Impact of Zinc(II) Chloride Contamination on Bentonites: Formation of Simonkolleite and Effects on Porosity and Chemical Composition
Abstract
1. Introduction
- Contamination of Na- and Ca-bentonites with ZnCl2 may lead to the formation of a new mineral phase—simonkolleite (Skl);
- Saturation of bentonites with ZnCl2 leads to changes in their chemical composition, which may reduce their sorption capacity and the soil specific surface area (SSA);
- The presence of Skl affects the porosity of the bentonites. This potentially alters their effectiveness in geotechnical barriers and poses a potential environmental threat.
2. Materials and Methods
2.1. Chemical Modification
2.2. Mineralogical Analysis
2.3. Thermal Analysis
2.4. Scanning Electron Microscopy
2.5. Chemical Analyses of Bentonites
2.6. Granulometric and Plastic Properties
2.7. Specific Surface Area and Porosity
- The water vapor sorption test (WST) according to Stępkowska [31] (SSAWST) (1),
- The Brunauer–Emmett–Teller (BET) method (SSABET) (2)
2.8. Data Quality Control
3. Results and Discussion
3.1. Mineralogical and Microstructural Analysis
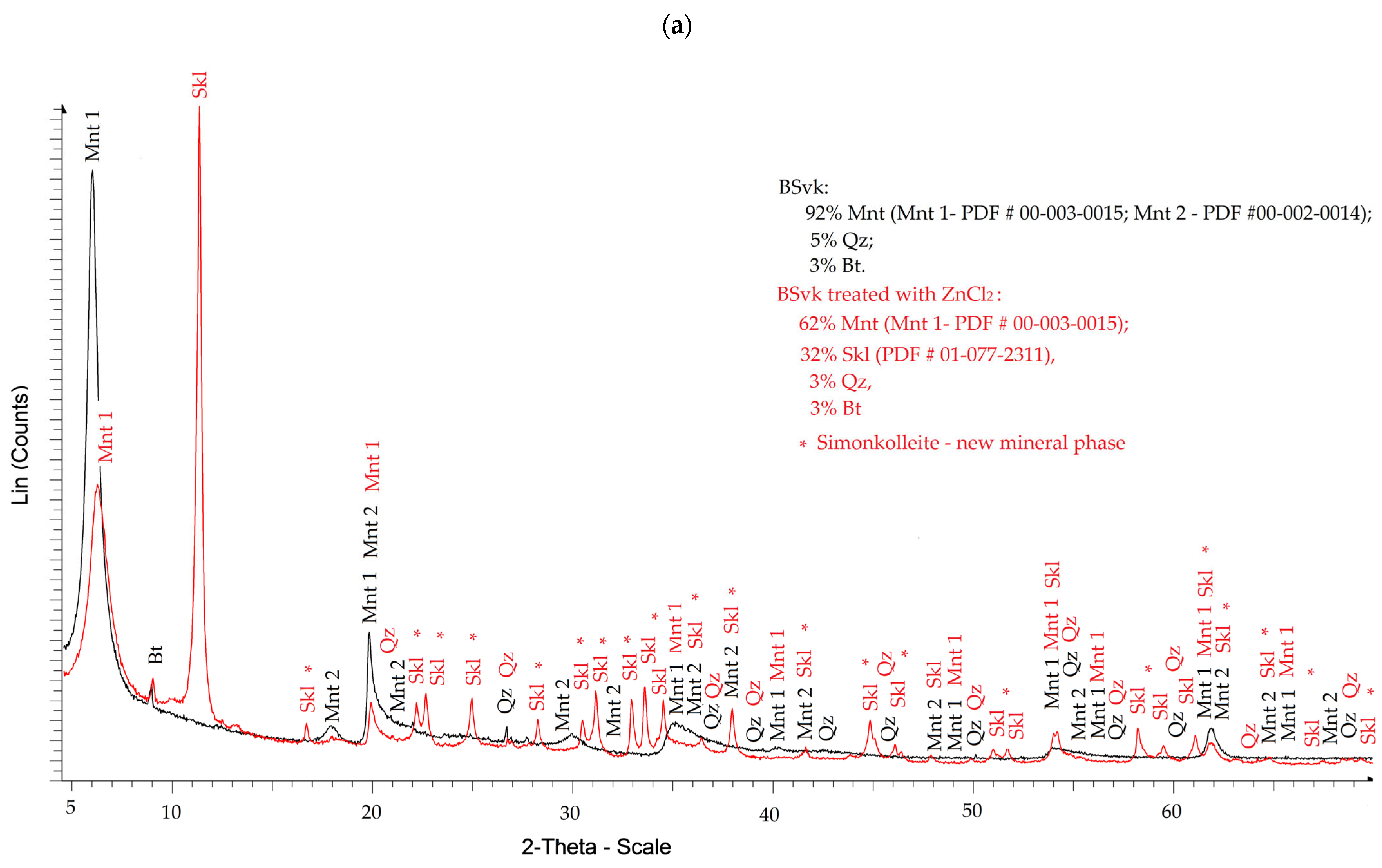
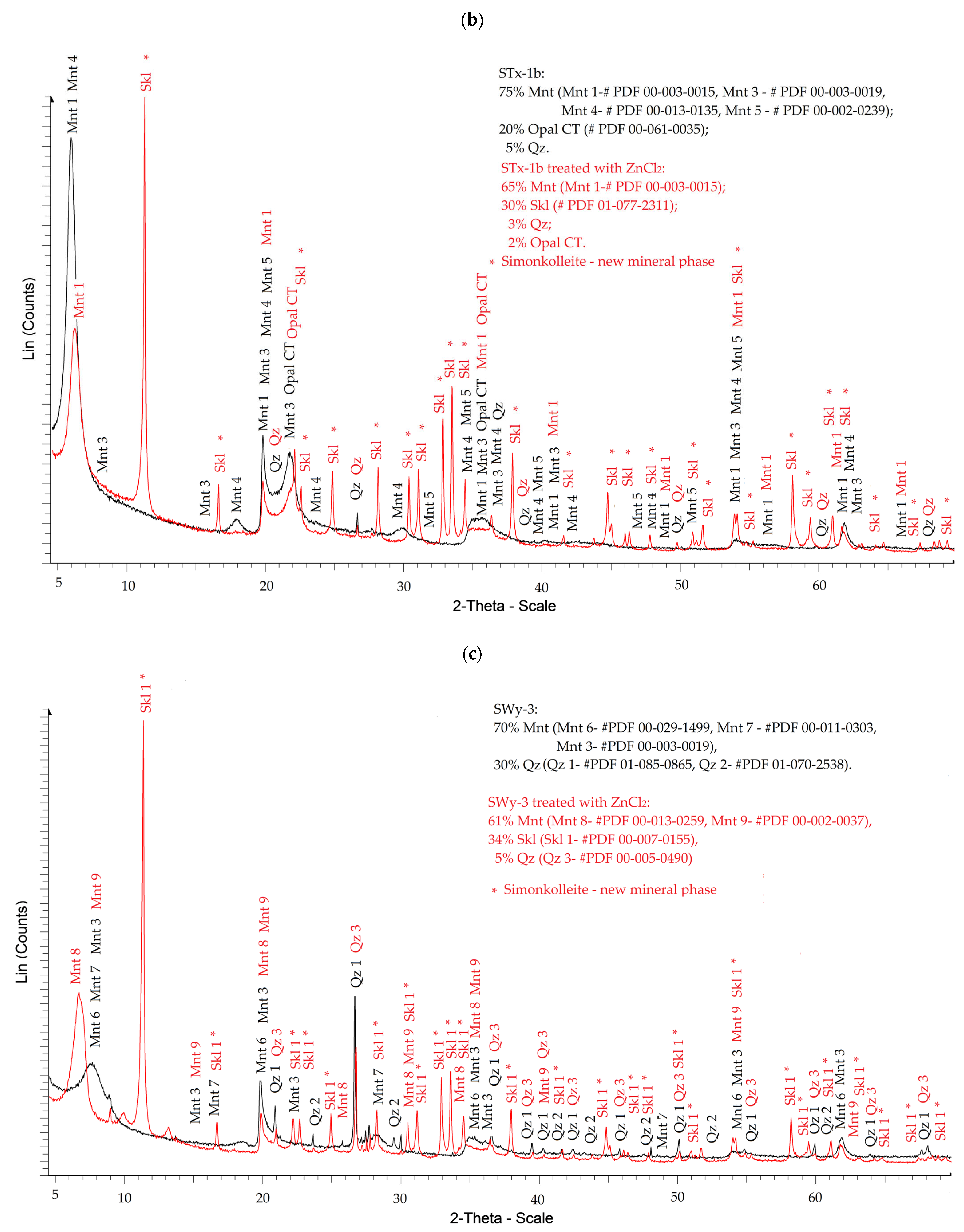
3.1.1. XRD
- Saturating montmorillonite (pH 7.92 ± 0.12) with a ZnCl2 solution (pH = 4.51 ± 0.01) causes only a slight increase in the suspension’s pH to 4.98 ± 0.11. Under these mildly acidic conditions, intensive ion exchange and partial dissolution of montmorillonite components occur. Chemical reactions, such as the formation of Zn(OH)2 according to Equation (4), take place primarily at the montmorillonite phase boundary or within its structure, where the local pH is higher.
- 2.
- The formation of Skl is most likely caused by Zn ions, which partially replace Al, Mg, Fe, and Si ions (as indicated by the XRF data described in the next section) in the Mnt structure, as well as SiO2, as shown by the XRD data (Figure 1). Only a portion of Mnt and SiO2 contributes to the formation of Skl, so the general reaction equation may proceed according to scheme (5).
