Adsorption of Butylparaben and Methylene Blue from Aqueous Solution Using Activated Carbon Derived from Oak Bark
Highlights
- Oak bark was used to produce activated carbon by physical activation.
- The activated carbon had surface areas between 247 and 696 m²/g.
- Butylparaben adsorption capacity ranged from 20 to 154 mg/g.
- Methylene blue adsorption capacity ranged from 13 to 224 mg/g
Abstract
1. Introduction
2. Materials and Methods
2.1. Activated Carbon Preparation
2.2. Characterisation of Activated Carbon
2.3. Adsorption Studies
3. Results and Discussion
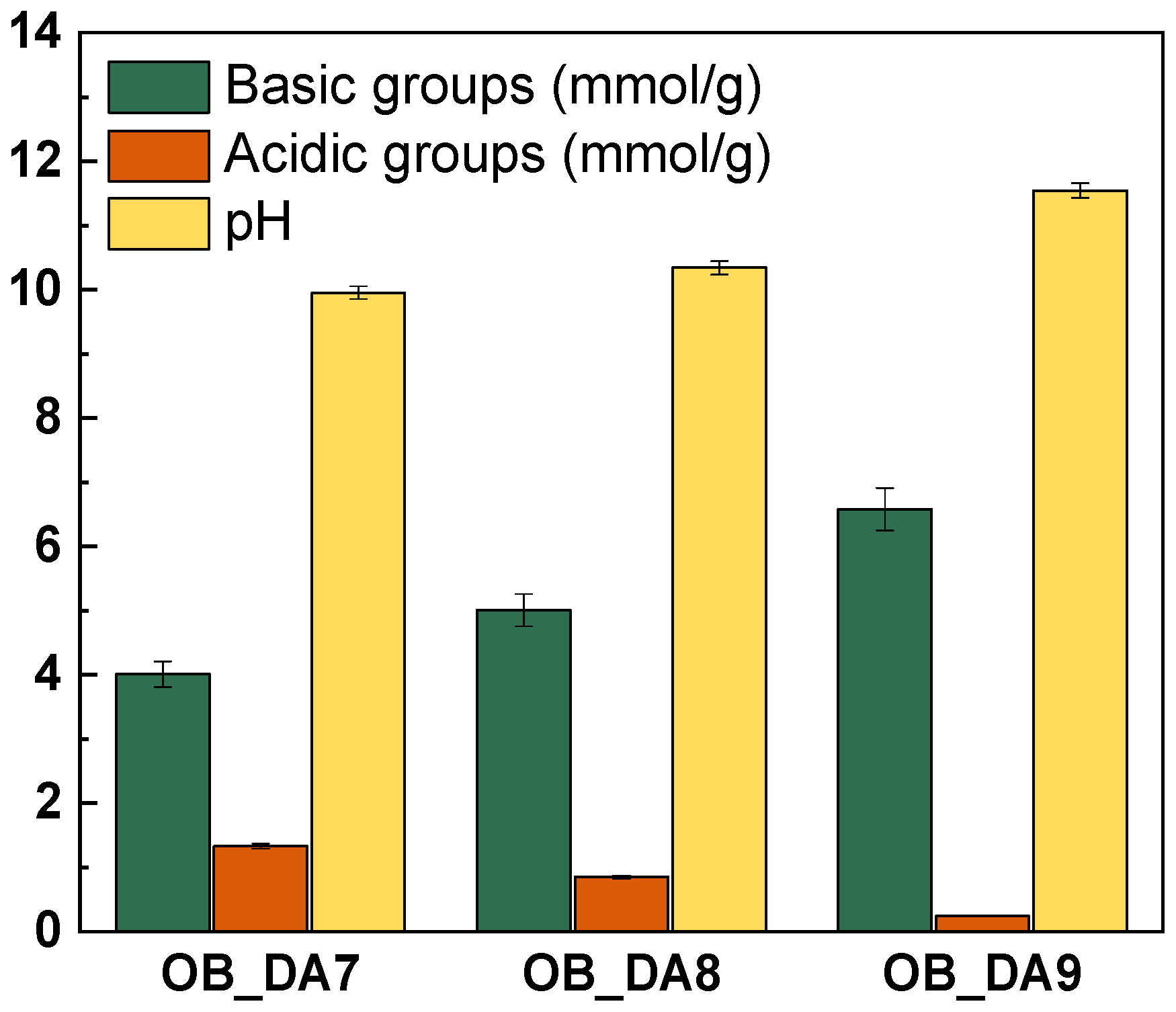
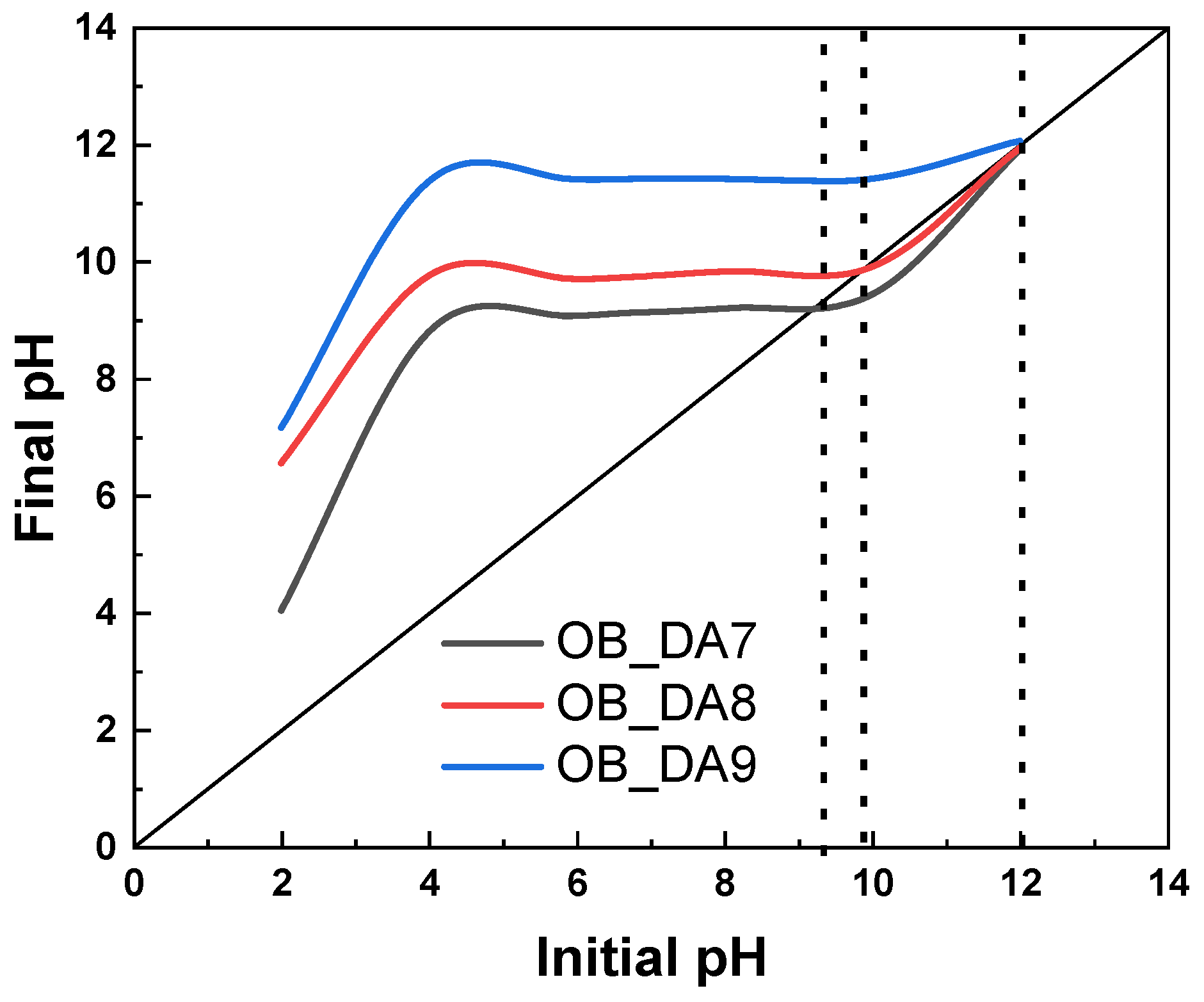
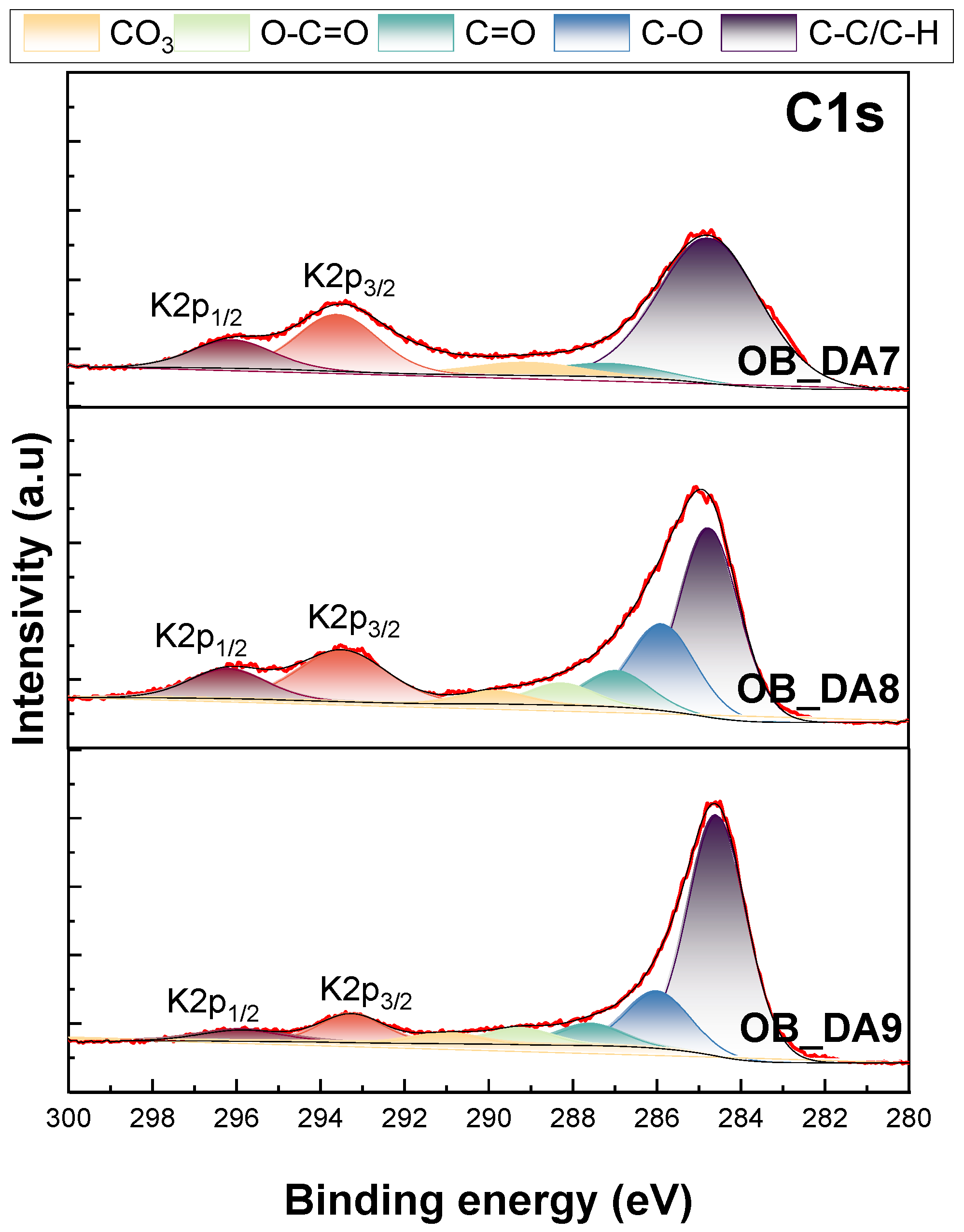
3.1. Physiochemical Characterization of the Activated Carbon
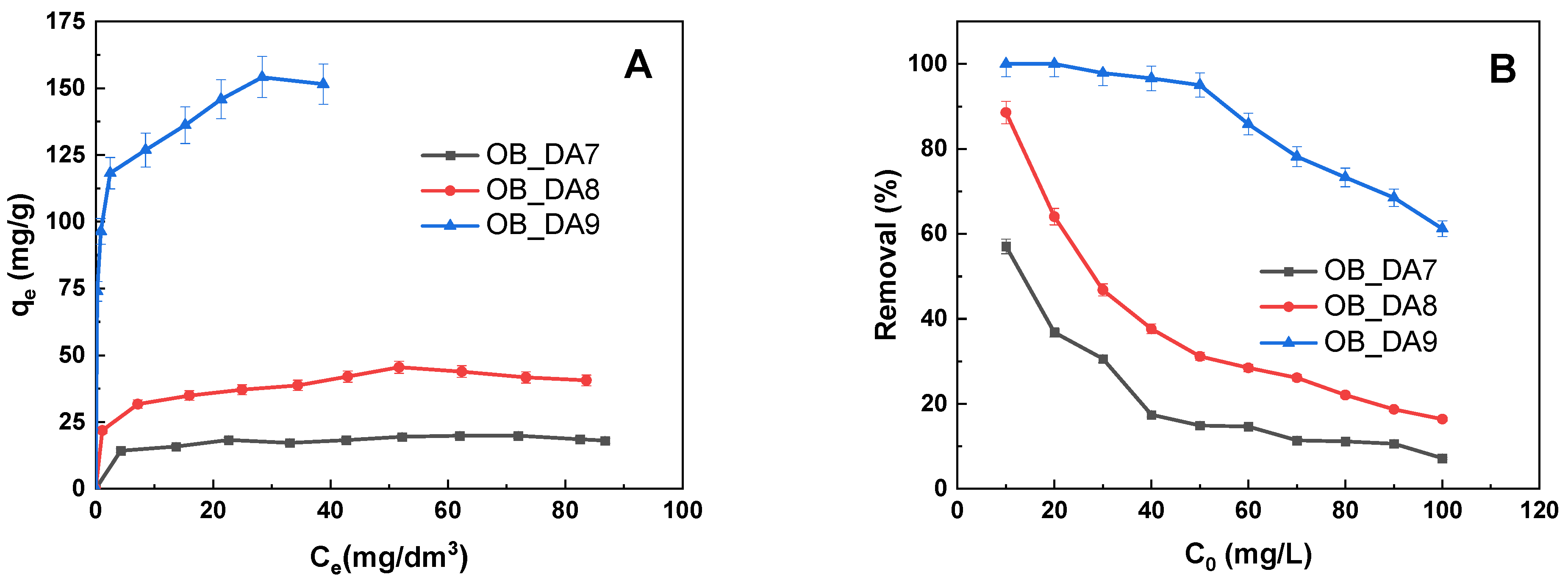
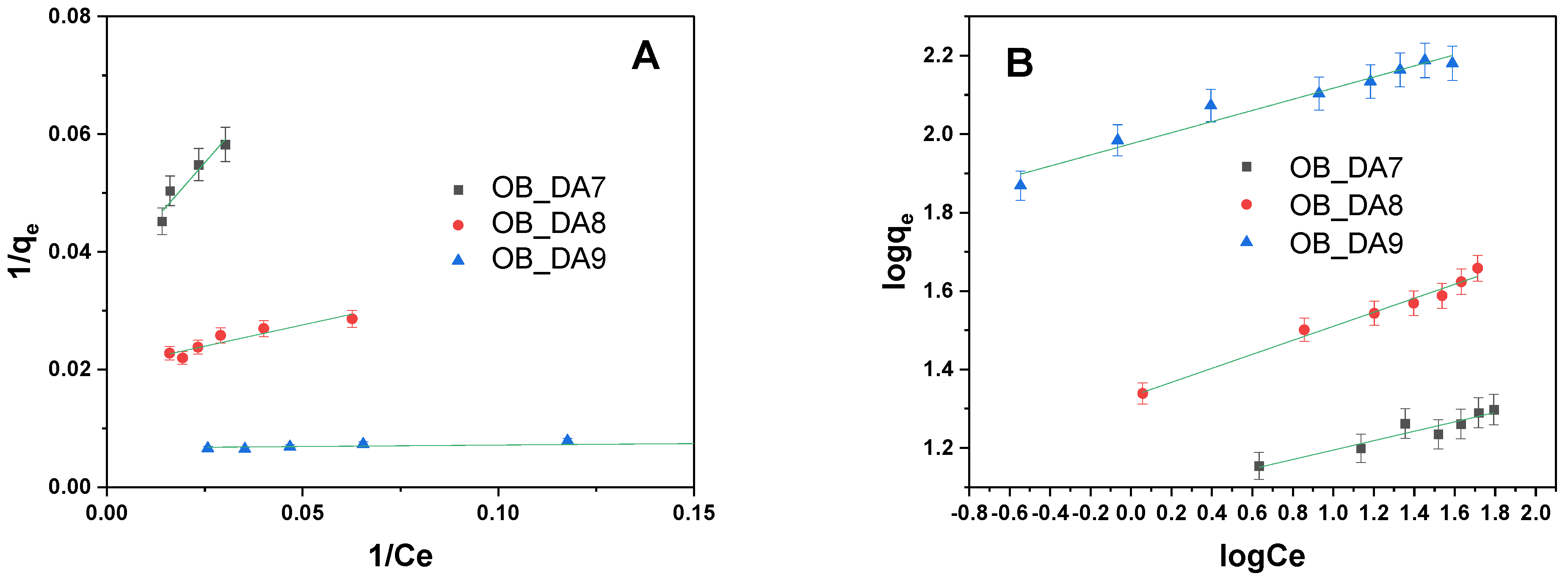
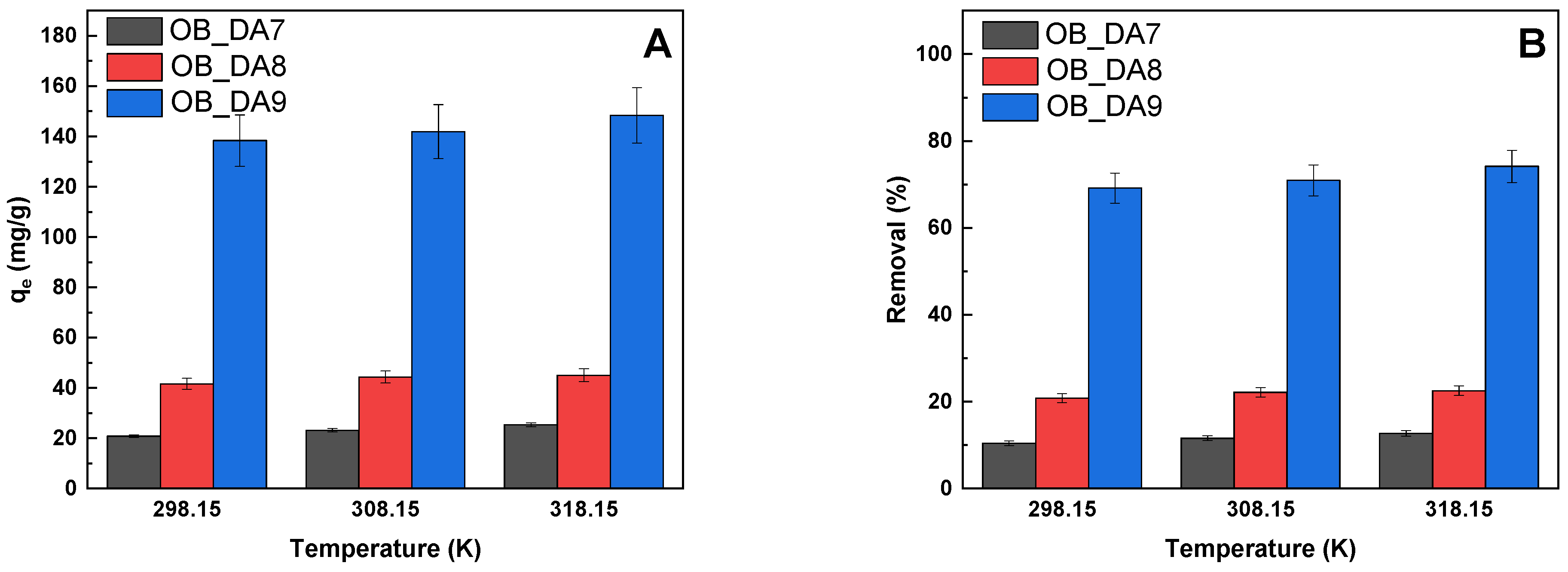
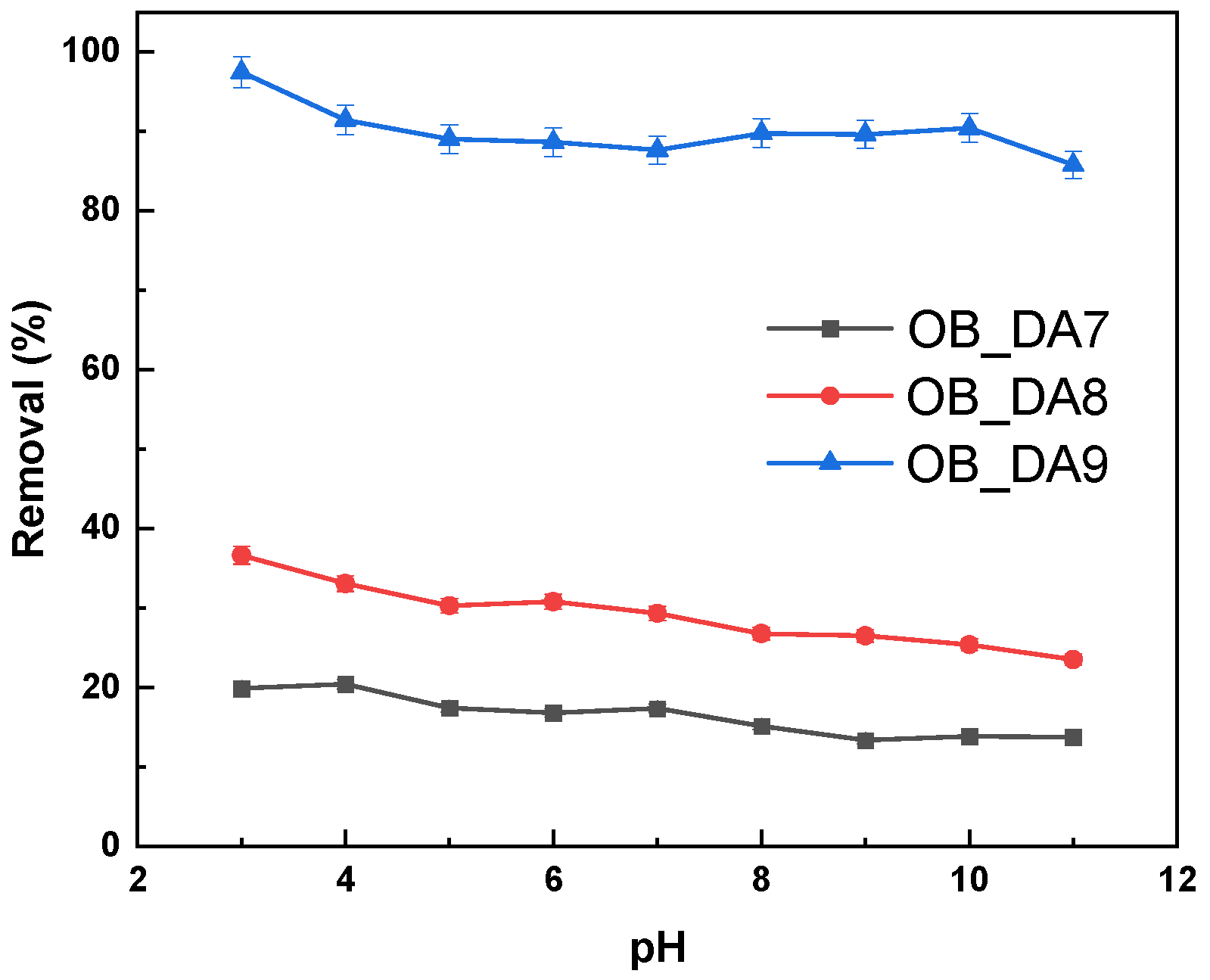
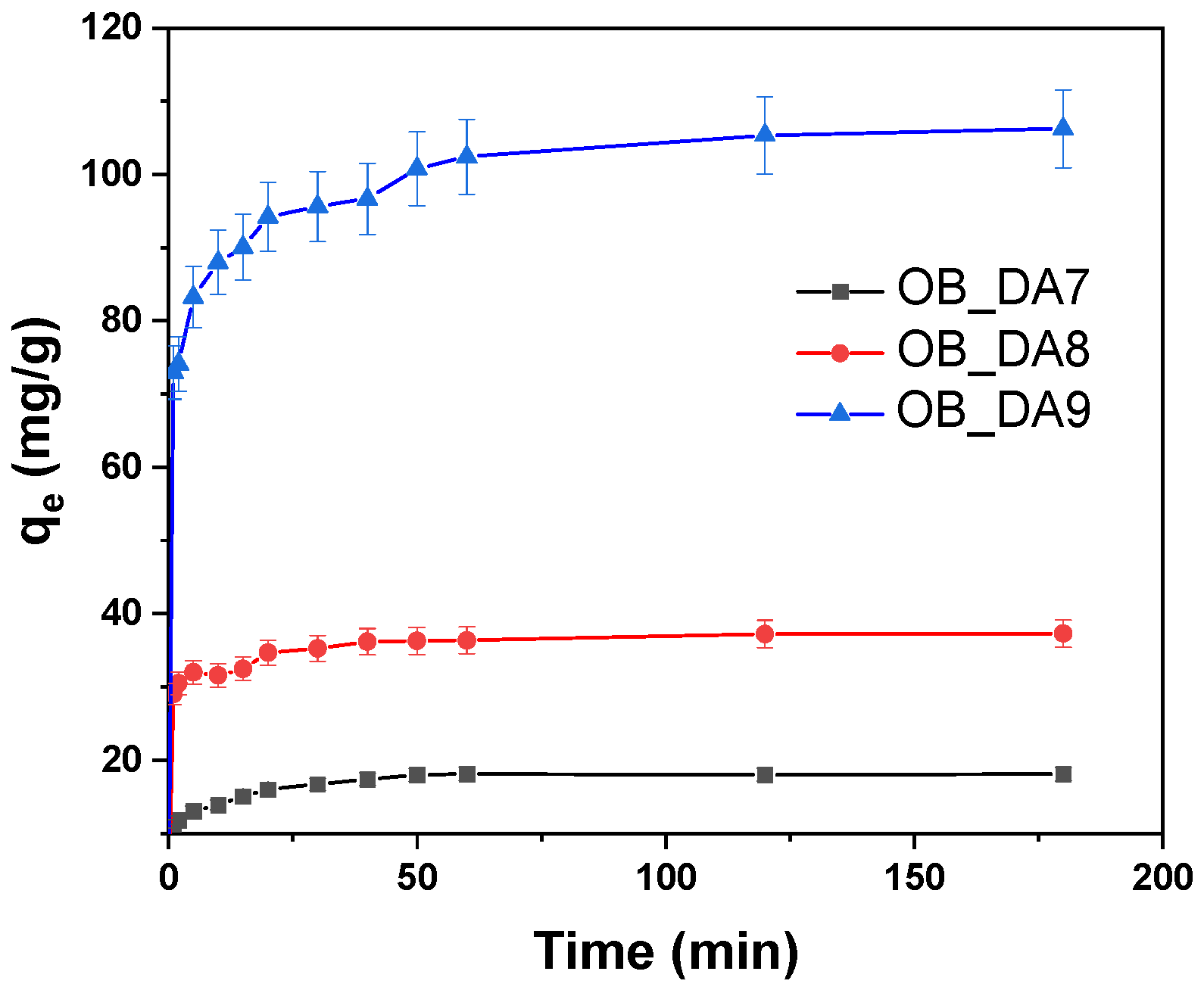
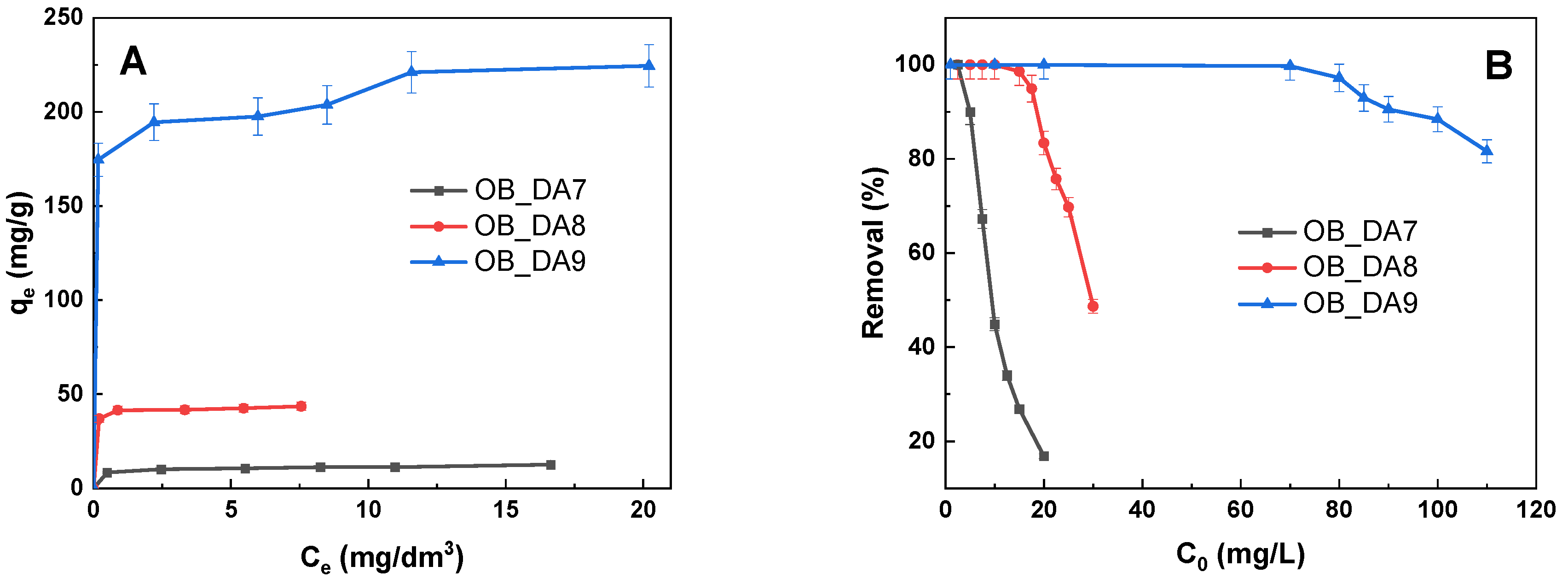
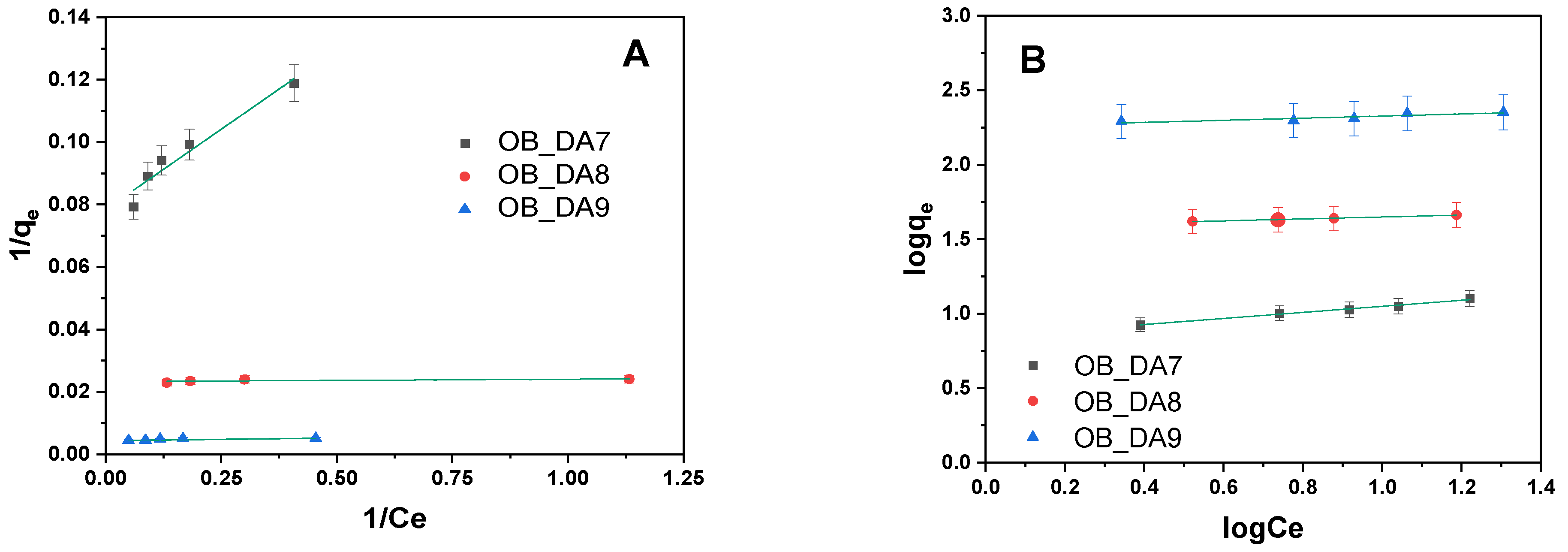
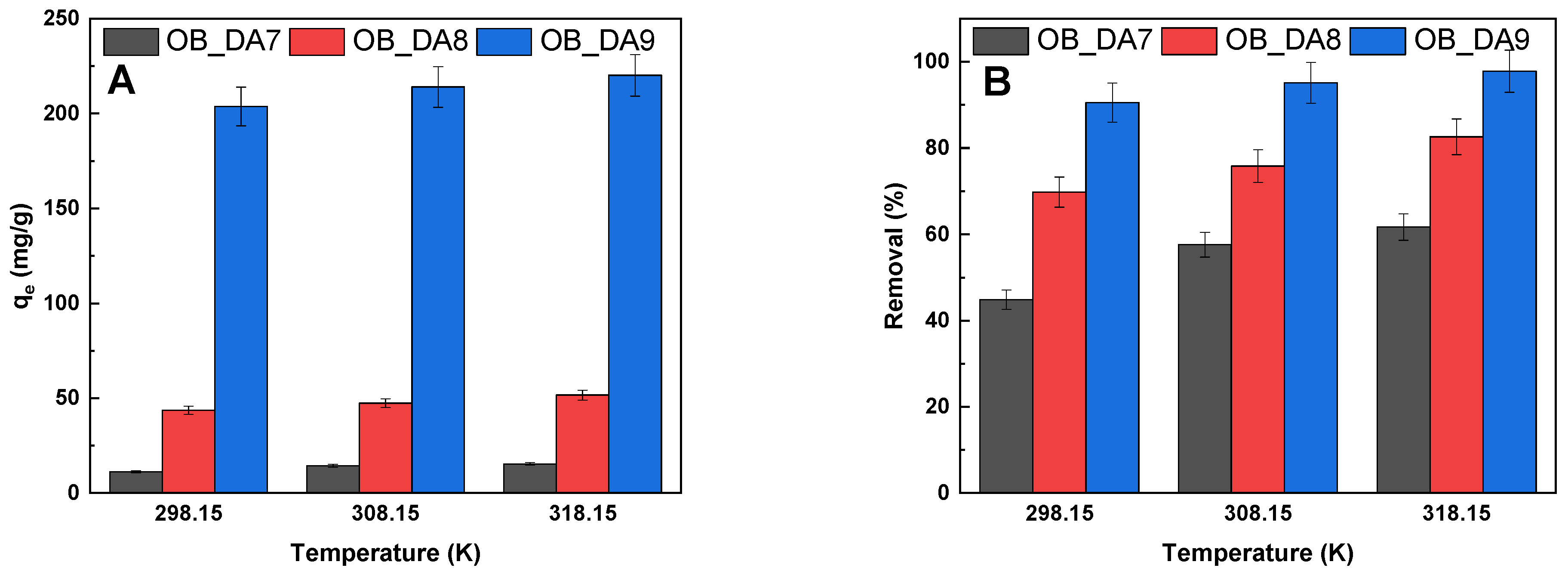
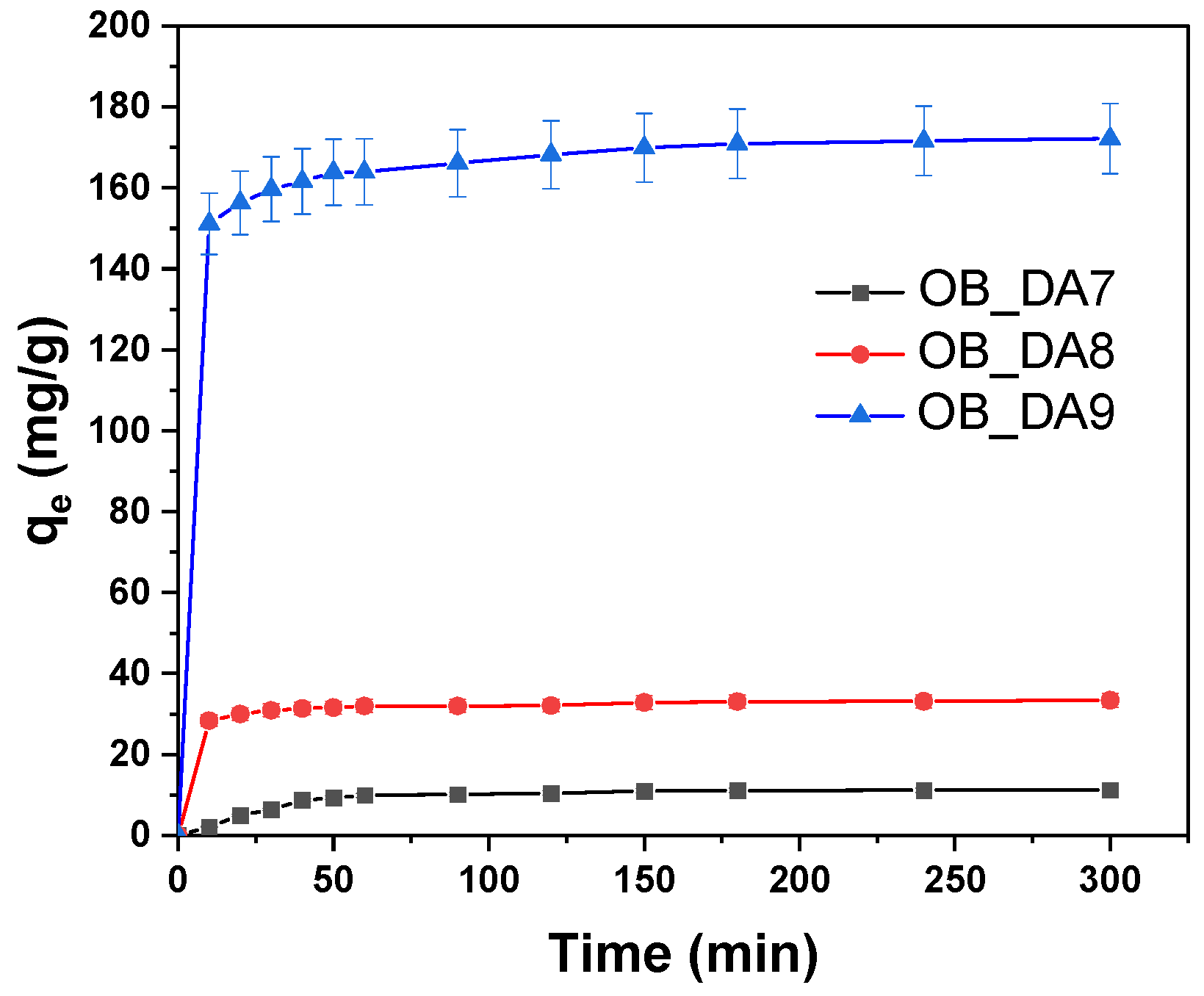
3.2. Adsorption of Butylparaben
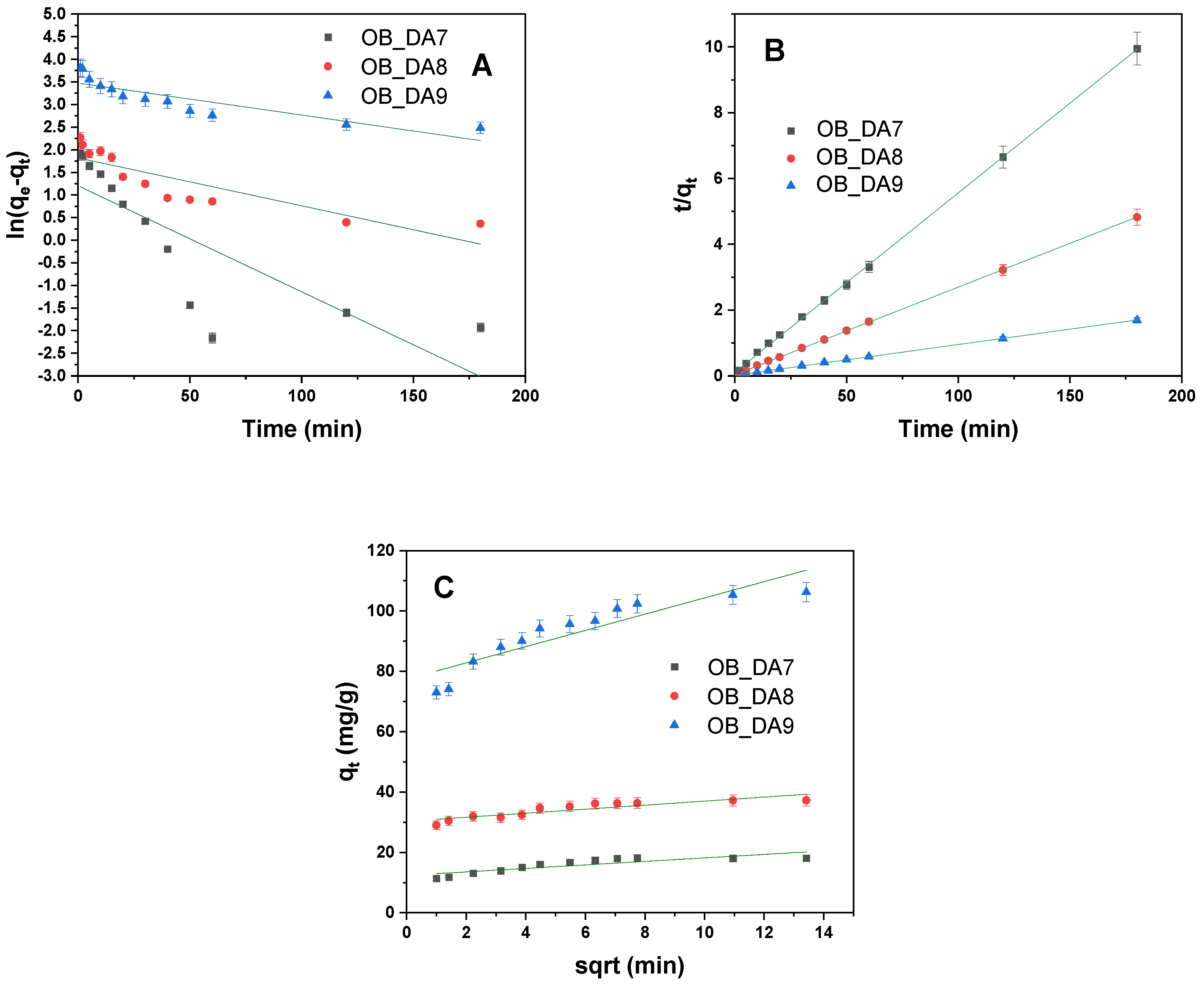
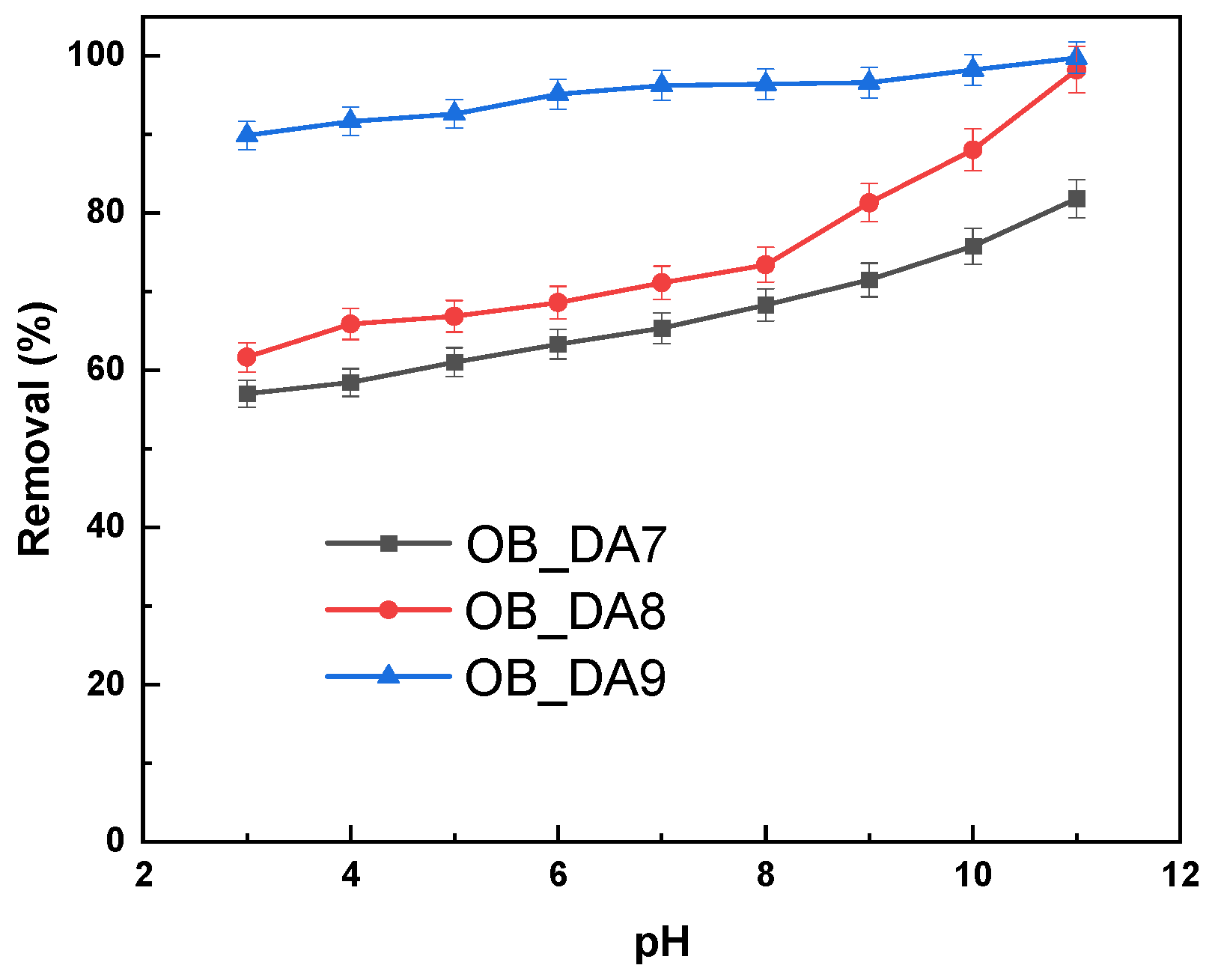
3.3. Adsorption of Methylene Blue
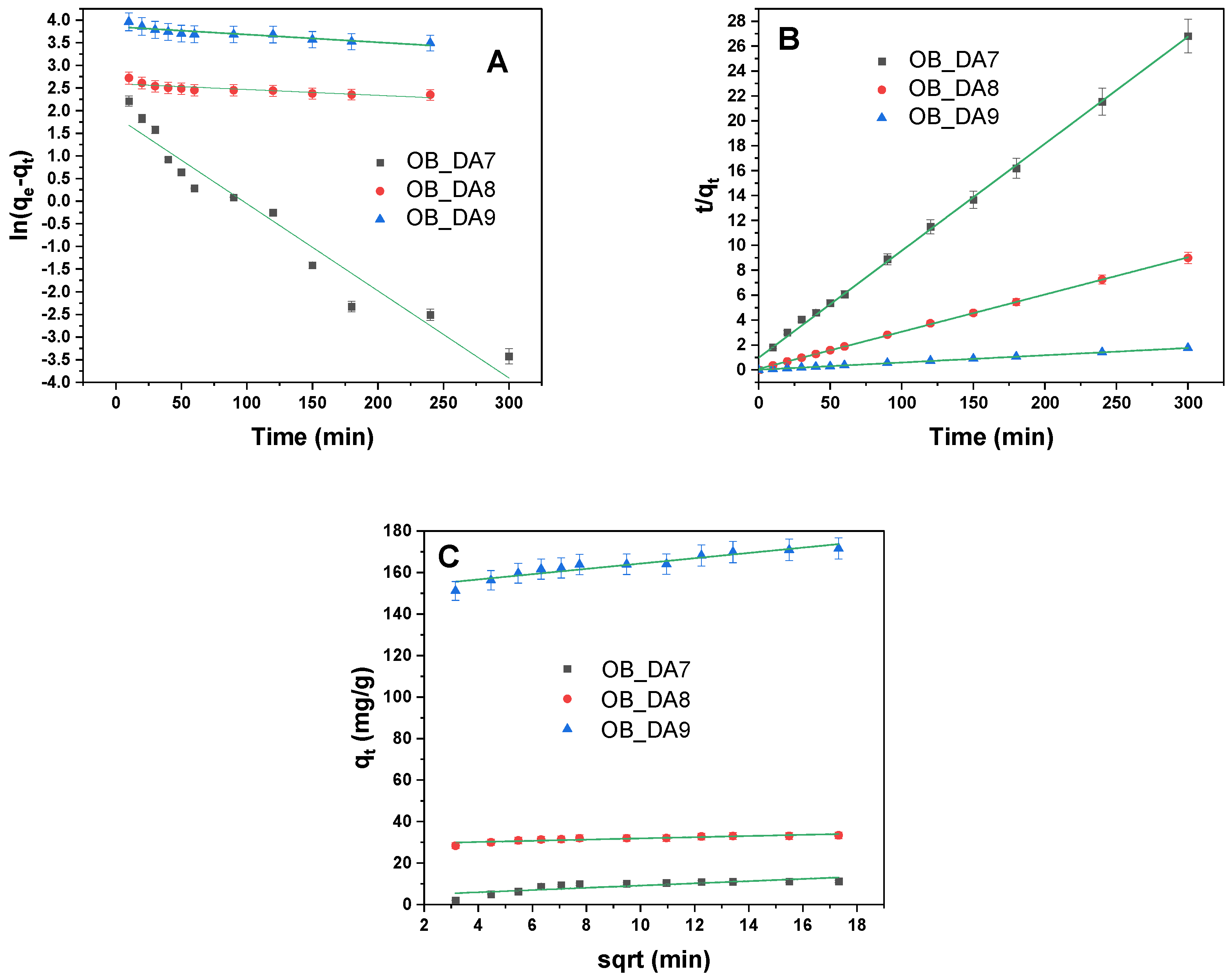
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Zoeller, R.T. EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [PubMed]
- Chen, Y.; Yang, J.; Yao, B.; Zhi, D.; Luo, L.; Zhou, Y. Endocrine disrupting chemicals in the environment: Environmental sources, biological effects, remediation techniques, and perspective. Environ. Pollut. 2022, 310, 119918. [Google Scholar] [CrossRef]
