Abstract
This systematic review aims to further current knowledge on the effects of microplastics from orthodontic clear aligners, identifying potential implications for human health and providing a basis for further research and development of alternative materials. A literature search to find all peer-reviewed citations relevant to the review topic was conducted in the following databases: PubMed, Scopus, Web of Science, and Cochrane Library on 31 December 2024. A manual search of grey literature was also performed. There were 62 citations retrieved by the search query, and 11 were selected for inclusion in the review. Four selected studies were in vitro, while seven were in vitro following intraoral material aging studies. Ten studies evaluated the surface morphology of the material after aging, among the mechanical characteristics assessed, while only one article evaluated the chemical characteristics and size of the microplastic particles released from the aligners after simulated in vitro use. Discussion: From the evaluation of the studies included in this review, it is possible to state that there is a gradual increase over time in the surface roughness of the material, and modifications occurred in the morphology and surface topography of the aligners. Furthermore, it emerged that dispersion of microplastics occurs during the use of different types of aligners, with microplastic particle sizes ranging from 5 to 20 μm The findings suggest that clear aligners may cause microplastic dispersion in saliva during therapy, and this could cause a problem for the general health of patients, due to the absorption or ingestion of these released molecules. Further research is needed to fully understand the extent of microplastics released from aligners and to find alternative materials that can reduce this occurrence.
1. Introduction
The treatment of dental malocclusions has been revolutionized, especially with the introduction of clear aligner therapy, which offers an aesthetic and comfortable alternative to traditional metal braces [1,2].
The clear aligner therapy usually consists of a series of tight-fitting clear plastic trays that cover the dentition [3]. The patient must always wear these trays except when eating and brushing teeth, and the protocol of use is 22 h a day, from 7 or 14 consecutive days for each aligner [4].
The composition of the material used to manufacture a clear aligner (CA) is determined by the manufacturing process, which can be divided into two types: the traditional vacuum thermoforming method with thermoplastic molding on physical models, and direct 3D printing without the use of intermediate physical models [5].
Although there are numerous CA systems on the market today, the thermoplastic molding method is still the most widely used to produce commercial clear aligners and in-house production aligners [6]. Thermoformed aligners represent a well-established and reliable solution with relatively low production costs, although they offer lower precision and involve a more complex manufacturing process. In contrast, 3D-printed aligners provide higher precision, advanced customization options, and more efficient production, though they come with higher initial costs and require certified biocompatible materials [6].
Thermoplastic polymers are frequently used in the production of clear aligners. Polyethylene terephthalate (PET) and its non-crystallizing amorphous copolymer, polyethylene terephthalate glycol (PETG), are widely used in the commercial production of clear aligners because of their excellent mechanical and optical properties [1,6,7]. Thermoplastic polyurethane (TPU), a highly versatile polymer composed mainly of di- and tri-isocyanates and polyols, is another extremely versatile polymer that has many beneficial properties, such as superior mechanical and elastomeric qualities, chemical and abrasion resistance, adhesion properties, and ease of processing [1,6,7].
Tera Harz TC-85 (Graphy, Seoul, Republic of Korea), a photopolymerizable polyester-urethane polymer, is currently the only commercially available 3D-printable material that satisfies the requirements for the direct 3D printing technique [6].
Thermoplastic polymers used as CA material are biocompatible and must be free of substances that could cause harmful local or systemic reactions [8,9]. However, long-term use of these devices in the oral cavity raises questions regarding their safety and potential risks to health [10]. Clear aligner materials are subject to aging and can be stressed by cyclic masticatory or parafunctional stresses, changes in salivary flow and composition, changes in the oral microbiota, and changes in the temperature of the oral cavity; these conditions affect their mechanical and physical properties [11]. The thermoplastic material may degrade chemically and physically when exposed to the oral environment for a long time [12]. In general, aging reduces fit accuracy, transparency, and biomechanical performance, which can compromise orthodontic outcomes if not managed appropriately [11].
