Suppressing Jahn–Teller Distortion in Manganese Oxides for High-Performance Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
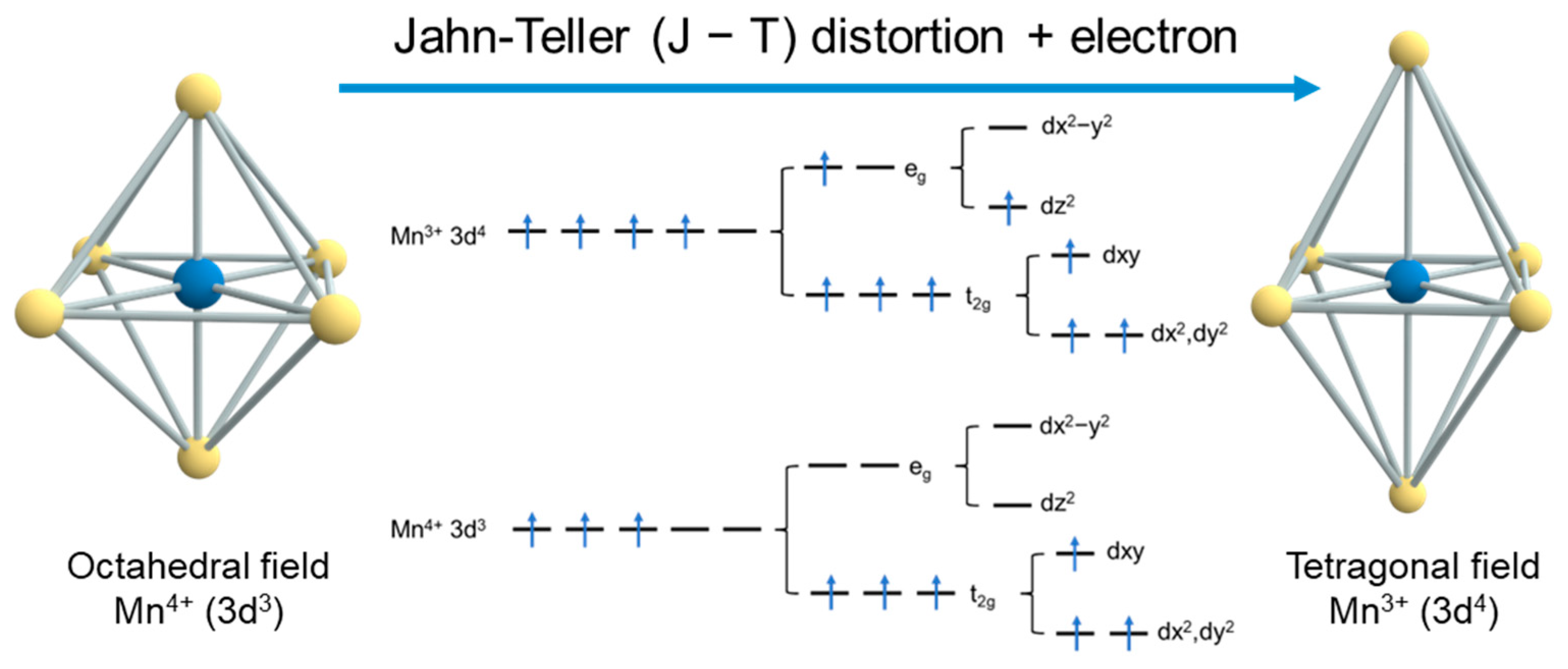
2. Fundamentals of Jahn–Teller Effect in MnOx
2.1. Electronic and Structural Basis
2.2. Impact of Jahn–Teller Effect on Electrochemical Implications in AZIBs
3. Mitigation Strategies to Suppress the Jahn–Teller Effect
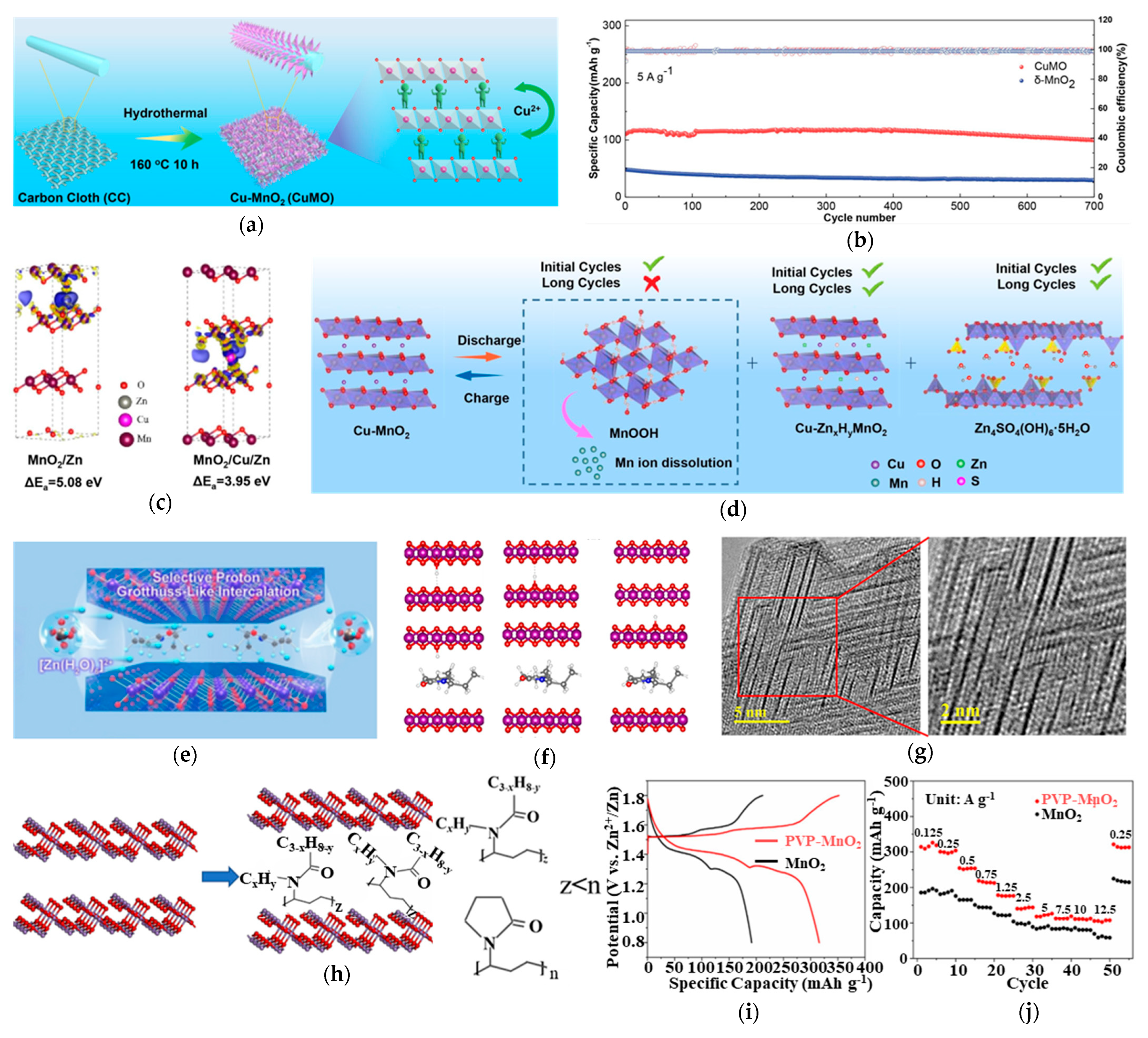
3.1. Strategic Cationic Doping for Structural Stabilization
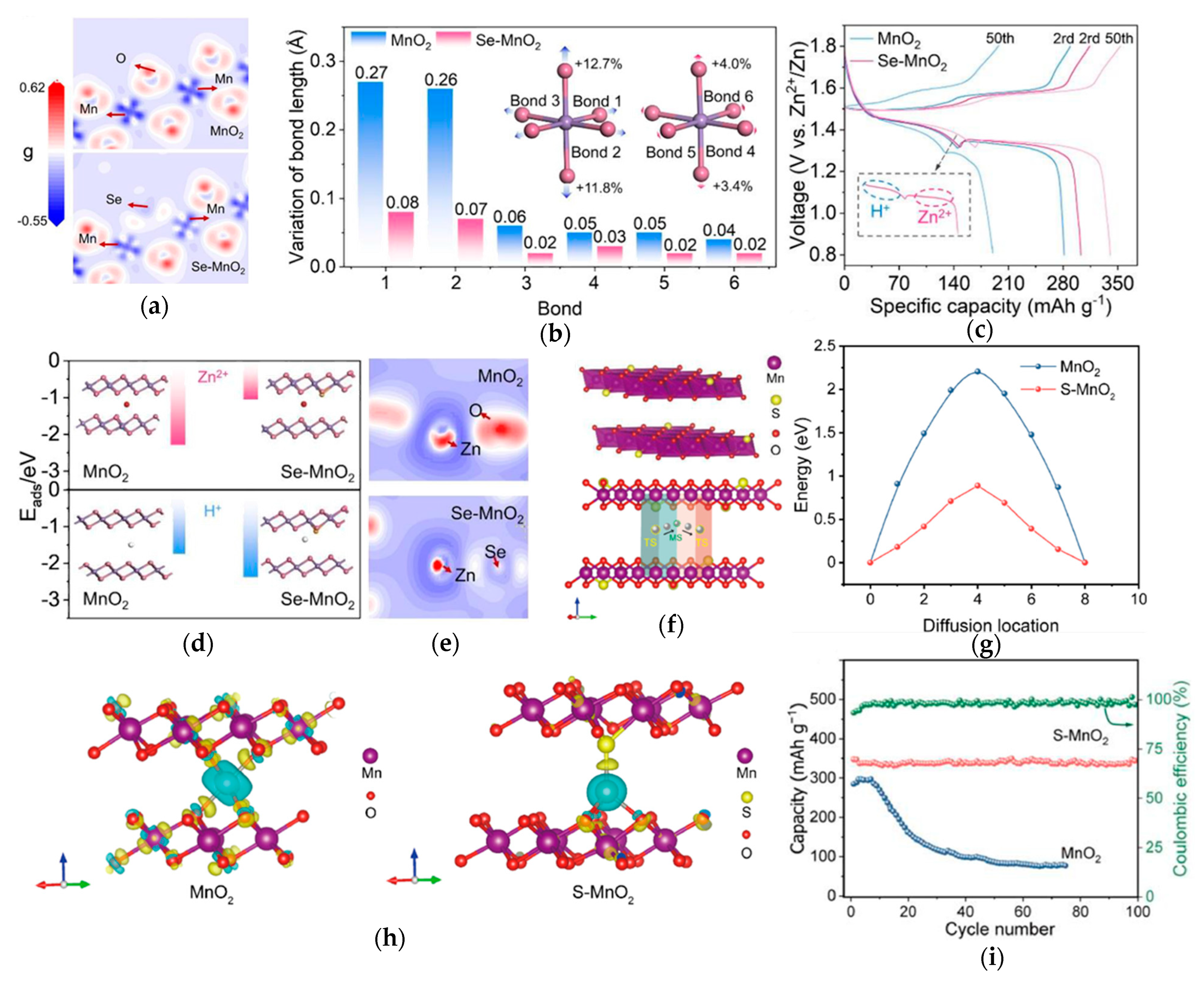
3.2. Anionic Doping for Enhanced Structural Resilience
3.3. Interlayer Engineering for Structural Stabilization
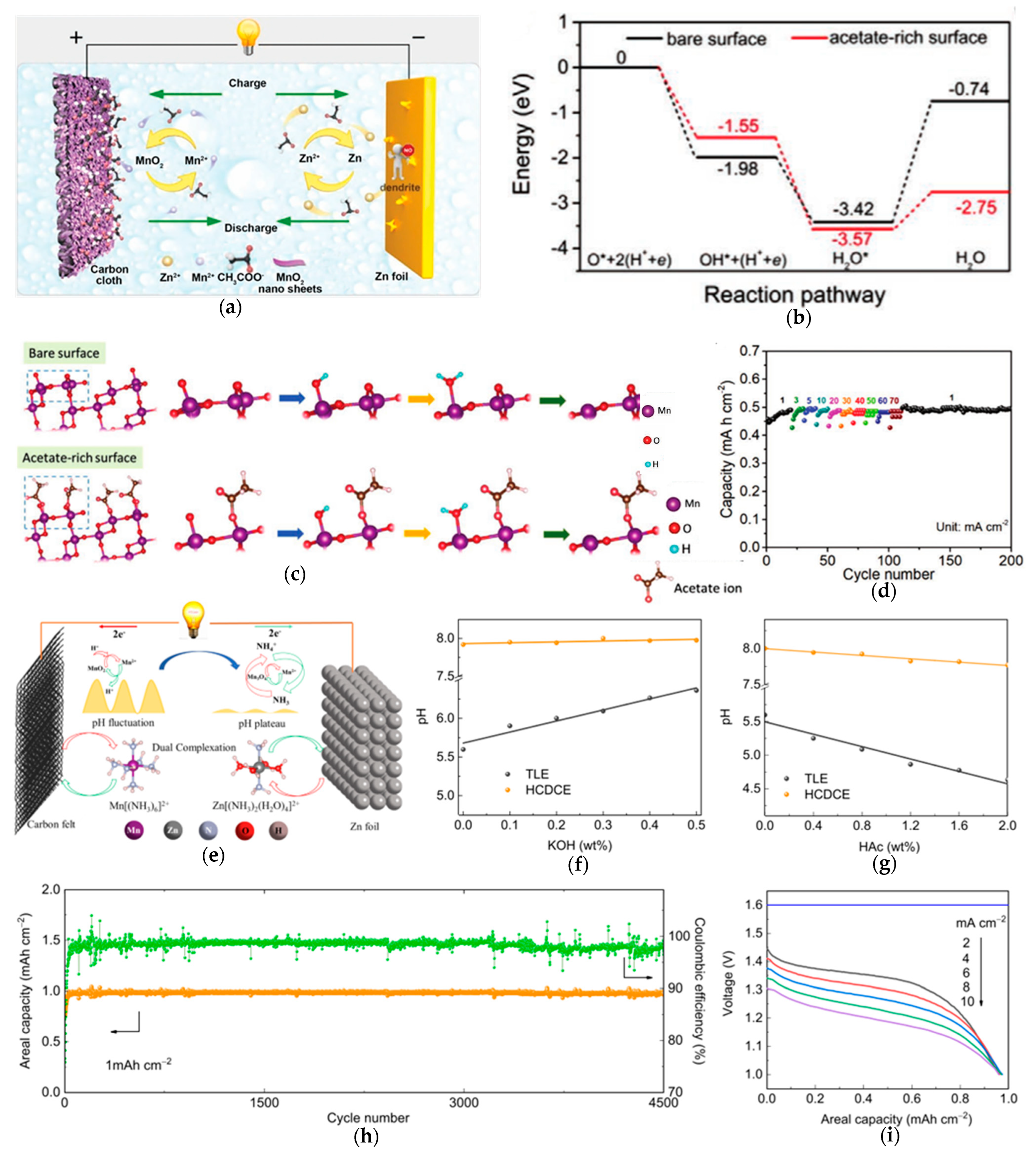
3.4. Hierarchical Surface–Interface Stabilization Strategies
3.5. Electrolyte Optimization in Suppressing Jahn–Teller Effect
4. Summary and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| HRTEM | High-resolution transmission electron microscope |
| SAED | Selected area electron diffraction |
| XRD | Phase analysis of X-ray diffraction |
| PVA | Polyvinyl alcohol |
References
- Liu, Y.; Han, S.-G.; Li, X.; Luo, Y.; Wu, Y.; Lin, X.; Zhu, Q.-L. Manganese dioxide cathode materials for aqueous zinc ion battery: Development, challenges and strategies. EnergyChem 2025, 7, 100152. [Google Scholar] [CrossRef]
- Xu, H.; Yang, W.; Li, M.; Liu, H.; Gong, S.; Zhao, F.; Li, C.; Qi, J.; Wang, H.; Peng, W.; et al. Advances in Aqueous Zinc Ion Batteries based on Conversion Mechanism: Challenges, Strategies, and Prospects. Small 2024, 20, 2310972. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Xu, B.; Zhao, J.; Fu, H. Advanced design for anti-freezing aqueous zinc-ion batteries. Energy Storage Mater. 2024, 70, 103490. [Google Scholar] [CrossRef]
- Yang, J.; Yao, G.; Li, Z.; Zhang, Y.; Wei, L.; Niu, H.; Chen, Q.; Zheng, F. Highly Flexible K-Intercalated MnO2/Carbon Membrane for High-Performance Aqueous Zinc-Ion Battery Cathode. Small 2023, 19, 2205544. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Yin, J.-W.; Liu, B.-C.; Wang, P.-F.; Liu, Z.-L.; Xie, Y.; Shu, J.; Zhang, Q.-Y.; Yi, T.-F. Construction of high-performance aqueous zinc-ion batteries by guest pre-intercalation MnO2-based cathodes. Adv. Colloid Interface Sci. 2025, 341, 103499. [Google Scholar] [CrossRef]
- Yan, L.; Han, Y.; Zhu, C.; Luo, L.; Qin, Y.; Yu, D.; Liu, B.; Zou, X.; Zhou, Y.; Xiang, B. Achieving high-performance aqueous Zn-ion storage through strong hydrogen bonding and charge redistribution by in-situ induced insertion of non-metallic ion clusters. Nano Energy 2024, 122, 109331. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.; Chen, X.; Bao, L.; Peng, J.; Li, X. Engineering d-band center of Mn to strengthen Mn–O bonding for long cycle life zinc-ion battery. J. Mater. Sci. Technol. 2025, 229, 203–212. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, C.; Chen, X.; Lu, Z.; Zhang, K.; Liu, Y.; Wang, J.; Han, G.; Xu, G. Modulating the Structure of Interlayer/Layer Matrix on δ-MnO2 via Cerium Doping-Engineering toward High-Performance Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2024, 14, 2304303. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Zhu, Y.; Du, W.; Liu, W.; Yao, M.; Wang, Y.; Qian, Y.; Feng, S. Unlocking the critical role of Mg doping in α-MnO2 cathode for aqueous zinc ion batteries. Chem. Eng. J. 2024, 485, 150077. [Google Scholar] [CrossRef]