- Rojas-Hucks, S.; Rodriguez-Jorquera, I.A.; Nimpstch, J.; Bahamonde, P.; Benavides, J.A.; Chiang, G.; Pulgar, J.; Galbán-Malagón, C.J. South American National Contributions to Knowledge of the Effects of Endocrine Disrupting Chemicals in Wild Animals: Current and Future Directions. Toxics 2022, 10, 735. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, B.R.; Shi, Y.J.; Chen, W.M.; Zhang, J.L. Environmental pollution of paraben needs attention: A study of methylparaben and butylparaben co-exposure trigger neurobehavioral toxicity in zebrafish. Environ. Pollut. 2024, 356, 124370. [Google Scholar] [CrossRef]
- Jobling, S.; Tyler, C.R. Endocrine disruption in wild freshwater fish. Pure Appl. Chem. 2003, 75, 2219–2234. [Google Scholar] [CrossRef]
- Bolujoko, N.B.; Ogunlaja, O.O.; Alfred, M.O.; Okewole, D.M.; Ogunlaja, A.; Olukanni, O.D.; Unuabonah, E.I. Occurrence and human exposure assessment of parabens in water sources in Osun State, Nigeria. Sci. Total Environ. 2022, 814, 152448. [Google Scholar] [CrossRef]
- Pereira, A.R.; Simões, M.; Gomes, I.B. Parabens as environmental contaminants of aquatic systems affecting water quality and microbial dynamics. Sci. Total Environ. 2023, 905, 167332. [Google Scholar] [CrossRef]
- Azizi, D.; Arif, A.; Blair, D.; Dionne, J.; Filion, Y.; Ouarda, Y.; Blais, J.F. A comprehensive review on current technologies for removal of endocrine disrupting chemicals from wastewaters. Environ. Res. 2022, 207, 112196. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Khan, I. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Ejairu, U.; Aderamo, A.T.; Olisakwe, H.C.; Esiri, A.E.; Adanma, U.M.; Solomon, N.O. Eco-friendly wastewater treatment technologies (concept): Conceptualizing advanced, sustainable wastewater treatment designs for industrial and municipal applications. Comp. Res. Rev. Eng. Technol. 2024, 2, 83–104. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. Int. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Vivekanandhan, S.; Das, O.; Romero Millán, L.M.; Klinghoffer, N.B.; Nzihou, A.; Misra, M. Biocarbon materials. Nat. Rev. Methods Prim. 2024, 4, 19. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the carbonization process on activated carbon properties from lignin and lignin-rich biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Zhu, L. Preparation, characterization and application of lignin-based activated carbon from black liquor lignin by steam activation. Chem. Eng. J. 2013, 228, 1074–1082. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Li, R.; Hu, D.; Lu, Y.; Luo, H.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb (II) and Cd (II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Serafin, J.; Cruz, O.F., Jr. Promising activated carbons derived from common oak leaves and their application in CO2 storage. J. Environ. Chem. Eng. 2022, 10, 107642. [Google Scholar] [CrossRef]
- Moreno-Marenco, A.R.; Giraldo, L.; Moreno-Piraján, J.C. Parabens Adsorption onto Activated Carbon: Relation with Chemical and Structural Properties. Molecules 2019, 24, 4313. [Google Scholar] [CrossRef]
- Moreno-Marenco, A.R.; Giraldo, L.; Moreno-Piraján, J.C. Adsorption of n-butylparaben from aqueous solution on surface of modified granular activated carbons prepared from African palm shell. Thermodynamic study of interactions. J. Environ. Chem. Eng. 2020, 8, 103969. [Google Scholar] [CrossRef]
- Atheba, P.; Allou, N.B.; Drogui, P.; Trokourey, A. Adsorption Kinetics and Thermodynamics Study of Butylparaben on Activated Carbon Coconut Based. J. Encapsul. Adsorpt. Sci. 2018, 8, 39–57. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Paluch, D.; Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Cielecka-Piontek, J.; Pietrzak, R. Fennel Seed Activated carbon: A Sustainable Approach for Methylene Blue Removal from Aqueous Solutions. Materials 2024, 17, 4350. [Google Scholar] [CrossRef] [PubMed]
