Synergistic Enhancement of Physicomechanical Performance and Microstructural Integrity in Hydrothermally Synthesized Autoclaved Lightweight Aggregates Through Quicklime–Fly Ash Blends
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
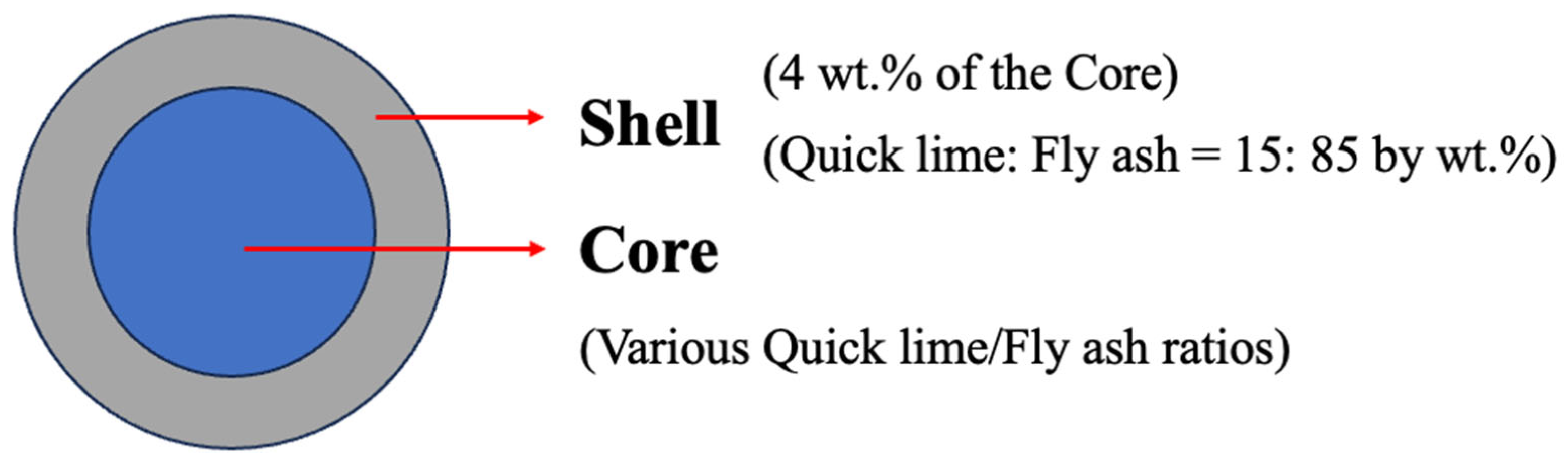
2.2. Sample Preparation
2.3. Testing
3. Results and Discussion
3.1. Characterization of Raw Materials
