Lightweight Artificial Aggregates Produced from Water Reservoir Sediment and Industrial Waste—Ecological and Technological Aspect
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Methods of Production of Lightweight Artificial Aggregates
2.3. Methods Analitical Raw Materials and Aggregates
3. Results and Discussion
3.1. Properties of Raw Materials Used to Produce Lightweight Aggregates
3.2. The Characteristics of the Obtained Lightweight Aggregates
3.3. Ecological Aspect of Lightweight Artificial Aggregates
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinti, G.; Bauza, V.; Clasen, T.; Medlicott, K.; Tudor, T.; Zurbrügg, C.; Vaccari, M. Municipal Solid Waste Management and Adverse Health Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4331. [Google Scholar] [CrossRef] [PubMed]
- Roleders, V.; Oriekhova, T.; Zaharieva, G. Circular Economy as a Model of Achieving Sustainable Development. Probl. Ekorozwoju 2022, 17, 178–185. [Google Scholar] [CrossRef]
- De Angelis, R. Circular economy: Laying the foundations for conceptual and theoretical development in management studies. Manag. Decis. 2021, 59, 1209–1227. [Google Scholar] [CrossRef]
- Chiang, P.F.; Zhang, T.; Claire, M.J.; Maurice, N.J.; Ahmed, J.; Giwa, A.S. Assessment of Solid Waste Management and Decarbonization Strategies. Processes 2024, 12, 1473. [Google Scholar] [CrossRef]
- Gomez, C.; Amelin, J.; Coulouma, G.; Gaab, J.; Dharumarajan, S.; Riotte, J.; Sekhar, M.; Ruiz, L. Reuse of bottom sediment from reservoirs to cropland is a promising agroecological practice that must be rationalized. Sci. Rep. 2025, 15, 7523. [Google Scholar] [CrossRef]
- Braga, B.B.; de Carvalho, T.R.A.; Brosinsky, A.; Foerster, S.; Medeiros, P.H.A. From waste to resource: Cost-benefit analysis of reservoir sediment reuse for soil fertilization in a semiarid catchment. Sci. Total Environ. 2019, 670, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Kazberuk, W.; Szulc, W.; Rutkowska, B. Use Bottom Sediment to Agriculture—Effect on Plant and Heavy Metal Content in Soil. Agronomy 2021, 11, 1077. [Google Scholar] [CrossRef]
- Tarnawski, M.; Baran, A.; Koniarz, T.; Wyrębek, M.; Grela, J.; Piszczek, M.; Koroluk, A. The possibilities of the environmental use of bottom sediments from the silted inlet zone of the Rożnów Reservoir. Geol. Geophys. Environ. 2018, 43, 335. [Google Scholar] [CrossRef]
- Almokdad, M.; Zentar, R. Characterization of recycled dredged Sediments: Toward circular economy in road construction. Constr. Build. Mater. 2023, 402, 132974. [Google Scholar] [CrossRef]
- Beddaa, H.; Ouazia, I.; Fraj, A.B.; Lavergne, F.; Torrenti, J.M. Reuse potential of dredged river sediments in concrete: Effect of sediment variability. J. Clean. Prod. 2020, 265, 121665. [Google Scholar] [CrossRef]
- Junakova, N.; Estokova, A.; Balintova, M.; Sicakova, A.; Junak, J. Sustainable cement mixtures based on sediments as a filler. MATEC Web Conf. 2024, 403, 06007. [Google Scholar] [CrossRef]
- Martellotta, A.M.N.; Petrella, A.; Gentile, F.; Levacher, D.; Piccinni, A.F. Reuse of Lake Sediments in Sustainable Mortar. Environments 2023, 10, 149. [Google Scholar] [CrossRef]
- Alloul, A.; Amar, M.; Benzerzour, M.; Abriak, N.E. Geopolymer mortar with flash-calcined sediments cured under ambient conditions. Constr. Build. Mater. 2023, 391, 131809. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Li, J.S.; Baek, K.; Ding, S.; Poon, C.S. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- Bhairappanavar, S.; Liu, R.; Shakoor, A. Eco-friendly dredged material-cement bricks. Constr. Build. Mater. 2021, 271, 121524. [Google Scholar] [CrossRef]
- Wan, Q.Q.; Ju, C.G.; Han, H.; Yang, M.; Li, Q.Q.; Peng, X.; Wu, Y. An extrusion granulation process without sintering for the preparation of aggregates from wet dredged sediment. Powder Technol. 2022, 396, 27–35. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, M.; Muslim, H.; Hou, T.; Bian, B.; Yang, Z.; Yang, W.; Zhang, L. Co-utilization of lake sediment and blue-green algae for porous lightweight aggregate (ceramsite) production. Chemosphere 2022, 287, 132145. [Google Scholar] [CrossRef]
- Deng, X.T.; Jian, R.L.; Chen, S.; Wang, X.S.; Wan, C.; Xue, Y.J.; Wang, T. Killing two birds with one stone: Preparation of ceramsite high-strength lightweight aggregate via co-sintering of dredged sediment and municipal solid waste incinerated fly ash. Constr. Build. Mater. 2023, 409, 134039. [Google Scholar] [CrossRef]
- Islam, N.; Sandanayake, M.; Muthukumaran, S.; Navaratna, D. Review on Sustainable Construction and Demolition Waste Management—Challenges and Research Prospects. Sustainability 2024, 16, 3289. [Google Scholar] [CrossRef]
- Garcia, J.C.; Caro, D.; Foster, G.; Pristera, G.; Gallo, F.; Tonini, D. Techno-Economic and Environmental Assessment of Construction and Demolition Waste Management in the European Union; European Commission: Luxembourg, 2024; JRC135470; Available online: https://data.europa.eu/doi/10.2760/721895 (accessed on 5 March 2025).
- United Nations Environment Programme; Yale Center for Ecosystems + Architecture. Building Materials and the Climate: Constructing a New Future. 2023. Available online: https://wedocs.unep.org/20.500.11822/43293 (accessed on 7 March 2025).
