Electrochemical Evaluation of New Ti-Based High-Entropy Alloys in Artificial Saliva with Fluoride: Implications for Dental Implant Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Alloy Preparation
2.2. Electrochemical Testing
2.3. Surface Characterization
3. Results
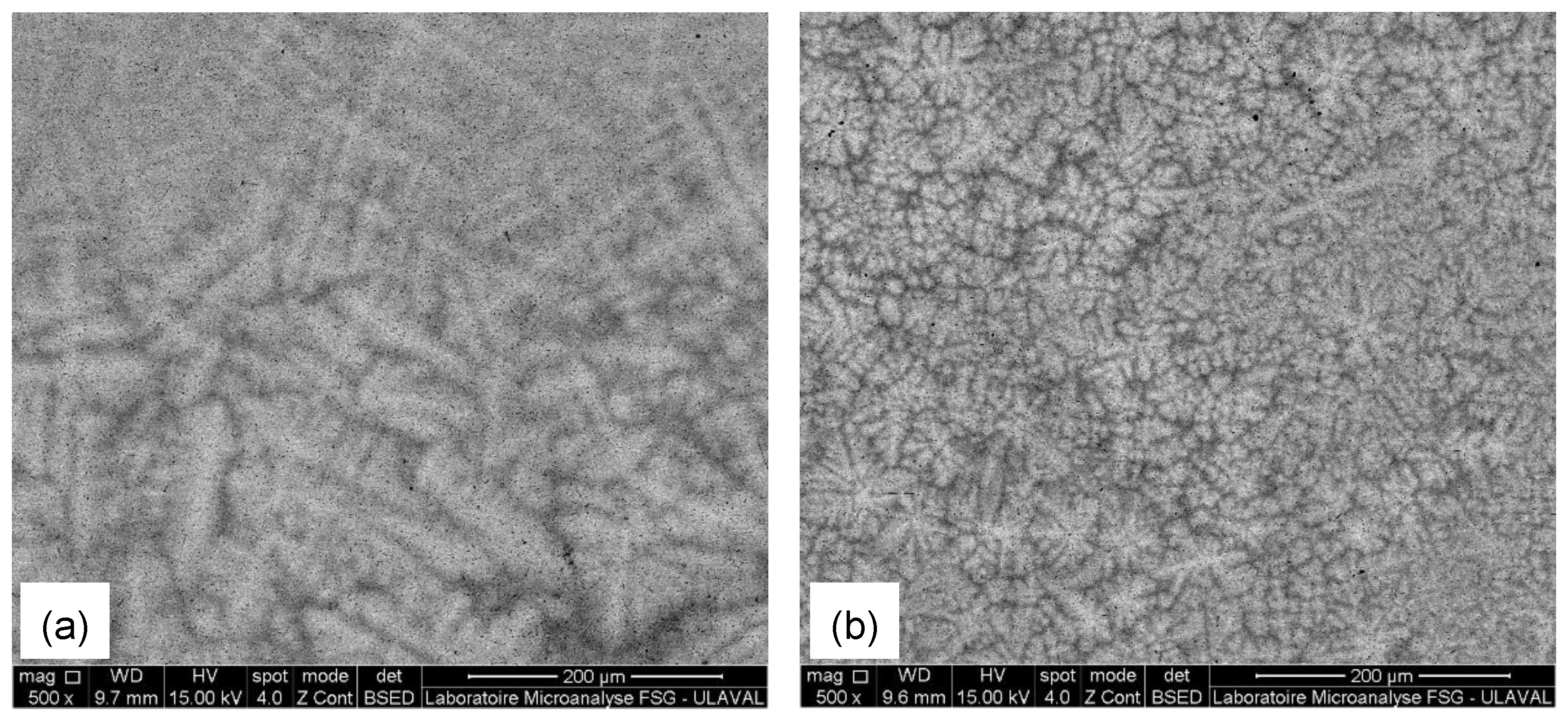
3.1. Microstructural Observation
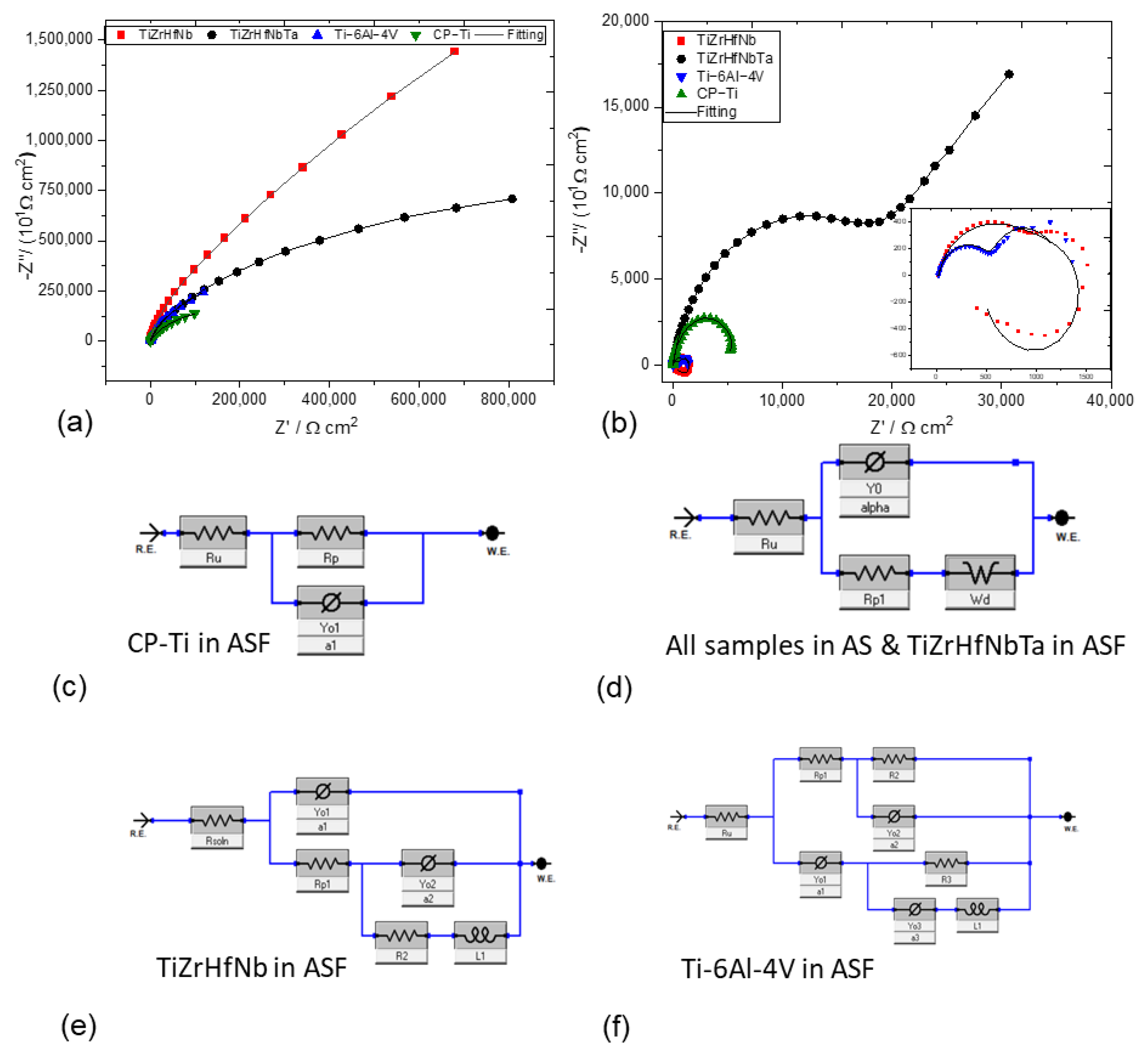
3.2. Electrochemical Impedance Spectroscopy
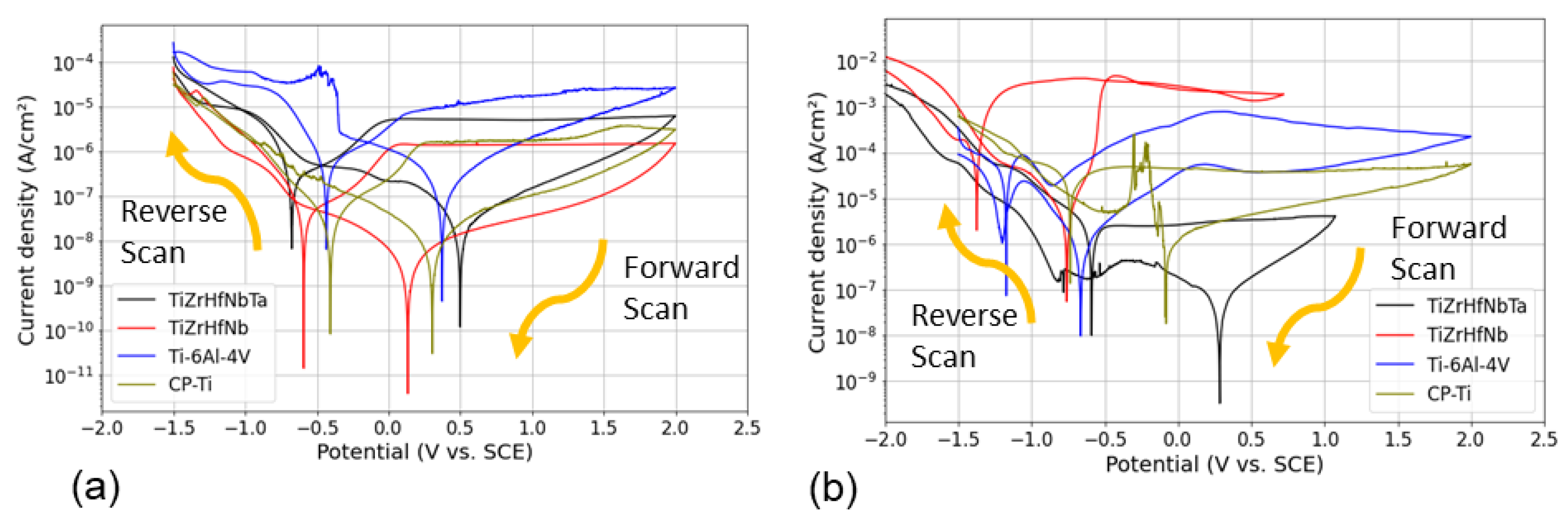
