Abstract
In this study, phosphogypsum waste collected from a factory dump in Kedainiai, Lithuania, was used for the first time as a starting material in the dissolution–precipitation synthesis of high-quality bioceramic calcium hydroxyapatite (Ca10(PO4)6(OH)2; CHA). The CHA powders were synthesized using the dissolution–precipitation method, employing phosphogypsum in four different conditions: untreated, dried at 100 °C, dried at 150 °C, and annealed at 1000 °C. Various phosphorus sources were used in the CHA synthesis process: Na2HPO4; a mixture of Na2HPO4 and NaH2PO4; or a combination of Na2HPO4, NaH2PO4, and NaHCO3. These mixtures were allowed to react at 80 °C for 48 h, 96 h, 144 h, and 192 h. X-ray diffraction (XRD) analysis revealed slight variations in the synthesized products depending on the specific starting materials used. Fourier transform infrared spectroscopy (FTIR) was conducted to confirm the structural characteristics of the synthesized CHA samples. The surface microstructure of the synthesized CHA samples differed notably from that of the raw phosphogypsum. All synthesized CHA samples exhibited Type IV nitrogen adsorption–desorption isotherms with H3-type hysteresis loops, indicating the presence of mesoporous structures, typically associated with slit-like pores or aggregates of plate-like particles. To the best of our knowledge, an almost monophasic CHA has been fabricated from phosphogypsum waste for the first time using a newly developed dissolution–precipitation synthesis method. A key challenge in the high-end market is the development of alternative synthesis technologies that are not only more environmentally friendly but also highly efficient. These findings demonstrate that phosphogypsum is a viable and sustainable raw material for CHA synthesis, with promising applications in the medical field, including the production of artificial bone implants.
1. Introduction
Synthetic calcium hydroxyapatite (Ca10(PO4)6(OH)2; CHA) is widely used as a bioceramic material to treat bone defects due to its chemical similarity to bone minerals, as well as its favorable biocompatibility and plasticity [1,2,3,4,5,6,7,8]. However, the quality of synthetic biomaterials largely depends on the overall characteristics and properties of the synthesized powders, including their density, purity, phase composition, crystallinity, particle size and distribution, morphology, and specific surface area [9,10]. Therefore, all of the aforementioned properties of bioceramics are highly influenced by the processing conditions, which play a crucial role in determining the crystallinity, crystal shape, crystal size, size distribution, and phase purity of the resulting powders.
Several methods have been employed for the synthesis of CHA. Calcium hydroxyapatite powders have been synthesized by using precipitation [11,12,13,14], hydrothermal [15,16,17,18,19], conventional solid-state reaction [14,19,20,21,22], mechanochemical [14,19,23,24,25,26], and sol–gel [4,9,27,28,29] methods. Overall, precipitation, hydrothermal, and sol–gel techniques can produce high-purity CHA powders with small particle sizes. However, important process variables including solution concentration, Ca/P ratio, pH, acid or complexing agent addition rate, stirring speed, temperature, reaction time, and atmospheric conditions should be controlled very carefully to obtain a monophasic end product. In standard procedures of solid-state reactions and mechanochemical methods, precursors are first milled and then calcined at very high temperatures, above 1000 °C, resulting in well-crystallized structures. However, it is important to note that powders usually exhibit heterogeneity, and are composed of irregularly shaped micron-sized grains. These methods suffer from the small diffusion of ions during the reactions following the formation of side phases.
Therefore, the synthesis method plays a critical role in controlling the physicochemical properties of CHA, and subsequently influence its mechanical and biological performance. A large number of review papers have been published on the synthesis of CHA from various natural sources, highlighting their advantages and disadvantages, their applications, and the essential properties of CHA for therapeutic applications [30,31,32,33,34]. Recent methods for extracting CHA from natural sources—such as mammalian, aquatic, or marine organisms, shells, eggshells, plants and algae, wood, animal bones, and minerals—have also been described. The use of plant-derived CHA at surgical sites can provide antifungal and antibacterial effects, helping to prevent infections in the affected area.
It was demonstrated that mollusk-derived hydroxyapatite is a widely available, cost-effective, sustainable, and low-impact biomaterial [35,36,37]. The results of previous studies [38,39] suggest that pufferfish teeth could be a natural alternative source for dental applications. The natural CHA produced from cow bone through ultrasound treatment followed by a calcination process at various temperatures and derived from marine resources could also be used for biomedical purposes [40,41].
An enormous number of scientific articles have been published on the potential of obtaining CHA from various wastes [42,43,44]. The utilization of eggshell waste as a raw material for the synthesis of CHA was suggested in several publications. In the initial preparation stage, calcium oxide was derived from eggshell waste and employed as a calcium precursor in order to prepare CHA via chemical precipitation with phosphoric acid [45,46]. CHA from eggshell wastes was also synthesized through mechanochemical activation and solid-sate reaction processes [47,48]. It was also demonstrated that shrimp shell waste, mussel shell waste, and other seafood shells could be a source of calcium to produce CHA [49,50,51,52]. Moreover, the potential use of bovine bone waste, fish waste, food products, sulphite waste, marble wastes, and other materials for the fabrication of CHA was suggested elsewhere [53,54,55,56,57]. Recently, we demonstrated for the first time that phosphogypsum waste could be successfully used as a precursor for the fabrication of CHA [58]. The company Lifosa, based in Lithuania, specializes in manufacturing phosphate-based fertilizers such as diammonium phosphate, monoammonium phosphate, and monocalcium phosphate. It produces phosphoric acid in house by processing apatite, which is then used in subsequent fertilizer production stages. One of the by-products generated during phosphoric acid synthesis is phosphogypsum. The factory site has accumulated a substantial amount of phosphogypsum waste, estimated at approximately 45 million tons. Although phosphogypsum is often classified as non-hazardous and is considered harmless to human health and the environment, its chemical composition can vary depending on the source of the phosphate raw material—apatite—used in the production process. To produce CHA and address potential disadvantages, such as those associated with solid-state reactions, appropriate synthesis methods must be proposed. A major challenge, particularly at the high end of the market, is to develop alternative synthesis technologies that are not only more environmentally friendly but also efficient and effective. The aim of this study is to optimize the dissolution–precipitation synthetic approach for the synthesis of high-quality CHA from phosphogypsum waste. In this paper, we present the results of the systematic characterization of an environmentally friendly dissolution–precipitation approach to CHA synthesis. The findings demonstrate that phosphogypsum is a viable and sustainable raw material for CHA synthesis, with promising applications for the production of artificial bone implants.