Considering that patients wear CAs for significantly prolonged periods of time, this degradation could promote the release of microplastic particles, potentially affecting oral health, or, once ingested or inhaled, they could accumulate in body tissues, potentially exposing patients to long-term adverse effects on overall health [13].
Microplastics are any synthetic solid particle or polymeric matrix, with regular or irregular shape and with size ranging from 1 μm to 5 mm, of either primary or secondary manufacturing origin, and which are insoluble in water [14].
Microplastics have been linked to several human health risks [15]. Recent studies indicate that these particles may be ingested or absorbed by oral tissues, potentially leading to a range of health risks and can inducing inflammatory responses and oxidative stress and possible DNA damage (genotoxicity) [16]. Some plastic additives or unreacted monomers may act as endocrine disruptors, interfering with hormonal balance, while long-term exposure could also impact the gut microbiota and immune system. Additionally, microplastics may carry adsorbed environmental toxins or heavy metals, contributing to bioaccumulation and increased toxic load in the body [15]. Such processes are linked to chronic diseases such as cardiovascular disease [17], respiratory disorders [18] and even some types of cancer [19]. In addition, the release of toxic chemicals from microplastics can exacerbate cellular damage by contributing to increased production of free radicals, unstable molecules that compromise cells in the human body, with potential impacts on cellular function and survival [20].
The effects of microplastics derived from orthodontic aligners are still being studied, and there are currently no specific guidelines to quantify safe exposure levels. However, evidence of inflammatory and toxic risks makes it urgent to better understand the effect of these particles on human health to inform both consumers and health professionals.
Based on these premises, the following research question for this systematic review was “Can clear aligners release microplastics that impact the patient’s overall health?” and the aim of the research was to deepen current knowledge on the release of microplastics from clear aligners, identifying potential implications for human health and providing a basis for further research and the development of alternative materials.
2. Materials and Methods
2.1. Protocol
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA-2020) criteria [21] were used in the formulation of this study. The protocol for this systematic review was registered on the INPLASY website (https://doi.org/10.37766/inplasy2025.5.0009) under the registration number INPLASY202550009, accessed on 5 May 2025.
2.2. Eligibility Criteria
Potentially relevant articles were selected based on these eligibility criteria: published in the period 2000–2024; written in the English language; abstract and full text available. The population, intervention, comparison, and outcome (PICO) approach was used to establish the inclusion criteria:
Population: All studies (in vivo or in vitro) investigating any thermoplastic CA material.
Intervention: Any type of orthodontic CA, any brand, material, and thickness, used or not used.
Comparison: A group of untreated patients used as a comparison group, or a control group compared to the experimental groups.
Outcome: Changes in the surface morphology of CA, release of microplastics, and adverse effects on the patient’s overall health.
Information sources, search strategy, and selection process:
A literature search to find all peer-reviewed papers relevant to the review topic was conducted in the following databases: PubMed, Scopus, Web of Science, and Cochrane Library on 31 December 2024. A manual search of grey literature was also performed.
The search strategy used for this systematic review was the keywords (“Clear aligner” OR “orthodontic aligner” OR “transparent aligners” OR “Invisalign”) AND (microplastic OR “micro plastic” OR nanoplastic OR nano plastic OR “surface morphology” OR “surface roughness”). The studies that were accessible in English were selected.
Based on the eligibility criteria applied, the search was performed by two researchers (D.A.A. and H.M.) who independently assessed the titles and abstracts of the retrieved articles. Subsequently, articles potentially satisfying the inclusion criteria for the review were retrieved in full text. Finally, a consensus was reached among the two reviewers (D.A.A. and H.M.) to include/exclude articles from the review.
2.3. Data Items and Collection Process
To identify the list of variables to be extracted, two researchers (D.A.A. and H.M.) collaborated to execute data extraction. The following data were extracted and organized in Microsoft Excel spreadsheets: title, authors, publication year, study design, sample size, observation period, outcome measures, assessment method, and results. The authors then conducted a consensus analysis of these findings so that they could be discussed in this review.