3.3. Cyclic Potentiodynamic Polarization
3.4. Mott–Schottky
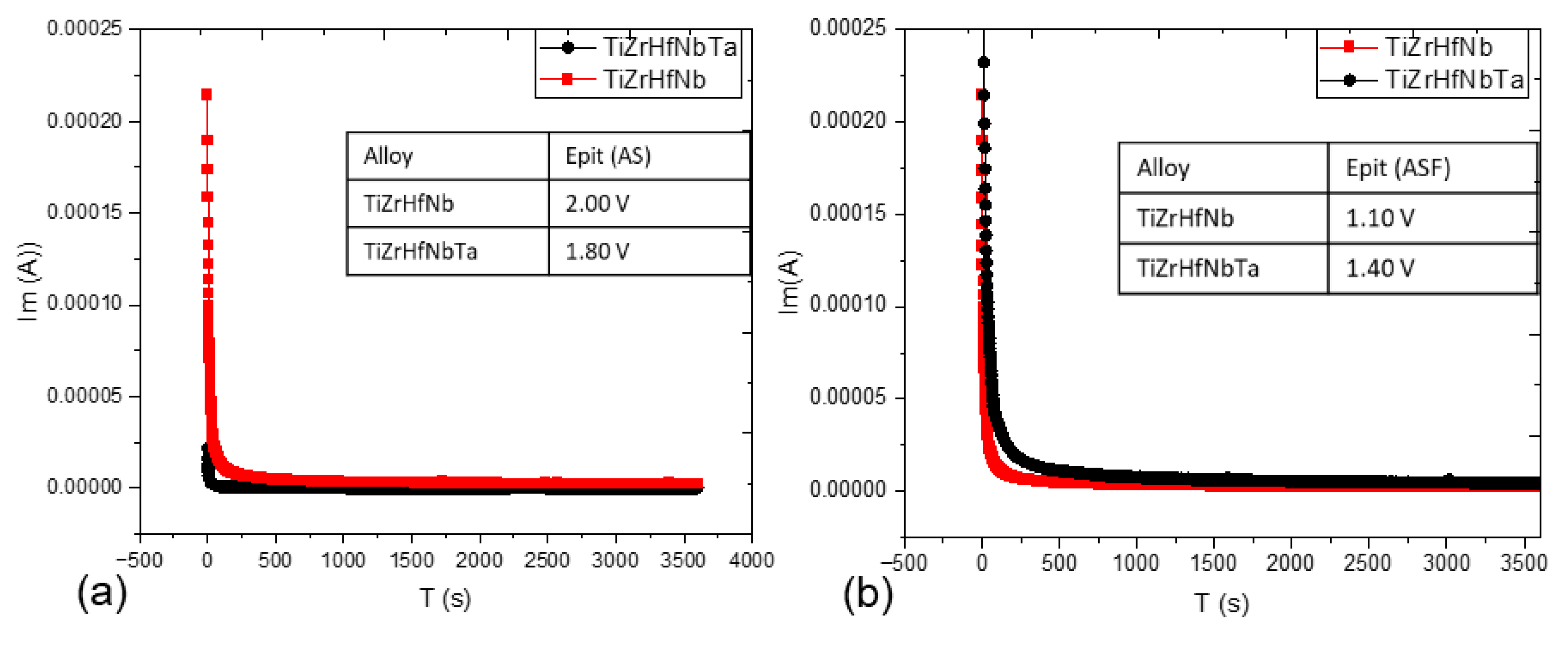
3.5. Potentiostatic Polarization
3.6. Post Corrosion AFM Observation
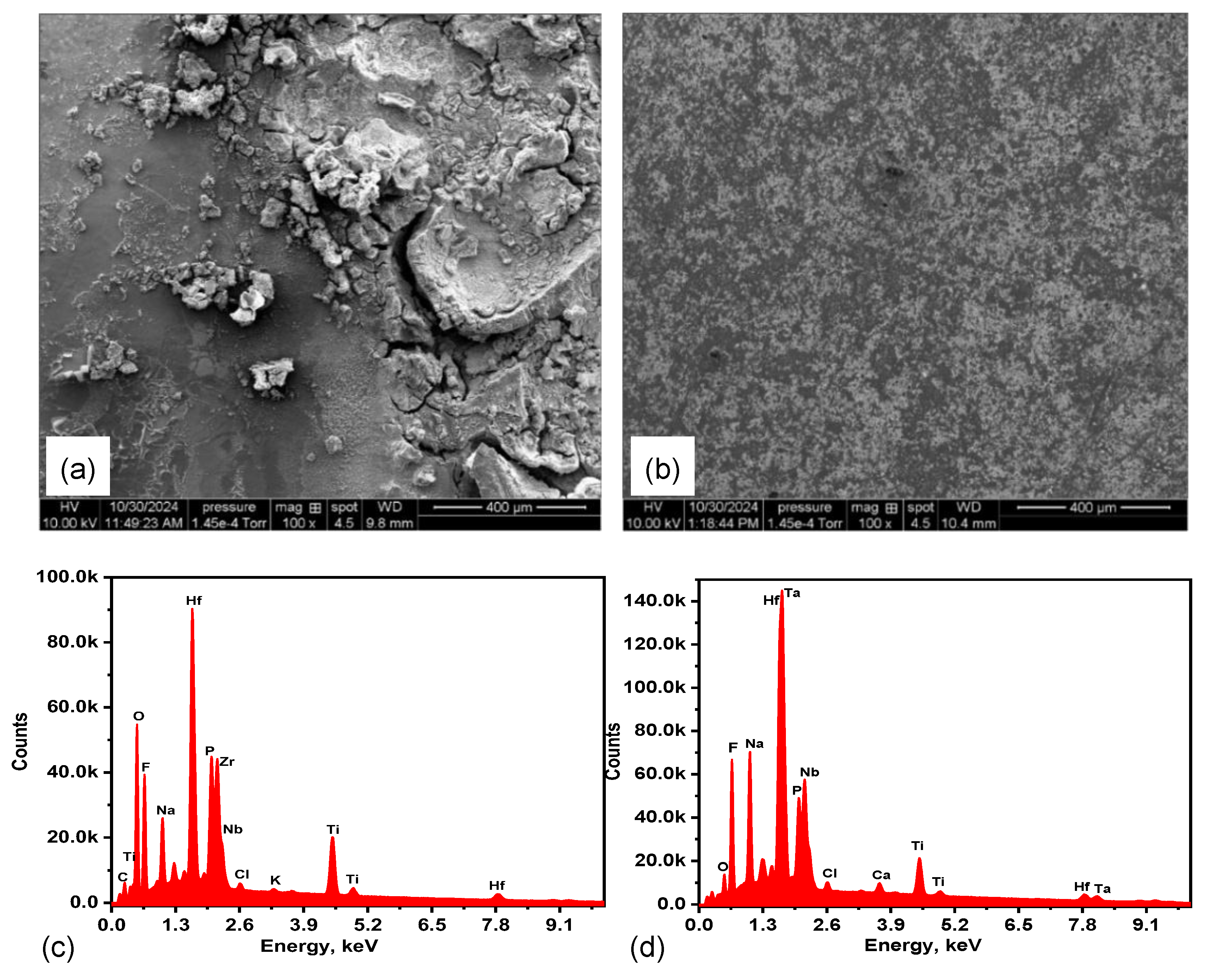
3.7. Post Corrosion SEM Observation
4. Discussion
- I.
