Preparation and Characterization of Materials Based on Graphene Oxide Functionalized with Fe, Mn, Ni, and Cu Oxides and Their Testing for the Removal of Water Pollutants
Highlights
- Synthesis of materials based on graphene oxide (GO) functionalized with different metal oxides (MnO2, Fe3O4, CuO, NiO);
- Potential applications in water decontamination;
- Valuable knowledge in the field of water treatment;
- Functionalization of GO with metal oxides modifies the material's structure, increasing its potential for environmental applications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials Synthesis
2.2.1. GO Synthesis
2.2.2. GO-MnO2 Synthesis
2.2.3. GO-Fe3O4 Synthesis
2.2.4. GO-Fe3O4-NiO Synthesis
2.2.5. GO-Fe3O4-CuO Synthesis
2.3. Materials Characterization
2.3.1. Powder X-Ray Diffraction (XRD)
2.3.2. Morphological Characterization
2.3.3. FTIR Analysis
2.3.4. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.3.5. Vibrating-Sample Magnetometry (VSM) Analysis
2.4. Preliminary Adsorption Tests
3. Results and Discussion
3.1. XRD Characterization
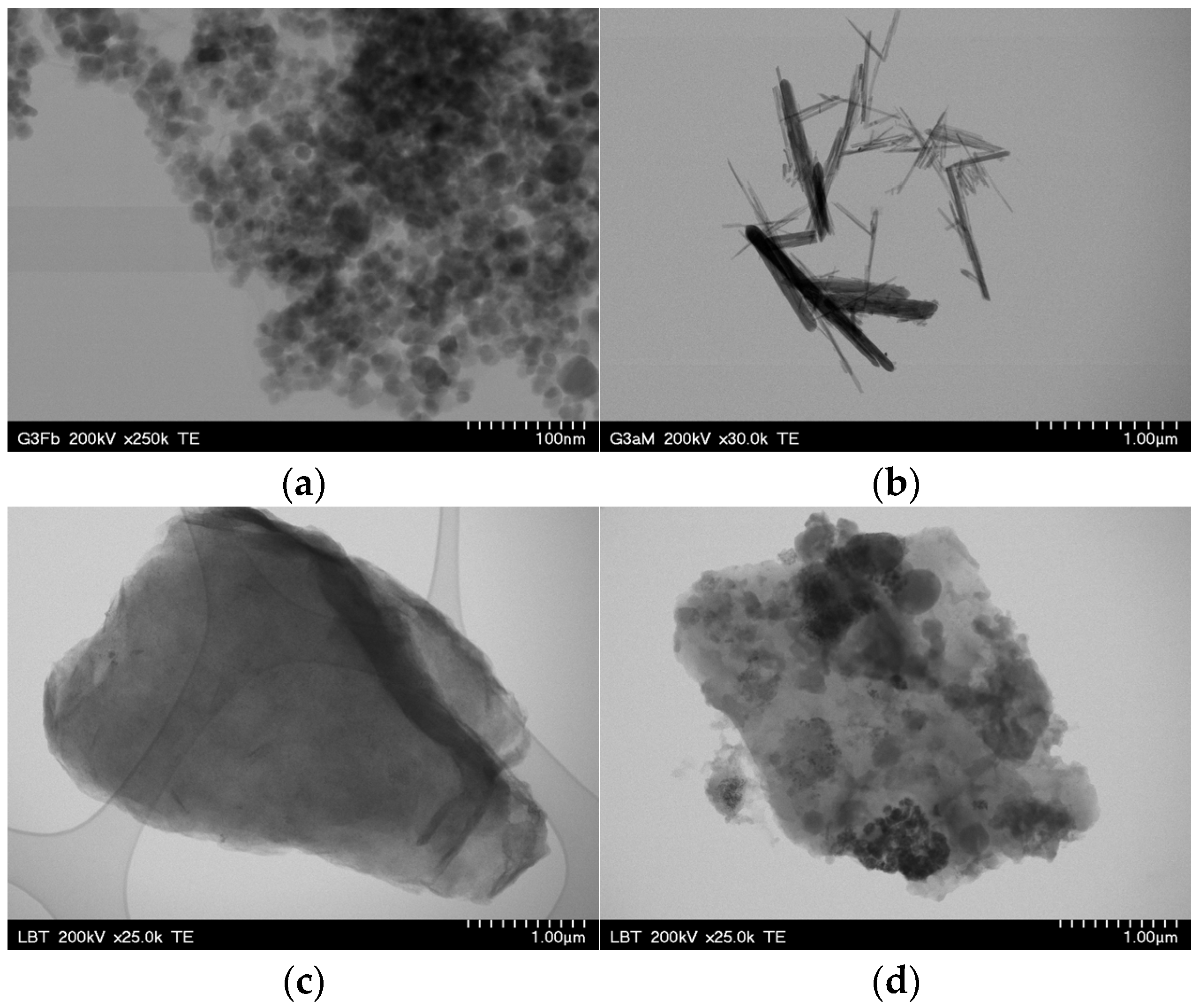
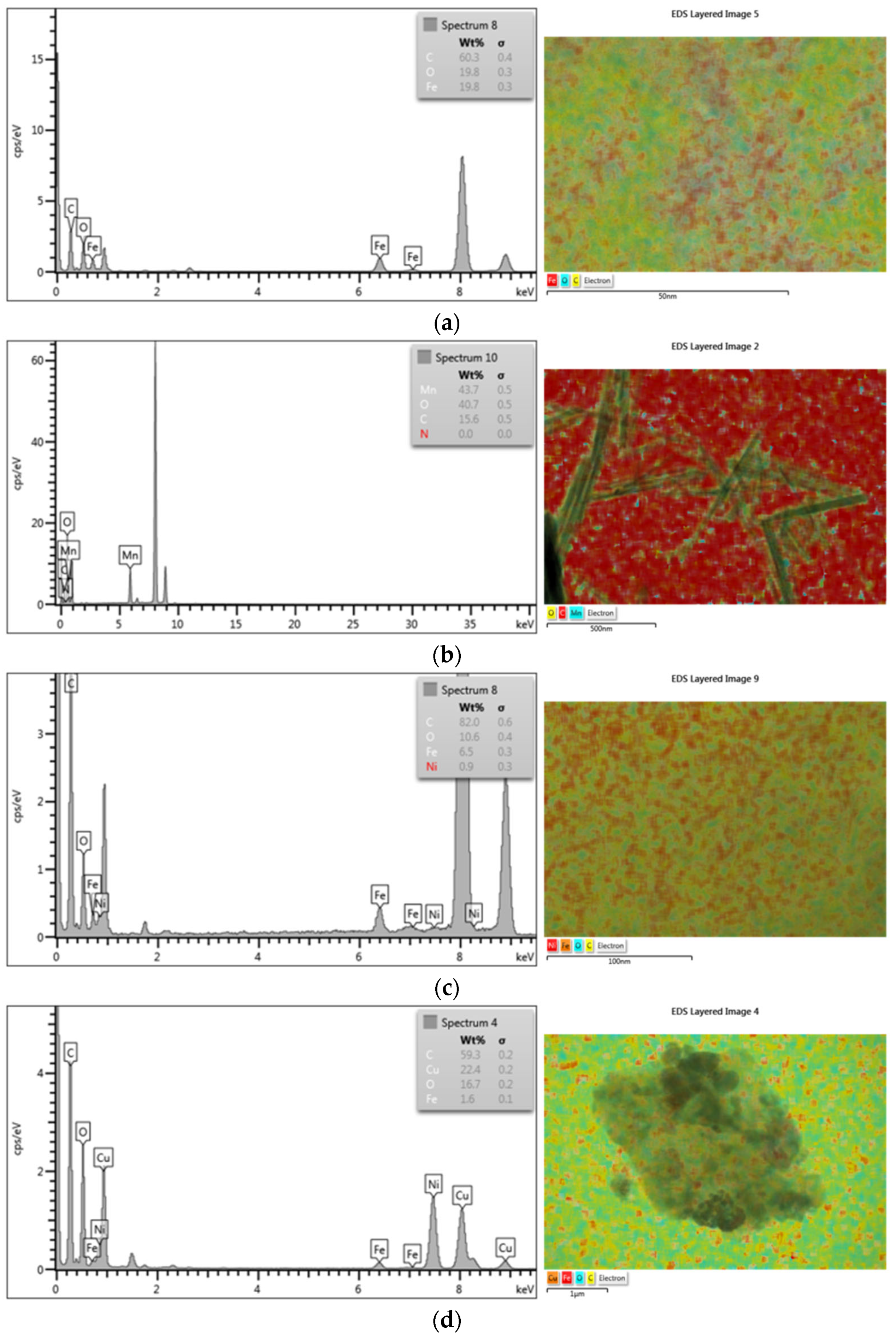
3.2. TEM Characterization
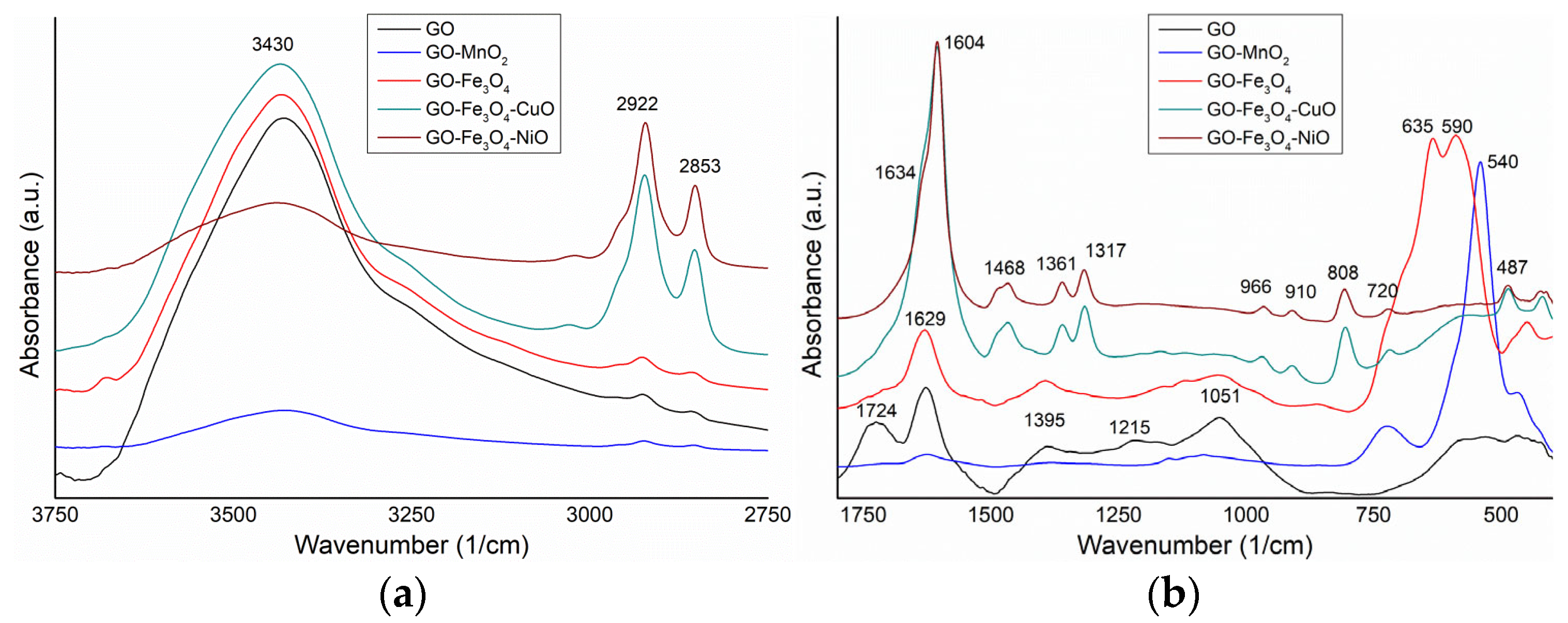
3.3. FTIR Characterization
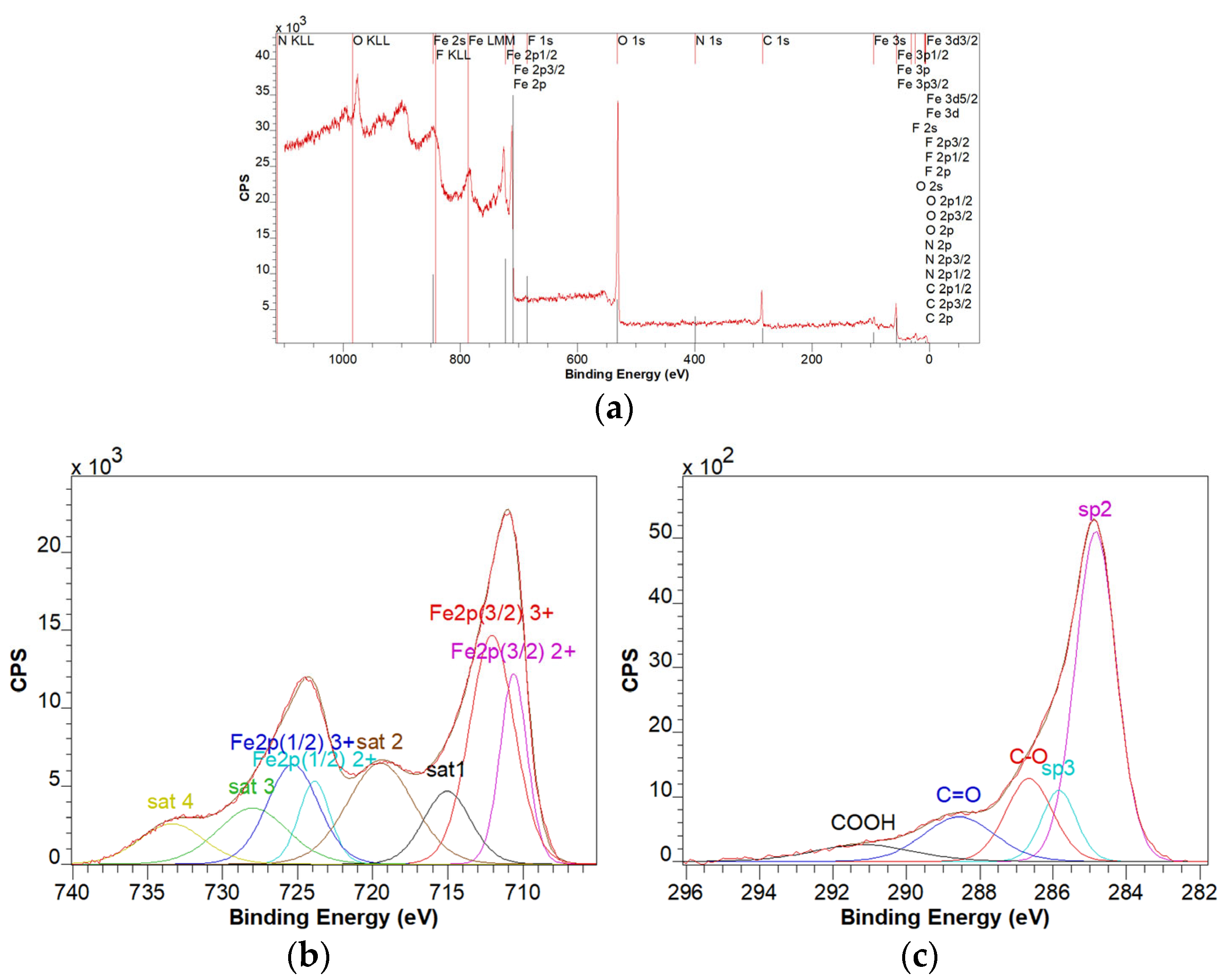
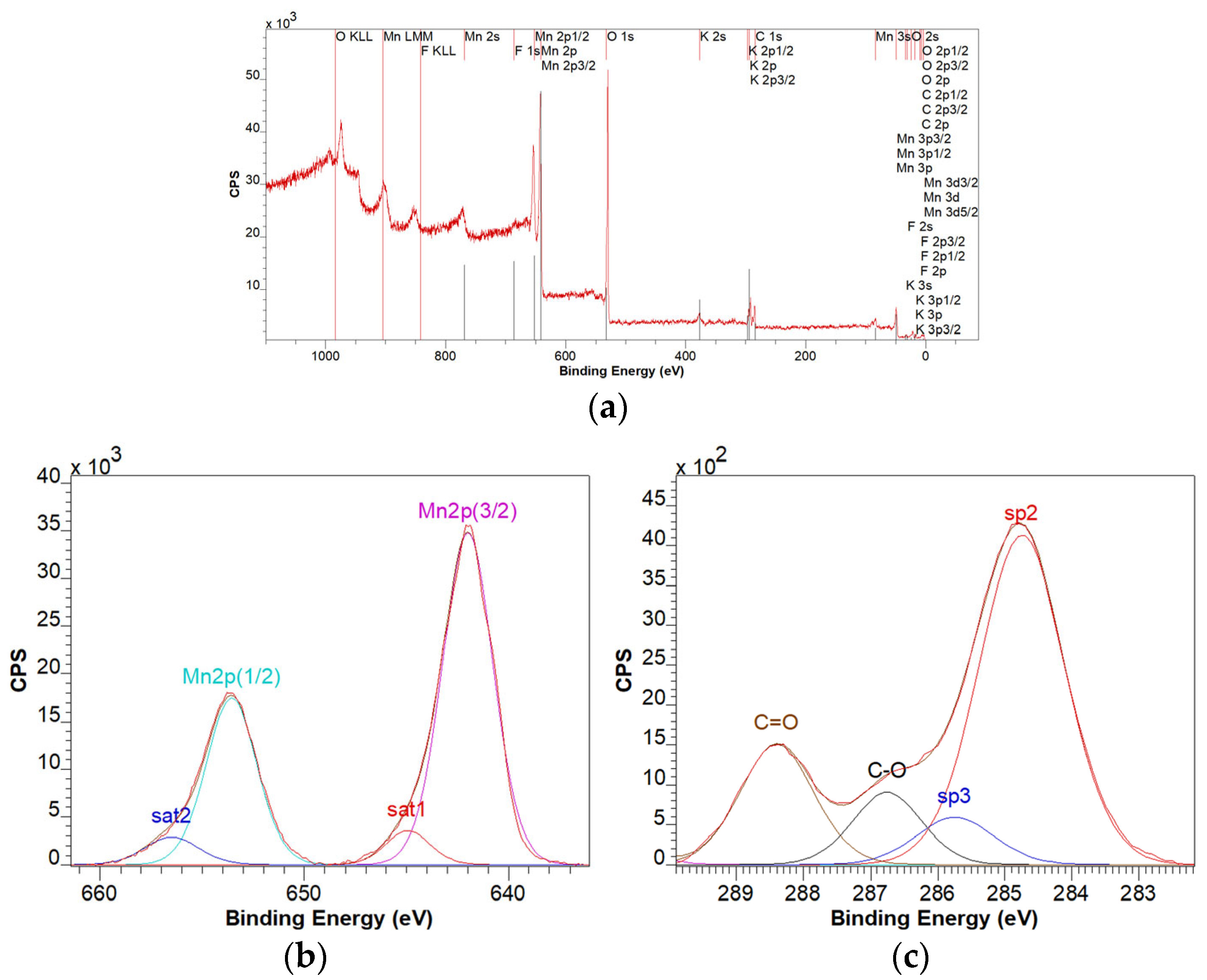
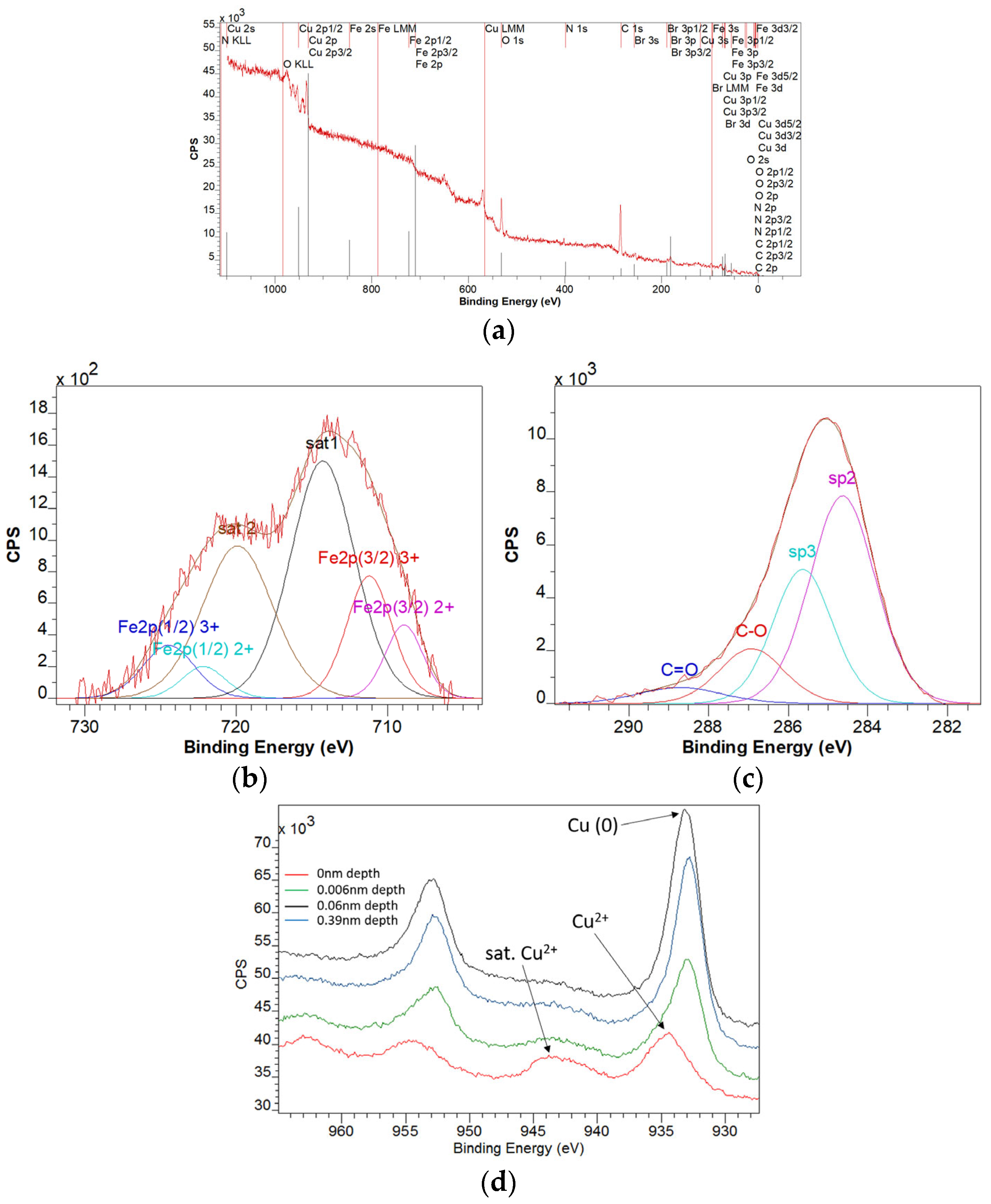
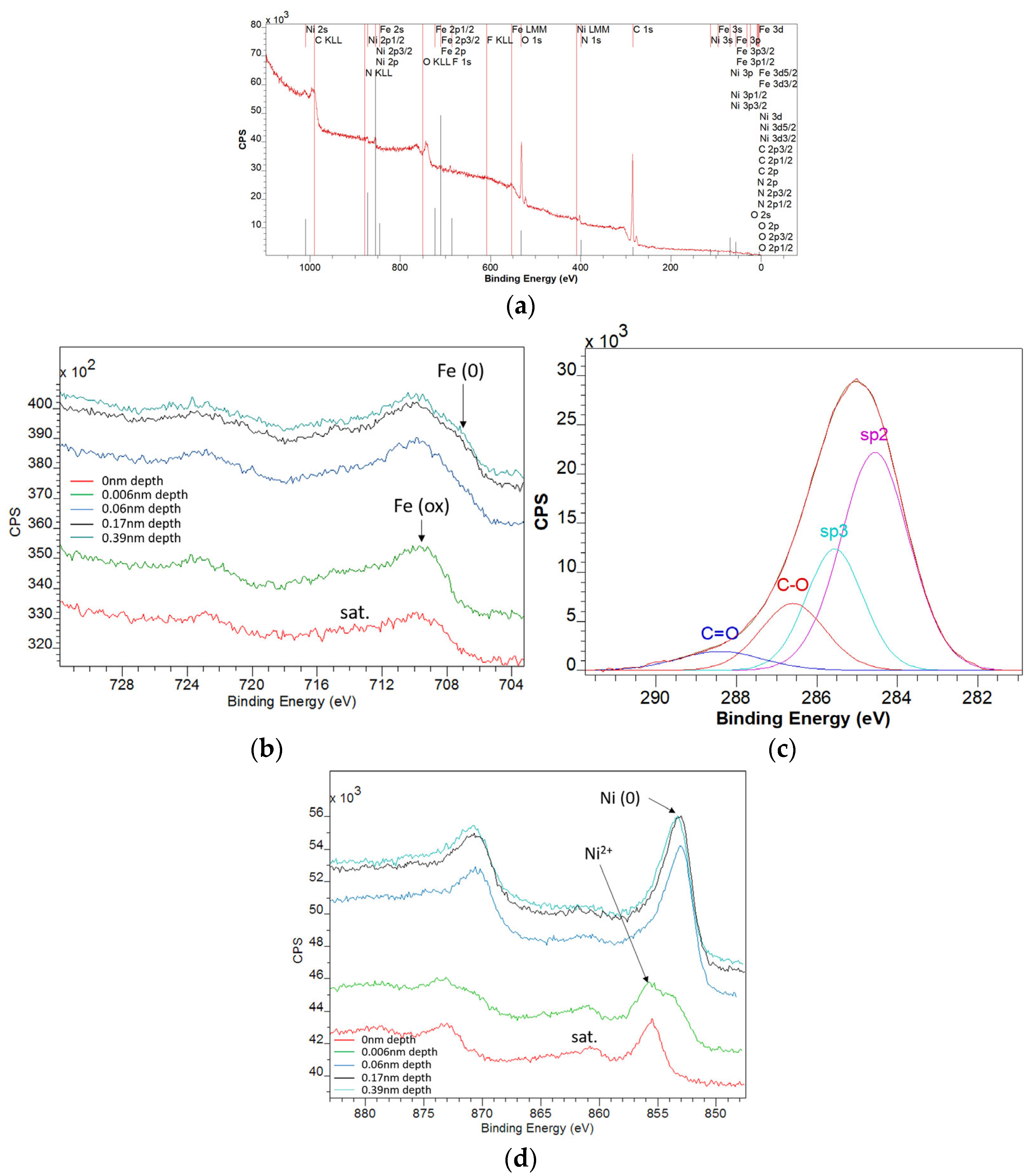
3.4. XPS Characterization
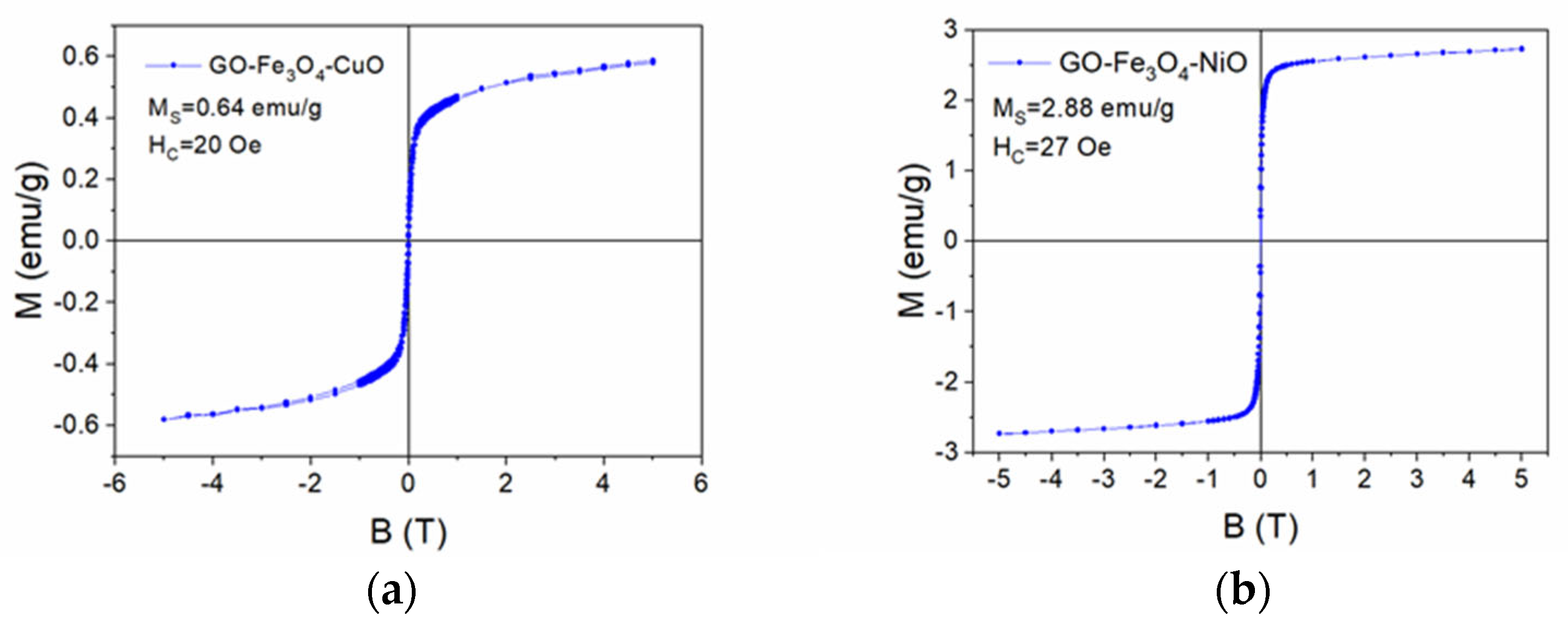
3.5. VSM Characterization
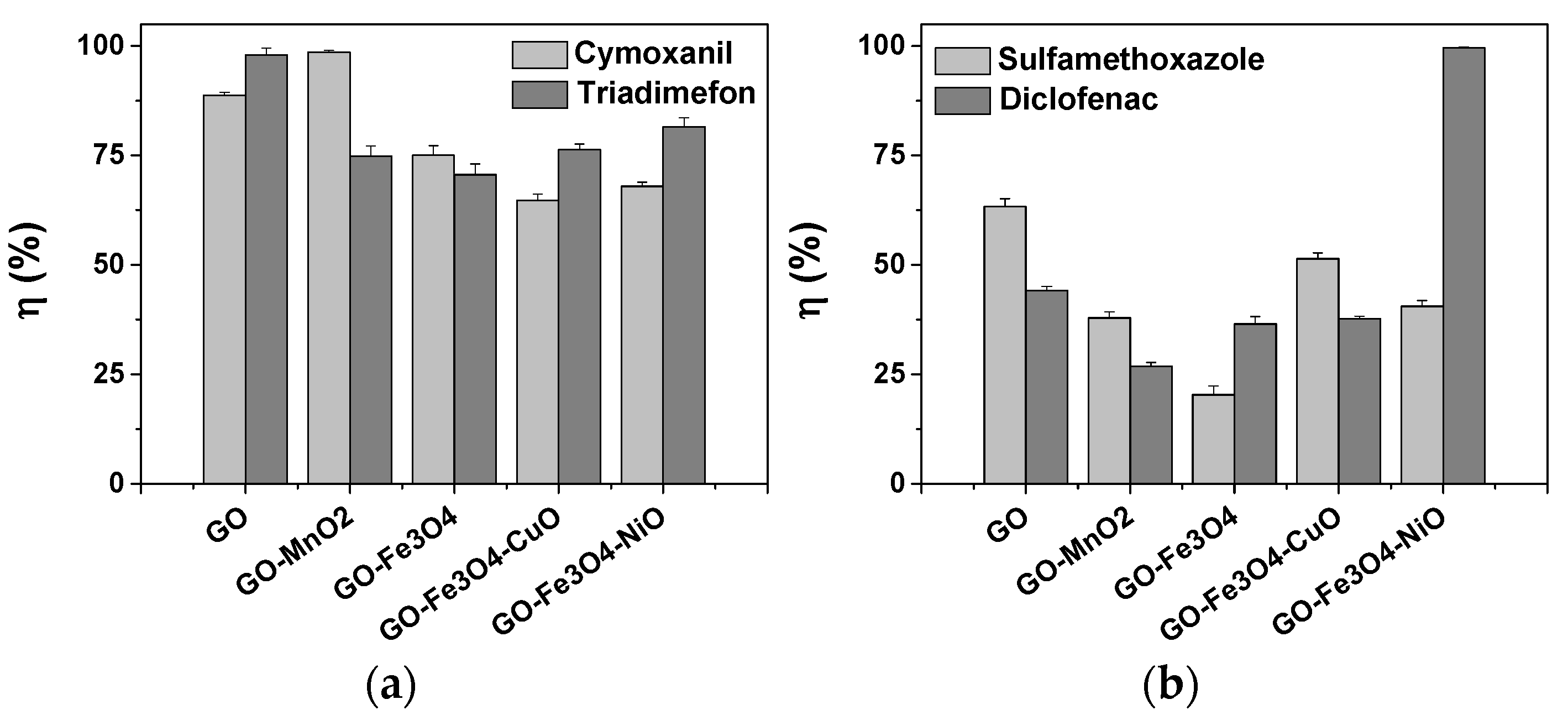
3.6. Removal of Pesticides and Drugs from Aqueous Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Ding, J.; Tian, D. Incorporation of MIL-101 (Fe or Al) into chitosan hydrogel adsorbent for phosphate removal: Performance and mechanism. J. Solid State Chem. 2022, 306, 122709. [Google Scholar] [CrossRef]
- Chowdury, M.S.K.; Park, Y.J.; Park, S.B.; Park, Y.-I. Two-dimensional nanostructured pristine graphene and heteroatom-doped graphene-based materials for energy conversion and storage devices. Sustain. Mater. Technol. 2024, 42, e01124. [Google Scholar] [CrossRef]
