Development of a Gold Nanoparticle Dispersion for Plasma Jet Printing on Solid Substrates
Abstract
1. Introduction
- Plasma jet printing inherently modifies the surface of the ceramic substrate through localised plasma treatment. This enhances surface energy and promotes strong adhesion of AuNPs—an essential factor when working with inert, non-porous substrates like ceramics [23];
- The plasma environment contributes to partial in situ sintering or activation of nanoparticles during deposition. This can reduce or even eliminate the need for high-temperature post-processing, which is especially valuable for maintaining the structural integrity of ceramic substrates or avoiding thermal mismatch issues [19,23];
- Unlike inkjet systems, which are sensitive to viscosity and prone to nozzle clogging when using nanoparticle dispersions, plasma jet systems are more robust and tolerant to a broader range of ink viscosities and particle sizes. This ensures reliable, consistent patterning without maintenance interruptions [11];
- Plasma jet printing supports fine feature resolution while maintaining a non-contact fabrication approach. This minimises mechanical damage and contamination risk on delicate or high-value substrates like technical ceramics [20].
2. Materials and Methods
2.1. AuNP Synthesis
2.2. Preparation of the AuNP Dispersion
2.3. Plasma Jet Printing of AuNP Dispersion on Al2O3
2.4. Characterisation of the AuNP Dispersion and AuNPs
2.4.1. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.4.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDS)
2.4.3. Transmission Electron Microscopy (TEM) and Energy Dispersive X-Ray Spectroscopy (EDS)
2.4.4. UV-Vis Spectroscopy
2.4.5. Dynamic Light Scattering (DLS) and Zeta Potential
2.4.6. Thermogravimetric Analysis (TGA)
2.4.7. Rheology Characteristics
2.4.8. Surface Roughness of the Printed Line
2.4.9. Statistical Analysis