Research designs and outcomes were used to categorize the studies. Text and tables were used to analyze and report the data, providing an accurate narrative-style descriptive overview.
2.4. Risk of Bias Assessment
As clinical trials were not included in the review, a quality evaluation, such as CASP (Critical Appraisal Skills Program) or a similar tool, could not be conducted. The CONSORT (Consolidated Standards of Reporting Trials) checklist adapted to in vitro studies of dental materials by Faggion et al. [22] was used to assess the quality of the articles.
The authors of the present systematic review used this checklist for the evaluation of the quality of the in vitro studies; items from 5 to 9 were eliminated from the checklist due to the selected studies being in vitro, so there is a low risk of bias and no need for randomization, and the sample size is not so important (Table 1).

Table 1.
Modified consort checklist for in vitro studies comparing different dental implant impression techniques.
3. Results
3.1. Study Selection
There were 62 citations retrieved by the search query. Following the removal of 28 duplicates and 8 articles that were clearly irrelevant based on their titles and abstracts, the complete texts of the 25 remaining articles were acquired. Fifteen were excluded for not being able to meet the inclusion criteria, and eleven were selected for inclusion in the review (Figure 1).
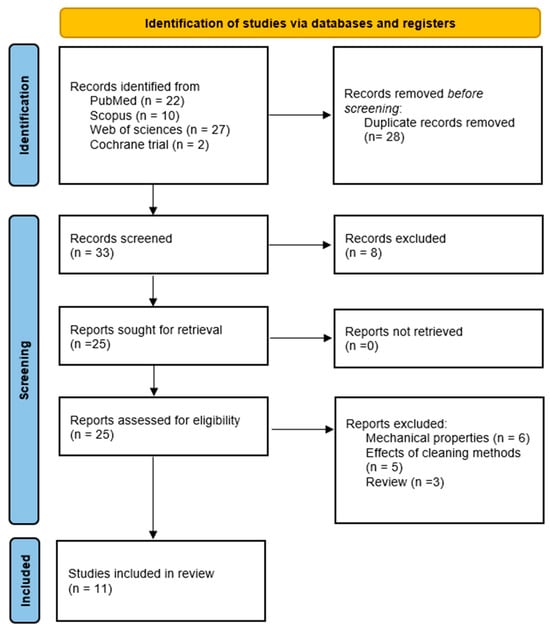
Figure 1.
PRISMA flow diagram of the study selection procedure.
3.2. Study Characteristics
Four selected studies were in vitro [23,24,25,26], while seven were in vitro following intra-oral material aging studies [27,28,29,30,31,32,33]. The included studies were mostly published within the last five years: four in 2024, three in 2023, one in 2020, and one in 2019—the oldest article being from 2004—and were conducted in several countries. The descriptive characteristics of the included studies are outlined in Table 2.

Table 2.
Descriptive characteristics of the included studies.
Ten studies evaluated the surface morphology of the material after aging, among the mechanical characteristics assessed, while only one article evaluated the chemical characteristics and size of the microplastic particles released from the aligners after simulated in vitro use [26].
3.3. Risk of Bias in Studies
The results of the quality assessment are summarized in Table 3. According to the modified CONSORT checklist used, three articles obtained a score of 80%; six articles obtained a score of 90%, and the rest 100%. All 11 studies were included in the present review because they obtained a score of 80% or more.

Table 3.
Results of the quality assessment of selected articles using the modified consort checklist for in vitro studies comparing different dental implant impression techniques.
3.4. Strategy of Data Synthesis
The narrative synthesis of data from the included studies will concentrate on two primary outcomes: (1) changes to the clear aligners’ surfaces that may indicate material degradation, and (2) the measurement of microplastic release during clinical usage. Methodologies, detection strategies, and published results will be compared using a structured thematic analysis with the goal of identifying recurring themes, methodological variations, and evidence gaps pertaining to the clinical behavior and environmental effects of aligners. Due to heterogeneity in study designs, detection methods, and outcome reporting, a meta-analysis was not feasible.
