Selective Ion Separation by Capacitive Deionization: A Comprehensive Review
Abstract
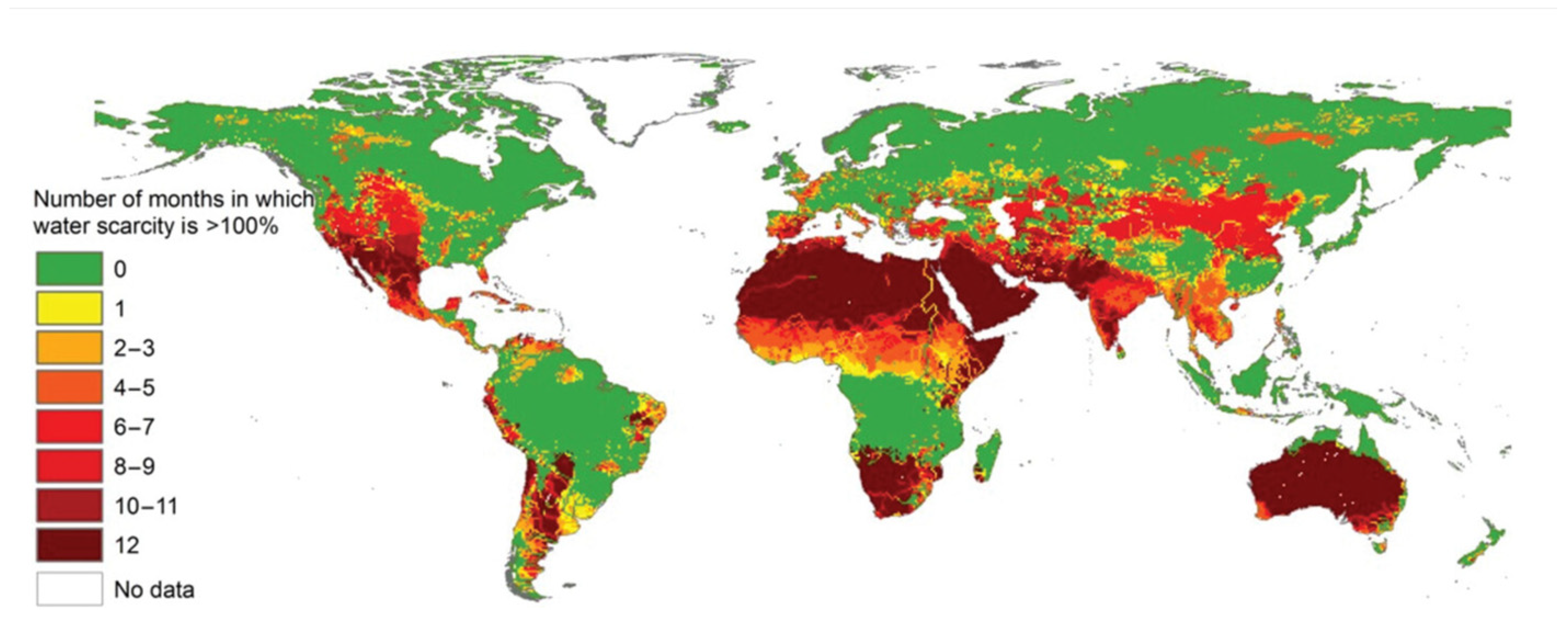
1. Introduction
2. Desalination Method
2.1. Pressure-Driven Desalination
2.2. Thermal Desalination
2.3. Electrokinetic Desalination
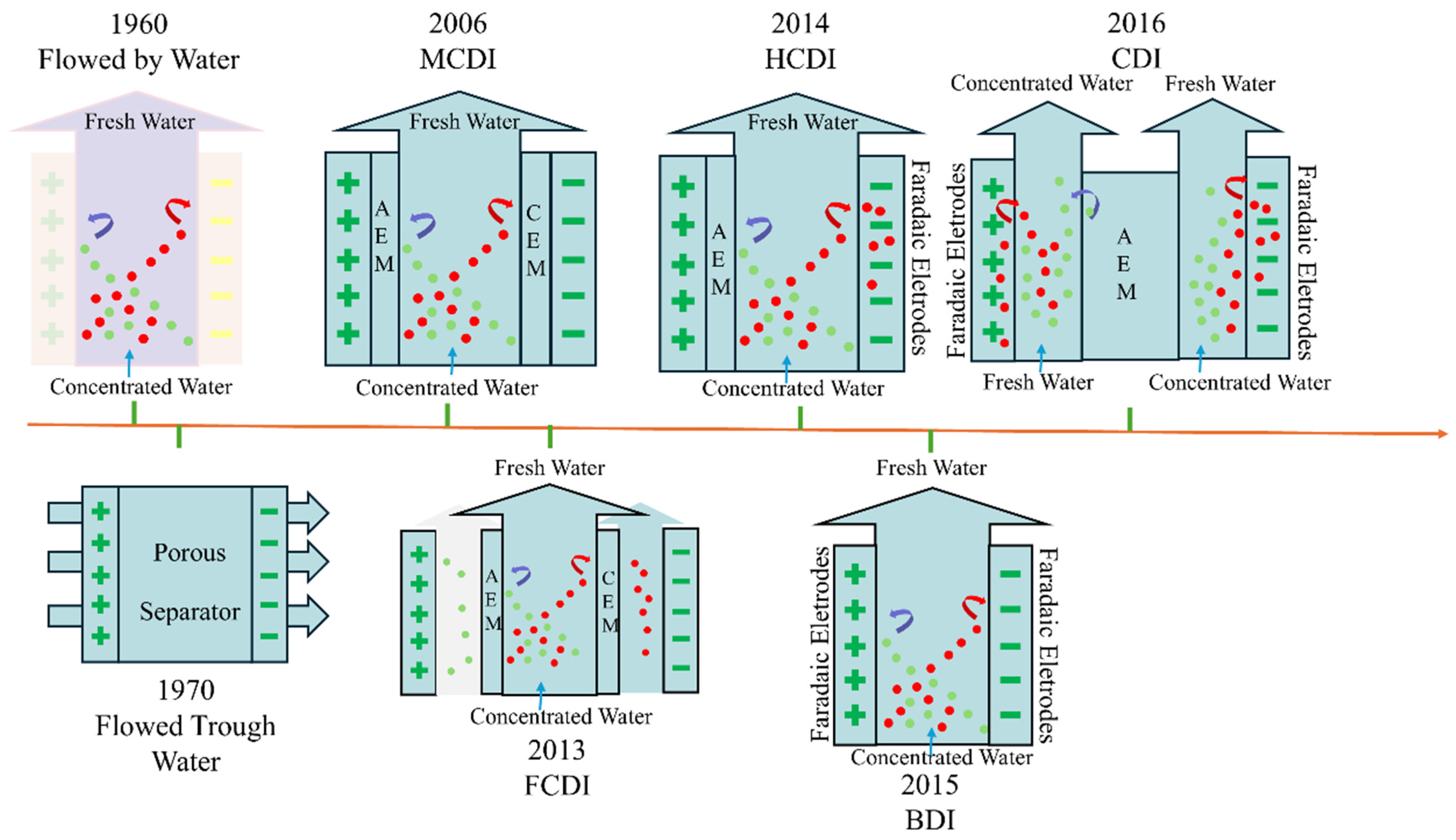
3. CDI Evolution and Working Principles
3.1. Evolution of CDI
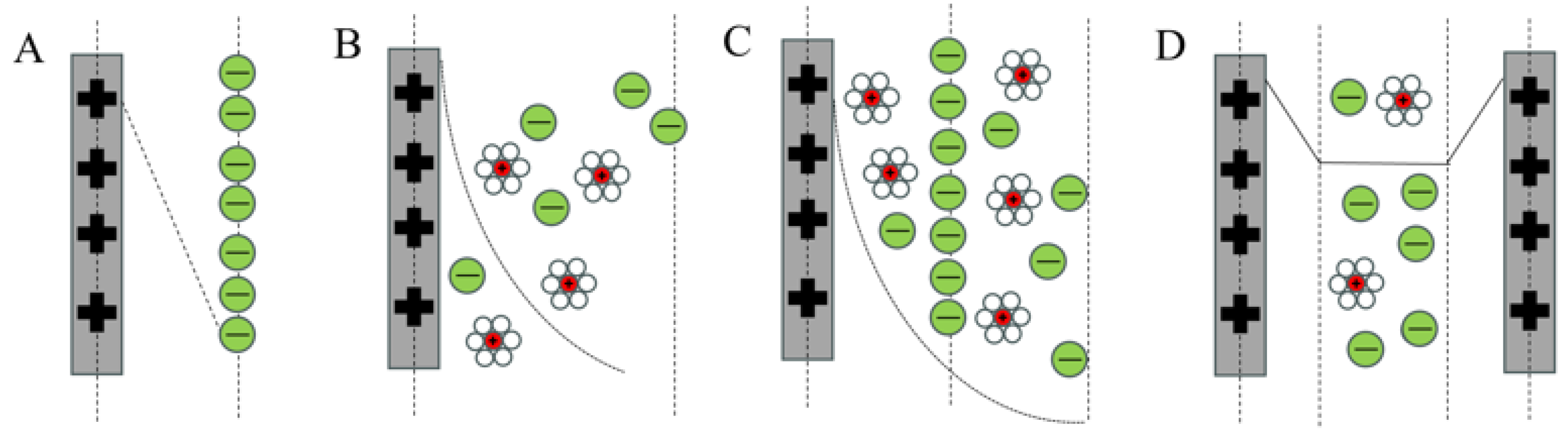
3.2. Working Principles of CDI
3.2.1. Electro-Absorption Principle
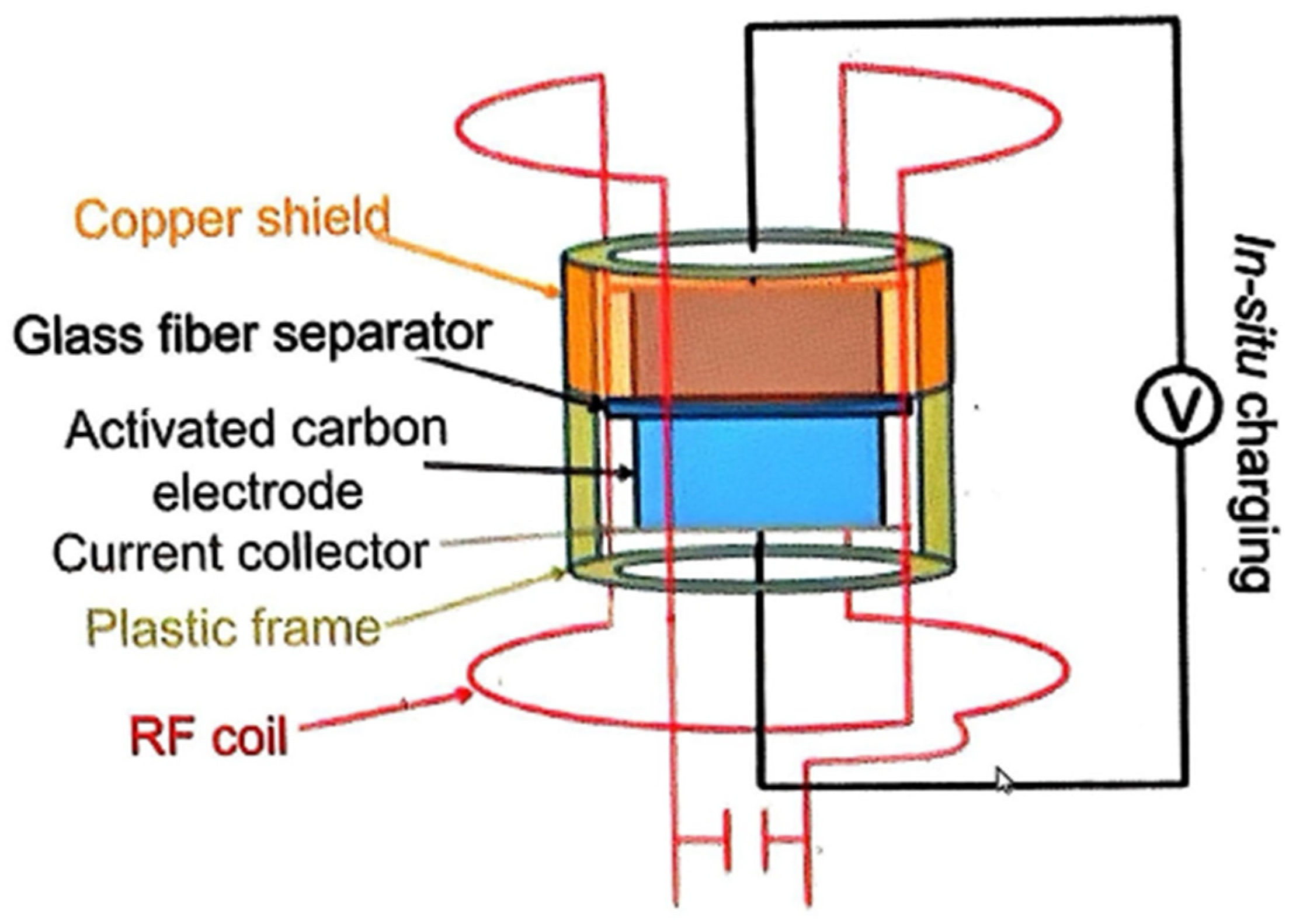
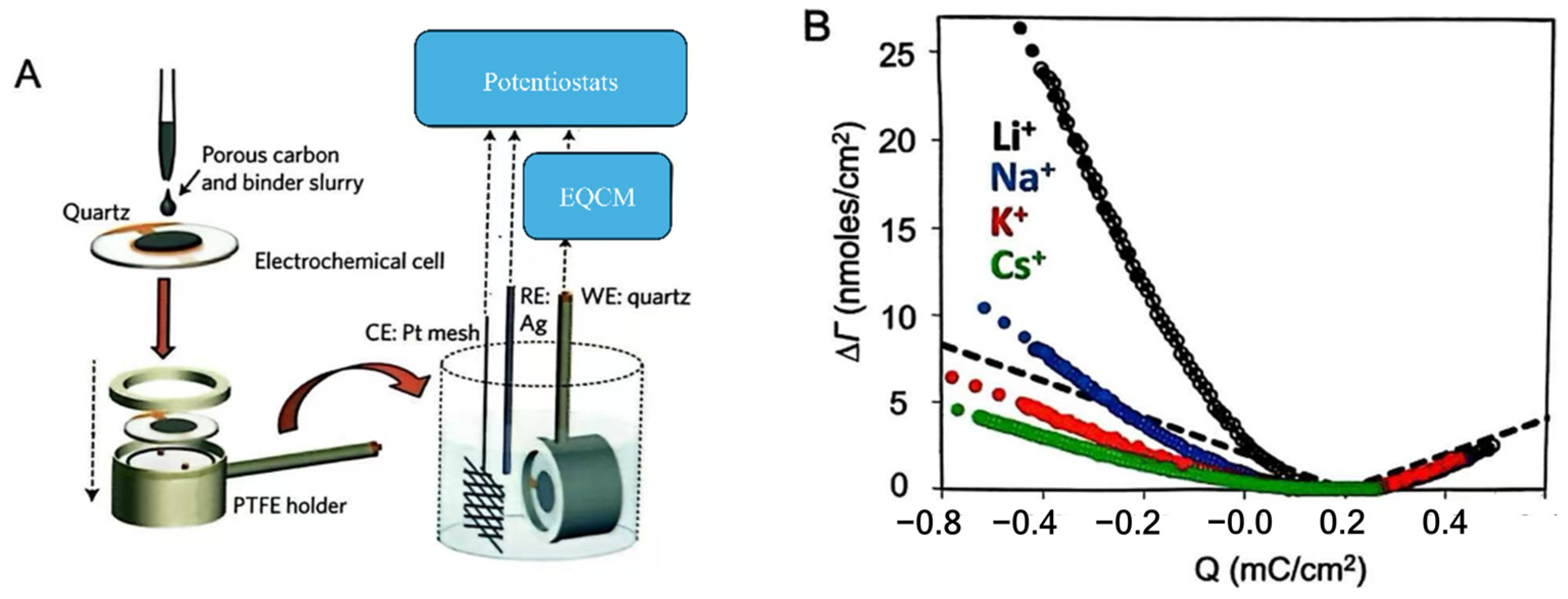
