Interfacial Behavior During Reactions Between Sn and Electroplated Co–Zn Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
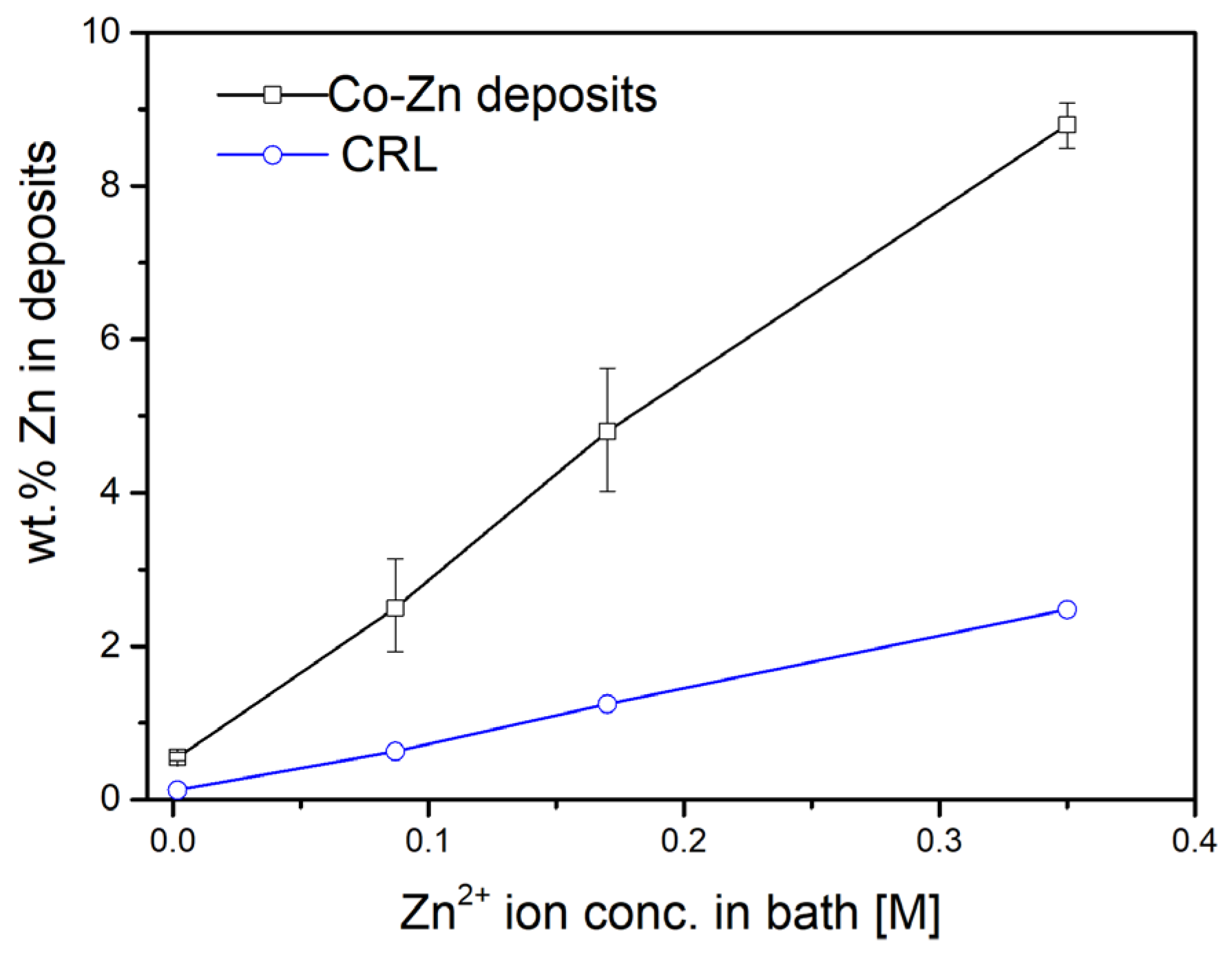
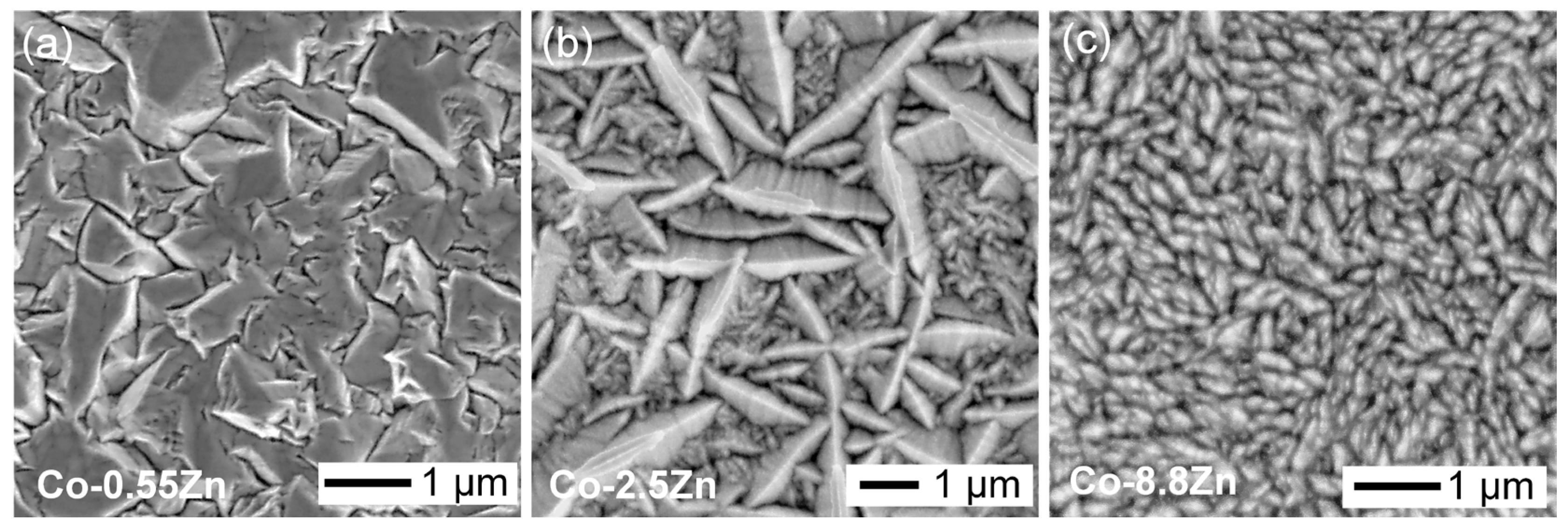
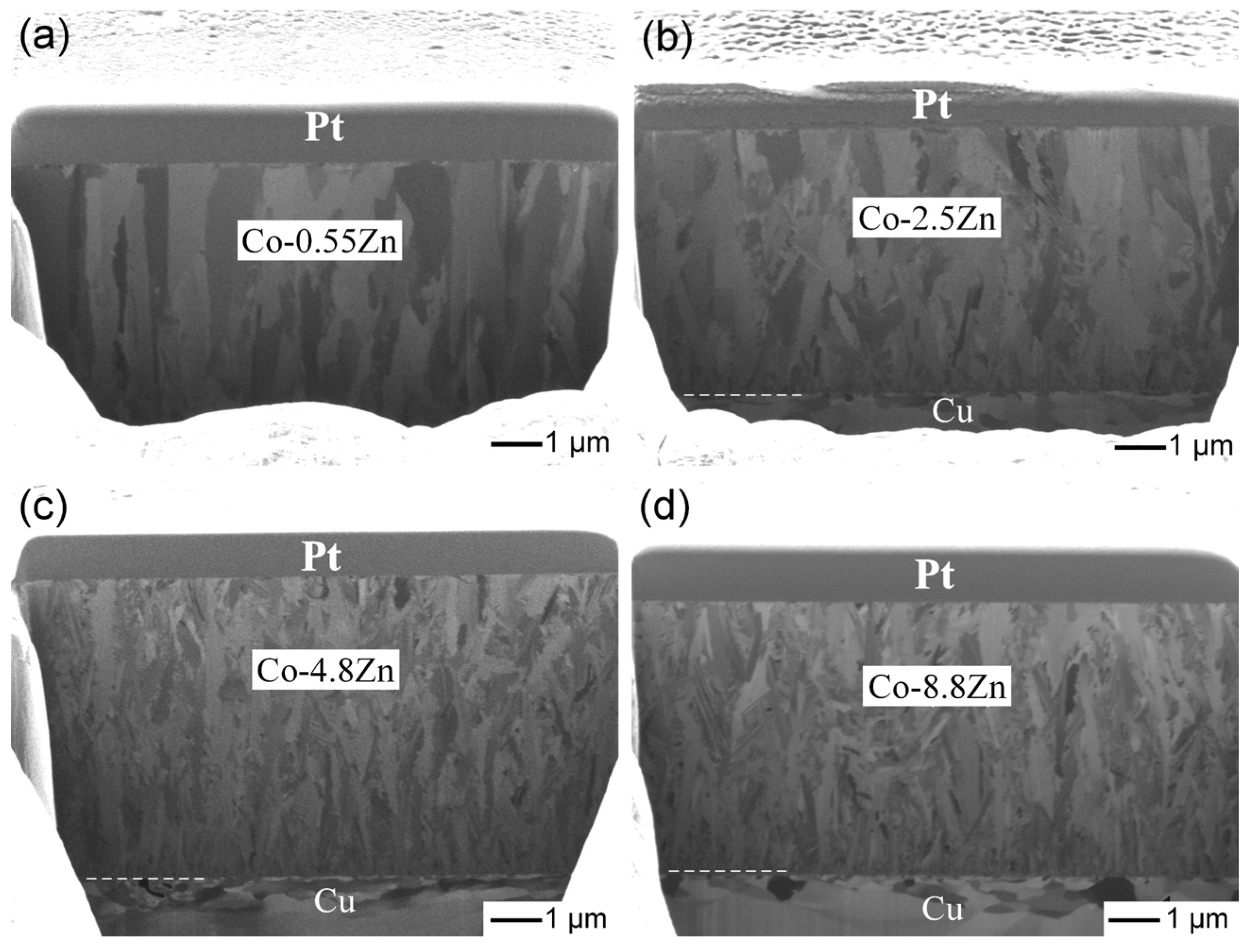
3.1. Characterization of Electroplated Co-Zn Coatings
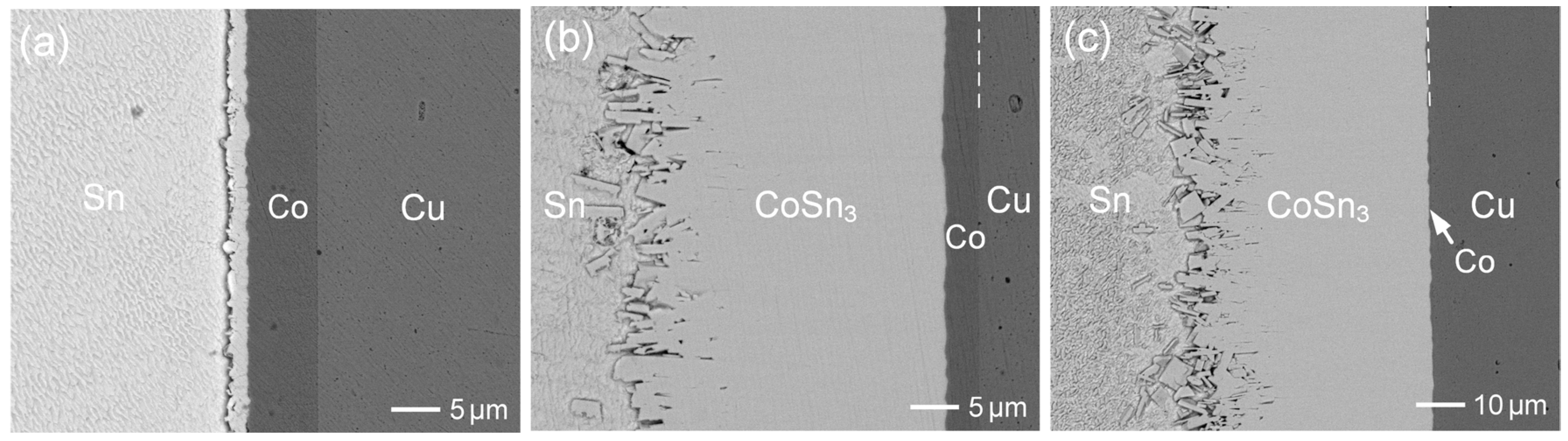
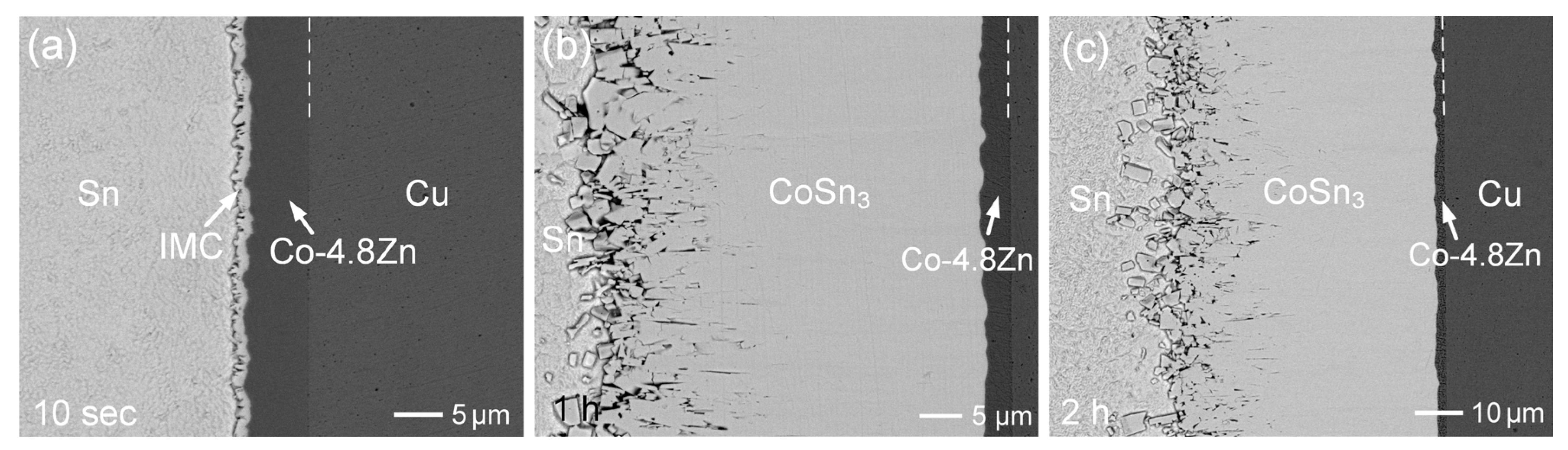
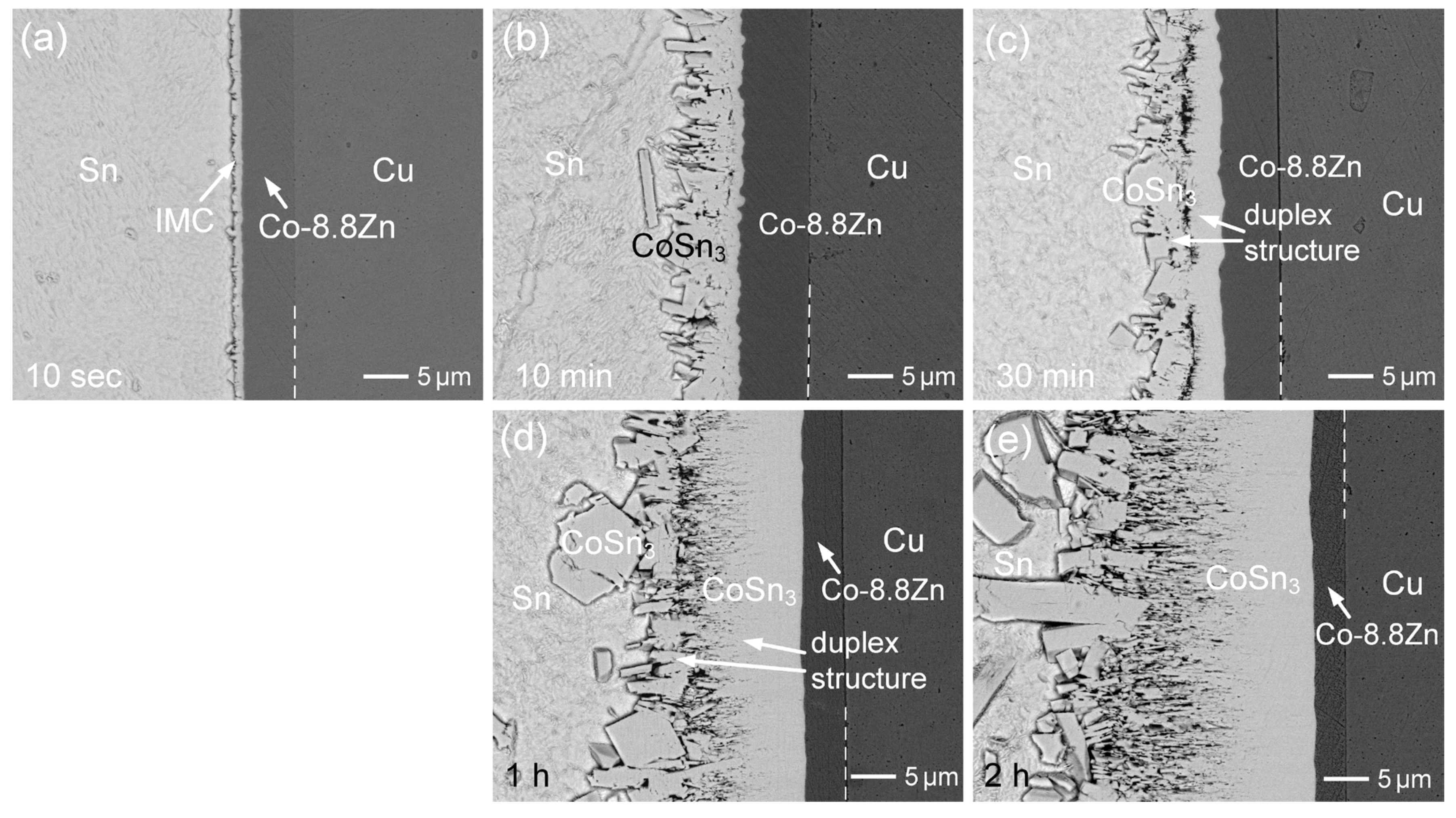
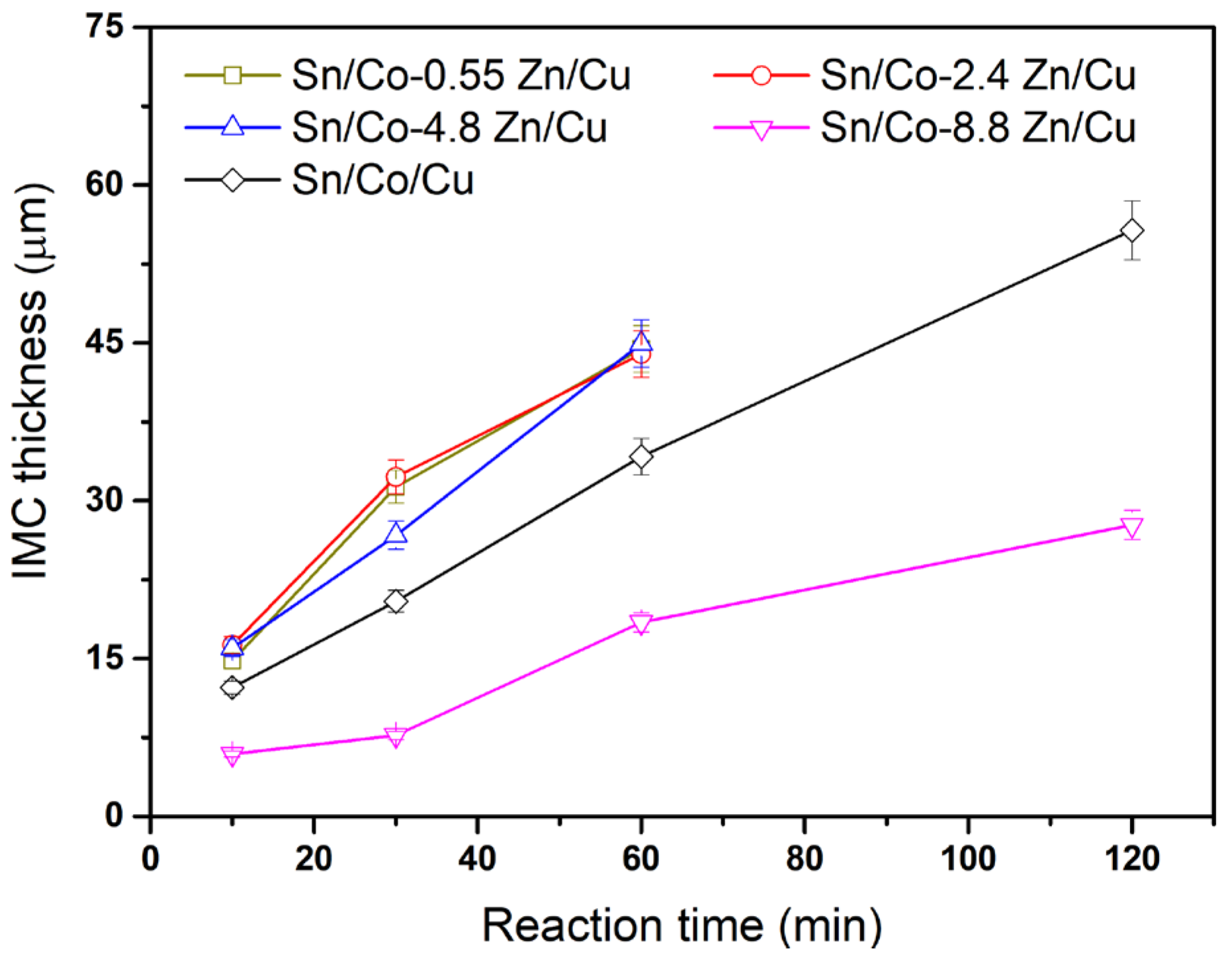
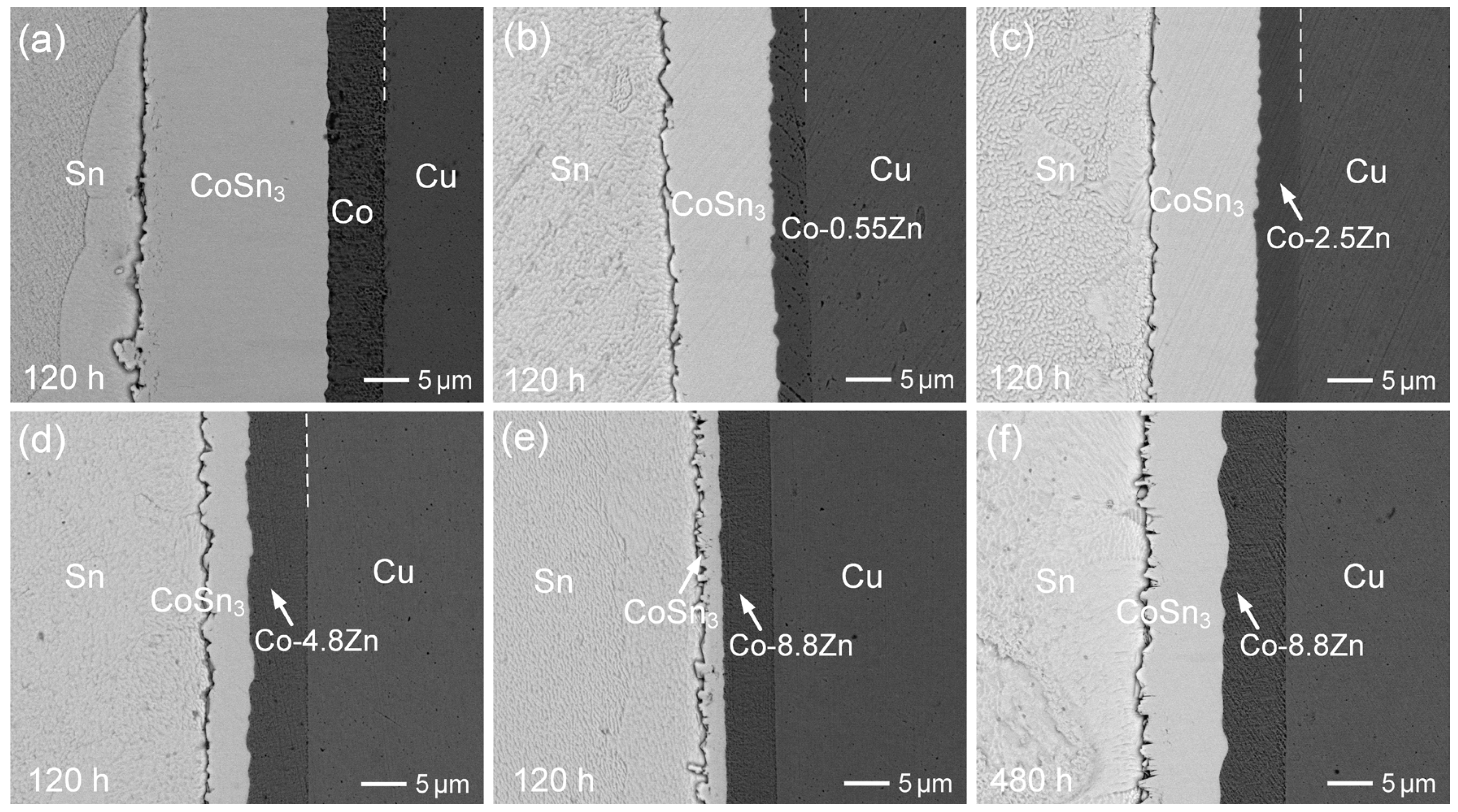
3.2. Interfacial Reactions of Co-Zn Deposits with Sn at 250 °C
3.3. Interfacial Reactions of Co-Zn Deposits with Sn at 160 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dele-Afolabi, T.T.; Ansari, M.N.M.; Azmah Hanim, M.A.; Oyekanmi, A.A.; Ojo-Kupoluyi, O.J.; Atiqah, A. Recent advances in Sn-based lead-free solder interconnects for microelectronics packaging: Materials and technologies. J. Mater. Res. Technol. 2023, 25, 4231–4263. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronics applications. Microelectron. Reliab. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Ma, H.; Suhling, J.C. A review of mechanical properties of lead-free solders for electronic packaging. J. Mater. Sci. 2009, 44, 1141–1158. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, J.; Wang, X.-N.; Zhou, X.-W.; Tang, X.-X.; Akira, K. Cu aggregation behavior on interfacial reaction of Sn-3.0Ag-0.5Cu/ENIG solder joints. Mater. Lett. 2023, 348, 134659. [Google Scholar] [CrossRef]
- Kim, J.; Jung, S.-B.; Yoon, J.-W. Effect of Ni(P) thickness in Au/Pd/Ni(P) surface finish on the electrical reliability of Sn–3.0Ag–0.5Cu solder joints during current-stressing. J. Alloys Compd. 2015, 622, 529–534. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.-L.; Sohn, Y. Diffusion barrier properties of the intermetallic compound layers formed in the Pt nanoparticles alloyed Sn-58Bi solder joints reacted with ENIG and ENEPIG surface finishes. Materials 2022, 15, 8419. [Google Scholar] [CrossRef]
- Wang, J.Y.; Tang, Y.K.; Yeh, C.Y.; Chang, P.J.; Lin, Y.X.; Lin, E.J.; Wu, C.Y.; Zhuang, W.X.; Liu, C.Y. Kinetics of Ni solid-state dissolution in Sn and Sn3.5Ag alloys. J. Alloys Compd. 2019, 797, 684–691. [Google Scholar] [CrossRef]
- Huang, L.C.; Zhang, Y.P.; Chen, C.M.; Hung, L.Y.; Wang, Y.P. Interfacial reactions between pure indium solder and Au/Ni metallization. J. Mater. Sci. Mater. Electron. 2022, 33, 13143–13151. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chen, H.-H.; Li, P.-Y.; Chu, P.-Y. Kinetic analysis of Ni5Zn21 growth at the interface between Sn-Zn solders and Ni. Intermetallics 2012, 22, 166–175. [Google Scholar] [CrossRef]
- Yao, J.; Li, C.; Shang, M.; Chen, X.; Wang, Y.; Ma, H.; Ma, H.; Liu, X. Diffusion barrier performance of Ni-W layer at Sn/Cu interfacial reaction. Materials 2024, 17, 3682. [Google Scholar] [CrossRef]
- Liu, L.; Shi, L.; Peng, J.; Jiang, B.; Liu, S.; Liu, C.; Chen, Z. Interfacial reaction between Sn–Ag solder and electroless Ni–Fe–P diffusion barriers with different internal microstructure. Mater. Res. Bull. 2022, 152, 111854. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Li, J.; Chen, Z. Comparison of interfacial reactions and isothermal aging of cone Ni–P and flat Ni–P with Sn3.5Ag solders. Appl. Surf. Sci. 2023, 625, 157219. [Google Scholar] [CrossRef]
- Humpston, G. Cobalt: A universal barrier metal for solderable under bump metallisations. J. Mater. Sci. Mater. Electron. 2010, 21, 584–588. [Google Scholar] [CrossRef]