3.5. Results of Individual Studies
The analysis of individual results was divided according to the study objective into aligner surface roughness and the release of microplastics after use.
3.5.1. Roughness Surface
In Vitro Study
Porojan et al. [25], Bhate et al. [23], and Mei et al. [24] performed in vitro studies to evaluate the variation of the surface of aligners in terms of surface roughness.
Poroijan et al. [25] used artificial saliva to simulate a 14-day aging process. The authors compared four types of PETG clear thermoplastic materials (Leone, Crystal, Erkodur, and Duran). The samples were submerged in artificial saliva with three different pH values: neutral (6.7), basic (8.3), and acid (4.3). The surface roughness was determined using a contact profilometer, and nanoroughness measurements were generated by three-dimensional profiles using an atomic force microscope (AFM). In terms of roughness, their findings indicate that following immersion and desiccation, the surfaces tended to be smoother on a microscale and more irregular on a nanoscale.
Bhate et al. [23] used distilled water to simulate a 14-day aging process by comparing clear aligners of PET-G (Duran material) and of TPU (Zendura material). Three distinct time points were used to measure the samples of the two groups: T0 was before thermoforming, T1 was after thermoforming, and T2 was after thermoforming and aging. Surface roughness was tested using a surface profilometer. According to their findings, the process of simulated intraoral aging affected the surface roughness in the TPU aligner.
Mei et al. [24] evaluated the biomechanical behavior of TPU aligners (Invisalign) by simulating aging in distilled water for 21 days. The biomechanical properties (flexural strength, translucency, surface roughness, hardness, and tensile strength) of the clear aligners were assessed each day. The surface roughness was measured using a profilometer. After seven and ten days, respectively, the clear aligners’ flexural strength and hardness dramatically declined. Throughout the 21 days of artificial aging, there was minimal variation in surface roughness, translucency, and tensile strength.
In Vitro Study Following Intra-Oral Material Aging
Eslami et al. [27] and Koletsi et al. [30] investigated the effects of 7 days of intraoral use on surface roughness in directly printed aligners (DPA) of resin (photopolymerizable polyester–urethane polymer) (Graphy, Seoul, Republic of Korea) and TPU (Invisalign).
Eslami et al. [27] used confocal laser scanning microscopy to investigate the effects of a week of intraoral use on the surface roughness characteristics of DPA and commercially manufactured Invisalign aligners compared to their unused control counterparts. The surface roughness parameters were examined using optical profilometry by Koletsi et al. [30]. According to both studies, directly printed aligners have a higher surface roughness increase than Invisalign.
Schuster et al. [32], Gracco et al. [29], Papadopoulou et al. [31], Fang et al. [28], and Lira et al. [33] evaluated TPU (Invisalign) aligners after intraoral use for 14 days. To observe the change in surface morphology, Schuster et al. [32] and Lira et al. [33] used an optical microscope (OM); Gracco et al. [29] and Fang et al. [28] used a scanning electron microscopy; and in the study of Papadopoulou et al. [31], an optical profilometer was used. According to these studies, Invisalign aligners retrieved from patients after the intraoral use for 14 days showed surface morphological changes in comparison to brand-new ones. The surface morphology of the used aligners showed microcracks, distortions, abrasion, and grooves. This decline occurred within the first week of clinical use and is not time-dependent.