3. Results
3.1. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
3.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy Analyses (EDS) Results of AuNPs
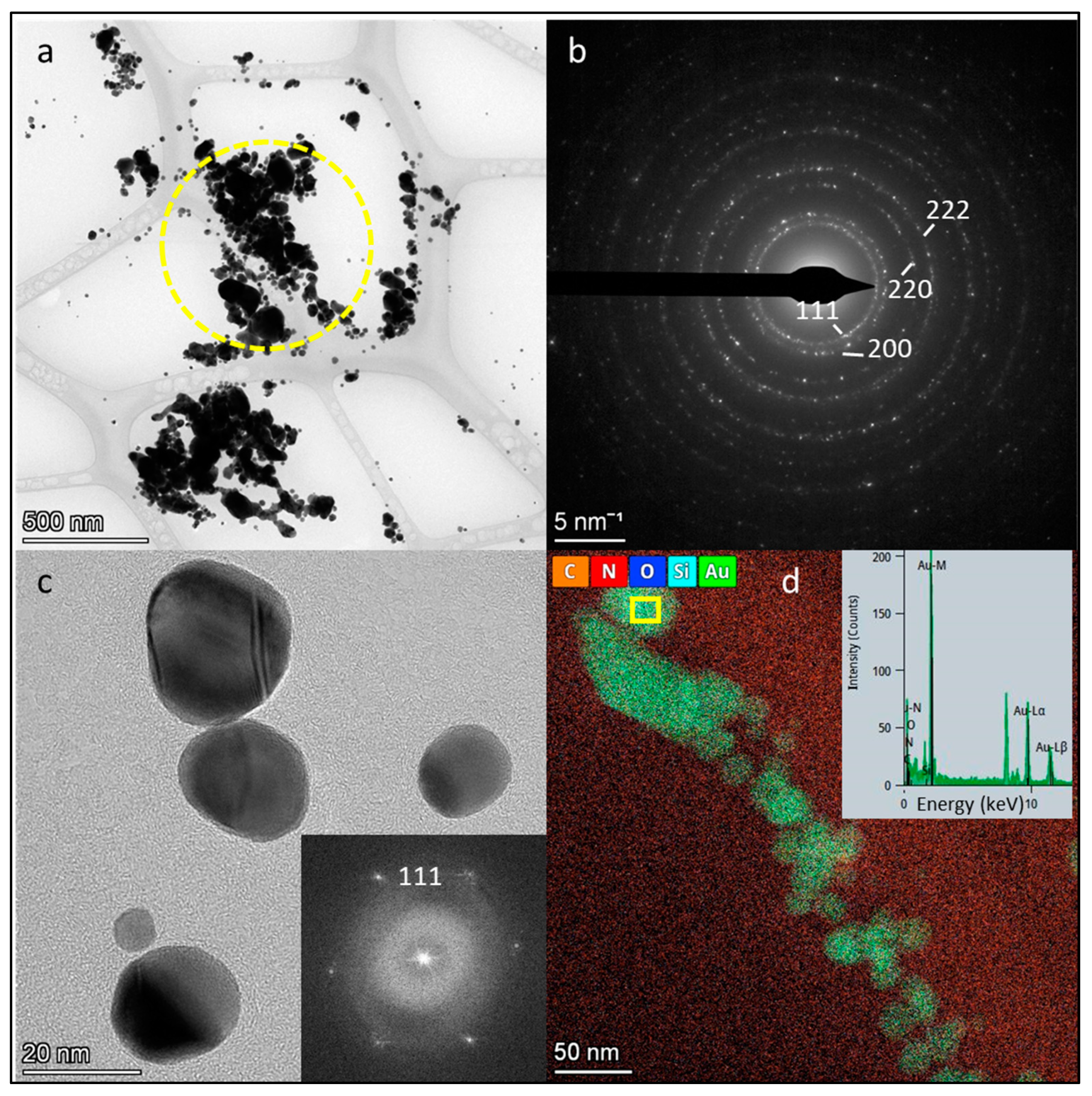
3.3. Transmission Electron Microscopy (TEM) and Selected Area Electron Diffraction (SAED), Scanning Transmission Electron Microscopy (STEM) and Energy Dispersive X-Ray Spectroscopy (EDS) Analyses of AuNPs
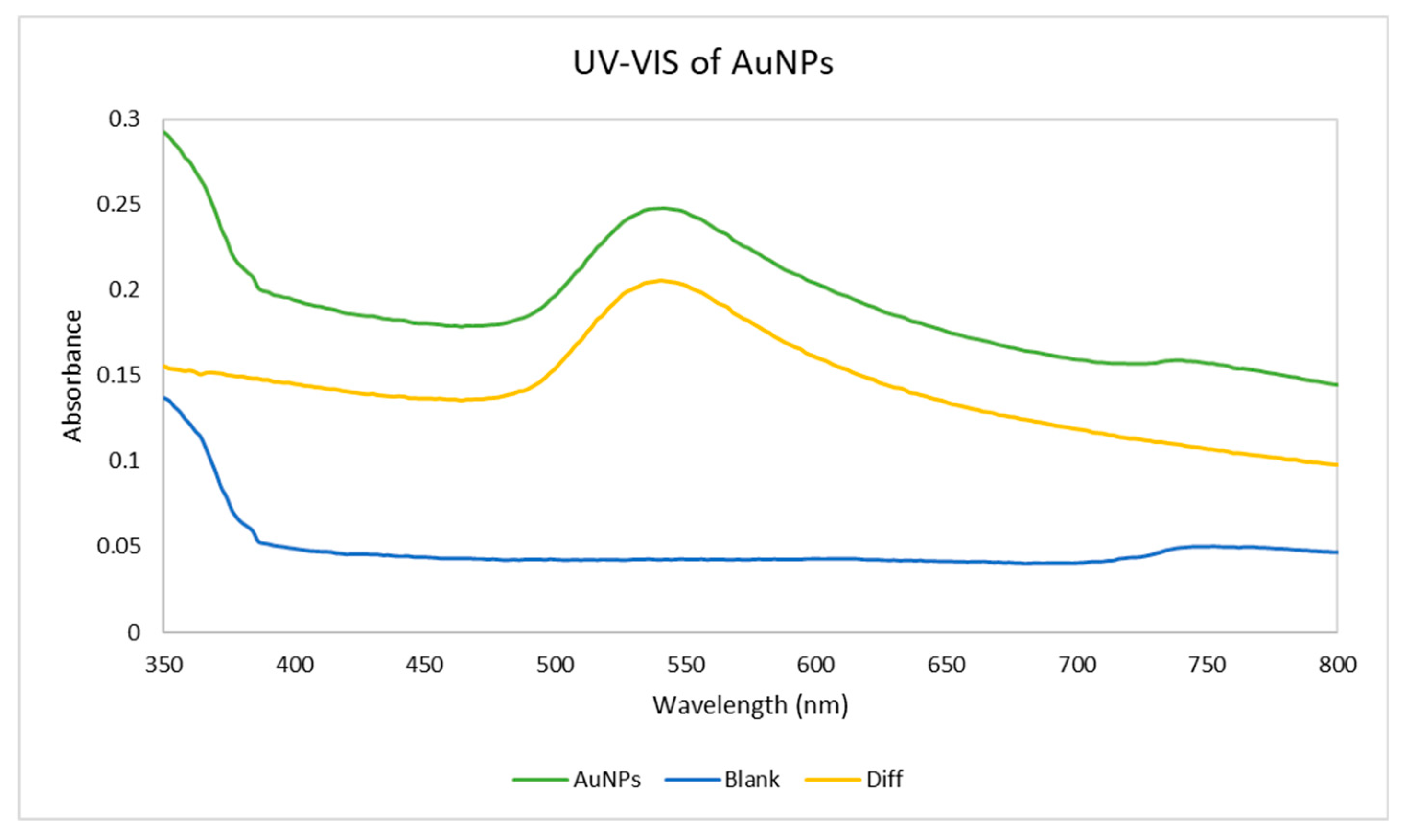
3.4. UV-Vis Spectroscopy
3.5. Dynamic Light Scattering Results (DLS)
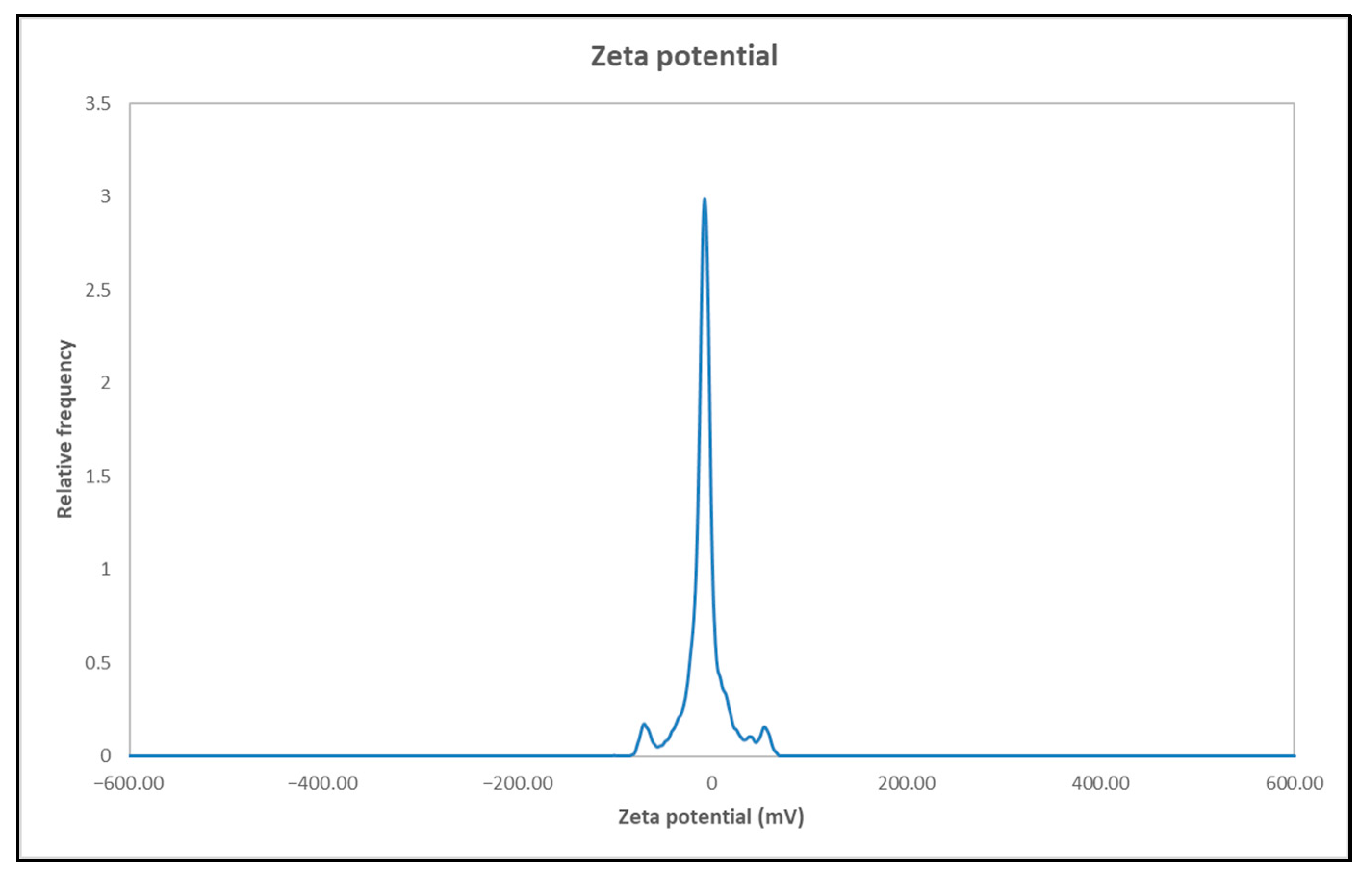
3.6. Zeta Potential Results
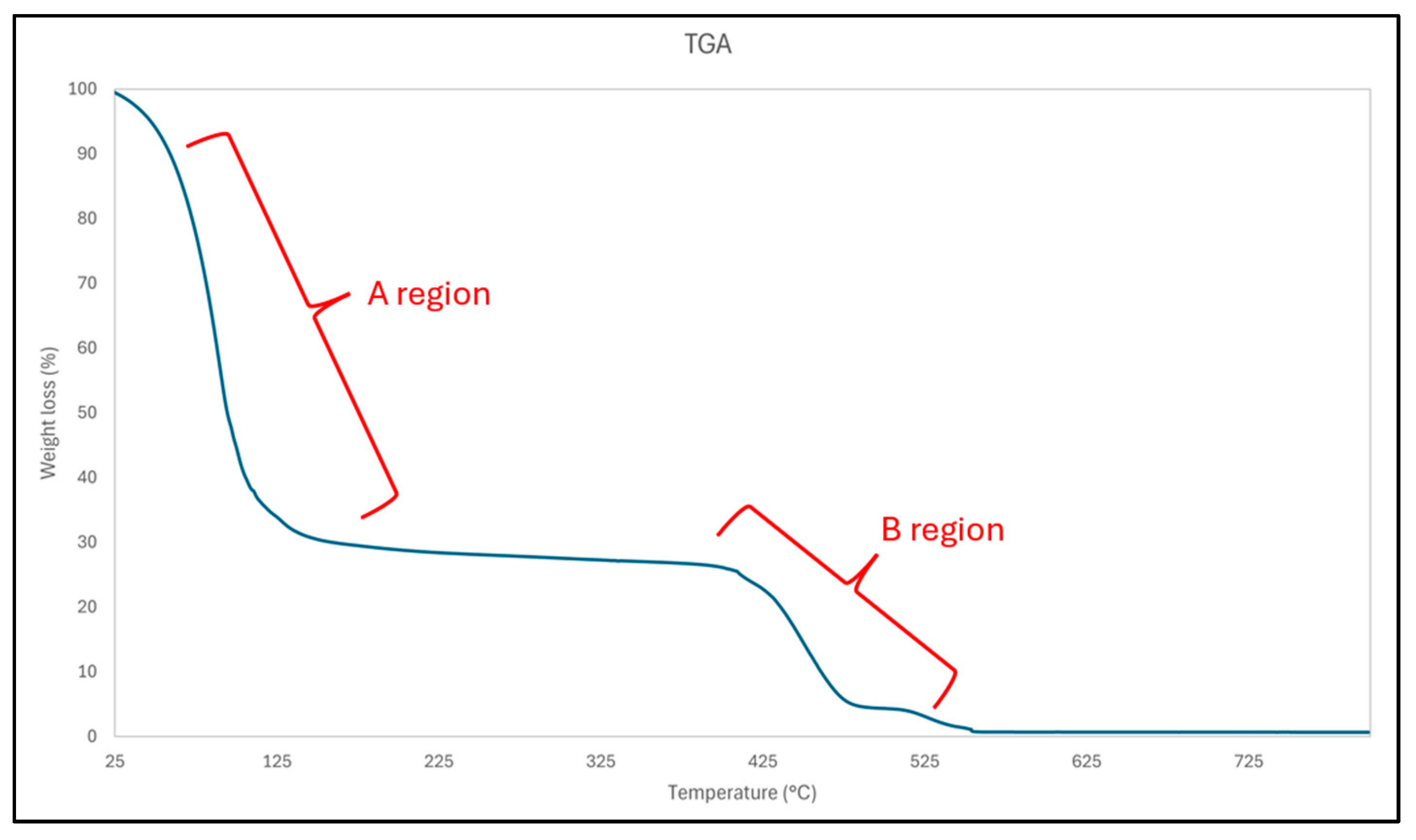
3.7. Thermogravimetric Analysis Results (TGA)
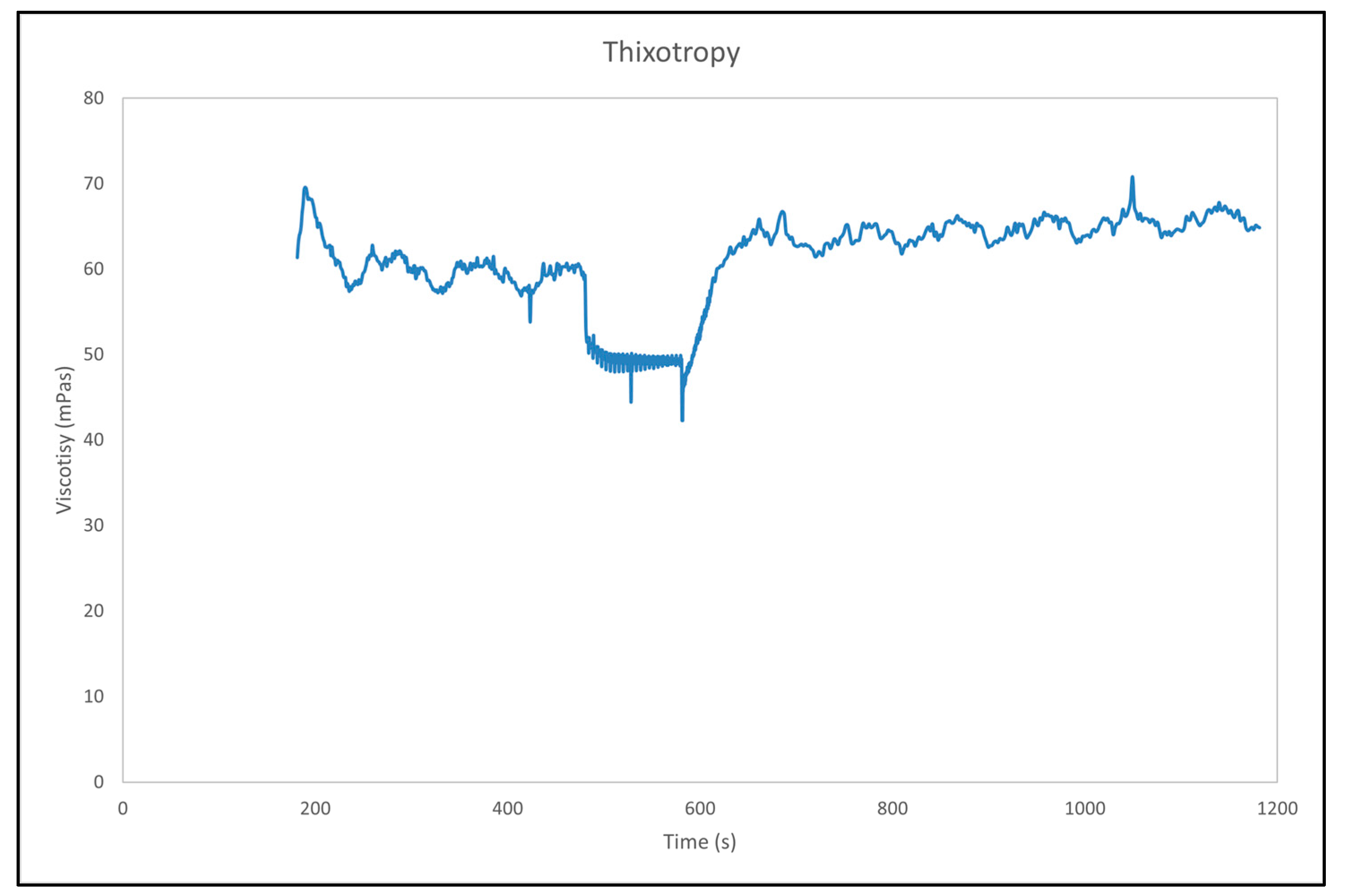
3.8. Rheology Measurement
3.9. Analysis of the Printed Lines
4. Discussion
5. Conclusions
- The synthesised AuNP dispersion is suitable for plasma jet printing, enabling the successful deposition of AuNP lines on Al2O3 with an achieved thickness of approximately 8 μm;
- The printed lines are purple in colour, confirming the presence of AuNPs; however, PVP remains present on the lines;
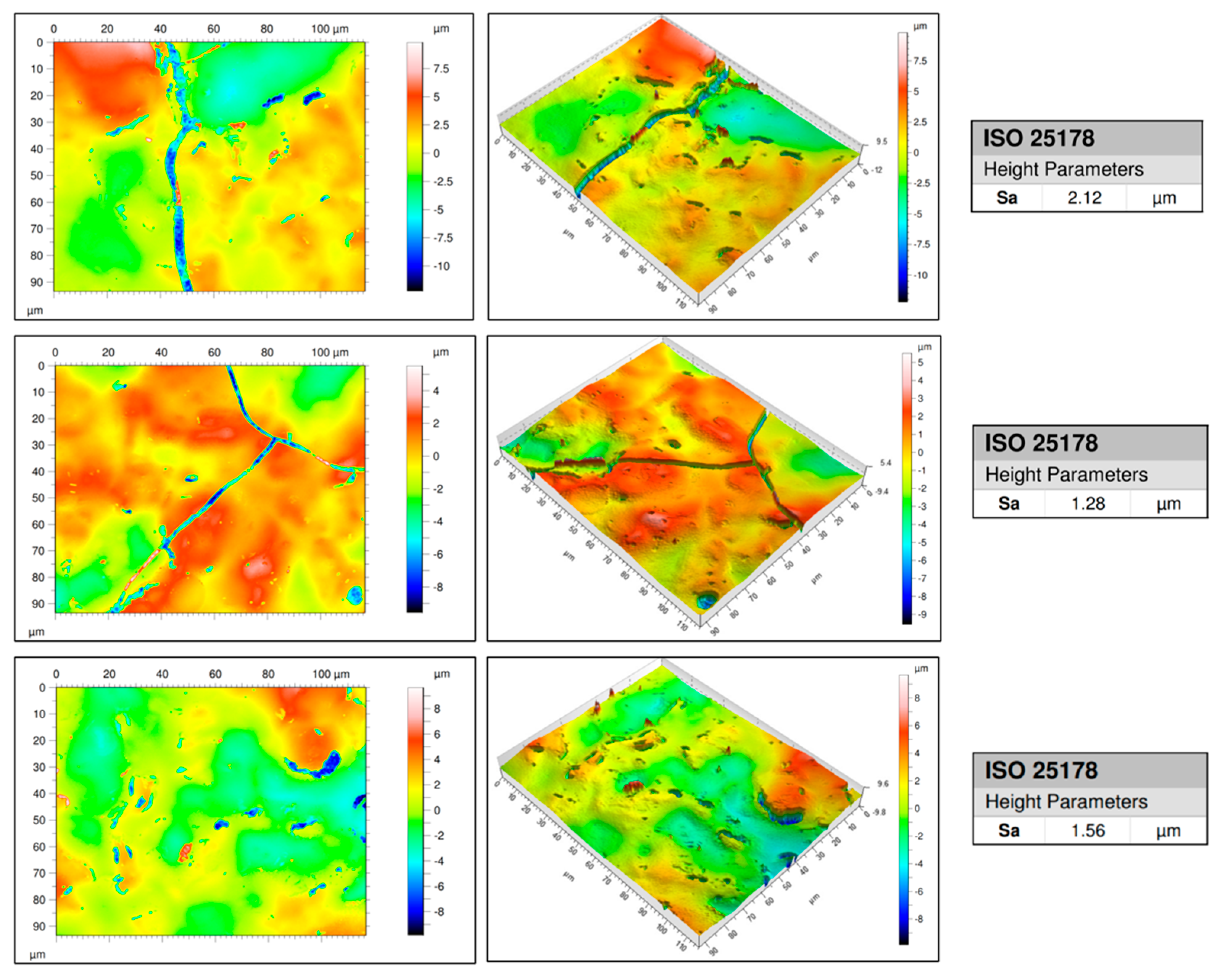
- The surface roughness of the printed lines ranged from 1.28 μm to 2.12 μm, thereby meeting the requirement for RF applications (<2.8 μm). This result demonstrates the potential for further development. The smoothness achieved is advantageous for applications in PCBs, particularly in high-frequency signal transmission, where surface quality is crucial.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Norman, A.; Das, S.; Rohr, T.; Ghidini, T. Advanced manufacturing for space applications. CEAS Space J. 2023, 15, 1–6. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous nanomaterials employed in the development of electrochemical sensors based on screen-printing technique—A review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for printed electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D printed electronics: Processes, materials and future trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Lemarchand, J.; Bridonneau, N.; Battaglini, N.; Carn, F.; Mattana, G.; Piro, B.; Zrig, S.; Noël, V. Challenges, Prospects, and Emerging Applications of Inkjet-Printed Electronics: A Chemist’s Point of View. Angew. Chem. Int. Ed. 2022, 61, e202200166. [Google Scholar] [CrossRef] [PubMed]
- Rezaga, B.F.Y.; Balela, M.D.L. Chemical sintering of Ag nanoparticle conductive inks at room temperature for printable electronics. J. Mater. Sci. Mater. Electron. 2021, 32, 17764–17779. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Aroche, A.F.; Schuck, A.; Lamberty, P.; Peter, C.R.; Hasenkamp, W.; Rocha, T.L.A.C. Silver nanoparticle conductive inks: Synthesis, characterization, and fabrication of inkjet-printed flexible electrodes. Sci. Rep. 2020, 10, 8878. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Li, Z.; Yang, J.; Lan, H. Preparation of nano silver paste and applications in transparent electrodes via electric-field driven micro-scale 3D printing. Nanomaterials 2020, 10, 107. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Ma, B.; Pei, Y.; Ai, C.; Yuan, L. Fabrication of micron-SiO2@nano-Ag based conductive line patterns through silk-screen printing. RSC Adv. 2014, 4, 47781–47787. [Google Scholar] [CrossRef]
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453. [Google Scholar] [CrossRef]
- Yan, K.; Li, J.; Pan, L.; Shi, Y. Inkjet printing for flexible and wearable electronics. APL Mater. 2020, 8, 120705. [Google Scholar] [CrossRef]
- Rao, C.H.; Avinash, K.; Varaprasad, B.K.S.V.L.; Goel, S. A Review on Printed Electronics with Digital 3D Printing: Fabrication Techniques, Materials, Challenges and Future Opportunities. J. Electron. Mater. 2022, 51, 2747–2765. [Google Scholar] [CrossRef]