2. Materials and Methods
Waste phosphogypsum obtained from the company Lifosa, disodium hydrogen phosphate (Na2HPO4, 98%, Merck, Darmstadt, Germany), sodium dihydrogen phosphate (NaH2PO4, 99%, Merck, Darmstadt, Germany), and sodium hydrogen carbonate (NaHCO3, 989%, Merck, Darmstadt, Germany) were used as starting materials for the fabrication of calcium hydroxyapatite (Ca10(PO4)6(OH)2; CHA) powders via a dissolution–precipitation reaction.
In order to assess the influence of the starting material on the formation of CHA, some phosphogypsum samples were prepared, which were taken from different parts of the raw material. CHA powders were subsequently synthesized by using the dissolution–precipitation method with untreated phosphogypsum, while other samples were dried at 100 °C or 150 °C, or annealed at 1000 °C. In the CHA synthesis process, a 1.00 g portion of waste phosphogypsum (i.e., untreated and subjected to drying at 100 °C or 150 °C, or annealing at 1000 °C) was placed in the reaction vessel. It was then mixed with various reagent solutions serving as phosphorus sources: (A) 100.0 mL of 1.00 M Na2HPO4 solution; (B) a mixture containing 50.0 mL of each 1.00 M Na2HPO4 and 1.00 M NaH2PO4; or (C) a mixture containing 50.0 mL of each 1.00 M Na2HPO4, 1.00 M NaH2PO4, and 1.00 M NaHCO3. The mixtures were left to react at 80 °C for 48, 96, 144, and 192 h. After synthesis, the liquid phase was decanted, and the resulting powders were rinsed with 500 mL of hot (~80 °C) deionized water, followed by several additional rinses using 250 mL of room-temperature deionized water. Finally, the vacuum-filtered product was dried at 80 °C for 2 h.
Phosphorus starting materials were selected based on findings reported in the literature. For instance, a mixture of Na2HPO4 and NaH2PO4 has been successfully employed in the synthesis of magnesium whitlockite via the dissolution–precipitation method [59]. Similarly, octacalcium phosphate blocks have been fabricated through a dissolution–precipitation reaction using Na2HPO4 as the phosphorus source [60]. Bone apatite is a form of carbonated calcium hydroxyapatite that typically contains 6–9 mass% carbonate within its apatitic structure [61]. Based on this, a mixture of Na2HPO4, NaH2PO4, and NaHCO3 was also used for the synthesis of calcium hydroxyapatite to better replicate the composition of natural bone apatite.
The prepared samples were characterized by powder X-ray diffraction (XRD) using a Rigaku MiniFlex II diffractometer (Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 1.541838 Å). The diffraction data were obtained by scanning in the 2θ range of 10–60° at a scan speed of 2°/min. Fourier transform infrared spectroscopy (FT-IR) was performed using an Alpha spectrometer (Bruker, Inc., Ettlingen, Germany) in the wavenumber range from 4000 to 450 cm−1, with a resolution of 4 cm−1. Product morphology was analyzed using field-emission scanning electron microscopy (SEM, SU-70, Hitachi, Tokyo, Japan). Particle size was evaluated using open-source Fiji (ImageJ2) software by randomly selecting a few hundred particles. Energy-dispersive X-ray (EDX) analysis of the samples was performed using a SEM Hitachi TM 3000 (Tokyo, Japan). The specific surface area was measured by using the Brunauer–Emmet–Teller (BET) method under vacuum for samples degassed at 120 °C with a N2 adsorption–desorption isotherm (at 77 K) using a Tristar II instrument (Norcross, GA, USA). The pore size distribution of the materials produced was obtained using the Barrett–Joyner–Halenda (BJH) method.
3. Results and Discussion
The powder XRD patterns of CHA samples synthesized from unheated phosphogypsum, as well as those dried at 100 °C or 150 °C and heated at 1000 °C using Na2HPO4 (A) in the dissolution–precipitation procedure, are presented in Figure 1.
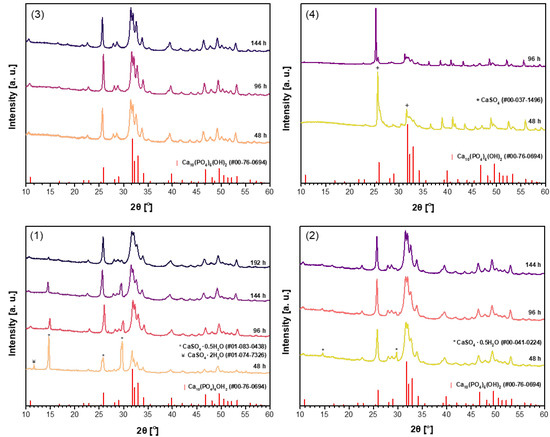
Figure 1.
XRD patterns of the CHA samples synthesized from unheated phosphogypsum (1), phosphogypsum dried at 100 °C (2) or 150 °C (3), or phosphogypsum heated at 1000 °C (4) using Na2HPO4 and different durations in the dissolution–precipitation procedure.
Evidently, the phase purity of the synthesized samples is dependent on the specific precursor utilized in the synthesis process. The more pronounced CHA phase is observed in the sample synthesized using unheated phosphogypsum (Figure 1(1)) only after a duration of 96 h. However, the diffraction lines of the CaSO4·0.5H2O secondary phase are visible even after treatment for 144 h. The impurity phase decreases significantly when increasing the reaction time to 192 h. When phosphogypsum dried at 100 °C (Figure 1(2)) was used as the starting material, very similar results are observed. Contrary, when phosphogypsum dried at 150 °C (Figure 1(3)) was used as the calcium source, the monophasic CHA was obtained after treatment for 96 h. With increasing synthesis time, no changes in XRD pattern are observed, indicating that all diffraction peaks belong to the desirable CHA phase. Obviously, the CaSO4 phase cannot be used as precursor for the synthesis of CHA by using dissolution–precipitation method (see Figure 1(4)).