Microplastic Release
Quinzi et al. [26] evaluated the potential release of microplastics from different PET-G aligners (Alleo; FlexiLigner; Lineo; Arc Angel, and Ortobel Aligner) and in TPU aligner (F22 Aligner; Invisalign) after 7 days of simulated aging process in artificial saliva and mechanical friction. The artificial saliva was filtered after seven days, and the filters were studied using Raman Microspectroscopy (RMS) and Scanning Electron Microscopy (SEM), respectively, to chemically characterize the polymeric matrix and to determine the size and quantity of the microplastics found. The authors found that all aligners studied released microplastics with a size range of 5 to 20 μm, with the highest number of microplastics in Arc Angel and the lowest in Invisalign. Microparticles with a diameter of 5–20 μm were found in all tested aligners and represented the largest group, with a percentage higher than 50%, except for F22 and Lineo (36% and 46%, respectively). A percentage range of 30–50% was detected for microplastics > 20 μm in all type of aligners, while microplastics < 5 μm were detected only in Alleo (17%), F22 (18%), and Invisalign (14%).
4. Discussion
This systematic review aimed to investigate the propensity of polymers used to produce clear aligners to fragment into microplastics during clinical intraoral use. The literature in this regard shows that during intraoral use, aligners suffer variations in their surfaces in the form of roughness, abrasion, microfractures, and grooves that are compatible with the release of material into the oral cavity in the form of microplastics.
Given the increasing use of clear aligners in orthodontic therapies in patients of all ages, it is becoming very interesting to investigate whether the release of microplastic material from these medical devices could be a risk to patients’ health.
The different thermoplastic materials used in the manufacture of clear aligners, which are mainly polymers of the thermoplastic polyurethane (TPU) and polyethylene terephthalate glycol (PET-G) types, have been described in the literature. However, different aligner companies use proprietary materials in the manufacture of aligners, so their exact composition is unknown [5]. The choice of thermoplastic polymer type depends on the material characteristics and properties that may affect the clinical performance of clear aligners during orthodontic treatment [1,8].
The protocol for using the aligners involves a use of 22 h a day from 7 to 14 consecutive days for each aligner [6]. Using aligners during all these hours inevitably leads to frequent contact between the upper and lower aligners, which results in wear or abrasion of the surfaces [34].
The studies included in this systematic review analyzed the changes in the surfaces of aligners after wear at 7 and 14 days, made of PETG, TPU, and DPA. The results show that after clinical use, there are surface changes as early as seven days in all types of aligners from different commercial companies, although Invisalign remains the most studied. Factors that can influence the modification of the surfaces of the aligners, in addition to the time of use—such as the type of diet, the oral cavity hygiene habits, the method of cleaning the aligners, and the influence of bad habits or behaviors such as bruxism—were not analyzed. However, these results suggest that if the surface of the aligner changes, it may release microparticles of material into the oral cavity.
Only Quinzi et al. [26] evaluated in vitro the effective dispersion of secondary microplastics from aligners of different commercial houses, made of PETG and TPU, subjected for 7 days to artificial chemicals and mechanical friction. Their results show that there is a release of microplastics of different sizes in a range between 5 to 20 μm, with different distributions depending on the material. Since Quinzi et al. [26] conducted a single 7-day assessment, it is not possible to quantify the amount of microplastics released day by day, nor the rate of release after day 7. New studies are needed to make these assessments.
Although studies on microplastics in the human body are limited, it has been shown in the literature that microplastics, when ingested, can cause damage to the digestive system, and that these particles can undergo structural changes by the microbiota of the intestinal system, also demonstrating an interaction with the system [35]. Before reaching the epithelium of the intestine, which is considered the main site of nutrient absorption, orally ingested plastic particles could pass through the epithelium of the stomach [36].
The size of microplastics is a determining factor in microplastics crossing the intestinal barrier. The larger ones remain in the intestinal lumen and are excreted via stool [37], but can cause disorders of the intestinal flora [38]. Smaller microplastics, up to a maximum size of 5–10 mm, can cross the intestinal barrier by endocytosis or by diffusion [13]. Microplastics can remain deposited in intestinal cells and cause inflammation and stress [13,38]. Only much smaller particles, up to 1.5 mm, could be distributed systemically through the bloodstream and potentially accumulate in various organs [37]. The presence of microplastics has been reported in the liver and has been associated with liver toxicity, and in the human spleen, but it is currently unclear whether microplastics can cause splenic dysfunction [39]. Particles circulating in the bloodstream can accumulate in the large arteries in the form of atheromatous plaques that correlate with cardiovascular events [17]. Microplastics can be considered an emerging risk factor for cardiovascular accidents, stroke, and death [40]. In addition to human organs, microplastics have been found in other human biological samples such as saliva, sputum, stool, urine, and breast milk; the presence of microplastics in these samples is due to passageways, storage, or excretion [16,37].