- Ramezani, M.; Rahmani, O. A review of recent progress in the graphene syntheses and its applications. Mech. Adv. Mater. Struct. 2024, 1–33. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Zheng, X.; Zhao, K.; Lin, Y.; Xu, X.; Long, H.; Zhang, Y.; Wang, X.; Chen, D.; et al. Recent advances in iron-based catalyst-driven persulfate activation for organic pollutant degradation. J. Water Process Eng. 2025, 71, 107423. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Chen, J.; Kashi, P.A.; Mohammadi, A. Dispersion strategies of nanomaterials in polymeric inks for efficient 3D printing of soft and smart 3D structures: A systematic review. Adv. Colloid Interface Sci. 2024, 333, 103285. [Google Scholar] [CrossRef] [PubMed]
- Perala, R.S.; Chandrasekar, N.; Balaji, R.; Alexander, P.S.; Humaidi, N.Z.N.; Hwang, M.T. A comprehensive review on graphene-based materials: From synthesis to contemporary sensor applications. Mater. Sci. Eng. R. Rep. 2024, 159, 100805. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A.; Zwamel, A.H.; Rachchh, N.; Ramachandran, T.; Shankhyan, A.; Karthikeyan, A.; Thatoi, D.N.; Gupta, D.; Formanova, S.; Alam, R.; et al. A review on graphene-based inorganic nanostructures: Synthesis, functionalization, and applications in photocatalytic degradation and electrochemical sensing of pollutants. Inorg. Chem. Comm. 2025, 177, 114398. [Google Scholar] [CrossRef]
- Badoni, A.; Thakur, S.; Vijayan, N.; Swart, H.C.; Bechelany, M.; Chen, Z.; Sun, S.; Cai, Q.; Chen, Y.; Prakash, J. Recent progress in understanding the role of graphene oxide, TiO2 and graphene oxide-TiO2 nanocomposites as multidisciplinary photocatalysts in energy and environmental applications. Cat. Sci. Technol. 2025, 15, 1702–1770. [Google Scholar] [CrossRef]
- Zafar, M.; Imran, S.M.; Iqbal, I.; Azeem, M.; Chaudhary, S.; Ahmad, S.; Kim, W.Y. Graphene-based polymer nanocomposites for energy applications: Recent advancements and future prospects. Results Phys. 2024, 60, 107655. [Google Scholar] [CrossRef]
