Influence of an Alkaline Activator and Mineral Admixture on the Properties of Alkali-Activated Recycled Concrete Powder-Foamed Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions
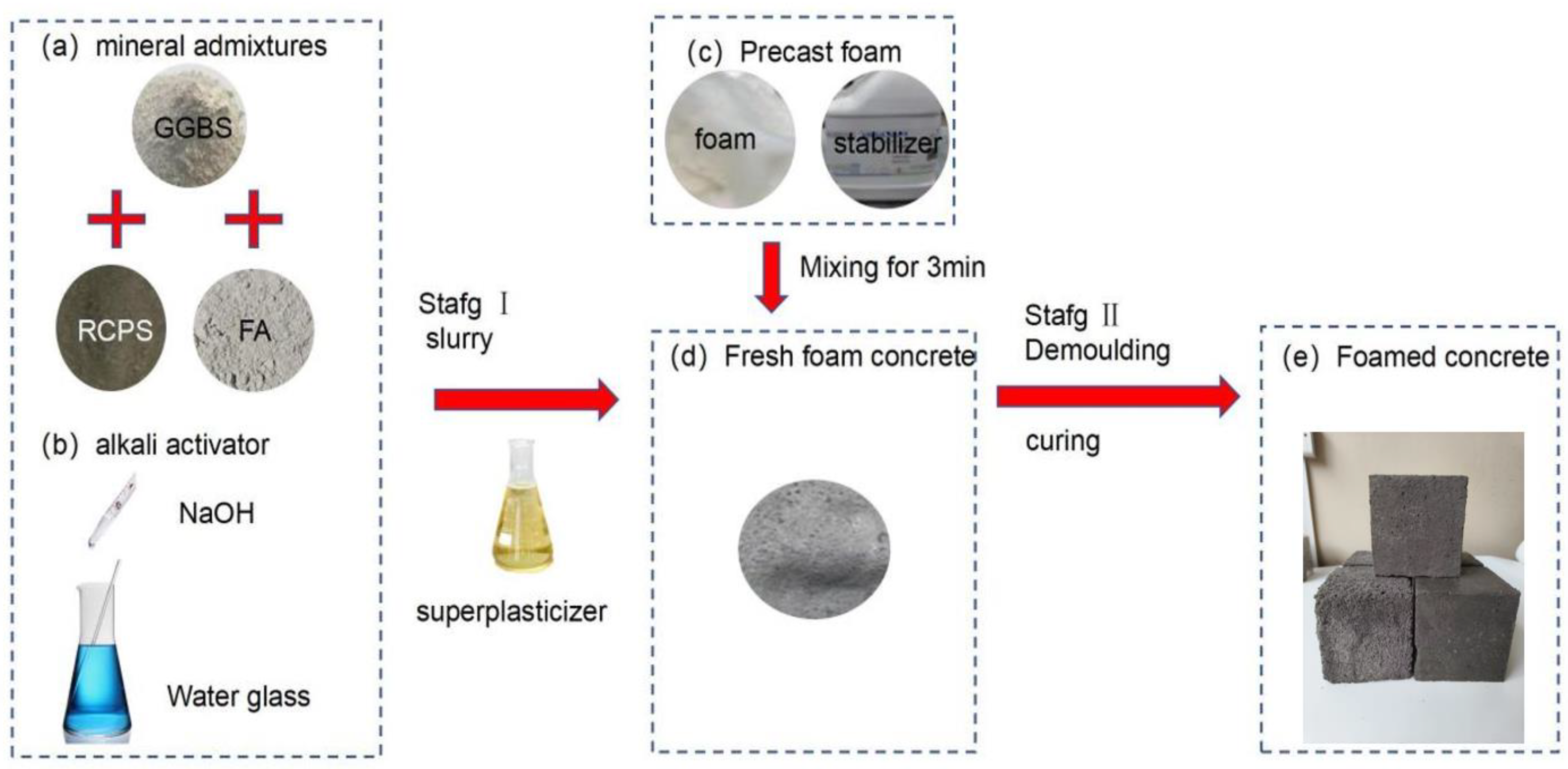
2.3. Sample Preparation
2.4. Compressive Strength Test
2.5. Dry Apparent Density Test
2.6. Water Absorption Test
2.7. Porosity
2.8. Thermal Conductivity Test
2.9. Drying Shrinkage
2.10. Micro-Structure Test
3. Results
3.1. The Effect of W/C Ratio on the Performance of ARCP-FC
3.1.1. Compressive Strength and Dry Apparent Density
3.1.2. Water Absorption
3.2. Effect of Na2O Content on the Performance of ARCP-FC
3.2.1. Compressive Strength and Dry Apparent Density
3.2.2. Water Absorption
3.2.3. Pore Structures
3.3. Effect of Mineral Admixtures on the Behavior of ARCP-FC
3.3.1. Compressive Strength and Dry Apparent Density
3.3.2. Water Absorption
3.4. Pore Structure and Thermal Conductivity
3.4.1. Pore Size Distribution
3.4.2. Average Pore Diameter and Pore Shape Factor
3.4.3. Thermal Conductivity
3.4.4. Drying Shrinkage
3.5. Micro-Structure
3.5.1. XRD
3.5.2. TG
3.5.3. SEM
4. Discussion
4.1. Alkali Activation Improves the Thickness of the Pore Wall
4.2. Fly Ash Improves Thermal Performance
4.3. Applications Prospect
5. Conclusions
- (1)
- Slag powder improved early strength but has an adverse effect on the functionality of ARCP-FC. Fly ash improved the deformation, and the pore was closed to the sphere, reducing the shrinkage and thermal conductivity. The optimal mixture of ARCP-FC was R60S20F20, which consisted of 60% recycled concrete powders, 20% slag, and 20% fly ash.
- (2)
- In the optimal mixture of ARCP-FC, the density, porosity, compressive strength, and thermal conductivity of ARCP-FC were 800 kg/m3, 59.1%, 4.1 MPa, and 0.1036 W/(m·K), respectively. ARCP-FC solved the contradiction between compressive strength and dry apparent density, making it a promising building material for external insulation boards and insulation layers.
- (3)
- Fly ash improved the deformation and the pore was closed to the sphere, reducing the shrinkage and thermal conductivity. ARCP-FC solved the contradiction between compressive strength and dry apparent density, making it a promising building materials for external insulation boards and insulation layers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miraldo, S.; Lopes, S.; Pacheco, T.F.; Lopes, A. Advantages and shortcomings of the utilization of recycled wastes as aggregates in structural concretes. Constr. Build. Mater. 2021, 298, 123729. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Razak, R.A. Fly ash-based geopolymer lightweight concrete using foaming agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.Q.; Wu, H.X.; Yang, D.Y.; Ma, Z.M. Reusing recycled powder as eco-friendly binder for sustainable GGBS-based geopolymer considering the effects of recycled powder type and replacement rate. J. Clean. Prod. 2022, 364, 132656. [Google Scholar] [CrossRef]