- Papamichael, I.; Voukkali, I.; Loizia, P.; Zorpas, A.A. Construction and demolition waste framework of circular economy: A mini review. Waste Manag. Res. 2023, 41, 1728–1740. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Letelier, V.; Suazo, A.; Bustamante, M.; Wenzel, B.; Ortega, J.M. Assessment of mortar with fine recycled aggregates and nanoparticles of titanium dioxide under accelerated carbonation. Constr. Build. Mater. 2024, 431, 136555. [Google Scholar] [CrossRef]
- Youssf, O.; Safaa Eldin, D.; Tahwia, A.M. Eco-Friendly High-Strength Geopolymer Mortar from Construction and Demolition Wastes. Infrastructures 2025, 10, 76. [Google Scholar] [CrossRef]
- Evangelista, L.; Guedes, M.; de Brito, J.; Ferro, A.C.; Pereira, M.F. Physical, chemical and mineralogical properties of fine recycled aggregates made from concrete waste. Constr. Build. Mater. 2015, 86, 178–188. [Google Scholar] [CrossRef]
- Hafez, H.; Kurda, R.; Kurda, R.; Al-Hadad, B.; Mustafa, R.; Ali, B.A. Critical Review on the Influence of Fine Recycled Aggregates on Technical Performance, Environmental Impact and Cost of Concrete. Appl. Sci. 2020, 10, 1018. [Google Scholar] [CrossRef]
- Pitarch, Á.M.; Piquer, A.; Reig, L.; Roig-Flores, M.; Albero, V.; Hernández-Figueirido, D.; Melchor-Eixea, A. Reutilization of Recycled CDW Sand in Mortars, Paving Blocks, and Structural Concrete. Appl. Sci. 2025, 15, 3652. [Google Scholar] [CrossRef]
- Gosk, E.; Kalinowska-Wichrowska, K.; Kosior-Kazberuk, M.; Yildiz, M.J.; Derpeński, Ł.; Zamojski, P.; Lipowicz, P. The Basic Properties of Lightweight Artificial Aggregates Made with Recycled Concrete Fines. Sustainability 2024, 16, 9134. [Google Scholar] [CrossRef]
- Kursula, K.; Mistri, A.; Illikainen, M.; Perumal, P. Utilization of fine concrete waste as a lightweight aggregate via granulation: Technical and environmental assessment. J. Clean. Prod. 2024, 434, 139938. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Nasrollahi, V.R.; Darajeh, N.; Lotfi Ghahroud, M.; Mehranzamir, K. Review on Waste-to-Energy Approaches toward a Circular Economy in Developed and Developing Countries. Processes 2023, 11, 2566. [Google Scholar] [CrossRef]
- Kasiński, S.; Dębowski, M. Municipal Solid Waste as a Renewable Energy Source: Advances in Thermochemical Conversion Technologies and Environmental Impacts. Energies 2024, 17, 4704. [Google Scholar] [CrossRef]
- Yang, D.; Kow, K.W.; Wang, W.; Meredith, W.; Zhang, G.; Mao, Y.; Xu, M. Co-treatment of municipal solid waste incineration fly ash and alumina-/silica-containing waste: A critical review. J. Hazard. Mater. 2024, 479, 135677. [Google Scholar] [CrossRef]
- Ren, J.; Liu, B.; Guo, J.; Liu, J.; Xing, F.; Zhu, H.; Zhao, L.; Mi, T. Bio-treatment of municipal solid waste incineration fly ash: A sustainable path for recyclability. J. Clean. Prod. 2024, 434, 139869. [Google Scholar] [CrossRef]
- Lam, C.H.; Ip, A.W.; Barford, J.P.; McKay, G. Use of incineration MSW ash: A review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef]
- Kanhar, A.H.; Chen, S.; Wang, F. Incineration Fly Ash and Its Treatment to Possible Utilization: A Review. Energies 2020, 13, 6681. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Rusănescu, M. Application of Fly Ash Obtained from the Incineration of Municipal Solid Waste in Agriculture. Appl. Sci. 2023, 13, 3246. [Google Scholar] [CrossRef]
- Zhang, Y.; Soleimanbeigi, A.; Likos, W.J.; Edil, T.B. Geotechnical and Leaching Properties of Municipal Solid Waste Incineration Fly Ash for Use as Embankment Fill Material. Transp. Res. Rec. 2016, 2579, 70–78. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, D.; Wang, J.; Wu, D.; Liu, J. The Performance Evaluation of Asphalt Mortar and Asphalt Mixture Containing Municipal Solid Waste Incineration Fly Ash. Materials 2022, 15, 1387. [Google Scholar] [CrossRef] [PubMed]
- Marieta, C.; Martín-Garin, A.; Leon, I.; Guerrero, A. Municipal Solid Waste Incineration Fly Ash: From Waste to Cement Manufacturing Resource. Materials 2023, 16, 2538. [Google Scholar] [CrossRef]
- Zhang, S.; Cheng, Y.; Wu, H.; Cong, J.; Zhou, Z.; Wei, D. Research on the Preparation of Dry Mixed Mortar Using Waste Incineration Fly Ash. Coatings 2024, 14, 1355. [Google Scholar] [CrossRef]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef]
- Collivignarelli, C.; Sorlini, S. Reuse of municipal solid wastes incineration fly ashes in concrete mixtures. Waste Manag. 2002, 22, 909–912. [Google Scholar] [CrossRef]
- Han, S.; Song, Y.; Ju, T.; Meng, Y.; Meng, F.; Song, M.; Lin, L.; Liu, M.; Li, J.; Jiang, J. Recycling municipal solid waste incineration fly ash in super-lightweight aggregates by sintering with clay and using SiC as bloating agent. Chemosphere 2022, 307, 135895. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, P.; Zeng, L.; Yu, L.; Li, D. Study on preparation of glass-ceramics from municipal solid waste incineration (MSWI) fly ash and chromium slag. J. Build. Eng. 2023, 68, 106080. [Google Scholar] [CrossRef]
- Juntarasakul, O.; Roongcharoen, P.; Tabelin, C.B.; Phengsaart, T. Municipal Solid Waste Incineration (MSWI) Fly Ash as a Potential Adsorbent for Phosphate Removal. Sustainability 2024, 16, 8869. [Google Scholar] [CrossRef]
- Xing, J.; Tang, Q.; Gan, M.; Ji, Z.; Fan, X.; Sun, Z.; Chen, X. Co-treatment of municipal solid waste incineration fly ash (MSWI FA) and municipal sludge: A innovative method to improve sludge dewatering with fly ash dechlorination. J. Environ. Manag. 2023, 332, 117403. [Google Scholar] [CrossRef]
- BS EN 13055:2016; Lightweight Aggregates. British Standards Institution (BSI): London, UK, 2016.
- Franus, M. Application of Waste Materials in Lightweight Aggregates, 1st ed.; Routledge: London, UK, 2023. [Google Scholar] [CrossRef]
- Hao, D.L.C.; Razak, R.A.; Kheimi, M.; Yahya, Z.; Abdullah, M.M.A.B.; Burduhos Nergis, D.D.; Fansuri, H.; Ediati, R.; Mohamed, R.; Abdullah, A. Artificial Lightweight Aggregates Made from Pozzolanic Material: A Review on the Method, Physical and Mechanical Properties, Thermal and Microstructure. Materials 2022, 15, 3929. [Google Scholar] [CrossRef]
- Owens, P.L.; Newman, J.B. Lightweight aggregate manufacture. Adv. Concr. Technol. 2003, 3, 7. [Google Scholar] [CrossRef]
- Franus, M.; Panek, R.; Madej, J.; Franus, W. The properties of fly ash derived lightweight aggregates obtained using microwave radiation. Constr. Build. Mater. 2019, 227, 116677. [Google Scholar] [CrossRef]
- Franus, M.; Barnat-Hunek, D.; Wdowin, M. Utilization of sewage sludge in the manufacture of lightweight aggregate. Environ. Monit. Assess. 2016, 188, 10. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Bąk, Ł.; Stachowicz, F. Unconventional materials from sewage sludge with a potential application in a road construction. Adv. Sci. Technol. Res. J. 2018, 12, 65–75. [Google Scholar] [CrossRef]
- Grygo, R.; Bujnarowski, K.; Prusiel, J.A. Using plastic waste to produce lightweight aggregate for RC structures. Econ. Environ. 2024, 89, 804. [Google Scholar] [CrossRef]
- Masłoń, A.; Cieśla, M.; Gruca-Rokosz, R.; Chutkowski, M.; Franus, M.; Kalinowska-Wichrowska, K. Method of Producing Aggregate Using Bottom Sediment and Aggregate Produced by This Method (In Polish: Sposób Wytwarzania Kruszywa Z Wykorzystaniem Osadu Dennego Oraz Kruszywo Wytworzone Tym Sposobem). Polish Patent Application No. P.451564, 25 March 2025. [Google Scholar]
- Masłoń, A.; Cieśla, M.; Gruca-Rokosz, R.; Chutkowski, M.; Franus, M.; Kalinowska-Wichrowska, K. Method of Producing Aggregate Using Bottom Sediment and Aggregate Produced by This Method (In Polish: Sposób Wytwarzania Kruszywa Z Wykorzystaniem Osadu Dennego Oraz Kruszywo Wytworzone Tym Sposobem). Polish Patent Application No. P.451565, 25 March 2025. [Google Scholar]
- PN-EN 15216:2022-03; Solid Components of the Environment—Determination of Total Dissolved Substance (TDS) in Water and Eluates. Polish Committee for Standardization: Warsaw, Poland, 2022.
- PN-ISO 6439:1994; Water Quality—Determination of Phenol Index. Polish Committee for Standardization: Warsaw, Poland, 1994.
- PN-EN 15935:2013-02; Sewage Sludge, Treated Biowaste, Soil and Waste—Determination of Loss on Ignition. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-EN 1097-3:2000; Tests for Mechanical and Physical Properties of Aggregates—Part 3: Determination of Loose Bulk Density and Voids. Polish Committee for Standardization: Warsaw, Poland, 2000.
- PN-EN 1097-11:2013; Tests for Mechanical and Physical Properties of Aggregates—Part 11: Determination of Compressibility and Confined Compressive Strength of Lightweight Aggregates. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-EN 1367-1:2007; Tests for Thermal and Weathering Properties of Aggregates—Part 1: Determination of Resistance to Freezing and Thawing. Polish Committee for Standardization: Warsaw, Poland, 2007.
