Differential lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells Following Co-Exposure to Silver Nanoparticles and Nanoplastic
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Characterization
2.2. Cell Culture
2.3. Neutral Red Assay
2.4. Analysis of lncRNA Expression by Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
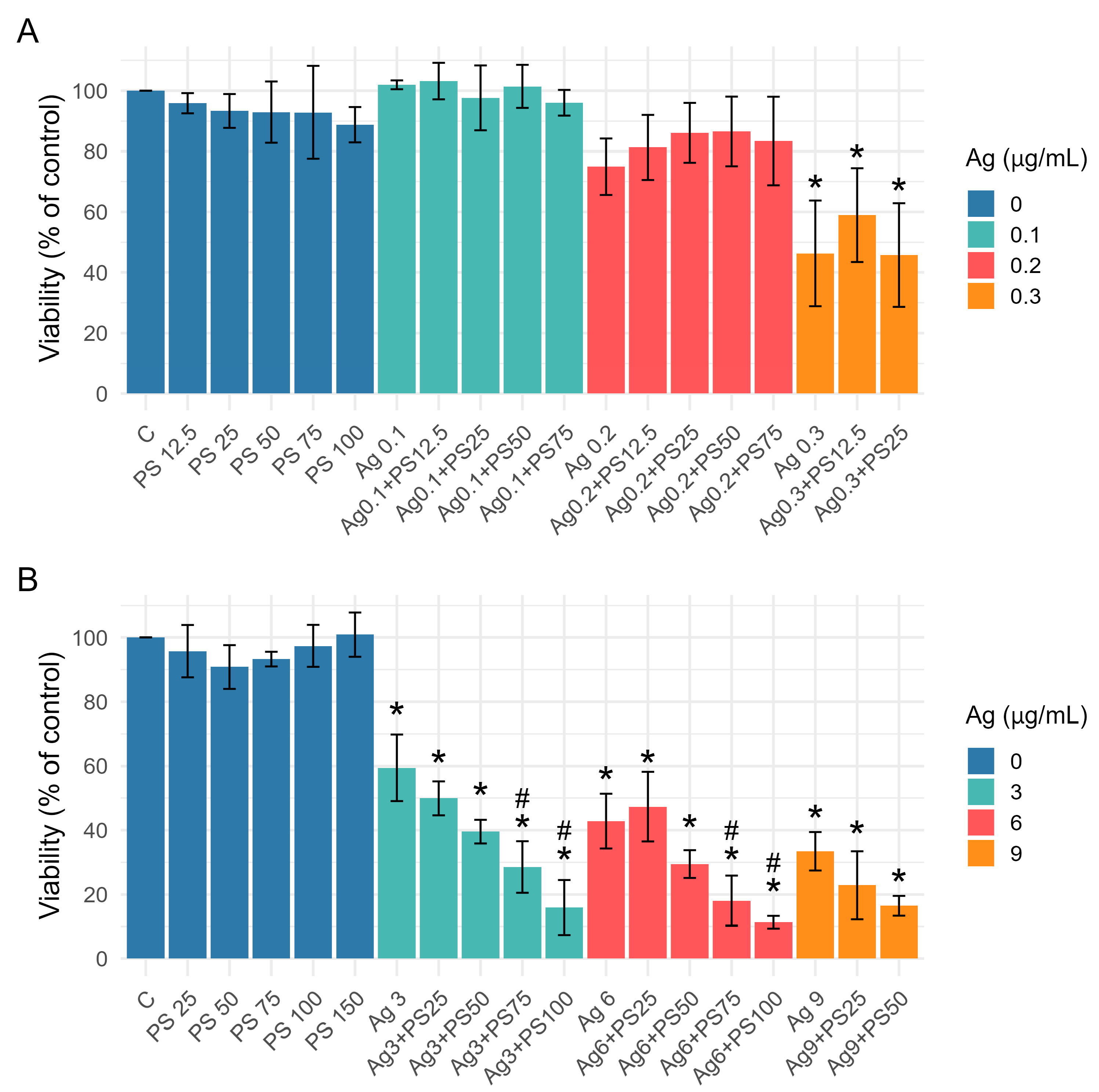
3.2. Effect of AgNPs and PSNPs Treatment on Viability of Undifferentiated and Differentiated LUHMES Cells
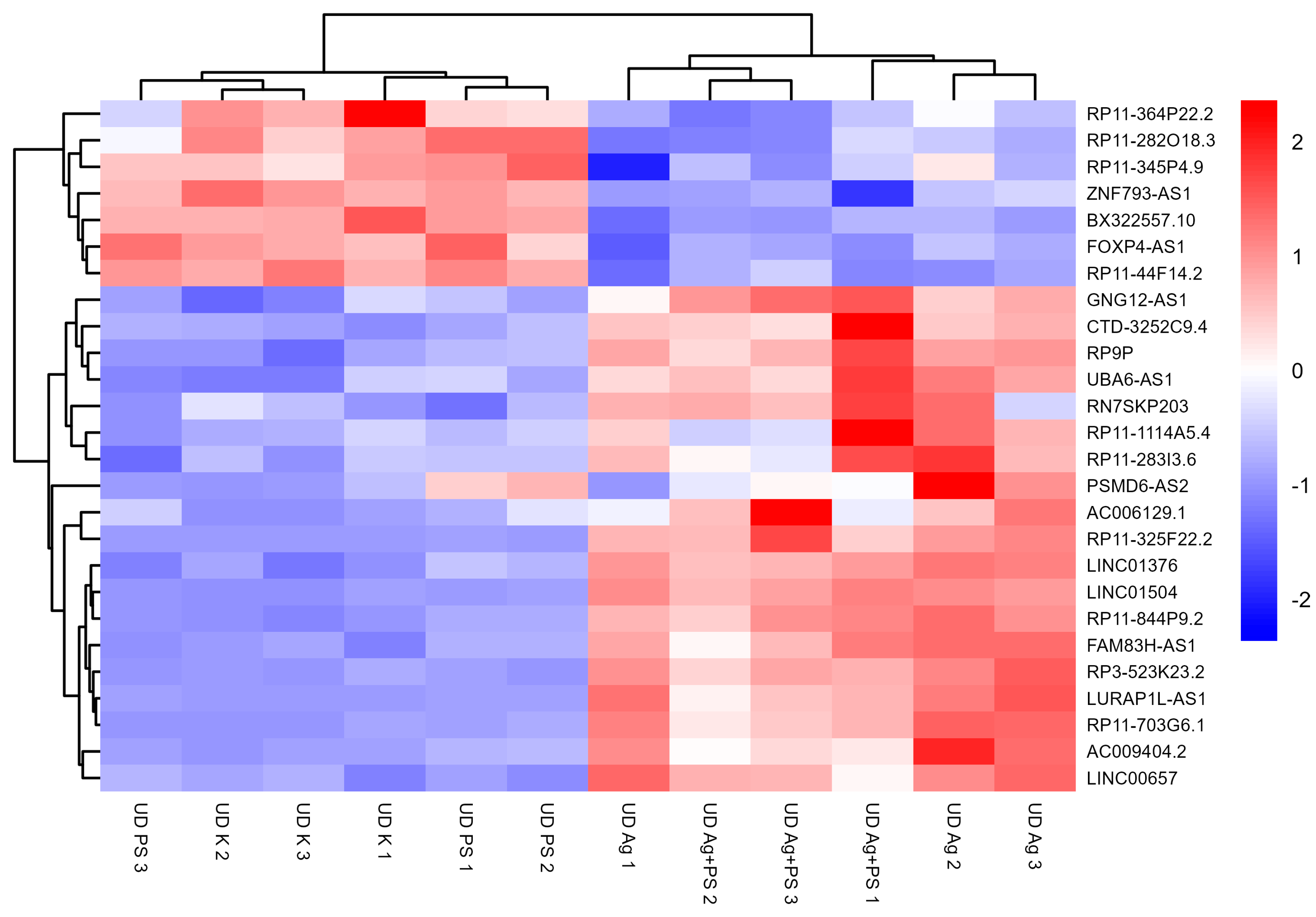
3.3. lncRNA Expression in Undifferentiated LUHMES Cells
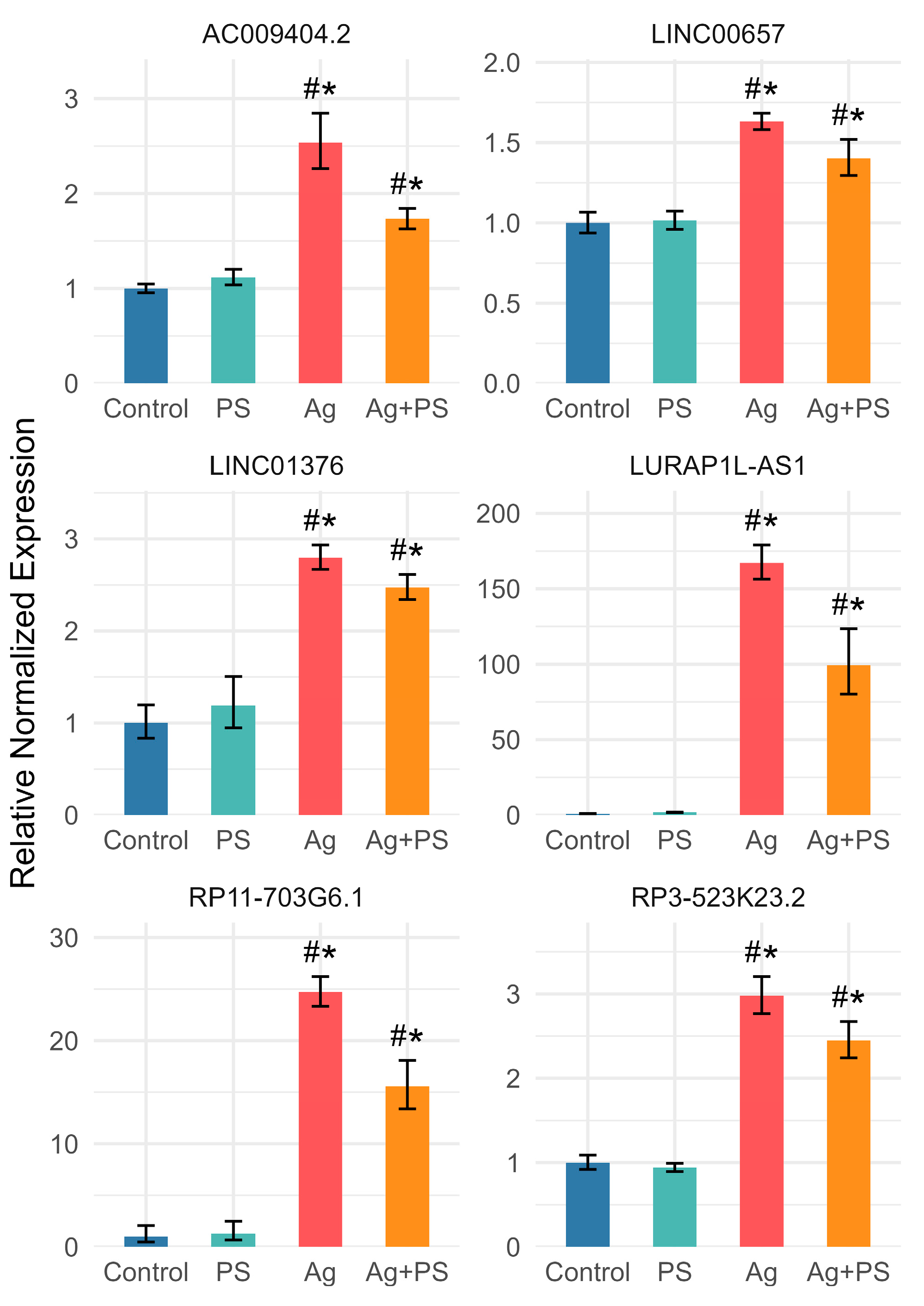
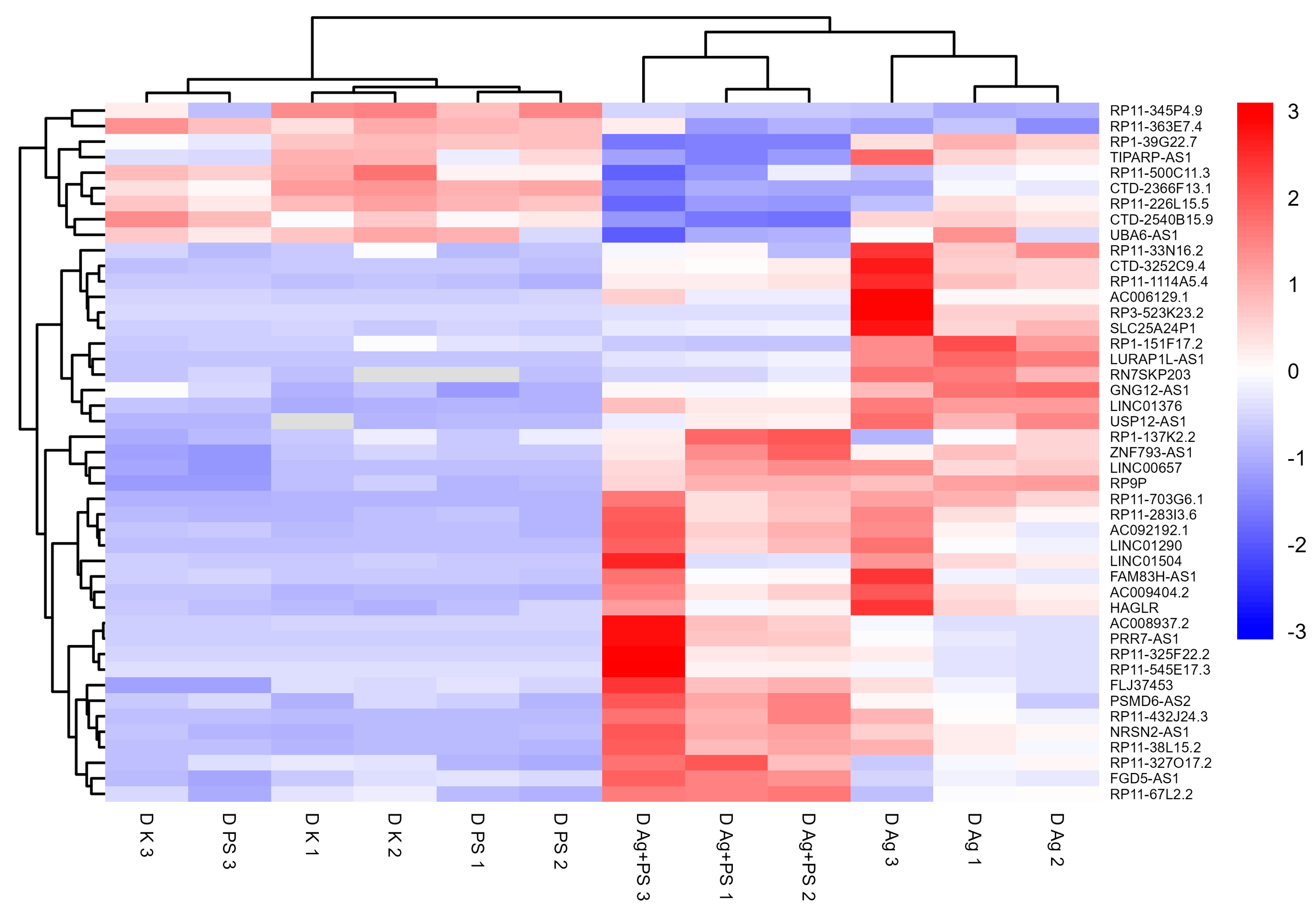
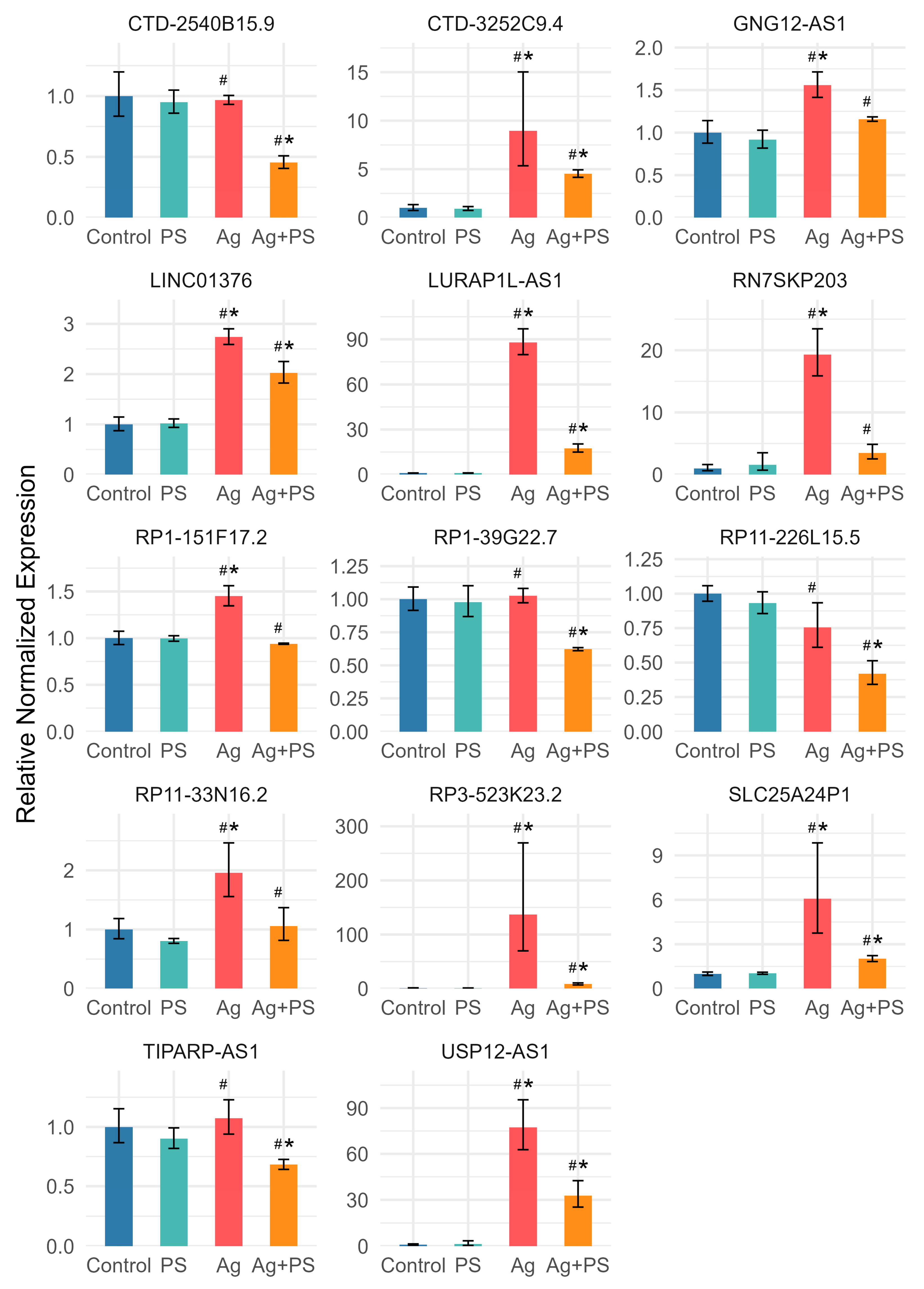
3.4. lncRNA Expression in Differentiated LUHMES Cells
3.5. Comparison of lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahra, Z.; Habib, Z.; Hyun, S.; Sajid, M. Nanowaste: Another Future Waste, Its Sources, Release Mechanism, and Removal Strategies in the Environment. Sustainability 2022, 14, 2041. [Google Scholar] [CrossRef]
- Shukla, S.; Khanna, S.; Khanna, K. Unveiling the Toxicity of Micro-Nanoplastics: A Systematic Exploration of Understanding Environmental and Health Implications. Toxicol. Rep. 2025, 14, 101844. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of Micro- and Nanoplastic Contamination in the Food Chain. Food Addit. Contam. Part. A 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic Contamination of the Food Chain: A Threat to Human Health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Li, H.; Zhu, L.; Ma, M.; Wu, H.; An, L.; Yang, Z. Occurrence of Microplastics in Commercially Sold Bottled Water. Sci. Total Environ. 2023, 867, 161553. [Google Scholar] [CrossRef]