- Bi, X.; Hu, X.; Jiang, X.; Li, Y. Mechanical properties of CoSn2 and α-CoSn3 intermetallic compounds: First-principles calculations and nano-indentation measurements. Appl. Phys. A 2019, 125, 217. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Chen, P.; Liu, J.; Hu, A.; Li, M. Effect of Co-W and Co-Fe-W diffusion barriers on the reliability of the solder/Cu interface during reflow conditions. Electron. Mater. Lett. 2024, 20, 517–524. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kuo, C.Y. Growth Kinetics of the solid-state interfacial reactions in the Sn–Cu/Co and Sn/Co–Cu couples. Mater. Chem. Phys. 2011, 130, 651–656. [Google Scholar] [CrossRef]
- Pan, H.-C.; Hsieh, T.-E. Diffusion barrier characteristics of electroless Co(W,P) thin film to lead-free Sn-Ag-Cu solder. J. Electrochem. Soc. 2011, 158, 123–129. [Google Scholar] [CrossRef]
- Wang, C.-H.; Huang, S.-E.; Chiu, C.-W. Influence of the P content on phase formation in the interfacial reactions between Sn and electroless Co(P) metallization on Cu substrate. J. Alloys Compd. 2015, 619, 474–480. [Google Scholar] [CrossRef]
- Lu, N.; Yang, D.; Li, L. Interfacial reaction between Sn-Ag-Cu solder and Co-P films with various microstructures. Acta Mater. 2013, 61, 4581–4590. [Google Scholar] [CrossRef]
