Synthesis of Porous Polymers by Nucleophilic Substitution Reaction of Polyamines and Monochlorotriazinyl-β-Cyclodextrin and Application to Dye Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Network Polymers
2.3. Adsorption Test of Dyes
2.4. Analytical Procedures
3. Results and Discussion
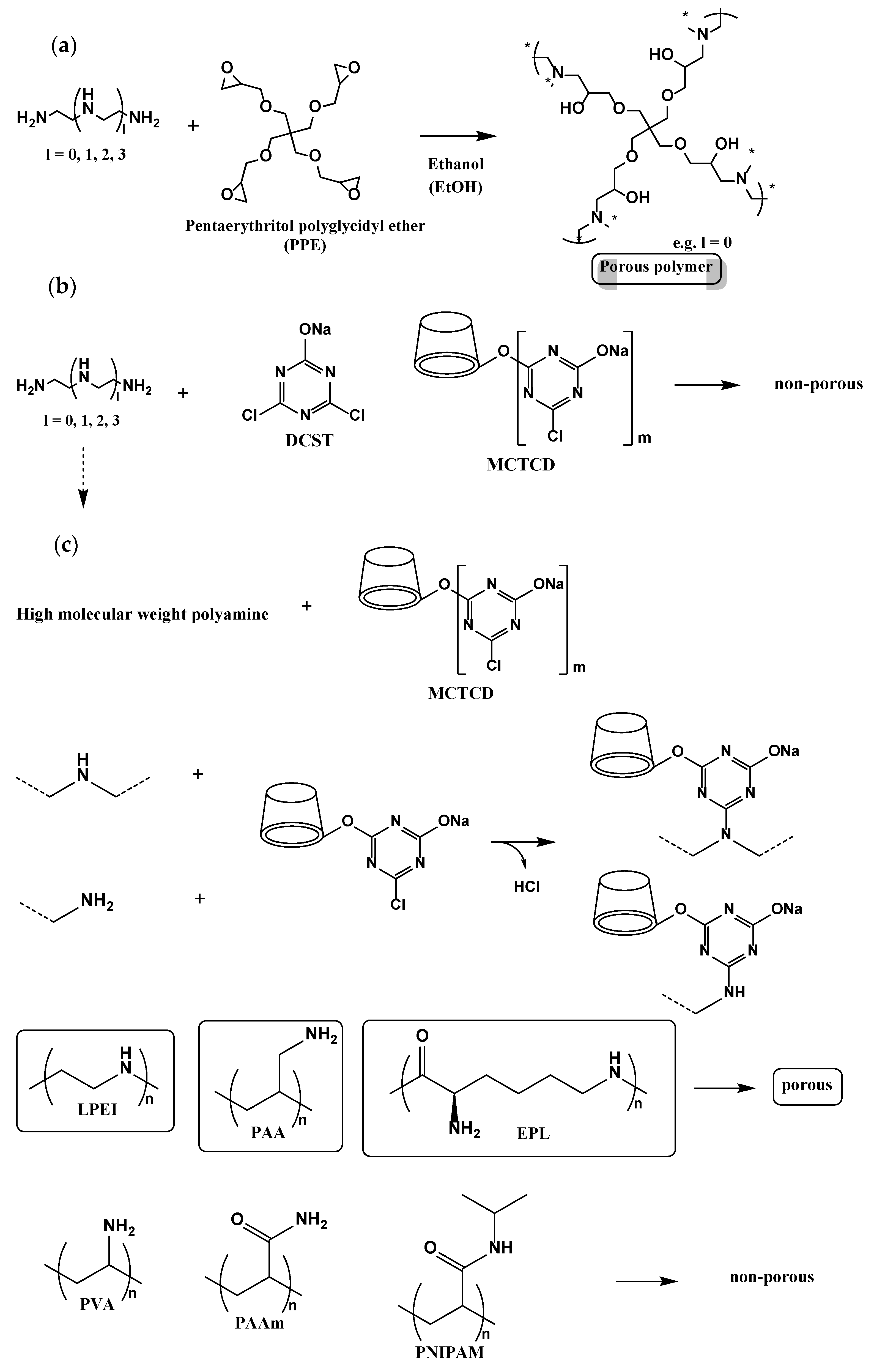
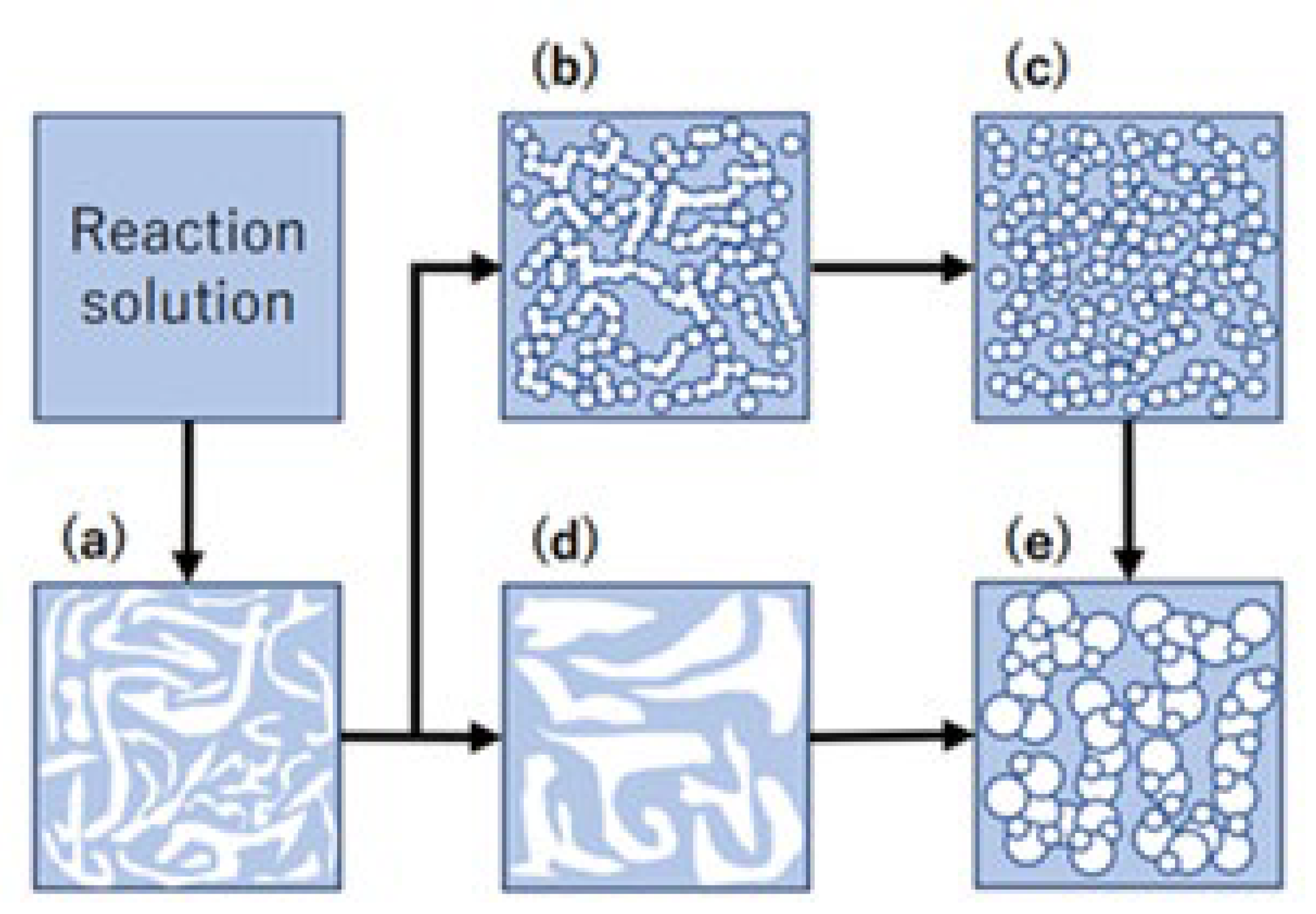
3.1. Synthesis of Network Polymers
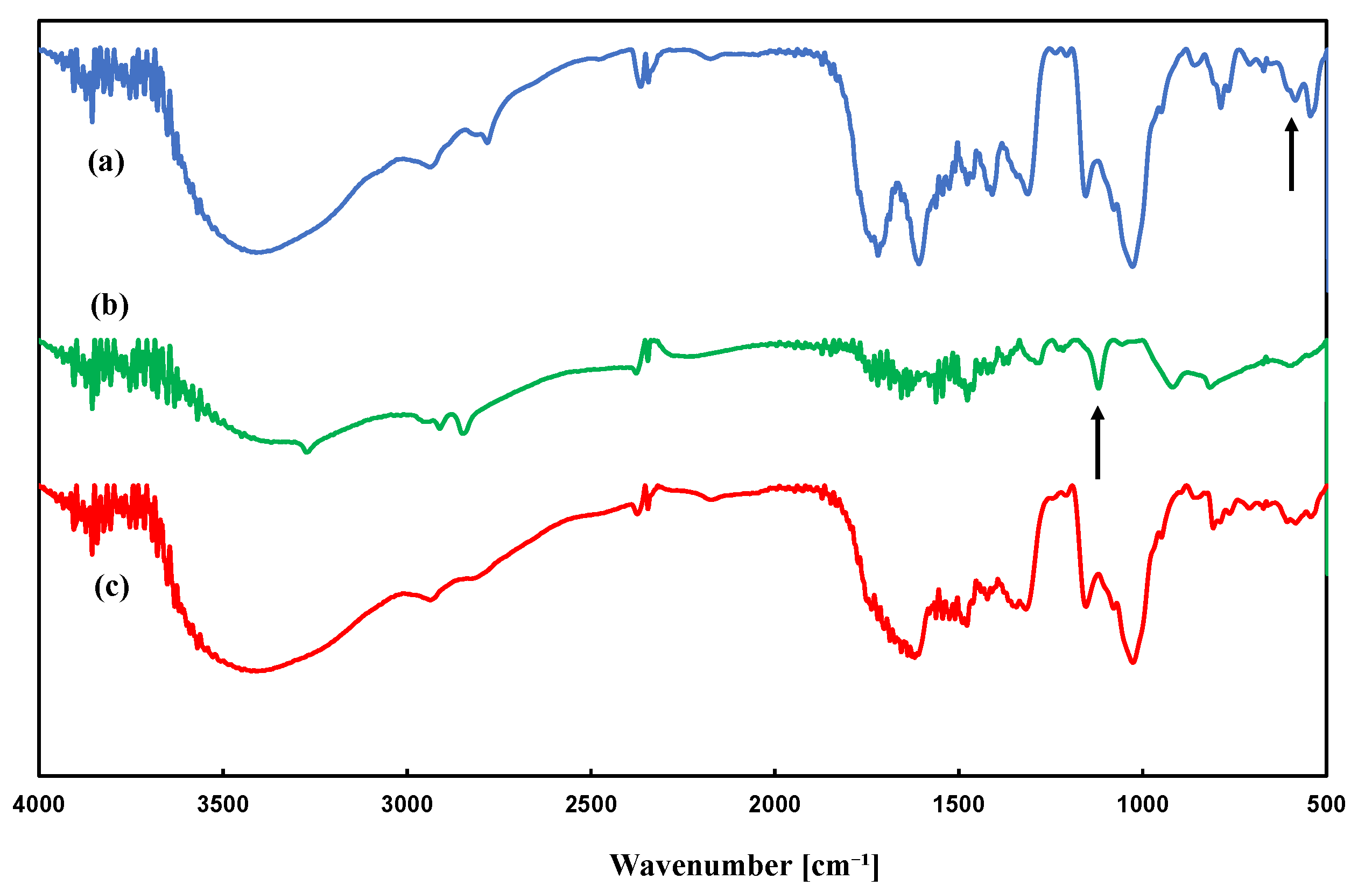
3.2. Structure of Porous Polymers
3.3. Mechanical Properties of Porous Polymes
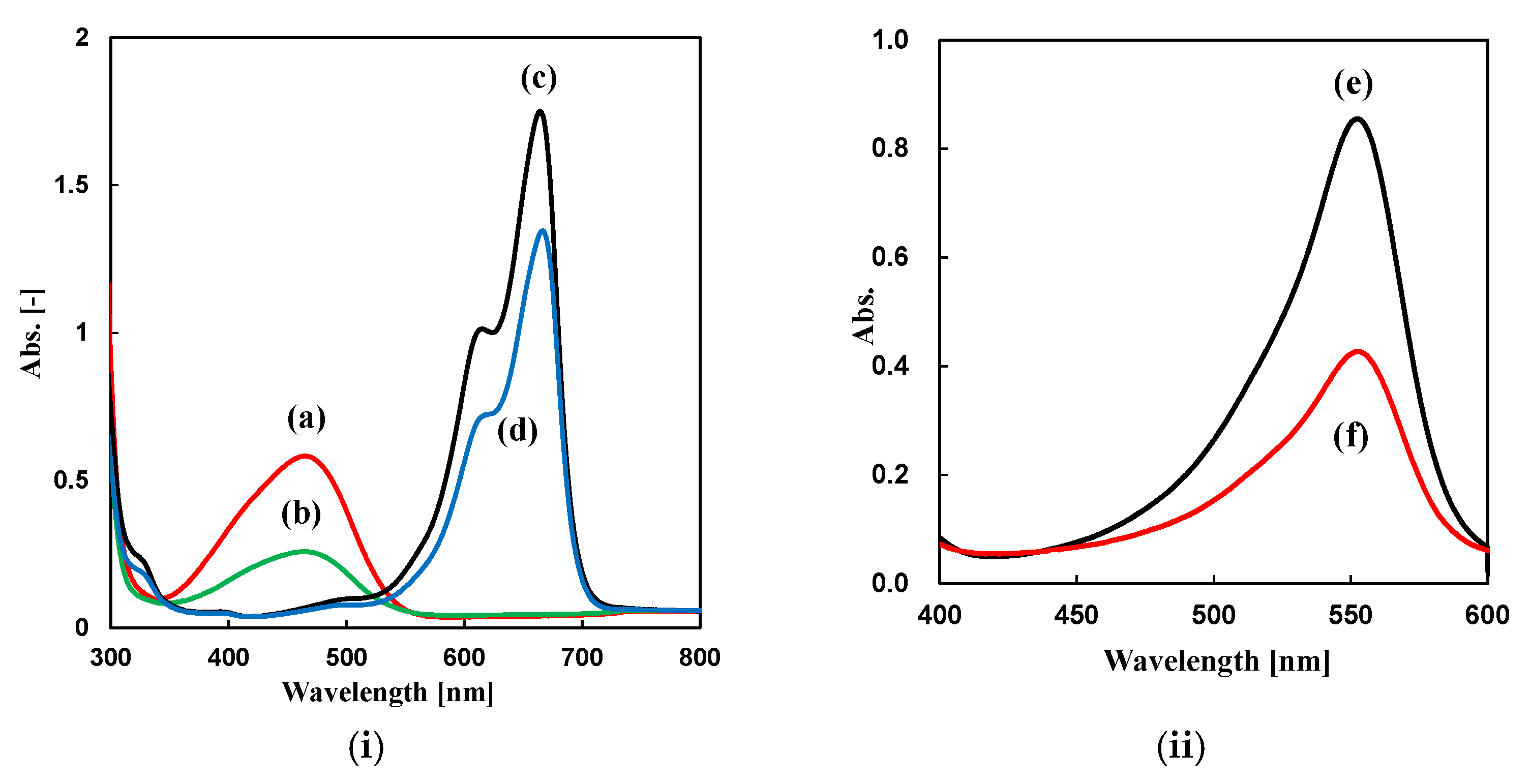
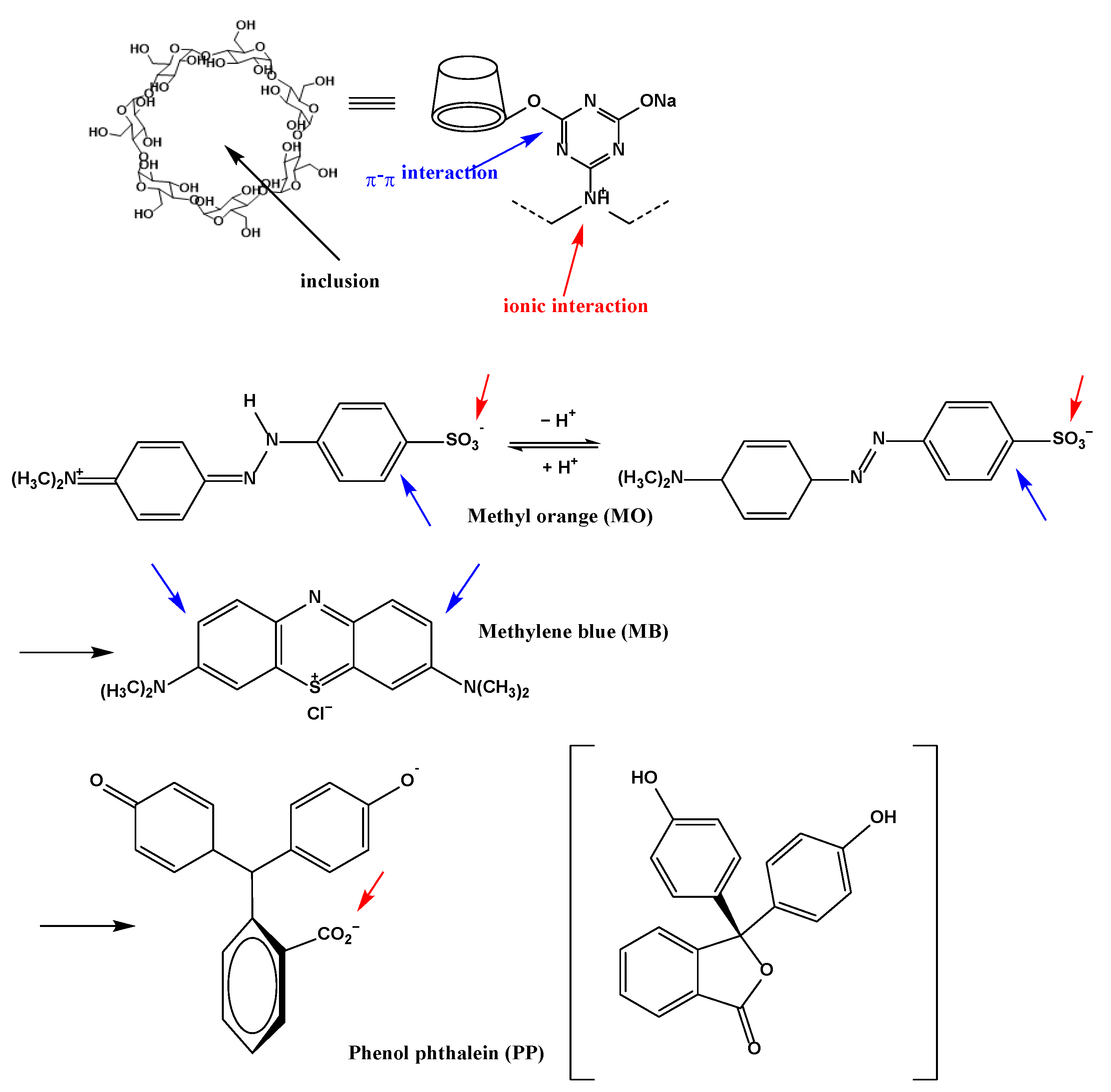
Adsorption of Dyes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steuerle, U.; Feuerhake, R. Aziridines. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Wagberg, L. Polyelectrolyte adsorption onto cellulose fibers—A review. Nord. Pulp Pap. Res. J. 2006, 15, 586–597. [Google Scholar] [CrossRef]
- Madkour, T.M. Polymer Data Handbook; Oxford University Press, Inc.: Oxford, UK, 1999; 490p. [Google Scholar]
- Vancha, A.R.; Govindaraju, S.; Parsa, K.V.L.; Jasti, M.; Gonzalez-Garcia, M.; Ballestero, R.P. Use of polyethyleneimine polymer in cell culture as attachment factor and lipofection enhancer. BMC Biotechnol. 