3.2. Physicomechanical Properties of Fly Ash Aggregates
3.2.1. Water Absorption
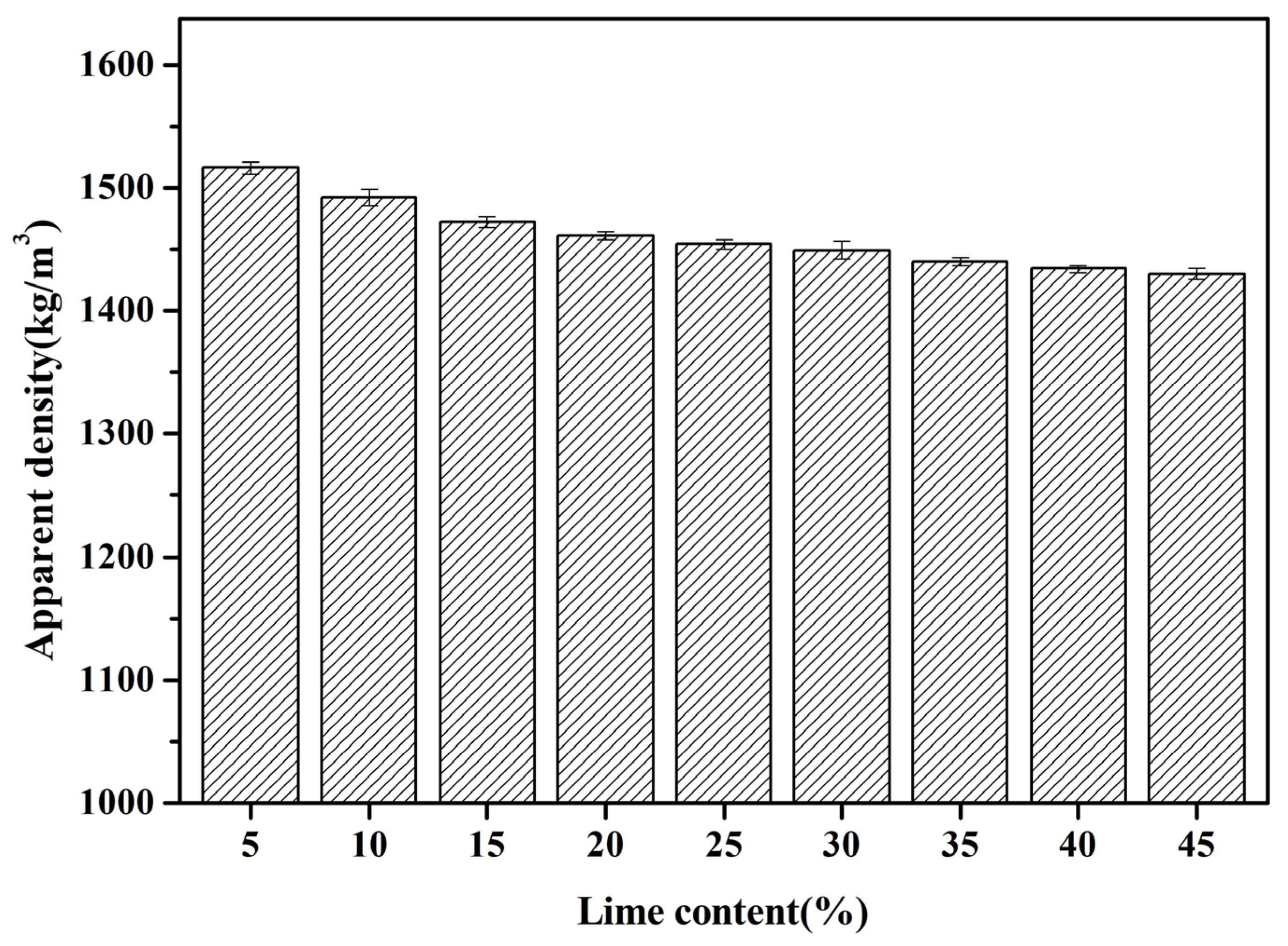
3.2.2. Apparent Density
3.2.3. Cylinder Compressive Strength
3.3. Micro-Structure Analysis
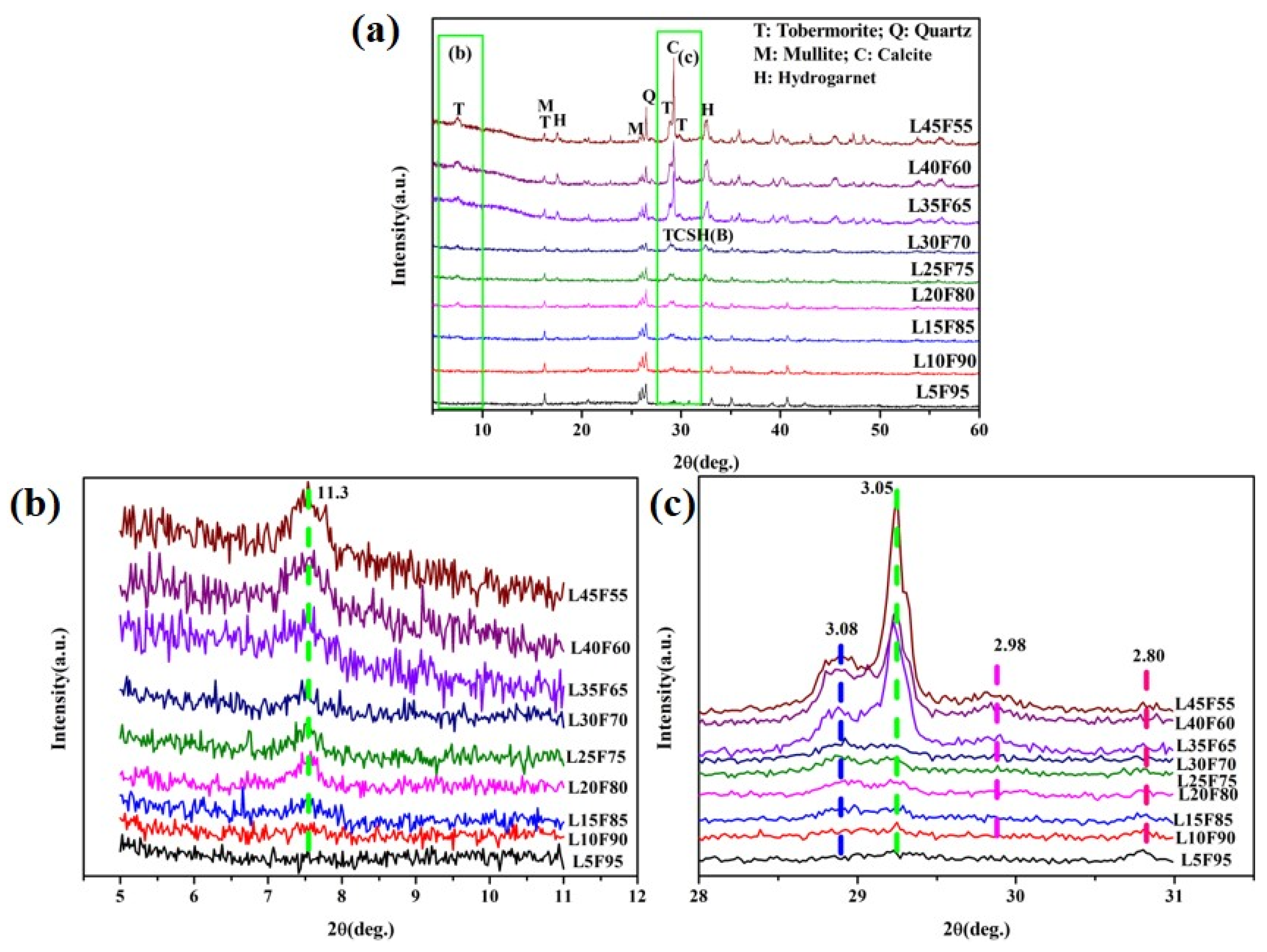
3.3.1. XRD Analysis
3.3.2. Insoluble Matter and Loss on Ignition
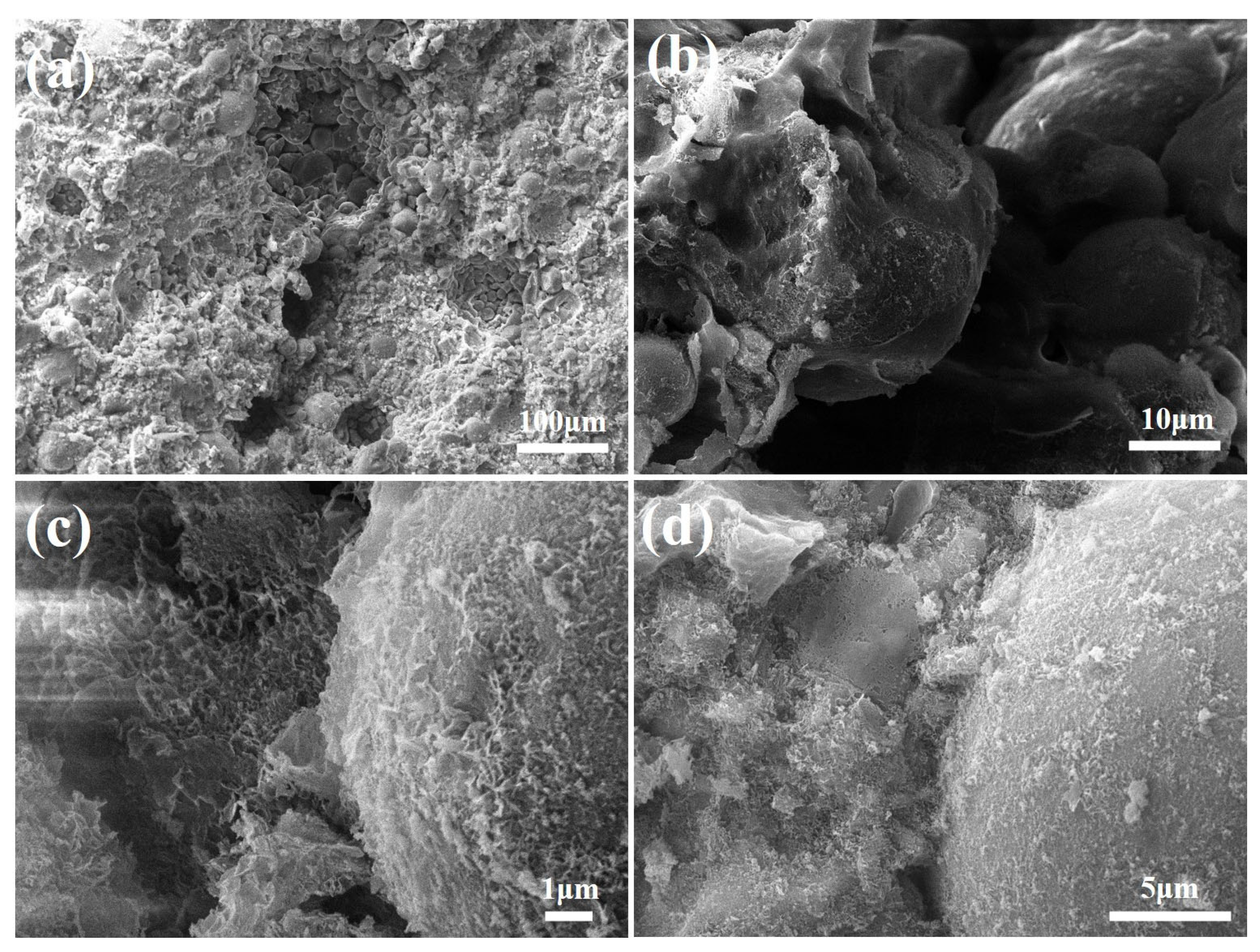