- In the AS solution—mildly corrosive
- II.
- In the ASF solution—aggressive, fluoride-containing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binhuraib, H.M.; Alhajrassi, S.K.; Bediwi, M.; Basudan, O.A.; Alfarsi, A.; Ahmad, J. Mini Dental Implants Retaining Overdenture on Old Patients with Atrophic Ridges. J. Healthc. Sci. 2024, 4, 1027. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and Biological Properties of Titanium and Its Alloys for Oral Implant with Preparation Techniques: A Review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Kim, J.E.; Yoon, Y.; Pae, A.; Lee, H.; Lee, K.W. Clinical Outcome of Narrow Diameter Dental Implants: A 3-Year Retrospective Study. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 26. [Google Scholar] [CrossRef]
- Long, F.; Badr, N.N.; Yao, Z.; Daymond, M.R. Towards Resolving a Long Existing Phase Stability Controversy in the Zr-H, Ti-H Systems. J. Nucl. Mater. 2020, 540, 152540. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Brechtl, J.; Choudhuri, D.; Zhang, Y.; Alam, T.; Ghazisaeidi, M.; Mills, M.J.; McDowell, D.L.; Neu, R.W. A Review of the Serrated-Flow Phenomenon and Its Role in the Deformation Behavior of High-Entropy Alloys. Metals 2020, 10, 1101. [Google Scholar] [CrossRef]
- Vinoth, A.; Datta, S. Computational Intelligence Based Design of Biomaterials. Comput. Methods Mater. Sci. 2022, 22, 229–262. [Google Scholar] [CrossRef]
- Mendis, S.; Xu, W.; Tang, H.P.; Jones, L.A.; Liang, D.; Thompson, R.; Choong, P.; Brandt, M.; Qian, M. Characteristics of Oxide Films on Ti-(10–75)Ta Alloys and Their Corrosion Performance in an Aerated Hank’s Balanced Salt Solution. Appl. Surf. Sci. 2020, 506, 145013. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.; Bakhsheshi-Rad, H.R.; Abdul Kadir, M.R.; Hamzah, E.; Abdul Khalil, H.P.S. Biocompatibility and Corrosion Resistance of Metallic Biomaterials. Corros. Rev. 2020, 38, 381–402. [Google Scholar] [CrossRef]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and Fabrication of Ti–Zr-Hf-Cr-Mo and Ti–Zr-Hf-Co-Cr-Mo High-Entropy Alloys as Metallic Biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of Non-Equiatomic Ti-Nb-Ta-Zr-Mo High-Entropy Alloys for Metallic Biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Sezanova, K.; Gergulova, R.; Shestakova, P.; Rabadjieva, D. Thermodynamic and Kinetic Studies of the Precipitation of Double-Doped Amorphous Calcium Phosphate and Its Behaviour in Artificial Saliva. Biomimetics 2024, 9, 455. [Google Scholar] [CrossRef]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A Review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, H.; Li, C.; Wang, L.; Lu, Y.; Zhao, Y.; Gao, H.; Zhou, H.; Lu, Z. Gradient Cell–Structured High-Entropy Alloy with Exceptional Strength and Ductility. Science 2021, 374, 984–989. [Google Scholar] [CrossRef]
- Cruz, R.; de Araújo, C.A.; Borges, A.P.B.; Censi, R.; Piva, E.; Pinto, L.M. Narrow-Diameter Implants versus Regular-Diameter Implants for Rehabilitation of the Anterior Region: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2021, 50, 674–682. [Google Scholar] [CrossRef]
- Tanji, A.; Fan, X.; Sakidja, R.; Liaw, P.K.; Hermawan, H. Niobium Addition Improves the Corrosion Resistance of TiHfZrNbx High-Entropy Alloys in Hanks’ Solution. Electrochim. Acta 2022, 424, 140651. [Google Scholar] [CrossRef]
- Li, Z.; Lai, W.; Tong, X.; You, D.; Li, W.; Wang, X. Design of TiZrNbTa Multi-Principal Element Alloys with Outstanding Mechanical Properties and Wear Resistance. Mater. Sci. Eng. A 2022, 845, 143203. [Google Scholar] [CrossRef]
- Maharaja, H.U. Electrochemical Assessment of Alumina-TiC Composite: A Potential Biomaterial. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2019. Available online: https://open.clemson.edu/all_dissertations/2451 (accessed on 23 April 2025).