- Khan, H. Graphene based semiconductor oxide photocatalysts for photocatalytic hydrogen (H2) production, a review. Int. J. Hydrog. Energy 2024, 84, 356–371. [Google Scholar] [CrossRef]
- Hu, H.; Ou, J.Z.; Xu, X.; Lin, Y.; Zhang, Y.; Zhao, H.; Chen, D.; He, M.; Huang, Y.; Deng, L. Graphene-assisted construction of electrocatalysts for carbon dioxide reduction. Chem. Eng. J. 2021, 425, 130587. [Google Scholar] [CrossRef]
- Pouthika, K.; Madhumitha, G. A mini review on recent advancements in metal oxide integrated nano-tubular inorganic clay mineral photocatalyst for organic pollutant degradation. Comments Inorg. Chem. 2025, 45, 30–62. [Google Scholar] [CrossRef]
- Abdel-Monem, R.A.; Khalil, A.M.; Darwesh, O.M.; Hashim, A.I.; Rabie, S.T. Antibacterial properties of carboxymethyl chitosan Schiff-base nanocomposites loaded with silver nanoparticles. J. Macromol. Sci. Part A 2020, 57, 145–155. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Khalil, A.M.; El-Shahat, M.; Khaireldin, N.Y.; Rabie, S.T. Antimicrobial activity of PVC-pyrazolone-silver nanocomposites. J. Macromol. Sci. Part A 2016, 53, 346–353. [Google Scholar] [CrossRef]
- Tiwari, G.; Devi, R.R.; Mahanta, S.P.; Raul, P.K.; Chatterjee, S.; Kamboj, D.V. Copper oxide nanoparticles modified activated carbon nanocomposite towards removal of tetracycline from waste water. Inorg. Chem. Commun. 2023, 152, 110687. [Google Scholar] [CrossRef]
- Su, P.; Chu, D.; Wang, L. Studies on catalytic activity of nanostructure Mn2O3, prepared by solvent-thermal method on degrading crystal violet. Mod. Appl. Sci. 2010, 4, 125–129. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Bai, Q. Flower-like hierarchical Ni-Zn MOF microspheres: Efficient adsorbents for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123795. [Google Scholar] [CrossRef]
- Arshad, A.; Iqbal, J.; Mansoor, Q. NiO-nanoflakes grafted graphene: An excellent photocatalyst and a novel nanomaterial for achieving complete pathogen control. Nanoscale 2017, 9, 16321–16328. [Google Scholar] [CrossRef] [PubMed]