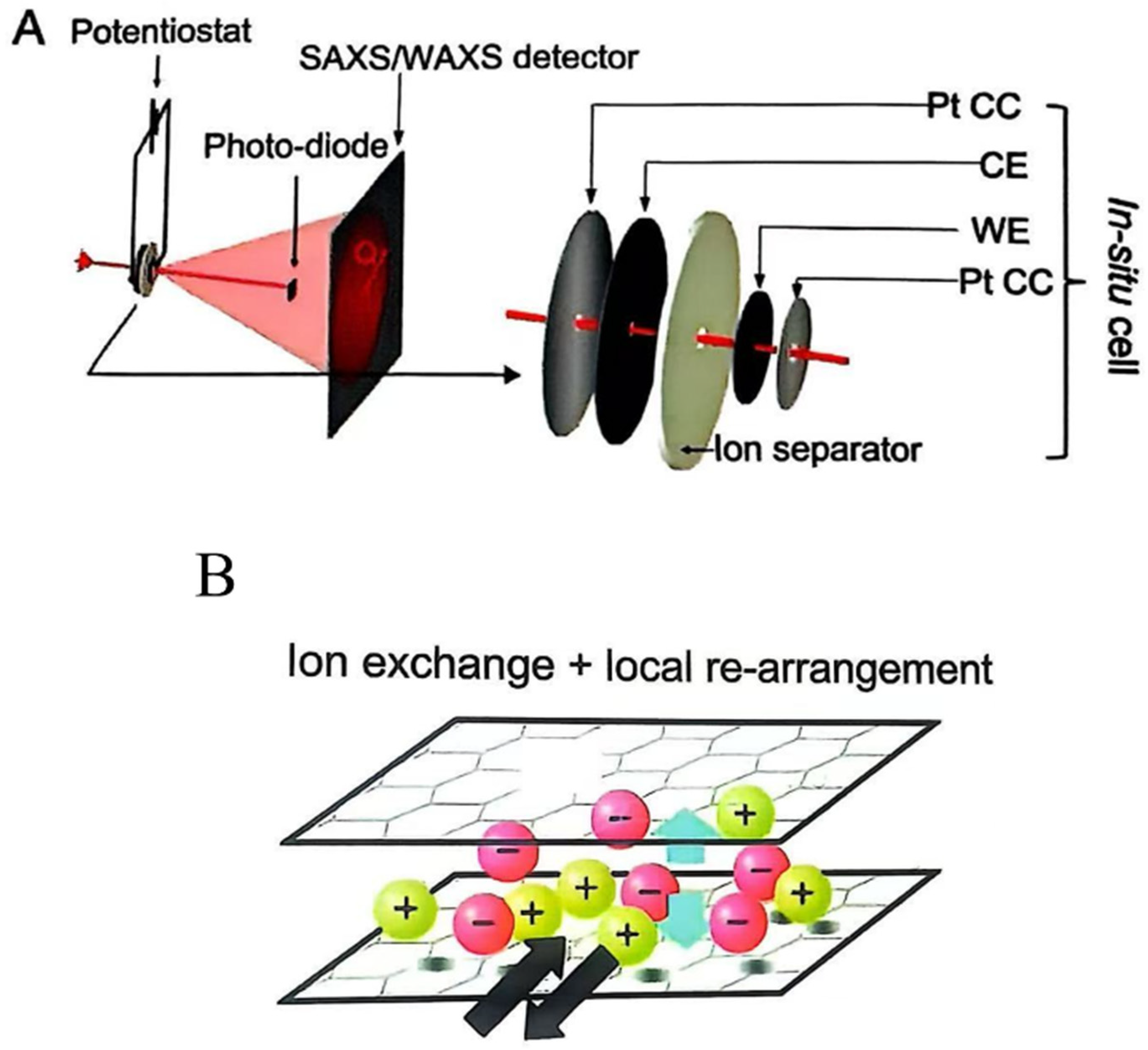
3.2.2. Advanced Characterization Techniques for Understanding Electrosorption
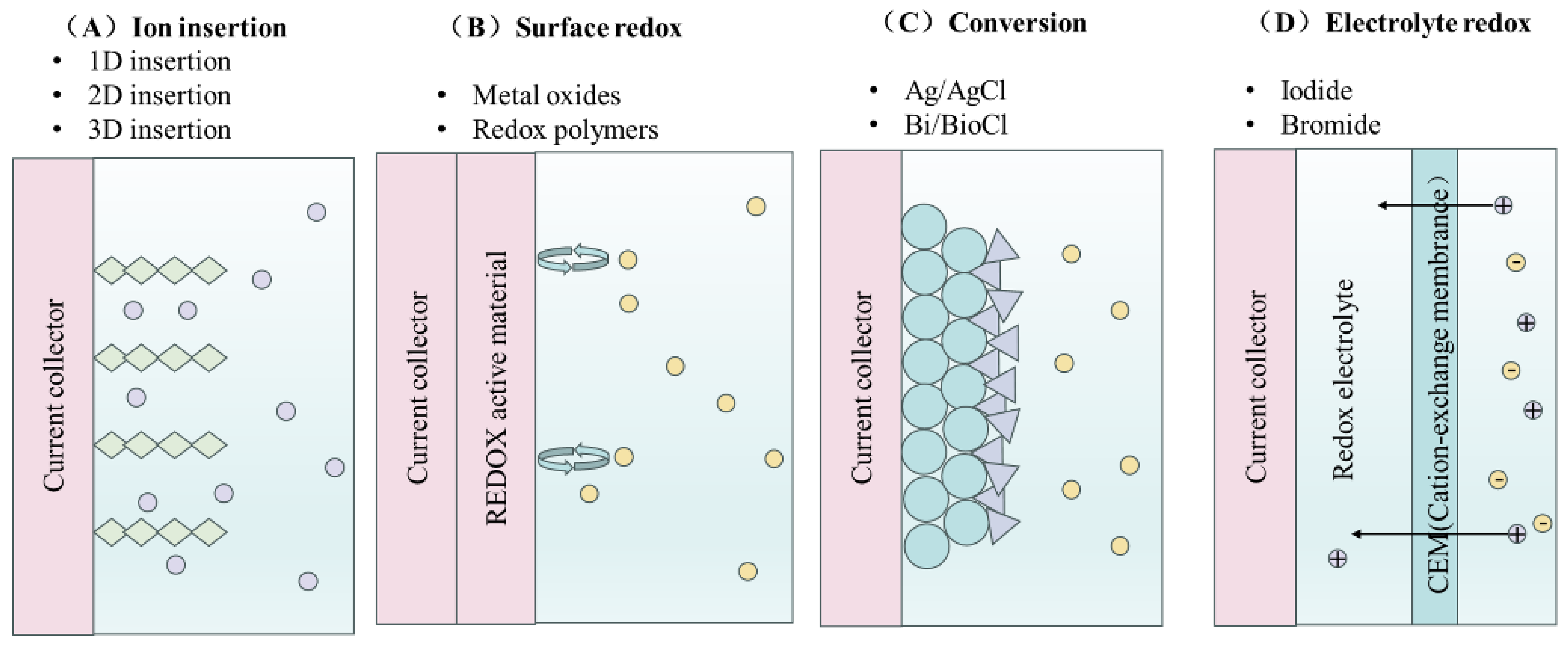
3.2.3. Faraday’s Principle of Ion Removal
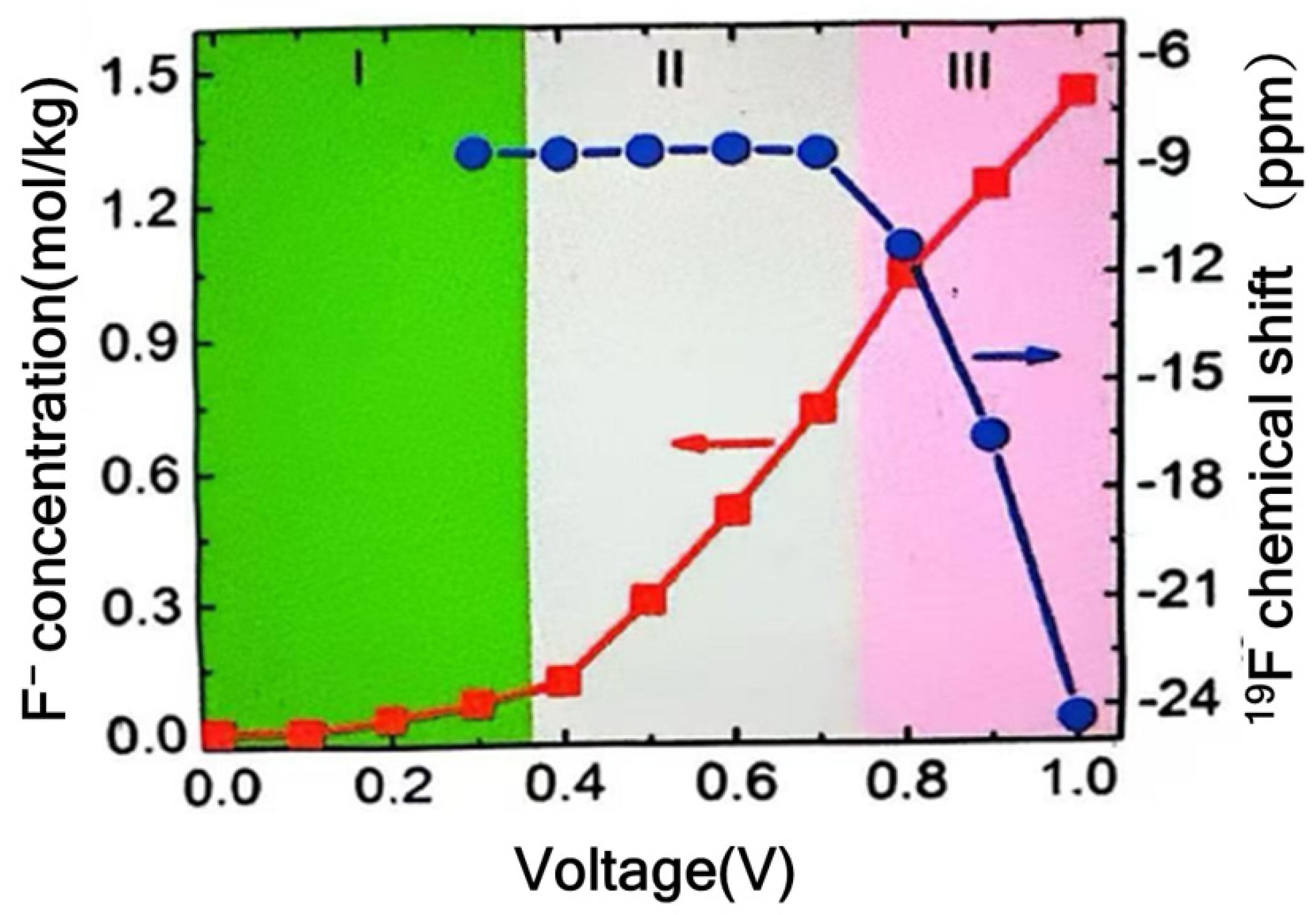
3.2.4. Advanced Characterization Techniques for Understanding Faradaic Storage
4. Performance Indicators and Electrode Materials
4.1. Performance Indicators
4.2. Electrode Materials
4.2.1. Capacitor Materials
4.2.2. Pseudo-Capacitor Materials
5. Ion-Selective Membrane
5.1. Cation-Exchange Membrane
5.2. Anion-Exchange Membrane
5.3. Ion-Exchange Resin Coating
6. Problems Faced and Solutions
6.1. Water Oxidation
6.2. Dissolved Oxygen Reduction
6.3. Electrode Fouling
7. Outlook
- Material discovery and design: The discovery of new materials can be accelerated through machine learning algorithms. For example, training models to predict the properties of different material combinations, such as capacitance values, conductivity, stability, etc., can help researchers find new materials with excellent properties. Machine learning can also be used to optimize the microstructure of materials, such as the pore distribution of porous carbon materials, to achieve higher specific capacitance.
- Manufacturing optimization: In the manufacturing process of capacitors, machine learning can be used to optimize process parameters such as temperature, pressure, reaction time, etc., to improve product quality and consistency. Three-dimensional printing technology combined with machine learning can enable a customized design of the internal structure to make them more suitable for specific application scenarios.