- Souza, J.C.; Barbosa, S.L.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. How Do Titanium and Ti6Al4V Corrode in Fluoridated Medium as Found in the Oral Cavity? An In Vitro Study. Mater. Sci. Eng. C 2015, 47, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ron, T.; Shirizly, A.; Aghion, E. Additive Manufacturing Technologies of High Entropy Alloys (HEA): Review and Prospects. Materials 2023, 16, 2454. [Google Scholar] [CrossRef]
- Aranda, V.A.; Figueroa, I.A.; Amigó, V.; González-Ojeda, R.; Lozada, O.; Vidilli, A.L.; Otani, L.B.; Gonzalez, G. Effect of Mo on High Entropy Ti-Nb-Zr-Ta Alloy: Phase Equilibria, Microstructure and Mechanical Properties. J. Alloy. Compd. 2023, 960, 170758. [Google Scholar] [CrossRef]
- Jahangiri, M. Influence of Cooling Rate During Solidification on Microstructural Features and γ′ Size and Morphology in Cast IN939 Superalloy. Int. J. Met. 2024, 18, 2357–2379. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Guo, Y.; Liu, Y.; Wang, Y.; Wang, L. Microstructure Modification and Ductility Improvement for TaMoNbZrTiAl Refractory High Entropy Alloys via Increasing Ti Content. Acta Metall. Sin. 2024, 37, 1186–1200. [Google Scholar] [CrossRef]
- Long, H.; Chen, Y.; Xu, G.; Liu, C.; Lin, L.; Chen, J. The Electronic Properties and Surface Chemistry of Passive Film on Reinforcement: Effect of Composition of Simulated Concrete Pore Solution. Constr. Build. Mater. 2022, 360, 129567. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Y.; Wang, J.; Li, F.; Liu, Z. Passivation Behavior of Chromium Alloyed High-Strength Rebar in Simulated Concrete Pore Solution. Metals 2024, 14, 859. [Google Scholar] [CrossRef]
- Boraei, N.F.E.; Elshamy, I.H.; Ibrahim, M.A. A Comparative Study on the Electrochemical Corrosion Behaviour of Biomedical β-Titanium Alloy with TiAlV and Titanium in Hank’s Physiological Solution and the Impact of Reactive Oxygen Species and Immersion Time. J. Bio-Tribo-Corros. 2024, 10, 38. [Google Scholar] [CrossRef]
- Cui, W.F.; Dong, Y.Y.; Bao, Y.C.; Qin, G.W. Improved corrosion resistance of dental Ti50Zr alloy with (TiZr)N coating in fluoridated acidic artificial saliva. Rare Met. 2021, 40, 2927–2936. [Google Scholar] [CrossRef]
- Muhammed, H.J. Electrochemical Corrosion Behavior of Metallic Biomaterials. Ph.D. Thesis, Antal Kerpely Doctoral School of Materials Science & Technology, University of Miskolc, Miskolc, Hungary, 2023. Available online: https://www.kerpely.uni-miskolc.hu/files/19672/Doktorandusz_Almanach_Vol_1_2022.pdf (accessed on 12 May 2025).
- Thakur, A.; Bansal, A.; Singh, R.; Sharma, A.; Dixit, A. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Panigrahi, K.; Mal, S.; Bhattacharyya, S. Deciphering Interfacial Charge Transfer Mechanisms in Electrochemical Energy Systems through Impedance Spectroscopy. J. Mater. Chem. A 2024, 12, 14334–14353. [Google Scholar] [CrossRef]
- Ikani, N.; Pu, J.H.; Cooke, K. Analytical Modelling and Electrochemical Impedance Spectroscopy (EIS) to Evaluate Influence of Corrosion Product on Solution Resistance. Powder Technol. 2024, 433, 119252. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, A.A.; Méndez-Rojas, M.A.; Chávez-Angel, E.; De León, A.S.; Lugo-Cervantes, E. Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution. Coatings 2024, 14, 1244. [Google Scholar] [CrossRef]
- Eliseev, E.A.; Yelisieiev, M.E.; Kalinin, S.V.; Morozovska, A.N. Observability of Negative Capacitance of a Ferroelectric Film: Theoretical Predictions. Phys. Rev. B 2022, 105, 174110. [Google Scholar] [CrossRef]
- Zhu, X.; Soult, M.C.; Wouters, B.; Mamme, M.H. Study of Solid-State Diffusion Impedance in Li-Ion Batteries Using Parallel-Diffusion Warburg Model. J. Electrochem. Soc. 2024, 171, 060539. [Google Scholar] [CrossRef]
- Ji, Y.; Schwartz, D.T. Second-Harmonic Nonlinear Electrochemical Impedance Spectroscopy: Part II. Model-Based Analysis of Lithium-Ion Battery Experiments. J. Electrochem. Soc. 2024, 171, 023504. [Google Scholar] [CrossRef]
- Mao, J.; Wang, E.; Wang, H.; Ouyang, M.; Chen, Y.; Hu, H.; Lu, L.; Ren, D.; Liu, Y. Progress in Metal Corrosion Mechanism and Protective Coating Technology for Interconnect and Metal Support of Solid Oxide Cells. Renew. Sustain. Energy Rev. 2023, 185, 113597. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Fan, X.-H.; Zhang, L. Pseudo-Passivation Mechanism of CoCrFeNiMo0.01 High-Entropy Alloy in H2S-Containing Acid Solutions. Corros. Sci. 2021, 179, 109146. [Google Scholar] [CrossRef]
- Tayyaba, Q.; Shahzad, M.; Butt, A.Q.; Rafi-ud-din; Khan, M.; Qureshi, A.H. The Influence of Electrophoretic Deposition of HA on Mg-Zn-Zr Alloy on Its In-Vitro Degradation Behaviour in the Ringer’s Solution. Surf. Coat. Technol. 2019, 375, 197–204. [Google Scholar] [CrossRef]
- Peng, S.; Xu, J.; Li, Z.; Jiang, S.; Xie, Z.-H.; Munroe, P. Electrochemical Noise Analysis of Cavitation Erosion Corrosion Resistance of NbC Nanocrystalline Coating in a 3.5 wt% NaCl Solution. Surf. Coat. Technol. 2021, 415, 127133. [Google Scholar] [CrossRef]
- Yang, J.; Kang, K.-M.; Oh, S.; Lee, Y.J.; Nah, Y.-C.; Kim, D.H. Enhancement of Long-Term Cyclic Durability of Electrochromic WO3 Thin Films via Ta2O5 Passivation. J. Alloy. Compd. 2025, 1013, 178572. [Google Scholar] [CrossRef]
- Dai, H.; Shi, S.; Yang, L.; Guo, C.; Chen, X. Recent Progress on the Corrosion Behavior of Metallic Materials in HF Solution. Corros. Rev. 2021, 39, 313–337. [Google Scholar] [CrossRef]
- Jarlöv, A. Corrosion Studies on Multicomponent TiZrNbTa Thin Films. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2020. Available online: https://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-427555 (accessed on 19 May 2025).