- Paluch, D.; Bazan-Wozniak, A.; Pietrzak, R. Methyl Red Adsorption on Activated carbon Obtained by Physical Activation of Caraway Seeds with Carbon Dioxide. ChemPhysChem 2024, 25, e202300821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W. Preparation and characteristics of activated carbon from wood bark and its use for adsorption of Cu (II). Mater. Sci. 2014, 20, 474–478. [Google Scholar] [CrossRef][Green Version]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.; Cadaval, T.R., Jr. Preparation of activated carbon from black wattle bark waste and its application for phenol adsorption. J. Environ. Chem. Eng. 2019, 7, 103396. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Chen, L.; Liu, Z.; Qin, J. Utilization of bark waste of Acacia mangium: The preparation of activated carbon and adsorption of phenolic wastewater. Ind. Crops Prod. 2021, 160, 113157. [Google Scholar] [CrossRef]
- Mahardiani, L.; Saputro, S.; Baskoro, F.; Zinki, N.M.; Taufiq, M. Facile synthesis of carboxylated activated carbon sample using green approach for water treatment. IOP. Conf. Ser. Mater. Sci. Eng. 2019, 578, 012003. [Google Scholar] [CrossRef]
- Christian, N.S.; Manga, N.H.; Raoul, T.T.D.; Gabche, A.S. Optimisation of activated carbon preparation by chemical activation of ayous sawdust, cucurbitaceae peelings and hen egg shells using response surface methodology. Int. Res. J. Pure Appl. Chem. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Kuwabara, A.; Kuroda, S.I.; Kubota, H. Polymer surface treatment by atmospheric pressure low temperature surface discharge plasma: Its characteristics and comparison with low pressure oxygen plasma treatment. Plasma Sci. Technol. 2007, 9, 181. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated activated carbon: A review. Biomass Convers. Biorefin. 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Correa-Navarro, Y.M.; Rivera-Giraldo, J.D.; Murcia-García, J.D. Isotherm and kinetic data for adsorption of butylparaben onto biochars derived from fique bagasse. Data Brief 2024, 57, 111113. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.L.; Wu, F.C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.; Zhu, J.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Heldreth, B. Amended safety assessment of parabens as used in cosmetics. Int. J. Toxicol. 2020, 39 (Suppl. S1), S5–S97. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Nowicki, P.; Gruszczynska, K.; Urban, T.; Wisniewska, T. Activated biocarbons obtained from post-fermentation residue as potential adsorbents of organic pollutants from the liquid phase. Physicochem. Probl. Miner. Process. 2022, 58, 146357. [Google Scholar]
- Nedjai, R.; Alkhatib, M.A.F.R.; Alam, M.Z.; Kabbashi, N.A. Adsorption of methylene blue onto activated carbon developed from baobab fruit shell by chemical activation: Kinetic equilibrium studies. IIUM Eng. J. 2021, 22, 31–49. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant adsorption isotherms: A review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.S.; Adegoke, K.A.; Sarumi, O.O.; Lameed, O.S. Functionalized locust bean pod (Parkia biglobosa) activated carbon for Rhodamine B dye removal. Heliyon 2019, 5, e02323. [Google Scholar] [CrossRef] [PubMed]
- Mussa, Z.H.; Al-Ameer, L.R.; Al-Qaim, F.F.; Deyab, I.F.; Kamyab, H.; Chelliapan, S.A. Comprehensive review on adsorption of methylene blue dye using leaf waste as a bio-sorbent: Isotherm adsorption, kinetics, and thermodynamics studies. Environ. Monit. Assess. 2023, 195, 940. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, T.; Ye, X.; Li, Q.; Guo, M.; Liu, H.; Wu, Z. Dye adsorption by resins: Effect of ionic strength on hydrophobic and electrostatic interactions. Chem. Eng. J. 2013, 228, 392–397. [Google Scholar] [CrossRef]
- Yin, Y.; Hou, X.; Wu, B.; Dong, J.; Yao, M. Heterogeneous Structured Nanomaterials from Carbon and Related Materials. Adv. Funct. Mater. 2024, 34, 2411472. [Google Scholar] [CrossRef]


| Dye | Chemical Formula | Structure | Mass (g/mol) | λmax (nm) |