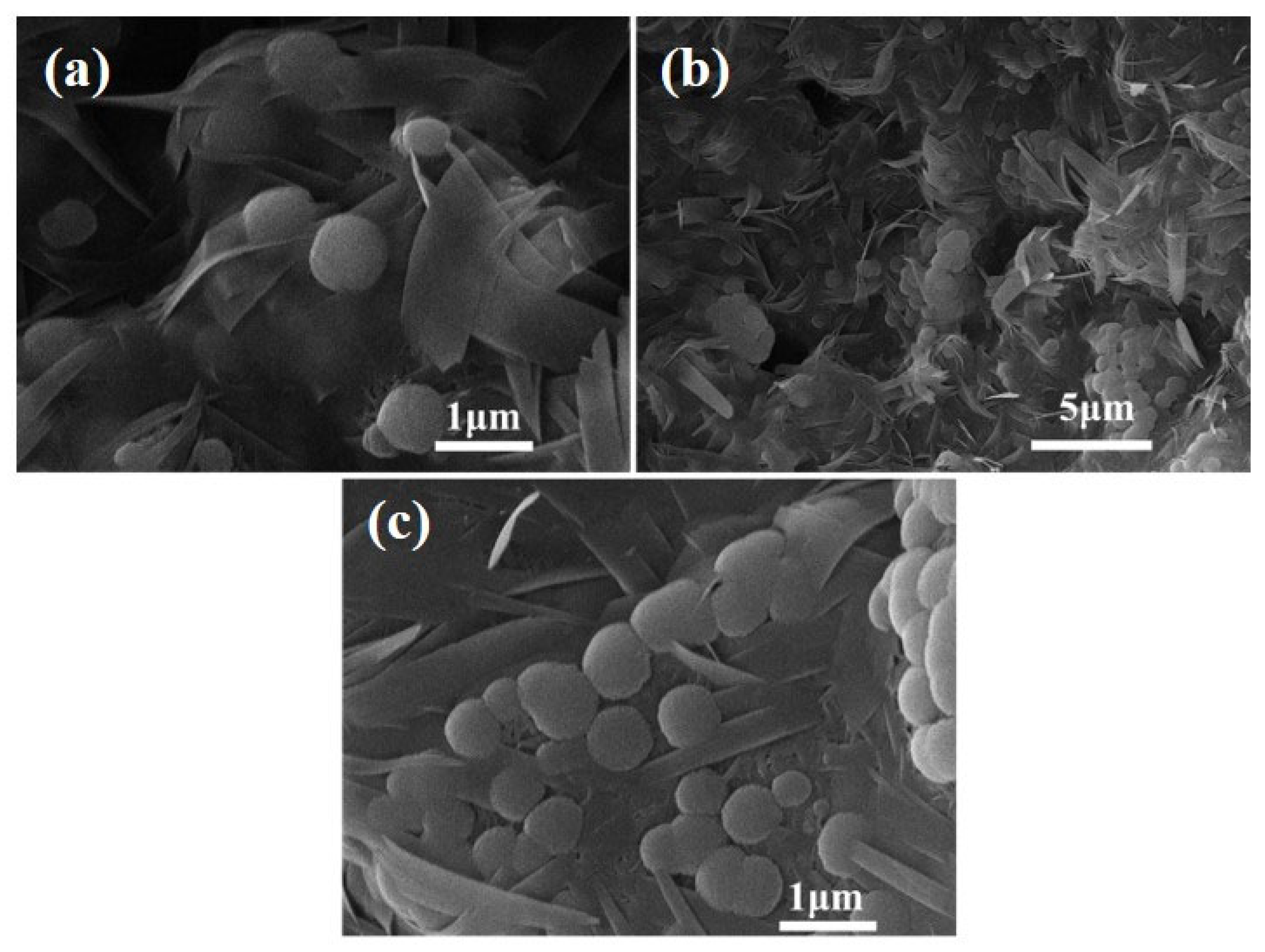
3.3.3. SEM Analysis
3.3.4. The Mechanism of Strength Development in FAAs
4. Conclusions
- (1)
- As the quicklime content increases from 5 wt.% to 25 wt.%, the enhancement in the filling effect of hydration products contributes to a gradual elevation in the cylinder compressive strength of the FAAs. However, beyond this threshold, specifically within the range of 25 wt.% to 45 wt.% quicklime content, a decrement in cylinder compressive strength is observed due to the diminishing micro-aggregate effect. The optimal balance between the filling effect and the micro-aggregate effect is achieved at a quicklime content of 25 wt.%, resulting in a peak cylinder compressive strength of 13 MPa.
- (2)
- Despite the augmentation in hydration product content with increasing quicklime addition, which positively impacts the filling effect, the resultant surge in water consumption during the granulation process leads to a reduction in initial defects within the FAAs. This phenomenon, in turn, results in a decrease in apparent density and a progressive increase in water absorption.