- Performance prediction and modeling: Based on historical experimental data, the machine learning model can predict the performance of capacitors under different conditions, such as temperature changes, the charge/discharge rate, and other factors on their life and performance. It can also help engineers to consider the impact of these factors at the design stage.
- Fault detection and diagnosis: Machine learning can be used to monitor the working status of capacitors, provide early warning of potential failures, extend service life, and reduce maintenance costs.
- Intelligent control of capacitors: In some application scenarios, such as power systems or battery management systems for electric vehicles, machine learning algorithms can be used to optimize the charging and discharging strategies of capacitors for optimal energy management. For example, researchers at Georgia Tech used supercomputers and machine learning techniques to accelerate the analysis of electronic materials, which helped them find ways to improve capacitors faster. Meanwhile, research has shown that a combination of 3D printing technology and machine learning can produce carbon micro-lattices with customized properties that can be used as energy storage elements for supercapacitors. These applications demonstrate the potential of machine learning to enhance the development, production, and application of capacitors, helping to advance capacitor technology.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.; Bodirsky, B.L.; Rijneveld, R.; Beier, F.; Bak, M.P.; Batool, M.; Droppers, B.; Popp, A.; van Vliet, M.T.H.; Strokal, M. A triple increase in global river basins with water scarcity due to future pollution. Nat. Commun. 2024, 15, 880. [Google Scholar] [CrossRef]
- Xu, X.; Eguchi, M.; Asakura, Y.; Pan, L.; Yamauchi, Y. Metal-organic framework derivatives for promoted capacitive deionization of oxygenated saline water. Energy Environ. Sci. 2023, 16, 1815–1820. [Google Scholar] [CrossRef]
- Sun, K.; Tebyetekerwa, M.; Wang, C.; Wang, X.; Zhang, X.; Zhao, X.S. Electrocapacitive Deionization: Mechanisms, Electrodes, and Cell Designs. Adv. Funct. Mater. 2023, 33, 2213578. [Google Scholar] [CrossRef]
- Ren, Y.; Nie, L.; Wang, L.; Wang, X. Capacitive deionization technology and its application in heavy metal separation and recovery. China Environ. Sci. 2023, 43, 6051–6062. [Google Scholar]
- Wang, L.; Zhong, Y.; Wang, H.; Malyi, O.I.; Wang, F.; Zhang, Y.; Hong, G.; Tang, Y. New Emerging Fast Charging Microscale Electrode Materials. Small 2024, 20, 2307027. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, X.; Yang, Y.; Liu, T.; Zhang, L. RGO-Coated MOF-Derived In2Se3 as a High-Performance Anode for Sodium-Ion Batteries. Acta Phys.-Chim. Sin. 2023, 39, 202210014. [Google Scholar]
- Gong, A.; Liu, Y.; Zhao, Y.; Li, K. Research progress on material modification and device improvement of capacitive deionization. Ind. Water Treat. 2021, 41, 14–19. [Google Scholar]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Imbrogno, J.; Belfort, G. Membrane Desalination: Where Are We, and What Can We Learn from Fundamentals? Annu. Rev. Chem. Biomol. Eng. 2016, 7, 29–64. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Pan, S.Y.; Snyder, S.W.; Lin, Y.J.; Chiang, P.C. Electrokinetic desalination of brackish water and associated challenges in the water and energy nexus. Environ. Sci.-Water Res. Technol. 2018, 4, 613–638. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Sea water desalination using electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- Suss, M.E.; Presser, V. Water Desalination with Energy Storage Electrode Materials. Joule 2018, 2, 10–15. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Remillard, E.M.; Shocron, A.N.; Rahill, J.; Suss, M.E.; Vecitis, C.D. A direct comparison of flow-by and flow-through capacitive deionization. Desalination 2018, 444, 169–177. [Google Scholar] [CrossRef]
- Yu, F.; Yang, Z.; Cheng, Y.; Xing, S.; Wang, Y.; Ma, J. A comprehensive review on flow-electrode capacitive deionization: Design, active material and environmental application. Sep. Purif. Technol. 2022, 281, 119870. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, K.K.; Eum, H.M.; Lee, C.W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; van der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef]
- Liu, M.; Xu, M.; Xue, Y.; Ni, W.; Huo, S.; Wu, L.; Yang, Z.; Yan, Y.-M. Efficient capacitive deionization using natural basswood-derived, freestanding, hierarchically porous carbon electrodes. Acs Appl. Mater. Interfaces 2018, 10, 31260–31270. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Ohs, B.; Hepp, K.A.; Femmer, R.; Wessling, M. Modeling continuous flow-electrode capacitive deionization processes with ion-exchange membranes. J. Membr. Sci. 2018, 546, 188–196. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Darawsheh, I.F.F.; Govindan, B.; Alhseinat, E.; Banat, F. Ag-Cu bimetallic nanoparticle decorated graphene nanocomposite as an effective anode material for hybrid capacitive deionization (HCDI) system. Electrochim. Acta 2019, 297, 1052–1062. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Xiao, D. Design of a novel asymmetric capacitive deionization device with high desalination performance. Environ. Sci.-Water Res. Technol. 2022, 8, 1938–1953. [Google Scholar] [CrossRef]
- Yong, T.; Li, Y.; Lu, J.; Lin, R.; Wu, J.; Zuo, X. Research progress of flow-electrode capacitive deionization technology. Ind. Water Treat. 2023, 43, 17–25. [Google Scholar]
- Lu, Q.; Liu, P.; Zhou, T.; Huang, R.; Zhang, K.; Hu, L.; Liu, R.; Ren, Z.; Wang, J.; Wang, X. Recent progress on electro-sorption technology for lithium recovery from aqueous sources. Nano Res. 2024, 17, 2563–2573. [Google Scholar] [CrossRef]
- Goodisman, J. Lippmann equation and gouy-chapman model. J. Chim. Phys. Phys.-Chim. Biol. 1975, 72, 143–147. [Google Scholar] [CrossRef]
- Oliva, C.; Zambrano, F.; Wolff, D. Adsorption of univalent and divalent-cations to planar bilayer-membranes containing sulfatids or phosphatiddylserine. Biochem. Int. 1985, 11, 863–868. [Google Scholar]
- Kim, J.; Rotenberg, B. Donnan equilibrium in charged slit-pores from a hybrid nonequilibrium molecular dynamics/monte carlo method with ions and solvent exchange. arXiv 2024, arXiv:2405.18957. [Google Scholar] [CrossRef] [PubMed]