It was demonstrated recently that for the synthesis of magnesium whitlockite (Ca18Mg2(HPO4)2(PO4)12) using the dissolution–precipitation method, a mixture of sodium phosphates (Na2HPO4 + NaH2PO4) as the phosphorus source was successfully used [59]. In order to reduce the synthesis time while obtaining a purer product, the same phosphate mixture was used in the synthesis of CHA in this work. Powder XRD patterns of the CHA samples synthesized from the same precursors using a mixture of Na2HPO4 and NaH2PO4 (B) in the dissolution–precipitation procedure are shown in Figure 2. Unfortunately, as can be seen from the XRD patterns depicted in Figure 2, the efficiency of CHA production could not be improved. Therefore, a third synthesis route was tested, where phosphogypsum was exposed to three reagents during synthesis, namely Na2HPO4, NaH2PO4, and NaHCO3 (C). XRD patterns of the resultant products are displayed in Figure 3. XRD analysis results show that the synthesis products are slightly different depending on which starting materials were used in the synthesis protocol. Interestingly, the duration of synthesis decreased to 48 h when CHA was synthesized using the phosphogypsum precursor dried at 150 °C (Figure 3(3)).
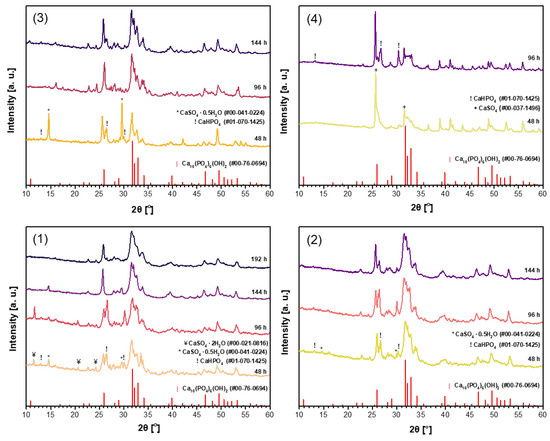
Figure 2.
XRD patterns of the CHA samples synthesized from unheated phosphogypsum (1), phosphogypsum dried at 100 °C (2) or 150 °C (3), or phosphogypsum heated at 1000 °C (4) using a mixture of Na2HPO4 + NaH2PO4 and different durations in the dissolution–precipitation procedure.
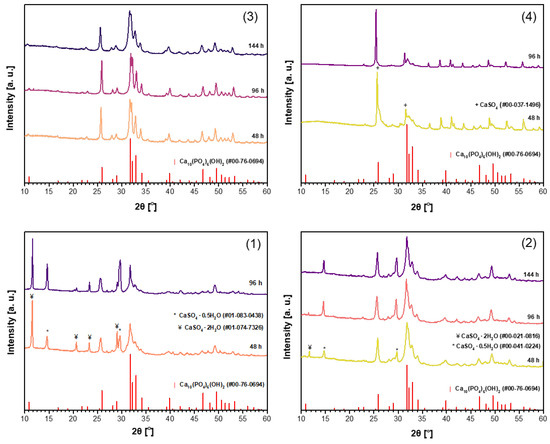
Figure 3.
XRD patterns of the CHA samples synthesized from unheated phosphogypsum (1), phosphogypsum dried at 100 °C (2) or 150 °C (3), or phosphogypsum heated at 1000 °C (4) using a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 and different durations in the dissolution–precipitation procedure.
In conclusion, the almost polycrystalline single-phase Ca10(PO4)6(OH)2 was obtained from waste phosphogypsum by using the dissolution–precipitation synthesis method with the phosphogypsum precursor dried at 100 °C and 150 °C as the Ca source and Na2HPO4 or a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 as the P source. These results highlight the influence of both the precursor drying temperature and the choice of phosphorus source on the phase purity of the final apatite product. The purest CHA samples were selected for further characterization. Miller indices have been assigned to the diffraction peaks observed in the XRD pattern of the CHA sample synthesized for 96 h from dried phosphogypsum at 150 °C, using a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 in the dissolution–precipitation process (Figure 3(3)). Nearly all diffraction lines have been indexed, confirming the formation of monophasic CHA (see Figure S1).
The tentative crystallite sizes for two representative samples were determined by using the Scherrer equation: τ = 0.9λ/Bcosθ, where τ is the mean crystallite size, λ is the X-ray wavelength, B is the line broadening at half maximum intensity (FWHM) (in radians), and θ is the Bragg angle. The calculated crystallite sizes for the samples obtained from dried phosphogypsum at 150 °C using either Na2HPO4 alone or a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 were 59.25 nm and 50.08 nm, respectively.
The FTIR spectra in the range of 4000–400 cm−1 of the representative and purest CHA samples synthesized for 144 h are presented in Figure 4. The strong absorption bands, which are further subdivided into multiple components within the range of 1170–955 cm−1, can be attributed to the distinct vibrational modes associated with the stretching vibrations of the P–O bonds in the PO43− ion [59]. The bands observed at approximately 600–465 cm−1 are associated with the bending vibrations of the P–O and O–P–O bonds in phosphate [59,62]. The presence of a broad absorption feature around 869 cm⁻1, linked to the P–O(H) vibrational mode of hydrogen phosphate, indicates a deficiency of calcium in the structure of the produced apatite-type material. The stretching vibrations of the O–H group in the CHA structure were detected as a broad absorption band in the 3575–3440 cm−1 region, along with a weaker band observed around 1680–1650 cm−1. In the case when a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 was used in the dissolution–precipitation synthesis procedure, the FTIR spectra of the resulting CHA samples revealed distinct absorption bands in the 1550–1360 cm−1 region at 1468 cm⁻1, 1456 cm⁻1, and 1417 cm⁻1. In biological and synthetic apatites, carbonate can substitute at two primary sites: the phosphate site (known as B-type substitution) or the hydroxyl (OH⁻) site (A-type substitution). The peaks observed in this study are consistent with B-type substitution, which is the most common form in biological apatite and typically results in bands around 1410–1470 cm⁻1 [62]. These findings allow us to conclude that in this synthesis route, carbonated hydroxyapatite (Ca10−x/2(PO4)6−x(CO3)x(OH)2) has formed [61,63]. Thus, FTIR analysis was performed to confirm the structural characteristics of the synthesized CHA sample. Moreover, the FTIR results clearly support the conclusions made from the XRD data.