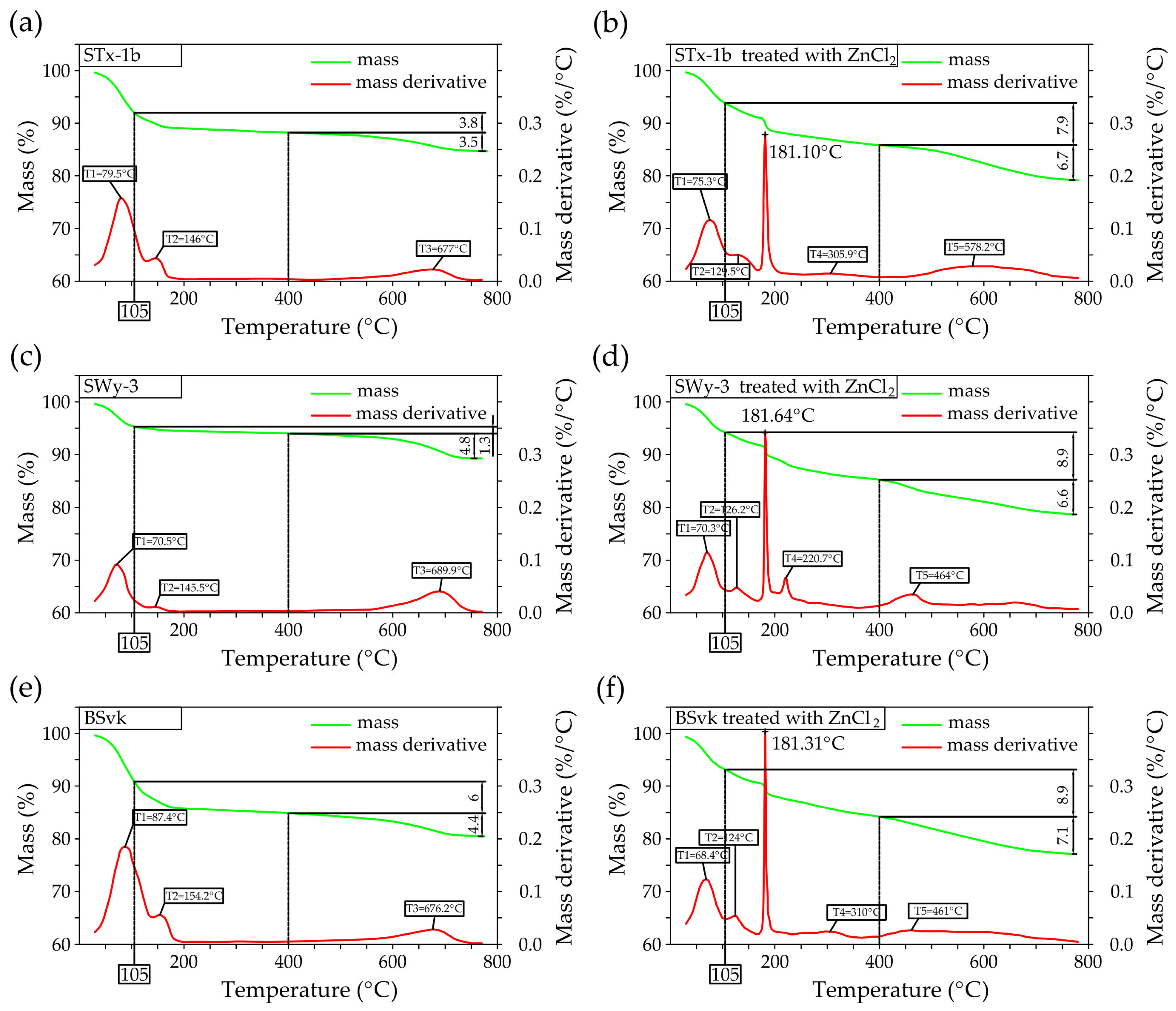
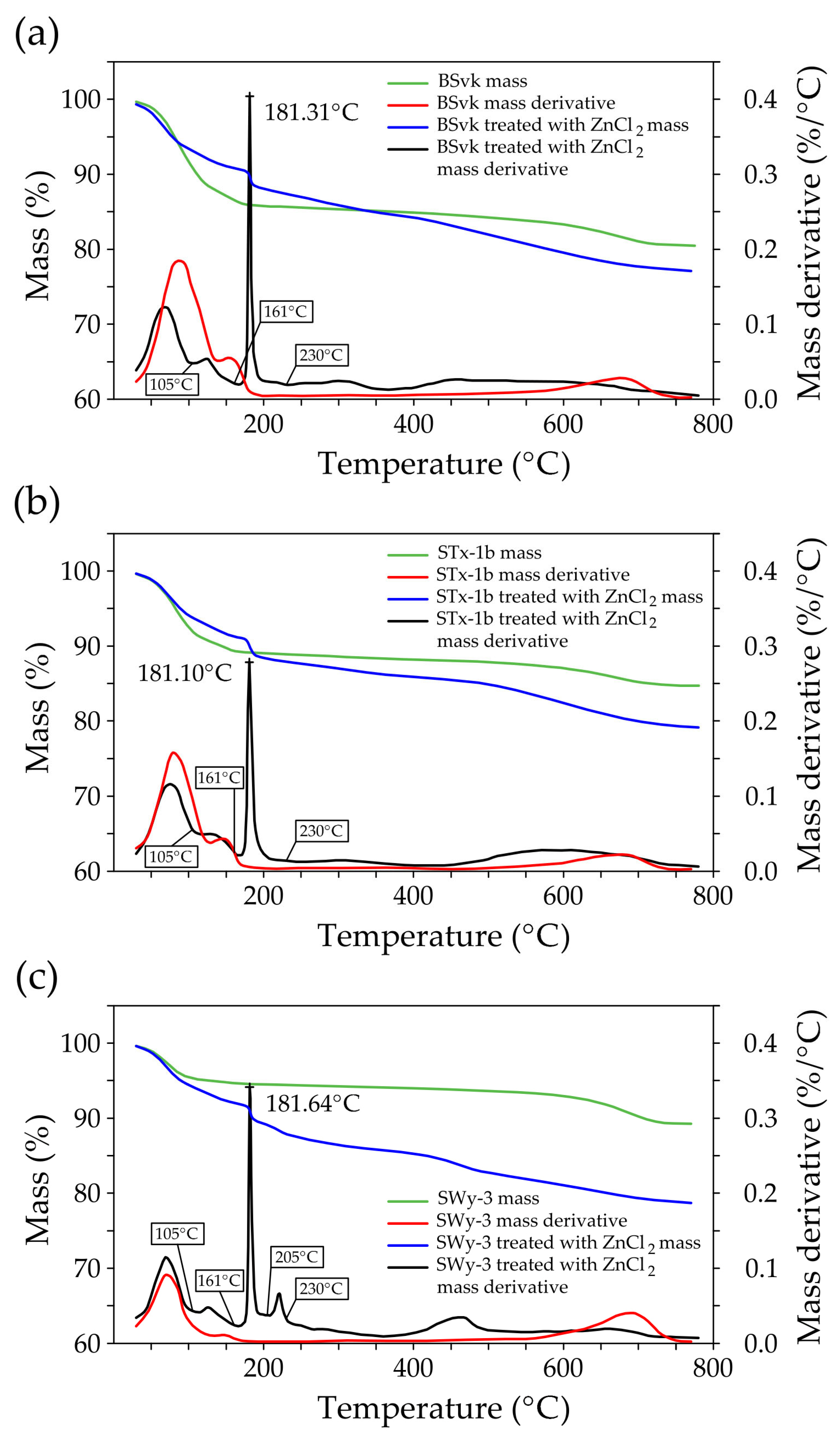
3.1.2. TGA
- Desorption (at ~75 °C): This stage corresponds to the release of physically adsorbed water (molecular water on or within clay mineral particles) due to weak interactions, such as van der Waals forces or hydrogen bonds.
- Dehydration (at ~150 °C): In this step, water that was desorbed from the mineral surface, potentially weakly bound by electrostatic forces or other physical interactions, is further released. This water may include interlayer water between the mineral layers, although it is not chemically bonded to the Mnt. In Zn-bentonites, the dehydration peak occurs at around 125 °C, which may indicate structural changes in the Mnt layers due to the interaction with Zn ions.
- Thermal decomposition between 550 °C and 800 °C can be attributed to dehydroxylation which refers to the release of chemically bound water associated with the mineral’s structure. This water is part of the hydroxyl groups (-OH) in the Mnt layers, forming part of the interlayer cations and the mineral structure.