- Cabrejos-Cardeña, U.; De-la-Torre, G.E.; Dobaradaran, S.; Rangabhashiyam, S. An Ecotoxicological Perspective of Microplastics Released by Face Masks. J. Hazard. Mater. 2023, 443, 130273. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A Threat to Human Health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.S.; Bai, Y.L.; Jin, C.H.; Na, J.; Zhang, R.; Gao, Y.; Pan, G.W.; Yan, L.J.; Sun, W. Evidence on Invasion of Blood, Adipose Tissues, Nervous System and Reproductive System of Mice After a Single Oral Exposure: Nanoplastics versus Microplastics. Biomed. Environ. Sci. 2022, 35, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, S.; Liu, J.; Liu, Z. The Effects of Micro- and Nanoplastics on the Central Nervous System: A New Threat to Humanity? Toxicology 2024, 504, 153799. [Google Scholar] [CrossRef]
- Sikorska, K.; Sawicki, K.; Czajka, M.; Kapka-Skrzypczak, L.; Kruszewski, M.; Brzóska, K. Adverse Effects of Non-Metallic Nanoparticles in the Central Nervous System. Materials 2023, 16, 7264. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K.; Gromadzka-Ostrowska, J.; Kruszewski, M. Nanoplastic Impact on the Gut-Brain Axis: Current Knowledge and Future Directions. Int. J. Mol. Sci. 2021, 22, 12795. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain Damage and Behavioural Disorders in Fish Induced by Plastic Nanoparticles Delivered through the Food Chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of Nanoparticles in the See-through Medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef]
- Yang, C.-S.; Chang, C.-H.; Tsai, P.-J.; Chen, W.-Y.; Tseng, F.-G.; Lo, L.-W. Nanoparticle-Based in Vivo Investigation on Blood−Brain Barrier Permeability Following Ischemia and Reperfusion. Anal. Chem. 2004, 76, 4465–4471. [Google Scholar] [CrossRef]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef]