- Choi, S.; Seo, J.K.; Park, H.; Park, J.H.; Kim, S.W.; Shin, S.W.; Bang, J.A.; Jung, K.-N.; Kim, B.G. A Multifunctional Crosslinked F-Free Binder for MnO2 Microparticle Thick Cathode in Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2417179. [Google Scholar] [CrossRef]
- Zhou, Z.; Tong, J.; Zou, X.; Wang, Y.; Bai, Y.; Yang, Y.; Li, Y.; Wang, C.; Liu, S. Ultra-low diffusion barrier tetramethyl ammonium cation-intercalated layered MnO2 for high-performance aqueous zinc-ion batteries. J. Mater. Chem. A 2024, 12, 10923–10931. [Google Scholar] [CrossRef]
- Ma, K.; Ge, S.; Fu, R.; Feng, C.; Zhao, H.; Shen, X.; Liang, G.; Zhao, Y.; Jiao, Q. Uncovering Se, P co-doping effect in MnO2 toward high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2024, 484, 149525. [Google Scholar] [CrossRef]
- Moon, H.; Ha, K.-H.; Park, Y.; Lee, J.; Kwon, M.-S.; Lim, J.; Lee, M.-H.; Kim, D.-H.; Choi, J.H.; Choi, J.-H.; et al. Direct Proof of the Reversible Dissolution/Deposition of Mn2+/Mn4+ for Mild-Acid Zn-MnO2 Batteries with Porous Carbon Interlayers. Adv. Sci. 2021, 8, 2003714. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhao, Y.; Zhao, Y.; Xu, M.; Liu, W.; Sun, X. Mo doping provokes two electron reaction in MnO2 with ultrahigh capacity for aqueous zinc ion batteries. Nano Res. 2023, 16, 2511–2518. [Google Scholar] [CrossRef]
- Ren, Y.; Meng, F.; Zhang, S.; Ping, B.; Li, H.; Yin, B.; Ma, T. CNT@MnO2 composite ink toward a flexible 3D printed micro-zinc-ion battery. Carbon Energy 2022, 4, 446–457. [Google Scholar] [CrossRef]
- Huang, H.; Feng, H.; He, Z.; Huang, Y.; Xu, J.; Hu, C.; Chen, Z.; Yang, Z.; Zhang, W. Enhancing Zn2+/H+ Joint Charge Storage in MnO2: Concurrently Tailoring Mn’s Local Electron Density and O’s p-Band Center. ACS Nano 2024, 18, 25601–25613. [Google Scholar] [CrossRef]
- Yu, X.; Qian, K.; Du, L.; Zhang, J.; Lu, N.; Miao, Z.; Li, Y.; Kobayashi, H.; Yan, X.; Li, R. A strong Jahn–Teller distortion in Mn3O4–MnO heterointerfaces for enhanced silver catalyzed formaldehyde reforming into hydrogen. Sustain. Energy Fuels 2022, 6, 3068–3077. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Sun, L.; Ding, Y.; Yao, Y.; Yao, L.; Drewett, N.E.; Zhang, W.; Tang, J.; Zheng, W. Jahn–Teller Distortion Induced Mn2+-Rich Cathode Enables Optimal Flexible Aqueous High-Voltage Zn-Mn Batteries. Adv. Sci. 2021, 8, 2004995. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhang, W.; Shi, Z.; Feng, Y.; Liu, C.; Fu, H.; Yong, Z.; Li, Q. Hydrogen-bond chemistry inhibits Jahn–Teller distortion caused by Mn 3d orbitals for long-lifespan aqueous Zn//MnO2 batteries. J. Mater. Chem. A 2024, 12, 25491–25503. [Google Scholar] [CrossRef]
- Wei, T.; Ren, Y.; Li, Z.; Zhang, X.; Ji, D.; Hu, L. Bonding interaction regulation in hydrogel electrolyte enable dendrite-free aqueous zinc-ion batteries from −20 to 60 °C. Chem. Eng. J. 2022, 434, 134646. [Google Scholar] [CrossRef]
