1. Introduction
Ultrahigh-strength steels (UHSSs) possess excellent strength, ductility, and toughness, making them widely used in critical aerospace applications such as bearings and aircraft landing gears [
1,
2,
3]. UHSSs can be classified into low-alloy UHSSs, secondary hardening UHSSs, maraging steels, and precipitation hardening stainless UHSSs based on their different strengthening mechanisms. Low-alloy UHSSs are primarily strengthened by a quenched and low-temperature-tempered martensitic matrix along with finely dispersed carbides [
4]. Secondary hardening UHSSs contain alloying elements such as Co, Ni, Mo, and Cr, with their strength mainly derived from the formation of fine, dispersed M
2C precipitations and high-density dislocations in lath martensite during tempering [
5]. Maraging steels achieve strengthening through the precipitation of intermetallic compounds, which is directly related to the types and amounts of alloying elements added [
6]. Precipitation hardening stainless UHSSs are primarily based on maraging steels but incorporate a high Cr content to enhance their corrosion resistance [
7].
As commercial demands for performance continue to rise, new ultrahigh-strength steels with higher strength and plasticity have been developed. Liu et al. [
8] employed a cold-rolling process in the production of medium-Mn steel to obtain a thin, lamellar dual-phase structure of martensite and austenite, followed by carbon redistribution to improve the austenite stability, achieving both high strength and high fracture toughness. He [
9] developed an ultrahigh-strength steel with a high dislocation density, where the microstructure was controlled via rolling to produce a metastable austenite embedded in a high-dislocation martensitic matrix, achieving a strength of 2200 MPa. Bhadeshia [
10] utilized Si to suppress carbide precipitation in bainitic steel, leading to the development of super nano-bainitic steel.
The type and size of the precipitations in UHSSs play a crucial role in determining their mechanical properties [
11]. Conventional UHSSs typically rely on a single type of precipitation for strengthening, such as M
2C precipitations in secondary hardening steels or intermetallic precipitations (e.g., Ni
3(Ti, Mo)) in maraging steels. However, these single-phase precipitations tend to nucleate preferentially at dislocations or defects, leading to an inhomogeneous distribution within the matrix, which limits the improvements in both the strength and ductility [
12]. Therefore, a key research challenge lies in reducing the size of precipitations and promoting their uniform dispersion throughout the matrix.
The excellent mechanical properties of high-Co-Ni UHSSs (such as AF1410 and AerMet100) primarily rely on the strengthening effects of nanoscale precipitations, with their precipitation mechanisms involving complex interactions between alloying elements and phase transformation kinetics. In ferritic matrix (BCC) UHSSs, precipitation strengthening is mainly achieved through second-phase particles (e.g., carbides and intermetallic compounds) impeding dislocation motion, with its effectiveness significantly influenced by the size, distribution, and coherency of the precipitations.
During the development of secondary hardening UHSSs and maraging steels, cobalt (Co) is considered a key alloying element for enhancing their strength [
13,
14]. In recent years, the specific strengthening mechanisms of Co have been continuously explored. Co exhibits high mutual solubility with Fe in the crystal structure and primarily exists in steel as a substitutional solid solution. Due to the similar atomic radii of Co and Fe, the lattice distortion caused by the Co solid solution is limited, resulting in relatively weak solid solution strengthening [
15,
16]. Speich [
17] found that in addition to its basic solid solution strengthening effect, Co also effectively delays dislocation recovery in the martensitic structure, promotes the fine and dispersed distribution of carbides, and enhances the secondary hardening peak. Perkas [
18] reported that in maraging steels, Co reduces the solubility of Mo and W in the matrix, leading to a greater number of finer precipitations. Floreen [
19] suggested that Co reduces the stacking fault energy of the body-centered cubic (BCC) lattice, increasing the dislocation density in the quenched state, which in turn provides more nucleation sites for precipitations. Gustafson used computational simulations to study the effect of Co on the coarsening of M
23C
6 carbides in P92 steel. The results showed that with the addition of 10% Co, the final average radius of the carbides after tempering at 600 °C for 30,000 h decreased by 30% [
20,
21].
At present, studies on the effect of Co on precipitations in UHSSs have mainly focused on individual precipitation types, such as M
2C precipitations in secondary hardening steels [
22,
23] and intermetallic compounds like Ni
3(Ti, Mo) in maraging steels [
24]. These single-phase precipitations tend to nucleate preferentially at dislocations or defects and exhibit a non-uniform distribution in the matrix, which limits the improvements in the steel’s strength–ductility balance [
12,
25]. Although the macroscopic strengthening effect of Co in steels has been observed, its influence at the nanoscale, particularly in systems containing multiple coexisting nanoscale precipitations, remains unclear. In this study, a dual-precipitation-strengthened steel was designed. Through simulation and computational analysis, the precipitation characteristics of steels with and without Co were investigated. The steel was developed based on these findings, achieving a maximum ultimate tensile strength of 2.3 GPa. The synergistic role of Co in the dual-nanoprecipitation system was analyzed through the detailed characterization of NiAl and M
2C.