- Hoelting, L.; Scheinhardt, B.; Bondarenko, O.; Schildknecht, S.; Kapitza, M.; Tanavde, V.; Tan, B.; Lee, Q.Y.; Mecking, S.; Leist, M.; et al. A 3-Dimensional Human Embryonic Stem Cell (hESC)-Derived Model to Detect Developmental Neurotoxicity of Nanoparticles. Arch. Toxicol. 2013, 87, 721–733. [Google Scholar] [CrossRef]
- Murali, K.; Kenesei, K.; Li, Y.; Demeter, K.; Környei, Z.; Madarász, E. Uptake and Bio-Reactivity of Polystyrene Nanoparticles Is Affected by Surface Modifications, Ageing and LPS Adsorption: In Vitro Studies on Neural Tissue Cells. Nanoscale 2015, 7, 4199–4210. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Matos, C.; Bellas, J.; Monteiro, S.M.; Félix, L. Microplastics Alone or Co-Exposed with Copper Induce Neurotoxicity and Behavioral Alterations on Zebrafish Larvae after a Subchronic Exposure. Aquat. Toxicol. 2021, 235, 105814. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Luzio, A.; Bellas, J.; Monteiro, S.M. Microplastics- and Copper-Induced Changes in Neurogenesis and DNA Methyltransferases in the Early Life Stages of Zebrafish. Chem.-Biol. Interact. 2022, 363, 110021. [Google Scholar] [CrossRef] [PubMed]
- Estrela, F.N.; Guimarães, A.T.B.; Araújo, A.P.d.C.; Silva, F.G.; da Luz, T.M.; Silva, A.M.; Pereira, P.S.; Malafaia, G. Toxicity of Polystyrene Nanoplastics and Zinc Oxide to Mice. Chemosphere 2021, 271, 129476. [Google Scholar] [CrossRef]
- Liu, X.; Yang, H.; Yan, X.; Xu, S.; Fan, Y.; Xu, H.; Ma, Y.; Hou, W.; Javed, R.; Zhang, Y. Co-Exposure of Polystyrene Microplastics and Iron Aggravates Cognitive Decline in Aging Mice via Ferroptosis Induction. Ecotoxicol. Environ. Saf. 2022, 233, 113342. [Google Scholar] [CrossRef]