- Dong, L.; Yang, W.; Yang, W.; Tian, H.; Huang, Y.; Wang, X.; Xu, C.; Wang, C.; Kang, F.; Wang, G. Flexible and conductive scaffold-stabilized zinc metal anodes for ultralong-life zinc-ion batteries and zinc-ion hybrid capacitors. Chem. Eng. J. 2020, 384, 123355. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Liu, R.; Xiao, H.; Liu, Y.; Wang, X.; Huang, Y.; Yuan, G. Molecular tailoring of MnO2 by bismuth doping to achieve aqueous zinc-ion battery with capacitor-level durability. Energy Storage Mater. 2022, 48, 212–222. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Yang, Z.; Zhou, X.; Qiu, D.; Qiu, H.; Huang, X.; Yu, Y. Unraveling the Role of Nitrogen-Doped Carbon Nanowires Incorporated with MnO Nanosheets as High Performance Cathode for Zinc-Ion Batteries. Energy Environ. Mater. 2023, 6, e12378. [Google Scholar] [CrossRef]
- Wang, W.; Black, A.P.; Liu, C.; Martin-Diaconescu, V.; Simonelli, L.; Tonti, D. High performance N-doped carbon nanosheet/MnO2 cathode derived from bacterial cellulose for aqueous Zn-ion batteries. J. Mater. Chem. A 2023, 11, 17272–17281. [Google Scholar] [CrossRef]
- Gou, L.; Li, J.; Liang, K.; Zhao, S.; Li, D.; Fan, X. Bi-MOF Modulating MnO2 Deposition Enables Ultra-Stable Cathode-Free Aqueous Zinc-Ion Batteries. Small 2023, 19, 2208233. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, C.; Yang, F.; Shan, L.; Tang, B.; Zhang, J.; Nan, Q.; Xie, Y.; Li, J.; Li, H.; et al. Spontaneous Interface Layer Engineering of Ag2Mn8O16 Cathode via Anodic Oxidation Strategy toward High-Performance Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2024, 9, 1063–1072. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Xiao, B.; Chen, J.; Yan, W.; Liu, Y.; Hu, C.; Chen, L.; Huang, J.; Peng, S. Interlayer Ag+ and In Situ Ag Metal Particles Synergistically Strengthening MnO2 Cathode Performance for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2023, 6, 1467–1474. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Unlocking Layered Double Hydroxide as a High-Performance Cathode Material for Aqueous Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2204320. [Google Scholar] [CrossRef]
- Peng, H.; Xia, F.; Zhang, C.; Zhuo, H.; Peng, X.; Song, P.; Sun, C.; Wu, J. The Coupling of Local Strain and K+-Ion Release Induced Phase Transition Heterogeneity in Tunnel MnO2. Adv. Funct. Mater. 2022, 32, 2113424. [Google Scholar] [CrossRef]
- Cui, Y.-f.; Zhuang, Z.-b.; Xie, Z.-l.; Cao, R.-f.; Hao, Q.; Zhang, N.; Liu, W.-q.; Zhu, Y.-h.; Huang, G. High-Energy and Long-Lived Zn–MnO2 Battery Enabled by a Hydrophobic-Ion-Conducting Membrane. ACS Nano 2022, 16, 20730–20738. [Google Scholar] [CrossRef]
- Ding, X.; Wen, Y.; Qing, C.; Wei, Y.; Wang, P.; Liu, J.; Peng, Z.; Song, Y.; Chen, H.; Rong, Q. Cr-induced enhancement of structural stability in δ-MnO2 for aqueous Zn-ion batteries. J. Alloys Compd. 2024, 986, 174041. [Google Scholar] [CrossRef]