- PN-EN 1097-6:2022-07; Tests on Mechanical and Physical Properties of Aggregates—Part 6: Determination of Grain Density and Absorbability. Polish Committee for Standardization: Warsaw, Poland, 2022.
- PN-EN 933-4:2008; The Standard for Tests for Geometrical Properties of Aggregates—Determination of Particle Shape. Shape index; Polish Committee for Standardization: Warsaw, Poland, 2008.
- PN EN 933-1:2012; Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method. Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-EN 12457-2:2006; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or With Size Reduction). Polish Committee for Standardization: Warsaw, Poland, 2006.
- Li, H.; Liu, G.; Cao, Y. Levels and Environmental Impact of PAHs and Trace Element in Fly Ash from a Miscellaneous Solid Waste by Rotary Kiln Incinerator, China. Nat. Hazards 2015, 76, 811–822. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, W.; Wang, Y.; Cheng, Q.; Yuan, X.; Li, Z.; Yang, J. Numerical Simulation of Combustion and Characteristics of Fly Ash and Slag in a “V-type” Waste Incinerator. Energies 2021, 14, 7518. [Google Scholar] [CrossRef]
- Gharpure, A.; Heim, J.W., II; Vander Wal, R.L. Characterization and Hazard Identification of Respirable Cement and Concrete Dust from Construction Activities. Int. J. Environ. Res. Public Health 2021, 18, 10126. [Google Scholar] [CrossRef] [PubMed]
- Gruszecka-Kosowska, A.; Wdowin, M.; Kosowski, T.; Klimek, A. An analysis of the chemistry, mineralogy and texture of waste dolomite powder used to identify its potential application in industry. Geol. Geophys. Environ. 2016, 41, 343. [Google Scholar] [CrossRef]
- Huts, A.; Konkol, J.; Marchuk, V. Granite Dust and Silica Fume as a Combined Filler of Reactive Powder Concrete. Materials 2024, 17, 6025. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef]
- Stempkowska, A.; Gawenda, T. Artificial lightweight aggregate made from alternative and waste raw materials, hardened using the hybrid method. Sci. Rep. 2024, 14, 16880. [Google Scholar] [CrossRef]
- Łach, M.; Kozub, B.; Bednarz, S.; Bąk, A.; Melnychuk, M.; Masłoń, A. The Influence of the Addition of Basalt Powder on the Properties of Foamed Geopolymers. Materials 2024, 17, 2336. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.K.; Gaurav, G.K.; Klemeš, J.J.; Sandhwar, V.K.; Pant, K.K.; Kumar, R. A critical review on sustainable hazardous waste management strategies: A step towards a circular economy. Environ. Sci. Pollut. Res. 2023, 30, 105030–105055. [Google Scholar] [CrossRef]