- Nishizawa, K.; Matsumoto, A.; Nakagawa, Y.; Sakuma, H.; Goto, S.; Fukumuro, N.; Yae, S. Comparison of electroless Ni-P and Co-W-P diffusion properties against GaAs substrate. J. Electron. Mater. 2023, 52, 4080–4090. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, J.; Xue, F.; Cao, R.; Wang, F. Microstructure, interfacial reactions and mechanical properties of Co/Sn/Co and Cu/Sn/Cu joints produced by transient liquid phase bonding. J. Mater. Sci. Mater. Electron. 2018, 29, 16388–16400. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, H.-C.; Lee, H.-Y.; Wu, A.-T. Co-P diffusion barrier for p-Bi2Te3 thermoelectric material. J. Electron. Mater. 2019, 48, 53–57. [Google Scholar] [CrossRef]
- Zhen, C.; Ma, L.; Liu, S.; Wang, Y.; Li, D.; Guo, F. Effect of P content on diffusion resistance and interfacial mechanical properties of crystalline Co–P coatings in solder joints. J. Mater. Sci. Mater. Electron. 2023, 34, 333. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kuo, C.-Y.; Huang, S.-E.; Li, P.-Y. Temperature effects on liquid-state Sn/Co interfacial reactions. Intermetallics 2013, 32, 57–63. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chen, S.-W. Sn/Co solid/solid interfacial reactions. Intermetallics 2008, 16, 524–530. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, H.Y.; Wu, A.T. Massive spalling and morphological change of intermetallic compound affected by adding Pd in Co-based surface finishes. J. Alloys Compd. 2013, 580, 195–200. [Google Scholar] [CrossRef]
- Wang, C.-H.; Huang, S.-E.; Li, K.-T. Inhibiting CoSn3 growth at the Sn/Co system by minor Zn addition. Intermetallics 2015, 56, 68–74. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kuo, C.-Y.; Guo, Y.-B. Effects of minor Cu, Ni and Ag additions on the reactions between Sn-based solders and Co substrate. JOM 2019, 71, 3023–3030. [Google Scholar] [CrossRef]
- Lodhi, Z.F.; Mol, J.M.C.; Hamer, W.J.; Terryn, H.A.; De Wit, J.H.W. Cathodic inhibition and anomalous electrodeposition of Zn–Co alloys. Electrochim. Acta 2007, 52, 5444–5452. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, J.; Liu, Z.; Yin, Y.; Qiao, C. Numerical and experimental investigation of the effect of current density on the anomalous codeposition of ternary Fe-Co-Ni alloy coatings. Materials 2022, 15, 6141. [Google Scholar] [CrossRef]
- Pandiyarajan, S.; Ganesan, M.; Liao, A.-H.; Manickaraj, S.S.M.; Huang, S.T.; Chuang, H.-C. Ultrasonic-assisted supercritical-CO2 electrodeposition of Zn-Co film for high-performance corrosion inhibition: A greener approach. Ultrason. Sonochem. 2021, 72, 105463. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.; Capel-Boute, C.; Decroly, C. Inhibition effect of zinc on the cathodic deposition of cobalt—I. Electrochemical and structural observations in sulphate solutions. Electrochim. Acta 1965, 10, 885–894. [Google Scholar] [CrossRef]
- Higashi, K.; Fukushima, H.; Urakawa, T.; Adaniya, T.; Matsudo, K. Mechanism of the electrodeposition of zinc alloys containing a small amount of cobalt. J. Electrochem. Soc. 1981, 128, 2081–2085. [Google Scholar] [CrossRef]
- Abou-Krisha, M.M. Electrochemical studies of zinc–nickel codeposition in sulphate bath. Appl. Surf. Sci. 2005, 252, 1035–1048. [Google Scholar] [CrossRef]
- WebElements. Available online: https://www.webelements.com (accessed on 28 May 2025).



| Component | Concentration (g/L or mL/L) | Concentration (M) |
|---|---|---|
| Cobalt sulfamate solution (Co(SO3NH2)2, Co > 180 g/L) | 500 mL/L | 1.527 M |
| Cobalt chloride (CoCl2) | 10 g/L | 0.077 M |
| Boric acid (H3BO3) | 40 g/L | 0.647 M |
| Adding Amount of ZnSO4·7H2O (Based on 200 mL of Co Electroplating Solution) | ZnSO4·7H2O Concentration (M) | Zn Content in Co-Zn Coatings (wt.%) | Average Zn Content and Notation |