- Alicanoglu, P.; Sponza, D. Removal of ciprofloxacin antibiotic with nano graphene oxide magnetite: Comparison of adsorption and photooxidation processes. In Proceedings of the 14th International Conference on Environmental Science and Technology, Rhodes, Grece, 3–5 September 2015. [Google Scholar]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Suhaimin, N.S.; Hanifah, M.F.R.; Azhar, M.; Jaafar, J.; Aziz, M.; Ismail, A.; Othman, M.; Rahman, M.A.; Aziz, F.; Yusof, N. The evolution of oxygen-functional groups of graphene oxide as a function of oxidation degree. Mater. Chem. Phys. 2022, 278, 125629. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Wen, X.; Jin, X.; Foller, T.; Joshi, R. Functional groups in graphene oxide. PCCP 2022, 24, 26337–26355. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-khalidi, A.; Abbas, J.K. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Ahmad, I.; Athar, M.S.; Muneer, M.; Altass, H.M.; Felemban, R.F.; Ahmed, S.A. Graphene oxide decorated BiOI/CdS nanocomposite: An efficient ternary heterostructure for photodegradation and adsorption study of organic pollutants. Surf. Interfaces 2024, 45, 103819. [Google Scholar] [CrossRef]
- Liaqat, M.; Iqbal, T.; Maryam, I.; Riaz, K.N.; Afsheen, S.; Sohaib, M.; Al-Zaqri, N.; Warad, I.; Al-Fatesh, A.S. Enhancing photocatalytic activity: Investigating the synthesis and characterization of BiVO4/Cu2O/graphene ternary nanocomposites. J. Photochem. Photobiol. A Chem. 2024, 446, 115122. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Dabur, V.A.; Rachmantyo, R.; Judawisastra, H.; Hu, C.; Wibowo, A. Carbon nitride-and graphene-based materials for the photocatalytic degradation of emerging water pollutants. Mater. Adv. 2024, 5, 2668–2688. [Google Scholar] [CrossRef]
- Das, A.; Adak, M.K. Kinetic and mechanistic way for photocatalytic degradation of pollutants from textile wastewater by graphene oxide supported nanocomposite. Next Mater 2024, 3, 100153. [Google Scholar] [CrossRef]
- Sereshti, H.; Amirafshar, A.; Kadi, A.; Nodeh, H.R.; Rezania, S.; Hoang, H.Y.; Barghi, A.; Vasseghian, Y. Isolation of organophosphate pesticides from water using gold nanoparticles doped magnetic three-dimensional graphene oxide. Chemosphere 2023, 320, 138065. [Google Scholar] [CrossRef]