- Regulation of the Minister of Economy of 16 July 2015 on the admission of waste for landfill. J. Laws 2015, 1277. (In Polish)
- Czop, M.; Łaźniewska-Piekarczyk, B. Evaluation of the Leachability of Contaminations of Fly Ash and Bottom Ash from the Combustion of Solid Municipal Waste before and after Stabilization Process. Sustainability 2019, 11, 5384. [Google Scholar] [CrossRef]
- Poranek, N.; Pizoń, J.; Łaźniewska-Piekarczyk, B.; Czajkowski, A.; Lagashkin, R. Recycle Option for Municipal Solid Waste Incineration Fly Ash (MSWIFA) as a Partial Replacement for Cement in Mortars Containing Calcium Sulfoaluminate Cement (CSA) and Portland Cement to Save the Environment and Natural Resources. Materials 2024, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, C.R.; Virdi, G.S. Properties of microstructure of lightweight aggregate produced from sintered sewage sludge ash. Resour. Conserv. Recycl. 2005, 45, 18–30. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Huang, C.-Y.; Chen, Y.-M. Lightweight aggregates from water reservoir sediment with added sodium hydroxide. Constr. Build. Mater. 2013, 46, 79–85. [Google Scholar] [CrossRef]
- Dachowski, R.; Komisarczyk, K. Determination of Microstructure and Phase Composition of Sand-Lime Brick after Autoclaving Process. Procedia Eng. 2016, 161, 747–753. [Google Scholar] [CrossRef][Green Version]
- Przychodzień, P.; Katzer, J. Properties of Structural Lightweight Aggregate Concrete Based on Sintered Fly Ash and Modified with Exfoliated Vermiculite. Materials 2021, 14, 5922. [Google Scholar] [CrossRef]
- Chen, H.-J.; Wang, S.-Y.; Tang, C.-W. Reuse of incineration fly ashes and reaction ashes for manufacturing lightweight aggregate. Constr. Build. Mater. 2010, 24, 46–55. [Google Scholar] [CrossRef]
- Wang, D.; Cui, C.; Chen, X.-F.; Zhang, S.; Ma, H. Characteristics of autoclaved lightweight aggregates with quartz tailings and its effect on the mechanical properties of concrete. Constr. Build. Mater. 2020, 262, 120110. [Google Scholar] [CrossRef]
- Zukri, A.; Nazir, R.; Said, K.N.M.; Moayedi, H. Physical and Mechanical Properties of Lightweight Expanded Clay Aggregate (LECA). MATEC Web Conf. 2018, 250, 01016. [Google Scholar] [CrossRef]