- Hassan, K.; Nine, M.J.; Tung, T.T.; Stanley, N.; Yap, P.L.; Rastin, H.; Yu, L.; Losic, D. Functional inks and extrusion-based 3D printing of 2D materials: A review of current research and applications. Nanoscale 2020, 12, 19007–19042. [Google Scholar] [CrossRef] [PubMed]
- Htwe, Y.Z.N.; Mariatti, M. Printed graphene and hybrid conductive inks for flexible, stretchable, and wearable electronics: Progress, opportunities, and challenges. J. Sci. Adv. Mater. Devices 2022, 7, 100435. [Google Scholar] [CrossRef]
- Jafarpour, M.; Nüesch, F.; Heier, J.; Abdolhosseinzadeh, S. Functional Ink Formulation for Printing and Coating of Graphene and Other 2D Materials: Challenges and Solutions. Small Sci. 2022, 2, 2200040. [Google Scholar] [CrossRef]
- Gandhiraman, R.P.; Jayan, V.; Han, J.W.; Chen, B.; Koehne, J.E.; Meyyappan, M. Plasma jet printing of electronic materials on flexible and nonconformal objects. ACS Appl. Mater. Interfaces 2014, 6, 20860–20867. [Google Scholar] [CrossRef]
- Doshi, P.; Li, Z.; Langer, F.; Gutierrez, D.H.; Benson, N.; Nordlund, D.; Senesky, D.G.; Gandhiraman, R.P. Plasma Jet Printing of Diamond and Silicon. IEEE J. Flex. Electron. 2024, 3, 252–258. [Google Scholar] [CrossRef]
- Hagger, O.S.J.; Parkes, M.A.; Estrin, F.L.; Agrotis, S.; Parkin, I.P.; Handoko, A.D.; Caruana, D.J. Additive metal printing on multi materials using an atmospheric pressure plasma jet on a 5-Axis platform. Mater. Des. 2025, 251, 113681. [Google Scholar] [CrossRef]
- Gandhiraman, R.P.; Singh, E.; Diaz-Cartagena, D.C.; Nordlund, D.; Koehne, J.; Meyyappan, M. Plasma jet printing for flexible substrates. Appl. Phys. Lett. 2016, 108, 123103. [Google Scholar] [CrossRef]
- Ramamurti, R.; Gandhiraman, R.P.; Lopez, A.; Doshi, P.; Nordlund, D.; Kim, B.; Meyyappan, M. Atmospheric pressure plasma printing of nanomaterials for IoT applications. IEEE Open J. Nanotechnol. 2020, 1, 47–56. [Google Scholar] [CrossRef]