- European Union. European Commission, EU Pesticides Database, Pesticide EU-MRLs Regulation (EC) No 396/2005 [WWW document]. Off. J. Eur. Union 2025. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 23 March 2025).
- Marsin, F.M.; Wan Ibrahim, W.A.; Nodeh, H.R.; Sanagi, M.M. New magnetic oil palm fiber activated carbon-reinforced polypyrrole solid phase extraction combined with gas chromatography-electron capture detection for determination of organochlorine pesticides in water samples. J. Chromatogr. A 2020, 1612, 460638. [Google Scholar] [CrossRef]
- Arulsoosairaj, D.A.; Muthu-Pandian, C.K.; Sengottayan, S.N. Phycogenic nanoparticles efficiently catalyse pesticide degradation through a novel metabolic pathway utilizing solar light. Chemosphere 2024, 369, 143877. [Google Scholar] [CrossRef]
- Rajak, P. Green nanomaterial-based sustainable analysis of contaminant remediation in wastewater: A bird’s-eye view on recent advances and limitations. Sustain. Chem. Environ. 2025, 100238. [Google Scholar] [CrossRef]
- Rajak, P. Metal-based nanoparticles and nanohybrids for sensing and remediation of environmental pesticides. Hybrid Adv. 2025, 10, 100485. [Google Scholar] [CrossRef]
- Chinnappa, K.; Ananthai, P.K.; Srinivasan, P.P.; Glorybai, D.C. Green synthesis of rGO-AgNP composite using Curcubita maxima extract for enhanced photocatalytic degradation of the organophosphate pesticide chlorpyrifos. Environ. Sci. Pollut. Res. 2022, 29, 58121–58132. [Google Scholar] [CrossRef]
- Dehghan, A.; Aliasghar, A.; Rahmati, R.; Delnavaz, M.; Khoshvaght, H. Green synthesis of ZnO/αFe2O3 nano-photocatalyst for efficient removal of carbamate pesticides in wastewater: Optimization, mineralization, and financial analysis. Korean J. Chem. Eng. 2024, 41, 249–269. [Google Scholar] [CrossRef]