- Yao, Y.Z.; Liu, C.; Zhang, W.; Liu, H.W.; Wang, T.L.; Wu, Y.W.; Li, X.; Chen, X.Q. Influence of vacuum and high-temperature on the evolution of mechanical strength and microstructure of alkali-activated lunar regolith simulant. J. Build. Eng. 2024, 97, 110709. [Google Scholar] [CrossRef]
- Li, M.K.; Bao, S.X.; Zhang, Y.M.; Huang, M.Y. Preparation and characterization of low-activity coal bottom ash-based cementitious materials via orthogonal experiment. J. Build. Eng. 2024, 96, 110495. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, W.S.; Ye, J.Y.; Chen, J.J.; Yan, F.L.; Ren, X.H.; Li, J. Mechanism of Na2CO3 on early properties of red mud-based alkali-activated cementitious materials. Constr. Build. Mater. 2024, 449, 138369. [Google Scholar] [CrossRef]
- Gu, Y.M.; Fang, Y.H.; You, D.; Gong, Y.F.; Zhu, C.H. Properties and microstructure of alkali-activated slag cement cured at below- and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Yin, J.M.; Kang, A.H.; Xiao, P.; Kou, C.J.; Gong, Y.F.; Xiao, C.H. Influences of spraying sodium silicate based solution/slurry on recycled coarse aggregate. Constr. Build. Mater. 2023, 337, 130924. [Google Scholar] [CrossRef]
- Rakhimova, N.; Shi, C.J. Upcycling of concrete wastes as precursors in alkali-activated materials: A review. Constr. Build. Mater. 2024, 436, 1136978. [Google Scholar] [CrossRef]
- Fort, J.; Vejmelkova, E.; Konakova, D.; Alblova, N.; Cachova, M.; Keppert, M.; Rovnanikova, P.; Cerny, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Kim, J. Sustainable construction exploration: A review of multi-recycling of concrete waste. Int. J. Environ. Res. 2024, 18, 103. [Google Scholar] [CrossRef]
- Zhou, Q.S.; Wang, W.; Noguchi, T. Recyclable calcium carbonate-based concrete: Utilizing calcium carbonate to bond recycled concrete fines through an in-situ heterogeneous dual-precipitation approach. Cem. Concr. Res. 2024, 186, 107679. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.G.; Gong, Y.F. Study on alkali-activated prefabricated building recycled concrete powder for foamed lightweight soils. Materials 2023, 16, 4167. [Google Scholar] [CrossRef] [PubMed]
- Li, M.G.; Tan, H.B.; He, X.Y.; Jian, S.W.; Li, G.Y.; Zhang, J.J.; Deng, X.F.; Lin, X.L. Enhancement in compressive strength of foamed concrete by ultra-fine slag. Constr. Build. Mater. 2023, 138, 104954. [Google Scholar] [CrossRef]