3. Results and Discussion
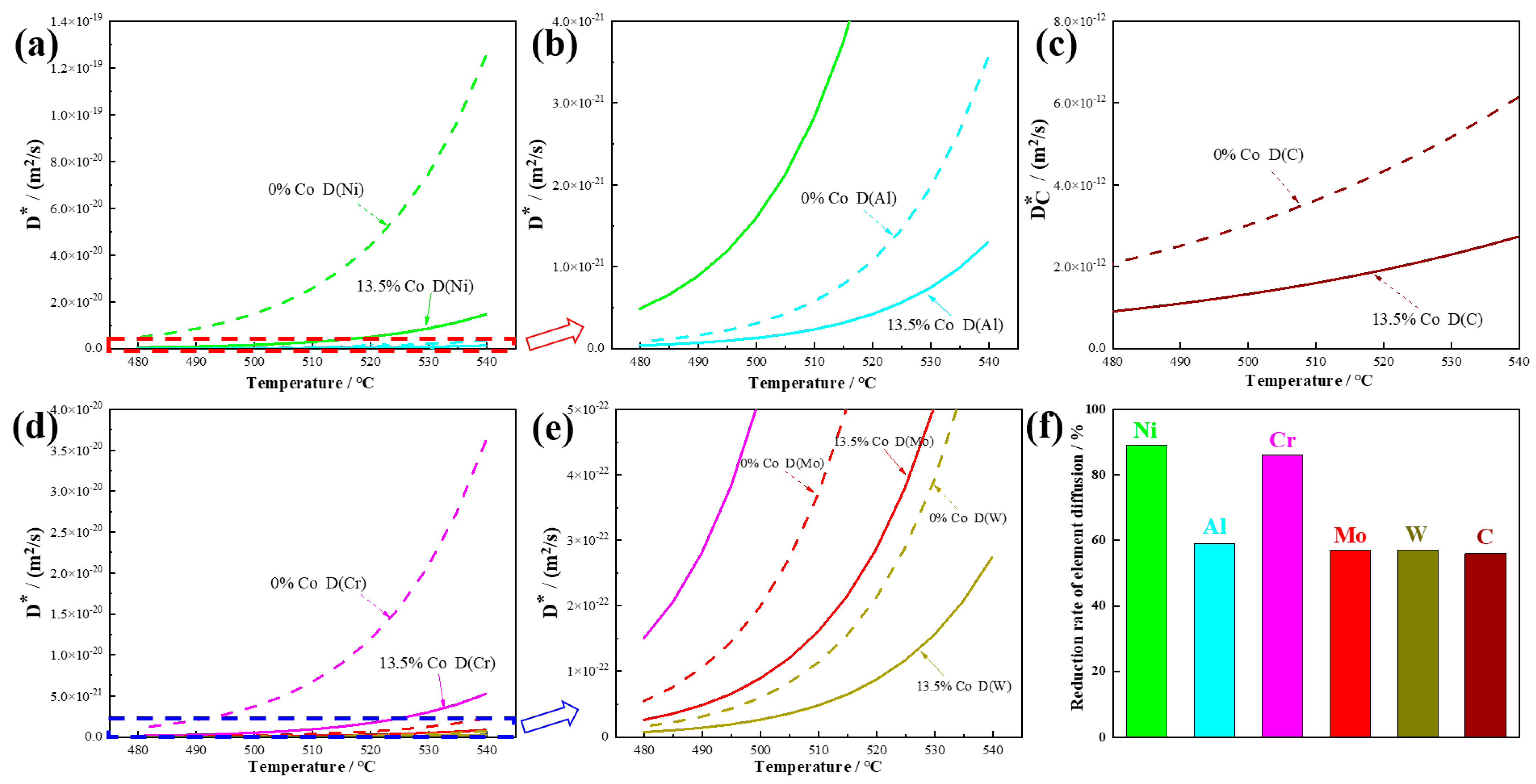
The simulation of the tracer diffusion coefficients for Ni, Al, C, Cr, Mo, and W is plotted as a function of the temperature for Co-free steel and 13.5% Co-containing steel in
Figure 2. It can be seen from
Figure 2a–e that when 13.5% Co was added to the steel, the tracer diffusion coefficients of all the elements were significantly reduced. The difference in the tracer diffusion coefficients between Co-free steel and 13.5% Co-containing steel became larger and larger as the temperature increased.
Figure 2f shows that 13.5% Co reduced the tracer diffusion coefficients of Al, Mo, W, and C in the matrix by almost 60% at a temperature of 510 °C. For Ni and Cr, the addition of 13.5% Co reduced their tracer diffusion coefficients by almost 95%. This indicates that the addition of Co to steel strongly suppressed the tracer diffusion coefficients of all the elements in the steel, hindering the self-diffusion rate and long-range diffusion effects of atoms. A decrease in the diffusion rate of precipitation atoms will affect the nucleation, distribution, and growth of precipitations [
26,
27].