- Pal, B.; Yasin, A.; Kaur, R.; Tebyetekerwa, M.; Zabihi, F.; Yang, S.; Yang, C.-C.; Sofer, Z.; Jose, R. Understanding electrochemical capacitors with in-situ techniques. Renew. Sustain. Energy Rev. 2021, 149, 111418. [Google Scholar] [CrossRef]
- Wang, H.; Forse, A.C.; Griffin, J.M.; Trease, N.M.; Trognko, L.; Taberna, P.-L.; Simon, P.; Grey, C.P. In Situ NMR Spectroscopy of Supercapacitors: Insight into the Charge Storage Mechanism. J. Am. Chem. Soc. 2013, 135, 18968–18980. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-X.; Xing, Y.-Z.; Liu, S.; Ling, Y.-C.; Kleinhammes, A.; Wu, Y. Dehydration of Ions in Voltage-Gated Carbon Nanopores Observed by in Situ NMR. J. Phys. Chem. Lett. 2015, 6, 5022–5026. [Google Scholar] [CrossRef]
- Levi, M.D.; Daikhin, L.; Aurbach, D.; Presser, V. Quartz Crystal Microbalance with Dissipation Monitoring (EQCM-D) for in-situ studies of electrodes for supercapacitors and batteries: A mini-review. Electrochem. Commun. 2016, 67, 16–21. [Google Scholar] [CrossRef]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Levi, M.D.; Salitra, G.; Levy, N.; Aurbach, D.; Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nat. Mater. 2009, 8, 872–875. [Google Scholar] [CrossRef]
- Levi, M.D.; Sigalov, S.; Aurbach, D.; Daikhin, L. In Situ Electrochemical Quartz Crystal Admittance Methodology for Tracking Compositional and Mechanical Changes in Porous Carbon Electrodes. J. Phys. Chem. C 2013, 117, 14876–14889. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Taberna, P.L.; Simon, P. Electrochemical Quartz Crystal Microbalance (EQCM) Study of Ion Dynamics in Nanoporous Carbons. J. Am. Chem. Soc. 2014, 136, 8722–8728. [Google Scholar] [CrossRef]
- Li, T.; Senesi, A.J.; Lee, B. Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev. 2016, 116, 11128–11180. [Google Scholar] [CrossRef]
- Prehal, C.; Weingarth, D.; Perre, E.; Lechner, R.T.; Amenitsch, H.; Paris, O.; Presser, V. Tracking the structural arrangement of ions in carbon supercapacitor nanopores using in situ small-angle X-ray scattering. Energy Environ. Sci. 2015, 8, 1725–1735. [Google Scholar] [CrossRef]
- Gibaud, A.; Xue, J.S.; Dahn, J.R. A small angle X-ray scattering study of carbons made from pyrolyzed sugar. Carbon 1996, 34, 499–503. [Google Scholar] [CrossRef]
- Chen, D.; Ding, D.; Li, X.; Waller, G.H.; Xiong, X.; El-Sayed, M.A.; Liu, M. Probing the Charge Storage Mechanism of a Pseudocapacitive MnO2 Electrode Using in Operando Raman Spectroscopy. Chem. Mater. 2015, 27, 6608–6619. [Google Scholar] [CrossRef]
- Richey, F.W.; Tran, C.; Kalra, V.; Elabd, Y.A. Ionic Liquid Dynamics in Nanoporous Carbon Nanofibers in Supercapacitors Measured with in Operando Infrared Spectroelectrochernistry. J. Phys. Chem. C 2014, 118, 21846–21855. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Wu, M.; Chi, F.; Liu, F.; Bai, J.; Cheng, H.; Li, C.; Qu, L. Maximization of Spatial Charge Density: An Approach to Ultrahigh Energy Density of Capacitive Charge Storage. Angew. Chem.-Int. Ed. 2020, 59, 14541–14549. [Google Scholar] [CrossRef]
- Srimuk, P.; Su, X.; Yoon, J.; Aurbach, D.; Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020, 5, 517–538. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Xiao, D.; Or, T.; Gao, R.; Li, Z.; Feng, M.; Shui, L.; Zhou, G.; Wang, X.; et al. Faradaic electrodes open a new era for capacitive deionization. Adv. Sci. 2020, 7, 2002213. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef]
- Smith, K.C.; Dmello, R. Na-Ion Desalination (NID) Enabled by Na-Blocking Membranes and Symmetric Na-Intercalation: Porous-Electrode Modeling. J. Electrochem. Soc. 2016, 163, A530–A539. [Google Scholar] [CrossRef]
- Shin, Y.-U.; Lim, J.; Boo, C.; Hong, S. Improving the feasibility and applicability of flow-electrode capacitive deionization (FCDI): Review of process optimization and energy efficiency. Desalination 2021, 502, 114930. [Google Scholar] [CrossRef]
- Augustyn, V.; Gogotsi, Y. 2D materials with nanoconfined fluids for electrochemical energy storage. Joule 2017, 1, 443–452. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Hong, T.Z.X.; You, L.; Zhou, K. Carbon-metal compound composite electrodes for capacitive deionization: Synthesis, development and applications. J. Mater. Chem. A 2019, 7, 26693–26743. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, G.; Li, Q.; Xue, H.; Pang, H. Non-noble metal-transition metal oxide materials for electrochemical energy storage. Energy Storage Mater. 2018, 15, 171–201. [Google Scholar] [CrossRef]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. Rsc Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Carpier, S.; Choi, J.; Zornitta, R.L.; Kim, C.; Aslan, M.; Presser, V. Confined redox reactions of lodide in carbon nanopores for fast and energy-efficient desalination of brackish water and seawater. ChemSusChem 2018, 11, 3460–3472. [Google Scholar] [CrossRef]
- Shi, W.H.; Liu, X.Y.; Deng, T.Q.; Huang, S.Z.; Ding, M.; Miao, X.H.; Zhu, C.Z.; Zhu, Y.H.; Liu, W.X.; Wu, F.F.; et al. Enabling Superior Sodium Capture for Efficient Water Desalination by a Tubular Polyaniline Decorated with Prussian Blue Nanocrystals. Adv. Mater. 2020, 32, e1907404. [Google Scholar] [CrossRef]
- Vafakhah, S.; Saeedikhani, M.; Huang, S.; Yan, D.; Leong, Z.Y.; Wang, Y.; Hou, L.; Guo, L.; Alvarado, P.; Yang, H.Y. Tungsten disulfide-reduced GO/CNT aerogel: A tuned interlayer spacing anode for efficient water desalination. J. Mater. Chem. A 2021, 9, 10758–10768. [Google Scholar] [CrossRef]
- Zhao, E.Y.; Nie, K.H.; Yu, X.Q.; Hu, Y.S.; Wang, F.W.; Xiao, J.; Li, H.; Huang, X.J. Advanced Characterization Techniques in Promoting Mechanism Understanding for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707543. [Google Scholar] [CrossRef]
- Jung, H.; Gerasopoulos, K.; Talin, A.A.; Ghodssi, R. A platform for in situ Raman and stress characterizations of V2O5 cathode using MEMS device. Electrochim. Acta 2017, 242, 227–239. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Zhang, J.; Saji, S.E.; Wibowo, A.A.; Rahman, S.; Truong, T.N.; Lu, Y.; Yin, Z.; Macdonald, D.; Nguyen, H.T. Twist-driven wide freedom of indirect interlayer exciton emission in MoS2/WS2 heterobilayers. Cell Rep. Phys. Sci. 2021, 2, 100509. [Google Scholar] [CrossRef]