- Hua, J.; Wu, X.; Xie, F.; Wu, M. Cr-doping δ-MnO2 as the cathode material for aqueous zinc-ion batteries. Surf. Interfaces 2025, 62, 106312. [Google Scholar] [CrossRef]
- Li, X.; He, D.; Zhou, Q.; Zhou, X.; Wang, Z.; Wei, C.; Shi, Y.; Hu, X.; Huang, B.; Yang, Z.; et al. Deciphering anomalous zinc ion storage in intermediate-state MnO2 during layer-to-tunnel structural transition. Energy Environ. Sci. 2024, 17, 9195–9204. [Google Scholar] [CrossRef]
- Lv, X.; Wang, Y.; Zhang, W.; Wang, T. High valence MnO2 as an aqueous zinc ion battery cathode prepared using a secondary hydrothermal method. Dalton Trans. 2024, 54, 1182–1190. [Google Scholar] [CrossRef]
- Wang, H.; Guo, R.; Ma, Y.; Zhou, F. Cross-Doped Mn/Mo Oxides with Core-Shell Structures Designed by a Self-Template Strategy for Durable Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2301351. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Du, X.; Zhang, M.; Cui, X.; Wang, Q.; Wang, H. CTAB controlled electrochemical deposition of manganese dioxide with enhanced performance in aqueous Zn-ion battery. Mater. Sci. Eng. B 2022, 282, 115777. [Google Scholar] [CrossRef]
- Qiaohui, D.; Yao, W.; Shuyu, D.; Denis, Y.W.Y. Facile electrode additive stabilizes structure of electrolytic MnO2 for mild aqueous rechargeable zinc-ion battery. J. Power Sources 2022, 528, 231194. [Google Scholar] [CrossRef]
- Carbery, W.P.; Farfan, C.A.; Ulbricht, R.; Turner, D.B. The phonon-modulated Jahn–Teller distortion of the nitrogen vacancy center in diamond. Nat. Commun. 2024, 15, 8646. [Google Scholar] [CrossRef]
- Wang, P.-F.; Jin, T.; Zhang, J.; Wang, Q.-C.; Ji, X.; Cui, C.; Piao, N.; Liu, S.; Xu, J.; Yang, X.-Q.; et al. Elucidation of the Jahn-Teller effect in a pair of sodium isomer. Nano Energy 2020, 77, 105167. [Google Scholar] [CrossRef]
- Waters, M.D.J.; Ng, Z.X.; Monahan, N.R.; Wörner, H.J. Ultrafast Imaging of the Jahn–Teller Topography in Carbon Tetrachloride. J. Am. Chem. Soc. 2023, 145, 7659–7666. [Google Scholar] [CrossRef]
- Miñarro, A.S.; Villa, M.; Casals, B.; Plana-Ruiz, S.; Sánchez, F.; Gázquez, J.; Herranz, G. Spin-orbit entanglement driven by the Jahn-Teller effect. Nat. Commun. 2024, 15, 8694. [Google Scholar] [CrossRef]
- Streltsov, S.V.; Khomskii, D.I. Jahn-Teller Effect and Spin-Orbit Coupling: Friends or Foes? Phys. Rev. X 2020, 10, 031043. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, H.; Li, J.; Hui, K.S.; Yu, Z.; Xu, H.; Dinh, D.A.; Ye, Z.; Zha, C.; Hui, K.N. Insights into the Jahn-Teller Effect in Layered Oxide Cathode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2024, 14, 2400461. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Zhao, X.; Hu, Y.; Li, T.; Liu, H.; Zhong, Y.; Jiao, L.; Liu, Y.; Chen, J. Intrinsic Distortion against Jahn-Teller Distortion: A New Paradigm for High-Stability Na-Ion Layered Mn-Rich Oxide Cathodes. Angew. Chem. Int. Ed. 2025, 64, e202423728. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Hada, M.; Ota, W.; Uesugi, F.; Sato, T. Localised surface plasmon resonance inducing cooperative Jahn–Teller effect for crystal phase-change in a nanocrystal. Nat. Commun. 2023, 14, 4471. [Google Scholar] [CrossRef] [PubMed]