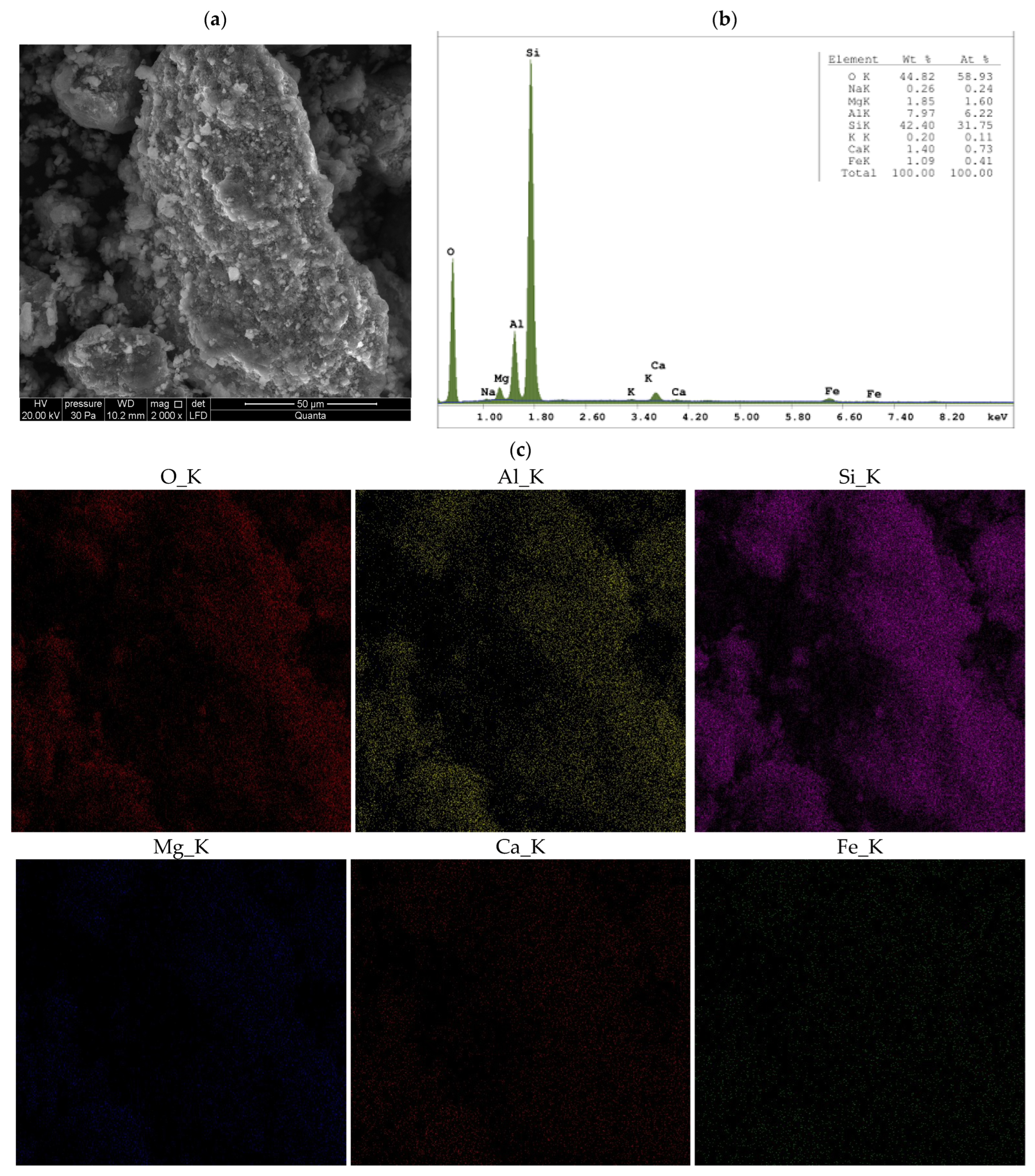
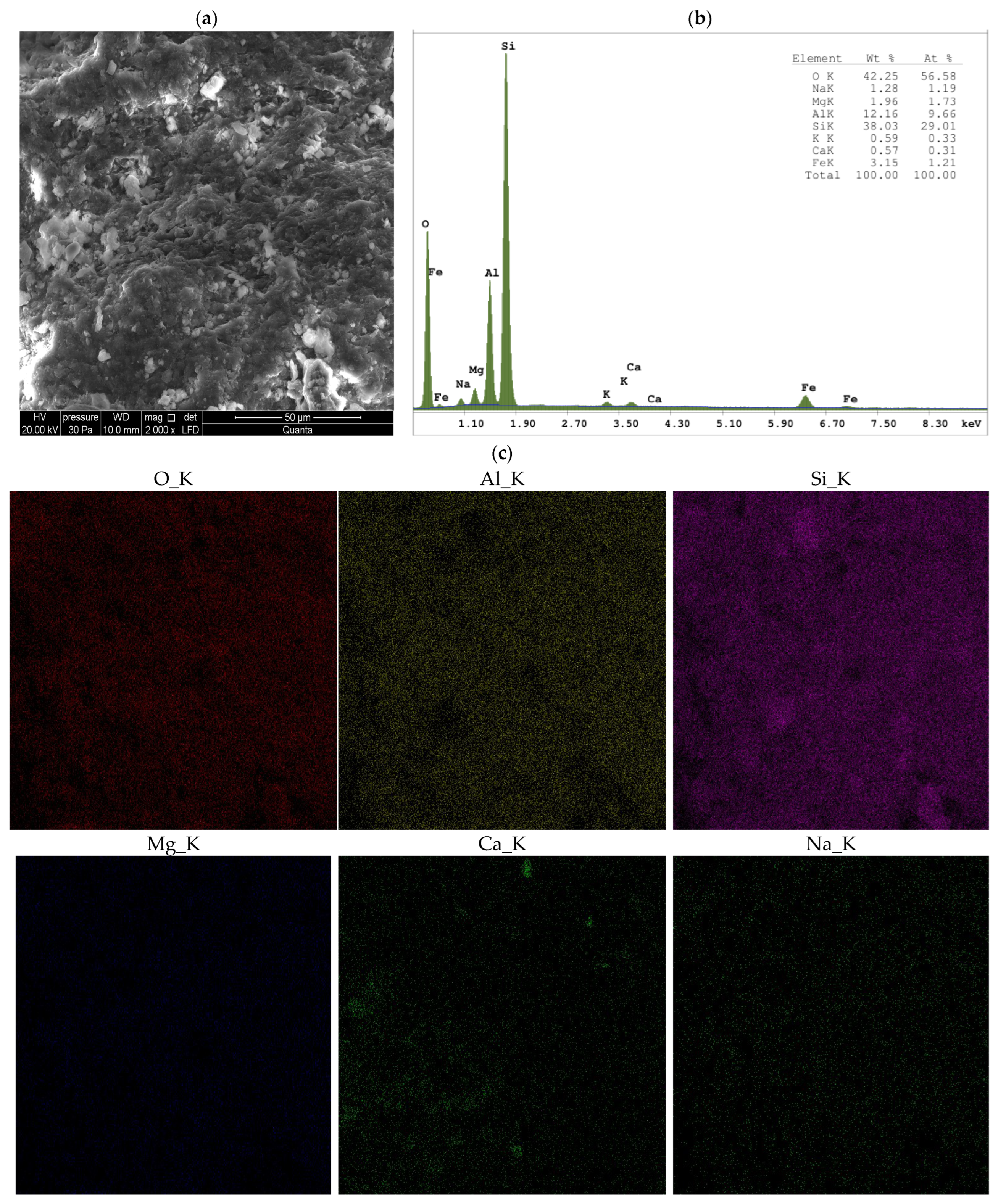
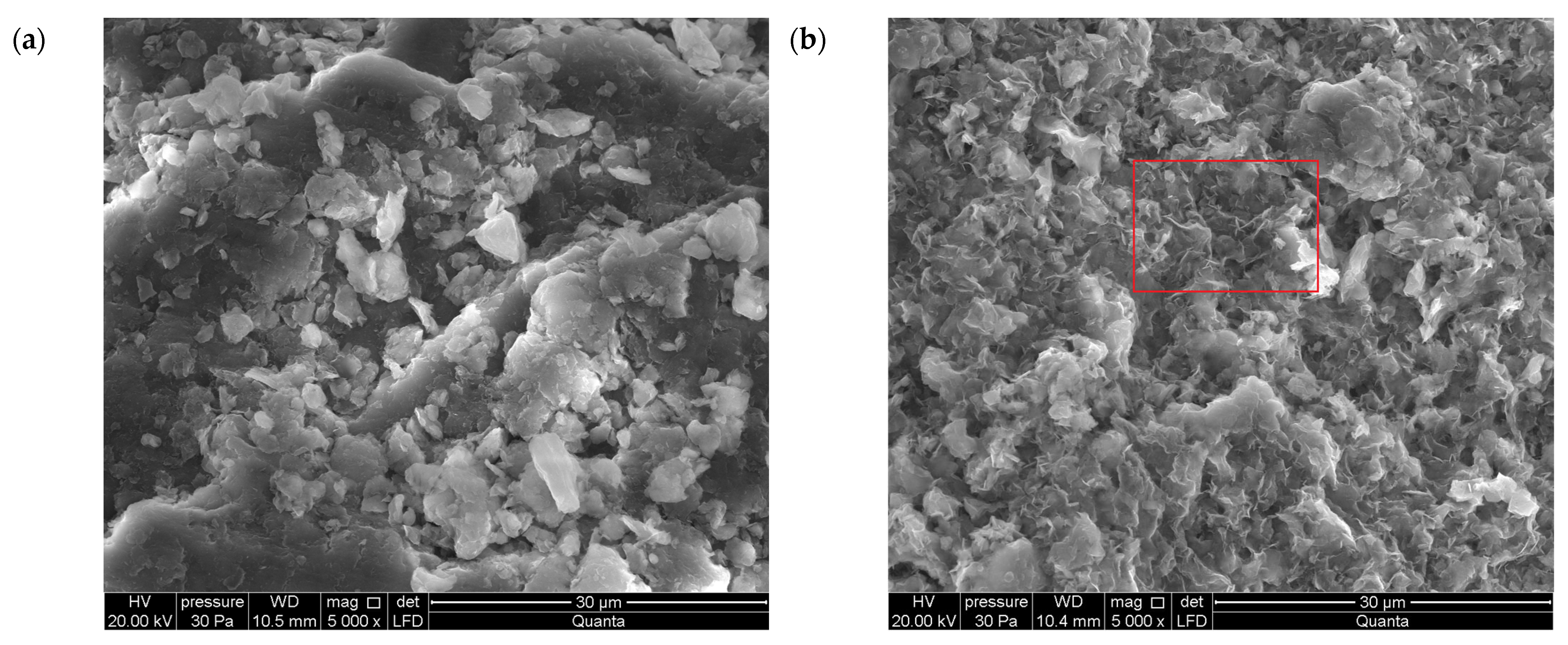
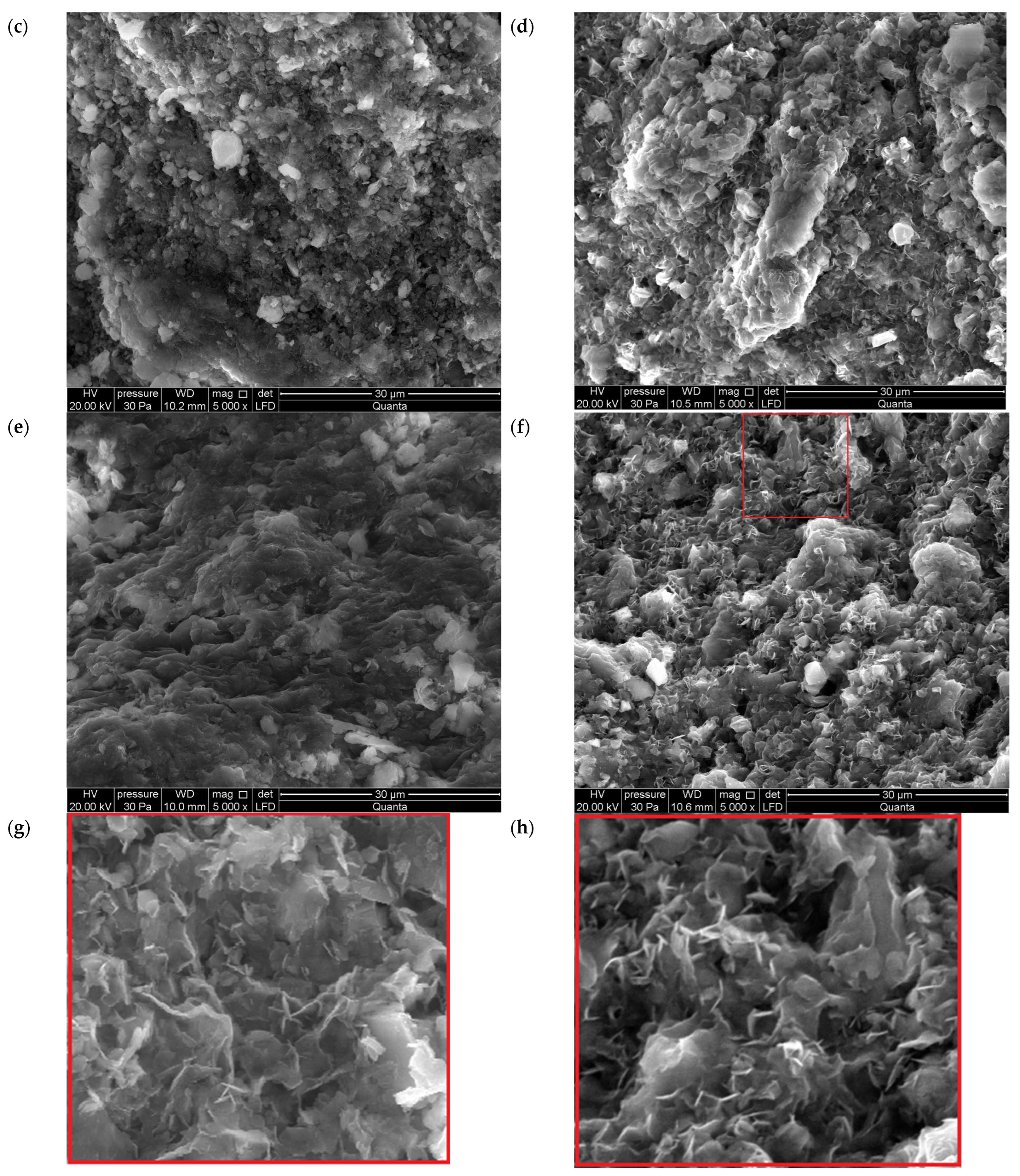
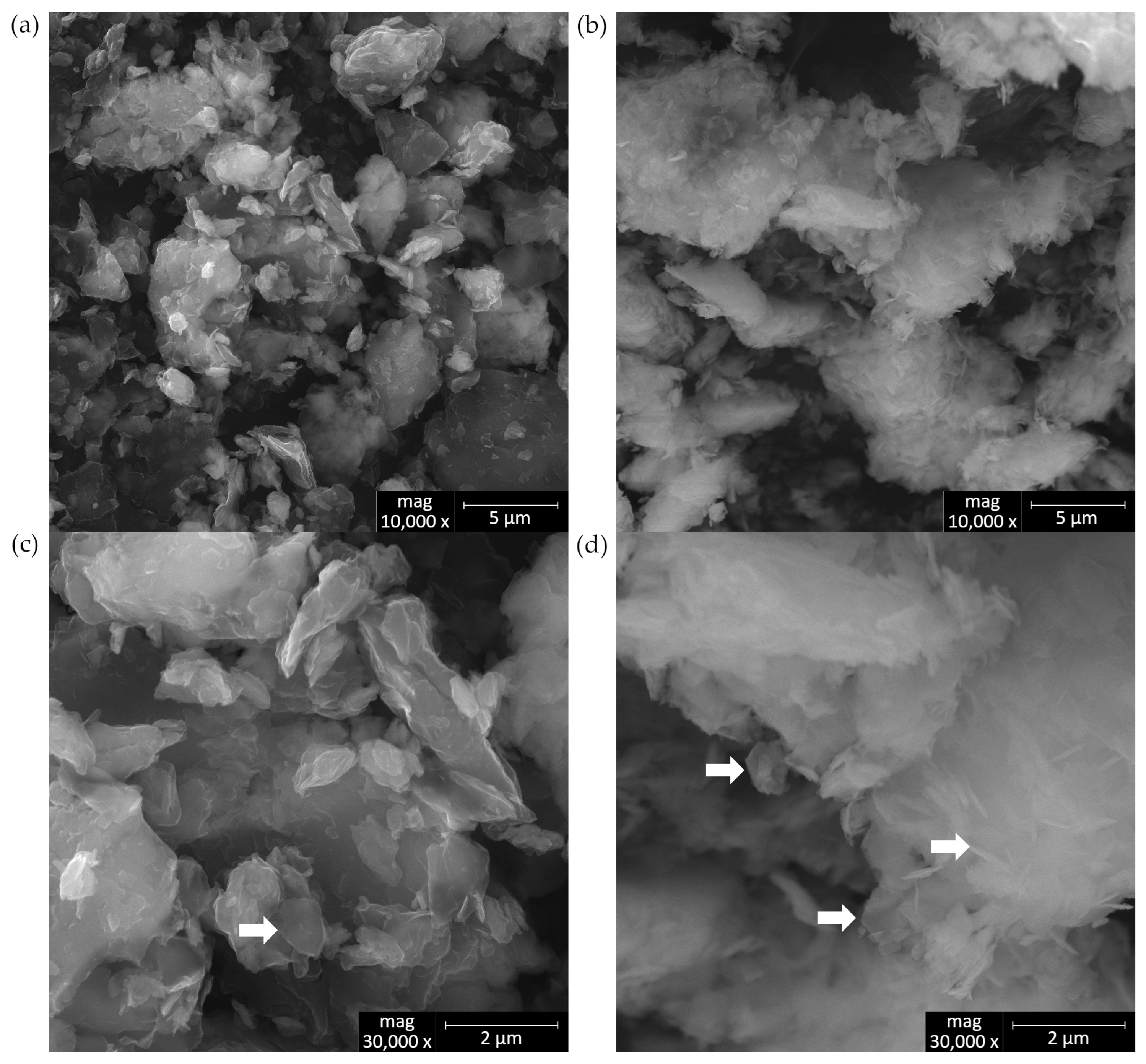
3.1.3. SEM
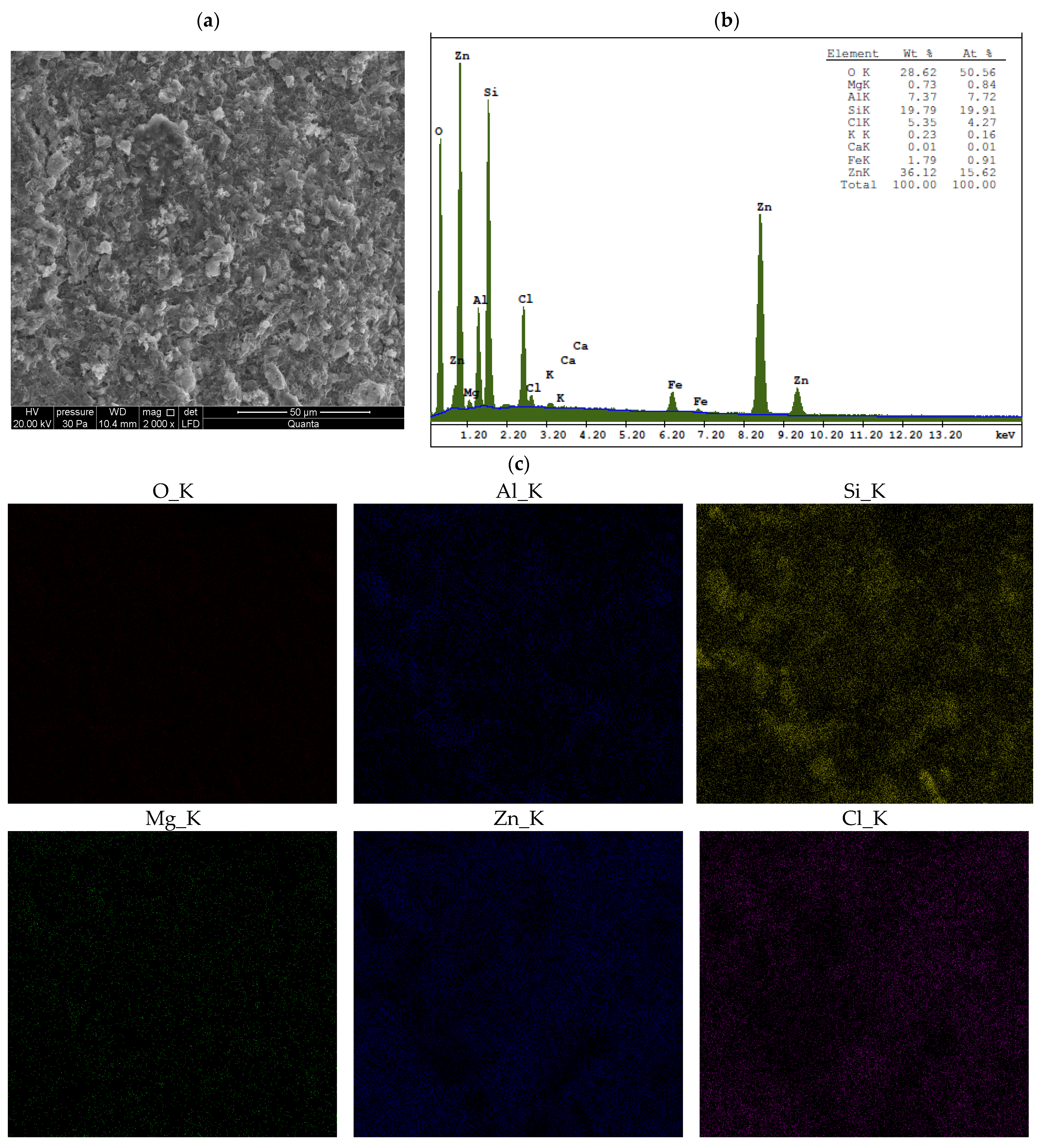
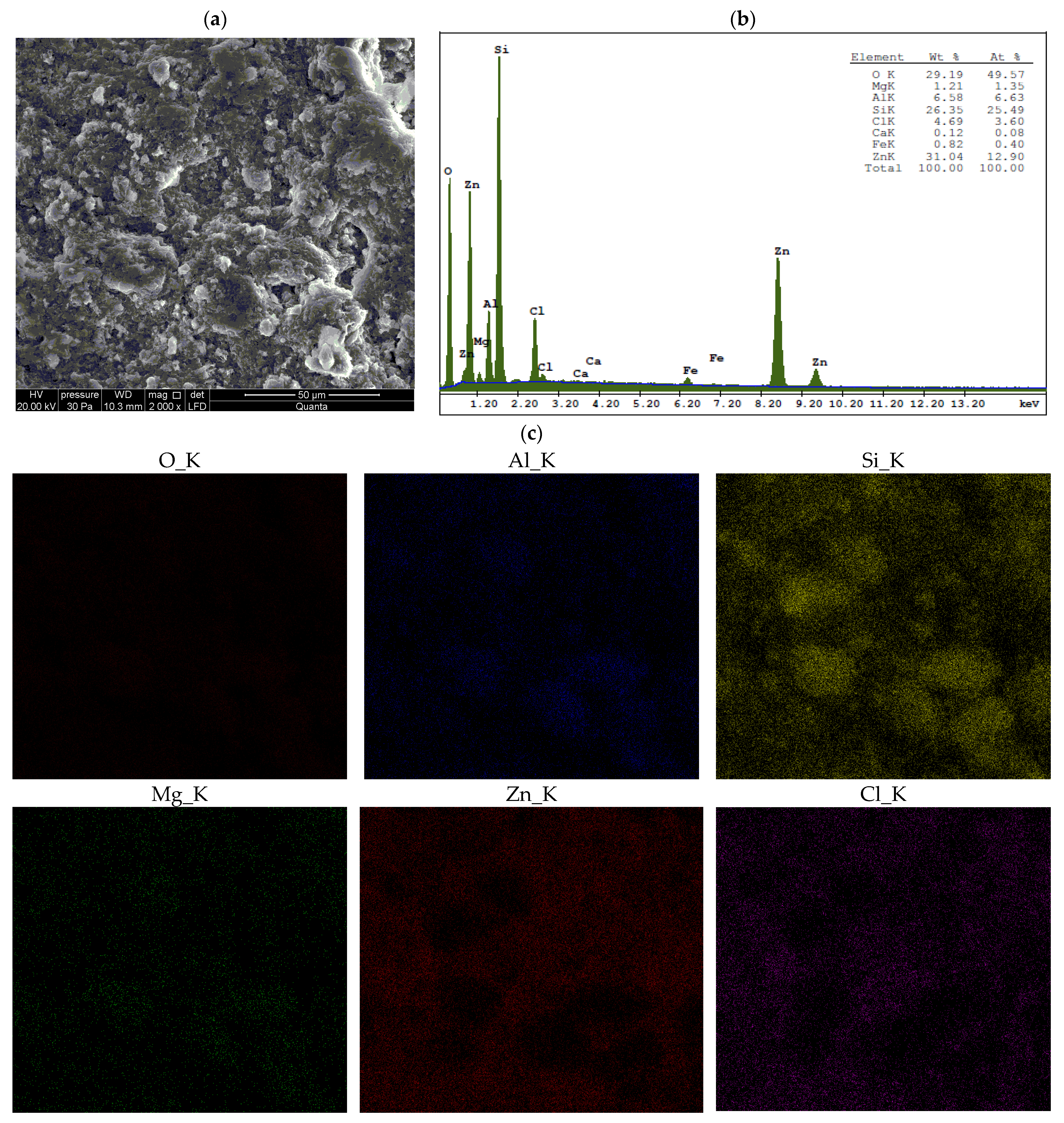
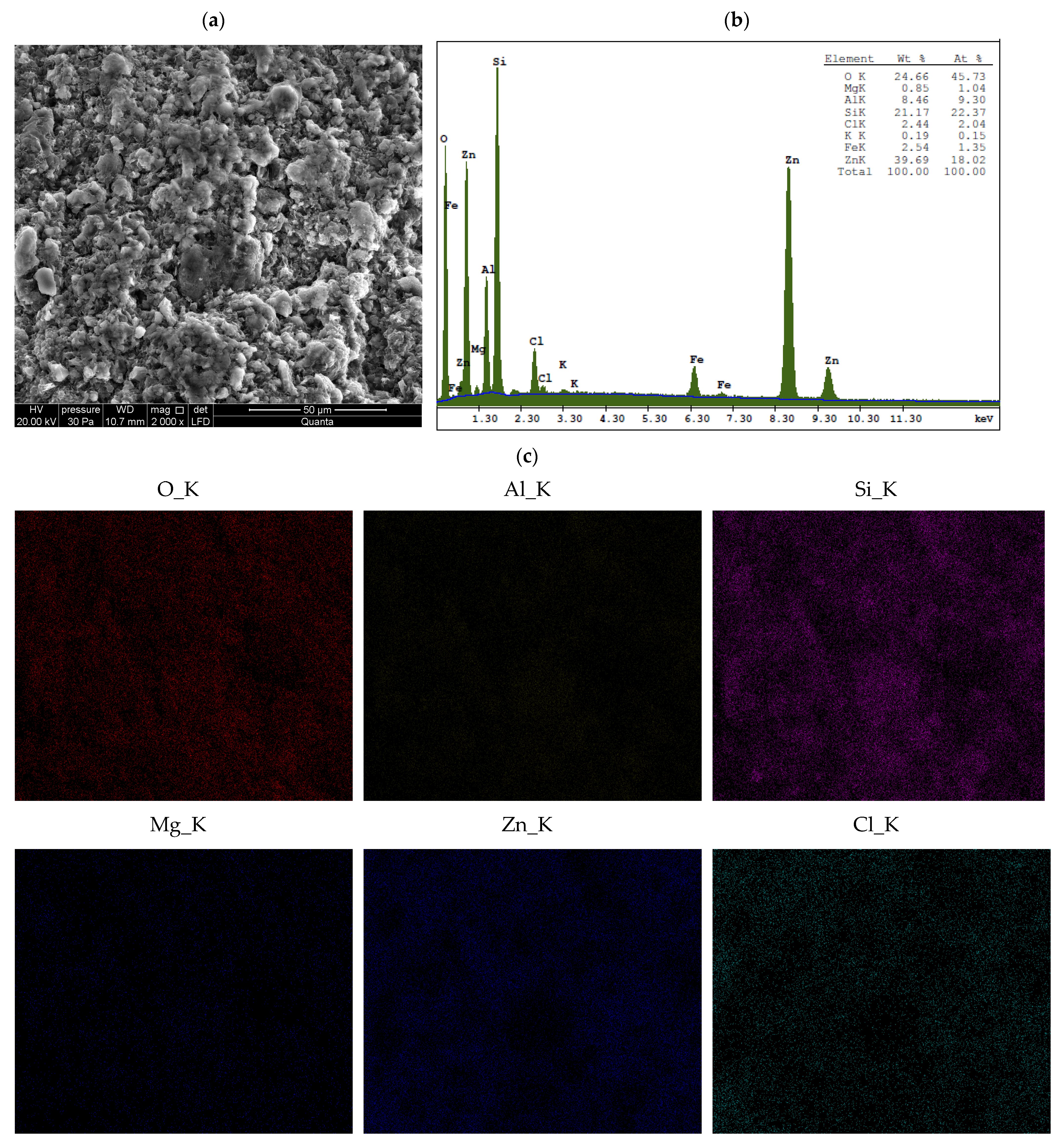
3.2. Chemical Analysis
3.3. Physical Analysis
3.3.1. Granulometric Composition, Plastic Parameters, and Soil Type
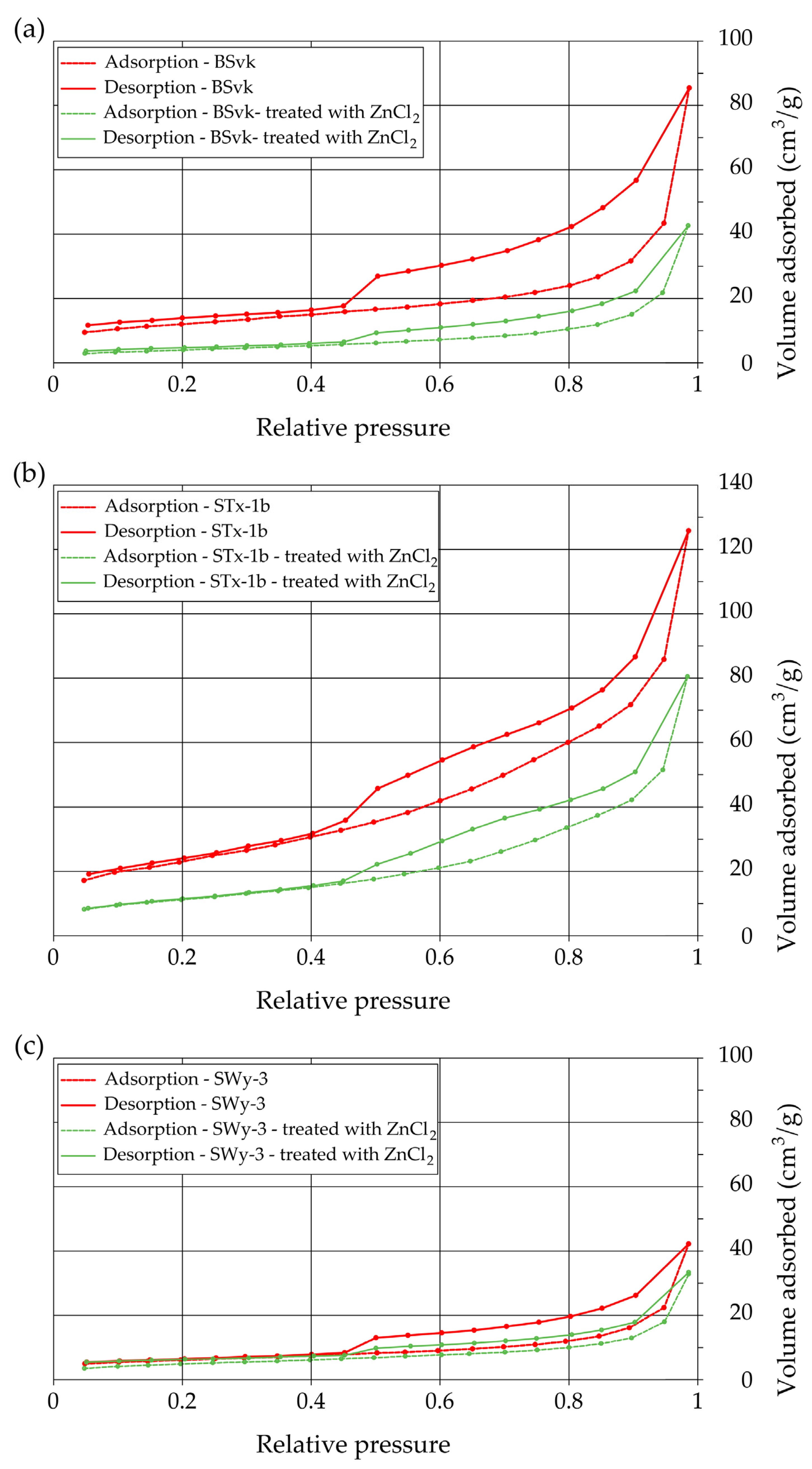
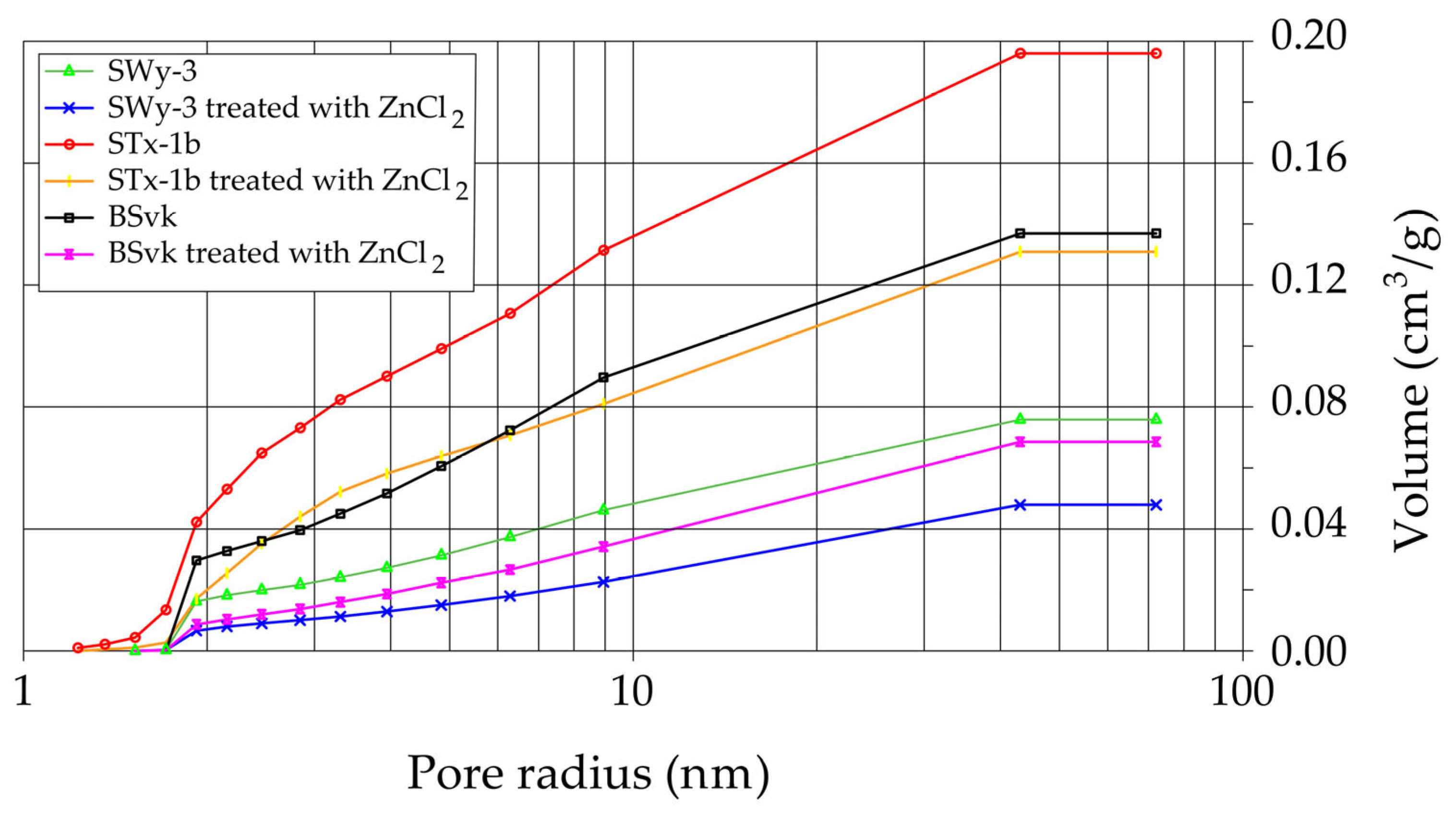
3.3.2. Specific Surface Area (SSA) and Porosity
- When Ca- or Na-bentonite with a pH of 7.92 ± 0.12 is treated with 1 M ZnCl2 (pH = 4.51 ± 0.01), the solution predominantly contains zinc ions (Zn2+) and chloride ions (Cl−). This treatment initiates an almost complete ion exchange process, in which Zn2+ ions replace Ca2+ or Na+ ions within the montmorillonite (Mnt) structure. As a result, the concentrations of Si, Al, Mg, and Fe decrease in all the studied bentonites. XRF analysis confirms that the content of Ca or Na approaches zero, indicating that the tetrahedral and octahedral layers of Mnt have undergone significant structural alterations. Unstable Mnt phases disappear in the XRD analysis.