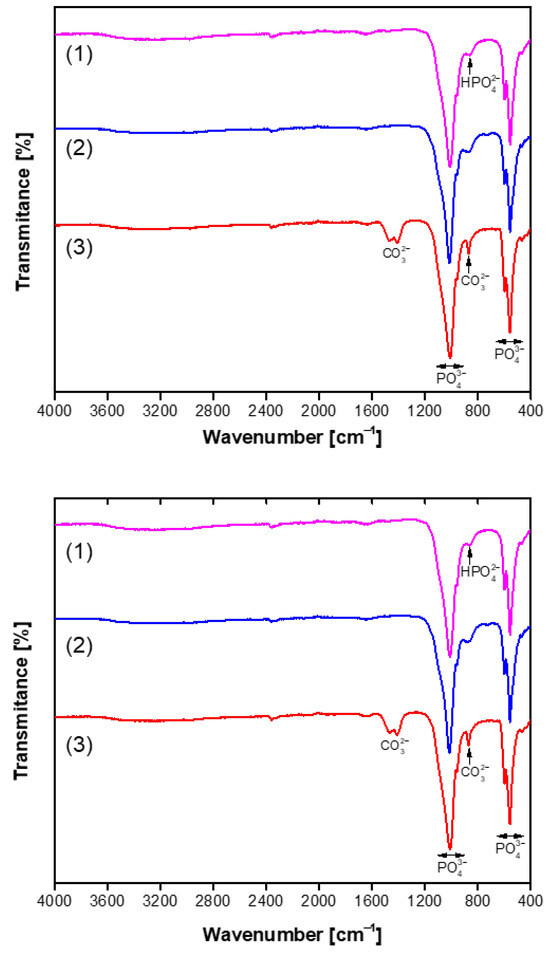
Figure 4.
FTIR spectra of CHA samples synthesized from phosphogypsum dried at 100 °C (bottom) and dried at 150 °C (top) and reacted with different phosphorus sources: Na2HPO4 (1), Na2HPO4 + NaH2PO4 (2), and a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 (3). The synthesis procedure was carried out for 144 h.
Considering the results obtained, the formation mechanisms of CHA using different phosphorus sources (A), (B), and (C) can be roughly expressed by the following chemical reactions, respectively:
10 CaSO4·0.5H2O + 6 Na2HPO4 → Ca10(PO4)6(OH)2 +8 NaHSO4 + 2 Na2SO4 + 3 H2O
10 CaSO4·0.5H2O + 4 Na2HPO4 + 2 NaH2PO4 → Ca10(PO4)6(OH)2 +10 NaHSO4 + 3 H2O
14 CaSO4·0.5H2O + 8 Na2HPO4 + 2 NaH2PO4 + 6 NaHCO3 → Ca10(PO4)6(OH)2 + Ca4(PO4)4(CO3)2(OH)2 + 4 NaHSO4 + 10 Na2SO4 + 4 CO + 11 H2O
SEM micrographs of the CHA samples synthesized from phosphogypsum dried at 100 °C using different phosphorus sources are presented in Figure 5.
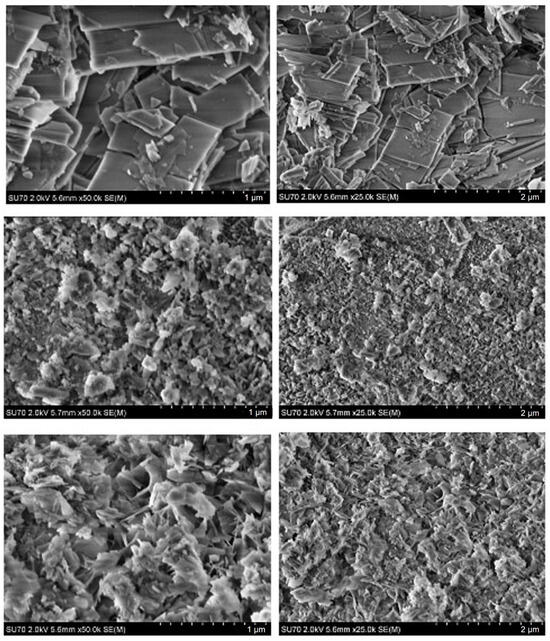
Figure 5.
SEM micrographs obtained at different magnifications of the CHA samples synthesized from phosphogypsum dried at 100 °C and reacted with different phosphorus sources: Na2HPO4 (bottom), Na2HPO4 + NaH2PO4 (middle), and Na2HPO4 + NaH2PO4 + NaHCO3 (top). The synthesis procedure was carried out for 144 h.
The SEM analysis reveals that the morphology of the surfaces of synthesized CHA is dependent on the phosphorus source. The SEM micrographs of the CHA sample synthesized using Na2HPO4 demonstrate a surface morphology composed of plate-like almost nanosized (~100–300 nm) differently oriented particles with a distinctive shape. The SEM images demonstrate that the surfaces of samples synthesized using the mixture of Na2HPO4 and NaH2PO4 are monolithic and predominantly composed of irregularly shaped (flower petals, plates, or rods) particles 50–200 nm in size. All these samples have visible pores on the surface, likely due to the release of gaseous products during synthesis. After NaHCO3 was added to the mixture of Na2HPO4 and NaH2PO4, a surface microstructure with dominant larger (about 0.5 µm) rectangular plate-like particles was observed. Interestingly, the surface microstructure of obtained the CHA samples is obviously different from that of the initial phosphogypsum, which is composed of larger and differently oriented 20–30 µm in size plate-like crystals and microrods [58].
SEM micrographs of the CHA samples synthesized from phosphogypsum dried at 150 °C using different phosphorus sources are presented in Figure 6.
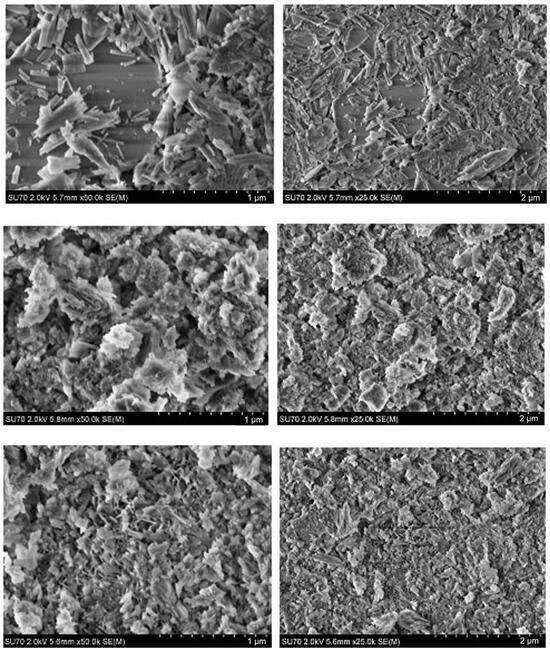
Figure 6.
SEM micrographs obtained at different magnifications of the CHA samples synthesized from phosphogypsum dried at 150 °C and reacted with different phosphorus sources: Na2HPO4 (bottom), Na2HPO4 + NaH2PO4 (middle), and Na2HPO4 + NaH2PO4 + NaHCO3 (top). The synthesis procedure was carried out for 144 h.