- Manzi, J.; Weltner, A.E.; Varghese, T.; McKibben, N.; Busuladzic-Begic, M.; Estrada, D.; Subbaraman, H. Plasma-jet printing of colloidal thermoelectric Bi2Te3 nanoflakes for flexible energy harvesting. Nanoscale 2023, 15, 6596–6606. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, I.; Hendriks, C.E.; Eckardt, R.; Kranenburg, J.M.; Perelaer, J.; Baumann, R.R.; Schubert, U.S. Argon plasma sintering of inkjet printed silver tracks on polymer substrates. J. Mater. Chem. 2009, 19, 3384–3388. [Google Scholar] [CrossRef]
- Estrin, F.L.; Hagger, O.S.J.; Sener, M.E.; Caruana, D.J. Metal Painting by Plasma Jet. Adv. Mater. Interfaces 2024, 11, 2400256. [Google Scholar] [CrossRef]
- Coonrod, J. Insertion Loss Comparisons of Common High Frequency PCB Constructions. In Proceedings of the IPC APEX EXPO Conference 2013, San Diego, CA, USA, 21 February 2013. [Google Scholar]
- Ghadimi, S.; Mazinani, S.; Bazargan, A.M.; Sharif, F. Effect of formulation and process on morphology and electrical conductivity of Ag-graphene hybrid inks. Synth. Met. 2021, 281, 116913. [Google Scholar] [CrossRef]
- Li, W.; Sun, Q.; Li, L.; Jiu, J.; Liu, X.Y.; Kanehara, M.; Minari, T.; Suganuma, K. The rise of conductive copper inks: Challenges and perspectives. Appl. Mater. Today 2020, 18, 100451. [Google Scholar] [CrossRef]
- Parvez, K.; Worsley, R.; Alieva, A.; Felten, A.; Casiraghi, C. Water-based and inkjet printable inks made by electrochemically exfoliated graphene. Carbon 2019, 149, 213–221. [Google Scholar] [CrossRef]
- Bishop, P.T.; Ashfield, L.J.; Berzins, A.; Boardman, A.; Buche, V.; Cookson, J.; Gordon, R.J.; Salcianu, C.; Sutton, P.A. Printed Gold for Electronic Applications. 2010. Available online: www.goldbulletin.org (accessed on 20 April 2025).