- Jin, Y.; Li, M.; Chen, F.; Wang, L.; Zhang, L.; Yang, Z.; Wang, N.; Fu, J.; Yu, Y.; Cheng, X.; et al. Secondary Pollution of Microplastic Hetero-Aggregates after Chlorination: Released Contaminants Rarely Re-Adsorbed by the Second-Formed Hetero-Aggregates. J. Hazard. Mater. 2023, 445, 130523. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Abass, D.; Villacorta, A.; Hernández, A.; Marcos, R. Antagonistic in Vivo Interaction of Polystyrene Nanoplastics and Silver Compounds. A Study Using Drosophila. Sci. Total Environ. 2022, 842, 156923. [Google Scholar] [CrossRef]
- Ilić, K.; Krce, L.; Rodriguez-Ramos, J.; Rico, F.; Kalčec, N.; Aviani, I.; Turčić, P.; Pavičić, I.; Vinković Vrček, I. Cytotoxicity of Nanomixture: Combined Action of Silver and Plastic Nanoparticles on Immortalized Human Lymphocytes. J. Trace Elem. Med. Biol. 2022, 73, 127004. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Ding, S.; Wei, Z.; Ma, Y. Biotoxicity of Silver Nanoparticles Complicated by the Co-Existence of Micro-/Nano-Plastics. Food Chem. Toxicol. 2024, 193, 115020. [Google Scholar] [CrossRef]
- Song, J.; Pu, Q.; Chen, C.; Liu, X.; Zhang, X.; Wang, Z.; Yan, J.; Wang, X.; Wang, H.; Qian, Q. Neurological Outcomes of Joint Exposure to Polystyrene Micro/Nanospheres and Silver Nanoparticles in Zebrafish. Environ. Health Perspect. 2025, 133, 57007. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, Y.; Wang, Y.; Wang, Y.; Sun, G.; Jiang, Y. Toxic Effects on Ciliates under Nano-/Micro-Plastics Coexist with Silver Nanoparticles. J. Hazard. Mater. 2024, 465, 133058. [Google Scholar] [CrossRef] [PubMed]
- Stępkowski, T.M.; Wasyk, I.; Grzelak, A.; Kruszewski, M. 6-OHDA-Induced Changes in Parkinson’s Disease-Related Gene Expression Are Not Affected by the Overexpression of PGAM5 in In Vitro Differentiated Embryonic Mesencephalic Cells. Cell Mol. Neurobiol. 2015, 35, 1137–1147. [Google Scholar] [CrossRef]