It is evident from the SEM images that the microstructural features of the synthesized CHA samples are almost identical to those presented in Figure 5. Thus, surface morphology depends mostly on the phosphorus source (Na2HPO4, or Na2HPO4 + NaH2PO4, or Na2HPO4 + NaH2PO4 + NaHCO3) used, but not on the initial drying temperature (100 °C or 150 °C) of the phosphogypsum which was used as the starting material. It could be concluded that dissolution–precipitation synthesis processing is responsible for the surface morphology of the final product.
The N2 adsorption–desorption isotherms of the CHA samples synthesized via the dissolution–precipitation method from phosphogypsum dried at 100 °C and reacted with Na2HPO4, phosphogypsum dried at 150 °C and reacted with Na2HPO4, and phosphogypsum dried at 150 °C and reacted with a mixed solution of Na2HPO4 + NaH2PO4 + NaHCO3 for 144 h are presented in Figure 7.
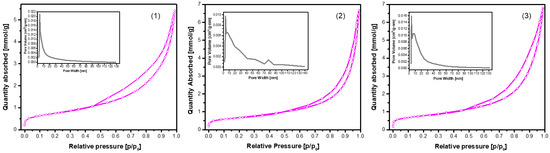
Figure 7.
N2 adsorption–desorption isotherms of the CHA samples synthesized via the dissolution–precipitation method from phosphogypsum dried at 100 °C and reacted with Na2HPO4 (1), phosphogypsum dried at 150 °C and reacted with Na2HPO4 (2), and phosphogypsum dried at 150 °C and reacted with a mixed solution of Na2HPO4 + NaH2PO4 + NaHCO3 (3). The synthesis procedure was carried out for 144 h. The inset shows the pore size distribution.
All samples exhibited Type IV isotherms with H3-type hysteresis loops, a characteristic feature of mesoporous materials with slit-like pores or plate-like particle aggregates. However, a notable variation in surface area, pore volume, and pore distribution was observed, indicative of the impact of synthesis conditions (see Figure 7 and Table 1).

Table 1.
Data derived from N2 adsorption–desorption measurements: SBET (specific surface area determined by the BET method), Sext (external surface area), Vμ (micropore volume), and Vp (total pore volume).
The sample obtained from phosphogypsum dried at 100 °C and subsequently reacted with Na2HPO4 exhibited a well-developed mesoporous structure, as evidenced by the presence of the highest SBET and Sext, in conjunction with a narrow pore size distribution. The close values of SBET and Sext indicate that the majority of the surface area is derived from mesopores. The low Vμ value indicates negligible microporosity. As demonstrated in sample (2), there was a notable similarity in SBET (58.7 m2/g) between the two samples. However, a significant disparity was observed in terms of Vp (0.21 cm3/g) and micropore contribution (Vμ = 0.0040 cm3/g). As demonstrated in sample (3), a notably lower SBET (15.9 m2/g) and Vp (0.067 cm3/g) were observed, despite the highest nitrogen uptake being exhibited at high relative pressures. This apparent contradiction can be attributed to the presence of larger mesopores or macropores, which contribute to gas volume but not significantly to surface area. The low external surface area (8.30 m2/g) and relatively small Vμ (0.0036 cm3/g) suggest limited textural development, possibly due to synthesis-related densification or partial pore blockage.
Figure 8 shows elemental mapping of the representative sample composed of plate-like shaped particles.
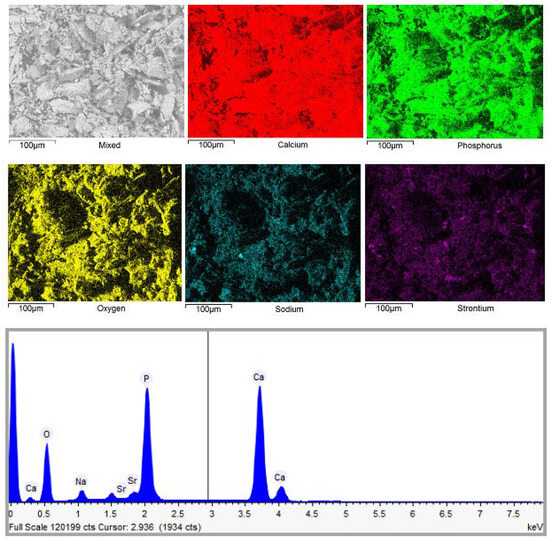
Figure 8.
The EDX elemental mapping and spectrum of the CHA sample synthesized via the dissolution–precipitation method from phosphogypsum dried at 150 °C and reacted with a mixed solution of Na2HPO4 + NaH2PO4 + NaHCO3. The synthesis procedure was carried out for 144 h.
In all cases, EDX elemental mapping demonstrates that all elements appear to be homogeneously distributed throughout the samples despite the differences in their preparation. The molar ratio of Ca and P in most of the cases corresponds to a ratio 1.67, which is characteristic of the ratio of these elements in calcium hydroxyapatite. A negligible amount of sodium (remaining from starting reagents) and strontium (initial presence in phosphogypsum waste) was also determined in the end products by EDX analysis. Since we did not observe Ca-rich, P-rich, or other metal-rich regions, we can conclude that the almost monophasic CHA could be fabricated by using the dissolution–precipitation synthesis method from phosphogypsum waste.
Finally, high-quality Ca10(PO4)6(OH)2 was successfully synthesized from phosphogypsum waste. This approach not only provides a valuable route for producing biomedical CHA but also aligns with the principles of sustainability by converting industrial by-products into high-value materials [64]. Utilizing phosphogypsum, a common waste product from phosphate fertilizer production, helps reduce environmental burden, promotes resource circularity, and supports cleaner production practices [65].