2004, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 2006, 58, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethyleneimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Rudolph, C.; Lausier, J.; Naundorf, S.; Muller, R.H.; Rosenecker, J. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J. Gene Med. 2000, 2, 269–278. [Google Scholar] [CrossRef]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and proton sponge hypothesis. J. Gene Med. 2004, 7, 657–663. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Koski, P. Polyethyleneimine is an effective permeabilizer of Gram-negative bacteria. Microbiology 1997, 143, 3193–3199. [Google Scholar] [CrossRef]
- Zhuk, D.S.; Gembitski, P.A.; Kargin, V.A. Advances in the Chemistry of Polyethyleneimine (Polyaziridine). Russ. Chem. Rev. 1965, 34, 515–526. [Google Scholar] [CrossRef]
- Gholami, L.; Mahmoudi, A.; Kazemi Oskuee, R.; Malaekeh-Nikouei, B. An overview of polyallylamine applications in gene delivery. Pharm. Dev. Tech. 2022, 27, 714–724. [Google Scholar] [CrossRef]
- Salvador, C.; Andreozzi, P.; Romero, G.; Loinaz, I.; Dupin, D.; Moya, S.E. Self-Assembled Oleic Acid-Modified Polyallylamines for Improved siRNA Transfection Efficiency and Lower Cytotoxicity. ACS Appl. Bio Mater. 2023, 6, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. Hybrid poly(allylamine hydrochloride)–graphene oxide microcapsules: Preparation, characterization and application in textiles with controlled release behavior. Mater. Adv. 2020, 1, 804–813. [Google Scholar] [CrossRef]