- Jaha, S.; Carvalheiras, J.; Mahmoudi, S.; Labrincha, J. Production of lightweight expanded aggregates from smectite clay, palygorskite-rich sediment and phosphate sludge. Clay Miner. 2024, 59, 85–99. [Google Scholar] [CrossRef]
- Ding, C.; Sun, T.; Shui, Z.; Xie, Y.; Ye, Z. Physical properties, strength, and impurities stability of phosphogypsum-based cold-bonded aggregates. Constr. Build. Mater. 2022, 331, 127307. [Google Scholar] [CrossRef]
- Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on substances particularly harmful to the aquatic environment and the conditions that must be met when introducing sewage into water or ground, as well as when discharging rainwater or meltwater into water or water facilities. J. Laws 2019. Item 1311 (In Polish)
- Tian, Y.; Liu, Y.; Xie, Y.-M.; Zhang, G.-Y.; Ye, H.-L.; Yan, D.-M.; Li, Y.-J.; Li, B.; Xue, H.-J.; Cai, Q. Coupled effects of chlorides and sulfates on steel reinforcement corrosion in concrete structures: A comprehensive review. Case Stud. Constr. Mater. 2025, 22, e04263. [Google Scholar] [CrossRef]


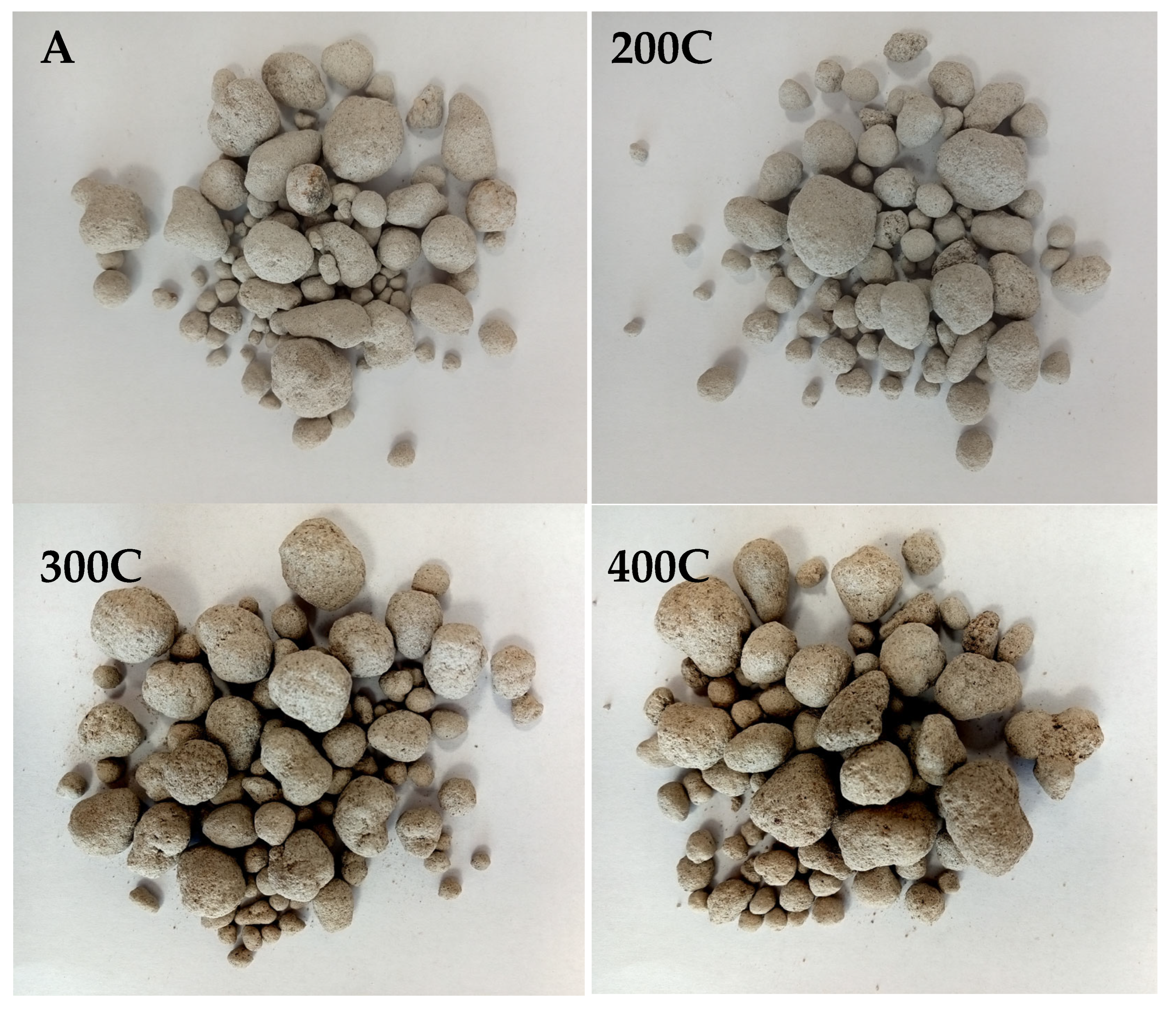
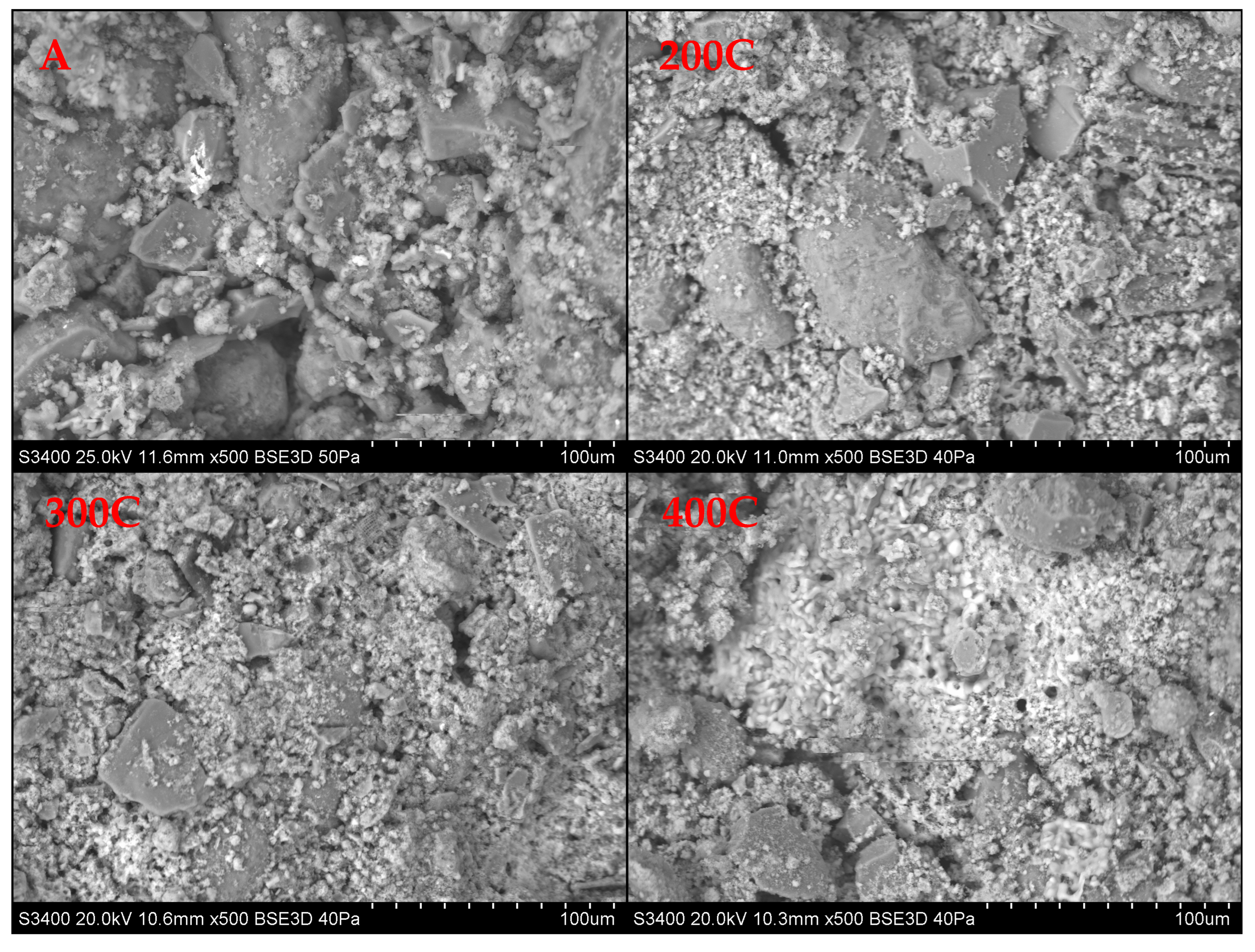
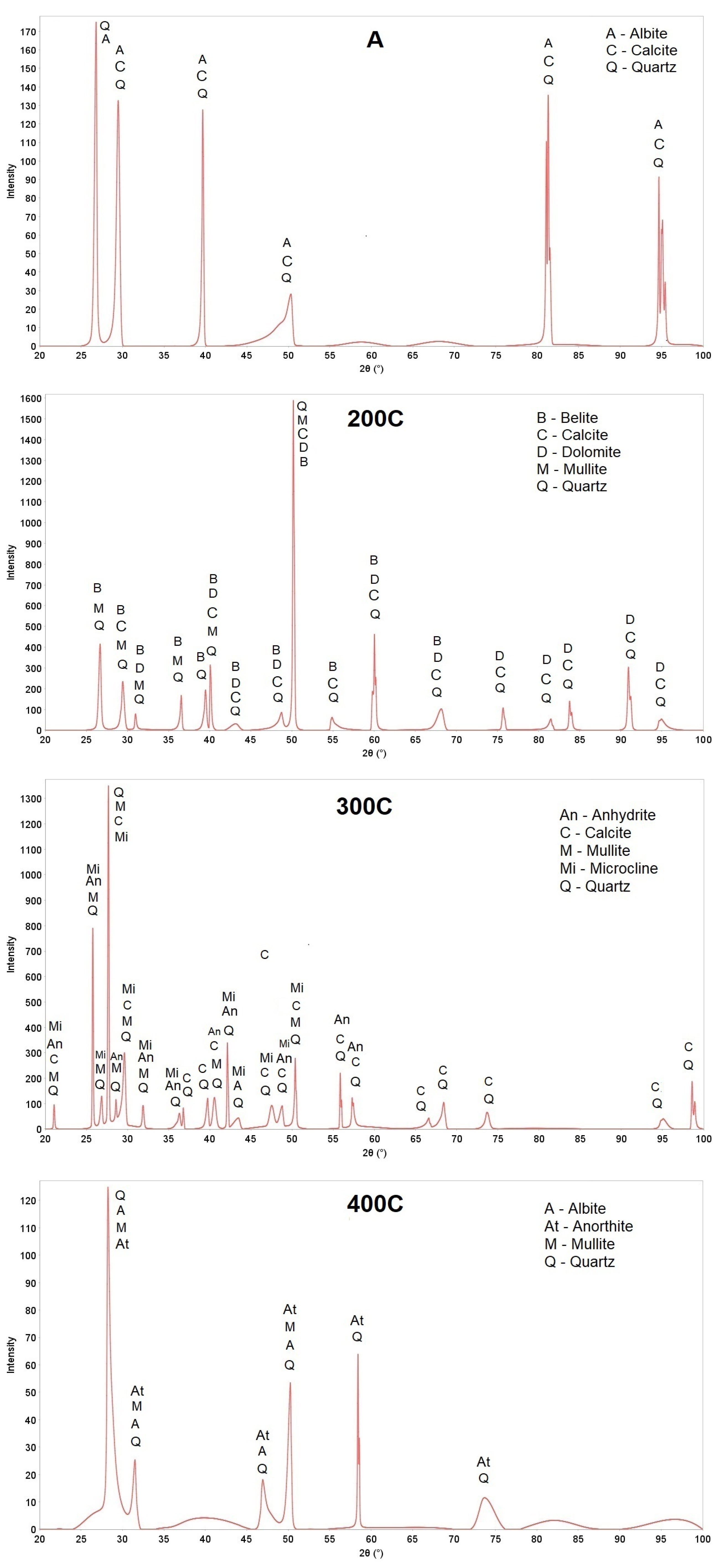
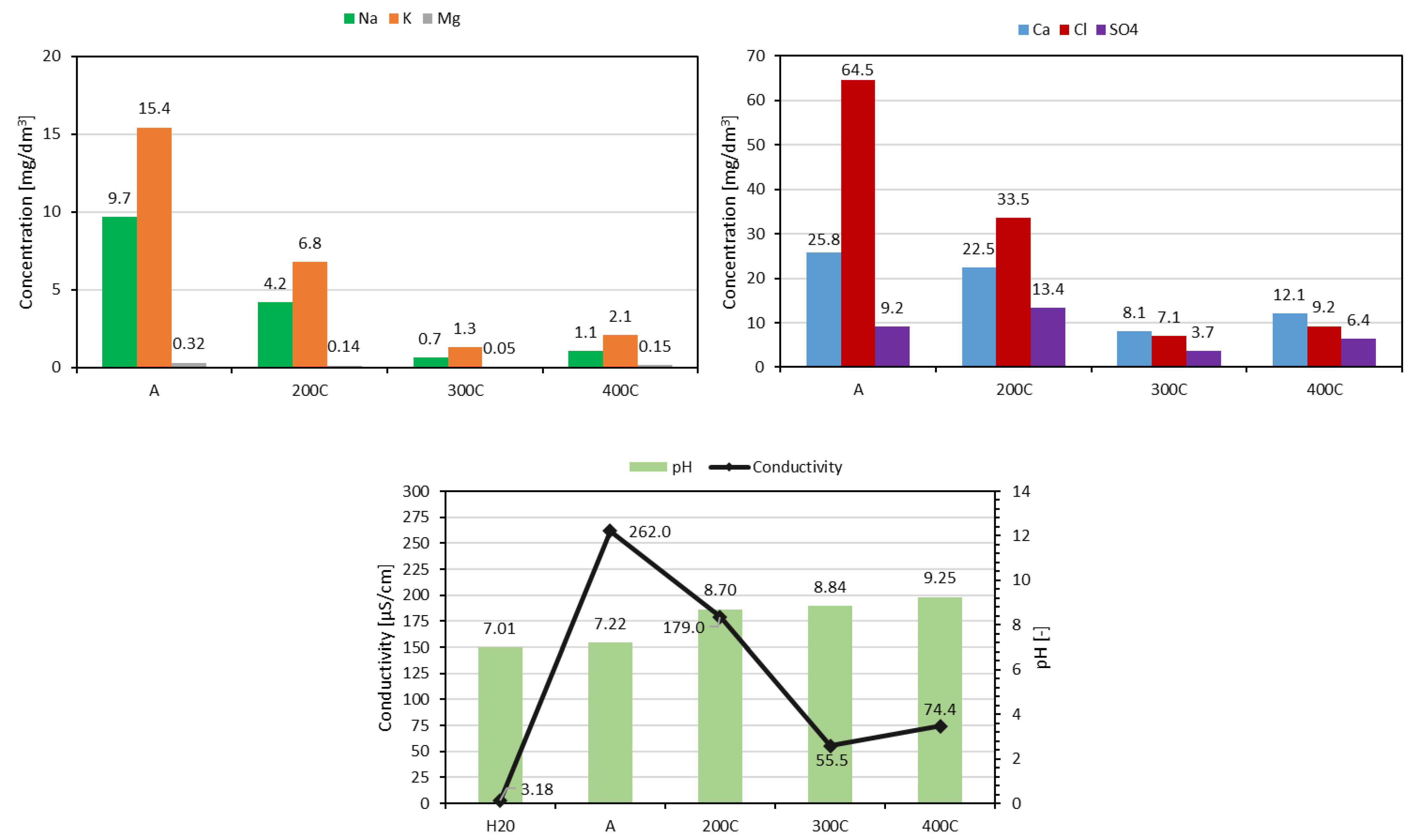
| Sample | Granulation | Drying (Temperature, Time) | Hardening (Type, Temperature, Time) |
|---|---|---|---|
| A | rotating dish granulator | shelf dryer 60 °C 4 h | Autoclave, 180 °C, 1 h, |
| 200C | Muffle oven, 200 °C, 1 h | ||
| 300C | Muffle oven, 300 °C, 1 h | ||
| 400C | Muffle oven, 400 °C, 1h |
| Parameter | Standard |
|---|---|
| Grain density | PN-EN 1097-3:2000 [60] |
| Bulk density | PN-EN 1097-3:2000 [60] |
| Loose bulk density | PN-EN 1097-3:2000 [60] |
| Porosity | PN-EN 1097-3:2000 [60] |
| Compressive strength | PN-EN 1097-11:2013 [61] |
| Frost resistance | PN-EN 1367-1:2007 [62] |
| Water absorption | PN-EN 1097-6:2022-07 [63] |
| Grain shape index | PN EN 933-4:2008 [64] |
| Grain composition | PN-EN 933-1:2012 [65] |
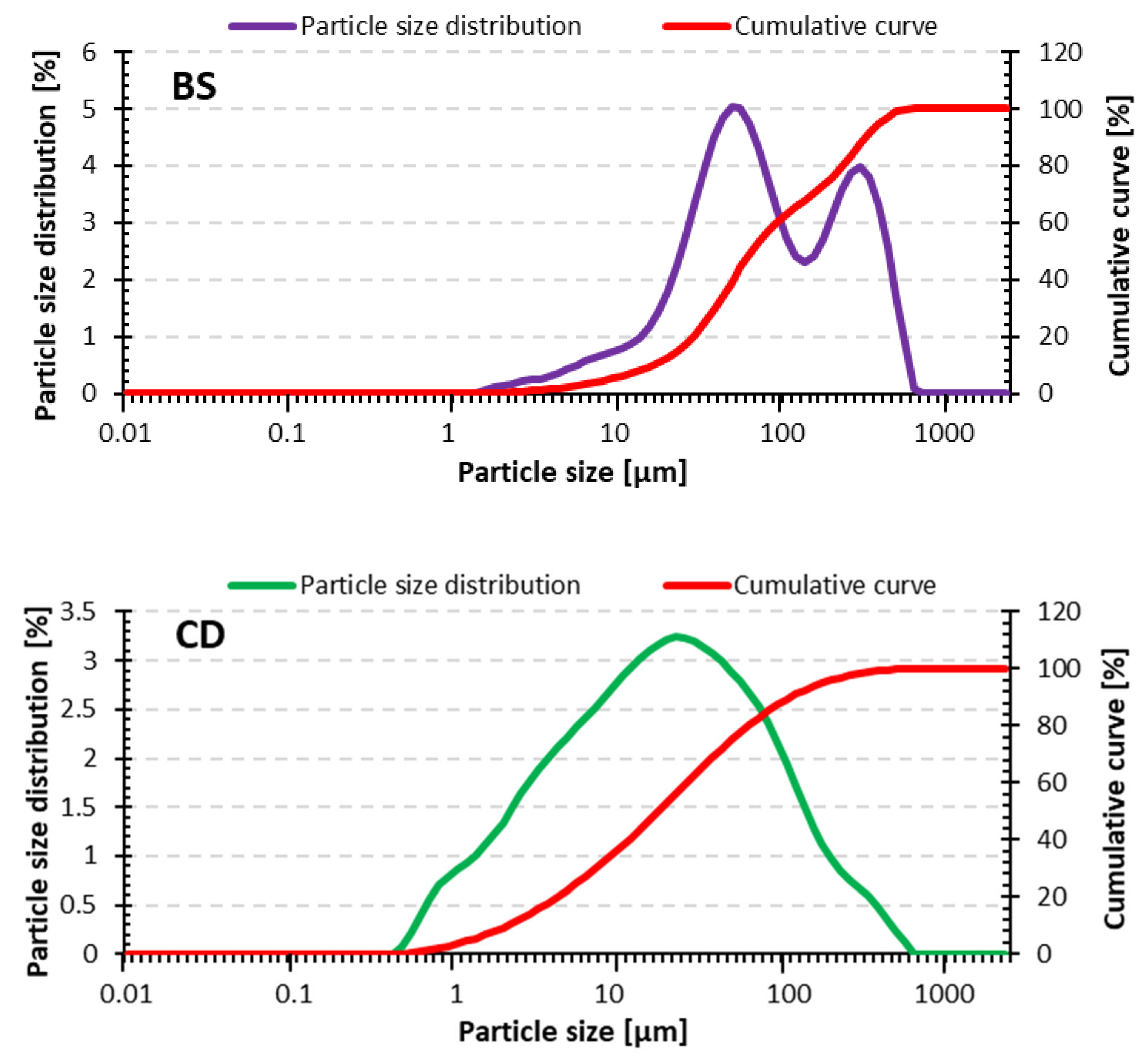
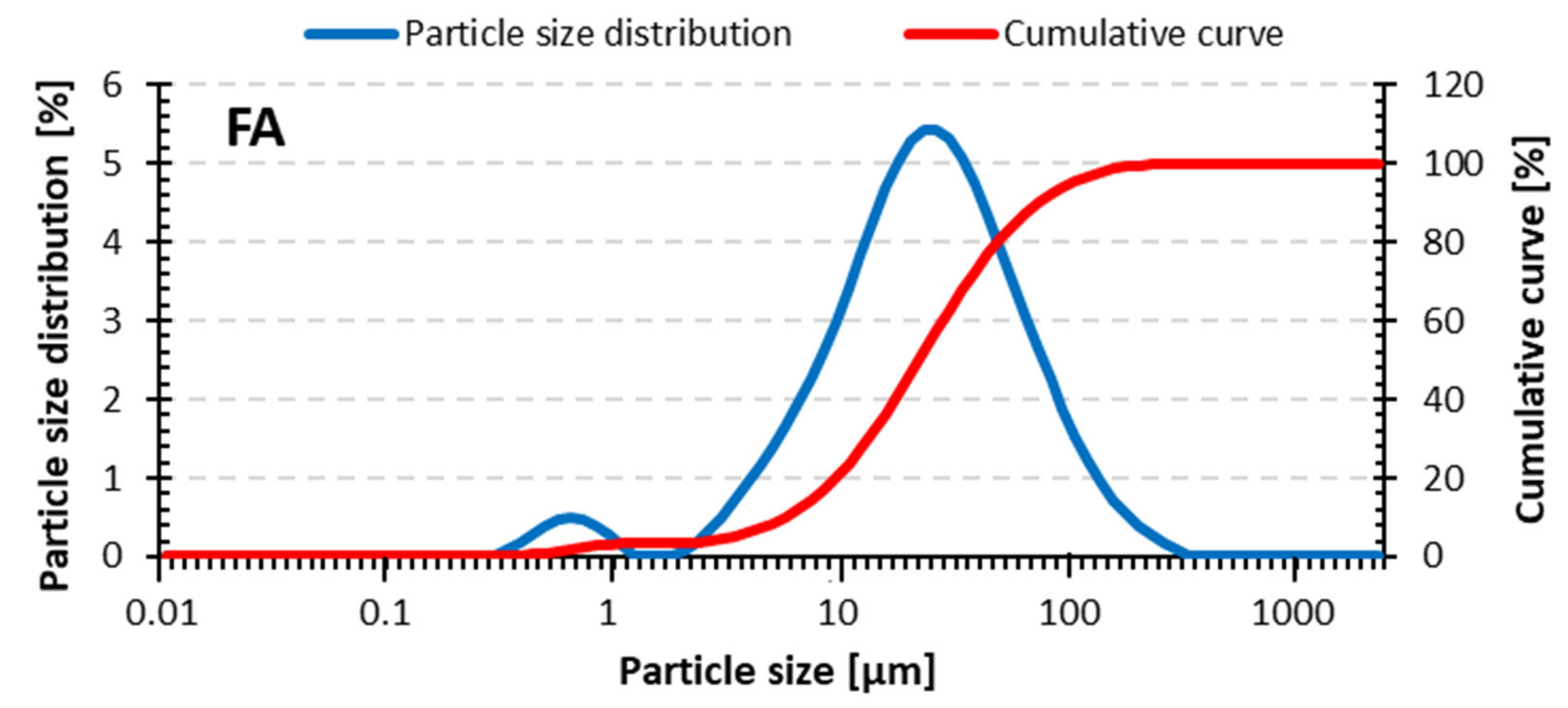
| Raw Material | D10 [μm] | D50 [μm] | D90 [μm] |
|---|---|---|---|
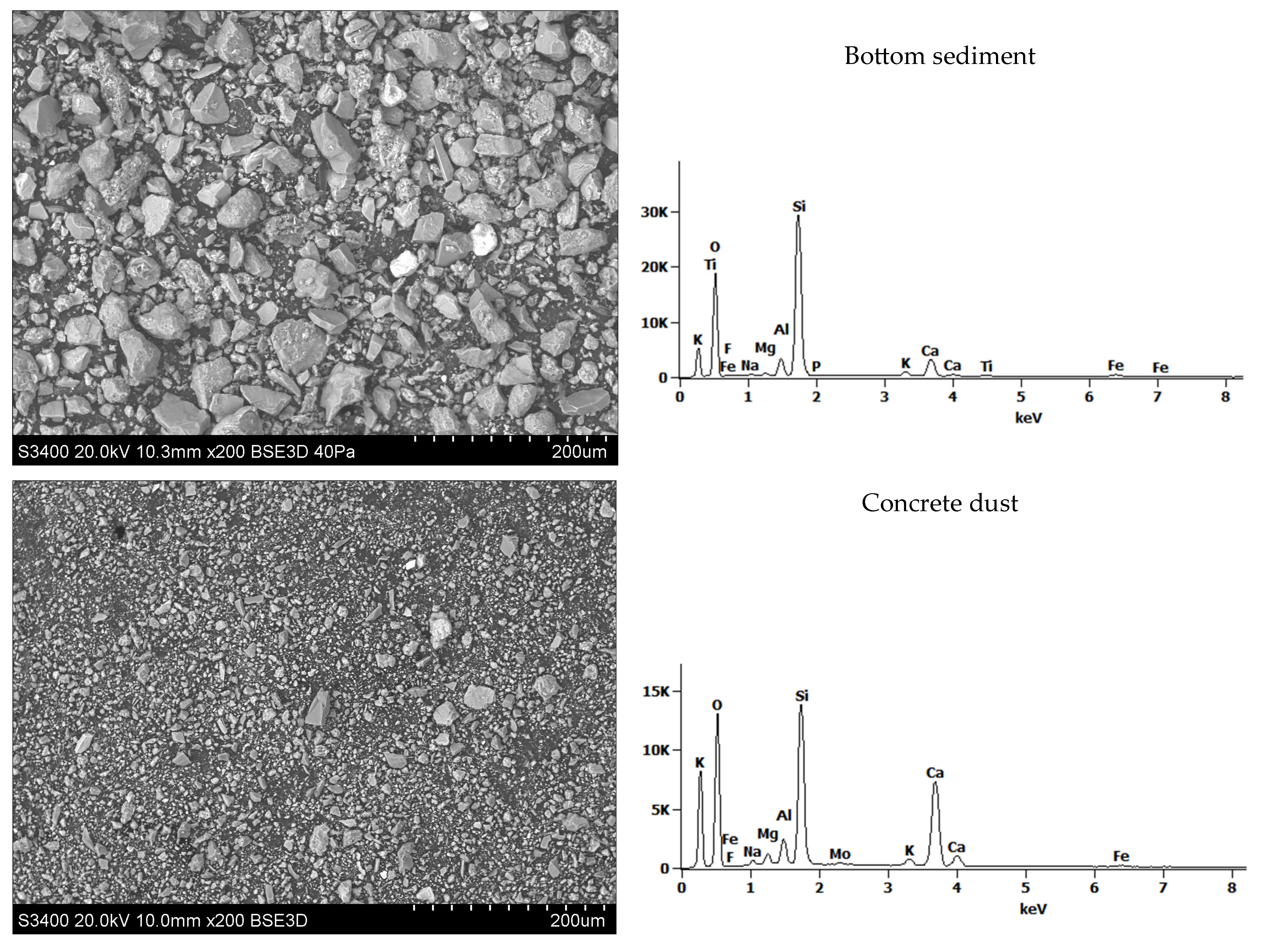
| Bottom sediment | 13.8 | 57.6 | 312.0 |
| Concrete dust | 2.21 | 17.7 | 100.0 |
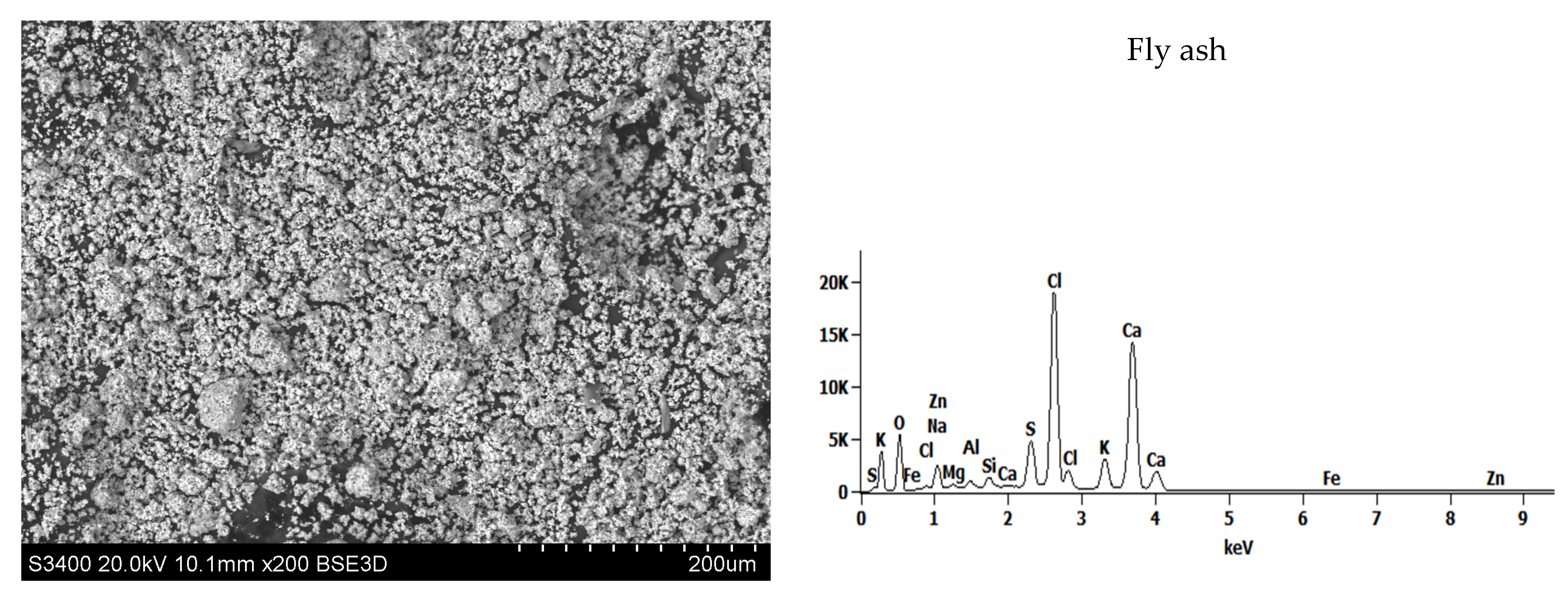
| Fly ash | 4.1 | 18.1 | 58.7 |
