Enhanced Reduction of Nitrate to Ammonia at the Co-N Heteroatomic Interface in MOF-Derived Porous Carbon
Abstract
1. Introduction
2. Experimental Procedure
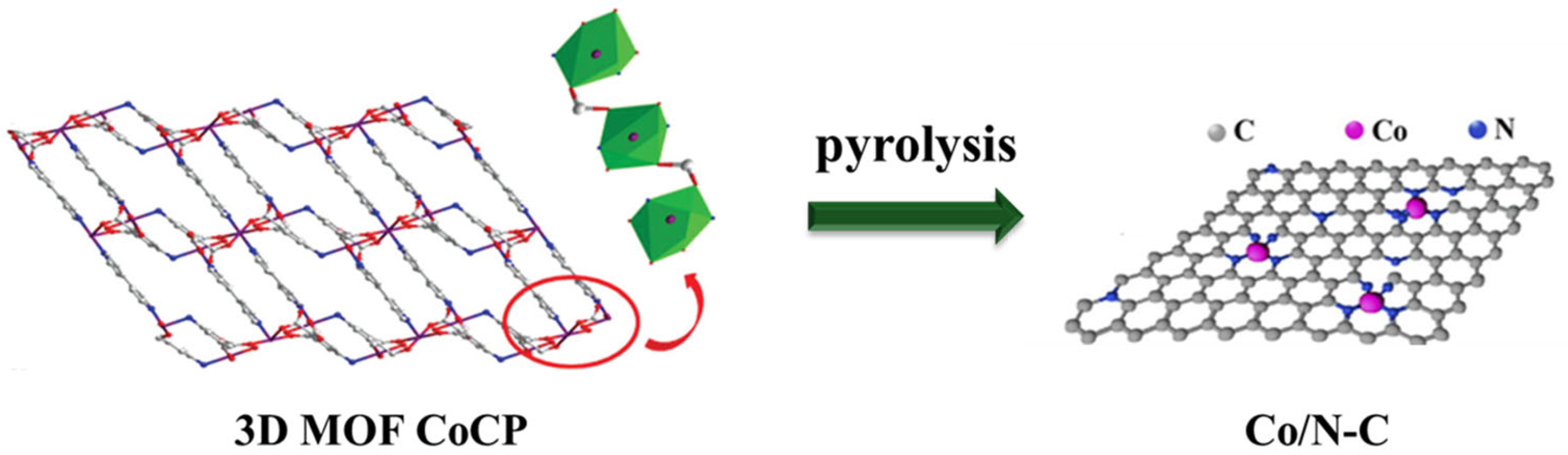
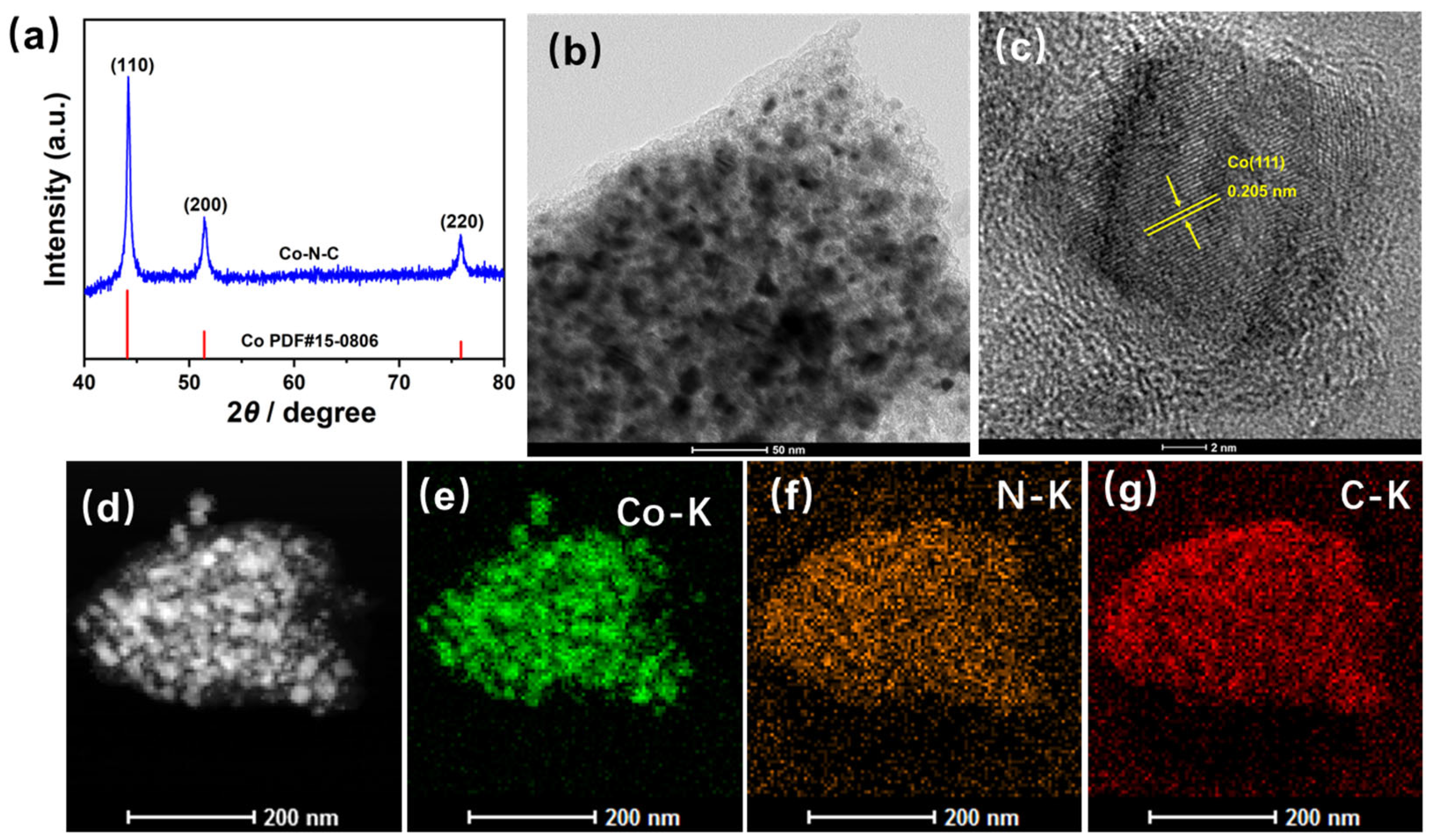
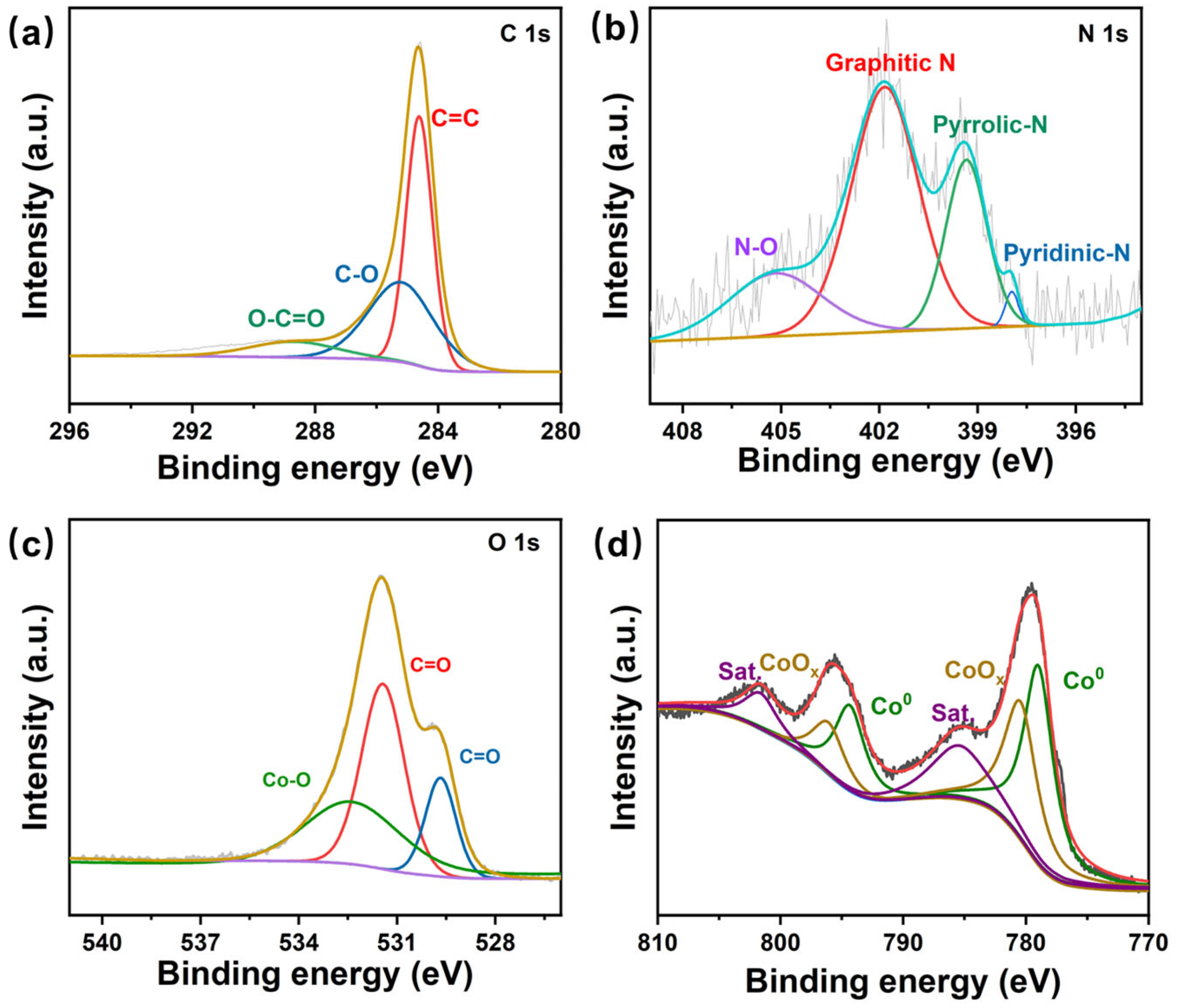
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Han, S.H.; Cheng, C.; Guo, C.; Li, T.; Yu, Y. Unveiling the Reaction Mechanism of Nitrate Reduction to Ammonia Over Cobalt-Based Electrocatalysts. J. Am. Chem. Soc. 2024, 146, 12976–12983. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Guan, Z.; Zhu, Y.; Guo, D.; Chen, X.; Wang, S. Highly Efficient Electrocatalytic Nitrate Reduction to Ammonia: Group VIII-Based Catalysts. ACS Nano 2024, 18, 27833–27852. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, J.; Kong, Y.; Zhang, Z.; Zhang, Z.; Li, S.; Tong, L.; Gao, X.; Zhang, J. Cu/CuxO/Graphdiyne Tandem Catalyst for Efficient Electrocatalytic Nitrate Reduction to Ammonia. Adv. Mater. 2024, 36, 2405660. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xiang, K.; Liu, Z.; Peng, Z.; Zou, Y.; Wang, S. Recent Advances in Upgrading of Low-Cost Oxidants to Value-Added Products by Electrocatalytic Reduction Reaction. Adv. Funct. Mater. 2022, 32, 2208212. [Google Scholar] [CrossRef]
- Xue, Z.-H.; Shen, H.-C.; Chen, P.; Pan, G.-X.; Zhang, W.-W.; Zhang, W.-M.; Zhang, S.-N.; Li, X.-H.; Yavuz, C.T. Boronization of Nickel Foam for Sustainable Electrochemical Reduction of Nitrate to Ammonia. ACS Energy Lett. 2023, 8, 3843–3851. [Google Scholar] [CrossRef]
- Yang, J.; Qi, H.; Li, A.; Liu, X.; Yang, X.; Zhang, S.; Zhao, Q.; Jiang, Q.; Su, Y.; Zhang, L.; et al. Potential-Driven Restructuring of Cu Single Atoms to Nanoparticles for Boosting the Electrochemical Reduction of Nitrate to Ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. [Google Scholar] [CrossRef]
- Yu, J.; Qin, Y.; Wang, X.; Zheng, H.; Gao, K.; Yang, H.; Xie, L.; Hu, Q.; He, C. Boosting electrochemical nitrate-ammonia conversion via organic ligands-tuned proton transfer. Nano Energy 2022, 103, 107705. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Wang, C.; Yao, J.; Tan, Z.; Yao, Z.; Li, C.; Guo, C. Active sites-rich layered double hydroxide for nitrate-to-ammonia production with high selectivity and stability. Chem. Eng. J. 2022, 434, 134641. [Google Scholar] [CrossRef]