- In addition to the structural changes in montmorillonite (Mnt), a new mineral phase, simonkolleite (Skl), forms during the contamination process. Chemical reactions take place primarily at the montmorillonite phase boundary or within its structure, where the local pH is higher. EDS analysis confirms elevated levels of Zn and Cl− in the contaminated bentonites, accompanied by a near-complete depletion of Ca or Na. This chemical transformation is a key indicator of Skl formation and has significant implications for the material’s physical properties, particularly the clay’s surface area and porosity.
- Upon lowering the pH to 6.78 ± 0.05, SEM imaging reveals the characteristic needle-like morphology and hexagonal platelets of simonkolleite (Skl), confirming the formation of this new mineral phase. XRD analysis further supports its presence, indicating that approximately 30% of the contaminated bentonite consists of Skl.
- Thermal analysis (TGA) confirms the presence of simonkolleite (Skl) and reveals thermal alterations in the montmorillonite (Mnt) structure resulting from physicochemical changes induced by ZnCl2 contamination. In particular, Zn2+ ions replacing Na+ in Na-bentonite accumulate within the interlayer spaces of Mnt, increasing the interlayer spacing and thereby enhancing the specific surface area for water (SSAWST). These structural modifications facilitate more efficient water flow through the material.
- In Ca-bentonite, Zn replacing Ca predominantly accumulates on the surface of Mnt particles, leading to a decrease in specific surface area (SSABET) and a reduction in surface area of mesopores (SABJH), and porosity. This reduction is also observed in Na-bentonite. Possible mechanisms contributing to these changes include particle aggregation, reduced access to micropores, and the blocking of micropores in Mnt by Skl. This hypothesis is supported by the higher SABJH values than SSABET, suggesting a higher proportion of mesopores in sorption. The aggregation, increasing effective diameter (d10), driven by processes like flocculation, swelling, dispersion, and salt crystallization, further reduces the ability of the material to adsorb water in micropores, which can increase its permeability.
4. Conclusions
- Contamination with 1 M ZnCl2 significantly alters the mineralogical composition and structural stability of bentonites, as evidenced by the formation of simonkolleite (~30%). These changes were confirmed by XRD, TGA, SEM, and complementary analytical techniques.
- Ion exchange between Zn2+ and Ca2+/Na+ within the montmorillonite structure leads to a marked reduction in Si, Al, Mg, and Fe content, along with the near-complete depletion of Ca and Na, as demonstrated by XRF and EDS data. EDS surface mapping revealed a uniform distribution of zinc and chlorine across the entire montmorillonite surface, including inter-aggregate spaces, supporting the formation of the simonkolleite phase.
- The extent of montmorillonite degradation depends on the specific type of bentonite and the montmorillonite phases present. BSvk and STx-1b bentonites (calcium forms) appear to undergo more advanced structural damage than SWy-3 (sodium form), as evidenced by the complete disappearance of certain montmorillonite reflections.
- The formation of Skl and the associated structural modifications cause a ~50% reduction in specific surface area and porosity, as determined by BET and BJH analyses, potentially compromising the material’s performance as a sealing barrier.