- Drosou, M.; Zahariou, G.; Pantazis, D.A. Orientational Jahn–Teller Isomerism in the Dark-Stable State of Nature’s Water Oxidase. Angew. Chem. Int. Ed. 2021, 60, 13493–13499. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Zhang, J.; Sheng, J.; Yang, F.; Wang, M.; Ni, J.; Jiang, H.; Li, Y. Jahn-Teller Effect Directed Bandgap Tuning of Birnessite for Pseudocapacitive Application. Energy Environ. Mater. 2022, 6, e12382. [Google Scholar] [CrossRef]
- Rui-Jie, L.; Xun-Lu, L.; Jie-Ying, D.; Jian, B.; Cui, M.; Chong-Yu, D.; Xin-Yin, C.; Xiao-Jing, W.; Yong-Ning, Z. Suppressing Jahn-Teller distortion and phase transition of K0.5MnO2 by K-site Mg substitution for potassium-ion batteries. Energy Storage Mater. 2022, 47, 408–414. [Google Scholar] [CrossRef]
- Zuo, C.; Hu, Z.; Qi, R.; Liu, J.; Li, Z.; Lu, J.; Dong, C.; Yang, K.; Huang, W.; Chen, C.; et al. Double the Capacity of Manganese Spinel for Lithium-Ion Storage by Suppression of Cooperative Jahn–Teller Distortion. Adv. Energy Mater. 2020, 10, 2000363. [Google Scholar] [CrossRef]
- Zuo, C.; Xiong, F.; Shao, Y.; Li, M.; Zhu, D.; Zhu, J.; Wang, J.; An, Q. Mitigating Jahn–Teller effect of MnO2 via charge regulation of Mn-local environment for advanced calcium storage. Energy Storage Mater. 2024, 72, 103763. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Li, H.; Dong, H.; Yan, C.; Meng, C.; Sang, Y.; Liu, H.; Guo, Y.-G.; Wang, S. Dynamic heterostructure design of MnO2 for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2024, 17, 3629–3640. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, J.; Chen, X.; Yu, X.; Li, L.; Bao, L.; Peng, J.; Li, X. Achieving ultra-long cycling life for MnO2 cathode: Modulating Mn3+ spin state to suppress Jahn–Teller distortion and manganese dissolution. Energy Storage Mater. 2025, 76, 104128. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, X.; Wang, R.; Jiao, Y.; Shu, Z.; Shan, S.; Bian, Y.; Lin, H.; Chen, J.; Xu, Y. Mn-O bond Engineering Mitigating Jahn-Teller Effects of Manganese Oxide for Aqueous Zinc-ion Battery Applications. Chem. Eng. J. 2024, 494, 152908. [Google Scholar] [CrossRef]
- Yang, B.; Li, K.; Yang, C.; Zhang, Y.; Gong, Y. Orbital hybridization and electron transfer triggered by V-dopant and symmetry breakage to stabilize low-valent Mn for zinc-ion batteries. Chem. Eng. J. 2025, 513, 163032. [Google Scholar] [CrossRef]
- Dai, Q.; Li, L.; Tu, T.; Zhang, M.; Song, L. An appropriate Zn2+/Mn2+ concentration of the electrolyte enables superior performance of AZIBs. J. Mater. Chem. A 2022, 10, 23722–23730. [Google Scholar] [CrossRef]
- Han, M.; Qin, L.; Liu, Z.; Zhang, L.; Li, X.; Lu, B.; Huang, J.; Liang, S.; Zhou, J. Reaction mechanisms and optimization strategies of manganese-based materials for aqueous zinc batteries. Mater. Today Energy 2020, 20, 100626. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Duan, Q.; Lee, P.-K.; Wang, S.; Yu, D.Y.W. Engineering cathode-electrolyte interface of graphite to enable ultra long-cycle and high-power dual-ion batteries. J. Power Sources 2020, 471, 228466. [Google Scholar] [CrossRef]