- Liu, H.; Lang, X.; Zhu, C.; Timoshenko, J.; Rüscher, M.; Bai, L.; Guijarro, N.; Yin, H.; Peng, Y.; Li, J.; et al. Efficient Electrochemical Nitrate Reduction to Ammonia with Copper-Supported Rhodium Cluster and Single-Atom Catalysts. Angew. Chem. Int. Ed. 2022, 61, e20220255. [Google Scholar]
- Liu, M.; Mao, Q.; Shi, K.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Electroreduction of Nitrate to Ammonia on Palladium–Cobalt–Oxygen Nanowire Arrays. ACS Appl. Mater. Interfaces 2022, 14, 13169–13176. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Z.; Zhou, S.; Han, C.; Kundi, V.; Kumar, P.V.; Thomsen, L.; Johannessen, B.; Peng, L.; Shan, Y.; et al. Surface Engineering on Ag-Decorated Co3O4 Electrocatalysts for Boosting Nitrate Reduction to Ammonia. ACS Catal. 2024, 14, 11231–11242. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Ren, T.; Wang, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Ultralow-content Pd in-situ incorporation mediated hierarchical defects in corner-etched Cu2O octahedra for enhanced electrocatalytic nitrate reduction to ammonia. Appl. Catal. B Environ. 2022, 306, 121094. [Google Scholar] [CrossRef]
- Li, H.; Xu, X.; Lin, X.; Chen, S.; He, M.; Peng, F.; Gao, F. Cooperative interaction between Cu and sulfur vacancies in SnS2 nanoflowers for highly efficient nitrate electroreduction to ammonia. J. Mater. Chem. A 2023, 11, 2014–2022. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, X.; Zhang, Z.; Chen, L.; Liu, W.; Tong, L.; Gao, X.; Zhang, J. Graphdiyne Enabled Nitrogen Vacancy Formation in Copper Nitride for Efficient Ammonia Synthesis. J. Am. Chem. Soc. 2024, 146, 14898–14904. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Hu, X.; Guo, J.; Liang, Y.; Gong, X.; Xiao, X.; Luo, L.; Wu, Z.; Qin, P. Mechanism of oxygen vacancy engineering CoOx/Fe3O4 regulated electrocatalytic reduction of nitrate to ammonia. J. Colloid Interface Sci. 2025, 683, 709–721. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, D.; Yao, J.; Zhu, K.; Zhao, H. Effects of crystal phase in cobalt-based perovskite for electro- reduction of nitrate to ammonia. Appl. Surf. Sci. 2025, 685, 161935. [Google Scholar] [CrossRef]