| Component, wt. % | BS | CD | FA |
|---|---|---|---|
| Na2O | n.o. | n.o. | 1.71 |
| MgO | 1.15 | 1.59 | 0.19 |
| Al2O3 | 9.36 | 2.85 | 0.53 |
| SiO2 | 65.53 | 34.40 | 1.48 |
| P2O5 | 0.32 | n.o. | n.o. |
| SO3 | 0.46 | 0.85 | 6.99 |
| Cl | n.o. | 0.03 | 15.59 |
| K2O | 2.82 | 1.08 | 3.89 |
| CaO | 5.31 | 39.39 | 29.82 |
| TiO2 | 0.80 | 0.19 | 0.30 |
| MnO | 0.14 | 0.06 | 0.05 |
| Fe2O3 | 5.15 | 2.13 | 0.58 |
| CuO | n.o. | n.o. | 0.04 |
| ZnO | 0.02 | 0.04 | 1.04 |
| Br2O3 | 0.04 | n.o. | 0.11 |
| SrO | 0.02 | 0.04 | 0.04 |
| ZrO2 | 0.06 | n.o. | n.o. |
| BaO | 0.02 | 0.03 | 0.02 |
| PbO | n.o. | n.o. | 0.13 |
| Component, mg/kg dm | BS | CD | FA |
|---|---|---|---|
| Total PAHs 1 | <0.01 | 0.085 | 0.3 |
| BTEX 2 | <0.1 | <0.1 | <0.1 |
| PCBs 3 | <0.001 | <0.001 | <0.001 |
| TOC 4 | 16,800 | 9936 | 7663 |
| C10–C40 hydrocarbons | 19 | 22 | 11 |
| As | <5 | <5 | <5 |
| Ba | 17.7 | 31.9 | 108 |
| Cd | <0.5 | <0.5 | 48.5 |
| Cr | 8.41 | 6.72 | 14.4 |
| Cu | <5 | 109 | 169 |
| Hg | <0.25 | <0.25 | 5.42 |
| Mo | <5 | <5 | <5 |
| Ni | <5 | 63.2 | 6.61 |
| Pb | <5 | <5 | 677 |
| Sb | <5 | <5 | 202 |
| Se | <5 | <5 | <5 |
| Zn | 12.6 | 16.1 | 5862 |
| Component, mg/kg dm | Raw Material | Limit Values [76] | ||||
|---|---|---|---|---|---|---|
| BS | CD | FA | Inert Waste | Waste Other than Inert and Hazardous | Hazardous Waste | |
| Ca | 360.6 | 584.3 | 63,374.0 | not limited | not limited | not limited |
| Mg | 40.52 | 7.3 | 8.65 | not limited | not limited | not limited |
| K | 27.25 | 461.1 | 41,598.9 | not limited | not limited | not limited |
| Na | 11.73 | 151.6 | 27,773.7 | not limited | not limited | not limited |
| Fl | <1 | 6.3 | 39 | 10.0 | 150.0 | 500.0 |
| Cl | 31 | 890 | 170,000 | 800.0 | 15,000.0 | 25,000.0 |
| SO4 | 300 | 300 | 14,000 | 1000.0 | 20,000.0 | 50,000.0 |
| As | <0.1 | <0.1 | <0.1 | 0.5 | 2.0 | 25 |
| Ba | 0.33 | 2.28 | 19.3 | 20.0 | 100.0 | 300.0 |
| Cd | <0.01 | <0.01 | <0.01 | 0.04 | 1.0 | 5.0 |
| Cr | <0.05 | 0.069 | <0.05 | 0.5 | 10.0 | 70.0 |
| Cu | <0.05 | <0.05 | <0.05 | 2.0 | 50.0 | 100.0 |
| Hg | <0.001 | <0.001 | <0.001 | 0.01 | 0.2 | 2.0 |
| Mo | <0.2 | <0.2 | 0.81 | 0.5 | 10.0 | 30.0 |
| Ni | <0.1 | <0.1 | <0.1 | 0.4 | 10.0 | 40.0 |
| Pb | <0.1 | <0.1 | <0.1 | 0.5 | 10.0 | 50.0 |
| Sb | <0.01 | <0.01 | <0.01 | 0.06 | 0.7 | 5.0 |
| Se | <0.1 | <0.1 | <0.1 | 0.1 | 0.5 | 7.0 |
| Zn | <0.2 | <0.2 | 2.92 | 4.0 | 50.0 | 200.0 |