- Souza, T.B.; Lima, V.M.E.; Araujo, L.F.R.; Neto, A.A.M. Alkali-activated slag cellular concrete with expanded polystyrene (EPS)–physical, mechanical, and mineralogical properties. J. Build. Eng. 2021, 44, 103387. [Google Scholar] [CrossRef]
- He, J.; Gao, Q.; Song, X.F.; Bu, X.L.; He, J.H. Effect of foaming agent on physical and mechanical properties of alkali-activated slag foamed concrete. Constr. Build. Mater. 2019, 226, 280–287. [Google Scholar] [CrossRef]
- Zhang, D.S.; Ding, S.; Ma, Y.; Yang, Q.N. Preparation and properties of foam concrete incorporating fly ash. Materials 2022, 15, 6287. [Google Scholar] [CrossRef] [PubMed]
- Masoule, M.S.T.; Bahrami, N.; Karimzadeh, M.; Mohasanati, B.; Shoaei, P.; Ameri, F.; Ozbakkaloglu, T. Lightweight geopolymer concrete: A critical review on the feasibility, mixture design, durability properties, and microstructure. Ceram. Int. 2022, 48, 10347–10371. [Google Scholar] [CrossRef]
- Li, P.W.; Wu, H.J.; Liu, Y.C.; Yang, J.M.; Fang, Z.S.; Lin, B.R. Preparation and optimization of ultra-light and thermal insulative aerogel foamed concrete. Constr. Build. Mater. 2019, 205, 529–542. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Isomoisio, H.; Karhu, M.; Lllikainen, M. Mechanical and acoustic properties of fiber-reinforced alkali-activated slag foam concretes containing lightweight structural aggregates. Constr. Build. Mater. 2018, 187, 371–381. [Google Scholar] [CrossRef]
- Hao, Y.F.; Yang, G.Z.; Liang, K.K. Development of fly ash and slag based high-strength alkali-activated foamed concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Yang, K.H.; Lee, K.H.; Song, J.K.; Gong, M.H. Properties and sustainability of alkali-activated slag foamed concrete. J. Clean. Prod. 2014, 68, 226–233. [Google Scholar] [CrossRef]
- Shi, J.Y.; Liu, Y.C.; Wang, E.L.; Wang, L.Z.; Li, C.Q.; Xu, H.J.; Zheng, X.M.; Yuan, Q. Physico-mechanical, thermal properties and durability of foamed geopolymer concrete containing cenospheres. Constr. Build. Mater. 2022, 325, 126841. [Google Scholar] [CrossRef]
- Alnahhal, A.M.; Alengaram, U.J.; Yusoff, S.; Darvish, P.; Srinivas, K.; Sumesh, M. Engineering performance of sustainable geopolymer foamed and non-foamed concretes. Constr. Build. Mater. 2022, 316, 125601. [Google Scholar] [CrossRef]
- Limbachiya, M.C.; Leelawat, T.; Dhir, R. Use of recycled aggregate in high-strength concrete. Mater. Struct. 2000, 33, 574–580. [Google Scholar] [CrossRef]
- Kim, J.; Jang, H. Closed-loop recycling of C&D waste: Mechanical properties of concrete with the repeatedly recycled C&D powder as partial cement replacement. J. Clean. Prod. 2022, 343, 130977. [Google Scholar]