- Jurko, L.; Bračič, M.; Hribernik, S.; Makuc, D.; Plavec, J.; Jerenec, F.; Žabkar, S.; Gubeljak, N.; Štern, A.; Kargl, R. Succinylation of Polyallylamine: Influence on Biological Efficacy and the Formation of Electrospun Fibers. Polymers 2021, 13, 2840. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Ye, W.; Zhou, X.; Xue, Z. Amine-Acrylate Michael Addition: A Versatile Platform for Fabrication of Polymer Electrolytes with Varied Cross-Linked Networks. ACS Appl. Polym. Mater. 2024, 6, 2041–2048. [Google Scholar] [CrossRef]
- Zou, W.; Lin, X.; Terentjev, E. Amine-Acrylate Liquid Single Crystal Elastomers Reinforced by Hydrogen Bonding. Adv. Mater. 2021, 33, 2101955. [Google Scholar] [CrossRef]
- Alaneed, R.; Golitsyn, Y.; Hauenschild, T.; Pietzsch, M.; Reichert, D.; Kressler, J. Network formation by aza-Michael addition of primary amines to vinyl end groups of enzymatically synthesized poly(glycerol adipate). Polym. Int. 2021, 70, 135–144. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernandez-Francos, X.; Serra, A.; Ramis, X. Sequential curing of amine-acrylate-methacrylate mixtures based on selective aza-Michael addition followed by radical photopolymerization. Eur. Polym. J. 2016, 84, 256–267. [Google Scholar] [CrossRef]