- Quantitative evaluation of hydraulic conductivity and mechanical strength in ZnCl2-contaminated bentonites;
- Long-term stability assessments under variable environmental conditions;
- Development of modified bentonite materials with improved resistance to potentially toxic metal-induced structural transformations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Mnt | Montmorillonite |
| Skl | Simonkolleite |
| SWy-3 | Na-bentonite from Wyoming, USA |
| STx-1b | Ca-bentonite from Texas, USA |
| BSvk | Ca-bentonite from Jelsovy Potok, Slovakia |
| PTMs | Potentially toxic metals |
| SSA | Specific surface area of clay |
| SA | Surface area of mesopores |
| CLY | Clay fraction |
| SIL | Silt fraction |
| d10 | Effective diameter—the particle diameter below which 10% of the mass is finer |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| XRF | X-ray fluorescence |
| EDS | Energy-Dispersive X-ray spectroscopy |
| BET | Brunauer–Emmett–Teller adsorption nitrogen method |
| BJH | Barrett–Joyner–Halenda method |
| WST | Water sorption test method |
Appendix A
| Soil Type | Mineral Phase | Key | Chemical Formula | PDF Number * |
|---|---|---|---|---|
| BSvk | Montmorillonite | Mnt 1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·H2O | 00-003-0015 ** |
| Mnt 2 | NaMgAlSiO2(OH)·H2O | 00-002-0014 | ||
| Quartz | Qz | SiO2 | 04-012-0490 | |
| Biotite | Bt | K(Mg,Fe)3(AlSi3O10)(OH)2 | 01-086-2115 | |
| STx-1b | Montmorillonite | Mnt 1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·xH2O | 00-003-0015 ** |
| Mnt 3 | Na-Al-Si-O-OH-H2O | 00-003-0019 | ||
| Mnt 4 | 15A Ca0.2(Al,Mg)2Si4O10(OH)2·4H2O | 00-013-0135 | ||
| Mnt 5 | CaMg2AlSi4(OH)2·H2O | 00-002-0239 | ||
| Opal CT | Opal CT | SiO2 | 00-061-0035 | |
| Quartz | Qz | SiO2 | 01-085-0457 | |
| SWy-3 | Montmorillonite | Mnt 6 | 22A Na0.3(Al,Mg)2 Si4O10(OH)2·H2O | 00-029-1499 ** |
| Mnt 7 | (Al(OH)2)0.33Al2(Si3.67O10)·8H2O | 00-011-0303 | ||
| Mnt 3 | Na-Al-Si-O-OH-H2O | 00-003-0019 | ||
| Quartz | Qz 1 | SiO2 | 01-085-0865 | |
| Qz 2 | SiO2 | 01-070-2538 | ||
| BSvk treated with ZnCl2 | Montmorillonite | Mnt 1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·xH2O | 00-003-0015 ** |
| Simonkolleite | Skl | Zn5(OH)8Cl2·H2O | 01-077-2311 | |
| Quartz | Qz | SiO2 | 04-005-0490 | |
| Biotite | Bt | K(Mg,Fe)3(AlSi3O10)(OH)2 | 01-086-2115 | |
| * STx-1b treated with ZnCl2 | Montmorillonite | Mnt 1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·xH2O | 00-003-0015 ** |
| Simonkolleite | Skl | Zn5(OH)8Cl2·H2O | 01-077-2311 | |
| Opal CT | Opal CT | SiO2 | 00-061-0035 | |
| Quartz | Qz | SiO2 | 01-085-0457 | |
| SWy-3 treated with ZnCl2 | Montmorillonite | Mnt 8 | 14A Na0.3(Al,Mg)2Si4O10(OH)2·xH2O | 00-013-0259 ** |
| Mnt 9 | AlSi2O6(OH)2 | 00-002-0037 | ||
| Simonkolleite | Skl | Zn5(OH)8Cl2·H2O | 00-007-0155 | |
| Quartz | Qz 3 | SiO2 | 00-005-0490 |

References
- Neretnieks, I.; Moreno, L. Ion Composition at Bentonite-Water Interface and Its Impact on Erosion; SKB Report P-20-26; Svensk Kärnbränslehantering AB (Swedish Nuclear Fuel and Waste Management Co.): Stockholm, Sweden, 2021; pp. 1–66. [Google Scholar]
- Geng, K.; Jin, J.; Chai, J.; Qin, Y. Adsorption characteristics and mechanism analysis of heavy metal Zn2+ by cement-soil and alkali activated slag-bentonite-soil. Case Stud. Constr. Mater. 2024, 21, e03583. [Google Scholar] [CrossRef]
- Huang, R.; Wu, L.; Wang, X.; Tang, N.; Gao, L.; Wang, A.; Lu, Y. Review on the effect of isomorphic replacement on the structure and application performance of typical clay minerals. Prog. Nat. Sci. Mater. Int. 2024, 34, 251–262. [Google Scholar] [CrossRef]
- Klik, B.; Holatko, J.; Jaskulska, I.; Gusiatin, M.Z.; Hammerschmiedt, T.; Brtnicky, M.; Liniauskienė, E.; Baltazar, T.; Jaskulski, D.; Kintl, A.; et al. Bentonite as a Functional Material Enhancing Phytostabilization of Post-Industrial Contaminated Soils with Heavy Metals. Materials 2022, 15, 8331. [Google Scholar] [CrossRef]
- Andriulaityte, I.; Valentukeviciene, M.; Zurauskiene, R. Research on the Reusability of Bentonite Waste Materials for Residual Chlorine Removal. Materials 2024, 17, 5647. [Google Scholar] [CrossRef] [PubMed]
- Mooshaee, M.R.; Sabour, M.R.; Kamza, E. The swelling performance of raw and modified bentonite of geosynthetic clay liner as the leachate barrier exposed to the synthetic E-waste leachate. Heliyon 2022, 8, e11937. [Google Scholar] [CrossRef] [PubMed]
- Egloffstein, T.A. Natural bentonites. Influence of the ion exchange and partial desiccation on permeability and self-healing capacity of bentonites used in GCL. Geotext. Geomembr. 2001, 19, 427–444. [Google Scholar] [CrossRef]
- Cui, Q.; Chen, B. Review of polymer-amended bentonite: Categories, mechanism, modification processes and application in barriers for isolating contaminants. Appl. Clay Sci. 2023, 235, 106869. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Tian, K. Hydraulic conductivity of bentonite-polymer geosynthetic clay liners to aggressive solid waste leachates. Geotext. Geomembr. 2024, 52, 900–911. [Google Scholar] [CrossRef]
- Somani, M.; Hölzle, I.; Datta, M.; Ramana, G.V. An investigation on mobility of heavy metals for assessing the reusability of soil-like material reclaimed from mining of municipal solid waste dumpsites. Waste Manag. 2023, 167, 113–121. [Google Scholar] [CrossRef]
- Bågenholm-Ruuth, E.; Sanchis-Sebastiá, M.; Hollinger, N.; Teleman, A.; Larsson, P.T.; Wallberg, O. Transforming post-consumer cotton waste textiles into viscose staple fiber using hydrated zinc chloride. Cellulose 2024, 31, 737–748. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Villegas, E.A.; Mansur, M.B. Management of solid wastes from steelmaking and galvanizing processes: A brief review. Resour. Conserv. Recycl. 2015, 102, 49–57. [Google Scholar] [CrossRef]
- Sun, W.J.; Tang, Q.T.; Lu, T.H.; Fan, R.-D.; Sun, G.-G.; Tan, Y.-Z. Adsorption performance of bentonite and clay for Zn(II) in landfill leachate. Geoenviron. Disasters 2024, 11, 4. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks, and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Oyourou, J.-N.; McCrindle, R.; Combrinck, S.; Fourie, C.J.S. Investigation of zinc and lead contamination of soil at the abandoned Edendale mine, Mamelodi (Pretoria, South Africa) using a field-portable spectrometer. J. S. Afr. Inst. Min. Metall. 2019, 119, 55–62. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Antonkiewicz, J.; Gruba, P.; Pająk, M. Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem. 2018, 16, 1143–1152. [Google Scholar] [CrossRef]
- Nartowska, E.; Podlasek, A.; Vaverková, M.D.; Koda, E.; Jakimiuk, A.; Kowalik, R.; Kozłowski, T. Mobility of Zn and Cu in Bentonites: Implications for Environmental Remediation. Materials 2024, 17, 2957. [Google Scholar] [CrossRef]
- Sahmoune, M.N. Evaluation of thermodynamic parameters for adsorption of heavy metals by green adsorbents. Environ. Chem. Lett. 2019, 17, 697–704. [Google Scholar] [CrossRef]
- Nartowska, E.; Podlasek, A.; Vaverková, M.D.; Koda, E.; Kanuchova, M.; Jakimiuk, A.; Gawdzik, J. Identification of factors affecting the properties of soils contaminated with Zn(II) and Cu(II) chlorides. J. Soils Sediments 2025, 25, 1175–1200. [Google Scholar] [CrossRef]
- Chai, W.; Huang, Y.; Su, S.; Han, G.; Liu, J.; Cao, Y. Adsorption behavior of Zn(II) onto natural minerals in wastewater. A comparative study of bentonite and kaolinite. Physicochem. Probl. Miner. Process. 2017, 53, 264–278. [Google Scholar] [CrossRef]
- Kaya, A.; Ören, A.H. Adsorption of zinc from aqueous solutions to bentonite. J. Hazard. Mater. 2005, 125, 183–189. [Google Scholar] [CrossRef]
- Cousy, S.; Gorodylova, N.; Svoboda, L.; Zelenka, J. Influence of synthesis conditions over simonkolleite/ZnO precipitation. Chem. Pap. 2017, 71, 2325–2334. [Google Scholar] [CrossRef]
- Moezzi, A.; Cortie, M.; McDonagh, A. Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide. Dalton Trans. 2016, 45, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Enobong, F.D.; Wang, C.; Li, C.; Dong, J.; Udoh, I.I.; Zhang, D.; Zhong, W.; Zhong, S. Evolution of corrosion degradation in galvanised steel bolts exposed to a tropical marine environment. J. Mater. Res. Technol. 2023, 27, 5177–5190. [Google Scholar] [CrossRef]
- Hsiao, Y.-F.; Hung, F.-Y.; Zhao, J.-R. Chlorination for all solid-state zinc-graphite battery: Investigate into the charge-discharge mechanism of zinc electrodes and sodium silicate electrolyte. Mater. Sci. Eng. B 2024, 301, 117146. [Google Scholar] [CrossRef]
- Oueslati, W.; Rhaiem, H.B.; Amara, A.B.H. Effect of relative humidity constraint on the metal exchanged montmorillonite performance: An XRD profile modeling approach. Appl. Surf. Sci. 2012, 261, 396–404. [Google Scholar] [CrossRef]
- PN-ISO 9297:1994; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). Polish Committee for Standardization: Warsaw, Poland, 1994.
- Sun, Q.; Peng, Y.; Georgolamprou, X.; Li, D.; Kiebach, R. Synthesis and characterization of a geopolymer/hexagonal-boron nitride composite for free forming 3D extrusion-based printing. Appl. Clay Sci. 2020, 199, 105870. [Google Scholar] [CrossRef]
- Torres-Rivero, K.; Bastos-Arrieta, J.; Fiol, N.; Florido, A. Chapter Ten—Metal and metal oxide nanoparticles: An integrated perspective of the green synthesis methods by natural products and waste valorization: Applications and challenges. In Comprehensive Analytical Chemistry, 1st ed.; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 94, pp. 433–469. [Google Scholar] [CrossRef]
- EN-ISO 17892-12:2018/Amd 2:2022; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 12: Determination of Liquid and Plastic Limits. International Organization for Standardization: Geneva, Switzerland, 2022.