- Li, X.; Rong, H.; Zhang, J.; Wang, D.; Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Liang, S.; Shan, L.; Xie, X.; Yi, Z.; Wang, Y.; Guo, S.; Zhou, J. Oxygen Defects in β-MnO2 Enabling High-Performance Rechargeable Aqueous Zinc/Manganese Dioxide Battery. Iscience 2020, 23, 100797. [Google Scholar] [CrossRef]
- Qu, W.; Cai, Y.; Chen, B.; Zhang, M. Heterointerface Engineering-Induced Oxygen Defects for the Manganese Dissolution Inhibition in Aqueous Zinc Ion Batteries. Energy Environ. Mater. 2024, 7, e12645. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Zhang, Y.; Liang, J.; Ren, L.; Fan, H.J.; Liu, W.; Sun, X. Vacancy-rich Al-doped MnO2 cathodes break the trade-off between kinetics and stability for high-performance aqueous Zn-ion batteries. Energy Environ. Sci. 2024, 17, 1279–1290. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene Scroll-Coated α-MnO2 Nanowires as High-Performance Cathode Materials for Aqueous Zn-Ion Battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Ye, J.-J.; Li, P.-H.; Hou, Z.; Zhang, W.; Zhu, W.; Jin, S.; Ji, H. Se-dopant Modulated Selective Co-Insertion of H+ and Zn2+ in MnO2 for High-Capacity and Durable Aqueous Zn-Ion Batteries. Angew. Chem. Int. Ed. 2024, 63, e202410900. [Google Scholar] [CrossRef]
- Cai, K.; Luo, S.-h.; Qian, L.; Meng, X.; Yan, S.-x.; Guo, J.; Wang, Q.; Ji, X.-b.; Zhou, X.-y. Three-dimensional porous composite Mn2O3@PPy as cathode material for zinc ion battery with high energy density. J. Power Sources 2023, 564, 232854. [Google Scholar] [CrossRef]
- Liu, L.; Ding, S.; Yao, L.; Liu, M.; Li, S.; Zhao, Q.; Qin, R.; Pan, F. In situ BaSO4 coating enabled activation-free and ultra-stable δ-MnO2 for aqueous Zn-ion batteries. Chem. Commun. 2023, 59, 6227–6230. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Zhao, Z.; Zhang, H.; Hu, X.; Lu, B.; Liang, S.; Zhou, J. In-situ positive electrode-electrolyte interphase construction enables stable Ah-level Zn-MnO2 batteries. Nat. Commun. 2025, 16, 2194. [Google Scholar] [CrossRef]
- Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C. Al-Intercalated MnO2 cathode with reversible phase transition for aqueous Zn-Ion batteries. Chem. Eng. J. 2021, 422, 130375. [Google Scholar] [CrossRef]
- Liu, X.; Yang, F.; Xu, W.; Zeng, Y.; He, J.; Lu, X. Zeolitic Imidazolate Frameworks as Zn2+ Modulation Layers to Enable Dendrite-Free Zn Anodes. Adv. Sci. 2020, 7, 2002173. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cai, C.; Ma, L.; Wang, J.; Mercer, M.P.; Liu, J.; Kramer, D.; Yu, X.; Xue, D.; Zhi, C.; et al. Tailoring MnO2 Cathode Interface via Organic–Inorganic Hybridization Engineering for Ultra-Stable Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2025, 15, 2402819. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Zhou, X.; Liu, Y. Amorphous aluminum-doped manganese oxide cathode with strengthened performance for aqueous zinc-ion batteries. J. Alloys Compd. 2025, 1012, 178502. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Lee, W.S.V.; Xue, J. Defect Engineering in Manganese-Based Oxides for Aqueous Rechargeable Zinc-Ion Batteries: A Review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Fu, W.