- Han, J.L.; Yan, T.T.; Shen, J.J.; Shi, L.Y.; Zhang, J.P.; Zhang, D.S. Capacitive Deionization of Saline Water by Using MoS2-Graphene Hybrid Electrodes with High Volumetric Adsorption Capacity. Environ. Sci. Technol. 2019, 53, 12668–12676. [Google Scholar] [CrossRef] [PubMed]
- Srimuk, P.; Lee, J.; Fleischmann, S.; Choudhury, S.; Jaeckel, N.; Zeiger, M.; Kim, C.; Aslan, M.; Presser, V. Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few-layered molybdenum disulfide. J. Mater. Chem. A 2017, 5, 15640–15649. [Google Scholar] [CrossRef]
- Zhang, Q.N.; Levi, M.D.; Dou, Q.Y.; Lu, Y.L.; Chai, Y.G.; Lei, S.L.; Ji, H.X.; Liu, B.; Bu, X.D.; Ma, P.J.; et al. The Charge Storage Mechanisms of 2D Cation-Intercalated Manganese Oxide in Different Electrolytes. Adv. Energy Mater. 2019, 9, 1802707. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Xu, K.L.; Liu, J.; Yan, Z.X.; Jin, M.; Xu, Z.H. Synthesis and use of hollow carbon spheres for electric double-layer capacitors. New Carbon Mater. 2021, 36, 794–809. [Google Scholar] [CrossRef]
- Tseng, Y.F.; Mofarah, S.S.; Zheng, X.; Arandiyan, H.; Wang, Y.; Abbasi, R.; Gao, Y.; Sorrell, C.C.; Koshy, P. Engineering of micro-mesoporous two-dimensional CeO2-based heterojunction oxides for energy storage applications. Surf. Interfaces 2023, 42, 103520. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J. CDI ragone plot as a functional tool to evaluate desalination performance in capacitive deionization. RSC Adv. 2015, 5, 1456–1461. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, H.; Wei, Y.; Huang, Y.; He, H.; Wang, Y.; Meng, Q.; Wu, W. Study of a mixed conductive layer fabricated by ion implantation and distribution Theory. Polymers 2023, 15, 270. [Google Scholar] [CrossRef]
- Eliad, L.; Salitra, G.; Soffer, A.; Aurbach, D. Ion sieving effects in the electrical double layer of porous carbon electrodes: Estimating effective ion size in electrolytic solutions. J. Phys. Chem. B 2001, 105, 6880–6887. [Google Scholar] [CrossRef]
- Macias, C.; Lavela, P.; Rasines, G.; Zafra, M.C.; Tirado, J.L.; Ania, C.O. Improved electro-assisted removal of phosphates and nitrates using mesoporous carbon aerogels with controlled porosity. J. Appl. Electrochem. 2014, 44, 963–976. [Google Scholar] [CrossRef]
- Gao, K.; Lin, X.; Yu, W.; Cheng, X.; Zhang, S.; Li, S.; Zhang, Z. Few-layered ReS2@CNTs as a high-performance cathode for aluminum-ion batteries. Adv. Mater. Interfaces 2022, 9, 2200635. [Google Scholar] [CrossRef]
- Jiao, S.; Zheng, J.; Li, Q.; Li, X.; Engelhard, M.H.; Cao, R.; Zhang, J.G.; Xu, W. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule 2018, 2, 110–124. [Google Scholar] [CrossRef]
- Oyarzun, D.I.; Hemmatifar, A.; Palko, J.W.; Stadermann, M.; Santiago, J.G. Ion selectivity in capacitive deionization with functionalized electrode: Theory and experimental validation. Water Res. X 2018, 1, 100008. [Google Scholar] [CrossRef]
- Chen, X.; Deng, W.; Miao, L.; Gao, M.; Ao, T.; Chen, W.; Ueyama, T.; Dai, Q. Selectivity adsorption of sulfate by amino-modified activated carbon during capacitive deionization. Environ. Technol. 2023, 44, 1505–1517. [Google Scholar] [CrossRef]
- Miao, L.; Deng, W.; Chen, X.; Gao, M.; Chen, W.; Ao, T. Selective adsorption of phosphate by carboxyl-modified activated carbon electrodes for capacitive deionization. Water Sci. Technol. 2021, 84, 1757–1773. [Google Scholar] [CrossRef]
- Liu, P.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Separation and recovery of heavy metal ions and salt ions from wastewater by 3D graphene-based asymmetric electrodes via capacitive deionization. J. Mater. Chem. A 2017, 5, 14748–14757. [Google Scholar] [CrossRef]
- Ji, Q.; An, X.; Liu, H.; Guo, L.; Qu, J. Electric Double-Layer Effects Induce Separation of Aqueous Metal Ions. Acs Nano 2015, 9, 10922–10930. [Google Scholar] [CrossRef]
- Deng, W.; Chen, Y.; Wang, Z.; Chen, X.; Gao, M.; Chen, F.; Chen, W.; Ao, T. Regulation, quantification and application of the effect of functional groups on anion selectivity in capacitive deionization. Water Res. 2022, 222, 118927. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Zhang, J.; Chen, G.; Wang, Z.; Wu, Z. Development of novel ZnZr-COOH/CNT composite electrode for selectively removing phosphate by capacitive deionization. Chem. Eng. J. 2022, 439, 135527. [Google Scholar] [CrossRef]
- Zuo, K.; Huang, X.; Liu, X.; Garcia, E.M.G.; Kim, J.; Jain, A.; Chen, L.; Liang, P.; Zepeda, A.; Verduzco, R.; et al. A Hybrid Metal-Organic Framework-Reduced Graphene Oxide Nanomaterial for Selective Removal of Chromate from Water in an Electrochemical Process. Environ. Sci. Technol. 2020, 54, 13322–13332. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Zhang, W.; Zheng, W. Origin of pseudocapacitance and achieving bulk pseudocapacitance. J. Chin. Ceram. Soc. 2024, 52, 442–453. [Google Scholar]
- Feng, J.; Bai, H.; Xue, Y.; Zhang, R.; Zhu, P.; Bu, D.; Dan, Z.; Li, W.; Lu, X. Recycling of iron and aluminum from drinking water treatment sludge for synthesis of a magnetic composite material (ALCS-Fe-Al) to remove fluoride from drinking water. Groundw. Sustain. Dev. 2020, 11, 100456. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.J.; Seo, D.-H.; Yeom, M.S.; Kang, K.; Kim, D.K.; Jung, Y. Ab initio study of the sodium intercalation and intermediate phases in Na0.44MnO2 for sodium-ion battery. Chem. Mater. 2012, 24, 1205–1211. [Google Scholar] [CrossRef]
- Tsai, S.W.; Cuong, D.V.; Hou, C.H. Selective capture of ammonium ions from municipal wastewater treatment plant effluent with a nickel hexacyanoferrate electrode. Water Res. 2022, 221, 118786. [Google Scholar] [CrossRef]
- Jiang, P.; Shao, H.; Chen, L.; Feng, J.; Liu, Z. Ion-selective copper hexacyanoferrate with an open-framework structure enables high-voltage aqueous mixed-ion batteries. J. Mater. Chem. A 2017, 5, 16740–16747. [Google Scholar] [CrossRef]
- Song, D.; Wang, S.; Liu, R.; Jiang, J.; Jiang, Y.; Huang, S.; Li, W.; Chen, Z.; Zhao, B. Ultra-small SnO2 nanoparticles decorated on three-dimensional nitrogen-doped graphene aerogel for high-performance bind-free anode material. Appl. Surf. Sci. 2019, 478, 290–298. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, X.; Li, Y.; Wang, K.; Lu, T.; Pan, L. Significantly improved stability of hybrid capacitive deionization using nickel hexacyanoferrate/reduced graphene oxide cathode at low voltage operation. Desalination 2019, 468, 114078. [Google Scholar] [CrossRef]