4. Conclusions
In this study, the dissolution–precipitation synthetic approach for the synthesis of high-quality calcium hydroxyapatite (Ca10(PO4)6(OH)2; CHA) from phosphogypsum waste was optimized. CHA powders were subsequently synthesized via the dissolution–precipitation method using untreated, dried at 100 °C, dried at 150 °C, or annealed at 1000 °C phosphogypsum. In the CHA synthesis process, various phosphorus sources were used (Na2HPO4; or mixture of Na2HPO4 and 1.00 M NaH2PO4; or mixture of 1.00 M Na2HPO4, 1.00 M NaH2PO4, and 1.00 M NaHCO3). The mixtures were left for 48 h, 96 h, 144 h, and 192 h, allowing the reaction to progress at 80 °C. The phase purity of the synthesized samples was dependent on the specific precursor utilized in the synthesis process. The polycrystalline almost single-phase Ca10(PO4)6(OH)2 was obtained from waste phosphogypsum by using the dissolution–precipitation synthesis method when the phosphogypsum precursor dried at 100 °C and 150 °C was used as the Ca source and Na2HPO4 or a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 was used as the P source. The SEM analysis reveals that the morphology of the surfaces of synthesized CHA is dependent on the phosphorus source. The SEM micrographs of the synthesized CHA sample using Na2HPO4 demonstrate a surface morphology composed of plate-like almost nanosized (~100–300 nm) differently oriented particles with a distinctive shape. The SEM images demonstrate that the surfaces of samples synthesized using the mixture of Na2HPO4 and NaH2PO4 are monolithic and predominantly composed of irregularly shaped (flower petals, plates, or rods) particles 50–200 nm in size. After NaHCO3 was added to the mixture of Na2HPO4 and NaH2PO4, the surface microstructure with dominant larger (about 0.5 µm) rectangular plate-like particles was observed. EDX elemental mapping demonstrates that all elements appear to be homogeneously distributed throughout the samples despite the differences in their preparation. The molar ratio of Ca and P in most of the cases corresponds to a ratio 1.67, which is characteristic of the ratio of these elements in calcium hydroxyapatite. In conclusion, the almost monophasic CHA could be fabricated by using the dissolution–precipitation synthesis method with phosphogypsum waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18122869/s1, Figure S1: XRD pattern of the CHA sample synthesized for 96 h from dried phosphogypsum at 150 °C using a mixture of Na2HPO4 + NaH2PO4 + NaHCO3 in the dissolution–precipitation procedure.
Author Contributions
Conceptualization, A.K. and I.G.; methodology, Z.S.; software, L.M.; validation, E.J.; formal analysis, E.J. and I.G.; investigation, E.J., L.M. and S.J.; resources, Z.S.; data curation, L.M., E.J. and I.G.; writing—original draft preparation, A.K.; writing—review and editing, A.K. and I.G.; visualization, I.G.; supervision, A.K.; project administration, Z.S.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goetz, W.; Papageorgiou, S.N. Molecular, cellular and pharmaceutical aspects of synthetic hydroxyapatite bone substitutes for oral and maxillofacial grafting. Curr. Pharmac. Biotechnol. 2017, 18, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Fukuda, N.; Kasugai, S.; Tachikawa, N.; Koyano, K.; Matsushita, Y.; Ogino, Y.; Ishikawa, K.; Miyamoto, Y. Maxillary Sinus Floor Augmentation Using Low-Crystalline Carbonate Apatite Granules with Simultaneous Implant Installation: First-in-Human Clinical Trial. J. Oral Maxillofac. Surg. 2019, 77, 985-e1. [Google Scholar] [CrossRef] [PubMed]
- Duta, L.; Grumezescu, V. The Effect of Doping on the Electrical and Dielectric Properties of Hydroxyapatite for Medical Applications: From Powders to Thin Films. Materials 2024, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based coatings—A review. Chemija 2020, 31, 25–41. [Google Scholar] [CrossRef]
- Gani, M.A.; Lee, G.; Ardianto, C.; Rantam, F.A.; Lestari, M.L.A.D.; Addimaysqi, R.; Adnyana, I.K.; Lee, K.; Khotib, J. Comparative study of bovine and synthetic hydroxyapatite in micro-and nanosized on osteoblasts action and bone growth. PLoS ONE 2025, 20, e0311652. [Google Scholar] [CrossRef]
- Lukaviciute, L.; Lukowiak, A.; Stankeviciute, Z.; Junka, A.; Mortimer, M.; Zarkov, A.; Yang, J.-C.; Ganceviciene, R.; Kareiva, A. Cytocompatible and antibacterial Fe-, Cu- and Zn-substituted calcium hydroxyapatite materials for skin applications. Ceram. Int. 2025, 51, 11286–11296. [Google Scholar] [CrossRef]
- de Andrade, R.; Paim, T.C.; Bertaco, I.; Naasani, L.S.; Buchner, S.; Kovarik, T.; Hajek, J.; Wink, M.R. Hierarchically porous bioceramics based on geopolymer-hydroxyapatite composite as a novel biomaterial: Structure, mechanical properties and biocompatibility evaluation. Appl. Mater. Today 2023, 33, 101875. [Google Scholar] [CrossRef]
- Montesissa, M.; Sassoni, E.; Boi, M.; Borciani, G.; Boanini, E.; Graziani, G. Synthetic or Natural (Bio-Based) Hydroxyapatite? A Systematic Comparison between Biomimetic Nanostructured Coatings Produced by Ionized Jet Deposition. Nanomaterials 2024, 14, 1332. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based biomaterials—A review of environmentally benign, simple and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Ahmed, L.O.; Omer, R.A. Hydroxyapatite biomaterials: A comprehensive review of their properties, structures, clinical applications, and producing techniques. Rev. Inorg. Chem. 2024, 44, 599–618. [Google Scholar] [CrossRef]
- Santos, M.H.; de Oliveira, M.; de Freitas Souza, L.P.; Mansur, H.S.; Vasconcelos, W.L. Synthesis control and characterization of hydroxyapatite prepared by wet precipitation process. Mater. Res. 2004, 7, 625–630. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Sokolova, M.; Putnins, A.; Kreicbergs, I.; Locs, J. Scale-Up of Wet Precipitation Calcium Phosphate Synthesis. Key Eng. Mater. 2014, 604, 216–219. [Google Scholar] [CrossRef]