- Wang, Z.; Yan, Y.; Wu, Y.; Huang, X.; Zhang, Y.; Su, Y.; Qiao, L. Corrosion and Tribocorrosion Behavior of Equiatomic Refractory Medium Entropy TiZr(Hf, Ta, Nb) Alloys in Chloride Solutions. Corros. Sci. 2022, 199, 110166. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; van der Merwe, J.W.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion Behavior of Titanium Alloys in Acidic and Saline Media: Role of Alloy Design, Passivation Integrity, and Electrolyte Modification. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Ji, P.F.; Li, B.; Chen, B.H.; Wang, F.; Ma, W.; Zhang, X.Y.; Ma, M.Z.; Liu, R.P. Effect of Nb Addition on the Stability and Biological Corrosion Resistance of Ti-Zr Alloy Passivation Films. Corros. Sci. 2020, 170, 108696. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.-H.; Fan, X.-H.; Jin, J.; Zhang, L.; Du, Y.-X. Corrosion Behavior and Surface Characterization of an Equiatomic CoCrFeMoNi High-Entropy Alloy under Various pH Conditions. J. Alloy. Compd. 2022, 900, 163432. [Google Scholar] [CrossRef]
- Kroll, R.; Henderson, Z.; Spencer, B.F.; Kaya, P.; Knoblauch, V.; Engelberg, D.L. Exploring the Influence of Mott–Schottky Acquisition Parameters on the Semiconduction Behaviour of Modified Native Aluminium Oxide Films. J. Electroanal. Chem. 2023, 939, 117481. [Google Scholar] [CrossRef]
- Sivula, K. Mott–Schottky Analysis of Photoelectrodes: Sanity Checks Are Needed. ACS Energy Lett. 2021, 6, 2549–2551. [Google Scholar] [CrossRef]
- Ning, Z.; Wen, D.; Zhou, Q.; Li, N.; Macdonald, D.D. Theoretical Explanation of Passivation Behavior in OFP-Cu and OF-Cu. Acta Mater. 2024, 271, 119882. [Google Scholar] [CrossRef]
- McHendrie, R.; Nguyen, N.H.; Nguyen, M.T.; Fallahnezhad, K.; Vasilev, K.; Truong, V.K.; Hashemi, R. Development of Novel Antibacterial Ti-Nb-Ga Alloys with Low Stiffness for Medical Implant Applications. J. Funct. Biomater. 2024, 15, 167. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Chu, Y.-H.; Zhang, L.; Li, R.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable Pitting Corrosion Behavior and Characteristics of Passive Film of Laser Powder Bed Fusion Produced Ti–6Al–4V in NaCl Solutions with Different Concentrations. Corros. Sci. 2023, 215, 111017. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Triveño Ríos, C.; Antunes, R.A. Electronic Properties of the Passive Films Formed on CoCrFeNi and CoCrFeNiAl High Entropy Alloys in Sodium Chloride Solution. J. Mater. Res. Technol. 2020, 9, 13879–13892. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Wang, G.; Kuang, Y.; Xiang, X.; Chen, X.; Cai, Q.; Wang, K.; Lv, X. Advances in the Visualization and Thermal Management of Electrochromic Materials. J. Mater. Chem. C 2024, 12, 15833–15854. [Google Scholar] [CrossRef]
- Li, G.; Du, M.; Wang, J.; Huang, G.; Ma, L. Effect of Hydrogen on Pitting Corrosion of 2205 Duplex Stainless Steel under Alternating Dry/Wet Marine Environment. Int. J. Hydrogen Energy 2023, 48, 17983–17994. [Google Scholar] [CrossRef]
- Feng, J.; Tang, Y.; Liu, J.; Zhang, P.; Liu, C.; Wang, L. Bio-High Entropy Alloys: Progress, Challenges, and Opportunities. Front. Bioeng. Biotechnol. 2022, 10, 977282. [Google Scholar] [CrossRef]
- Xu, J.; Cai, L.; Lian, J.; Liao, Y. Corrosion-Resistant Behavior and Structural Optimization of Ti/Cr, Ti/Cu, Cr/Cu, Ti/Cr/Cu Multimetallic Layer Coatings in Simulated Seawater. J. Phys. D Appl. Phys. 2024, 57, 155501. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Chen, P.; Liu, X.; Hao, J.; Yang, H. Electrochemical Corrosion Behavior and Passive Film Properties of Fe2Ni2CrV0.5Nbx (x = 0.2, 0.4, 0.6, 0.8) Eutectic High-Entropy Alloy Coating Prepared by Laser Cladding. Surf. Interfaces 2024, 55, 105317. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Peng, Z. Review and Perspective on Additive Manufacturing of Refractory High Entropy Alloys. Mater. Today Adv. 2024, 22, 100497. [Google Scholar] [CrossRef]
- Tan, M.Y. Localized Corrosion in Complex Environments; John Wiley & Sons: Hoboken, NJ, USA, 2023; ISBN 978-1-119-77860-8. [Google Scholar] [CrossRef]
- Dehury, R.K.; Kumar, R.; Joshi, Y.G.; Gupta, V. Elemental Influence on Oxidation Behaviour of Cantor-Based and Refractory High Entropy Alloys: A Critical Review. Adv. Eng. Mater. 2024, 26, 2302124. [Google Scholar] [CrossRef]