- Zornitta, R.L.; Ruotolo, L.A.M. Corrigendum to “Simultaneous analysis of electrosorption capacity and kinetics for CDI desalination using different electrode configurations” [Chem. Eng. J. 332 (2018) 33–41]. Chem. Eng. J. 2018, 348, 1063. [Google Scholar] [CrossRef]
- Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and Transport Properties of Novel MOF/PIM-1/MOF Sandwich Membranes for Gas Separation. Membranes 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Omosebi, A.; Gao, X.; Landon, J.; Liu, K. Asymmetric Electrode Configuration for Enhanced Membrane Capacitive Deionization. Acs Appl. Mater. Interfaces 2014, 6, 12640–12649. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sahin, S.; Gamaethiralalage, J.G.; Zornitta, R.L.; de Smet, L.C.P.M. Simultaneous, monovalent ion selectivity with polyelectrolyte multilayers and intercalation electrodes in capacitive deionization. Chem. Eng. J. 2022, 432, 128329. [Google Scholar] [CrossRef]
- Nnorom, N.C.; Rogers, T.; Jain, A.; Alazmi, A.; Elias, W.C.; DuChanois, R.M.; Flores, K.; Gardea-Torresdey, J.L.; Cokar, M.; Elimelech, M.; et al. Sulfonated polymer coating enhances selective removal of calcium in membrane capacitive deionization. J. Membr. Sci. 2022, 662, 120974. [Google Scholar] [CrossRef]
- Kim, J.; Jain, A.; Zuo, K.; Verduzco, R.; Walker, S.; Elimelech, M.; Zhang, Z.; Zhang, X.; Li, Q. Removal of calcium ions from water by selective electrosorption using target-ion specific nanocomposite electrode. Water Res. 2019, 160, 445–453. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Choi, J.-H. Enhancement of nitrate removal from a solution of mixed nitrate, chloride and sulfate ions using a nitrate-selective carbon electrode. Desalination 2013, 320, 10–16. [Google Scholar] [CrossRef]
- Uzun, H.I.; Debik, E. Economical approach to nitrate removal via membrane capacitive deionization. Sep. Purif. Technol. 2019, 209, 776–781. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, J.-H. Selective removal of nitrate ion using a novel composite carbon electrode in capacitive deionization. Water Res. 2012, 46, 6033–6039. [Google Scholar] [CrossRef]
- Gan, L.; Wu, Y.; Song, H.; Zhang, S.; Lu, C.; Yang, S.; Wang, Z.; Jiang, B.; Wang, C.; Li, A. Selective removal of nitrate ion using a novel activated carbon composite carbon electrode in capacitive deionization. Sep. Purif. Technol. 2019, 212, 728–736. [Google Scholar] [CrossRef]
- Zuo, K.; Kim, J.; Jain, A.; Wang, T.; Verduzco, R.; Long, M.; Li, Q. Novel Composite Electrodes for Selective Removal of Sulfate by the Capacitive Deionization Process. Environ. Sci. Technol. 2018, 52, 9486–9494. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chai, L.; Liu, M.; Shu, Y.; Li, Q.; Wang, Y.; Qiu, D. Effect of the electronegativity on the electrosorption selectivity of anions during capacitive deionization. Chemosphere 2018, 195, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Rajabzadeh, S.; Tijing, L.; Shon, H.K. Advances in capacitive deionization (CDI) systems for nutrient recovery from wastewater: Paving the path towards a circular economy. Desalination 2024, 583, 117695. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)—Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef]
- Balaji Wright, A.; Wu, J.; Shocron, A.N.; Dana, A.G.; Suss, M.; Mani, A. Understanding degradation of capacitive deionization cells: Full-cell simulations with anode corrosion. Desalination 2024, 587, 117924. [Google Scholar] [CrossRef]
- Cohen, I.; Avraham, E.; Bouhadana, Y.; Soffer, A.; Aurbach, D. The effect of the flow-regime, reversal of polarization, and oxygen on the long term stability in capacitive de-ionization processes. Electrochim. Acta 2015, 153, 106–114. [Google Scholar] [CrossRef]
- Shapira, B.; Avraham, E.; Aurbach, D. Side reactions in capacitive deionization (CDI) processes: The role of oxygen reduction. Electrochim. Acta 2016, 220, 285–295. [Google Scholar] [CrossRef]
- Lee, J.-H.; Bae, W.-S.; Choi, J.-H. Electrode reactions and adsorption/desorption performance related to the applied potential in a capacitive deionization process. Desalination 2010, 258, 159–163. [Google Scholar] [CrossRef]
- Srimuk, P.; Ries, L.; Zeiger, M.; Fleischmann, S.; Jaeckel, N.; Tolosa, A.; Kruener, B.; Aslan, M.; Presser, V. High performance stability of titania decorated carbon for desalination with capacitive deionization in oxygenated water. Rsc Adv. 2016, 6, 106081–106089. [Google Scholar] [CrossRef]
- Celebanska, A.; Opallo, M. Layer-by-layer gold-ceramic nanoparticulate electrodes for electrocatalysis. Chemelectrochem 2016, 3, 1629–1634. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts. J. Hazard. Mater. 2013, 244–245, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liang, H.; Bai, L.; Liu, B.; Zhu, X.; Wang, J.; Xing, J.; Ren, N.; Li, G. Desalination performance and fouling mechanism of capacitive deionization: Effects of natural organic matter. J. Electrochem. Soc. 2020, 167, 043501. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.; Lee, S.; Lee, K.-B.; Jung, K.-W.; Choi, J.-W. Pretreatment for capacitive deionization: Feasibility tests using activated filter media and granule activated carbon filtration. J. Ind. Eng. Chem. 2021, 93, 253–258. [Google Scholar] [CrossRef]
- Xu, Q.; He, R.; Li, Y. Problems and Mistakes for Electron Transfer Mechanism in Z-Scheme Photocatalytic System. Acta Phys.-Chim. Sin. 2023, 39, 2211009. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Yuan, L.; Zhao, R.; Qin, B.; Zhang, F.; Ren, L.; Yang, H.; Yuan, M. Selective Ion Separation by Capacitive Deionization: A Comprehensive Review. Materials 2025, 18, 1107. https://doi.org/10.3390/ma18051107
Xu F, Yuan L, Zhao R, Qin B, Zhang F, Ren L, Yang H, Yuan M. Selective Ion Separation by Capacitive Deionization: A Comprehensive Review. Materials. 2025; 18(5):1107. https://doi.org/10.3390/ma18051107
Chicago/Turabian StyleXu, Fanyi, Ling Yuan, Rui Zhao, Bing Qin, Feng Zhang, Liming Ren, Hailun Yang, and Menglei Yuan. 2025. "Selective Ion Separation by Capacitive Deionization: A Comprehensive Review" Materials 18, no. 5: 1107. https://doi.org/10.3390/ma18051107
APA StyleXu, F., Yuan, L., Zhao, R., Qin, B., Zhang, F., Ren, L., Yang, H., & Yuan, M. (2025). Selective Ion Separation by Capacitive Deionization: A Comprehensive Review. Materials, 18(5), 1107. https://doi.org/10.3390/ma18051107