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Majerič, P.; Bračič, M.; Anžel, I.; Rudolf, R. Gold inks for inkjet printing on photo paper: Complementary characterization. Nanomaterials 2021, 11, 599. [Google Scholar] [CrossRef]
- Varghese, T.V.; Eixenberger, J.; Rajabi-Kouchi, F.; Lazouskaya, M.; Francis, C.; Burgoyne, H.; Wada, K.; Subbaraman, H.; Estrada, D. Multijet Gold Nanoparticle Inks for Additive Manufacturing of Printed and Wearable Electronics. ACS Mater. Au 2024, 4, 65–73. [Google Scholar] [CrossRef]
- Reiser, B.; González-García, L.; Kanelidis, I.; Maurer, J.H.M.; Kraus, T. Gold nanorods with conjugated polymer ligands: Sintering-free conductive inks for printed electronics. Chem. Sci. 2016, 7, 4190–4196. [Google Scholar] [CrossRef]
- Qin, Y.; Ouyang, X.; Lv, Y.; Liu, W.; Liu, Q.; Wang, S. A Review of Carbon-Based Conductive Inks and Their Printing Technologies for Integrated Circuits. Coatings 2023, 13, 1769. [Google Scholar] [CrossRef]
- Le Porcher, B.; Rieu, M.; Viricelle, J.P. Development of Gold Inks for Inkjet Printing of Gas Sensors Electrodes on Plastic Support. Electronics 2024, 13, 2110. [Google Scholar] [CrossRef]
- Juric, D.; Hao, H.; Ermantraut, E.; Glaser, K.; Eberhardt, W.; Zimmermann, A. Laser Irradiation for Improving the Wetting of Nanoparticle Gold Inks for Printed Electronics. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 325–331. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Štager, V.; Albreht, B. Process for the Production of Gold Nanoparticles by Modified Ultrasonic Spray Pyrolysis. SI26000A, 30 November 2021. [Google Scholar]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and their Advanced Energy, Environmental and Biomedical Applications. Chem. Asian J. 2021, 16, 720–742. [Google Scholar] [CrossRef]