- Lotharius, J.; Falsig, J.; van Beek, J.; Payne, S.; Dringen, R.; Brundin, P.; Leist, M. Progressive Degeneration of Human Mesencephalic Neuron-Derived Cells Triggered by Dopamine-Dependent Oxidative Stress Is Dependent on the Mixed-Lineage Kinase Pathway. J. Neurosci. 2005, 25, 6329–6342. [Google Scholar] [CrossRef]
- Zuberek, M.; Stępkowski, T.M.; Kruszewski, M.; Grzelak, A. Exposure of human neurons to silver nanoparticles induces similar pattern of ABC transporters gene expression as differentiation: Study on proliferating and post-mitotic LUHMES cells. Mech. Ageing Dev. 2018, 171, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.K.; Covarrubias, S.; Carpenter, S. The How and Why of lncRNA Function: An Innate Immune Perspective. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2020, 1863, 194419. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-Coding RNA Networks in Cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Vance, K.W.; Ponting, C.P. Transcriptional Regulatory Functions of Nuclear Long Noncoding RNAs. Trends Genet. 2014, 30, 348–355. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Tierney, L.; Cady, N.C.; Murray, T.M.; Chittur, S.V.; Reliene, R. Surface Coatings Alter Transcriptional Responses to Silver Nanoparticles Following Oral Exposure. NanoImpact 2020, 17, 100205. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, S.; Zhang, W. Epigenetic Effects of Silver Nanoparticles and Ionic Silver in Tetrahymena Thermophila. Sci. Total Environ. 2021, 768, 144659. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Zhang, Y.-X.; Liu, S.-Z.; Reza, A.M.M.T.; Wang, J.-L.; Li, L.; Cai, H.-Q.; Zhong, P.; Kong, I.-K. Multiple RNA Profiling Reveal Epigenetic Toxicity Effects of Oxidative Stress by Graphene Oxide Silver Nanoparticles In-Vitro. Int. J. Nanomed. 2023, 18, 2855–2871. [Google Scholar] [CrossRef]