- Naga, N.; Inose, D.; Ishida, T.; Kubota, K.; Nageh, H.; Nakano, T. Synthesis of polymer networks by means of addition reactions of tri-amine and poly(ethylene glycol) diacrylate or diglycidyl ether compounds. Polym. Bull. 2021, 78, 2745–2763. [Google Scholar] [CrossRef]
- Grate, J.W.; Mo, K.F.; Daily, M.D. Triazine-Based Sequence-Defined Polymers with Side-Chain Diversity and Backbone–Backbone Interaction Motifs. Angew. Chem. Int. Ed. 2016, 55, 3925–3930. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Sasaki, H.; Shibata, H.; Tsukamoto, T.; Oishi, Y. Synthesis of Semiaromatic Polyguanamines from Tetraazacalix[2]arene[2]-triazine Dichloride with Aliphatic Diamines. J. Photopolym. Sci. Technol. 2024, 37, 7–13. [Google Scholar] [CrossRef]
- Ravi, S.; Kim, J.; Choi, Y.; Han, H.H.; Wu, S.; Xiao, R.; Bae, Y.S. Metal-Free Amine-Anchored Triazine-Based Covalent Organic Polymers for Selective CO2 Adsorption and Conversion to Cyclic Carbonates Under Mild Conditions. ACS Sustain. Chem. Eng. 2023, 11, 1190–1199. [Google Scholar] [CrossRef]
- Mizuno, S.; Asoh, T.; Takashima, Y.; Harada, A.; Uyama, H. Cyclodextrin cross-linked polymer monolith for efficient removal of environmental pollutants by flow-through method. Polym. Degrad. Stab. 2019, 160, 136–141. [Google Scholar] [CrossRef]