- Kumar, R.; Mukherji, S. Assessment of photocatalytic efficiency of graphene oxide–TiO2 nanocomposite for removal of binary mixtures of organophosphorus pesticides from water. ACS EST Water 2024, 4, 4075–4082. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, J.; Chen, H.; Zhao, Q.; Hou, J.; Yan, J.; Wang, H.; Ding, L.; Ren, N. Fast determination of sulfonamides from egg samples using magnetic multiwalled carbon nanotubes as adsorbents followed by liquid chromatography-tandem mass spectrometry. Food Chem. 2013, 140, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Song, Y.; Hu, M.; Xu, X.; Zhang, H.; Yu, A.; Ma, Q.; Wang, Z. Determination of sulfonamides in butter samples by ionic liquid magnetic bar liquid-phase microextraction high-performance liquid chromatography. Anal. Bioanal. Chem. 2015, 407, 569–580. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernandez-Borges, J.; Afonso, M.M.; Palenzuela, J.A.; Rodriguez-Delgado, M.A. Comparison between magnetic and non magnetic multi-walled carbon nanotubes-dispersive solid-phase extraction combined with ultra-high performance liquid chromatography for the determination of sulfonamide antibiotics in water samples. Talanta 2013, 116, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into anorganophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, H.; Chen, R.; Pham-Huy, C.; Hui, X.; He, H. Adsorptive removal of trace sulfonamide antibiotics bywater-dispersible magnetic reduced graphene oxide-ferrite hybrids from wastewater. J. Chromatogr. B 2016, 1029–1030, 106–112. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Advanced graphene-based technologies for antibiotic removal from wastewater: A review (2016–2024). C-J. Carbon Res. 2024, 10, 92. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Orrell-Trigg, R.; Truong, Y.B.; Cozzolino, D.; Truong, V.K.; Chapman, J. Wastewater depollution of textile dyes and antibiotics using unmodified and copper oxide/zinc oxide nanofunctionalised graphene oxide materials. Environ. Sci. Adv. 2022, 1, 456–469. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef] [PubMed]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef]