- Tao, L.; Chen, X.; Sun, J.; Wu, C. Silver Nanoparticles Achieve Cytotoxicity against Breast Cancer by Regulating Long-Chain Noncoding RNA XLOC_006390-Mediated Pathway. Toxicol. Res. 2021, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhao, B.; Chen, M.; Liu, Y.; Xu, M.; Wang, Z.; Liu, S.; Zhang, C. Nrf-2-Driven Long Noncoding RNA ODRUL Contributes to Modulating Silver Nanoparticle-Induced Effects on Erythroid Cells. Biomaterials 2017, 130, 14–27. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, W.; Zhang, Y.; Ning, J.; Pang, Y.; Hu, H.; Chen, M.; Wu, M.; Wang, M.; Yang, P.; et al. Comprehensive Analysis of lncRNA-mRNA Expression Profiles in Depression-like Responses of Mice Related to Polystyrene Nanoparticle Exposure. Toxics 2023, 11, 600. [Google Scholar] [CrossRef]
- Yang, S.; Ge, Y.; Zhang, T.; Yin, L.; Pu, Y.; Chen, Z.; Liang, G. Dynamic Non-Coding RNA Biomarker Reveals Lung Injury and Repair Induced by Polystyrene Nanoplastics. Environ. Int. 2025, 195, 109266. [Google Scholar] [CrossRef]
- Qu, M.; Zhao, Y.; Zhao, Y.; Rui, Q.; Kong, Y.; Wang, D. Identification of Long Non-Coding RNAs in Response to Nanopolystyrene in Caenorhabditis Elegans after Long-Term and Low-Dose Exposure. Environ. Pollut. 2019, 255, 113137. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Pöltl, D.; Genewsky, A.; Weng, M.; Waldmann, T.; Schildknecht, S.; Leist, M. Rapid, Complete and Large-Scale Generation of Post-Mitotic Neurons from the Human LUHMES Cell Line. J. Neurochem. 2011, 119, 957–971. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Gao, C.-H.; Yu, G.; Cai, P. ggVennDiagram: An Intuitive, Easy-to-Use, and Highly Customizable R Package to Generate Venn Diagram. Front. Genet. 2021, 12, 706907. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty Heatmaps, R Package Version 1.0.12. 2018. Available online: https://github.com/raivokolde/pheatmap (accessed on 4 June 2025).