|---|---|---|---|---|
| Methylene blue | [C16H18N3S] + Cl− | 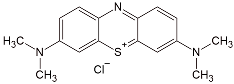 | 319.85 | 665 |
| Butylparaben | C11H14O3 | 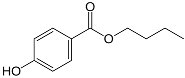 | 194.23 | 254 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Iodine Number (mg/g) | Yield (%) | ||
|---|---|---|---|---|---|---|---|
| Total | Micropore | Total | Micropore | ||||
| OB_DA7 | 247 | 152 | 0.480 | 0.086 | 3.80 | 277 | 49.24 |
| OB_DA8 | 452 | 198 | 0.958 | 0.107 | 4.81 | 369 | 43.52 |
| OB_DA9 | 696 | 141 | 1.445 | 0.077 | 6.35 | 741 | 38.91 |
| Sample | Ndaf | Cdaf | Hdaf | Sdaf | Odaf* | % Ash |
|---|---|---|---|---|---|---|
| Oak bark | 0.42 | 47.55 | 6.08 | 0.14 | 45.80 | 1.40 |
| OB_DA7 | 1.59 | 83.63 | 0.31 | 0.78 | 13.69 | 12.72 |
| OB_DA8 | 1.72 | 80.17 | 0.32 | 0.80 | 17.00 | 15.58 |
| OB_DA9 | 1.71 | 73.65 | 0.27 | 0.80 | 23.57 | 17.23 |
| Element | OB_DA7 | OB_DA8 | OB_DA9 |
|---|---|---|---|
| O | 6.02 | 8.02 | 10.79 |
| C | 75.07 | 73.59 | 71.31 |
| K | 18.91 | 18.39 | 17.90 |
| Precursor | Activator | Activation Time (min) | Activation Temperature (°C) | Sorption Capacity (mg/g) | Source |
|---|---|---|---|---|---|
| Oak bark | CO2 | 60 | 700 | 20 | This study |
| CO2 | 60 | 800 | 46 | ||
| CO2 | 60 | 900 | 154 | ||
| Fique bagasse | NaOH | 120 | 800 | 21 | [32] |
| Coconut shell | CO2 | 180 | 800 | 7.5 | [20] |
| African palm shells | CO2 | 360 | 900 | 144 | [19] |
| Model | Parameters | Sample | ||
|---|---|---|---|---|
| OB_DA7 | OB_DA8 | OB_DA9 | ||
| qexp (mg/g) | 20 | 46 | 154 | |
| Langmuir | KL (L/mg) | 0.048 | 0.140 | 0.047 |
| qm (mg/g) | 28 | 49 | 150 | |
| R2 | 0.909 | 0.864 | 0.749 | |
| Adj2 | 0.864 | 0.830 | 0.686 | |
| Freundlich | KF (mg/g(L/mg)1/n) | 11.877 | 16.658 | 94.497 |
| 1/n | 0.120 | 0.179 | 0.142 | |
| R2 | 0.911 | 0.985 | 0.962 | |
| Adj2 | 0.893 | 0.982 | 0.955 | |
| Sample | Temperature (K) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/mol × K) |