- (3)
- The water absorption capacity of the FAAs, measured after 1 h, falls within the range of 14.1% to 23.98%, while the 24 h absorption varies between 20.58% and 26.37%. The apparent density, ranging from 1430 kg/m3 to 1516 kg/m3, aligns with the lightweight material criteria. Furthermore, when the quicklime content is within the range of 10 wt.% to 45 wt.%, the FAAs satisfy the specifications for high-strength lightweight aggregates, as outlined in the national standards.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Hu, H.; Wang, X.; Liu, H.; Dong, L.; Luo, G.; Zhao, Y.; Yao, H. A critical review on lead migration, transformation and emission control in Chinese coal-fired power plants. J. Environ. Sci. 2023, 124, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Li, W.; Nie, Y.; Wang, F.; Bian, R.; Wang, H.; Wang, Y.; Gong, Z.; Lu, J.; et al. Solidification/stabilization pre-treatment coupled with landfill disposal of heavy metals in MSWI fly ash in China: A systematic review. J. Hazard. Mater. 2024, 478, 135479. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-F.; Quan, C.-Q.; Jiao, C.-J. Experimental study of chloride resistance of polypropylene fiber reinforced concrete with fly ash and modeling. Materials 2021, 14, 4417. [Google Scholar] [CrossRef]
- Hou, H.; Su, L.; Guo, D.; Xu, H. Resource utilization of solid waste for the collaborative reduction of pollution and carbon emissions: Case study of fly ash. J. Clean. Prod. 2023, 383, 135449. [Google Scholar] [CrossRef]
- Shirin, S.; Jamal, A.; Emmanouil, C.; Singh, V.P.; Yadav, A.K. Assessment and characterization of waste material used as backfilling in an abandoned mine. Int. J. Coal Prep. Util. 2023, 43, 1402–1410. [Google Scholar] [CrossRef]
- Kumar, P.; Dinakar, P.; Saravanan, T.J. Development and Mechanical Performance of Self-Compacting Lightweight Aggregate Concrete Using Sintered Fly Ash Aggregates. J. Mater. Civ. Eng. 2024, 36, 04024174. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Qiao, Z.; Deng, H.; Xu, L.; Wu, K.; Yang, Z.; Tang, L. The toxic leaching behavior of MSWI fly ash made green and non-sintered lightweight aggregates. Constr. Build. Mater. 2023, 373, 130809. [Google Scholar] [CrossRef]
- Geng, J.; Niu, S.; Han, K.; Wang, Y.; Zhu, J.; Yang, Z.; Liu, J.; Zhang, H.; Sun, X.; Liang, B.; et al. Properties of artificial lightweight aggregates prepared from coal and biomass co-fired fly ashes and sewage sludge fly ash. Ceram. Int. 2024, 50, 28609–28618. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Luo, H.; Wang, H.; Chen, L.; Liu, M.; Tang, M.; He, B.-J. Preparation and properties of alkali-activated red mud-based artificial lightweight aggregates. Constr. Build. Mater. 2024, 449, 138304. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, F.; Khushnood, R.A.; Amjad, H.; Ahmad, F. Feasibility Study of Expanded Clay Aggregate Lightweight Concrete for Nonstructural Applications. Adv. Civ. Eng. 2024, 2024, 8263261. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, R.; Zhang, P.; Cui, Y.; Xue, S.; Song, Q.; Ma, Y. Thermal resistance, water absorption and microstructure of high-strength self-compacting lightweight aggregate concrete (HSSC-LWAC) after exposure to elevated temperatures. Constr. Build. Mater. 2023, 365, 130071. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Qiao, Z.; Xu, L.; Wu, K.; Li, P.; Yang, Z. Effect of curing time and temperature on the mechanical properties of green and ultra-high-strength non-sintered aggregate via autoclave technology. Constr. Build. Mater. 2023, 374, 130874. [Google Scholar] [CrossRef]