- Dziendzikowska, K.; Węsierska, M.; Gromadzka-Ostrowska, J.; Wilczak, J.; Oczkowski, M.; Męczyńska-Wielgosz, S.; Kruszewski, M. Silver Nanoparticles Impair Cognitive Functions and Modify the Hippocampal Level of Neurotransmitters in a Coating-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 12706. [Google Scholar] [CrossRef]
- Kruszewski, M.; Brzoska, K.; Brunborg, G.; Asare, N.; Dobrzyńska, M.; Dušinská, M.; Fjellsbø, L.M.; Georgantzopoulou, A.; Gromadzka-Ostrowska, J.; Gutleb, A.C.; et al. Chapter Five—Toxicity of Silver Nanomaterials in Higher Eukaryotes. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 5, pp. 179–218. [Google Scholar]
- Domenech, J.; Cortés, C.; Vela, L.; Marcos, R.; Hernández, A. Polystyrene Nanoplastics as Carriers of Metals. Interactions of Polystyrene Nanoparticles with Silver Nanoparticles and Silver Nitrate, and Their Effects on Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 859. [Google Scholar] [CrossRef]
- Pierce, S.E.; Tyson, T.; Booms, A.; Prahl, J.; Coetzee, G.A. Parkinson’s Disease Genetic Risk in a Midbrain Neuronal Cell Line. Neurobiol. Dis. 2018, 114, 53–64. [Google Scholar] [CrossRef]
- Tüshaus, J.; Kataka, E.S.; Zaucha, J.; Frishman, D.; Müller, S.A.; Lichtenthaler, S.F. Neuronal Differentiation of LUHMES Cells Induces Substantial Changes of the Proteome. Proteomics 2021, 21, 2000174. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, G.; Liu, X.; Tang, T.-S.; Guo, C.; Liu, H. Polymerases and DNA Repair in Neurons: Implications in Neuronal Survival and Neurodegenerative Diseases. Front. Cell. Neurosci. 2022, 16, 852002. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.L.; Cui, J.Y. Long Non-Coding RNAs: A Novel Paradigm for Toxicology. Toxicol. Sci. 2017, 155, 3–21. [Google Scholar] [CrossRef]
- Miguel, V.; Lamas, S.; Espinosa-Diez, C. Role of Non-Coding-RNAs in Response to Environmental Stressors and Consequences on Human Health. Redox Biol. 2020, 37, 101580. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Jing, Z.; Yuan, X.; Zhang, J.; Huang, X.; Zhang, Z.; Liu, J.; Zhang, M.; Oyang, J.; Zhang, Y.; Zhang, Z.; et al. Chromosome 1 Open Reading Frame 190 Promotes Activation of NF-κB Canonical Pathway and Resistance of Dendritic Cells to Tumor-Associated Inhibition in Vitro. J. Immunol. 2010, 185, 6719–6727. [Google Scholar] [CrossRef]
- Ren, X.; Li, L.; Wu, J.; Lin, K.; He, Y.; Bian, L. PDGF-BB Regulates the Transformation of Fibroblasts into Cancer-Associated Fibroblasts via the lncRNA LURAP1L-AS1/LURAP1L/IKK/IκB/NF-κB Signaling Pathway. Oncol. Lett. 2021, 22, 537. [Google Scholar] [CrossRef]