- Krishnan, A.; Krishnan, A.V.; Ajith, A.; Shibli, S.M.A. Influence of Materials and Fabrication Strategies in Tailoring the Anticorrosive Property of Superhydrophobic Coatings. Surf. Interfaces 2021, 25, 101238. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Z.; Wang, Z.; Xiang, C.; Pu, J.; Sun, G.; Han, E.-H. Insights into the Passivity and Electrochemistry of CoCrFeMnNi High Entropy Alloy Fabricated by Underwater Laser Direct Metal Deposition. Corros. Sci. 2024, 237, 112289. [Google Scholar] [CrossRef]
- Zohdy, K.M.; El-Sherif, R.M.; El-Shamy, A.M. Corrosion and Passivation Behaviors of Tin in Aqueous Solutions of Different pH. J. Bio Tribo Corros. 2021, 7, 74. [Google Scholar] [CrossRef]
- Mohapatra, B.D.; Sulka, G.D. Review of Anodic Tantalum Oxide Nanostructures: From Morphological Design to Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 13865–13892. [Google Scholar] [CrossRef] [PubMed]
- Fragou, S.; Eliades, T. Effect of Topical Fluoride Application on Titanium Alloys: A Review of Effects and Clinical Implications. Pediatr. Dent. 2010, 32, 99–105. [Google Scholar] [PubMed]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A Review of Passivity Breakdown on Metal Surfaces: Influence of Chloride- and Sulfide-Ion Concentrations, Temperature, and pH. Emergent Mater. 2021, 4, 1187–1203. [Google Scholar] [CrossRef]
- Rafique, M.M.A. Bulk Metallic Glasses and Their Composites: Additive Manufacturing and Modeling and Simulation; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2021. [Google Scholar]
- Rao, P.; Singh, S.; Kumar, K.; Pandel, U.; Srivastava, C. Understanding the Evolution of Microstructure, Phase Homogeneity, Electrochemical Characteristics, and Surface Chemistry of Electrodeposited MnCrCoFeNiCu High Entropy Alloy Coating with Reinforcement of Carbon Nanotubes. Surf. Coat. Technol. 2023, 471, 129912. [Google Scholar] [CrossRef]
- Taratuta, A.; Lisoń-Kubica, J.; Paszenda, Z.; Szewczenko, J.; Kazek-Kęsik, A.; Opilski, Z.; Szindler, M.; Szindler, M.; Lukaszkowicz, K.; Dyner, A.; et al. Influence of Passive Layer Fabrication Method on Physicochemical and Antimicrobial Properties of the Ta2O5 Layer on NiTi Alloy. Vacuum 2023, 214, 112187. [Google Scholar] [CrossRef]
- Russo, F. Innovative Composite Enamel Coatings with Improved Abrasion Resistance and Mechanical Properties. Ph.D. Thesis, University of Trento, Trento, Italy, 2023. Available online: https://hdl.handle.net/11572/381529 (accessed on 23 April 2025).
- Aimone, P.; Moser, K. Working with Tantalum and Tantalum Alloys. In Proceedings of the CORROSION 2003, San Diego, CA, USA, 16–20 March 2003. [Google Scholar] [CrossRef]
- Kadiri, M.; Tanji, A.; Fan, X.; Liaw, P.K.; Mahlia, T.I.; Hermawan, H. Corrosion of TiHfZrNbx high-entropy alloys in a simulated condition of proton exchange membrane water electrolyser. Electrochim. Acta 2025, 521, 145925. [Google Scholar] [CrossRef]
- Li, J.; Zhu, C.; Cao, F.; Sun, Q. Nanoscale Pitting Corrosion of Commercially Pure Ti in Solution Containing Fluoride Ion. Mater. Chem. Phys. 2024, 323, 129650. [Google Scholar] [CrossRef]
- Tanji, A.; Fan, X.; Sakidja, R.; Liaw, P.K.; Hermawan, H. Role of Niobium on the Passivation Mechanisms of TiHfZrNb High-Entropy Alloys in Hanks’ Simulated Body Fluid. J. Funct. Biomater. 2024, 15, 305. [Google Scholar] [CrossRef] [PubMed]





| Element | Fractional Calculation | EDX Measurement | |||||
|---|---|---|---|---|---|---|---|
| Molar Fraction (m) | Atomic Fraction (at. %) | Atomic Weight (Ar) | Ar × at. % | Weight Fraction (wt. %) | Atomic Fraction (at. %) | Weight Fraction (wt. %) | |
| TiZrHfNb | |||||||
| Ti | 1 | 29.41 | 47.87 | 14.07 | 13.49 | 31.95 | 15.13 |
| Zr | 1 | 29.41 | 91.22 | 26.83 | 25.70 | 29.10 | 26.26 |
| Hf | 1 | 29.41 | 178.49 | 52.48 | 50.32 | 26.96 | 47.60 |
| Nb | 0.4 | 11.76 | 92.91 | 10.92 | 10.48 | 11.99 | 11.09 |
| Total | 3.4 | 100 | 104.3 | 100 | 100 | 100 | |
| TiZrHfNbTa | |||||||
| Ti | 1 | 20.00 | 47.87 | 9.57 | 8.09 | 21.89 | 8.88 |
| Zr | 1 | 20.00 | 91.22 | 18.24 | 15.42 | 17.89 | 13.91 |
| Hf | 1 | 20.00 | 178.49 | 35.70 | 30.17 | 17.83 | 21.75 |