| TDS | 1900 | 39,200 | 322,000 | 4000.0 | 60,000.0 | 100,000.0 |
| DOC | 250 | 169 | 443 | 500.0 | 800 | 1000.0 |
| Volatile Phenols | <0.25 | 0.78 | 0.47 | 1.0 | not limited | not limited |
| Sample | O | Ca | Si | Na | Mg | Al | K | Fe | Cl | S | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 33.9 | 37.7 | 13.7 | 0.5 | 0.7 | 1.6 | 1.7 | 0.9 | 5.1 | 3.2 | 0.6 |
| 200C | 34.4 | 33.9 | 15.2 | 1.5 | 0.6 | 1.8 | 2.0 | 1.0 | 6.8 | 2.4 | 0.9 |
| 300C | 33.0 | 34.2 | 14.1 | 0.8 | 0.5 | 1.6 | 3.0 | 1.3 | 9.0 | 2.3 | 0.6 |
| 400C | 31.4 | 36.7 | 11.45 | 1.8 | 0.5 | 1.5 | 3.3 | 1.3 | 9.6 | 2.0 | 0.4 |
| Parameter | Unit | A | 200C | 300C | 400C |
|---|---|---|---|---|---|
| Grain density | g/cm3 | 1.74 ± 0.05 | 1.69 ± 0.08 | 1.79 ± 0.07 | 1.76 ± 0.03 |
| Bulk density | g/cm3 | 1.02 ± 0.03 | 0.92 ± 0.02 | 0.92 ± 0.03 | 0.90 ± 0.03 |
| Specific density | g/cm3 | 2.62 ± 0.06 | 2.63 ± 0.05 | 2.61 ± 0.05 | 2.64 ± 0.07 |
| Total porosity | % | 33.6 ± 2.03 | 36.0 ± 1.49 | 31.5 ± 0.89 | 33.4 ± 1.21 |
| Open-pore porosity | % | 16.8 ± 2.89 | 22.6 ± 1.60 | 20.3 ± 2.31 | 19.9 ± 6.48 |
| Compressive strength | MPa | 0.684 ± 0.23 | 0.663 ± 0.26 | 0.609 ± 0.18 | 0.270 ± 0.10 |
| Frost resistance | % | 4.45 | 4.60 | 4.82 | 8.32 |
| Water absorption | % | 9.63 ± 1.92 | 13.43 ± 1.43 | 11.35 ± 4.44 | 11.30 ± 3.80 |
| Grain shape index | % | 9.1 | 10.2 | 8.5 | 12.0 |
| Component, mg/kg dm | A | 200C | 300C | 400C |
|---|---|---|---|---|
| Total PAHs | <0.01 | 0.02 | 0.02 | <0.01 |
| BTEX | <0.01 | <0.01 | <0.01 | <0.01 |
| PCBs | <0.001 | <0.001 | <0.001 | <0.001 |
| TOC | 6460.0 | 6844.0 | 6620.0 | 6543.0 |
| C10–C40 hydrocarbons | 8.5 | 6.2 | 4.25 | 2.64 |
| As | <5 | <5 | <5 | <5 |
| Ba | 39.2 | 39.1 | 39.4 | 39.2 |
| Cd | 9.2 | 9.3 | 9.4 | 9.4 |
| Cr | 8.4 | 8.5 | 8.5 | 8.4 |
| Cu | 73.15 | 72.9 | 73.1 | 73.30 |
| Hg | 1.1 | 1.0 | 1.1 | 1.1 |
| Mo | <5 | <5 | <5 | <5 |
| Ni | 26.4 | 26.6 | 25.9 | 25.9 |
| Pb | 130.6 | 130.8 | 1307 | 130.5 |
| Sb | 40.6 | 40.8 | 40.5 | 40.7 |
| Se | <5 | <5 | <5 | <5 |
| Zn | 1116.8 | 1116.6 | 1117.1 | 1116.8 |
| Component, mg/kg dm | A | 200C | 300C | 400C |
|---|---|---|---|---|
| Ca | 14,552.0 | 14,084.3 | 13,184.2 | 15,988.7 |
| Mg | 43.0 | 41.7 | 49.4 | 50.1 |
| K | 6771.6 | 7339.4 | 7106.6 | 7871.5 |
| Na | 4770.1 | 5130.0 | 4953.3 | 5551.1 |
| Fl | 4.4 | 5.2 | 6.3 | 8.1 |