- Tang, Q.; Ma, Z.M.; Wu, H.X.; Wang, W. The utilization of eco-friendly recycled powder from concrete and brick waste in new concrete: A critical review. Cem. Concr. Compos. 2020, 114, 103807. [Google Scholar] [CrossRef]
- JG/T 266-2011; Foamed Concrete. Ministry of Housing and Urban-Rural Development: Beijing, China, 2011.
- GB/T 5486-2008; Test Methods for Inorganic Rigid Thermal Insulation. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. National Development and Reform Commission: Beijing, China, 2011.
- Hilal, A.A.; Thom, N.H.; Dawson, A.R. On entrained pore size distribution of foamed concrete. Construct. Build. Mater. 2015, 75, 227–233. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Alengaram, U.J.; Yap, S.P. Mechanical strength and permeation properties of high calcium fly ash-based geopolymer containing recycled brick powder. J. Build. Eng. 2020, 32, 101655. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Kioupis, D.; Skaropoulou, A.; Tsivilis, S.; Kakali, G. Valorization of brick and glass CDWs for the development of geopolymers containing more than 80% of Wastes. Minerals 2020, 10, 672. [Google Scholar] [CrossRef]
- Sahin, O.; Llcan, H.; Atesli, A.T.; Kul, A.; Yildirim, G.; Sajmaran, M. Construction and demolition waste-based geopolymers suited for use in 3-dimensional additive manufacturing. Cem. Concr. Compos. 2021, 121, 104088. [Google Scholar] [CrossRef]
- Huo, W.W.; Zhu, Z.D.; Chen, W.; Zhang, J.; Kang, Z.Z.; Pu, S.Y.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.R.; Tong, X.F.; Gong, Y.F. Investigation of mineral admixtures on mechanical properties of alkali-activated recycled concrete powders cement. Buildings 2022, 12, 1204. [Google Scholar] [CrossRef]
- Xu, J.; Kang, A.; Wu, Z.G.; Gong, Y.F.; Xiao, P. Evaluation of workability, microstructure and mechanical properties of recycled powder geopolymer reinforced by waste hydrophilic basalt fiber. J. Clean. Prod. 2023, 396, 136514. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C.J. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Shi, D.; Ye, J.Y.; Zhang, W.S.; Shen, W.G. Properties, mineralogy and microstructure evolution of 4-year calcium silicate slag-based alkali-activated material. Constr. Build. Mater. 2019, 212, 569–577. [Google Scholar] [CrossRef]
- Dang, J.T.; Tang, X.S.; Xiao, J.Z.; Han, A.H. Influence of alkaline activator and precursor on the foam characterization and alkali-activated foamed concrete properties. Cem. Concr. Compos. 2024, 145, 105341. [Google Scholar] [CrossRef]
- Bayraktar, O.Y.; Ozel, H.B.; Benli, A.; Yilmazoglu, M.U.; Turkel, I.H.; Dal, B.B.; Sevik, H.K.; Kaplan, G. Sustainable foam concrete development: Enhancing durability and performance through pine cone powder and fly ash incorporation in alkali-activated geopolymers. Constr. Build. Mater. 2024, 457, 139422. [Google Scholar] [CrossRef]



