Although studies have demonstrated that microplastics are common in breast milk, the discovery of microplastics in meconium and infants’ stool raises additional concerns because, since they have been identified in the placenta, it is thought that they may be able to pass through the placental barrier, enter the fetal bloodstream, eventually make their way to the fetal intestine, and then be expelled in meconium [16,37].
Another aspect is the type of polymer that is most frequently deposited in tissues and detected in biological samples. The characteristics of microplastics— shape, size, color, and type of polymer—have been picked up by Roslan et al. [16] in their systematic review. They suggest that polypropylene and polyethylene microplastics can accumulate in various human tissues, but are most abundant in the digestive tract, placenta, and lungs. PET microplastics have been detected in blood vessels, heart, liver, spleen, lung tissue, placenta, breast milk, meconium, and newborn feces, while TPU has been reported in pulmonary tissue, placenta, breast milk, meconium, newborn feces, and stool.
The microparticles released by transparent aligners processed in PETG and TPU, according to the study by Quinzi et al. [26], have a size between 3 and 95 μm, with a higher percentage between 5 and 20 μm. Particles of this size could, as described above, cross the intestinal barrier and deposit in various organs, with a potential risk to patients’ health.
Among the limitations of this systematic review is that the potential release of microplastics during clinical use of clear aligners is only supported by in vitro studies, which do not consider all the real-world conditions to which clear aligners are subjected during intraoral use. Another limitation is represented by the fact that most of the studies included in the review are based on the evaluation of aligners from a few commercial houses, particularly Invisalign, but nowadays, many commercial companies on the market produce aligners, and there are also homemade aligners with non-standardized procedures. It should also be considered that many commercial houses use proprietary material to produce aligners, so there is no absolute knowledge of the type of polymer used.
Considering the limitations reported and the quality of the studies included, this review has clinical implications regarding the possible correlation between exposure to aligner microplastics and the health risk to the patient wearing them. The results suggest that the use of aligners should be reduced in pregnant and breastfeeding women. The protocols of aligner use must be reviewed, in light of the results obtained, to reduce the time of use of each aligner, to limit as much as possible the exposure to microplastics.
Based on these findings, further research is needed on the actual release of plastic microparticles from clear aligners. Although new studies should be conducted to evaluate other possible factors that may affect surface modification and subsequent release of material during clinical use of aligners.
It would also be interesting to assess whether different usage protocols can vary the potential release of microparticles and, to the same extent, to study whether new manufacturing materials can reduce the potential risk of microplastic release in the oral cavity.
5. Conclusions
The findings suggest that clear aligners may cause microplastic dispersion in saliva during therapy, and this could cause a problem for the general health of patients, due to the absorption or ingestion of these released molecules. Further research is needed to fully understand the extent of microplastics released from aligners and to find alternative materials that can reduce this occurrence.