- Anandan, D.; Jaiswal, A.K. Synthesis methods of hydroxyapatite and biomedical applications: An updated review. J. Austral. Ceram. Soc. 2024, 60, 663–679. [Google Scholar] [CrossRef]
- Riman, R.E.; Suchanek, W.L. Hydrothermal synthesis of advanced ceramic powders. Adv. Sci. Technol. 2006, 45, 184–193. [Google Scholar]
- Aminian, A.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Bakhshi, F.; Gorjipour, F.; Farzadi, A.; Moztarzadeh, F.; Schmücker, M. Synthesis of silicon-substituted hydroxyapatite by a hydrothermal method with two different phosphorous sources. Ceram. Int. 2011, 37, 1219–1229. [Google Scholar] [CrossRef]
- Nagata, F.; Yamauchi, Y.; Tomita, M.; Kato, K. Hydrothermal synthesis of hydroxyapatite nanoparticles and their protein adsorption behavior. J. Ceram. Soc. Jpn. 2013, 121, 797–801. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Batch hydrothermal synthesis of nanocrystalline, thermostable hydroxyapatite at various pH and temperature levels. Inorg. Chem. Commun. 2023, 157, 111301. [Google Scholar] [CrossRef]
- Kawsar, M.; Hossain, M.S.; Alam, M.K.; Bahadur, N.M.; Shaikh, M.A.A.; Ahmed, S. Synthesis of pure and doped nano-calcium phosphates using different conventional methods for biomedical applications: A review. J. Mater. Chem. B. 2024, 12, 3376–3391. [Google Scholar] [CrossRef]
- Ezerskyte-Miseviciene, A.; Kareiva, A. Everything old is new again: A reinspection of solid-state method for the fabrication of high quality calcium hydroxyapatite bioceramics. Mendel. Commun. 2019, 29, 273–275. [Google Scholar] [CrossRef]
- Viana, J.R.; Mathias, A.A.M.; Santos, A.O.D.; Filho, P.D.F.; Graca, M.P.F.; Valent, M.A.; da Silva, C.C. Comparative analysis of solid state hydroxyapatite synthesis. Matéria 2020, 25, e-12588. [Google Scholar]
- Javadinejad, H.R.; Ebrahimi-Kahrizsangi, R. Thermal and kinetic study of hydroxyapatite formation by solid-state reaction. Int. J. Chem. Kinet. 2021, 53, 583–595. [Google Scholar] [CrossRef]
- Rhee, S.H. Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials 2002, 23, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zhang, Q.; Saito, F. Mechanochemical synthesis of hydroxyapatite from Ca(OH)2-P2O5 and CaO-Ca(OH)2-P2O5 mixtures. J. Mater. Sci. 2000, 35, 5401–5405. [Google Scholar] [CrossRef]
- Amirthalingam, N.; Deivarajan, T.; Paramasivam, M. Mechano chemical synthesis of hydroxyapatite using dolomite. Mater. Lett. 2019, 254, 379–382. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M.; Lu, W.; Monmaturapoj, N. Synthesis and characterization of biomimetic bioceramic nanoparticles with optimized physicochemical properties for bone tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 1654–1666. [Google Scholar] [CrossRef]
- Jeyhani, N.; Masaeli, E.; Mirahmadi-Zare, S.Z.; Alirezaei, S.; Shoaraye-Nejati, A. Effect of precursor on structural and antibacterial behaviour of hydroxyapatite/silver nanocomposites. Mater. Technol. 2022, 37, 1086–1096. [Google Scholar] [CrossRef]
- Brochu, B.M.; Sturm, S.R.; Goncalves, J.A.K.D.; Mirsky, N.A.; Sandino, A.I.; Panthaki, K.Z.; Panthaki, K.Z.; Nayak, V.V.; Daunert, S.; Witek, L.; et al. Advances in Bioceramics for Bone Regeneration: A Narrative Review. Biomimetics 2024, 9, 690. [Google Scholar] [CrossRef]
- Liu, C.R.; Xu, M.C.; Wang, Y.C.; Yin, Q.Y.; Hu, J.; Chen, H.; Sun, Z.W.; Liu, C.; Li, X.Y.; Zhou, W.J.; et al. Exploring the potential of hydroxyapatite-based materials in biomedicine: A comprehensive review. Mater. Sci. Eng. R Rep. 2024, 161, 100870. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Osuchukwu, O.A.; Anisiji, E.O.; Lawal, M.Y.; Mohammed, S.A.; Ibitoye, O.I.; Oni, P.G.; Aderibigbe, V.D.; Aina, T.; Oyebode, D.; et al. In-depth review of synthesis of hydroxyapatite biomaterials from natural resources and chemical regents for biomedical applications. Arab. J. Chem. 2024, 17, 106010. [Google Scholar] [CrossRef]
- Veluswamy, R.; Balasubramaniam, G.; Natarajan, M.; Krishnaswamy, M.; Chinnappan, B.A.; Nagarajan, S.; Subramanian, B.; Velauthapillai, D. Multifunctional and sustainable hydroxyapatite from natural products for biomedical and industrial applications—A comprehensive review. Sust. Chem. Pharm. 2024, 41, 101653. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Abdullah, M.M.A.; Rahim, S.Z.A.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabialek, M. Synthesis methods of hydroxyapatite from natural sources: A review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Koshy, N.A.S.M.P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Muntean, F.L.; Olariu, I.; Marian, D.; Olariu, T.; Petrescu, E.L.; Olariu, T.; Draghici, G.A. Hydroxyapatite from Mollusk Shells: Characteristics, Production, and Potential Applications in Dentistry. Dent. J. 2024, 12, 409. [Google Scholar] [CrossRef]
- Trinkunaite-Felsen, J.; Prichodko, A.; Semasko, M.; Skaudzius, R.; Beganskiene, A.; Kareiva, A. Synthesis and characterization of iron-doped/substituted calcium hydroxyapatite from seashells Macoma balthica (L.). Adv. Powder Technol. 2015, 26, 1287–1293. [Google Scholar] [CrossRef]
- Alkaron, W.; Almansoori, A.; Balazsi, K.; Balazsi, C. Hydroxyapatite-Based Natural Biopolymer Composite for Tissue Regeneration. Materials 2024, 17, 4117. [Google Scholar] [CrossRef]
- Dogdu, S.A.; Turan, C.; Depci, T.; Ayas, D. Natural hydroxyapatite obtained from pufferfish teeth for potential dental application. J. Ceram. Process. Res. 2021, 22, 356–361. [Google Scholar]
- Dogdu, S.A.; Turan, C.; Depci, T.; Bahceci, E.; Sangun, K.; Ayas, D. Hydroxyapatite production and characterization from four pufferfish species teeth. J. Ceram. Process. Res. 2024, 25, 85–91. [Google Scholar]