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Plasma-induced ε-MnO2 based aqueous zinc-ion batteries and their dissolution-deposition mechanism. J. Mater. Sci. Technol. 2022, 127, 206–213. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Qiao, R.; Dai, X.; Jing, W.; Song, J.; Chen, Y.; Guo, S.; Sun, J.; Tan, Q.; et al. Binder-free flexible zinc-ion batteries: One-step potentiostatic electrodeposition strategy derived Ce doped-MnO2 cathode. Chem. Eng. J. 2022, 431, 133387. [Google Scholar] [CrossRef]
- Dong, H.; Liu, R.; Hu, X.; Zhao, F.; Kang, L.; Liu, L.; Li, J.; Tan, Y.; Zhou, Y.; Brett, D.J.L.; et al. Cathode–Electrolyte Interface Modification by Binder Engineering for High-Performance Aqueous Zinc-Ion Batteries. Adv. Sci. 2023, 10, 2205084. [Google Scholar] [CrossRef]
- Li, J.; Ma, D.; Zhang, Q.; Cao, Y.; Wang, J.; Wang, T.; Liu, N.; Ma, C.; Zhang, T.; Zhao, Q.; et al. Optimized integration of intercalation and conversion behaviors for Cu2O/MnO2 hybrid cathodes of zinc ion batteries. J. Energy Chem. 2025, 107, 212–220. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, P.; Yang, H.; Min, H.; Niu, M.; Jin, S.; Jiang, Y.; Pan, Z.; Yan, J.; Shen, X.; et al. Manipulating intercalation-extraction mechanisms in structurally modulated δ-MnO2 nanowires for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133687. [Google Scholar] [CrossRef]
- Guo, C.; Tian, S.; Chen, B.; Liu, H.; Li, J. Constructing α-MnO2@PPy core-shell nanorods towards enhancing electrochemical behaviors in aqueous zinc ion battery. Mater. Lett. 2020, 262, 127180. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Wang, J.-Y.; Wang, P.; Liu, Z.; Zhu, Y.-R.; Shu, J.; Yi, T.-F. Construction of high-voltage aqueous Zn-MnO2 batteries based on polar small-molecule organic acid-induced MnO2/Mn2+ reactions. Chem. Eng. J. 2025, 507, 160415. [Google Scholar] [CrossRef]
- Liang, X.; Liu, X.; Wang, P.; Guo, Z.; Chen, X.; Yao, J.; Li, J.; Gan, Y.; Lv, L.; Tao, L.; et al. Ion-exchange induced Ni doping of α-MnO2 cathode with structural modification for aqueous zinc ion batteries. J. Power Sources 2025, 635, 236518. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Ding, Y.; Xue, W.; Chen, K.; Yang, C.; Feng, Q.; Zheng, D.; Xu, W.; Wang, F.; Lu, X. Sodium Ion Pre-Intercalation of δ-MnO2 Nanosheets for High Energy Density Aqueous Zinc-Ion Batteries. Nanomaterials 2023, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Kang, L.; Berry-Gair, J.; McColl, K.; Li, J.; Dong, H.; Jiang, H.; Wang, R.; Corà, F.; Brett, D.J.L.; et al. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J. Mater. Chem. A 2020, 8, 22075–22082. [Google Scholar] [CrossRef]