Author Contributions
Conceptualization, M.H. and A.A.D.S.; methodology, A.A.D.S.; software, M.H.; validation, G.G.; formal analysis, A.A.D.S.; investigation, M.H.; resources, A.A.D.S.; data curation, G.G.; writing—original draft preparation, A.A.D.S. and M.H.; writing—review and editing, A.A.D.S. and M.H.; visualization, G.G.; supervision, G.G.; project administration, G.G; funding acquisition, G.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Putrino, A.; Barbato, E.; Galluccio, G. Clear aligners: Between evolution and efficiency—A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 2870. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, G.; De Stefano, A.A.; Horodynski, M.; Impellizzeri, A.; Guarnieri, R.; Barbato, E.; Di Carlo, S.; De Angelis, F. Efficacy and Accuracy of Maxillary Arch Expansion with Clear Aligner Treatment. Int. J. Environ. Res. Public Health 2023, 20, 4634. [Google Scholar] [CrossRef] [PubMed]
- Kau, C.H.; Soh, J.; Christou, T.; Mangal, A. Orthodontic Aligners: Current Perspectives for the Modern Orthodontic Office. Medicina 2023, 59, 1773. [Google Scholar] [CrossRef]
- Alwafi, A.; Bichu, Y.M.; Avanessian, A.; Adel, S.M.; Vaid, N.R.; Zou, B. Overview of systematic reviews and meta-analyses assessing the predictability and clinical effectiveness of clear aligner therapy. Dent. Rev. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Alwafi, A.; Liu, X.; Andrews, J.; Ludwig, B.; Bichu, A.Y.; Zou, B. Advances in orthodontic clear aligner materials. Bioact. Mater. 2022, 22, 384–403. [Google Scholar] [CrossRef]
- Hartshorne, J.; Brian Wertheimer Bellville, M.; Africa, S. Emerging insights and new developments in clear aligner therapy: A review of the literature. AJO-DO Clin. Companion 2022, 2, 311–324. [Google Scholar] [CrossRef]
- Gold, B.P.; Siva, S.; Duraisamy, S.; Idaayath, A.; Kannan, R. Properties of Orthodontic Clear Aligner Materials—A Review. J. Evol. Med. Dent. Sci. 2021, 10, 3288–3294. [Google Scholar] [CrossRef]
- Macrì, M.; Murmura, G.; Varvara, G.; Traini, T.; Festa, F. Clinical Performances and Biological Features of Clear Aligners Materials in Orthodontics. Front. Mater. 2022, 9, 819121. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef]
- Martina, S.; Rongo, R.; Bucci, R.; Razionale, A.V.; Valletta, R.; D’Antò, V. In vitro cytotoxicity of different thermoplastic materials for clear aligners. Angle Orthod. 2019, 89, 942–945. [Google Scholar] [CrossRef]
- Bucci, R.; Rongo, R.; Levatè, C.; Michelotti, A.; Barone, S.; Razionale, A.V.; D’antò, V. Thickness of orthodontic clear aligners after thermoforming and after 10 days of intraoral exposure: A prospective clinical study. Prog. Orthod. 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.; J, M.; George, S.A. Bisphenol-A release from thermoplastic clear aligner materials: A systematic review. J. Orthod. 2023, 50, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.B.; Stock, V.; Cara-Carmona, J.; Lisicki, E.; Shopova, S.; Fessard, V.; Braeuning, A.; Sieg, H.; Böhmert, L. Micro- and nanoplastics—Current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020, 2, 4350–4367. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Wu, P.; Lin, S.; Cao, G.; Wu, J.; Jin, H.; Wang, C.; Wong, M.H.; Yang, Z.; Cai, Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard. Mater. 2022, 437, 129361. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Anuar, S.T.; Yusof, K.M.K.K.; Lai, L.A.; Brentnall, T. Detection of microplastics in human tissues and organs: A scoping review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Uogintė, I.; Vailionytė, A.; Skapas, M.; Bolanos, D.; Bagurskienė, E.; Gruslys, V.; Aldonytė, R.; Byčenkienė, S. New evidence of the presence of micro- and nanoplastic particles in bronchioalveolar lavage samples of clinical trial subjects. Heliyon 2023, 9, e19665. [Google Scholar] [CrossRef]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro(nano)plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Yang, Z.; DeLoid, G.M.; Zarbl, H.; Baw, J.; Demokritou, P. Micro- and nanoplastics (MNPs) and their potential toxicological outcomes: State of science, knowledge gaps and research needs. NanoImpact 2023, 32, 100481. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. he PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract 2012, 12, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bhate, M.; Nagesh, S. Assessment of the Effect of Thermoforming Process and Simulated Aging on the Mechanical Properties of Clear Aligner Material. Cureus 2024, 16, e64933. [Google Scholar] [CrossRef]
- Mei, L.; Jin, C.; Na, A.; Marra, J.; Guan, S.; Choi, J. Biomechanical aging behaviour of clear aligners. Australas. Orthod. J. 2024, 40, 60–66. [Google Scholar] [CrossRef]
- Porojan, L.; Toma, F.R.; Gherban, M.I.; Vasiliu, R.D.; Matichescu, A. Surface Topography of Thermoplastic Appliance Materials Related to Sorption and Solubility in Artificial Saliva. Biomimetics 2024, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Quinzi, V.; Orilisi, G.; Vitiello, F.; Notarstefano, V.; Marzo, G.; Orsini, G. A spectroscopic study on orthodontic aligners: First evidence of secondary microplastic detachment after seven days of artificial saliva exposure. Sci. Total. Environ. 2023, 866, 161356. [Google Scholar] [CrossRef]
- Eslami, S.; Kopp, S.; Goteni, M.; Dahmer, I.; Sayahpour, B. Alterations in the surface roughness and porosity parameters of directly printed and Invisalign aligners after 1 week of intraoral usage: An in vivo prospective investigation. Am. J. Orthod. Dentofac. Orthop. 2024, 165, 73–79. [Google Scholar] [CrossRef]
- Fang, D.; Li, F.; Zhang, Y.; Bai, Y.; Wu, B.M. Changes in mechanical properties, surface morphology, structure, and composition of Invisalign material in the oral environment. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 745–753. [Google Scholar] [CrossRef]
- Gracco, A.; Mazzoli, A.; Favoni, O.; Conti, C.; Ferraris, P.; Tosi, G.; Guarneri, M.P. Short-term chemical and physical changes in Invisalign appliances. Australas. Orthod. J. 2009, 25, 34–40. [Google Scholar] [CrossRef]
- Koletsi, D.; Panayi, N.; Laspos, C.; E Athanasiou, A.; Zinelis, S.; Eliades, T. In vivo aging-induced surface roughness alterations of Invisalign and 3D-printed aligners. J. Orthod. 2023, 50, 352–360. [Google Scholar] [CrossRef]
- Papadopoulou, A.K.; Cantele, A.; Polychronis, G.; Zinelis, S.; Eliades, T. Changes in roughness and mechanical properties of invisalign appliances after one- and two-weeks use. Materials 2019, 12, 2406. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Eliades, G.; Zinelis, S.; Eliades, T.; Bradley, T.G. Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 725–728. [Google Scholar] [CrossRef]
- Lira, L.F.; Otero Amaral Vargas, E.; Moreira da Silva, E.; Nunes da Silva Meirelles Dória Maia, J.; Elzubair, A.; Siqueira de Morais, L.; Alvaro de Souza Camargo, S., Jr.; Serra, G.; Gomes de Souza, M.M. Effect of oral exposure on chemical, physical, mechanical, and morphologic properties of clear orthodontic aligners. Am. J. Orthod. Dentofac. Orthop. 2023, 164, e51–e63. [Google Scholar] [CrossRef] [PubMed]
- Bakdach, W.M.M.; Haiba, M.; Hadad, R. Changes in surface morphology, chemical and mechanical properties of clear aligners during intraoral usage: A systematic review and meta-analysis. Int. Orthod. 2022, 20, 100610. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. Vitr. 2016, 31, 126–136. [Google Scholar] [CrossRef]
- Basri, K.S.; Daud, A.; Astuti, R.D.P.; Basri, K. Detection of exposure to microplastics in humans: A systematic review. Sci. Found. Spiroski 2021, 9, 275–280. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total. Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Duggal, B.; Kumar, G. Cardiotoxicity of Microplastics: An Emerging Cardiovascular Risk Factor. Curr. Cardiol. Rev. 2025, 21, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).