- Ortega-Córdova, R.; Sánchez-Carillo, K.; Carrasco-Saavedra, S.; Ramírez-García, G.; Pérez-García, M.G.; Soltero-Martínez, J.F.A.; Mota-Morales, J.D. Polyvinylpyrrolidone-mediated synthesis of ultra-stable gold nanoparticles in a nonaqueous choline chloride–urea deep eutectic solvent. RSC Appl. Interfaces 2024, 1, 600–611. [Google Scholar] [CrossRef]
- Loría-Bastarrachea, M.I.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Vázquez-Torres, H.; Ávila-Ortega, A. A TG/FTIR study on the thermal degradation of poly(vinyl pyrrolidone). J. Therm. Anal. Calorim. 2011, 104, 737–742. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147–148, 214–227. [Google Scholar] [CrossRef]
- Diaz, F.; Stopić, S.; Friedrich, B. Scaling Up of Nanopowder Collection in the Process of Ultrasonic Spray Pyrolysis. World Met. Erzmetall 2017, 70, 97–101. [Google Scholar]
- Workie, A.B.; Ningsih, H.S.; Shih, S.J. An comprehensive review on the spray pyrolysis technique: Historical context, operational factors, classifications, and product applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Ardekani, S.R.; Aghdam, A.S.R.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A comprehensive review on ultrasonic spray pyrolysis technique: Mechanism, main parameters and applications in condensed matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Milad, G. Minimizing Signal Transmission Loss in High-Frequency Circuits, Uyemura International Corporation, July 2020. Available online: https://www.uyemura.com/pdfs/UIC-HF-story-July2020.pdf (accessed on 20 April 2025).
- Ihle, M.; Reinhardt, K.; Gierth, P. Printed Electronics for High Frequencies: 6G Wireless Communication Technology and Millimeter Wave Radar. Fraunhofer Institute for Ceramic Technologies and Systems, 10 May 2022. Available online: https://www.ikts.fraunhofer.de/content/dam/ikts/downloads/whitepaper/whitepaper-printed-electronics-for-high-frequencies.pdf (accessed on 20 April 2025).
