich Vereshchagin, Tatyana Amanovna Adyrbayeva, and Yelena Sergeevna Dubinina. 2025. "Mineral-Forming Effect of the Joint Participation of Natural Infusible Calcium Silicate and Dust-like Silica in Ceramic Compositions" Materials 18, no. 13: 2991. https://doi.org/10.3390/ma18132991
APA StyleKurbanbayev, M. Y., Yessimov, B. O., Vereshchagin, V. I., Adyrbayeva, T. A., & Dubinina, Y. S. (2025). Mineral-Forming Effect of the Joint Participation of Natural Infusible Calcium Silicate and Dust-like Silica in Ceramic Compositions. Materials, 18(13), 2991. https://doi.org/10.3390/ma18132991