- Li, X.; Jiao, Y.; Cui, Y.; Dai, C.; Ren, P.; Song, C.; Ma, X. Synergistic Catalysis of the Synthesis of Ammonia with Co-Based Catalysts and Plasma: From Nanoparticles to a Single Atom. ACS Appl. Mater. Interfaces 2021, 13, 52498–52507. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, Y.-G.; Xue, Q.; Li, F.-M.; Zhao, G.-T.; Jin, P.-J.; Li, S.-N.; Chen, Y. Efficient Nitrate-to-Ammonia Electroreduction at Cobalt Phosphide Nanoshuttles. ACS Appl. Mater. Interfaces 2021, 13, 45521–45527. [Google Scholar] [CrossRef]
- Ni, J.; Yan, J.; Li, F.; Qi, H.; Xu, Q.; Su, C.; Sun, L.; Sun, H.; Ding, J.; Liu, B. Atomic Co–P Catalytic Pair Drives Efficient Electrochemical Nitrate Reduction to Ammonia. Adv. Energy Mater. 2024, 14, 2400065. [Google Scholar] [CrossRef]
- Xie, L.; Liu, Q.; Li, X.; Wang, G.; Ren, Z.; Qiu, H.; Sun, X.; Kong, Q. Electrochemical Reduction of Nitrate to Ammonia on an In Situ–Derived Co3O4@CoBi Core–Shell Nanoarray. ACS Appl. Nano Mater. 2023, 7, 951–956. [Google Scholar] [CrossRef]
- Wang, J.; Cai, C.; Wang, Y.; Yang, X.; Wu, D.; Zhu, Y.; Li, M.; Gu, M.; Shao, M. Electrocatalytic Reduction of Nitrate to Ammonia on Low-Cost Ultrathin CoOx Nanosheets. ACS Catal. 2021, 11, 15135–15140. [Google Scholar] [CrossRef]
- Paul, S.; Sarkar, S.; Adalder, A.; Kapse, S.; Thapa, R.; Ghorai, U.K. Strengthening the Metal Center of Co–N4 Active Sites in a 1D–2D Heterostructure for Nitrate and Nitrogen Reduction Reaction to Ammonia. ACS Sustain. Chem. Eng. 2023, 11, 6191–6200. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, M.; Lai, C.; Cheng, M.; Xu, F.; Ma, D.; Li, L.; Yan, H.; Sun, H.; Fan, X.; et al. Metal-organic frameworks derived low-cost Cu-doped Co3O4 for efficient reduction of ultra-low nitrate concentrations to ammonia. Chem. Eng. J. 2024, 493, 152543. [Google Scholar] [CrossRef]