- Naga, N.; Kusakabe, M.; Nakano, T. Synthesis and morphology control of a tetra-functional epoxy/polyethylenepolyamine monolithic porous polymers aspiring to selective molecular adsorption. J. Polym. Res. 2025, 32, 29. [Google Scholar] [CrossRef]
- Naga, N.; Miyazaki, Y.; Nakano, T. Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound. Separations 2025, 12, 94. [Google Scholar] [CrossRef]
- Yuan, J.J.; Jin, R.H. Fibrous Crystalline Hydrogels Formed from Polymers Possessing a Linear Poly(ethyleneimine) Backbone. Langmuir 2005, 21, 3136–3145. [Google Scholar] [CrossRef]
- Sutekin, S.D.; Demirci, S.; Kurt, S.B.; Guven, O.; Sahiner, N. Tunable fluorescent and antimicrobial properties of poly(vinyl amine) affected by the acidic or basic hydrolysis of poly(N-vinylformamide). J. Appl. Polym. Sci. 2021, 138, 51234. [Google Scholar] [CrossRef]
- Zhang, F.; Islam, M.S.; Berry, R.M.; Tam, K.C. β-Cyclodextrin-Functionalized Cellulose Nanocrystals and Their Interactions with Surfactants. ACS Omega 2019, 4, 2102–2110. [Google Scholar] [CrossRef]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Naga, N.; Miyanaga, T.; Wang, Y.; Nakano, T. Synthesis and properties of σ-π conjugated porous polymers obtained with Mizoroki–Heck reaction of tetra vinyl cyclic siloxane with dibromo fluorene. J. Polym. Sci. 2020, 58, 2301–2309. [Google Scholar] [CrossRef]