- Garg, S.P.; Kumar, V.; Mishra, L.; Dumee, R.S. Sharma, Environmental Health and Society, 1st ed.; Academic Publication: Cambridge, MA, USA, 2021; Chapter 17; pp. 188–206. [Google Scholar]
- Jurado, A.; Vázquez-Suñé, E.; Pujades, E. Urban groundwater contamination by non-steroidal anti-inflammatory drugs. Water 2021, 13, 720. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C.-U. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- da Rocha Medeiros, G.; da Silva Pereira Júnior, A.; Galvão, F.M.F.; do Nascimento, J.H.O.; Tinôco, J.D. Optimization of diclofenac sodium adsorption onto graphene nanosheets: Capacity, kinetics, isotherms and removal. Desalination Water Treat. 2022, 271, 176–191. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opriș, O.; Gutoiu, S.; Leostean, C.; Lazar, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef] [PubMed]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opriș, O. Application of CNT-COOH/MnO2/Fe3O4 nanocomposite for the removal of cymoxanil from aqueous solution: Isotherm and kinetic studies. Anal. Lett. 2023, 56, 216–230. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opriș, O. Devrinol and triadimefon removal from aqueous solutions using CNT-COOH/MnO2/Fe3O4 nanocomposite. J. Iran. Chem. Soc. 2022, 19, 2031–2039. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Tarekegne, A.H.; Worku, D.A. Synthesis and characterization of reduced graphene oxide(rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. 2019, 2019, 5058163. [Google Scholar]
- Young, G.S.; Tran, Q.T.; Ok-Ja, Y.; Il-Yung, S.; Nae-Eung, L. Nanocomposites of reduced graphene oxide nanosheets and conducting polymer for stretchable transparent conducting electrodes. J. Mater. Chem. 2012, 22, 23759–23766. [Google Scholar]
- Mylarappa, M.; Lakshmi, V.V.; Mahesh, K.R.V.; Nagaswarupa, H.P.; Raghavendra, N. A facile hydrothermal recovery of nano sealed MnO2 particle from waste batteries: An advanced material for electrochemical and environmental applications. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012178. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef]