To investigate the effect of Co on the precipitation behavior in steel, the TC-PRISMA precipitation module in Thermo-Calc was employed to simulate the size, distribution, and number density of MxC (x = 2 and 6) precipitations during aging at 510 °C for two alloy systems: a Co-free steel (Fe-0.26C-14.2Ni-1.2Al-2.1Cr-1.8Mo-0.6W, wt.%) and a 13.5 wt.% Co-containing steel (Fe-0.26C-13.5Co-14.2Ni-1.2Al-2.1Cr-1.8Mo-0.6W, wt.%). The TC-PRISMA module is based on the Langer–Schwartz theory and the Kampmann–Wagner numerical method, which are used to model the nucleation and growth of precipitations in multiphase systems under arbitrary heat treatment conditions. The growth behavior of the precipitations in the steel was analyzed using the classical CAJ model, and the calculations involved Equations (1)–(8), as shown below [
28].
In Equation (1), υ represents the interface velocity, and n is the number of components. C denotes the volume concentration of components at the interface between the matrix and the precipitation; M is the mobility of the atoms in the matrix phase; and μ refers to the chemical potential at the interface. ζ is the effective diffusion distance factor in Equation (3). Ω denotes the dimensionless supersaturation in Equation (4). ΔGm is the driving force for precipitation in Equation (6). Xi(r) represents the composition along the tie-line in the matrix phase in Equation (7). In Equation (8), <r> is the average equivalent radius of the precipitations, and Nv is the number density of the precipitations.
The simulation results for the carbides in the steel are shown in
Figure 3 [
29].
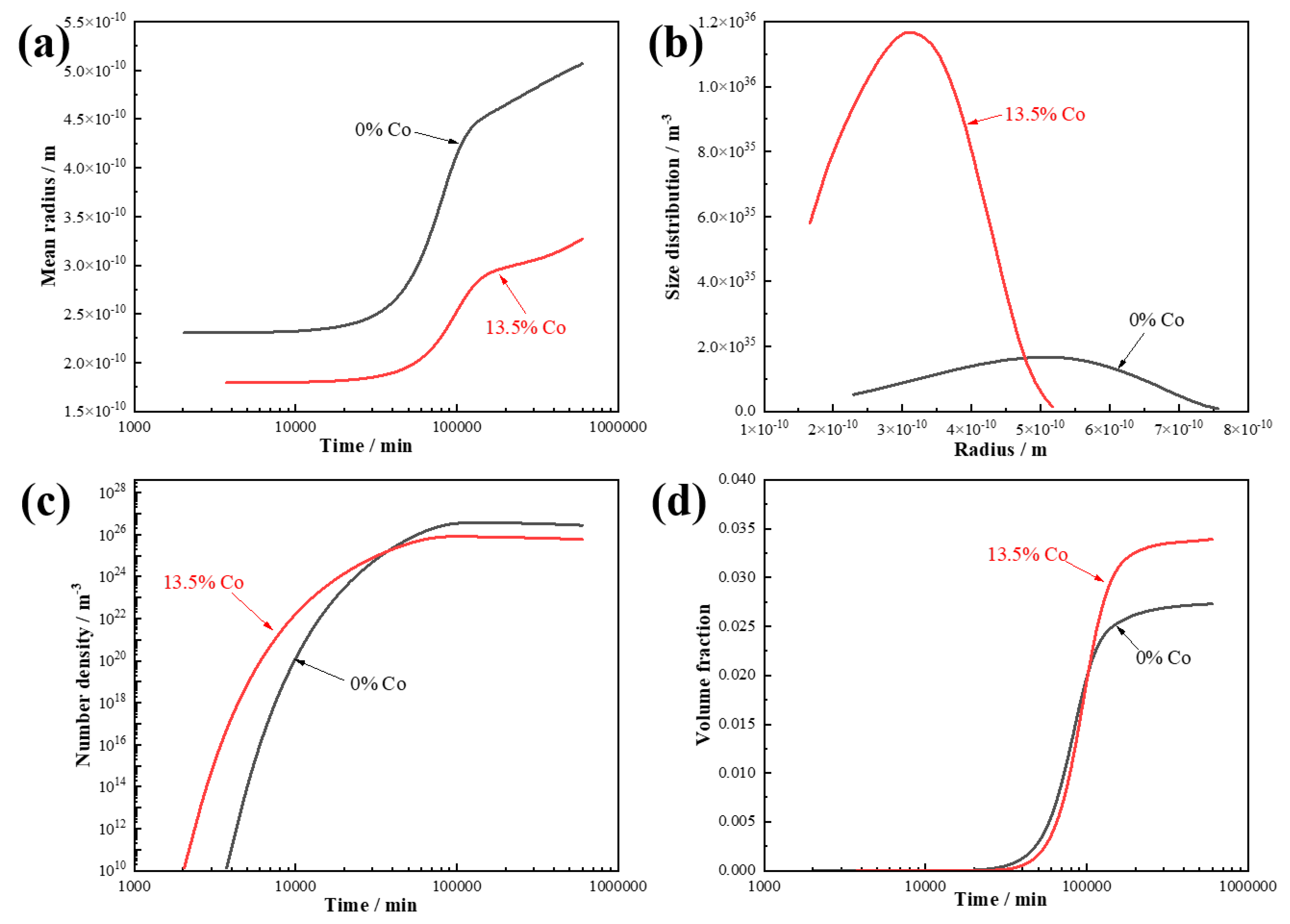
Figure 3a presents the relationship between the aging time and the equivalent radius of the carbides for both steels. It can be observed that the carbides in the 13.5 wt.% Co steel had a smaller equivalent radius, and the onset of significant coarsening was delayed.