- Yang, H.; Niu, S.; Guo, M.; Xue, Y. Molecular Mechanisms of Silver Nanoparticle-Induced Neurotoxic Injury and New Perspectives for Its Neurotoxicity Studies: A Critical Review. Environ. Pollut. 2024, 362, 124934. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Lin, Y.; Zou, X.; Li, Z.; Liao, Y.; Du, M.; Zhang, H. Endothelial Cell Injury and Dysfunction Induced by Silver Nanoparticles through Oxidative Stress via IKK/NF-κB Pathways. Biomaterials 2014, 35, 6657–6666. [Google Scholar] [CrossRef]
- Stępkowski, T.M.; Brzóska, K.; Kruszewski, M. Silver Nanoparticles Induced Changes in the Expression of NF-κB Related Genes Are Cell Type Specific and Related to the Basal Activity of NF-κB. Toxicol. Vitr. 2014, 28, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Zhang, X.; Cai, H.; Zhang, C.; Qu, H.; Liu, L.; Zhang, M.; Fu, J.; Zhang, J.; et al. Oxidative Stress Activates NORAD Expression by H3K27ac and Promotes Oxaliplatin Resistance in Gastric Cancer by Enhancing Autophagy Flux via Targeting the miR-433-3p. Cell Death Dis. 2021, 12, 90. [Google Scholar] [CrossRef]
- Lei, N.; Kong, P.; Chen, S.; Wang, Q.; Tang, X.; Liu, F. Upregulated NORAD Is Implicated in Apoptosis, Inflammation, and Oxidative Stress in Ulcerative Colitis through the Nuclear Factor-κappaB Signaling. Eur. J. Gastroenterol. Hepatol. 2022, 34, 630–639. [Google Scholar] [CrossRef]
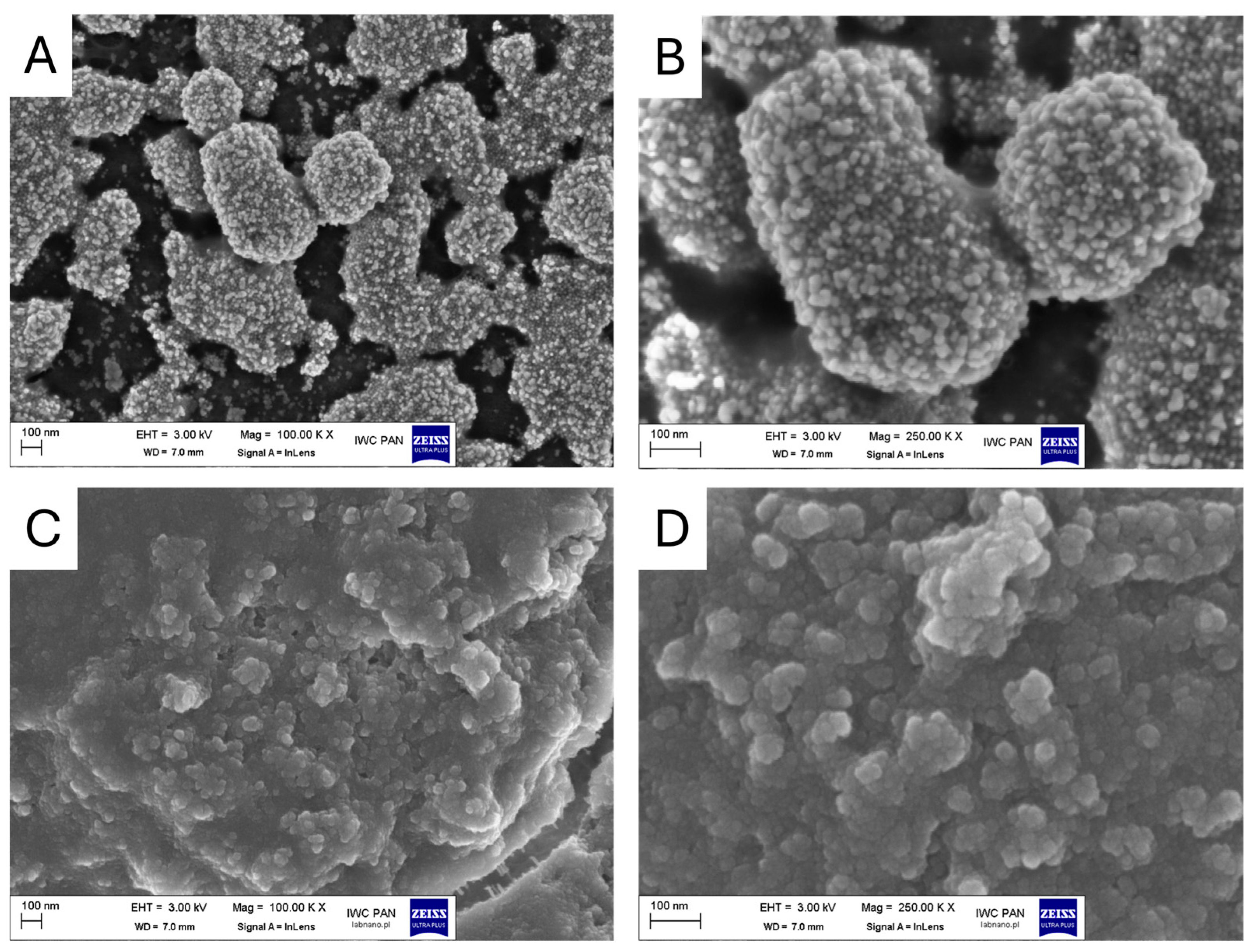


| Medium | Parameter | PSNPs | AgNPs | PSNPs + AgNPs |
|---|---|---|---|---|
| PBS | Mean (nm) | 159 ± 18 | 251 ± 47 | 233 ± 18 |
| Mode (nm) | 120 ± 21 | 182 ± 14 | 170 ± 11 | |
| DMEM/F-12 | Mean (nm) | 92 ± 1 | 280 ± 32 | 289 ± 50 |
| Mode (nm) | 87 ± 7 | 233 ± 57 | 281 ± 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzóska, K.; Czerwińska, M.; Kruszewski, M. Differential lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells Following Co-Exposure to Silver Nanoparticles and Nanoplastic. Materials 2025, 18, 2690. https://doi.org/10.3390/ma18122690
Brzóska K, Czerwińska M, Kruszewski M. Differential lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells Following Co-Exposure to Silver Nanoparticles and Nanoplastic. Materials. 2025; 18(12):2690. https://doi.org/10.3390/ma18122690
Chicago/Turabian StyleBrzóska, Kamil, Malwina Czerwińska, and Marcin Kruszewski. 2025. "Differential lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells Following Co-Exposure to Silver Nanoparticles and Nanoplastic" Materials 18, no. 12: 2690. https://doi.org/10.3390/ma18122690
APA StyleBrzóska, K., Czerwińska, M., & Kruszewski, M. (2025). Differential lncRNA Expression in Undifferentiated and Differentiated LUHMES Cells Following Co-Exposure to Silver Nanoparticles and Nanoplastic. Materials, 18(12), 2690. https://doi.org/10.3390/ma18122690