- Naga, N.; Yamada, K.; Moriyama, K.; Kudoh, S.; Nagami, Y.; Nageh, H.; Nakano, T. Synthesis of network polymers by photo-initiated thiol-ene reaction between multi-functional thiol and poly(ethylene glycol) diacrylate. Polym. Bull. 2022, 79, 2411–2427. [Google Scholar] [CrossRef]
- Naga, N.; Inagaki, A.; Narita, T.; Nakano, T. Quaternary ammonium ionene porous polymers via Menschutkin reaction of methylated polyethyleneimine and dibromoalkanes. Mater. Chem. Phys. 2023, 297, 127409. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, S.; Zhou, S.; Wang, D.; Zhang, Y.; Hao, X.; Liu, G.; Liu, P.; Gu, P. Preparation of sulfonate-grafted tetraphenylethylene-based hyper-cross-linked polymers for efficient adsorption of organic dyes in aqueous solutions. ACS Appl. Polym. Mater. 2024, 6, 10615–10624. [Google Scholar] [CrossRef]
- Onder, A.; Ozay, H. Highly efficient removal of methyl orange from aqueous media by amine functional cyclotriphosphazene submicrospheres as reusable column packing material. Chem. Eng. Process. Process Intensif. 2021, 165, 108427. [Google Scholar] [CrossRef]
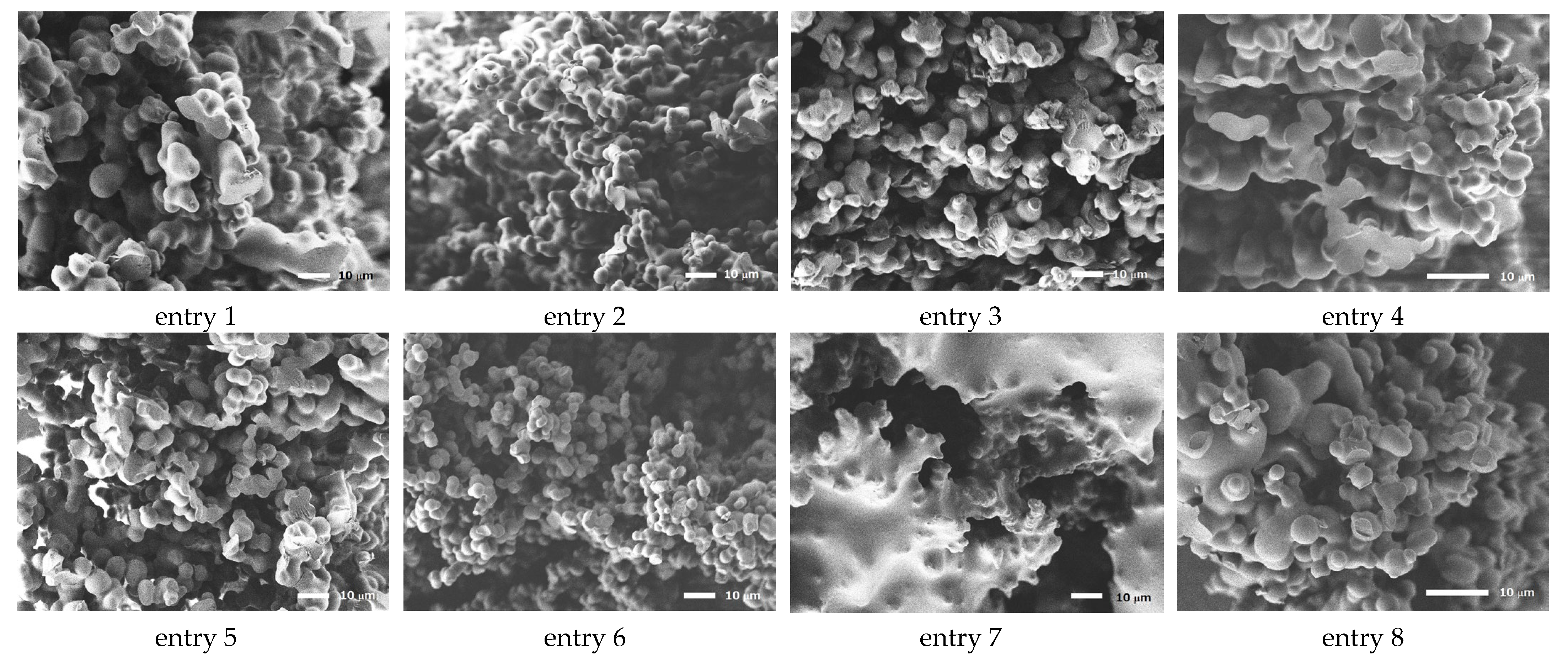
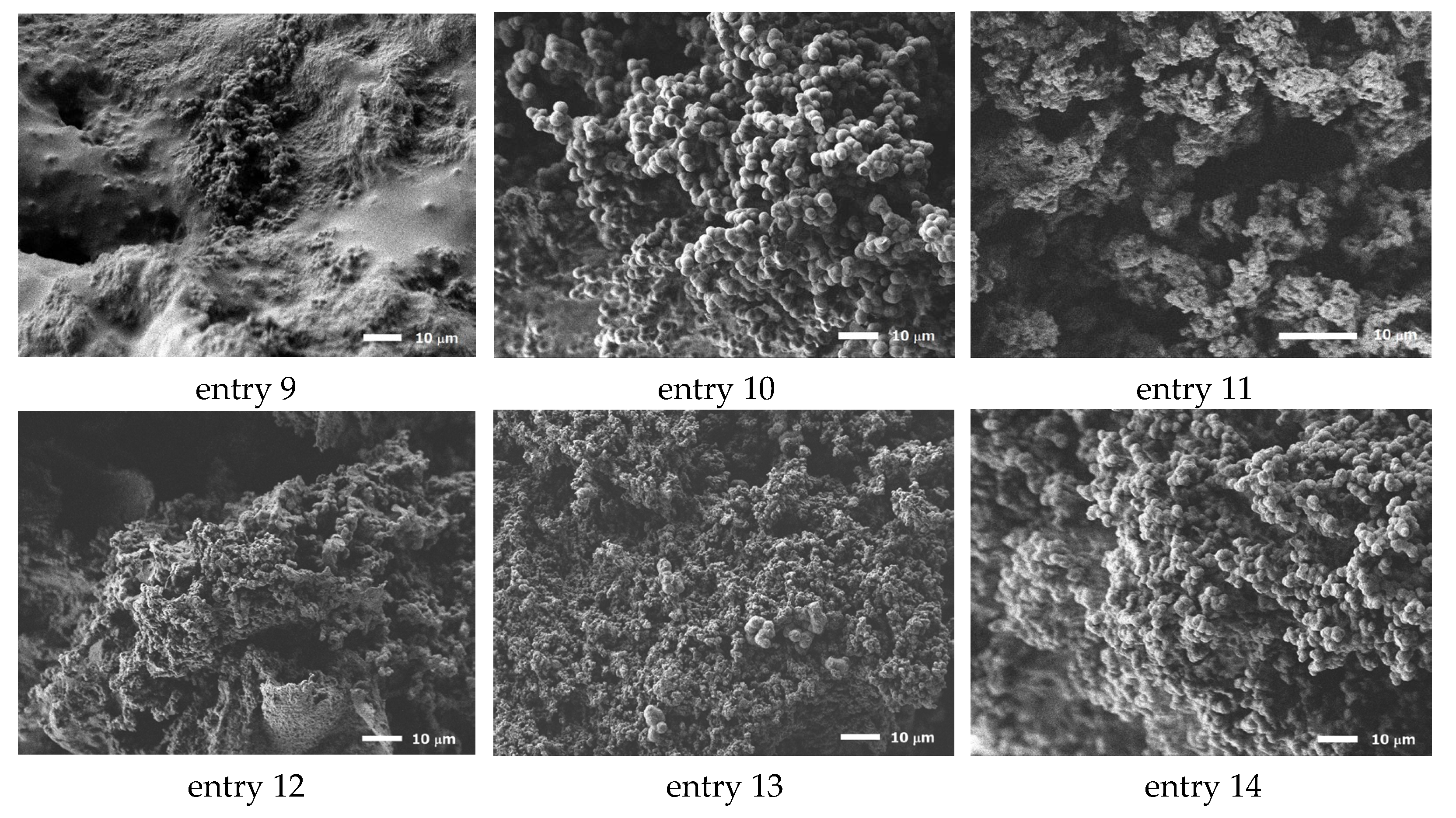


| Entry | Polyamine | Solvent (wt%/wt%) | [MCT]/[NH] mol/mol | Material Conc. wt% | Morphology 1 | Average Diameter μm | SD 2 | CV 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | LPEI50K | MeOH(20)/H2O(80) | 0.5 | 30 | C | 6.03 | 1.44 | 0.24 |