Figure 3b illustrates the size distribution of the carbides in the two steels. The results indicate that the addition of 13.5 wt.% Co significantly refined the size distribution of the carbides. The evolution of the number density and volume fraction of the carbides with the aging time are shown in
Figure 3c,d. Compared to the Co-free steel, the 13.5 wt.% Co steel exhibited a higher density of carbides when the ageing time were less than 10,000 min. After 600,000 min of aging, the volume fraction of the carbides in the Co-containing steel was 1.2 times that in the Co-free steel.
Overall, the simulation results suggest that the addition of 13.5 wt.% Co can reduce the carbide size, improve the size distribution, increase the number density, and enhance the volume fraction. Although the computational approach can predict the precipitation trends of carbides, it is typically based on models of relatively large precipitations, making it difficult to accurately reflect the behavior of nanoscale precipitations in actual steels. Therefore, while the simulations provide useful guidance for alloy design, discrepancies still exist between the predicted results and the actual microstructure. Moreover, the simulations primarily captured changes in the size, number density, and volume of precipitations after approximately 1000 min of aging. In real aging processes, local variations in the Co concentration across different regions of the steel may also influence the precipitation behavior.
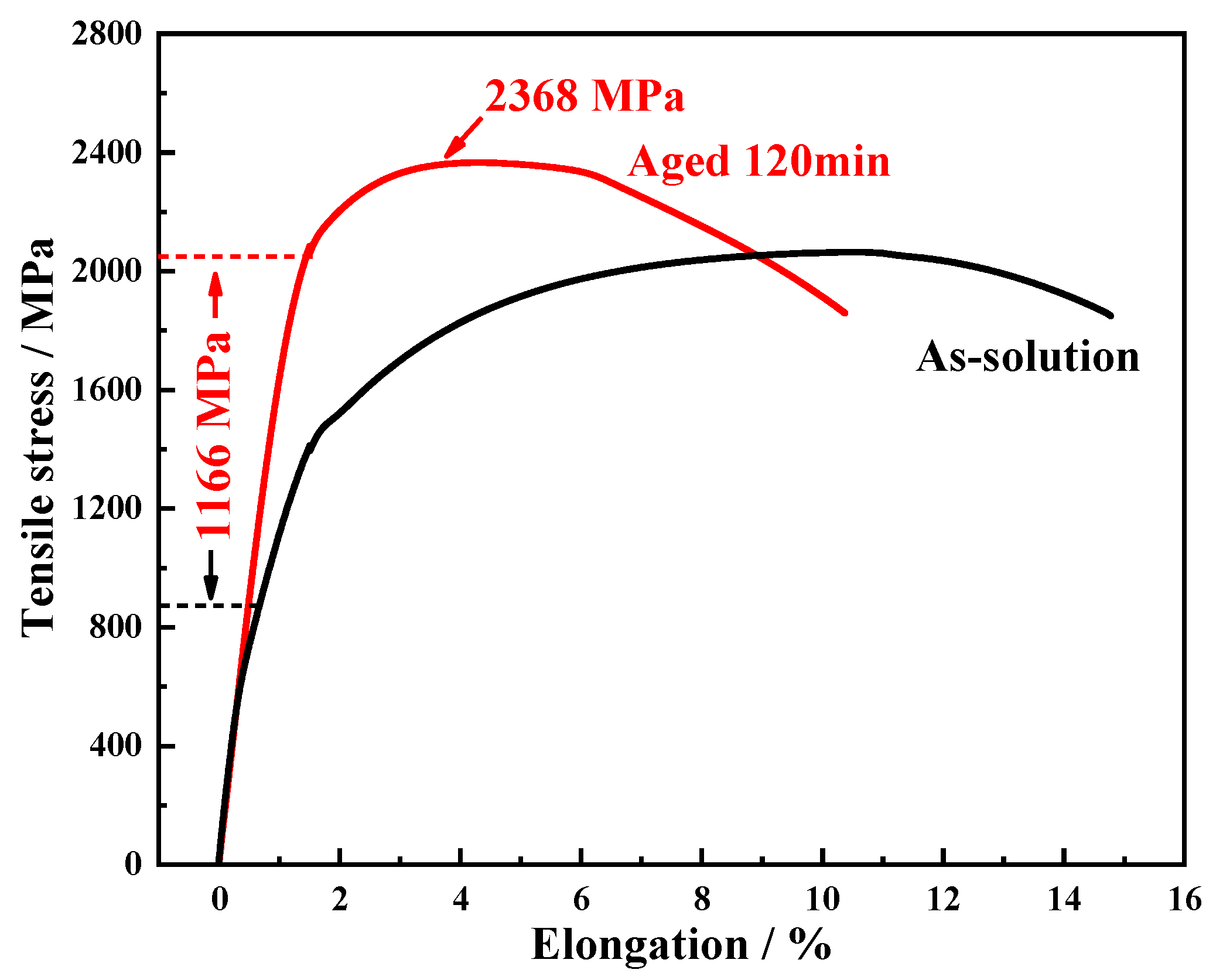
Following the above simulations and calculations, a steel alloy containing 13.5 wt.% Co was fabricated to investigate the influence of Co on nanoscale precipitations and its strengthening effect. Tensile tests were conducted under different heat treatment conditions, and the resulting engineering stress–strain curves are shown in
Figure 4. In the solution-treated condition, the ultimate tensile strength (UTS) was 2054 ± 5 MPa, with a total elongation of 13.5 ± 0.7%. After an aging treatment, the UTS increased to 2368 ± 3 MPa; however, the total elongation decreased to 10.4 ± 0.6%. Moreover, a significant increase in the yield strength—by 1166 MPa—was observed after the aging treatment. These results indicate that under the Co-containing condition, the nanoscale precipitations exhibited a strong hardening effect.