- Arun, K.J.; Batra, A.K.; Krishna, A.; Bhat, K.; Aggarwal, M.D.; Fran, P.J.J. Surfactant free hydrothermal synthesis of copper oxide nanoparticles. Am. J. Mater. Sci. 2015, 5, 36–38. [Google Scholar]
- Sharma, P.K.; Singh, M.K.; Sharma, G.D.; Agrawal, A. NiO nanoparticles: Facile route synthesis, characterization and potential towards third generation solar cell. Mater. Today Proc. 2021, 43, 3061–3065. [Google Scholar] [CrossRef]
- Deng, H.; Yao, L.; Huang, Q.A.; Su, Q.; Zhang, J.; Zhang, F.; Du, G. Facile assembly of a S@carbon nanotubes/polyaniline/graphene composite for lithium–sulfur batteries. RSC Adv. 2017, 7, 9819–9825. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opriș, O.; Stegarescu, A.; Lung, I.; Porav, A.S.; Kacso, I.; Borodi, G.; Leoștean, C.; Pană, O.; Soran, M.-L. Preparation and Characterization of Materials Based on Graphene Oxide Functionalized with Fe, Mn, Ni, and Cu Oxides and Their Testing for the Removal of Water Pollutants. Materials 2025, 18, 2735. https://doi.org/10.3390/ma18122735
Opriș O, Stegarescu A, Lung I, Porav AS, Kacso I, Borodi G, Leoștean C, Pană O, Soran M-L. Preparation and Characterization of Materials Based on Graphene Oxide Functionalized with Fe, Mn, Ni, and Cu Oxides and Their Testing for the Removal of Water Pollutants. Materials. 2025; 18(12):2735. https://doi.org/10.3390/ma18122735
Chicago/Turabian StyleOpriș, Ocsana, Adina Stegarescu, Ildiko Lung, Alin Sebastian Porav, Irina Kacso, Gheorghe Borodi, Cristian Leoștean, Ovidiu Pană, and Maria-Loredana Soran. 2025. "Preparation and Characterization of Materials Based on Graphene Oxide Functionalized with Fe, Mn, Ni, and Cu Oxides and Their Testing for the Removal of Water Pollutants" Materials 18, no. 12: 2735. https://doi.org/10.3390/ma18122735
APA StyleOpriș, O., Stegarescu, A., Lung, I., Porav, A. S., Kacso, I., Borodi, G., Leoștean, C., Pană, O., & Soran, M.-L. (2025). Preparation and Characterization of Materials Based on Graphene Oxide Functionalized with Fe, Mn, Ni, and Cu Oxides and Their Testing for the Removal of Water Pollutants. Materials, 18(12), 2735. https://doi.org/10.3390/ma18122735