- Lu, J.-X. Recent advances in high strength lightweight concrete: From development strategies to practical applications. Constr. Build. Mater. 2023, 400, 132905. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Yang, F.; Zhang, W.; Xu, L.; Wu, K.; Tang, L.; Yang, Z. Resourceful utilization of quarry tailings in the preparation of non-sintered high-strength lightweight aggregates. Constr. Build. Mater. 2022, 334, 127444. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Cai, J.; Tian, Q.; Liu, R.; Zhang, J.; Du, Z. Comparison of waste glass and fly ash as silica sources for autoclaved materials. Constr. Build. Mater. 2024, 455, 139154. [Google Scholar] [CrossRef]
- Liao, S.-K.; Lu, W.-C.; Chen, Y.-L.; Shen, Y.-H. Production of autoclaved aerated concrete by using municipal solid waste incinerator fly ash as an alternative raw material. Case Stud. Constr. Mater. 2024, 21, e03914. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Jiao, C.-J.; Zhang, X.-C.; Yang, Y.; Chen, X.-F. Study on the microstructure of recycled aggregate concrete strengthened by the nano-SiO2 soaking method. Structures 2023, 58, 105388. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. Microstructure and physical properties of concrete containing recycled aggregates pre-treated by a nano-silica soaking method. J. Build. Eng. 2022, 51, 104363. [Google Scholar] [CrossRef]
- Lu, C.-G.; Zhang, X.-C.; Chen, X.-F. Maximizing Nano-Silica Efficiency in Laboratory-Simulated Recycled Concrete Aggregate via Prior Accelerated Carbonation: An Effective Strategy to Up-Cycle Construction Wastes. Molecules 2024, 29, 5995. [Google Scholar] [CrossRef]
- Ma, H.; Cui, C.; Li, X.; Sun, Z. Study of high performance autoclaved shell-aggregate from propylene oxide sludge. Constr. Build. Mater. 2011, 25, 3030–3037. [Google Scholar] [CrossRef]
- GB/T 17431.2; Lightweight Aggregates and Its Test Methods—Part 2: Test Methods for Lightweight Aggregates. National Standards of People’s Republic of China: Beijing, China, 2010.
- Ulusoy, U. A Review of Particle Shape Effects on Material Properties for Various Engineering Applications: From Macro to Nanoscale. Minerals 2023, 13, 91. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, J.; Ma, P.; Meng, H.; Cheng, F.; Luo, J. Influence of quick lime on pore characteristics of high-temperature zone in iron ore sinter based on XCT technology. J. Mater. Res. Technol. 2021, 15, 4475–4486. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, H.; Zhang, J.; Li, W.; Liu, J.; Liu, B.; Zhang, S. High strength porous ceramics and its potential in adsorption and building materials: A short process to co-disposal secondary aluminum dross and quicklime. Constr. Build. Mater. 2023, 395, 132292. [Google Scholar] [CrossRef]
- Jia, Y.; Ding, M.; Lin, Y.; Wang, L.; Wei, Y.; Han, S. Effect of pozzolanic slurry coating containing nano-alumina on macro-micro properties of recycled brick aggregate. Constr. Build. Mater. 2025, 459, 139752. [Google Scholar] [CrossRef]
- GB/T 17431.1; Lightweight Aggregates and Its Test Methods—Part 1: Lightweight Aggregates. National Standards of People’s Republic of China: Beijing, China, 2010.
- Ra, J.; Shin, S.; Kim, J. Experimental Study on the Properties of Autoclave Curing High-Strength Concrete According to CaO/SiO2 Ratio. Appl. Sci. 2023, 13, 6190. [Google Scholar] [CrossRef]