- Stępkowska, E.T. Simple method of crystal phase water, specific surface and clay mineral content estimation in natural clays. Stud. Geotechn. 1973, IV, 21–36. [Google Scholar]
- Asabina, E.A.; Zhukova, A.I.; Sedov, V.A.; Pet’kov, V.I.; Osaulenko, D.A.; Markova, E.B.; Fukina, D.G.; Koshkin, V.A. Catalytic characteristics of NASICON-type phosphates with rare earth elements in ethanol conversion. Solid State Sci. 2025, 162, 107865. [Google Scholar] [CrossRef]
- Borinelli, J.B.; Portillo-Estrada, M.; Costa, J.O.; Pajares, A.; Blom, J.; Hernando, D.; Vuye, C. Emission reduction agents: A solution to inhibit the emission of harmful volatile organic compounds from crumb rubber modified bitumen. Constr. Build. Mater. 2024, 411, 134455. [Google Scholar] [CrossRef]
- Chipera, S.J.; Bish, D.L. Baseline studies of the Clay Minerals Society source. Clays: Powder x-ray diffraction analyses. Clays Clay Miner. 2001, 49, 398–409. [Google Scholar] [CrossRef]
- Castellini, E.; Malferrari, D.; Bernini, F.; Brigatti, M.F.; Castro, G.R.; Medici, L.; Mucci, A.; Borsari, M. Baseline Studies of the Clay Minerals Society Source Clay Montmorillonite STx-1b. Clays Clay Miner. 2017, 65, 220–233. [Google Scholar] [CrossRef]
- Bagheri, M.; Lothenbach, B.; Shakoorioskooie, M.; Scrivener, K. Effect of different ions on dissolution rates of silica and feldspars at high pH. Cem. Concr. Res. 2022, 152, 106644. [Google Scholar] [CrossRef]
- Voora, V.K.; Al-Saidi, W.A.; Jordan, K.D.J. Density Functional Theory Study of Pyrophyllite and M-Montmorillonites (M = Li, Na, K, Mg, and Ca): Role of Dispersion Interactions. J. Phys. Chem. A 2011, 115, 9695–9703. [Google Scholar] [CrossRef]
- Zhu, K.; He, Y.; He, Q.; Lou, W.; Zhang, Z.; Zhang, K. Effects of ionic strength and bentonite ratio on the migration of Cr(VI) in clayey soil-bentonite engineered barrier. Environ. Sci. Pollut. Res. 2024, 31, 45310–45325. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Jiang, W.; Li, X.; Dai, Y.; Jia, H. Understanding the sorption behaviors of heavy metal ions in the interlayer and nanopore of montmorillonite: A molecular dynamics study. J. Hazard. Mater. 2021, 416, 125976. [Google Scholar] [CrossRef] [PubMed]
- Gorodylova, N.; Cousy, S.; Šulcová, P. Thermal transformation of layered zinc hydroxide chloride. J. Therm. Anal. Calorim. 2017, 127, 675–683. [Google Scholar] [CrossRef]
- Qu, S.; Hadjittofis, E.; Malaret, F.; Hallett, J.; Smith, R.; Sedransk Campbell, K. Controlling simonkolleite crystallisation via metallic Zn oxidation in a betaine hydrochloride solution. Nanoscale Adv. 2023, 5, 2437–2452. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Mao, X.; Yao, Y.; Li, J.; Shen, Z.; Tu, K.-N.; Liu, Y. Effect of WC thickness on the microstructure and properties of WC-C/DLC coated 304 steel. Appl. Surf. Sci. 2025, 704, 163418. [Google Scholar] [CrossRef]
- Nartowska, E.; Kozłowski, T. The effect of the concentration of copper ions on the unfrozen water content in bentonites measured with the use of DSC method. Minerals 2022, 12, 632. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Soil Taxonomy: A Basic system of Soil Classification for Making and Interpreting Soil Surveys; U.S. Department of Agriculture: Washington, DC, USA, 1999.
- EN-ISO 14688-1; Geotechnical Investigation and Testing—Identification and Classification of Soil—Part 1: Identification and Description. International Organization for Standardization: Geneva, Switzerland, 2002.
- EN-ISO 10390:2021; Water quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2021.
- Tang, S.; She, D.; Wang, H. Effect of salinity on soil structure and soil hydraulic characteristics. Can. J. Soil Sci. 2020, 101, 62–73. [Google Scholar] [CrossRef]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Kabir, M.H.; Kafy, A.-A.; Rahaman, Z.A.; Rahman, M.T. Geotechnical properties and applicability of bentonite-modified local soil as landfill and environmental sustainability liners. Environ. Sustain. Indic. 2023, 18, 100241. [Google Scholar] [CrossRef]
- Wei, L.; Li, Y.; Noguera, D.R.; Zhao, N.; Song, Y.; Ding, J.; Zhao, Q.; Cui, F. Adsorption of Cu2+ and Zn2+ by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Viisanen, Y.; Lbadaoui-Darvas, M.; Alvarez Piedehierro, A.; Welti, A.; Nenes, A.; Laaksonen, A. Water vapor adsorption-desorption hysteresis due to clustering of water on nonporous surfaces. Langmuir 2024, 40, 20311–20321. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Seo, C.; Yun, T.; Joo, Y.; Jo, H.Y. Prediction of Na- and Ca-montmorillonite contents and swelling properties of clay mixtures using Vis-NIR spectroscopy. Geoderma 2023, 430, 116294. [Google Scholar] [CrossRef]
- Yamamoto, O.; Nagashima, M.; Nakata, Y.; Udagawa, E. The significant potential of Simonkolleite powder for deep wound healing under a moist environment: In vivo histological evaluation using a rat model. Bioengineering 2023, 10, 375. [Google Scholar] [CrossRef]
| Chemical Analysis | Element | BSvk (Ca Form) | BSvk Treated with ZnCl2 | STx-1b (Ca Form) | STx-1b Treated with ZnCl2 | SWy-3 (Na Form) | SWy-3 Treated with ZnCl2 |
|---|---|---|---|---|---|---|---|
| ICP-OES & [17] | [mg/kg dry mass] | ||||||
| Ca | 11,945 ± 140 | 2778 ± 31 | 11,802 ± 101 | 2985 ± 23 | 8282 ± 57 | 4526 ± 34 | |
| Na | 1151 ± 9 | 1204 ± 22 | 1970 ± 18 | 885 ± 16 | 10,086 ± 81 | 995 ± 9 | |
| Zn | 64.54 ± 0.69 | 17,857 ± 89 | 73.68 ±0.27 | 16,153 ± 75 | 163.66 ± 1.50 | 44,463 ± 124 | |
| XRF * | Ca | 1.14 | 0.02 | 1.33 | 0.03 | 1.02 | 0.04 |
| Mg | 2.32 | 0.76 | 2.15 | 0.72 | 1.74 | 0.53 | |
| K | 0.19 | 0.09 | 0.14 | 0.06 | 0.42 | 0.18 | |
| Na | 0.17 | − | 0.31 | − | 1.22 | − | |
| Al | 8.42 | 3.68 | 7.16 | 2.85 | 8.31 | 3.39 | |
| Si | 24.29 | 11.38 | 37.20 | 15.17 | 27.14 | 11.73 | |
| Fe | 1.67 | 0.99 | 0.82 | 0.41 | 2.55 | 1.42 | |
| Cl | − | 2.16 | − | 2.15 | − | 2.88 | |
| XRF (potentially toxic metals) | Zn | − | 21.22 | − | 21.24 | − | 21.01 |
| Mn | 0.0511 | 0.0273 | 0.0134 | 0.0062 | 0.0148 | 0.0044 | |
| Co | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Ni | 0.0010 | <0.0001 | 0.0009 | <0.0001 | 0.0010 | <0.0001 | |
| Cu | 0.0006 | <0.0001 | 0.0005 | <0.0001 | 0.0006 | <0.0001 | |
| Cd | <0.00005 | <0.00002 | <0.00005 | <0.00002 | <0.00002 | <0.00002 | |
| Pb | 0.0028 | 0.0020 | 0.0012 | 0.0028 | 0.00367 | 0.00113 | |
| Properties | BSvk | BSvk Treated with ZnCl2 | STx-1b | STx-1b Treated with ZnCl2 | SWy-3 | SWy-3 Treated with ZnCl2 | ||
|---|---|---|---|---|---|---|---|---|
| Soil classification $ | USDA | silt loam | silt | silt loam | silt | clay loam | silt | |
| EN-ISO 14688-1 | clayey silt | silt | clayey silt | clayey silt | clay | silt | ||
| pHKCl(-) * | 7.82 | 6.76 | 7.88 | 6.75 | 8.05 | 6.84 | ||
| alkaline | neutral | alkaline | neutral | alkaline | neutral | |||
| Granulometric ** | CLY (%) | 19.8 | 8.9 | 18.5 | 10.7 | 42.6 | 6.6 | |
| SIL (%) | 80.2 | 90.2 | 81.5 | 87 | 57.4 | 87 | ||
| SA (%) | 0.0 | 0.9 | 0.0 | 2.3 | 0.3 | 6.4 | ||
| d10 (μm) | 1.33 | 2.18 | 1.39 | 1.91 | 0.96 | 2.83 | ||
| Plasticity § | PL (%) | 46 | 61 | 44 | 73 | 35 | 54 | |
| LL (%) | 165 | 119 | 142 | 101 | 519 | 104 | ||
| Sorption/desorption # | SABJH (m2/g) | 58.80 | 22.19 | 96.27 | 57.21 | 31.24 | 15.33 | |
| SSABET (m2/g) | 41.39 | 14.38 | 82.06 | 40.55 | 23.30 | 17.16 | ||
| SSAWST (m2/g) | 671 | 557 | 568 | 538 | 307 | 516 | ||
| w95 (%) | 30.14 | 20.10 | 29.08 | 22.67 | 21.67 | 17.05 | ||
| Porosity ϵ | total (cm3/g) | 0.137 | 0.069 | 0.196 | 0.130 | 0.076 | 0.048 | |
| Pore radius | Dv (nm) | 1.921 | 1.923 | 1.931 | 1.925 | 1.924 | 1.920 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartowska, E.; Stępień, P.; Kanuchova, M. Impact of Zinc(II) Chloride Contamination on Bentonites: Formation of Simonkolleite and Effects on Porosity and Chemical Composition. Materials 2025, 18, 2933. https://doi.org/10.3390/ma18132933
Nartowska E, Stępień P, Kanuchova M. Impact of Zinc(II) Chloride Contamination on Bentonites: Formation of Simonkolleite and Effects on Porosity and Chemical Composition. Materials. 2025; 18(13):2933. https://doi.org/10.3390/ma18132933
Chicago/Turabian StyleNartowska, Edyta, Piotr Stępień, and Maria Kanuchova. 2025. "Impact of Zinc(II) Chloride Contamination on Bentonites: Formation of Simonkolleite and Effects on Porosity and Chemical Composition" Materials 18, no. 13: 2933. https://doi.org/10.3390/ma18132933
APA StyleNartowska, E., Stępień, P., & Kanuchova, M. (2025). Impact of Zinc(II) Chloride Contamination on Bentonites: Formation of Simonkolleite and Effects on Porosity and Chemical Composition. Materials, 18(13), 2933. https://doi.org/10.3390/ma18132933