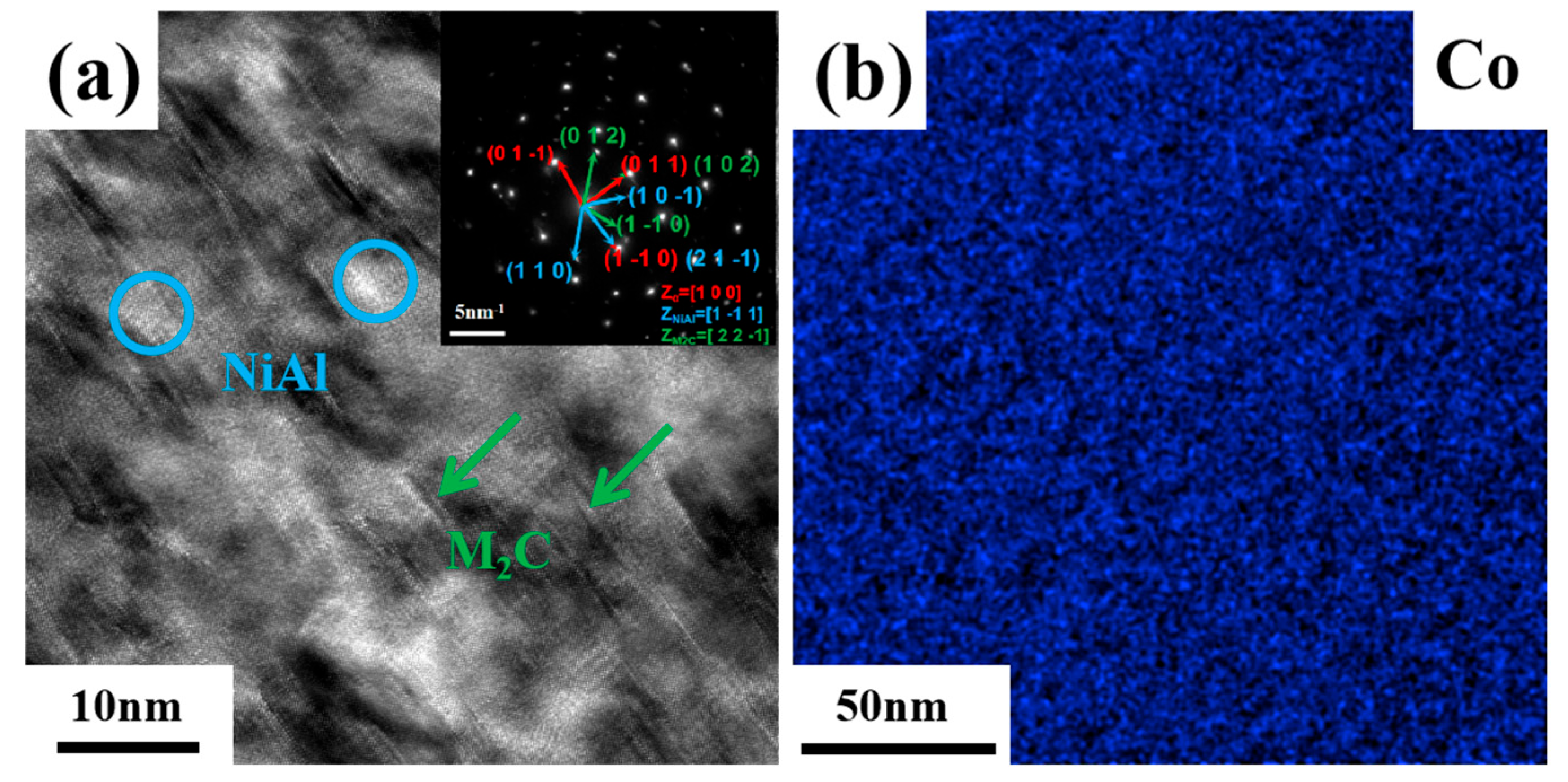
Figure 5 illustrates TEM images of the sample aged at 510 °C for 120 min. Two kinds of NiAl and M
2C precipitations were uniformly distributed in the martensitic matrix. As shown in
Figure 5b, the Co element was uniformly distributed in the martensitic matrix. The upper-right corner of
Figure 5a shows the corresponding Fast Fourier Transform (FFT) pattern. The red arrow along the [100]-zone axis shows the diffraction points of martensite. The blue arrow along the [1-11]-zone axis identifies the diffraction points of NiAl, while the green arrow along the [22-1]-zone axis corresponds to the diffraction points of M
2C.