| Cl | 31,721.1 | 35,344.2 | 35,237.1 | 39,314.8 |
| SO4 | 11,816.4 | 9080.6 | 6932.9 | 12,050.9 |
| As | <0.01 | <0.01 | <0.01 | <0.01 |
| Ba | 3.2 | 3.8 | 4.12 | 4.55 |
| Cd | <0.01 | <0.01 | <0.01 | <0.01 |
| Cr | <0.05 | <0.05 | <0.05 | <0.05 |
| Cu | <0.05 | <0.05 | <0.05 | <0.05 |
| Hg | <0.001 | <0.001 | <0.001 | <0.001 |
| Mo | 0.26 | 0.24 | 0.22 | 0.30 |
| Ni | <0.01 | <0.01 | <0.01 | <0.01 |
| Pb | <0.01 | <0.01 | <0.01 | <0.01 |
| Sb | <0.001 | <0.001 | <0.001 | <0.001 |
| Se | <0.1 | <0.1 | <0.1 | <0.1 |
| Zn | 0.48 | 0.44 | 0.61 | 0.68 |
| TDS | 45,482.6 | 51,247.5 | 68,592.4 | 71,258.5 |
| DOC | 84.6 | 86.3 | 72.8 | 186.5 |
| Volatile Phenols | 0.3 | 0.34 | 0.28 | 0.25 |
| Component, mg/dm3 | A | 200C | 300C | 400C | Limit Values [88] |
|---|---|---|---|---|---|
| Cl | 3172.1 | 3534.4 | 3523.7 | 3931.5 | 1000.0 |
| SO4 | 1181.6 | 908.1 | 693.3 | 1205.1 | 500.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masłoń, A.; Cieśla, M.; Gruca-Rokosz, R.; Bichajło, L.; Nowotnik, A.; Pytel, M.; Gancarczyk, K.; Chutkowski, M.; Potoczek, M.; Franus, M.; et al. Lightweight Artificial Aggregates Produced from Water Reservoir Sediment and Industrial Waste—Ecological and Technological Aspect. Materials 2025, 18, 2563. https://doi.org/10.3390/ma18112563
Masłoń A, Cieśla M, Gruca-Rokosz R, Bichajło L, Nowotnik A, Pytel M, Gancarczyk K, Chutkowski M, Potoczek M, Franus M, et al. Lightweight Artificial Aggregates Produced from Water Reservoir Sediment and Industrial Waste—Ecological and Technological Aspect. Materials. 2025; 18(11):2563. https://doi.org/10.3390/ma18112563
Chicago/Turabian StyleMasłoń, Adam, Maksymilian Cieśla, Renata Gruca-Rokosz, Lesław Bichajło, Andrzej Nowotnik, Maciej Pytel, Kamil Gancarczyk, Marcin Chutkowski, Marek Potoczek, Małgorzata Franus, and et al. 2025. "Lightweight Artificial Aggregates Produced from Water Reservoir Sediment and Industrial Waste—Ecological and Technological Aspect" Materials 18, no. 11: 2563. https://doi.org/10.3390/ma18112563
APA StyleMasłoń, A., Cieśla, M., Gruca-Rokosz, R., Bichajło, L., Nowotnik, A., Pytel, M., Gancarczyk, K., Chutkowski, M., Potoczek, M., Franus, M., & Kalinowska-Wichrowska, K. (2025). Lightweight Artificial Aggregates Produced from Water Reservoir Sediment and Industrial Waste—Ecological and Technological Aspect. Materials, 18(11), 2563. https://doi.org/10.3390/ma18112563