|---|---|---|---|---|
| OB_DA7 | 298.15 | 3.06 | 8.82 | 19.351 |
| 308.15 | 2.86 | |||
| 318.15 | 2.67 | |||
| OB_DA8 | 298.15 | 1.04 | 3.97 | 9.899 |
| 308.15 | 0.87 | |||
| 318.15 | 0.84 | |||
| OB_DA9 | 298.15 | –4.27 | 9.72 | 46.837 |
| 308.15 | –4.63 | |||
| 318.15 | –5.21 |
| Model | Parameters | Sample | ||
|---|---|---|---|---|
| OB_DA7 | OB_DA8 | OB_DA9 | ||
| qe (mg/g) | 18 | 37 | 111 | |
| Pseudo-first-order | k1 (1/min) | 1.63 × 10−6 | 7.36 × 10−6 | 4.93 × 10−6 |
| qe/cal (mg/g) | 3 | 6 | 32 | |
| R2 | 0.662 | 0.747 | 0.751 | |
| Adj2 | 0.643 | 0.724 | 0.739 | |
| Pseudo-second-order | k2 (g/mg × min) | 2.7 × 10−2 | 1.8 × 10−2 | 4.0 × 10−3 |
| qe/cal (mg/g) | 18 | 38 | 108 | |
| R2 | 0.999 | 0.999 | 0.999 | |
| Adj2 | 0.999 | 0.999 | 0.999 | |
| Intraparticle diffusion | kid (mg/g × min1/2) | 0.58 | 0.67 | 0.58 |
| C (mg/g) | 12 | 30 | 77 | |
| R2 | 0.744 | 0.797 | 0.825 | |
| Adj2 | 0.719 | 0.776 | 0.807 | |
| Precursor | Activator | Activation Time (min) | Activation Temperature (°C) | Sorption Capacity (mg/g) | Source |
|---|---|---|---|---|---|
| Oak bark | CO2 | 60 | 700 | 13 | This study |
| CO2 | 60 | 800 | 44 | ||
| CO2 | 60 | 900 | 224 | ||
| Sludge | - | 120 | 550 | 24 | [36] |
| Barley malt bagasse | CO2 | 60 | 800 | 161 | [37] |
| Baobab fruit shell | KOH | 60 | 500 | 114 | [39] |
| Model | Parameters | Sample | ||
|---|---|---|---|---|
| OB_DA7 | OB_DA8 | OB_DA9 | ||
| qexp (mg/g) | 13 | 44 | 224 | |
| Langmuir | KL (L/mg) | 0.762 | 3.758 | 2.586 |
| qmax (mg/g) | 13 | 45 | 229 | |
| R2 | 0.945 | 0.943 | 0.999 | |
| Adj2 | 0.927 | 0.886 | 0.999 | |
| Freundlich | KF (mg/g(L/mg)1/n) | 7.011 | 38.367 | 180.090 |
| 1/n | 0.203 | 0.065 | 0.071 | |
| R2 | 0.989 | 0.985 | 0.798 | |
| Adj2 | 0.985 | 0.978 | 0.731 | |
| Sample | Temperature (K) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/mol × K) |
|---|---|---|---|---|
| OB_DA7 | 298.15 | −1.76 | 27.01 | 96.98 |