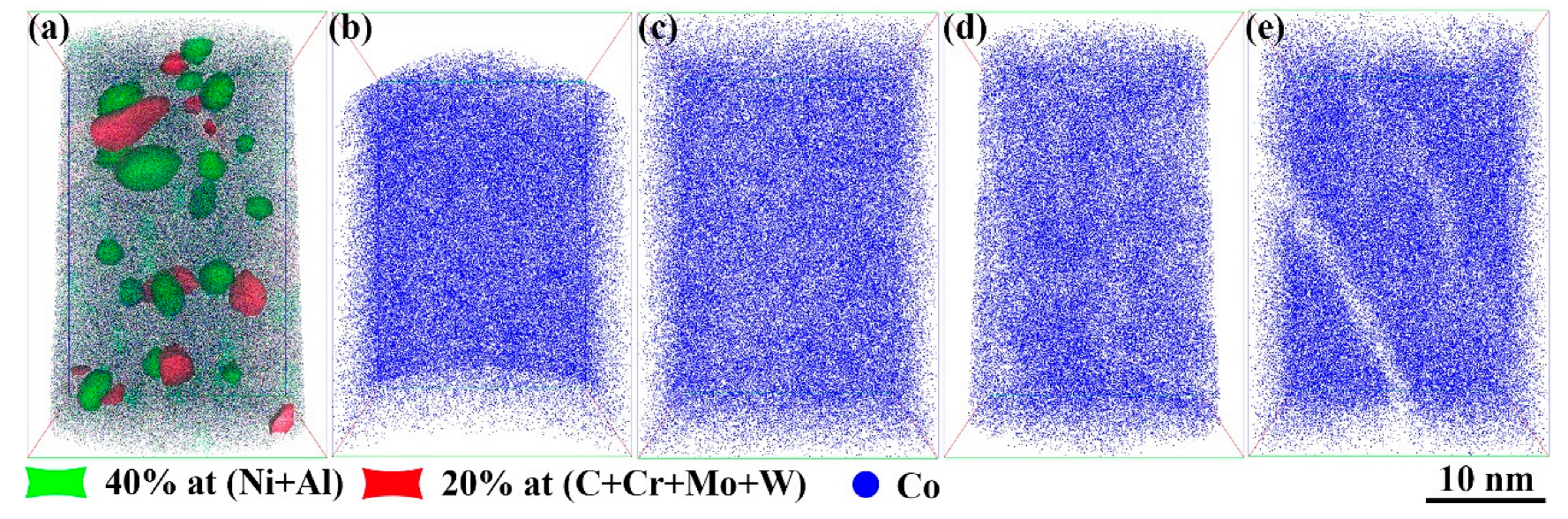
Figure 6 presents a three-dimensional reconstruction of the precipitations in the steel and the distribution of the Co atoms under different aging conditions.
Figure 6a shows a reconstructed image of nanoscale NiAl and M
2C after 2 h of aging treatment. The fine, spherical, and closely packed precipitations were identified as NiAl, defined by iso-concentration surfaces of 40 at.% for both Ni and Al. The precipitations rich in C, Cr, Mo, and W were identified as M
2C, made up of iso-concentration surfaces of 20 at.% for C, Cr, Mo, and W. The number density of the NiAl precipitations was approximately 1.35 × 10
24 m
−3, with sizes ranging from 1 to 8 nm. The number density of the M
2C precipitations was about 0.83 × 10
24 m
−3, with lengths between 2 and 10 nm. The NiAl precipitations, typically spherical and small (approximately 2–5 nm in diameter), were uniformly distributed within the matrix, with a number density of about 0.9 × 10
24 m
−3. The M
2C precipitations, mainly short, rod-like structures (approximately 2–3 nm in diameter and 2–5 nm in length), were primarily located around the NiAl precipitations, with a number density of approximately 0.5 × 10
24 m
−3. The M
2C precipitations were often connected at their interfaces to adjacent NiAl particles, indicating that the NiAl phase influenced the nucleation sites and mechanisms of the M
2C precipitations.