- Liu, P.; Yan, J.; Huang, H.; Song, W. Cu/Co bimetallic conductive MOFs: Electronic modulation for enhanced nitrate reduction to ammonia. Chem. Eng. J. 2023, 466, 143134. [Google Scholar] [CrossRef]
- Shi, Q.; Cheng, M.; Liu, Y.; Wang, J.; Zhang, G.; Li, L.; Du, L.; Wang, G.; Liu, H. In-situ generated MOFs with supportive LDH substrates and their derivatives for photo-electrocatalytic energy production and electrochemical devices: Insights into synthesis, function, performance and mechanism. Coord. Chem. Rev. 2024, 499, 215500. [Google Scholar] [CrossRef]
- Lv, Y.; Ke, S.W.; Gu, Y.; Tian, B.; Tang, L.; Ran, P.; Zhao, Y.; Ma, J.; Zuo, J.L.; Ding, M. Highly Efficient Electrochemical Nitrate Reduction to Ammonia in Strong Acid Conditions with Fe2M-Trinuclear-Cluster Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202305246. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Wang, M.; Zhang, L.; Jiang, Y.; Qian, T.; Xiong, J.; Yang, C.; Yan, C. In Situ Construction of Metal–Organic Frameworks as Smart Channels for the Effective Electrocatalytic Reduction of Nitrate at Ultralow Concentrations to Ammonia. ACS Catal. 2023, 13, 9125–9135. [Google Scholar] [CrossRef]
- He, H.; Wen, H.-M.; Li, H.-K.; Zhang, H.-W. Recent advances in metal–organic frameworks and their derivatives for electrocatalytic nitrogen reduction to ammonia. Coord. Chem. Rev. 2022, 471, 214761. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, M.; Ghorbani-Asl, M.; Zhang, J.; Ly, K.H.; Liao, Z.; Chen, G.; Wei, Y.; Biswal, B.P.; Zschech, E.; et al. Boosting the Electrocatalytic Conversion of Nitrogen to Ammonia on Metal-Phthalocyanine-Based Two-Dimensional Conjugated Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 19992–20000. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, H.; Huang, T.; Sun, Y.; Pang, H. Research Progress on the Application of MOF and MOF-Based Materials in Nitrogen Reduction. Adv. Sustain. Syst. 2024, 8, 2400225. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.-D.; Zhao, H.; Zou, Y.; Zhang, Z.-Y.; Liu, J.; Wang, J.; Gu, Z.-G.; Yan, X. High-valent cobalt active sites derived from electrochemical activation of metal-organic frameworks for efficient nitrate reduction to ammonia. Appl. Catal. B Environ. 2024, 340, 123237. [Google Scholar] [CrossRef]