| 308.15 | −3.13 | |||
| 318.15 | −3.68 | |||
| OB_DA8 | 298.15 | −4.34 | 28.35 | 109.55 |
| 308.15 | −5.28 | |||
| 318.15 | −6.54 | |||
| OB_DA9 | 298.15 | −7.87 | 61.01 | 230.90 |
| 308.15 | −9.96 | |||
| 318.15 | −12.50 |
| Model | Parameters | Sample | ||
|---|---|---|---|---|
| OB_DA7 | OB_DA8 | OB_DA9 | ||
| qe (mg/g) | 11 | 44 | 204 | |
| Pseudo-first-order | k1 (1/min) | 8.02 × 10−5 | 5.38 × 10−6 | 7.17 × 10−6 |
| qe/cal (mg/g) | 6.50 | 13 | 47 | |
| R2 | 0.952 | 0.721 | 0.829 | |
| Adj2 | 0.945 | 0.690 | 0.810 | |
| Pseudo-second-order | k2 (g/mg × min) | 3.76 × 10−3 | 1.66 × 10−2 | 2.19 × 10−2 |
| qe/cal (mg/g) | 12 | 43 | 199 | |
| R2 | 0.998 | 0.999 | 0.999 | |
| Adj2 | 0.998 | 0.999 | 0.999 | |
| Intraparticle diffusion | kid (mg/g × min1/2) | 0.53 | 0.23 | 1.28 |
| C (mg/g) | 3.83 | 29.00 | 152 | |
| R2 | 0.683 | 0.809 | 0.893 | |
| Adj2 | 0.652 | 0.790 | 0.883 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paluch, D.; Wolski, R.; Bazan-Wozniak, A.; Pietrzak, R. Adsorption of Butylparaben and Methylene Blue from Aqueous Solution Using Activated Carbon Derived from Oak Bark. Materials 2025, 18, 2984. https://doi.org/10.3390/ma18132984
Paluch D, Wolski R, Bazan-Wozniak A, Pietrzak R. Adsorption of Butylparaben and Methylene Blue from Aqueous Solution Using Activated Carbon Derived from Oak Bark. Materials. 2025; 18(13):2984. https://doi.org/10.3390/ma18132984
Chicago/Turabian StylePaluch, Dorota, Robert Wolski, Aleksandra Bazan-Wozniak, and Robert Pietrzak. 2025. "Adsorption of Butylparaben and Methylene Blue from Aqueous Solution Using Activated Carbon Derived from Oak Bark" Materials 18, no. 13: 2984. https://doi.org/10.3390/ma18132984
APA StylePaluch, D., Wolski, R., Bazan-Wozniak, A., & Pietrzak, R. (2025). Adsorption of Butylparaben and Methylene Blue from Aqueous Solution Using Activated Carbon Derived from Oak Bark. Materials, 18(13), 2984. https://doi.org/10.3390/ma18132984