Figure 6b–e show the distribution of the Co atoms in the solution-treated state and after aging for 30 min, 120 min, and 600 min, respectively. In the solution-treated state, the Co atoms were uniformly distributed. After 30 min of aging, no significant change in the Co distribution was observed. However, by 2 h, localized low-concentration regions of Co began to appear, and by 10 h, these low-concentration zones became more pronounced. These areas corresponded to regions occupied by rod-like precipitations, indicating that Co atoms were expelled into the matrix as the precipitations grew. These results demonstrate that although Co was not a primary constituent of the precipitations, it played a critical role in the nucleation and growth of NiAl and M
2C.
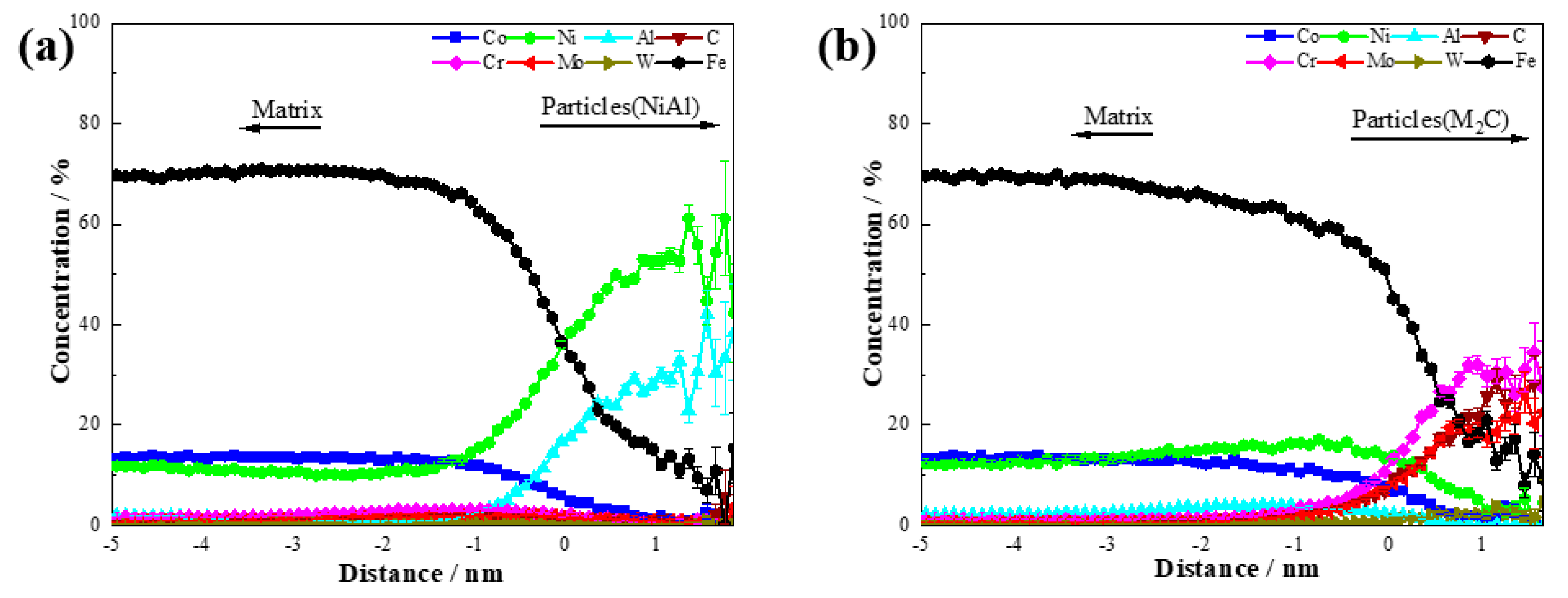
The concentration distributions of NiAl and M
2C in the steel after aging at 510 °C for 120 min are shown in
Figure 7. The core composition of the NiAl precipitations was approximately 61.1 ± 11.5 at.% Ni, 33.3 ± 11.1 at.% Al, and 0 at.% Co. For M
2C, the core composition consisted of 27.3 ± 9.5 at.% Cr, 22.7 ± 8.9 at.% Mo, 4.5 ± 4.4 at.% W, 27.3 ± 9.5 at.% C, and 0 at.% Co. These results indicate that during the nucleation process of NiAl and M
2C, Co atoms were excluded from the precipitations. Although Co did not enter the precipitations, it played a crucial role in the nucleation and growth of both NiAl and M
2C as a non-precipitating element.