- Cui, W.; Lu, W.; Zhang, Y.; Lin, G.; Wei, T.; Jiang, L. Gold nanoparticle ink suitable for electric-conductive pattern fabrication using in ink-jet printing technology. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 35–41. [Google Scholar] [CrossRef]
- Feng, J.; Gao, Y.; Zhang, F.; Ma, M.; Gu, Y.; Liu, Z.; Chen, K. Effects of organic binder on rheological behaviors and screen-printing performance of silver pastes for LTCC applications. J. Mater. Sci. Mater. Electron. 2022, 33, 10774–10784. [Google Scholar] [CrossRef]




| Sample Name | mg/mL Au |
|---|---|
| AuNPs | 2.81 |
| AuNP dispersion | 0.246 |
| Results | AuNPs |
|---|---|
| Hydrodynamic diameter | 180 nm |
| Polydispersity index | 25% |
| Standard Deviation | 2.93 nm |
| Spectrum | C | O | Al | Au |
|---|---|---|---|---|
| Spectrum 1 | 19.74 | 51.64 | 27.21 | / |
| Spectrum 2 | 75.23 | 24.59 | / | 0.18 |
| Spectrum 3 | 76.42 | 22.88 | / | 0.70 |
| Spectrum 4 | 83.80 | 15.18 | / | 1.02 |
| Spectrum 5 | 75.29 | 24.05 | / | 0.33 |
| Spectrum 6 | 5.62 | 56.46 | 37.91 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kresnik, L.; Majerič, P.; Feizpour, D.; Rudolf, R. Development of a Gold Nanoparticle Dispersion for Plasma Jet Printing on Solid Substrates. Materials 2025, 18, 2713. https://doi.org/10.3390/ma18122713
Kresnik L, Majerič P, Feizpour D, Rudolf R. Development of a Gold Nanoparticle Dispersion for Plasma Jet Printing on Solid Substrates. Materials. 2025; 18(12):2713. https://doi.org/10.3390/ma18122713
Chicago/Turabian StyleKresnik, Lan, Peter Majerič, Darja Feizpour, and Rebeka Rudolf. 2025. "Development of a Gold Nanoparticle Dispersion for Plasma Jet Printing on Solid Substrates" Materials 18, no. 12: 2713. https://doi.org/10.3390/ma18122713
APA StyleKresnik, L., Majerič, P., Feizpour, D., & Rudolf, R. (2025). Development of a Gold Nanoparticle Dispersion for Plasma Jet Printing on Solid Substrates. Materials, 18(12), 2713. https://doi.org/10.3390/ma18122713