- Long, Y.; Pu, K.; Yang, Y.; Huang, H.; Fang, H.; Shen, D.; Geng, H.; Ruan, J.; Gu, F. Preparation of High-strength ceramsite from municipal solid waste incineration fly ash and clay based on CaO-SiO2-Al2O3 system. Constr. Build. Mater. 2023, 368, 130492. [Google Scholar] [CrossRef]
- Kamseu, E.; Alzari, V.; Rosa, R.; Nuvoli, D.; Sanna, D.; Mariani, A.; Leonelli, C. Marble wastes recycling: Design and synthesis of low-temperature calcium silicate hydrate under various CaO:SiO2 ratio and alkalinity. Materialia 2021, 20, 101224. [Google Scholar] [CrossRef]
- Chen, X.-F.; Jiao, C.-J. Effect of construction wastes on the rheo-physical behavior of photocatalytic mortar. Case Stud. Constr. Mater. 2022, 16, e01049. [Google Scholar] [CrossRef]
- Alaskar, A.; Alshannag, M.; Higazey, M. Mechanical properties and durability of high-performance concrete internally cured using lightweight aggregates. Constr. Build. Mater. 2021, 288, 122998. [Google Scholar] [CrossRef]
- Roufael, G.; Beaucour, A.-L.; Eslami, J.; Hoxha, D.; Noumowé, A. Influence of lightweight aggregates on the physical and mechanical residual properties of concrete subjected to high temperatures. Constr. Build. Mater. 2021, 268, 121221. [Google Scholar] [CrossRef]







| Chemical Composition | Fly Ash (%) | Cement (%) |
|---|---|---|
| SiO₂ | 49.7 | 21.3 |
| Al₂O₃ | 32 | 5.1 |
| Fe₂O₃ | 5.6 | 3.2 |
| CaO | 7.2 | 63.5 |
| MgO | 2.1 | 3.1 |
| K₂O | 1.4 | 1.2 |
| Na₂O | 0.3 | 0.4 |
| SO₃ | 0.2 | 1.3 |
| LOI | 1.5 | 0.9 |
| Notation | Core (%) | Shell (%) | Water (%) | ||
|---|---|---|---|---|---|
| Quicklime | Fly Ash | Quicklime | Fly Ash | ||
| L5F95 | 5 | 95 | 15 | 85 | 35.15 |
| L10F90 | 10 | 90 | 15 | 85 | 41.80 |
| L15F85 | 15 | 85 | 15 | 85 | 42.55 |
| L20F80 | 20 | 80 | 15 | 85 | 45.55 |
| L25F75 | 25 | 75 | 15 | 85 | 48.15 |
| L30F70 | 30 | 70 | 15 | 85 | 52.05 |
| L35F65 | 35 | 65 | 15 | 85 | 57.75 |
| L40F60 | 40 | 60 | 15 | 85 | 58.15 |
| L45F95 | 45 | 55 | 15 | 85 | 68.40 |
| Notation | CaO/SiO2 a | R (wt.%) | CaO/SiO2 b |
|---|---|---|---|
| L5F95 | 0.15 | 70.94 | 0.53 |
| L10F90 | 0.26 | 63.83 | 0.72 |
| L15F85 | 0.38 | 48.71 | 0.74 |
| L20F80 | 0.51 | 45.72 | 0.94 |
| L25F75 | 0.66 | 39.48 | 1.09 |
| L30F70 | 0.83 | 30.22 | 1.19 |
| L35F65 | 1.03 | 25.57 | 1.38 |
| L40F60 | 1.26 | 17.94 | 1.54 |
| L45F55 | 1.53 | 14.96 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Zhang, X.-C.; Peng, Y. Synergistic Enhancement of Physicomechanical Performance and Microstructural Integrity in Hydrothermally Synthesized Autoclaved Lightweight Aggregates Through Quicklime–Fly Ash Blends. Materials 2025, 18, 2739. https://doi.org/10.3390/ma18122739
Chen X-F, Zhang X-C, Peng Y. Synergistic Enhancement of Physicomechanical Performance and Microstructural Integrity in Hydrothermally Synthesized Autoclaved Lightweight Aggregates Through Quicklime–Fly Ash Blends. Materials. 2025; 18(12):2739. https://doi.org/10.3390/ma18122739
Chicago/Turabian StyleChen, Xue-Fei, Xiu-Cheng Zhang, and Ying Peng. 2025. "Synergistic Enhancement of Physicomechanical Performance and Microstructural Integrity in Hydrothermally Synthesized Autoclaved Lightweight Aggregates Through Quicklime–Fly Ash Blends" Materials 18, no. 12: 2739. https://doi.org/10.3390/ma18122739
APA StyleChen, X.-F., Zhang, X.-C., & Peng, Y. (2025). Synergistic Enhancement of Physicomechanical Performance and Microstructural Integrity in Hydrothermally Synthesized Autoclaved Lightweight Aggregates Through Quicklime–Fly Ash Blends. Materials, 18(12), 2739. https://doi.org/10.3390/ma18122739