- Shao, Z.; Han, X.; Liu, Y.; Xu, W.; Wu, Q.; Xie, Q.; Zhao, Y.; Hou, H. Metal-dependent photocatalytic activity and magnetic behaviour of a series of 3D Co–Ni metal organic frameworks. Dalton Trans. 2019, 48, 6191–6197. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, J.; Liu, Q.; Dong, K.; Yue, L.; Wei, P.; Luo, Y.; Liu, Q.; Li, N.; Tang, B.; et al. Co nanoparticle-decorated pomelo-peel-derived carbon enabled high-efficiency electrocatalytic nitrate reduction to ammonia. Chem. Commun. 2022, 58, 4259–4262. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, S.; Liu, J.; Jiang, Y.; Zhang, Y.; Wang, G.; Zhang, H. High-performance electrocatalytic nitrate reduction into ammonia using a chitosan regulated Co nanocatalyst. Inorg. Chem. Front. 2024, 11, 8371–8376. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, M.; Zhai, L.; Wang, Y.; Han, D.; Chen, P.; Qin, N.; Mi, L.; Yang, L. Co-N heteroatomic interface engineering in peanut Shell-Derived porous carbon for enhanced oxygen reduction reaction. J. Colloid Interface Sci. 2022, 622, 971–977. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Zhao, C.; Yin, X.; Zhao, Y. Co, N co-doped porous carbons as high-performance oxygen reduction electrocatalysts. New Carbon Mater. 2021, 36, 209–218. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn–Air Batteries and Water Splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, L.; Ruther, R.E.; Hamers, R.J. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat. Mater. 2013, 12, 836–841. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 12, 3634. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Y.; Wang, M.; Liu, S.; Wang, Z.; Qian, T.; Yan, C. Optimizing Intermediate Adsorption via Heteroatom Ensemble Effect over RuFe Bimetallic Alloy for Enhanced Nitrate Electroreduction to Ammonia. Adv. Energy Mater. 2023, 13, 2301409. [Google Scholar] [CrossRef]