| 2 | LPEI50K | MeOH(20)/H2O(80) | 1.0 | 30 | C | 3.98 | 0.80 | 0.20 |
| 3 | LPEI500K | MeOH(20)/H2O(80) | 0.5 | 30 | C | 4.78 | 0.93 | 0.19 |
| 4 | LPEI500K | MeOH(20)/H2O(80) | 1.0 | 30 | C | 3.38 | 0.96 | 0.28 |
| 5 | LPEI50K | MeOH(10)/H2O(90) | 0.5 | 30 | C | 4.87 | 0.97 | 0.20 |
| 6 | LPEI50K | MeOH(10)/H2O(90) | 1.0 | 30 | C | 3.46 | 0.62 | 0.18 |
| 7 | LPEI500K | MeOH(10)/H2O(90) | 0.5 | 30 | D | n.d. 4 | ||
| 8 | LPEI500K | MeOH(10)/H2O(90) | 1.0 | 30 | C | 3.29 | 0.10 | 0.30 |
| 9 | PAA1600 | H2O | 0.5 | 20 | D | n.d. 4 | ||
| 10 | PAA1600 | H2O | 0.5 | 30 | G | 2.95 | 0.40 | 0.14 |
| 11 | PAA1600 | H2O | 1.0 | 30 | D | n.d. 4 | 0.13 | 0.20 |
| 12 | PAA3000 | H2O | 0.5 | 20 | D | n.d. 4 | ||
| 13 | PAA3000 | H2O | 0.5 | 30 | G | 0.58 | 0.31 | 0.53 |
| 14 | PAA3000 | H2O | 1.0 | 30 | G | 2.36 | 0.16 | 0.067 |
| 15 | EPL | H2O | 0.5 | 30 | C | 9.51 | 2.95 | 0.31 |
| 16 | EPL | H2O | 1.0 | 30 | D | 5.99 | 2.23 | 0.37 |
| Entry | Polyamine | Bulk Density | Young’s Modulus kPa | Breaking Stress kPa | Breaking Strain % |
|---|---|---|---|---|---|
| 1 | LPEI50K | 0.384 | 3308 | 758.7 | 8.2 |
| 3 | LPEI500K | 0.421 | 3796 | 844.8 | 4.2 |
| 5 | LPEI50K | 0.279 | 831.8 | 807.2 | 36.4 |
| 7 | LPEI500K | 0.434 | 2103 | 539.7 | 5.6 |
| 10 | PAA1600 | 0.315 | 318.4 | 649.6 | 26.4 |