| Material | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | TiO2 | Na2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| RCPs | 34.3 | 42.1 | 8.2 | 6.2 | 4.5 | 1.2 | 1.0 | 1.0 | 1.5 |
| Slag | 30.0 | 38.1 | 13.6 | 0.6 | 12.5 | 0.4 | 0.6 | 0.3 | 3.9 |
| Fly ash | 61.9 | 2.4 | 28.8 | 2.5 | 0.8 | 1.5 | 1.04 | 0.3 | 0.76 |
| No. | Density (kg/m3) | RCPs (%) | Slag (%) | W/C Ratio | Na2O (%) | Foam Content (%) |
|---|---|---|---|---|---|---|
| FC1 | 600 | 50 | 50 | 0.4 | 6 | 5 |
| FC2 | 50 | 50 | 0.45 | 6 | ||
| FC3 | 50 | 50 | 0.50 | 6 | ||
| FC4 | 800 | 50 | 50 | 0.4 | 6 | 3.4 |
| FC5 | 50 | 50 | 0.45 | 6 | ||
| FC6 | 50 | 50 | 0.50 | 6 | ||
| FC7 | 1000 | 50 | 50 | 0.4 | 6 | 1.8 |
| FC8 | 50 | 50 | 0.45 | 6 | ||
| FC9 | 50 | 50 | 0.50 | 6 |
| No. | Density (kg/m3) | RCPs (%) | Slag (%) | W/C Ratio | Na2O (%) | Foam Content (%) |
|---|---|---|---|---|---|---|
| FCN1 | 600 | 50 | 50 | 0.45 | 6 | 5 |
| FCN2 | 50 | 50 | 0.45 | 8 | ||
| FCN3 | 50 | 50 | 0.45 | 10 | ||
| FCN4 | 800 | 50 | 50 | 0.45 | 6 | 3.4 |
| FCN5 | 50 | 50 | 0.45 | 8 | ||
| FCN6 | 50 | 50 | 0.45 | 10 | ||
| FCN7 | 1000 | 50 | 50 | 0.45 | 6 | 1.8 |
| FCN8 | 50 | 50 | 0.45 | 8 | ||
| FCN9 | 50 | 50 | 0.45 | 10 |
| No. | RCPs (%) | Slag (%) | Fly Ash (%) | W/C Ratio | Na2O (%) |
|---|---|---|---|---|---|
| R60S40 | 60 | 40 | 0 | 0.45 | 6 |
| R60S30F10 | 60 | 30 | 10 | 0.45 | 6 |
| R60S20F20 | 60 | 20 | 20 | 0.45 | 6 |
| R50S50 | 50 | 50 | 0 | 0.45 | 6 |
| R50S40F10 | 50 | 40 | 10 | 0.45 | 6 |
| R50S30F20 | 50 | 30 | 20 | 0.45 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Liu, C.; Zhao, Z.; Wu, Z.; Wu, B. Influence of an Alkaline Activator and Mineral Admixture on the Properties of Alkali-Activated Recycled Concrete Powder-Foamed Concrete. Materials 2025, 18, 2567. https://doi.org/10.3390/ma18112567
Gong Y, Liu C, Zhao Z, Wu Z, Wu B. Influence of an Alkaline Activator and Mineral Admixture on the Properties of Alkali-Activated Recycled Concrete Powder-Foamed Concrete. Materials. 2025; 18(11):2567. https://doi.org/10.3390/ma18112567
Chicago/Turabian StyleGong, Yongfan, Chao Liu, Zhihui Zhao, Zhengguang Wu, and Bangwei Wu. 2025. "Influence of an Alkaline Activator and Mineral Admixture on the Properties of Alkali-Activated Recycled Concrete Powder-Foamed Concrete" Materials 18, no. 11: 2567. https://doi.org/10.3390/ma18112567
APA StyleGong, Y., Liu, C., Zhao, Z., Wu, Z., & Wu, B. (2025). Influence of an Alkaline Activator and Mineral Admixture on the Properties of Alkali-Activated Recycled Concrete Powder-Foamed Concrete. Materials, 18(11), 2567. https://doi.org/10.3390/ma18112567