|---|---|---|---|
| 0.1 g | 0.00174 M | 0.51–0.62 | Co-0.55 wt.%Zn |
| 0.5 g | 0.0087 M | 1.93–3.14 | Co-2.5 wt.%Zn |
| 1.0 g | 0.0174 M | 4.02–5.62 | Co-4.8 wt.%Zn |
| 2.0 g | 0.035 M | 8.49–9.08 | Co-8.8 wt.%Zn |
| Material | CTE (×10−6 K−1) |
|---|---|
| Co | ~13 |
| Cu | ~16.5 |
| Sn (β-Sn) | ~22 |
| Zn | 30.2 |
| Co-Zn alloys (estimated) | ~14–16 (depending on Zn content) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Lin, C.-Y. Interfacial Behavior During Reactions Between Sn and Electroplated Co–Zn Alloys. Materials 2025, 18, 2680. https://doi.org/10.3390/ma18122680
Wang C-H, Lin C-Y. Interfacial Behavior During Reactions Between Sn and Electroplated Co–Zn Alloys. Materials. 2025; 18(12):2680. https://doi.org/10.3390/ma18122680
Chicago/Turabian StyleWang, Chao-Hong, and Che-Yang Lin. 2025. "Interfacial Behavior During Reactions Between Sn and Electroplated Co–Zn Alloys" Materials 18, no. 12: 2680. https://doi.org/10.3390/ma18122680
APA StyleWang, C.-H., & Lin, C.-Y. (2025). Interfacial Behavior During Reactions Between Sn and Electroplated Co–Zn Alloys. Materials, 18(12), 2680. https://doi.org/10.3390/ma18122680