| Nb | 1 | 20.00 | 92.91 | 18.58 | 15.71 | 20.64 | 16.34 |
| Ta | 1 | 20.00 | 180.95 | 36.19 | 30.61 | 21.89 | 33.75 |
| Total | 5 | 100 | 118.28 | 100 | 100 | 100 | |
| Compound | NaCl | KCl | CaCl2·2H2O | Na2S·9H2O | NaH2PO4·2H2O | Urea |
|---|---|---|---|---|---|---|
| Concentration (g/L) | 0.4 | 0.4 | 0.795 | 0.005 | 0.69 | 1 |
| Parameter | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V |
|---|---|---|---|---|---|---|---|---|
| AS Solution | ASF Solution | |||||||
| Ru (Ω) | 25.10 | 25.69 | 24.35 | 25.50 | 34.37 | 35.75 | 35.24 | 36.00 |
| Rp1 (Ω) | 3634.30 | 3269.50 | 2822.50 | 2500.70 | 1030.48 | 3569.90 | 2134.26 | 661.46 |
| Y01 (×10−6 S·sa) | 4.63 | 6.51 | 3.54 | 3.23 | 1.03 | 2.37 | 3.73 | 2.32 |
| a1 | 0.96 | 0.83 | 0.94 | 0.81 | 0.86 | 0.87 | 0.86 | 0.84 |
| Wd (×10−6 S·s½) | 1.23 | 12.3 | 17 | 19.3 | – | 199 | – | – |
| L (H) | – | – | – | – | 530.34 | – | – | 427.2 |
| R2 (Ω) | – | – | – | – | 576.53 | – | – | 576.54 |
| Y02 (S·sa) | – | – | – | – | 0.03 | – | – | 0.03 |
| a2 | – | – | – | – | – | – | – | 0.82 |
| Parameter | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V |
|---|---|---|---|---|---|---|---|---|
| AS Solution | ASF Solution | |||||||
| icorr (nA·cm−2) | 26.98 | 18.38 | 84.20 | 71.91 | 61.92 | 40.70 | 74.36 | 101.04 |
| Ecorr (mV) | 147.70 | 300.10 | 275.2 | 289 | −650.0 | 285.20 | 250.10 | −663.40 |
| Epass (V) | 0.20 | 0.10 | 0.05 | 0.30 | −0.20 | 0.30 | 0.10 | −0.10 |
| Epit (V) | 2.00 | 1.80 | 1.20 | 1.60 | 1.10 | 1.40 | 1.20 | 0.50 |
| Ea/c (V) | −0.40 | −0.30 | −0.35 | −0.25 | 0.80 | 0.90 | 0.80 | 0.70 |
| Parameter | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V | TiZrHfNb | TiZrHfNbTa | CP-Ti | Ti-6Al-4V |
|---|---|---|---|---|---|---|---|---|
| AS Solution | ASF Solution | |||||||
| Slope (×1015) | −0.43 | −0.44 | −0.02 | 1.37 | −0.04 | 9.65 | 2.08 | −0.23 |
| ND (×1020 cm−3) | −2.6 | −0.18 | −4.7 | 81 | −2.3 | 0.11 | 0.05 | −4.8 |
| Semiconductor type | P-type | P-type | P-type | N-type | P-type | N-type | N-type | N- to P-type |
| Sample | Ra (nm) | ||
|---|---|---|---|
| Prior | AS Solution | ASF Solution | |
| TiZrHfNb | 0.61 | 1.53 | 106.77 |
| TiZrHfNbTa | 6.49 | 2.58 | 50.17 |
| Element | TiZrHfNb | TiZrHfNbTa | ||
|---|---|---|---|---|
| At. % | Wt. % | At. % | Wt. % | |
| O (K) | 15.10 | 2.64 | 21.87 | 3.91 |
| Ta (M) | - | - | 10.16 | 20.57 |
| Zr (L) | 24.06 | 23.99 | 18.39 | 18.76 |
| Nb (L) | 10.23 | 10.39 | 12.78 | 13.28 |
| Ti (K) | 25.08 | 13.13 | 20.53 | 11.00 |
| Hf (L) | 25.54 | 49.84 | 16.27 | 32.47 |
| Element | TiZrHfNb | TiZrHfNbTa | ||
|---|---|---|---|---|
| At. % | Wt. % | At. % | At. % | |
| C (K) | 7.84 | 2.57 | 5.07 | 1.19 |
| O (K) | 38.28 | 16.73 | 7.27 | 2.27 |
| F (K) | 17.76 | 9.22 | 24.85 | 9.23 |
| Na (K) | 6.34 | 3.98 | 16.31 | 7.33 |
| Hf (M) | 5.41 | 26.39 | 7.46 | 26.03 |
| Ta (M) | - | - | 5.04 | 17.83 |
| P (K) | 3.68 | 3.11 | 12.23 | 7.41 |
| Nb (L) | 4.39 | 11.15 | 9.88 | 17.94 |
| Cl (K) | 0.45 | 0.44 | 0.57 | 0.39 |
| Ca (K) | - | - | 1.65 | 1.29 |
| Ti (K) | 10.56 | 13.81 | 9.69 | 9.07 |
| Zr (L) | 4.89 | 12.17 | - | - |
| K (K) | 0.4 | 0.42 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slama, H.; Tayyaba, Q.; Kadiri, M.; Hermawan, H. Electrochemical Evaluation of New Ti-Based High-Entropy Alloys in Artificial Saliva with Fluoride: Implications for Dental Implant Applications. Materials 2025, 18, 2973. https://doi.org/10.3390/ma18132973
Slama H, Tayyaba Q, Kadiri M, Hermawan H. Electrochemical Evaluation of New Ti-Based High-Entropy Alloys in Artificial Saliva with Fluoride: Implications for Dental Implant Applications. Materials. 2025; 18(13):2973. https://doi.org/10.3390/ma18132973
Chicago/Turabian StyleSlama, Hanine, Qanita Tayyaba, Mariya Kadiri, and Hendra Hermawan. 2025. "Electrochemical Evaluation of New Ti-Based High-Entropy Alloys in Artificial Saliva with Fluoride: Implications for Dental Implant Applications" Materials 18, no. 13: 2973. https://doi.org/10.3390/ma18132973
APA StyleSlama, H., Tayyaba, Q., Kadiri, M., & Hermawan, H. (2025). Electrochemical Evaluation of New Ti-Based High-Entropy Alloys in Artificial Saliva with Fluoride: Implications for Dental Implant Applications. Materials, 18(13), 2973. https://doi.org/10.3390/ma18132973