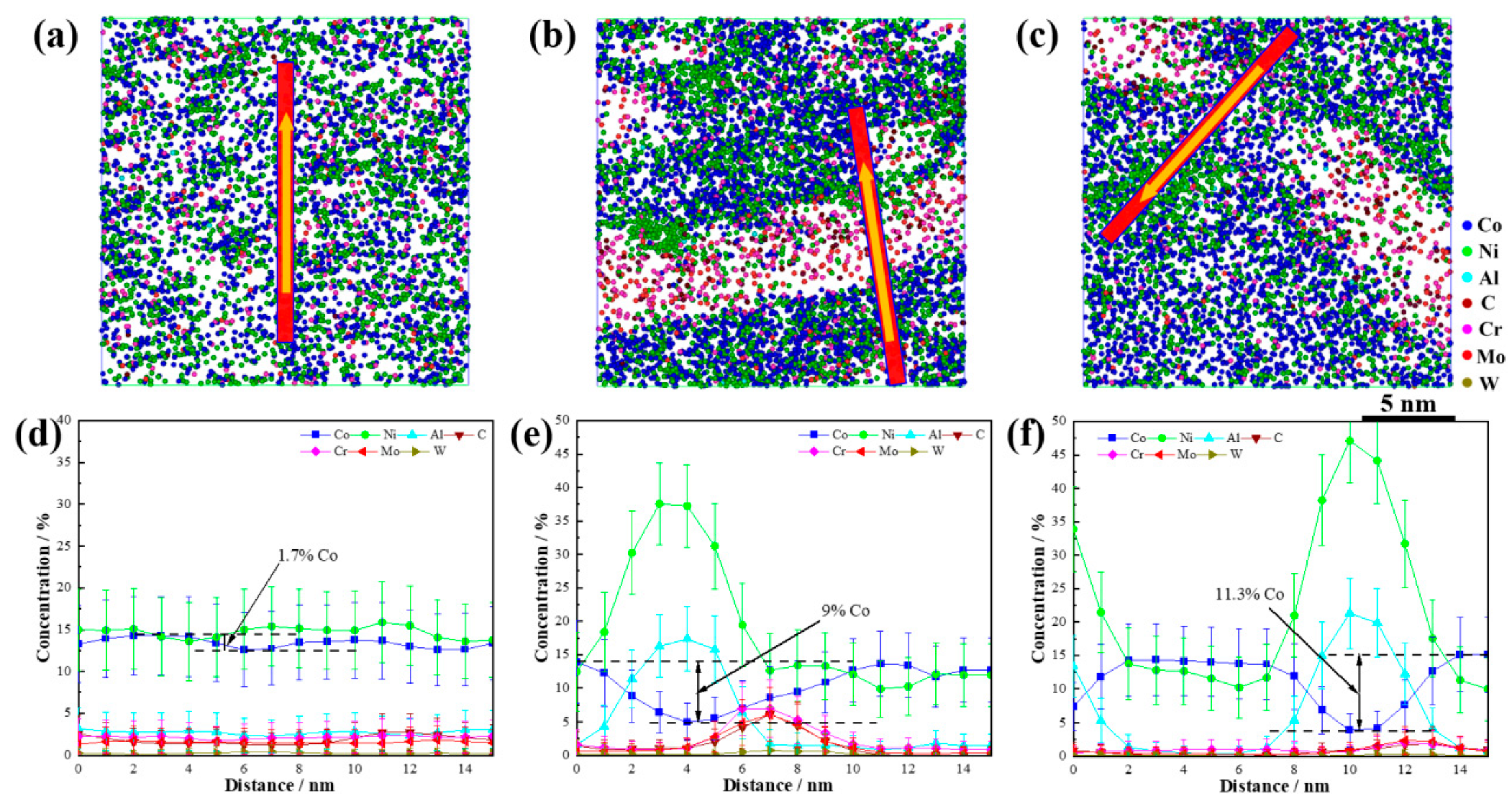
To investigate the synergistic effect of Co on the dual nanoscale precipitations, cross-sectional analyses of the precipitations at various aging stages were performed, as shown in
Figure 8. In the solution-treated state, the Co concentration fluctuations were minimal, with an average value of approximately 12.9% and a maximum difference between the high- and low-concentration areas of only 1.7%. After aging for 120 min, the Co content in the core of the NiAl precipitations decreased to 5%, and in the M
2C precipitations it decreased to 8.5%. The highest Co concentration at the phase boundary was 13.9%. These results show that with an increasing aging time, the Co atoms were gradually expelled from the precipitations into the matrix. The maximum Co concentration difference between the precipitations and the matrix was 9%. After 600 min of aging treatment, the precipitations began to coarsen slightly yet still maintained their nanoscale size. At this stage, the Co content in the NiAl core further decreased to 3.8%, and it decreased to 7.6% in M
2C. The highest Co concentration at the boundary of the precipitations was 15.2%, resulting in a maximum Co concentration difference of 11.3% between the precipitations and the matrix.
Based on the simulation results, it can be concluded that Co suppressed the diffusion of the solute atoms that formed the precipitations, promoting the short-range segregation of these atoms in the matrix. This effect induced the uniform nucleation of the precipitations and improved their distribution and number density [
30,
31]. As the precipitations nucleated and grew, Co atoms were gradually released into the surrounding matrix. The regions of high Co concentrations around the precipitations hindered the long-range diffusion of atoms away from the precipitation–matrix interface, thereby retarding precipitation coarsening [
32]. Even after long-term aging treatment, the precipitations maintained a high number density and nanoscale size. Thus, Co exhibited a synergistic strengthening effect in the newly designed steel.