- Lü, F.; Zhao, S.; Guo, R.; He, J.; Peng, X.; Bao, H.; Fu, J.; Han, L.; Qi, G.; Luo, J.; et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy 2019, 61, 420–427. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Quan, X.; Fan, X.; Chen, S.; Yu, H.; Zhao, H.; Zhang, Y.; Zhao, J. Facile Ammonia Synthesis from Electrocatalytic N2 Reduction under Ambient Conditions on N-Doped Porous Carbon. ACS Catal. 2023, 13, 16286. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, K.; Cong, L.; Yu, Z.; Qu, L.; Huang, W. Facile synthesis of a Ru-dispersed N-doped carbon framework catalyst for electrochemical nitrogen reduction. Catal. Sci. Technol. 2020, 10, 1336–1342. [Google Scholar] [CrossRef]
- Zhang, S.; Li, M.; Li, J.; Song, Q.; Liu, X. High-ammonia selective metal–organic framework–derived Co-doped Fe/Fe2O3 catalysts for electrochemical nitrate reduction. Proc. Natl. Acad. Sci. USA 2022, 119, e2115504119. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Liu, G.; Wu, S.; Xu, R. Bimetallic MOF derived nickel nanoclusters supported by nitrogen-doped carbon for efficient electrocatalytic CO2 reduction. Nano Res. 2022, 16, 4546–4553. [Google Scholar] [CrossRef]
- Xuan, C.; Hou, B.; Xia, W.; Peng, Z.; Shen, T.; Xin, H.L.; Zhang, G.; Wang, D. From a ZIF-8 polyhedron to three-dimensional nitrogen doped hierarchical porous carbon: An efficient electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 10731–10739. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Wang, S.-M.; Han, Z. MOF-templated nitrogen-doped porous carbon materials as efficient electrocatalysts for oxygen reduction reactions. Inorg. Chem. Front. 2017, 4, 1231–1237. [Google Scholar] [CrossRef]
- Yang, S.-C.; Muthiah, B.; Chang, J.-W.; Tsai, M.-D.; Wang, Y.-C.; Li, Y.-P.; Kung, C.-W. Support effect in metal–organic framework-derived copper-based electrocatalysts facilitating the reduction of nitrate to ammonia. Electrochim. Acta 2024, 492, 144348. [Google Scholar] [CrossRef]