- Wu, H.; Yan, C.; Xu, L.; Xu, N.; Wu, X.; Jiang, Z.; Zhu, S.; Diao, G.; Chen, M. Super Flexible Cathode Material with 3D Cross-Linking System Based on Polyvinyl Alcohol Hydrogel for Boosting Aqueous Zinc Ion Batteries. ChemElectroChem 2022, 9, e202200288. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, R.; Wang, Y.; Yue, J.; Yang, J.; Wang, X.; Wu, C.; Bai, Y. Hybrid Superlattice-Triggered Selective Proton Grotthuss Intercalation in δ-MnO2 for High-Performance Zinc-Ion Battery. Angew. Chem. Int. Ed. 2023, 62, e202313163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xu, X.; Liu, Y.; Tan, L.; Zhou, F.; Xu, Z.; Hu, R. Facile plasma treated β-MnO2@C hybrids for durable cycling cathodes in aqueous Zn-ion batteries. J. Alloys Compd. 2020, 827, 154273. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, J.; Mao, J.; Hao, J.; Wang, Z.; Zhou, S.; Ling, C.D.; Guo, Z. Toward a Reversible Mn4+/Mn2+ Redox Reaction and Dendrite-Free Zn Anode in Near-Neutral Aqueous Zn/MnO2 Batteries via Salt Anion Chemistry. Adv. Energy Mater. 2020, 10, 1904163. [Google Scholar] [CrossRef]
- Huang, J.; Chi, X.; Wu, J.; Liu, J.; Liu, Y. High-concentration dual-complex electrolyte enabled a neutral aqueous zinc-manganese electrolytic battery with superior stability. Chem. Eng. J. 2022, 430, 133058. [Google Scholar] [CrossRef]



| Cathode | Specific Capacity | Cycling Performance | Dissolved Mn2+ | Refs. |
|---|---|---|---|---|
| δ-MnO2 | 125 mAh g−1 at 0.2 A g−1 | 14.3% after 200 cycles at 1 A g−1 | 2.5 mg L−1 after 50 cycles | [61] |
| α-MnO2/MGS | 382.2 mAh g−1 at 0.3 A g−1 | 94% after 3000 cycles at 3 A g−1 | 0.42 mg L−1 after 1 cycle | [62] |
| Se-MnO2 | 386 mAh g−1 at 0.1 A g−1 | 78% after 5000 cycles at 3 A g−1 | 0.71 mg L−1 after 300 cycles | [63] |
| Al-MnO2 | 379 mAh g−1 at 0.2 A g−1 | 87% after 1000 cycles at 1 A g−1 | 0.12 mg L−1 after 50 cycles | [61] |
| δa-MnO2 | 175 mAh g−1 at 0.5 A g−1 | 91% after 500 cycles at 1 A g−1 | 0.54 mg L−1 after 100 cycles | [60] |
| Mn2O3@PPy | 353.9 mAh g−1 at 0.5 A g−1 | 82% after 500 cycles at 1 A g−1 | 0.27 mg L−1 after 200 cycles | [64] |
| BMO | 348 mAh g−1 at 0.1 A g−1 | 60% after 2000 cycles at 1 A g−1 | 0.015 mg L−1 after 100 cycles | [65] |
| δ-MnO2 (ZS-DOP electrolyte) | 160 mAh g−1 at 1 A g−1 | 80% after 70 cycles at 7.5 mA g−1 | 1.2 mg L−1 after 100 cycles | [66] |
| AMO | 400 mAh g−1 at 0.1 A g−1 | 94.5% after 2000 cycles at 2 A g−1 | 0.25 mg L−1 after 300 cycles | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Huang, M.; Song, M.; Zhou, W.; Tan, H. Suppressing Jahn–Teller Distortion in Manganese Oxides for High-Performance Aqueous Zinc-Ion Batteries. Materials 2025, 18, 2817. https://doi.org/10.3390/ma18122817
Duan J, Huang M, Song M, Zhou W, Tan H. Suppressing Jahn–Teller Distortion in Manganese Oxides for High-Performance Aqueous Zinc-Ion Batteries. Materials. 2025; 18(12):2817. https://doi.org/10.3390/ma18122817
Chicago/Turabian StyleDuan, Jiangfeng, Man Huang, Ming Song, Weijia Zhou, and Hua Tan. 2025. "Suppressing Jahn–Teller Distortion in Manganese Oxides for High-Performance Aqueous Zinc-Ion Batteries" Materials 18, no. 12: 2817. https://doi.org/10.3390/ma18122817
APA StyleDuan, J., Huang, M., Song, M., Zhou, W., & Tan, H. (2025). Suppressing Jahn–Teller Distortion in Manganese Oxides for High-Performance Aqueous Zinc-Ion Batteries. Materials, 18(12), 2817. https://doi.org/10.3390/ma18122817