| 13 | PAA3000 | 0.389 | 1681 | 1049 | 9.3 |
| Dye | Mechanism | Adsorption Efficiency % | |
|---|---|---|---|
| MO | Ionic interaction | π-π interaction | 50.9 |
| MB | β-CD inclusion | π-π interaction | 24.9 |
| PP | Ionic interaction | β-CD inclusion | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naga, N.; Hiura, R.; Nakano, T. Synthesis of Porous Polymers by Nucleophilic Substitution Reaction of Polyamines and Monochlorotriazinyl-β-Cyclodextrin and Application to Dye Adsorption. Materials 2025, 18, 2588. https://doi.org/10.3390/ma18112588
Naga N, Hiura R, Nakano T. Synthesis of Porous Polymers by Nucleophilic Substitution Reaction of Polyamines and Monochlorotriazinyl-β-Cyclodextrin and Application to Dye Adsorption. Materials. 2025; 18(11):2588. https://doi.org/10.3390/ma18112588
Chicago/Turabian StyleNaga, Naofumi, Risa Hiura, and Tamaki Nakano. 2025. "Synthesis of Porous Polymers by Nucleophilic Substitution Reaction of Polyamines and Monochlorotriazinyl-β-Cyclodextrin and Application to Dye Adsorption" Materials 18, no. 11: 2588. https://doi.org/10.3390/ma18112588
APA StyleNaga, N., Hiura, R., & Nakano, T. (2025). Synthesis of Porous Polymers by Nucleophilic Substitution Reaction of Polyamines and Monochlorotriazinyl-β-Cyclodextrin and Application to Dye Adsorption. Materials, 18(11), 2588. https://doi.org/10.3390/ma18112588