- Akindoyo, J.O.; Ghazali, S.; Beg, M.D.H.; Jeyaratnam, N. Characterization and Elemental Quantification of Natural Hydroxyapatite Produced from Cow Bone. Chem. Eng. Technol. 2019, 42, 1805–1815. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S. Hydroxyapatite Derived from Marine Resources and their Potential Biomedical Applications. Biotechnol. Bioproc. Eng. 2021, 26, 312–324. [Google Scholar] [CrossRef]
- Yong, P.; Macaskie, L.E.; Sammons, R.L.; Marquis, P.M. Synthesis of nanophase hydroxyapatite by a Serratia sp from waste-water containing inorganic phosphate. Biotechnol. Lett. 2004, 26, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, K.A.; Yatim, N.; Ali, N.; Rastegari, H. Recycling phosphorus and calcium from aquaculture waste as a precursor for hydroxyapatite (HAp) production: A review. Environ. Sci. Pollut. Res. 2022, 29, 46471–46486. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoaty, A.; Mousa, S. Synthesis and characterization of hydroxyapatite nanoparticles from calcium hydroxide fouled with gases evolved from smokestack of glass industry. Sci. Rep. 2024, 14, 10969. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Tangnorawich, B. Utilization of eggshell waste as raw material for synthesis of hydroxyapatite. Coll. Polym. Sci. 2015, 293, 2477–2483. [Google Scholar] [CrossRef]
- Razak, A.; Isa, N.M.; Adzila, S. Synthesis of Calcium Phosphate Extracted from Eggshell Waste through Precipitation Method. Biointerface Res. Appl. Chem. 2021, 11, 15058–15067. [Google Scholar]
- Ghouse, R.M.; Natasha, A.N.; Shahedan, N.F.; Ramesh, S.; Bang, L.T. The Properties of Hydroxyapatite Derived from Carbonate Eggshell Waste Through Ball Milling and Heat Treatment Method. Int. J. Integr. Eng. 2024, 16, 112–120. [Google Scholar] [CrossRef]
- Idulhaq, M.; Mudigdo, A.; Utomo, P.; Wasita, B.; Trapsilantya, M.E. Structural Comparison of Hydroxyapatite from Clam Shell Waste and Eggshell Waste Compared to Commercial Synthetic Hydroxyapatite. Malays. Ortop. J. 2024, 18, 27–31. [Google Scholar]
- Zakaria, K.A.; Yatim, N.I.; Ali, N.; Lananan, F.; Kasan, N.A. Extracting valuable compounds from shrimp shell waste: Recovery of high-quality as calcium-centric resources for hydroxyapatite production. J. Aust. Ceram. Soc. 2024, 60, 1019–1029. [Google Scholar] [CrossRef]
- Irfa’i, M.A.; Schmahl, W.W.; Pusparizkita, Y.M.; Muryanto, S.; Prihanto, A.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Hydrothermally synthesized-nanoscale carbonated hydroxyapatite with calcium carbonates derived from green mussel shell wastes. J. Mol. Struct. 2024, 1306, 137837. [Google Scholar] [CrossRef]
- Nunez, D.; Elgueta, E.; Varaprasad, K.; Oyarzun, P. Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater. Lett. 2018, 230, 64–68. [Google Scholar] [CrossRef]
- Bee, S.L.; Hamid, Z.A.A. Hydroxyapatite derived from food industry bio-wastes: Syntheses, properties and its potential multifunctional applications. Ceram. Int. 2020, 46, 17149–17175. [Google Scholar] [CrossRef]
- Irfa’i, M.A.; Muryanto, S.; Pusparizkita, Y.M.; Prihanto, A.; Vaquer, A.S.; Schmahl, W.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Calcination-based direct extraction of hydroxyapatite from bovine bone waste. Environ. Technol. 2024, 45, 6249–6261. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; da Silva, V.H.P.; Ruiz, P.L.M.; Ussui, V.; Lazar, D.R.R.; Renno, A.C.M.; Ribeiro, D.A. Physico-chemical characterization and biocompatibility of hydroxyapatite derived from fish waste. J. Mech. Behav. Biomed. Mater. 2018, 80, 137–142. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Zarkov, A.; Tsuru, K.; Ishikawa, K.; Kareiva, A. A novel synthetic approach for the calcium hydroxyapatite from the food products. J. Sol-Gel Sci. Technol. 2019, 91, 63–71. [Google Scholar] [CrossRef]
- Takei, T.; Imazawa, N.; Miura, A.; Kumada, N.; Ogihara, K. Conversion of calcium sulfite waste to hydroxyapatite. Powder Technol. 2013, 237, 400–405. [Google Scholar] [CrossRef]
- Algamal, Y.; Khalil, N.M.; Saddiq, A.; Baghdadi, A.M. Antimicrobial activity of Hydroxyapatite nanoparticles prepared from marble wastes. Main Group Chem. 2022, 21, 865–873. [Google Scholar] [CrossRef]
- Bajorinaite, E.; Michailova, L.; Jureviciute, S.; Sokol, D.; Stankeviciute, Z.; Grigoraviciute, I.; Kareiva, A. Initial evaluation of waste phosphogypsum for its use as a precursor for bioceramic materials. Chemija 2024, 35, 35–44. [Google Scholar] [CrossRef]
- Raiseliene, R.; Linkaite, G.; Zarkov, A.; Kareiva, A.; Grigoraviciute, I. Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor. Materials 2024, 17, 788. [Google Scholar] [CrossRef]
- Sugiura, Y.; Munar, M.L.; Ishikawa, K. Fabrication of octacalcium phosphate block through a dissolution-precipitation reaction using a calcium sulphate hemihydrate block as a precursor. J. Mater. Sci. Mater. Med. 2018, 29, 151. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef]
- Garskaite, E.; Alinauskas, L.; Drienovsky, M.; Krajcovic, J.; Cicka, R.; Palcut, M.; Jonusauskas, L.; Malinauskas, M.; Stankeviciute, Z.; Kareiva, A. Fabrication of composite of nanocrystalline carbonated hydroxyapatite (cHAP) with polylactic acid (PLA) and its surface topographical structuring with direct laser writing (DLW). RSC Adv. 2016, 6, 72733–72743. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated hydroxyapatite as bone substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Sanchez-Urzua, J.M.; Mendoza, P.G.Y.G.Y.; Navarro-Marquez, M.; Zayas-Olivares, A.; Gutierrez-Uribe, J.A.; Ortega-Lara, W.; Cervantes-Aviles, P. Recovery of calcium from maize Lime-Cooking wastewater as hydroxyapatite for biomedical applications. Sep. Purif. Technol. 2025, 365, 132777. [Google Scholar] [CrossRef]
- Deng, Q.L.; Bai, J.; Luo, C.L.; Liao, X.Z.; He, Q.R.; Tan, H.B.; Dong, F.Q.; Chen, W.L.; Jiang, J.L. Preparation of calcium-based cementitious material by decomposing phosphogypsum as the sole calcium source utilizing biomass synergized with iron accelerators. Proc. Saf. Environ. Prot. 2025, 195, 106775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).