- Lei, F.; Xu, W.; Yu, J.; Li, K.; Xie, J.; Hao, P.; Cui, G.; Tang, B. Electrochemical synthesis of ammonia by nitrate reduction on indium incorporated in sulfur doped graphene. Chem. Eng. J. 2021, 426, 131317. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Guo, H.; Li, M.; Yang, Y.; Luo, R.; Liu, W.; Zhang, F.; Tang, C.; Yang, G.; Zhou, Y. Self-Supported Pd Nanorod Arrays for High-Efficient Nitrate Electroreduction to Ammonia. Small 2023, 19, 2207743. [Google Scholar] [CrossRef]
- Wu, K.; Sun, C.; Wang, Z.; Song, Q.; Bai, X.; Yu, X.; Li, Q.; Wang, Z.; Zhang, H.; Zhang, J.; et al. Surface Reconstruction on Uniform Cu Nanodisks Boosted Electrochemical Nitrate Reduction to Ammonia. ACS Mater. Lett. 2022, 4, 650–656. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.; Liang, Y.; Jiang, H.; Li, Z.; Wu, S.; Gao, Z.; Cui, Z.; Zhu, S. Dynamically Restructuring Nanoporous Cu–Co Electrocatalyst for Efficient Nitrate Electroreduction to Ammonia. ACS Catal. 2024, 14, 12251–12259. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Y.; Wang, C.; Ling, Y.; Yu, Y.; Zhang, B. Boosting Selective Nitrate Electroreduction to Ammonium by Constructing Oxygen Vacancies in TiO2. ACS Catal. 2020, 10, 3533–3540. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Du, S.; Huang, Z.; Liu, N.; Shao, Z.; Qin, N.; Wang, Y.; Wang, H.; Ni, Z.; Yang, L. Enhanced Reduction of Nitrate to Ammonia at the Co-N Heteroatomic Interface in MOF-Derived Porous Carbon. Materials 2025, 18, 2976. https://doi.org/10.3390/ma18132976
Liu J, Du S, Huang Z, Liu N, Shao Z, Qin N, Wang Y, Wang H, Ni Z, Yang L. Enhanced Reduction of Nitrate to Ammonia at the Co-N Heteroatomic Interface in MOF-Derived Porous Carbon. Materials. 2025; 18(13):2976. https://doi.org/10.3390/ma18132976
Chicago/Turabian StyleLiu, Jing, Shuo Du, Zibin Huang, Ning Liu, Zhichao Shao, Na Qin, Yanjie Wang, Hongfang Wang, Zhihui Ni, and Liping Yang. 2025. "Enhanced Reduction of Nitrate to Ammonia at the Co-N Heteroatomic Interface in MOF-Derived Porous Carbon" Materials 18, no. 13: 2976. https://doi.org/10.3390/ma18132976
APA StyleLiu, J., Du, S., Huang, Z., Liu, N., Shao, Z., Qin, N., Wang, Y., Wang, H., Ni, Z., & Yang, L. (2025). Enhanced Reduction of Nitrate to Ammonia at the Co-N Heteroatomic Interface in MOF-Derived Porous Carbon. Materials, 18(13), 2976. https://doi.org/10.3390/ma18132976




